Abstract
Microbial fuel cells (MFCs) are a technology that can be applied to both the wastewater treatment and bioenergy generation. This work discusses the contribution of improvements regarding the configurations, electrode materials, membrane materials, electron transfer mechanisms, and materials cost on the current and future development of MFCs. Analysis of the most recent scientific publications on the field denotes that dual-chamber MFCs configuration offers the greatest potential due to the excellent ability to be adapted to different operating environments. Carbon-based materials show the best performance, biocompatibility of carbon-brush anode favors the formation of the biofilm in a mixed consortium and in wastewater as a substrate resembles the conditions of real scenarios. Carbon-cloth cathode modified with nanotechnology favors the conductive properties of the electrode. Ceramic clay membranes emerge as an interesting low-cost membrane with a proton conductivity of 0.0817 S cm−1, close to that obtained with the Nafion membrane. The use of nanotechnology in the electrodes also enhances electron transfer in MFCs. It increases the active sites at the anode and improves the interface with microorganisms. At the cathode, it favors its catalytic properties and the oxygen reduction reaction. These features together favor MFCs performance through energy production and substrate degradation with values above 2.0 W m−2 and 90% respectively. All the recent advances in MFCs are gradually contributing to enable technological alternatives that, in addition to wastewater treatment, generate energy in a sustainable manner. It is important to continue the research efforts worldwide to make MFCs an available and affordable technology for industry and society.
Keywords: Anode, Cathode, Membranes, Electron transfer mechanism, Materials cost, Microbial fuel cell
Anode; Cathode; Membranes; Electron transfer mechanism; Materials cost; Microbial fuel cell
1. Introduction
Water and energy have a close connection. Water is required for all sources of energy production, including the electrical energy production, and energy is necessary for the disposal of water and the treatment of wastewater [1]. Water is an essential natural resource for humans and all life in the earth. It is an important component in ecosystems health, food production, socio-economic progress, and energy production. Water and sanitation systems must work together to ensure the human health and development [2].
However, according to The United Nations and Water Security and Sustainable Management Report 2020 [3] data, 1.8 million people lack of safe managed sanitation services. Moreover, more than 80 percent of wastewater returns to ecosystems without being treated. This wastewater discharged without any treatment generates negative effects on human health, natural environment, and global economics, in both, local population and far-away population from the pollution source. According to the World Health Organization [4], it is estimated that in middle-and-low development countries, 842,000 of annual deaths are related to wastewater and sanitation. From the environmental point of view, untreated or partially treated wastewater generates contamination of surface water, soil and groundwater. When it is discharged into natural water bodies such as lakes and rivers, this water can infiltrate into aquifers and deteriorate the quality of fresh water. Also, the untreated wastewater that reaches the oceans contributes increasing the number of de-oxygenated dead zones. The marine ecosystem damage is estimated to reach an area of 245,000 . This has a direct impact on the economy of the fishing industry, livelihoods, and food chains. Therefore, poor water quality interferes with economic development [5].
On the other hand, access to energy is key to social development. According to the British Petroleum Statistical Review of World Energy data from 2020 [6], the global energy consumption grew at a rate of 1.3 percent. This growth was less than the growth reported in 2018 with 2.8 percent. Although the energy production was led by natural gas and renewable sources, global carbon dioxide emissions keep growing. While the growth rate 0.5 percent in 2019 was less than the annual average of 1.1 percent reported since 2010, it is still imperative to try to curb its growth. Notice that, is the most abundant greenhouse gas that is mainly generated by burning fossil fuel and it is directly related to the global temperature and sea-level rise, sudden weather changes, and other adverse effects of unprecedented scale [7]. The world population continues to increase, and fossil fuels are being over-exploited faster than new sources are being discovered. It is important to develop green energies to reduce the negative effects of fossil fuel [8].
Therefore, wastewater treatment and alternative energy production is of main concern worldwide, as a society. Treatment of wastewater not only reduces pollutants from the water, but also enables the reuse of water [9]. A significant fraction of the world's energy demand can be obtained from wastewater, which contains an average chemical energy of 1.9 kWh m−3 stored as organic compounds, as long as it is converted into useful and economic energy [10], [11], [12], [13]. It has been estimated that domestic wastewater approximately contains 9 times the amount of energy that is used to treat it [14]. Additionally, the development of renewable energy sources contributes to the reduction of greenhouse gas emissions and their associated negative effects. All this is reflected in positive effects on health, society, economics, and natural environment.
An emerging technology that has aroused great interest among the scientific community due to its great potential to treat wastewater and generate bioenergy, are the microbial fuel cells (MFCs). These devices use bacteria as a catalyst to oxidize organic and inorganic matter and generate electrical current. Bacteria degrade the substrate contained in the wastewater, generate protons, and release electrons and carbon dioxide. Typical configuration of MFCs consists of an anode chamber, a cathode chamber, a membrane between the chambers, and an external electrical circuit. The released electrons flow from the anode (negative terminal) to the cathode (positive terminal), through a conductive material [15]. The electrons, protons, and oxygen present in the cathode (conventional configuration) react to form water [16].
In recent years, there have been important advances in MFCs research. Some studies have reported applications of MFCs at pilot-scales and field-scales (30 L [17], 200 L [18], 225 L [19], 1,000 L [20]) that allow to visualize the potential of MFCs in more realistic scenarios and the importance of further work on scaling up. The aim of this study is to review and analyze the most recent scientific publications on MFCs technology. Particular emphasis is done on the analysis of the configurations, anode, cathode and membrane materials, as well as the mechanisms for electron transfer and their influence on MFC performance. In addition, the study discusses the strategies implemented to improve those elements, and the effect they have on the ability of MFCs to treat wastewater and generate bioenergy. Furthermore, a cost analysis of the main MFCs materials (anode, cathode and membrane) is conducted. Finally, the areas with the greatest potential to promote the development of MFCs effectively and economically are identified.
2. Trends of microbial fuel cell research
In 1911 Michael C. Potter [21] made the first observations that relate the electrical energy with the metabolic activity of bacteria. In 1962 Davis and Yarbrough Jr. [22] carried out experiments on a microbial fuel cell using a hydrocarbon as food to generate electrical energy. Then in 1983 in an effort to construct a better MFCs, Bennetto, et al. [23] evaluated a microbial fuel cell and perform potentiometric and amperometric measurements. They used an MFC with glucose as a substrate, and microorganisms Escherichia coli or yeast in the anode, and investigated the catalytic effects of thionine and resorufin as a redox mediator. Subsequent MFCs investigations were done with the use of chemical mediators or electron shuttles. It was not until 1999, that MFC investigations began to be conducted without the use of mediators.
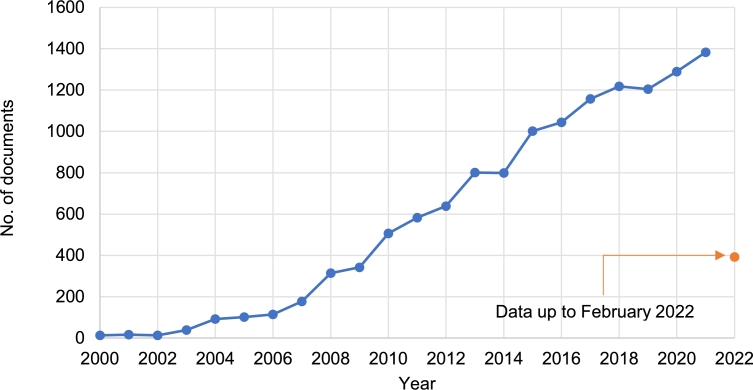
As Kim, et al. [25] did in their work in which they evaluated an MFC type biosensor for lactate using a metal-reducing bacterium without an electrochemical mediator. Then it was recognized that the mediators were not required and consequently increased the interest in MFCs [26]. In the following years, the interest in MFCs continue to rise. Fig. 1 shows the trend in the number of publications regarding MFCs. It is observed that between 2000 and 2005, published MFCs research did not exceed 100 articles per year, and the trend remained upward until 2007 with 177 publications. This slightly increase could be attributed to the disclosure of the feasibility of working MFCs without the use of chemical mediators. After that, a tremendous increase is observed in the following 17 years, in 2021 with 1,383 and in the first two months of 2022 with 392 published articles [24]. This confirms that the research and technology have captured the attention of the scientific community, due to the capability of the MFCs to transform organic waste into electricity [27].
Figure 1.
Publishing trend of MFCs scientific research reports (Data from Scopus Elsevier). [24].
The growing participation of the scientific community in the development of MFCs is also reflected in the increase of MFCs review articles. Table 1 shows a summary of the main subjects discussed in 100 review articles, published between 2015 and 2022.
Table 1.
Main subjects discussed in MFC review articles between 2015 to 2022.
| Anode | Cathode | Membrane | Cost | Electron Acceptor | Electron Transfer | Biofouling | Configuration | Microorganisms | Hydrogen | Substrate | Models | Catalyst | Nanomaterials | Applications | Scaling up | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ■ | [28], [29], [30], [31], [32] | |||||||||||||||
| ■ | ■ | ■ | ■ | [33] | ||||||||||||
| ■ | ■ | ■ | [34] | |||||||||||||
| ■ | ■ | [35], [36], [37] | ||||||||||||||
| ■ | [38], [39], [40], [41], [42] | |||||||||||||||
| ■ | [43], [44] | |||||||||||||||
| ■ | [45], [46], [47], [48], [49] | |||||||||||||||
| ■ | [50], [51], [52], [53], [54], [55], [56] | |||||||||||||||
| ■ | [57], [58] | |||||||||||||||
| ■ | [59] | |||||||||||||||
| ■ | [60], [61], [62] | |||||||||||||||
| ■ | ■ | ■ | ■ | [63] | ||||||||||||
| ■ | ■ | [64] | ||||||||||||||
| ■ | ■ | [65] | ||||||||||||||
| ■ | ■ | ■ | ■ | [66] | ||||||||||||
| ■ | ■ | ■ | [67] | |||||||||||||
| ■ | ■ | ■ | [68] | |||||||||||||
| ■ | ■ | [69] | ||||||||||||||
| ■ | [70] | |||||||||||||||
| ■ | [71], [72] | |||||||||||||||
| ■ | ■ | [73], [74] | ||||||||||||||
| ■ | ■ | [75], [76] | ||||||||||||||
| ■ | [77] | |||||||||||||||
| ■ | ■ | ■ | [78] | |||||||||||||
| ■ | ■ | ■ | [79] | |||||||||||||
| ■ | ■ | ■ | ■ | [80], [81] | ||||||||||||
| ■ | ■ | [82] | ||||||||||||||
| ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | [83] | ||||||||
| ■ | ■ | ■ | [84] | |||||||||||||
| ■ | ■ | ■ | [85] | |||||||||||||
| ■ | ■ | [86] | ||||||||||||||
| ■ | ■ | [87] | ||||||||||||||
| ■ | ■ | ■ | ■ | [88] | ||||||||||||
| ■ | ■ | ■ | [89], [90] | |||||||||||||
| ■ | ■ | ■ | ■ | [91] | ||||||||||||
| ■ | ■ | [92], [93] | ||||||||||||||
| ■ | [94] | |||||||||||||||
| ■ | [95] | |||||||||||||||
| ■ | ■ | ■ | [96] | |||||||||||||
| ■ | ■ | ■ | ■ | [97] | ||||||||||||
| ■ | ■ | ■ | ■ | ■ | ■ | ■ | [98] | |||||||||
| ■ | ■ | ■ | ■ | ■ | ■ | [99] | ||||||||||
| ■ | ■ | ■ | ■ | [100] | ||||||||||||
| ■ | ■ | ■ | ■ | [101] | ||||||||||||
| ■ | ■ | ■ | ■ | ■ | ■ | ■ | [102] | |||||||||
| ■ | ■ | ■ | ■ | ■ | [103], [104] | |||||||||||
| ■ | ■ | ■ | ■ | ■ | [105] | |||||||||||
| ■ | ■ | [106] | ||||||||||||||
| ■ | ■ | ■ | ■ | ■ | ■ | [107], [108] | ||||||||||
| ■ | ■ | ■ | ■ | ■ | ■ | [109] | ||||||||||
| ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | [110] | ||||||||
| ■ | ■ | ■ | [111] | |||||||||||||
| ■ | ■ | ■ | ■ | ■ | [112] | |||||||||||
| ■ | ■ | ■ | ■ | [113] | ||||||||||||
| ■ | ■ | ■ | ■ | [114] | ||||||||||||
| ■ | ■ | ■ | [115] | |||||||||||||
| ■ | [116] | |||||||||||||||
| ■ | ■ | ■ | ■ | [117] | ||||||||||||
| ■ | [118] | |||||||||||||||
| ■ | ■ | ■ | [27] | |||||||||||||
| ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | [119] | ||||||
| ■ | ■ | ■ | ■ | ■ | [120] | |||||||||||
| ■ | ■ | ■ | [121] | |||||||||||||
| ■ | ■ | ■ | ■ | ■ | ■ | [122] | ||||||||||
| ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | [123] | ||||||||
| ■ | ■ | ■ | [124] | |||||||||||||
| ■ | ■ | ■ | ■ | ■ | ■ | [125] | ||||||||||
| 37% | 41% | 21% | 8% | 23% | 9% | 3% | 31% | 27% | 9% | 12% | 3% | 8% | 9% | 22% | 14% | Discussion frequency |
In recent years, the number of review articles has increased, which is associated with the impact generated by COVID-19 worldwide. In this period, access to research centers may have been scarce or nonexistent due to health restrictions. Therefore, literature review emerged as a safest option to work during this time. In Table 1 the subjects with the highest and lowest frequency of discussion are clearly and precisely identified. On one hand, there are commonly reviewed subjects such as those related to the anode, the cathode and the MFCs configurations, with frequencies of 38%, 44% and 31%, respectively. These high percentages are attributed to the fact that electrodes and configurations are essential in the construction of MFCs and any slight modification on these elements affects the MFCs performance. On the other hand, some of the subjects with less discussion are related to costs, electron acceptor and biofouling, with frequencies of 9%, 9% and 2%, respectively. These subjects can be considered as indirect MFCs factors; however, its low discussion frequency does not mean less relevance in the MFCs performance. On the contrary, these are research areas that could contribute importantly on the MFCs performance. Analyzing the frequency of topics discussed in reports included in Table 1, as well as their direct link with MFCs performance, the issues discussed in this work were selected. Thus, even though MFCs configurations, anode, cathode and membrane materials are the main subjects reported in previous review papers, they were selected because their great contribution on the MFCs performance. Additionally, subjects such as biofouling, electron acceptor, electron transfer and costs, are discussed in this review. The aim is to contribute on the identification of progress trends, as well as gaps and challenges that will be addressed in the coming years to maximize the benefits of MFCs and continue advancing in their development and application in the real world.
3. MFC configurations
Figure 2, Figure 3 show some MFCs configurations, including the most commonly used, and their main advantages and challenges are highlighted.
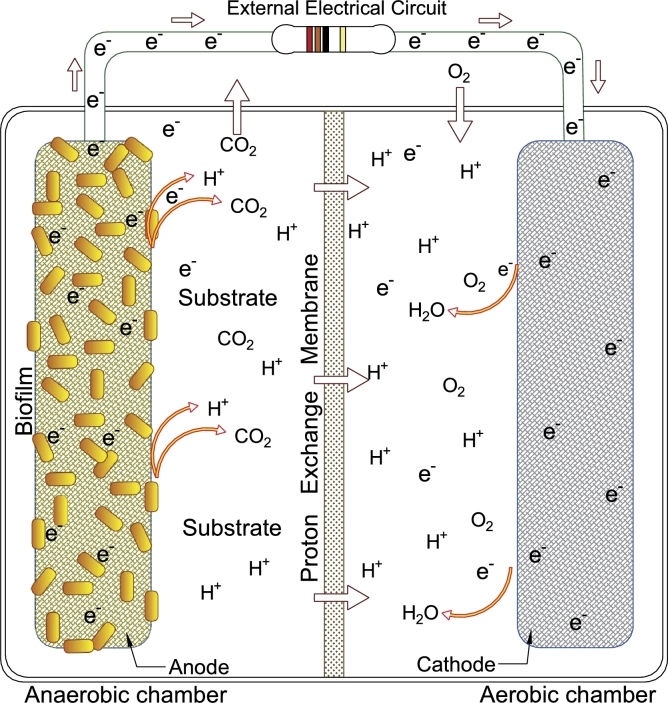
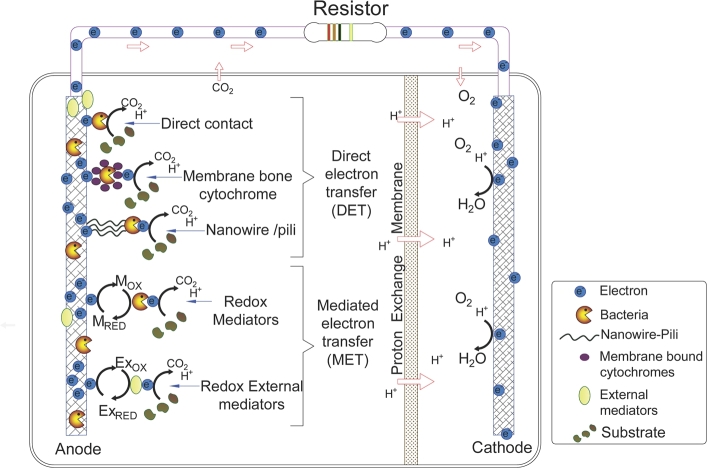
Figure 2.
Schematic representation of a dual-chamber microbial fuel cell.
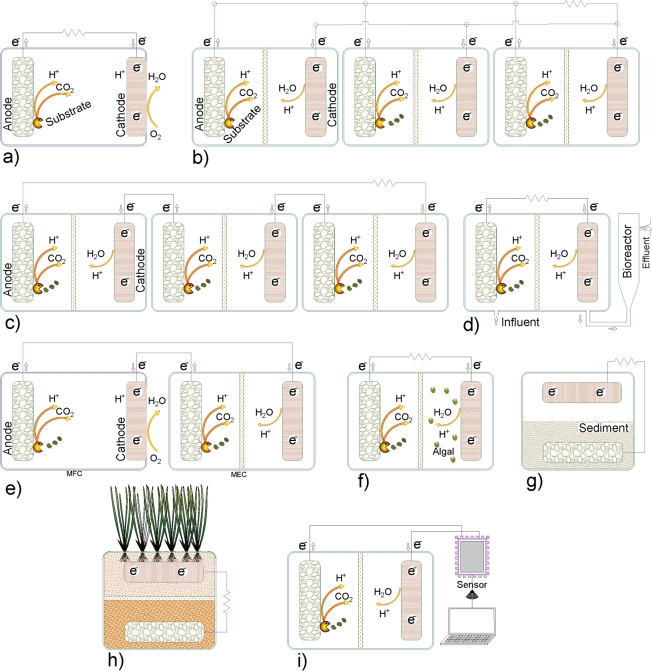
Figure 3.
Examples of microbial fuel cell configurations. a) Single-chamber MFC [146], b) Stacked MFC connected in parallel, c) Stacked MFC connected in series [131], d) MFC + Bioreactor [133], e) MFC + Microbial electrolysis cell [135], f) Microalgal-MFC, g) Benthic microbial fuel cell [138], h) Constructed wetland + MFC, i) Biosensor-MFC.
Dual-chamber microbial fuel cell (DC-MFC) is the typical architecture reported in the literature (Fig. 2). The DC-MFCs are integrated with an anodic chamber, a cathodic chamber, a proton exchange membrane (PEM) between the chambers, an external electrical circuit, and microorganisms in the anode. The main advantage of this configuration is to keep the liquids of the cathodic chamber and the anodic chamber separated by a membrane. The anodic chamber is under anaerobic conditions, to promote microbial growth and to avoid that the oxygen inhibits electricity generation. Microorganisms adhere to the anode surface and form a biofilm of exoelectrogenic microorganisms, which carry out the bio-electrochemical reactions to oxidize organic matter via metabolic reactions. This anaerobic digestion release electrons, protons, and carbon dioxide to the solution. The protons flow from the anodic chamber into the cathodic chamber through the proton exchange membrane. The cathodic chamber is characterized to work under aerobic conditions, since the oxygen acts as an electron acceptor resulting in the formation of water [108].
Single-chamber microbial fuel cell (SC-MFC) is another type of configuration (Fig. 3-a), which consists of a single chamber where the anode and the cathode are placed in opposite sides. The cathode is located with one face in direct contact with the electrolyte and the opposite face in direct contact with air. The most important characteristic of SC-MFC is their operation without the use of any separators or PEM. Some of the main advantages of using SC-MFC are reduction of the overall system cost, favor mass transfer from the anode to the cathode, and reduced of the chamber volume [126], [127]. A challenge to address in these configurations is the formation of biofouling on the surface of the water-side air cathode. Some efforts have already done, for example Rossi et al. [128] implemented a magnet on both sides of the cathode to daily clean the biofouling from the water-side surface, which results in an increase of the SC-MFC performance. The use of cathode catalyst agents as anti-biofouling is also another mechanism than can be employed to solve this problem [129], [130].
The stacking MFCs are another type of configurations, this consist of a connection of several MFCs, they can be connected in series, in parallel, and series/parallel (Fig. 3-b and 3-c). The objective of this configuration is to increase the power generation. The main disadvantage of using this configuration is the presence of losses due to the connections between the MFCs, which is reflected in the difference between the final voltage and the sum of the individual voltages. However, recent studies recommend the use of this design as a feasible alternative on larger scales [131], [132].
There are also combinations of MFCs with other types of technologies, their main objective is to improve the performance of the different technologies involved. The enhancing strategies include the use of MFC energy production for the operation of coupling technology, and the use of a coupling technology to improve the quality of the wastewater treatment. In the literature are reported combination such as: i) MFC + anaerobic fluidized bed bioreactor with the advantage of increasing the wastewater treatment efficiencies with a lower energy consumption [133]; ii) MFC + anaerobic digestion to produce biohydrogen and biomethane, and improving wastewater degradation [134]; iii) MFC + microbial electrolysis cell [135]; and iv) MFC + ammonia electrolysis cell [132], these last two with the aim of producing biohydrogen [16], [26] (Fig. 3-d and 3-e).
Microalgae technology has also been used in MFCs [136], [137]. Several advantages of using this combination are reported: i) microalgae can be used in the anodic and cathodic chamber, ii) microalgae can supply oxygen to the cathodic chamber, iii) microalgae contribute to the nutrients removal, iv) helps to the mitigation, v) helps to the carbohydrates-proteins-lipids accumulation and vi) promote biomass production (Fig. 3-f).
Benthic microbial fuel cell (BMFC) [138], this configuration is mainly used for marine applications. BMFCs operate in the benthic zone of the ocean (lowest level of a body of water, including the sediment surface bed). The cathode is immersed in seawater and the anode is introduced into the anoxic sediment surface bed. The use of freshwater sediment has also been reported [139]. This technology can be used to provide energy to small devices like marine sensors in remotes location, or even for remote sensors in environments with dangerous conditions [140]. Some challenges faced by BMFCs are the cost of the electrodes and the low power generation, which has limited it to be marketable (Fig. 3-g).
Constructed wetland microbial fuel cell (CW-MFC) or plant microbial fuel cell (Fig. 3-h) is also a type of system used to treat organic matter of wastewater. This configuration is characterized for the use of wetland plants, soils and microorganisms in their design. Examples for these plants are macrophyte (aquatic plants) such as Pharagmites australis [141] and rhizodeposits (roots of plants). The CW-MFC combine physical, chemical and biological process to treat wastewater and produce energy. An advantage of this system is its low-cost of the CW mechanism, besides, it has been reported the improvement in the efficiency of wastewater treatment and energy production when CW systems are coupled with MFCs. However, a constraint in its operation is the emission of greenhouse gases from CW, such as , methane () and nitrous oxide (). Then, research on the performance of CW-MFC coupling still requires further investigation.
Biosensor-MFC configuration (Fig. 3-i) is developed from the need of on-site monitoring of water or wastewater quality. In this configuration, the biosensor captures the output voltage coming from the MFC and interprets it as a result representing the concentration of a specific parameter. The output signal of MFCs is directly related to the electron transfer. This electron transfer depends on the behavior of the biofilm on the anode, which is susceptible to variations in the concentrations of the species in the medium. Parameters that have been controlled with this configuration of MFCs include P-nitrophenol [142], cadmium [143], chromium, benzoylecgonine (a metabolic product of cocaine) [144], biochemical oxygen demand [145], volatile fatty acids. The biosensor could be implemented in SC-MFC or DC-MFC depend on the purpose of each study. The use of micro-scale MFCs (proximal to >25 mL) is common in studies reporting the use of biosensor-MFCs. Therefore, it is considered that there is still a wide field for the development of this technology. Particularly with the development of larger scale prototypes to increase the volume of wastewater treatment and simultaneously energy production.
Table 2 shows a compendium of several MFC configurations, in which the main characteristics and their efficiency in terms of degraded organic matter and energy produced is highlighted. The Table identifies different MFC configurations and the frequency of their use, for instance, the configurations with the highest usage rate are DC-MFC [147], [148], [149], [150], [151], [152], [153], [154], [155], followed by SC-MFC [16], [84], [126], [127], [132], [146], [156], [157], [158]. On one hand, the main advantage of using DC-MFC configuration, as previously mentioned, is allowing the liquid of the anodic and cathodic chamber to be separated using a PEM, which favors the reaction in each chamber. On the other hand, the advantage of using SC-MFC configuration is the cost savings, since its configuration does not include the PEM. Another relevant data showed in Table 2 are the energy production and the substrate removal, which allow identifying additional advantages between the use of different MFC configurations. The studies carried out with DC-MFC configurations report the highest rate of energy production that oscillate between 2,100 mW m−2 to 3,600 mW m−2 [148], [149], [154], [159]. Followed by studies using SC-MFC configurations reporting power densities of 400 mW m−2 to 1,500 mW m−2 [126], [127], [156]. The table also includes some MFC configurations with a reduced number of publications and a low energy production such as: i) stacking MFCs that report a power density of 1,287 mW m−2 [131] and 536 mW m−2 [132]; ii) combination of MFC with anaerobic fluidized bed membrane bioreactor reports 89 mW m−2 [133]; iii) MFC + Anaerobic digestion reports 1.98 mW m−2 [134]; iv) MFC + Microbial electrolysis cell reports 343 mA m−2 [135]; v) MFC + microalgal reports 466.9 mW m−3 [136] and 54.48 mW m−2 [137]; vi) and for specifics uses in marine environments the BMFC configuration reports power density of 190 mW m−2 [138].
Table 2.
MFC configurations, electrode materials, membrane materials and performance.
| Design | Resistance | Material |
Substrate | Biofilm | %COD | Energy | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| Ω | Anode | Cathode | Membrane | Removal | |||||
| DC-MFC | 100 | Plain Graphite | Plain Graphite | Ultrex, membrane international | Glucose | Mixed consortium | 85.0 | 3,600.0 mW m−2 | [159] |
| DC-MFC | 0-10 KΩ | Graphene- modified plain stainless-steel mesh | Carbon paper | Nafion 112, DuPont | Glucose | Escherichia coli | - | 2,668.0 mW m−2 | [154] |
| DC-MFC | 1,000 | Stainless steel-based with a thin layer of graphene | Carbon felt | Ultrex, membrane international | Domestic wastewater | Mixed consortium | - | 2,143.0 mW m−2 | [149] |
| DC-MFC | 100 | Graphite | Graphite | Nafion 117, DuPont | Domestic wastewater | Mixed consortium | 95.0 | 2,100.0 mW m−2 | [148] |
| SC-MFC | 100 | Graphite fiber brush | Carbon Cloth | - | Acid elutriatione | Mixed consortium | 93.0 | 1,553.0 mW m−2 | [127] |
| DC-MFC | - | Chitosan/ vacuum - stripped Graphene | - | - | - | Mixed consortium | - | 1,530.0 mW m−2 | [152] |
| DC-MFC | 500 | GO | Carbon felt | Nafion 117, DuPont | Domestic wastewater | Mixed consortium | - | 1,390.0 mW m−2 | [151] |
| 3-DC-MFC series | 300 | Carbon felt | Carbon felt | Ultrex CMI 7,000 membrane international | Wastewater -Sodium Acetate | Mixed consortium | 57.6 | 1,287.0 mW m−2 | [131] |
| SC-MFC | 1,000 | Carbon Cloth | GO-Zn/Co | - | Acetate | Mixed consortium | - | 773.0 mW m−2 | [156] |
| 4-SC-MFC serie | 1,000 | Carbon fibers brush | Platinum layer | - | Glucose- ammonium mixture | Mixed consortium | 86.9 | 536.0 mW m−2 | [132] |
| SC-MFC | 218 | Carbon paper | Carbon paper | - | Acetate | Mixed consortium | 99.0 | 506.0 mW m−2 | [126] |
| SC-MFC | 1,000 | Carbon fibers brush | Platinum layer | - | Glucose- ammonium mixture | Mixed consortium | 99.1 | 414.0 mW m−2 | [132] |
| SC-MFC | 1,000 | Carbon paper | Carbon paper | - | Butyrate | Mixed consortium | 98.0 | 305.0 mW m−2 | [126] |
| SC-MFC | 980 | Graphite fiber brush | Carbon Cloth | - | Raw piggery waste | Mixed consortium | 84.0 | 192.0 mW m−2 | [127] |
| DC-MFC | 100 | Carbon felt | Titanium Plate | Nafion 117, DuPont | Glucose | Mixed consortium | - | 156.0 mW m−2 | [155] |
| 4-SC-MFC serie | 1,000 | Carbon fibers brush | Platinum layer | - | Landfill leachate | Mixed consortium | 62.6 | 143.0 mW m−2 | [132] |
| SC-MFC | 0.4 to 100 | Graphite fiber/ Ti | Stainless steel | – | wastewater | Mixed consortium | 82.0 | 101.0 mW m−2 | [146] |
| SC-MFC-AFMB | Graphite fiber brushes with a titanium wire core | Carbon cloth | - | Domestic wastewater | Mixed consortium | 92.5 | 89.0 mW m−2 | [133] | |
| SC-MFC | 33 – 22 KΩ | Carbon paper | Carbon Cloth | Nafion 117, DuPont | Domestic wastewater | Mixed consortium | 42.0 | 72.0 mW m−2 | [16] |
| DC-MFC | 100 | Carbon felt | Titanium Plate | Nafion 117, DuPont | Acetate | Mixed consortium | - | 64.3 mW m−2 | [155] |
| DC-MFC | 100 | Carbon felt | Titanium Plate | Nafion 117, DuPont | Propianate | Mixed consortium | - | 58.0 mW m−2 | [155] |
| DC-MFC | 100 | Stainless steel | Carbon felt | - | Synthetic wastewater | Mixed consortium /bio-cathode algae Chlorella vulgaris | 75 | 54.48 mW m−2 | [137] |
| DC-MFC | 100 | Carbon felt | Titanium Plate | Nafion 117, DuPont | Butyrate | Mixed consortium | - | 51.4 mW m−2 | [155] |
| DC-MFC | 100 | Carbon felt | Carbon felt | SBC-600 | Synthetic wastewater | Mixed consortium | 81.0 | 41.08 mW m−2 | [160] |
| SC-MFC | - | Carbon fiber graphite brush | Carbon Cloth coated with platinum black | Nafion 112, DuPont | Synthetic wastewater | Lactobacillus pentosus | 42-58 | 5.04 mW m−2 | [157] |
| SC-MFC +AD | 1 MΩ - 100 | Graphite plate | Graphite plate | - | Domestic wastewater | Mixed consortium | 25.2 | 1.98 mW m−2 kg | [134] |
| DC-MFC | 100 | Crumpled graphene-modified | Carbon brush | Ultrex CMI 7,000 membrane international | Synthetic wastewater | Mixed consortium | - | 3,600 mW m−3 | [153] |
| PMFC | 500 | Carbon brush | Carbon brush | Nafion 117, DuPont | Synthetic wastewater | Mixed consortium /bio-cathode algae Chlorella vulgaris | 93.2 | 466.9 mW m−3 | [136] |
| SSM-MFCs | 562 | Carbon Fiber Veil | AC-PTFE | - | Urine | Mixed consortium | - | 15.74 μ W cm−3 | [161] |
| MFC | - | Roughened Glassy Carbon plates | - | - | - | Shewanella oneidensis | - | 40μA cm−2 | [162] |
| SC-MFC+ MEC | 10 | Carbon paper | Carbon paper | - | Propionate | Mixed consortium | - | 343 mA cm−2 | [135] |
| SC-MFC+ MEC | 1,000 | Carbon paper | Carbon paper | - | Propionate | Mixed consortium | - | 81 mA cm−2 | [135] |
| DC-MFC | - | Carbon Cloth | Carbon Cloth | Internal Nanoscale polypyrrole | Molasses wastewater | Mixed consortium | 25.24 | 0.0173 V | [147] |
| DC-MFC | - | - | - | - | Synthetic wastewater | Sulfate- reducing bacteria | 30-40 | - | [150] |
| 4-SC-MFC series + AEC | 1,000 | Pt/N-rGO/ Carbon felt | Mo2C/N-rGO/ Carbon felt | Nafion 117, DuPont | Glucose- ammonium | Mixed consortium | 70.7 | - | [132] |
DC=Dual Chamber, SC=Single Chamber, MEC=Microbial Electrolysis Cell, AD=Anaerobic Digestion, AFMB=Anaerobic fluidized bed membrane bioreactor, AEC=Ammonia Electrolysis Cell, PMFC=Microalgal-based photoautotrophic microbial fuel cell, GO=graphene oxide, SSM-MFCs=self-stratifying microbial fuel cells, AC-PTFE=activated carbon with polytetrafluoroethylene
Regarding the ability of MFCs to degrade the substrate, the information in Table 2 shows different percentages of substrate removal (this data is only reported in some studies). For instance, for DC-MFC configurations a substrate removal between 25% to 95% [147], [148] is reported, for SC-MFC values between 42% and 99% [126], [134], and for MFC + Microalgal the removal varies from 75% to 93% [136], [137].
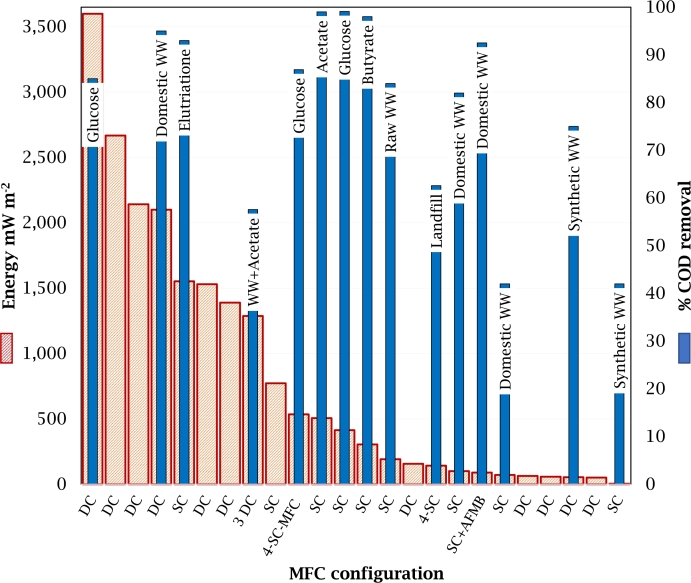
Fig. 4 summarizes graphically the different MFC configurations and the link to their performance. It is observed that the use of DC-MFCs reports the highest power generation values, followed by the use of SC-MFC configurations. Furthermore, if the ability to degrade the substrate is considered, the same trend of high removal rate is observed for these configurations. However, the SC-MFC configurations present the highest COD removal values when the substrate used are acetate or butyrate. Also, MFC + microalgal configuration provides good removal of substrate, as well as nutrients; however, bioenergy production is lower in comparison with the previous configurations. Therefore, based on this analysis, it can be inferred that the DC-MFC configuration is the best option for further improvement.
Figure 4.
Performance of different MFC configurations. DC = Dual-chamber MFC; SC = Single-chamber MFC; AFMB = Anaerobic fluidized bed membrane bioreactor; WW = Wastewater.
4. Materials of MFC components
Among the main elements of a DC-MFC include the anode, the cathode, and the membrane. It is important to make a good selection of these elements to maximize the power generation, the coulombic efficiency (CE), the ratio of the total number of electrons transferred to the anode from the substrate and the maximum possible number of electrons if the entire substrate were degraded and produce current), and to favor the degradation of organic matter [15]. In the following sections analysis and discussion of the components of MFCs is done.
4.1. Electrodes
As already mentioned, the electrodes have a direct effect on MFCs performance. Among the electrode materials, the most common reported are carbon-base materials and metal-base materials.
Carbon-based materials are commonly used to make MFCs electrodes because of their i) low cost due to their high availability; ii) high surface area; iii) excellent biocompatibility, these last two facilitate bacterial adhesion and allow the formation of a uniform biofilm when the material is used as anode; iv) chemical inertness; v) high conductivity that serve to transfer the electrical energy; and vi) good stability such as high melting point that allows its use at high temperatures without changes [163], [164]. Examples of them are carbon cloth, carbon paper, carbon fibers brush, carbon felt, graphite plate, graphite fibers brush and graphite felt, graphite granules, rods, foam, reticulated vitreous carbon, glassy carbon, activated carbon cloth [165], and in recent years graphene. This last one is a newly created material that is formed from a layer of graphite. Graphene is a material with a low production cost and also high porosity. Since the isolation of graphene in 2004, researchers have found a variety of unique properties [76] such as high electron transfer rate and large active surface area, excellent biocompatibility and strong resistance to corrosion [166]. Graphene is a two-dimensional carbon nanomaterial, consisting of a single layer of cohesion carbon atoms through sp2 hybridization bonds, with a structure similar to that of a honeycomb. There are also publications reporting its use in electrochemical energy-storage devices [167]. This material has a special structure, it allows electrons to move freely across the plane in delocalized orbitals [168].
Metal-based materials are also used to make MFCs electrodes, some of them are titanium, platinum, stainless steel, copper, nickel, gold, cobalt and silver. These materials are used mainly for the different properties that favor the MFCs performance. For example, platinum is one of the most commonly used material as cathode catalyst, due to the rapid kinetics of the oxidation-reduction reaction [169]. Titanium has excellent corrosion resistance [170]. Silver, copper and gold have excellent conductivity 62.1 MS m−1, 58.5 MS m−1 and 44.2 MS m−1, respectively [171], [172]. Then it is possible to say that metal-based electrodes provide great advantages in the performance of MFCs. However, these metal-based materials have significant limitations, making them difficult to use for larger-scale projections. For example, platinum [165], titanium, gold, and silver are very expensive. Besides titanium and cobalt has low biocompatibility [172]. Platinum tends to be toxic when the substrate is wastewater and can harm microorganisms, furthermore, the catalytic activity of Pt can be significantly decreased when sulfur-containing pollutants are present. And cooper and stainless steel are susceptible to corrosion that limited their biocompatibility [170], [173], [174].
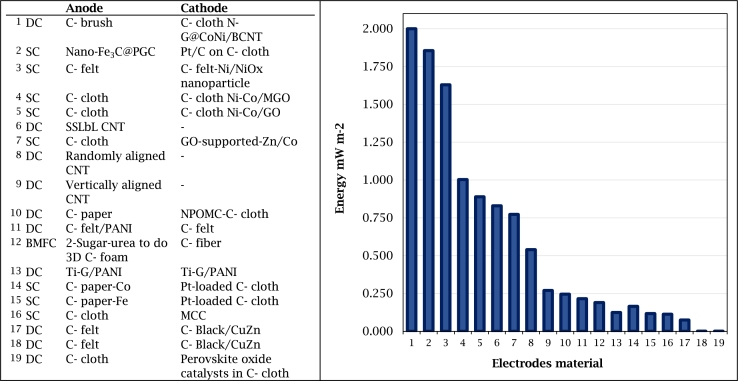
The scientific community has implemented different mechanisms to enhance the performance of MFCs electrodes made of either carbon-based or metal-based materials. Some reported techniques include the use of doping, coating, synthesis, or nanotechnology. Regarding the use of nanostructures, they are mainly used in carbon-based materials to improve hydrogen and electricity production [8]. These techniques have been used with specific objectives of improving the anode and the cathode, Table 3 and Fig. 5 show some relevant results reported by several authors and there are analyzed below.
Table 3.
Electrode materials, configurations and performance of MFCs.
| Anode | Cathode | Design | Volume | Power Densities | Reference |
|---|---|---|---|---|---|
| Carbon brush | Carbon cloth N-G@CoNi/BCNT | DC-MFC | 270 mL | 2.000 W m−2 | [177] |
| Nano-Fe3C@PGC | Pt/C on carbon cloth | SC-MFC | 28 mL | 1.856 W m−2 | [176] |
| Carbon felt | Carbon felt-Ni/NiOx nanoparticle | SC-MFC | 100 mL | 1.630 W m−2 | [169] |
| Carbon cloth | Carbon cloth Ni-Co/MGO | SC-MFC | - | 1.003 W m−2 | [173] |
| Carbon cloth | Carbon cloth Ni-Co/GO | SC-MFC | - | 0.889 W m−2 | [173] |
| SSLbL CNT | - | DC-MFC | μL | 0.830 W m−2 | [140] |
| Carbon cloth | GO-supported-Zn/Co | SC-MFC | 28 mL | 0.773 W m−2 | [156] |
| Randomly aligned CNT | - | DC-MFC | μL | 0.540 W m−2 | [140] |
| Vertically aligned CNT | - | DC-MFC | μL | 0.270 W m−2 | [140] |
| Carbon paper | NPOMC-Carbon cloth | DC-MFC | cc 10 mL | 0.245 W m−2 | [178] |
| Carbon felt/PANI | Carbon felt | DC-MFC | 250 mL | 0.216 W m−2 | [175] |
| 2-Sugar-urea to do 3D Carbon foam | Carbon fiber | BMFC | 1000 mL | 0.190 W m−2 | [138] |
| Ti-G/PANI | Ti-G/PANI | DC-MFC | 1400 mL | 0.124 W m−2 | [179] |
| Carbon paper-Co | Pt-loaded carbon cloth | SC-MFC | 80 mL | 0.165 W m−2 | [163] |
| Carbon paper-Fe | Pt-loaded carbon cloth | SC-MFC | 80 mL | 0.117 W m−2 | [163] |
| Carbon cloth | MCC | SC-MFC | 520 mL | 0.113 W m−2 | [180] |
| Carbon felt | Carbon Black/CuZn | DC-MFC | cc150 mL | 0.075 W m−2 | [181] |
| Carbon felt | Carbon Black/CuZn | DC-MFC | 25,000 mL | 0.00032 W m−2 | [181] |
| Carbon cloth | Perovskite oxide catalysts in Carbon cloth | DC-MFC | 450 mL | 0.00139 W m−2 | [182] |
| - | Fe-AAPyr catalyst in RRDE | SC-MFC | 125 mL | 262μW cm−2 | [183] |
| Graphite fiber brush | Fe-BZIM-AB RRDE | SC-MFC | 28 mL | 162μW cm−2 | [184] |
| Graphite fiber brush | Fe-ABZIM RRDE | SC-MFC | 28 mL | 159μW cm−2 | [184] |
| Graphite fiber brush | Activated carbon | SC-MFC | 28 mL | 100μW cm−2 | [184] |
| 3D N-doped-GA | Carbon cloth | DC-MFC | 25 mL | 750.0 W m−3 | [11] |
| Fe/N/S-doped CT | Fe/N/S-doped CT | SC-MFC | 28 mL | 479.0 W m−3 | [185] |
| Multilayer Carbon flet/N-doped-CNT/PANI/MnO2 | 5-graphite rod | DC-MFC | – | 13.8 W m−3 | [186] |
| SSLbL CNT | DC-MFC | 12.5 μL | 3,320.0 W m−3 | [140] | |
| 8-TNs-modified | 1-TNs-modified | DC-MFC | ac800 mL cc100 mL | 12.7 A m−2 | [170] |
| Carbon veil | Conductive latex | SC-MFC | 15 mL | 0.092 W | [187] |
PANI=polyaniline; CNT=carbon nanotubes; MCC=modified clay cup; TNs=titanium dioxide nanotubes; cc=cathodic chamber; ac=anodic chamber; MGO=silanefunctionalised graphene oxide; GO=graphene oxide; CT=carbon tubes; BMFC=benthic MFC; RRDE=rotating ring disk electrode; Fe-AAPyr=Fe-Aminoantipyrine; Nano-Fe3C@PGC=iron carbide nanoparticles dispersed in porous graphitized carbon; GA=graphaerogel; Fe-ABZIM=Iron nitrate with aminobenzimidazole; Fe-BZIM=Iron nitrate with benzimidazole; NPOMC=Nitrogen-phosphorus-doped mesoporous carbon; AB=airbreathing; G/PANI=graphene/polyaniline SSLbL=spin-spray layer by layer; BCNT=bamboo-like carbon nanotube
Figure 5.
Electrodes material and MFC performance.
Anode. This is an essential component in MFCs, some of its main functions are as a support for bacteria and the conduction of electrons. The efficiency of the anode is directly related with the generation of electricity of the MFCs. The most important characteristics that should be taken into an account in the selection of the anode material are: available surface area, roughness, conductivity, biocompatibility, microorganisms and substrates [26], [154].
Carbon-base materials as an anode are widely used and different investigations focus their objectives on the improvement of these materials through their modification [138], [162], [175] (Table 3 and Fig. 5). Pushkar et al. [138] evaluated a sugar-urea carbon foam as anode in a BMFC. They used 1.5L of sediment and seawater from Dumas seashore, Surat, Gujrat, India. The sugar-urea carbon foam anode was submerged into the sediment. In the cathodic electrolyte a carbon fiber cathode was placed, and an aerator was used to supply oxygen. The external electrical circuit was operated with 100 Ω and 10 Ω resistors. They reported a maximum power density of 0.190 W m−2 that was higher than the results obtained with carbon felt 0.0085 W m−2. They attributed this result to the synthesized carbon foam anode that provided a large surface area, hydrophilicity and biocompatibility properties. Particularly the good biocompatibility was attributed to the presence of functional groups as -CN, -OH and -CO, these groups provide polarity to the anode and favor the attachment of exoelectrogens microorganisms. Rajesh et al. [175] also used a carbon-base material, they evaluated a carbon felt coating with polyaniline (PANI) as the anode in a DC-MFC with a pretreated inoculum using Chaetoceros, and synthetic wastewater as substrate. Their results showed a maximum power density of 0.216 W m−2 and an improvement on the CE by 17% and generating a total of 42.45% compared with the unmodified anode and without pretreatment inoculum. Ye et al. [162] evaluated carbon plates as an anode, through the physical modification of the surface. They compared two glassy carbon plates with different uniform roughness, 10 and 100 nanometers each. Their results show that the rougher surface of the electrode increases the biomass growth; therefore, it produces higher power densities, 40 μ A cm−2 than the smoother electrode, 10 μ A cm−2. The three techniques applied for modifying carbon-base materials (synthesized, coating, and physical modification) show the possibility to potentiate some anode characteristics such as the surface area, the biocompatibility and the roughness. These characteristics favor the microbial adhesion, electrogenic activity and reduce the charge transfer resistance. Comparison of these studies shows that the use of carbon felt coating with PANI as anode produced the highest power density. However, it is interesting to evaluate a new scenario that combines the advantage obtained from each study. For instance, evaluation of urea-sugar carbon foam as anode on DC-MFC with a pretreated inoculum and synthetic wastewater, could be interesting research, taking advantage of the low cost and natural origin of this anode material and the good performance with a pretreated inoculum. Thus, expanding the application of such material in DC-MFC beyond it use in benthic zones.
Metal-based materials are also widely reported as anode material. Baudler et al. [172] evaluated the biocompatibility of different materials (gold, silver, copper, nickel, cobalt, titanium and stainless steel) to be used in an MFC. Their results showed that copper was the material with the highest biocompatibility with the electrochemically active bacteria. And even with the antimicrobial properties of cooper, bacteria were able to tolerate and adapt. However, it is important to note that the advances in carbon-based materials reported in recent studies offer even greater advantages compared to metal-based materials.
Nanomaterials are another type of material used as anode. In the literature, different applications from non-structure to carbon-base and metal-base materials are reported, such as carbon nanotubes (CNT), nanoparticles of graphene (GN), PANI, 3D graphene-based material,Ti-TiO2, and nanoparticles of metals. The last are commonly used as carbon-base anode coating, example of these metal nanoparticles are Fe, Zn, Ni, Co, and Cu [11], [151], [163]. Some studies that have used the nanotechnology in carbon-base material are those conducted by Hu et al., Ren at al., and Hou et al. [140], [151], [176] (Table 2, Table 3).
Hu et al. [176] reported the use of iron carbide nanoparticles dispersed in porous graphitized carbon (Nano-Fe3C@PGC) as anode and carbon cloth with catalyst Pt/C as cathode, connected with a 1,000 Ω external loading resistance. They used a 28 mL SC-MFC, inoculated with activated anaerobic sludge. Their results showed a maximum power density of 1.856 W m−2 that was better than the results on the same conditions but using carbon felt with 0.487 W m−2. These results were attributed to the electrocatalytic activity and conductivity provided by the iron carbide nanoparticles (Nano-Fe3C@PGC) anode. Ren at al. [140] evaluated a CNT anode on a micro-scale 25 μL DC-MFC. They used an inoculum (Geobacter) obtained from a MEC and sodium acetate as substrate. Their results showed an improvement with the use of spin-spray layer by layer (SSLbL)CNT anode with a power density of 0.83 W m−2 greater than that of the bare gold anode control of 0.48 W m−2. Additionally, a grater biofilm thickness was observed in CNT than in the bare gold, 9.0 μm and 1.8 μm, respectively. Therefore, it can be said that the SSLbL-CNT anode favors the attraction of exoelectrogenic microorganisms due to a higher biocompatibility. Hou et al. [151] tested a reducing graphene oxide coating with PANI nano-fibers anode on a DC-MFC. They obtained a maximum power density of 1.390 W m−2. This result was attributed to the high conductivity of graphene and the large surface area provided by PANI.
The use of nanotechnology in metal-base material was reported by Feng et al. and Su et al. [170], [188] (Table 3 and Fig. 5). Feng et al. [170] reported in their work the evaluation of modified titanium electrodes in DC-MFC. The modification consisted of the onsite growth of titanium dioxide nanotubes (TNs) on the surface of electrodes. They reported a maximum current density of 12.7 A m−2, which was higher than that provided by the bare titanium electrode. It was also observed coverage of almost 100% biofilm on the TNs anode surface, different from the poor coverage in the bare titanium. The nanoscale titanium tubes provided an increased and rougher electrode surface area, and favored its biocompatibility, hydrophilicity, and conductivity. Su et al. [188] used the TiO2@TiN nanocomposite as anode to develop a hybrid biofilm with Shewanella Ioihica in a DC-MFC. Their results showed a maximum power density of 0.064 W m−2, which was higher than the 0.0334 W m−2 of DC-MFC with pure biofilm; this improvement was attributed to the nanocomposite that favored the biocompatibility and conductivity of the anode.
In the analysis of the application of nanotechnology in carbon-based and metal-based materials, it was found that their use does favor them. This translates into better performance as electrodes in MFCs, where energy production is the parameter commonly used to quantify these improvements. However, it can highlight that the nanotechnology applied in carbon-based materials presented the best energy production efficiencies in MFCs. The work of Hou et al. [151] reported a maximum power density of 1.39 W m−2 using graphene with PANI, which represents a large difference in contrast to the achieved maximum power density of 0.064 W m−2 using TiO2@TiN nanocomposite [188]. Therefore, it can be suggested that the application of nanotechnology in carbon-based materials used as electrodes in MFCs could be a promising alternative for future research.
As already discussed, the selection of the anode material and the different techniques to increase its performance are very important. However, the microorganisms and substrate selection also play a crucial role on the anode performance. Therefore, some of the most important consideration to carry out a successful selection are discussed below. Microorganisms form the biofilm and they are responsible for degrading the substrate, as well as generating the energy. The commonly used way to quantify the ability of microorganisms to degrade the substrate is through the reduction of Chemical Oxygen Demand (COD). Substrate degradation is carry out by the action of anaerobic microorganisms present in the anodic chamber, which are exoelectrogenic and also called electroactive [189]. They can be divided into two general types, mixed cultures, and pure cultures. The first one contain various types of microorganisms especially present in wastewater, and the advantage of using them are low cost, presence of self-mediators and good performance [127], [148], [149], [152]. The main challenges to overcome is the internal microbial competition reaction, which is reflected in the undesirable reactions such as methanogenesis that transform the organic matter into methane and CO2 instead into CO2 and electrons [14], which reduces electricity generation. The second type, pure cultures, contain only one type of microorganisms for example, Escherichia coli [154], Shewanella oneidensis [162], Lactobacillus pentosus [157], among others. The advantage of the use of this type of pure cultures is the reduction of reaction between microorganism and substrate. Also, allows the study of specific objectives such as biocompatibility analysis, electron transfer mechanisms and substrate degradation [163], [169].
Once the MFCs consortium has been selected, it is necessary to select the substrate and analyze the effect on the MFCs performance. Some examples of substrates are: acetate, glucose, propionate, butyrate, sucrose, complex substrates such as synthetic wastewater, animal waste, and real wastewater. In Table 2 is possible to identify different types of substrates and microorganism. On one hand, studies using mixed cultures reported the highest rates of COD removal on the order of 99%, 98% and 93%, for acetate, butyrate and acid elutriatione; and reached power densities of 506 mW m−2, 1,553 mW m−2 and 305 mW m−2, respectively [126], [127]. Other works have presented the highest power densities using glucose as substrate, even when their COD removal rates are not the highest as mentioned in the previous paragraph. In their work, Rabaey et al. [159] used glucose in a DC-MFC with a mixed consortium, and they reported a maximum power density of 3,600 mW m−2 and a COD removal of 85%. Follow by the works that used domestic wastewater as substrate which presented power densities between 1,287 mW m−2 and 2,143 mW m−2, and the highest COD removal of 95% for 2,100 mW m−2 [131], [148], [149], [151]. Regarding the use of animal waste, Chandrasekhar et al. [127] reported 84% of COD removal and 192 mW m−2 of energy production, both lower than the other substrates. On the other hand, investigations using pure cultures report percentages of COD removal between 30% and 50% and power density of 5.04 mW m−2 [131], [157]. But some of them using Escherichia coli reports high energy production about 2,700 mW m−2 and 80% of COD removal [154]. Therefore, according to this analysis the use of mixed cultures offers greater advantages even on a laboratory scale. Regarding the substrate, the use of glucose reports the highest energy production following by wastewater. However, if the objective is to apply MFCs in real scenarios, the most certain suggestion is to use wastewater as an energy source with a mixed culture (usually found in wastewater). These conditions allow generating very similar or equal scenarios to which the MFCs will be subjected in practical situations. In addition, the percentages of COD removal and energy production will also be similar to those expected at larger scales, which will allow better decisions-making.
The studies revised and summarized in Table 2, Table 3 and Fig. 5 provide evidence that even when several strategies have been evaluated to improve the anode performances, still do not represent a significant breakthrough in MFC performance, compared to unmodified anode materials. However, it was possible to identify opportunities to continue the research in order to improve carbon-based anode materials such as the use of nanotechnology. Additionally, the use of mixed cultures as biomass and wastewater as substrate is desirable.
Cathode. In the cathode occurs an electrochemical reduction reaction in which an oxidant is reduced [184]. The most relevant properties that are searched in the cathode are catalytic qualities, high surface area and high conductivity [190], [191], and some of the main challenges on cathode materials to face include poor oxygen reduction reaction, low catalytic activity, and biofouling [137], [169], [183]. A detailed review of the studies focused on improving the cathode properties and enhancing the MFCs efficiency through different strategies is done.
Regarding the catalytic properties of the cathode, there are two types biotic and abiotic catalysts, both can be used in neutral conditions. The biotic catalysts are mainly bacteria and enzymes. However, bacteria catalysis for oxygen reduction reaction (ORR) is sluggish and enzymes are active and selective but expensive and no eco-friendly. On the other hand, there are abiotic catalyst that are commonly used because are durable and resistant to most pollutants. Examples of them are platinum group metal (PGM) catalysts, platinum group metal-free (PGM-free) catalysts, and carbonaceous materials [161], [183], [184]. PGM cathode catalysts were commonly used in MFC in the past decades [187]. Currently due to the low durability and high cost of platinum-based materials, scientific efforts have been made to develop alternative catalysts [161]. PGM-free catalysts have been reported to be efficient for catalyzing oxygen in neutral media [187]. Examples of these catalysts based on transition metals include Mn, Fe, Co and Ni, and although their durability and performance are high, their cost is also high, making it difficult to project their use on a large-scale [161], [178]. Carbonaceous materials have been used as cathode catalysts in MFCs due to its low cost. Some examples of these materials are activated carbon (AC), modified activated carbon, AC mixed with polytetrafluoroethylene (PTFE) and pasted on a stainless steel mesh, carbon black, carbon black modified with aerogel, carbon nanotubes (CNT), carbon nanofibers (CNF), and graphene [161], [184], [187].
Santoro et al., Mecheri et al., and Song et al. [178], [183], [184] (Table 3 and Fig. 5) conducted researches with catalytic modified materials as cathodes. Santoro et al. [183] evaluated the effect of the catalyst loading of the cathode in the MFC power generation. Their results showed that a rotating ring disk cathode with 10 mg cm−2 of Fe-Aminoantipyrine catalyst in a SC-MFC achieved 262 μW cm−2 of maximum power density. They suggested that a high catalyst load results in high power output of the MFC, but they recommend continuing the research to reduce the cost of the catalyst. Mecheri et al. [184] also, carried out a similar work in which they used iron nitrate with benzimidazole (Fe-BZIM) and iron nitrate with aminobenzimidazole (Fe-ABZIM) as cathode catalyst, tested in rotating ring disk electrode (RRDE). They used graphite fiber brush as anode and a 28 mL SC-MFC connected with an external resistance of 1 kΩ. They reported a maximum power density of 162 μW cm−2 for the catalysts Fe-BZIM integrated into an air-breathing (AB). This is higher than the power density of Fe-ABZIM 159 μW cm−2, and the activated carbon control, but not higher than the maximum power density obtained by Santoro et, al [183] as is shown in Table 3 and Fig. 5. Song et al. [178] evaluated a nitrogen-phosphorus-doped mesoporous carbon (NPOMC) as a cathode catalyst in a DC-MFC. Their results showed a maximum power density of 245.8 mW m−2, which represented 75% of the power density obtained by Pt/C catalyst 329.8 mW m−2. They observed that the mesoporous structural catalyst provides a large surface area with a higher electrochemical charge transfer. Although the power density was lower than that obtained with the Pt/C catalyst cathode, a comparison with the Santoro and Mecheri studies mentioned in the previous paragraph shows that the power density obtained by the NPOMC of Song was higher. In addition, the most relevant achievement of this study was the low cost of the NPOMC catalyst which represented less than 5% of the price of the Pt/C catalyst.
Similar study conducted by Huo et al. [177]reported the development of a catalyst based on N-doped bamboo-like carbon nanotube (BCNT) and CoNi-alloy N-G@CoNi/BCNT. Then, they evaluated this catalyst in a DC-MFC in a 130 mL anodic chamber and 140 mL cathodic chamber. Ultrex CMI-7000 was used as cation exchange membrane, carbon brush as anode, and carbon cloth with catalyst as cathode. Their results showed a maximum power density of 2.0 W m−2 for N-G@CoNi/BCNT, this value was lower than the obtained with Pt/C of 2.6 W m−2. However, the power density obtained with this N-G@CoNi/BCNT catalyst was one of the highest reported among those that use carbon-base cathode catalysts in MFC (Table 3 and Fig. 5). It was shown that the N-G@CoNi/BCNT catalyst increased the surface area of the cathode, which favored its electrocatalytic properties, and promoted oxygen reduction reactions. The most outstanding achievement of this study was the low cost of the catalyst compared with the Pt/C, this trend of decreasing costs can also be observed in the work by Song [178] mentioned in the previous paragraph.
The use of nanotechnology in cathodes to improve the MFC performance is another strategy that has been reported [156], [169], [173]. Yang et al. [156] evaluated a modified cathode with graphene oxide (GO) - supported zinc cobalt oxides (GO-Zn/Co) nanocomposites in a SC-MFC. Their results reported an increase in the performance of the electrode with a power density of 0.773 W m−2. They attributed that the (GO-Zn/Co) nanocomposites generated an antibacterial activity on the cathode surface that inhibits the formation of microorganisms on its surface (biofouling). Papiya et al. [173] also evaluated the use of GO cathodes modified with nanocomposites in SC-MFC. The GO were grafted with g-amino propyl tri-ethoxy silane (APTES), and Ni and Co nanoparticles deposited on GO and silane-functionalised graphene oxide (MGO) matrices. They reported the highest power density of 1.003 W m−2 in the Ni-Co/MGO cathode as it is shown in the Table 3. Choi et al. [169] fabricated a nanocatalysts PGM-free cathode, consisted of the deposition of a thin layer of Ni/NiOx nanoparticles on the carbon-felt material. Then they evaluated a carbon-felt Ni/NiOx performance in an MFC, their results showed a power density of 1.631 W m−2 that was higher than a carbon-felt of Pt/C cathode at the same condition with 0.489 W m−2. These results are attributed to the fact that the Ni/NiOx nanoparticles provided a higher surface area and an increase in the active sites of the cathode, which favored the ORR.
New electrode materials have been also tested as an alternative to improve the cathode performance. Liu et al. [180] evaluated clay cathode in an MFC to remove cooper and generated electricity (Table 3 and Fig. 5). They used carbon cloth as anode, and modified clay cup as cathode that was produced by sintering at 1,000∘C temperature. The 520 mL SC-MFC was inoculated with a mixed consortium from a wastewater treatment plant, and sodium acetate was used as a substrate. The anode and the cathode were connected by an external electrical circuit integrated by a 1 kΩ resistor and 0.4 mm titanium wire. They reported a maximum power density of 0.1137 W m−2 and a copper removal of 96.5%. Although, the cooper removal was close to 100%, the power generation was low. Therefore, further research is needed to improve the performances of clay as electrode material. The application of these strategies translates into improvements in the performance of the cathode, as well as in mitigating some cathode challenges such as poor oxygen reduction reaction, low catalytic activity, and biofouling.
Regarding biofouling, it is a limitation that occurs more frequently in single-chamber MFCs and affect the overall MFCs performance. In order to avoid this problem and allow the correct cathode function, it is important to identify its causes and apply possible mitigation techniques. Biofouling consists in a biofilm formed by heterotrophic microorganisms attached in the surface of the material [84]. As already mentioned, its presence is common in membrane-less MFCs, and this is because the cathode is directly in contact with the substrate. The biofouling causes an increase in the charge transfer and ohmic resistance of the cathodes and decreases their performance [187]. Some of the strategies used to mitigate the biofouling problem are the following: i) design adaptation, the reconfiguration of MFC should allow removing the cathode to clean the biofouling and should prevent the contact between the air and the anode; ii) modification of the surface cathode, this strategy could delay the biofouling; iii) clean the electrode, the electrode can be washed with a concentrated acid solution [84], and also the washing of cathode can be conducted periodically to minimize the formation of biofouling [146]. Biofouling is also caused by fungi that can be growth on the cathode surface. This type of biofouling is reported in some studies [181]. They reported the growth of fungi on the surface cathode after 50 days of operation. At the same time, they observed a small decrease in the current production. They use Fluconazole as fungicide, which is spread over the entire cathode surface to mitigate the problem. After this, a positive result is observed with an increase in the current of the system.
Analysis of these studies allow the identification of the benefits obtained by the use of some techniques that improve cathode performance. Among these, the studies focused on the improvement of the catalytic cathode properties are the most reported. This trend can be attributed to the observed increase in ORR when improvements in the catalytic properties of the cathode are obtained, and consequently promotes energy production in the MFCs. In addition, it is important to note that in several studies it was observed that the Pt/C catalyst produced the best energy yield, one of the main reasons for these results is that the use of Pt/C catalyst favors the one-step ORR that occurs with the reduction of oxygen through 4 e− [192], [193]. However, the economic feasibility of using other types of catalysts was identified as the main advantage. Consequently, in order to be a priority for large-scales MFCs systems, more efforts in the development of other catalysts must be done to replace platinum. Regarding the development of alternative cathode materials, such as clay, must be also considered as an opportunity area. Even though the low MFC energy production reported, because of the ability of clay to remove some toxicants in the water such as cooper. Nevertheless, more research should be conducted in this respect. Finally, nanotechnology enhances the cathode performance and increase the energy production; furthermore, nanocomposites generate antibacterial activity on the cathode surface that inhibits the formation of biofouling on its surface.
4.2. Separators
A separator (most commonly, a membrane) is a component that connects and separates both chambers of the dual-chamber MFCs for keeping the anode and cathode liquids separated. Furthermore, it allows protons to permeate from the anodic to cathodic chamber and allows the separation of hydrogen gas (), oxygen (), and substrate [26]. Membrane performance is usually measured with factors such as proton conductivity, permeability to water, ion transport number, biofouling, internal resistance and, mechanical strength, chemical resistance and oxygen diffusion [160], [194]. The surface of the membrane also has an effect in the membrane performance and the power generation in MFCs systems. Oh et al. [15] reported that when the surface area of the membrane increases, the power density increases as well. They reported 45.0 mW m−2 for a proton exchange membrane (PEM) area of 3.5 cm2; 68.0 mW m−2 for a PEM area of 6.2 cm2; and 190.0 mW m−2 for a PEM area of 30.6 cm2. Thus, a desired property of a proton exchange membrane is a large surface area.
Currently, several membranes materials have been reported that function as separators in MFC systems. Commercial membrane materials include Nafion membranes, Ultrex membranes and Zirfon membranes to name a few.
The commonly used membrane is developed by Nafion DuPont Inc. USA, due to its accessibility and good performance. This Nafion perfluorinated membrane has sulfonic acid groups that favor the proton conductivity, transfer protons from the anodic chamber to the cathodic chamber, is selective to small cations, has high permeability to water, and avoid the pass of electrons [195], [196], [197].
Before the Nafion membrane is used, an activation treatment is necessary, which activates its conductive capacity, eliminate possible organic molecules absorbed from the air, and hydrates the membrane. This activation can be carried out with strong acid solutions for example nitric, sulfuric, perchloric, phosphoric and hydrochloric acids [198]. However, the standard activation membrane method uses 1 molar sulfuric acid solution, which continues being the most widely used [197].
The performance of MFC systems using Nafion membrane is variable, for instance Zhang et al. [154] reported a power generation of 2,668 mW m−2 in MFC using Nafion membrane 112, DuPont, and López-Zavala et al. [148] reported a power generation of 2,100 mW m−2 in MFC using Nafion membrane 117, DuPont (Table 2). However, this power density cannot be attributed only to the use of these Nafion membranes but to the participation of the whole MFC system.
The mains limitations to the use of this Nafion material are the high cost, oxygen leakage, substrate loss and biofouling. Additionally, if the substrate in the anode chamber is wastewater, or leaches, it is possible to find the presence of other cation species with concentrations higher than that of the protons, which can interfere with the transport of protons and cause a decrease in the performance of the MFCs [165], [195], [196], [199].
Zirfon is another membrane used in MFC. It is an anion exchange membrane with 15 wt% of polysulfone and 85 wt% of ZrO2. Some studies have reported a good performance of its use. For example, Hernandez-Flores et al. [199] compared the performance of Nafion membrane 117 and Zirfon membrane in a SC-MFC, using leachate as substrate. The highest volumetric power (10,380 mW m−3) was obtained with the use of Zirfon membrane rather than the Nafion membrane. They attributed the low result using the Nafion membrane to the alkalinity of its substrate. Sevda et al. [200] compared Zirfon membrane and Fumasep membrane in an SC-MFC air cathode. And they also reported better performance with the use of Zirfon, measured through the maximum power density (424.5 mW −2). They attributed their results to the lower resistance observed with Zirfon compared with Fumasep. Even with these results, Zirfon is not a commonly used membrane in MFCs. Therefore, it is suggested to continue its evaluation with different scenarios (substrates, MFCs configurations, etc.) to be considered as a possible replacement for the Nafion membrane.
Other commercial membranes have also been used in MFCs, some examples of them and the main results obtained are shown in the Table 2. For example, Rabaey et al. [159] reported a power density of 3,600 mW m−2 and Hou et al [149] reported a power density of 2,143.0 mW m−2, both in MFC systems using Ultrex membrane. Mehravanfar et al. [131] reported a power density of 1,287.0 mW m−2, and Xiao et al. [153] reported a power density of 3,600.0 mW m−3 in MFC systems using Ultrex CMI 7,000 membrane international. Although the objectives of these studies were not to specifically evaluate the Ultrex membrane performance and its behavior in MFCs, it was possible to observe energy production equal to or higher than those reported in MFCs using Nafion membrane. Even though high energy production has been reported in MFCs using the Ultrex membrane, as well as with Zirfon membrane, the use of these membranes is still lower than the use of the Nafion membrane. Furthermore, these improvements, as well as those already mentioned with the Nafion membrane, cannot be attributed only to the use of the Ultrex membrane and more detailed studies are necessary to be conducted. Some of the strategies carried out by the scientific community to overcome the challenges of using commercial membranes (especially Nafion) in MFCs are membrane modification or the development of new membrane materials.
In the past years, other types of membranes have also been evaluated. For example J-cloth membrane, dynamic membrane, anion exchange membrane and ultrafiltration membrane. J-cloth membrane is a type of coarse pore filter, that can be configured with layers of cloth. This type of membrane has low ohmic resistance, reduces the oxygen diffusion and favors the ion transport. Even with some good results reported by Fan et al. [201] years ago, it is important to overcome the challenges of its use. For example, a disadvantage of this J-cloth membrane is the biodegradability of the cloth, making it difficult to work with for a long time. Li et al. [202] compared the performance of different membranes such as dynamic membrane, anion exchange membrane, cation exchange membrane, ultrafiltration membrane and J-cloth membrane. The dynamic membrane is composed of a nylon support which serves for the adhesion of filamentous bacteria and vorticellidae-like protozoa. The advantage of these microorganisms is that they reduce the oxygen transfer from the cathodic to the anodic chamber by consuming dissolved oxygen. In this study the use of the dynamic membrane presented the highest power density (3,377 mW m−3). In addition, another advantage was the low acquisition cost, compared to the other membranes. It is important to consider these results and evaluate its feasibility in other MFCs configurations.
Ceramic material with different composition has also reported in the development of low-cost membrane. Jadhav et al. [203] reported the use of a ceramic material as PEM, they reported that the main advantages of its use were the low manufacturing cost compared to the Nafion membrane acquisition cost. In addition, the clay membrane allowed the anodic potential to remain stable throughout the test in a DC-MFC with a sodium hypochlorite (NaOCl) catholyte. This behavior is due to the fact that the clay membrane prevents the free passage of anions from the cathodic to the anodic chamber, since the clay mineral provides a negatively charged surface. Hasani-Sadrabadi et al. [195] evaluated a ceramic material of a modified PEM in an MFC. The membrane was prepared based on montmorillonite, first a montmorillonite was organically modified with 2-acrylamido-d-methylpropanesulfonic acid (AMPS); then, the resulting product was incorporated into Nafion solution; finally, they evaluated its performance in an MFC. Their results showed a power density of 88.0 mW m−2 that was higher than the 39.0 mW m−2 for conventional Nafion 117 (Table 4). Das et al. [204] also used montmorillonite in a clayware ceramic membrane. They fabricated three different membranes, using 20% of montmorillonite then pre-treated each with acid, with neutral water and with alkali. This last membrane pre-treated with alkai showed the best performance of the MFCs compared to the other membranes. They obtained a maximum power density of 83.5 mW m−2, a COD removal of 88%, and a coulombic efficiency of 10.2%. These results indicate increased proton transport and decreased diffusion oxygen and substrate across the membrane. Daud et al. [194] also evaluated a ceramic material as PEM in an MFC. They used raw clay to fabricate a porous clay earthenware membrane in a DC-MFC with 16 mL of volume in each chamber, plain graphite felt anode, graphite felt cathode, synthetic wastewater, mixed consortium and a 100 Ω of external resistor. They reported a maximum power density of 2,250 mW m−2, a CE of 44%, and a COD removal of 99%. These results were higher than the obtained using Nafion 117 membrane, maximum power density of 650 mW m−2, a CE of 23 % and a COD removal of 91%. Cheraghipoor et al. [205] evaluated two ceramic membranes in a DC-MFC. They fabricated these membranes using leached and non-leached soil, and added different concentrations of silicon dioxide (SiO2) to each membrane. They reported that membrane porosity, as well as proton diffusion and conductivity, are properties that influence the performance of MFCs. These characteristics were favored in the membrane made from the leached soil, and the addition of SiO2 did not benefit this improvement. They obtained a low internal resistance of 52.81 Ω, a current density of 1,535 mA m−2, a power density of 20.18 W m−3 a coulombic efficiency of 83% and a COD removal of 93.1%. Like Raychaudhuri et al. [206] also evaluated a ceramic membrane modified with silica. They fabricated a clayware ceramic membrane and added different concentration of silica. Their results showed that using a membrane modified with 30% of silica improve the performance of the MFCs. They obtained a maximum power density of 791.72 mW m−3, a coulombic efficiency of 35.77% and a 76.2% COD removal. These results were attributed to reduced oxygen diffusion, and the increased proton transfer.
Table 4.
Membrane proton conductivity.
| Membrane | Proton conductivity (S cm−1) | Reference |
|---|---|---|
| Nafion AMPS-MMT | 0.0817 | [195] |
| Clayware-20%MT-alkali | 0.0179 | [204] |
| Nafion 117 | 0.0810 | [195] |
| Nafion 117 | 0.0900 | [160] |
| Nafion 117 | 0.0185 | [147] |
| TiO2/SiO2 | 0.0166 | [147] |
| Nanoscale polypyrrole | 0.0208 | [147] |
| SBC-600 | 0.0700 | [160] |
AMPS = 2-acrylamido-d-methylpropanesulfonic acid;
MMT = modified montmorillonite
The eco-friendly materials used to manufacture membranes is another line of research with high potential to be developed. An example of organic membrane material (biochar) was presented by Chakraborty et al. [160]. They reported a power density of 41.08 mW m−2 using a membrane (SBC-600) from food waste. First, they used the pyrolysis process at 600∘C, then, sulphonation from poly vinyl alcohol based matrix, and finally evaluated the SBC-600 performance in an MFC. Although lower power density values than the Nafion 117 membrane were obtained in this research, it was possible to develop a low-cost PEM.
Recently, the nanotechnology has also been used to enhance the PEM performance. Fan et al. [147] obtained a potential of 0.0173 V using internal nanoscale polypyrrole membrane, and also reported a higher proton conductivity using this membrane than the conductivity of Nafion membrane data included in their work (Table 4). However, if the conductivity of this nanoscale polypyrrole membrane is compared with the conductivity of Nafion 117 reported by other authors such as those in Table 4, nanoscale polypyrrole would be positioned as a low conductivity membrane. Therefore, further studies are required.
Regarding biofouling, this problem that can appear in the membrane as a thick layer of microorganism attached in the positively-charged surface of the PEM, which clog the membrane and limit the migration of () ions to the cathode. Recent solutions that were reported in the literature to overcome the biofouling include the development of biochar membrane materials. Chakraborty et al. [160] reported that the use of an SBC-600 membrane that provided a negative surface charge and hydrophilic nature matrix that can act as a protection against the biofouling.
The main limitation of commercial Nafion membrane is well known, its expensive acquisition cost which limits the feasibility of scaling up MFCs systems, while continues to lead as the most widely used membrane in MFCs, followed by the Ultrex membrane. This trend could be attributed to the fact that the properties of these commercial membranes favor the performance of MFCs to a greater extent. This can be observed in some studies that use DC-MFCs configurations and include these Nafion or Ultrex membranes. The results showed the highest energy yield values compared to studies using other membranes or in SC-MFCs without membrane (Table 2). However, these results cannot be attributed only to the type of membrane used, so further studies are needed to broaden the understanding of the effect obtained by the use of different commercial membranes on MFCs.
Regarding the use of alternative PEM, significant progress has been accomplished: i) the use of new membrane materials such as the biochar from food waste; ii) the use of nanotechnology such as nanoscale polypyrrole PEM; iii) the use of Zirfon membrane, j-cloth and dynamic membrane. iv) the use of ceramic materials such as montmorillonite, leached soil, silica and clay membrane. Even with the great efforts made, the gap between the efficiency obtained with new or modified membranes and commercial membranes is high. The main benefit identified by using alternative membranes is the low production cost compared to commercial membranes price. The montmorillonite membrane modified with 2-acrylamido-d-methylpropanesulfonic acid, reported one of the highest values of proton conductivity, 0.0817 S cm−1, followed by the SBC-600 membrane with 0.0700 S cm−1. And the clay membrane showed the highest power density of 2,250 mW m−2, which were higher than the results obtained with the Nafion membrane. Therefore, ceramic materials top the list of materials to develop competitive membranes with the greatest potential for low-cost, high-performance membranes.
5. Electron transfer mechanism
In MFCs systems, oxidation and reduction reactions take place here and the Oxidation-Reduction Potential (ORP) can be used to measure the intensity of the systems to accept electrons (reduce) or donate electrons (oxidize). The oxidation reaction is carried out by the anaerobic digestion of exoelectrogenic microorganisms (also called electroactive or electrogenic) attached to the anode [189]. These electroactive microorganisms obtain energy from the oxidation of a substrate that has a low redox potential and released or donated electrons. Electrons are transferred to the cathode through the external electrical circuit to be reduced by a final electron acceptor with a more positive redox potential [207].
An advantage in these systems is that microorganisms have the ability to regenerate and adapt easily to different environmental and substrate conditions. In the anode, microorganisms degrade substrates such as acetate, glucose, sucrose [208], domestic wastewater [209] (as expressed by Eq. (1), (2), (3) and (4), respectively), lactate, ethanol, synthetic wastewater, animal wastewater, and food waste, then the resulting products are carbon dioxide, electrons, and protons. In the cathode, oxygen (the most widely used electron acceptor due to its high redox potential of 1.229V) is reduced by protons and electrons to form water [76], [108], [127], [166], [210].
| (1) |
| (2) |
| (3) |
| (4) |
The electrons released for the anaerobic digestion of exoelectrogenic microorganisms is commonly known as extracellular electron transfer (EET), which allows electron transfer (ET) between the intracellular metabolism (living system) and external solid materials, electrodes or minerals (non-living systems) [26], [140], [211], [212]. In MFCs systems is essential the conductivity of the biofilm to produce a high energy, and the transfer of electrons is directly related to electricity production [213], [214], [215]. In the past decade the mechanisms for electron transfer were less well understood, but currently is well known that there are different physiological mechanisms of electrons transfer that can involve mediators, pili-nanowires, shuttles and membrane-bound [216].
Fig. 6 shows the schematic representation of these mechanisms which are divided in direct and indirect transfer. Direct electron transfer (DET) is carried out by a direct physical contact between the electrode material and the cell. DET can occur with the participation of membrane-bone cytochrome c (cyt c) and/or pili-nanowires produced by exoelectrogenic bacteria. An advantage of DET has recently been reported, indicating that this mechanism is more adaptive to changes in organic loads or in the presence of contaminants [217]. In MFC the primary pathway of EET is through cytochrome c [218]. The membrane-bound cytochromes contain redox active proteins, and it is possible to transport electrons to the surface of the anode material where the exoelectrogenic microorganisms are attached. The nanowires or pili are conductive tails that transport electrons at distant of 10 μm from the biofilm to the surface of the anode [211], [219], [220]. Previous studies have reported that Geobacter sulfurreducens [212], [214], [221], [222] and Shewanella oneidensis [215] increase their electron transfer ability through the use of nanowires. These microorganisms produce conductive protein filaments (pili/nanowires) as an electrical connection between the surface of the materials and the cell to extracellular electron transfer [223], [224]. Furthermore, in recent years Yalcin et al. [224] identified that nanowires can be modified by changing the pH or applying an electric field in order to improve the conductivity properties.
Figure 6.
Mechanisms of electron transfer by microbial digestion in the anode.
Indirect transfer is made with the help of external soluble redox-mediators (artificial) added to the system or redox-mediators (metabolites) produced by the same microorganisms, even some pollutants present in the medium can act as electron mediators [112], [163]. This electron mediators transfers electrons between an external acceptor-donor and a microorganism. The properties that mediators must have for proper function are: reversible electron transfer reactions, good stability in both oxidation and reduction form, solubility, high negative potential, ease of movement across the bacterial membrane, and long service life. Phenothiazine produced by Pseudomonas aeruginosa [225], thionine [226], humic acids, quinones produced by Lactococcus lactis, flavins produced by S. Omiedensis, etc. are examples of mediated electron transfer (MET) [211]. Some limitations on artificial METs are possible instability and toxicity, making them less attractive to use. One way to explain the EET is by means of Marcus theory, which establishes that the Gibbs energy and the distance between the donor and acceptor are directly related to the rate constant for electron transfer (Eq. (5)). This theory considers electron transfer ; where D is a donor species and A an acceptor species in solution.
| (5) |
where is the Gibbs energy of activation, β is a constant that depends on the medium where the electron travel from donor to acceptor, r is the distance between D and A. According with this, the EET become more efficient as the distance between donor and acceptor decreases. As well as the Gibbs energy becomes more negative [227].
EET in the MFCs is a complex phenomenon in which exoelectrogenic microorganisms can involve one or more of these ET mechanisms [211], [219]. Catalyst microorganisms in the ET are inexpensive, but often suffer from low turnover [184]. Additionally, the main problem is the low electron transfer that impact in the MFC performance. For this reason, the scientific community has invested time in developing different strategies to improve the ET performance, such as controlling environment parameters (pH, temperature, etc.), electrode design, as well as studies of microorganisms and biofilm formation and conductivity [188], [214]. Another important strategy is to add functional groups on the electrodes materials [187], which improves the electrical conductivity and promotes the flow of the release electrons from the site of generation to the cathode.
The modification of the electrodes has been used as strategy to increase the transfer of electrons as reported by Yang et al. [228]. They modeled the EET mechanism using the Marcus theory, based on this they modified the electrodes, specifically the used a mesoporous carbon-modified cathode and high surface area and then evaluated their performance on the MFC. Their results showed a two-fold improvement in power density of 1.18 W m−2 compared to a control MFC using bare carbon cathode. They attributed this to the structure and shape of the porous cathode that allows better electron transfer because it has more active reaction sites.
Zhu et al. [229] and Li et al. [230] also reported an EET improvement with the modification of the anode. Zhu et al. evaluated a bio-reduced graphene oxide (GO)(br-GO) modified carbon felt anode. This modification provided the anode a three-dimensional structure, which improved the attached of the microorganisms (Shewanella putrefaciens) and enhanced the EET between the anode and bacteria. They confirmed an increased on the potential from 0.071V (control) to 0.517V, and a maximum power density of 240.2 mW m−2, four times higher than the control. Li et al. [230] reported the use of the carbon cloth with polydopamine and reduced GO (CC-PDA-rGO) as anode in an MFC. They obtained a maximum power density of 2,047.0 mW m−2 as well as a significant improvement in the EET. These results can be achieved due to the high electrochemically active sites that rGO provided to the material surface and that favored EET, as well as the hydrophilic properties of PDA that strengthen the adherence between the biofilm and the anode.
Another strategy to enhance EET is through the use of eco-friendly, nature feedstock bio-based products, such as that reported by Hemalatha et al. [231]. They used deoiled Azolla pinnata biomass (DAB) to produce substrate and fabricated an anode. First, they obtained biochar from DAB by pyrolysis at 800 ∘C and this biochar was used to fabricate the anode. Then, they obtained reducing sugar from the hydrolysis of DAB which was used as substrate. Finally, they evaluated these anode and substrate in an MFC. Their results showed a maximum power density of 105 mW m−2 with 65.6% COD reduction. They identified the increase of the ET due to the improvement of the complex IV cytochrome c couples (cytochrome Cox (Cyt Cox) / cytochrome Crd (Cyt Crd)). These improvements were attributed to the use of Azolla, which increased bioelectrogenic activity on the surface anode and decreased electron losses.
Other studies have been focused on the use of nanotechnology in the cathode to enhance the EET. Liang et al. [232] documented the use of nanoparticles of nitrogen-doped porous carbon (NC) encapsulating CoO and MgO nanoparticles (CoO/MgO@NC) as better cathode catalyst in an air-cathode MFC. The study shows a maximum power density of 2,258.0 ± 70 mW m−2, which is 58.3 % higher than the control NC. The higher power density can be attributed to the use of the CoO/MgO@NC catalyst that promoted the ET, reduced internal resistance, provided more active sites, and increased the ORR. Liang et al. [233] also, carried out a similar study synthesizing, optimizing and measuring a nitrogenous mesoporous carbon coated with Co and Cu nanoparticles to modify activated carbon as a cathode catalyst in an air-cathode MFCs. The air-cathode was composed first, by a gas diffusion layer; then, by a mesh titanium as current collector; finally, by the catalytic layer (the air-cathode was fabricated according to rolling method reported by Dong et al. [234]). They obtained a power density results of 2,033.0 mW m−2, which was 147.0 % higher than the power density obtained by bare activated carbon. They attributed their result to the action of the pyridinic-N, cobalt and copper nanoparticles of the catalyst that improved the oxygen reduction reaction, increased the active sites, provided a high surface area of catalyst, promoted electron transfer and enhanced the electrochemical performance of the cathode. Su et al. [188] reported an improvement in the electron-transfer at bacteria-electrode interface with modifications in the anode and additionally on the biofilm. They formed a hybrid biofilm with Shewanella loihica as biomass and the addition of TiO2@TiN nanocomposite, then tested in a DC-MFC. Their results showed a maximum power density of 64.4 mW m−2 which represents a 92.8% increase compared with the pure biofilm test. They attributed these results to the addition of TiO2@TiN nanocomposites that favored the formation of high conductivity, decreased resistance to charge transfer, and increased secretion of MET (flavin and cytochromec). Therefore, these approaches showed that TiO2@TiN nanocomposite altered the metabolism of Shewanella loihica which stimulated both direct and indirect electron transfer.
Evaluation of anolytes and mediators to increase electron transfer has also been reported [218], [226], [235]. Wang et al. [218] reported the effect of anolityc nitrate at relatively low concentrations in the enhancement of the electricity generation capability of MFCs. The study proved that nitrite could react as the co-matrix on the electrode (in concentrations of less than 60 mg L−) and promote electricity generation. Precisely, it transfers electrons to the anode throughout the nitrite-to-nitrate conversion, supporting electricity. However, conversely, at higher concentrations, nitrite inhibits the action of the electrogenic bacteria, lowering the production of cytochrome c and extracellular polymeric substances; and therefore, the coulombic efficiency suffers a decrease as well. Wu et al. [235] reported the use of the micropollutant sulfamethoxazole (SMX) as mediator, a type of antibiotic, which is capable of increasing the activity of the electroactive biofilm in an MFC, acting as an agonist in improving the regulation of the electroactive biofilm; and to extend the energy production duration. The results showed that the maximum power density is upgraded by 18.0% to 40.0 W m−3, in comparison with the absence of SMX in the MFC. Therefore, the SMX as a micropollutant, can boost the performance of electroactive biofilms and be degraded at the same time. Choi et al. [226] used a thionine mediator and presented an assessment of the effect of temperature and ethanolic stresses on the coulombic efficiency of an MFCs. They used a DC-MFC, with a vitreous carbon anode, platinum plate cathode, Nafion cation exchange membrane, Proteus vulgaris biomass, thionine transfer mediator, and 560 Ω external resistor. Their results showed that coulombic efficiency and the thionine were very sensitive to temperature changes and decreased dramatically at high temperatures. However, membrane lipids were able to adapt to these changes and allowed the electron transfer mediator to be more impermeable.
On the other hand, some studies have focused their interest on improving the transfer of electrons through the evaluation of different catholytes and different final electron acceptors. In MFCs systems the oxygen is the most suitable electron acceptor due to its high oxidation potential (+1.229V) towards the reduction reaction, natural availability, sustainability, and low cost. The electrochemical oxygen reduction reaction is carried out in the DC-MFCs and MFCs air-cathode. Some of the challenges to overcome to make MFCs feasibly to field-scales is the low energy production, which is typically limited due the kinetic problems of the ORR and deficient ion transfer on the cathode [183], [184].
The ORR can be carried out in function of the electrolyte pH in alkaline and in acidic media, high activation overpotentials and sluggish kinetics. Table 5 shows the thermodynamic reaction potential for ORR. In alkaline electrolyte the following reactions occur: i) 4e− reduction of O2 to OH− ii) 2e− reduction of O2 to HO and OH−. These reactions take place if the proton concentration is low, and can increase the pH of the solution due to the accumulation of OH−. Whereas in acidic electrolyte the pathways can be: i) 4e− reduction of O2 to H2O; ii) 2e− reduction of O2 to H2O2 [183], [236]. When the proton concentration is high, the reduction of oxygen is carried out by these reactions. And an intermediate product of incomplete oxygen reduction is , therefore, is undesired during the ORR process [184], [237].
Table 5.
ORR pathways in aqueous electrolytes and thermodynamic potentials at standard conditions [192], [193], [236], [238], [239].
| Electrolyte | Electrons | Reaction | Potential (V) | ||
|---|---|---|---|---|---|
| Alkaline | Direct way | 4e− | O2 + 2H2O + 4e− | →4OH− | +0.401 |
| Indirect way | 2e− | O2 + H2O + 2e− | -0.065 | ||
| 2e− | →3OH− | +0.867 | |||
| Acidic | Direct way | 4e− | O2 + 4H+ + 4e− | →2H2O | +1.229 |
| Indirect way | 2e− | O2 + 2H+ + 2e− | →H2O2 | +0.670 | |
| 2e− | H2O2 + 2H+ + 2e− | →2H2O | +1.770 | ||
| Chemical decomposition | 2H2O2 | →2H2O + O2 | +1.776 | ||
Nevertheless, the maximum power output in the MFC is limited to a certain value, which is not completely available. The power can be calculated with the current, the open circuit voltage (OCV), and the potential of the oxidation-reduction reaction in the anode and cathode. This OCV represent the difference potential between the anode and the cathode [189]. If it is considered the oxidation of the sodium acetate in the anode and the oxygen reduction to water in the cathode (Eq. (1)), the associated OCV is 1.0 V, but this maximum potential is not completely available. Recent studies suggest that to increase the power production is better to connect several small MFCs, than increase the size of an individual system [131].
One of the strengths that underline the potential of the MFCs technology is the ability to adapt to different operating conditions, which has allowed the successful use of other electron acceptors. In the literature also is reported the use of other electron acceptors, such as manganese dioxide, nitrobenzene, ferric iron, hydrogen phosphate, hydrogen peroxide, ferricyanide [226], copper chloride, potassium hexacyanoferrate (K3[Fe(CN)6]), perchlorate and nitrates , hydrochloric acid (HCl), sodium hypochlorite (NaOCl), and nitric oxide (NO) [11], [148], [159], [240], [241], [242]. Among these, (K3[Fe(CN)6]) is the most widely used, one of the main reasons is the low overpotential generated when carbon-based material is used as cathode [120] Additionally, the ferric ion complex increased electron transfer rates [243].
Table 6 shows some oxidation-reduction reactions that can occur in the MFCs systems, these reactions depend on the available substrate to be oxidized in the anodic chamber, as well as on the final electron acceptor available on the cathodic chamber. The table also indicate the potential under standard conditions and other conditions.
Table 6.
Potentials of oxidation and reduction reactions for different substrates and final electron acceptors.
| Electrode | Reaction | E0 (V) | pH | Reference | |
|---|---|---|---|---|---|
| Anode | 2H+ + 2e− | →H2 | 0 | pH=7 | [26] |
| Anode | →CH3COO− + 4H2O | 0.187 | pH=7 | [26], [210] | |
| Anode | →CH3COO− + 3H2O | 0.130 | pH=7 | [26] | |
| Anode | 6CO2 + 24H+ + 24e− | →C6H12O6 + 6H2O | 0.014 | pH=7 | [26], [166] |
| Cathode | 2O2 + 8H+ + 8e− | →4H2O | 1.230 | pH=7 | [84], [210] |
| Cathode | O2 + 4H+ + 4e− | →2H2O | 1.229 | pH=7 | [26], [192], [232] |
| [244], [245] | |||||
| Cathode | O2 + 2H+ + 2e− | →H2O2 | 0.695 | pH=7 | [192], [245] |
| Cathode | O2 + 2H+ + 2e− | →H2O2 | 0.695 | pH=10 | [26] |
| Cathode | 0.361 | pH=7 | [26] | ||
| Cathode | MnO2(S) + 4H+ + 2e− | →Mn2+ + 2H2O | 1.229 | pH=7 | [26] |
| Cathode | →MnO2 + 2H2O | 1.680 | pH=7 | [244] | |
| Cathode | →MnO2 + 2H2O | 1.680 | pH=3.5 | [26] | |
| Cathode | Mg(OH)2 + 2H+ + 4e− | →Mg2+ + 2H2O | - | pH=7 | [232], [246] |
| Cathode | 2NO + O2 + e− | - | pH=7 | [242] | |
| Cathode | NO + H+ + e− | →HNO | - | pH=7 | [242] |
| Cathode | 2HOCl + 2H+ + 2e− | →Cl2 + 2H2O | - | pH=7-8 | [241] |
| Cathode | →Cr3+ + 4H2O | 1.330 | pH=7-8 | [243] | |
| Cathode | →Br− + 3H2O | 1.440 | pH=7-8 | [247] | |
| Cathode | BrO− + 2H+2e− | →Br− + H2O | - | pH=3 | [247] |
| Cathode | →MnO2 + 2H2O | 1.700 | pH=3.5 | [26] | |
| T=303∘K | |||||
Several studies with MFCs have reported improvements on the power densities and the electron transfer when they use non-oxygen electron acceptors. For instance, Lawson et al. [248] used ferricyanide as catholyte on graphite fiber brush cathode in a DC-MFC and reported a power density of 2.46 W m−2, higher than 1.33 W m−2 obtained with the use of an air-cathode. Jadhav et al. [241] (Table 6) used NaOCl as catholyte and reported a maximum power density of 0.148 W m−2 which was better than 0.017 W m−2 obtained with oxygen as electron acceptor. As well as a lower electron transfer coefficient with NaOCl as catholyte than with oxygen, they attributed to the fact that less energy is required in activation for ET using NaOCl. Shi et al. [242] (Table 6), evaluated NO and O2 as electron acceptors, they reported a maximum power density of 1.23 W m−2, which was better than 0.710 W m−2 using O2 as electron acceptors, their results also showed that electron transfer was favored. Dai et al. [247] (Table 6), tested sodium bromate as a cathodic electron acceptor in a dual-chamber MFC. They tested different concentration of sodium bromate (from 10 to 100mM) as catholyte and the catholyte pH was adjusted (for 7.0, 3.0, 5.0 and 10.0 values) with the addition of hydrochloric acid (100 mM) and sodium hydroxide (100 mM). The results showed that the power generation increased as the concentrations of sodium bromate did; and that the optimum concentration was 100mM, with a maximum power density of 1.491 W m−3, a voltage output of 0.538 V and a higher exchange current density that means a faster electron transfer were obtained at a 3.0 pH value.
Hydrochloric acid (HCl) is another catholyte used in a DC-MFC, as was reported by López-Zavala at el. [148] They carried out the assessment of an MFC with graphite electrodes, Nafion 117 PEM, 0.1 M HCl used as catholyte, and wastewater used as anolyte, and both chambers under oxygen-free conditions. Their results showed a COD removal of 95%, and a maximum power density of 2.1 W m−2. These results were attributed to the diffusion of HCl from the cathodic chamber to the anodic chamber through the semipermeable PEM. The diffusion of HCl allowed the pH of the anolyte to decrease from approximately 7 to 3, which enhanced hydrolysis and inhibited methanogenic activity, thus enhancing anaerobic degradation and energy production.
Some pollutant compounds have also been evaluated as electron acceptors. For instance, Kim et al. [249] reported the use of wastewater with Cr (VI) from electroplating processes as MFC catholyte. They used a bio-polar membrane (which allows dissociating water to H+ in cathodic chamber and OH− in anodic chamber) to stimulate the Cr (VI) as the final electron acceptor and obtained a maximum power density of 0.151 W m−2. Finally, Cr (VI) was reduced and precipitated in the cathodic chamber, which favors its removal.
Analysis of these scientific reports highlights the influence of conductivity in the biofilm on the effective performance of MFCs. An extensive study carried out on the isolated strains such as Geobacter sulfurreducens and Shewanella oneidensis has identified them as strains with high conductive properties. The high conductive properties are due to the natural production of conductive pili/nanowires for extracellular electron transfer. However, there is still a gap in the knowledge of ET in mixed consortium systems. MFCs operate in large numbers with mixed consortia biofilms, therefore, it is important to study them in depth to understand their contribution to electron transfer, and which influences energy production. Furthermore, different types of exoelectrogenic microorganisms can also generate networks that allow them to carry out electron exchange through the simultaneous interaction of ET mechanisms. Electrodes, substrate and catholyte compositions have been the main avenues of study in order to favor ET and energy production in MFCs systems. The main strategies include the following: i) The importance of the redox potential in MFCs. This can be used to deduce the oxidation-reduction reactions that may be occurring in the MFCs. It can also be used to identify the tendency to donate or receive electrons in the cathodic and anodic chambers, as well as to identify the operational conditions that favors ET and thus the overall performance of the MFC system. ii) Modification of the materials used as anodes. In these studies, it was observed that the modifications of the anode can increase their active sites, as well as their surface area. Therefore, the adherence of electrogenic microorganism and the electron transfer are significantly favored. It is worth noting that the studies that have used this strategy have reported the highest power density. iii) The use of nanotechnology both in anodes and cathodes. Among the advantages reported in studies using this strategy are improved catalytic properties of the cathode, improved ORR, increased ET, as well as favored the interface between the microorganisms and the anode. Together result in an improved the energy production of the MFCs. iv) The use of bio-base materials both in anodes and cathodes. It was observed that the main achievement of this strategy lies in the improving of ET and the decrease of electron losses by developing eco-friendly and low-cost materials, but with lower power density output compared to those mentioned above. v) The use of specific anolytes. In these studies were reported the use of nitrate, but with some limitations for ET linked to anolyte concentrations. The use of anolytes containing micropollutants such as sulfamethoxazole. This strategy has great potential for further development and application in the treatment of specific industrial wastewater in which some contaminants can be used to increase the ET of the MFCs systems and at the same time degrade them. vi) The use of non-oxygen final electron acceptors. Several electron acceptors have been reported with lower and higher magnitude of ET enhancement.
It was noted that their incorporation can be as catholytes or as gases. Studies have been carried out under different pH conditions and have reported different improvements in power densities compared to those using oxygen. Among electron acceptors, HCl and ferricyanide stands out for their high power density production. The use of industrial wastes in the cathodic chamber has also been reported to utilize the micropollutants as a final electron acceptor, such as Cr (VI) which can also be successfully degraded. This information highlights the versatility of MFCs and their ability to adapt to different scenarios that can improve their ET and therefore, their performance. Some triggers for the successful development of MFCs are the purpose of their use, the substrate to be used, the energy production expected, as well as easily accessible operating conditions. If the purpose is to increase the power density, it is suggested to deepen the use of nanotechnology in the electrodes as well as the use of HCl as a catholyte, since these are strategies with a favorable potential for further research. And if the objective is the degradation of specific pollutants, it is suggested to continue with the improvement to degrade pharmaceuticals such as sulfamethoxazole in the anodic chamber or conversion of heavy metals such as Cr to less toxic form in the cathodic chamber, of which high removal rates have been reported, and which have simultaneously increased bioenergy production.
6. Costs of electrodes and proton exchange membrane
The high cost of potential energy production is another bottleneck that limits the MFC systems to be scalable as a feasible technology for industry and society. The MFC systems require of proper designs, construction, operation, and maintenance to function. Each stage involves a cost and can be identified the followings: cost of construction, construction materials, electrodes, membrane, substrate, inoculum preparation, catholyte, energy for stirring, aeration, temperature, workforce, etc. The scientific community has shown great interest in improving the performance of electrodes, membrane, and the configuration of the MFCs, which represent the main fraction of the overall cost of the systems. One of the challenges is to obtain an efficient MFC in both the technical and economic aspects [131] that can be offered as a commercial alternative to treat wastewater and the simultaneous energy production.
A wide variety of MFCs electrodes material has currently been reported. The bioanode materials and the ORR catalyst cathode materials represent a large percent of the overall cost of MFCs [169]. Rozendal et al. [250] have reported an estimate of the cost of bioelectrochemical systems and MFCs are considered part of them. They assumed a laboratory-scale system with a graphite felt anode, a platinum-catalysed cathode and a laboratory membrane such as Nafion. Under this scheme they reported that the anode represented 9.4%, the cathode 47% and the membrane 38%, which placed the cathode as the most expensive element of the system. However, as already mentioned in previous paragraphs, the use of low-cost electrodes and membrane materials with competitive performance compared to commonly used materials have been reported. Therefore, an analysis of recent cost data for the main electrode and membrane materials is presented below to provide an overview of the economic feasibility of MFCs systems.
As already mentioned, the metal-based electrode materials are used due to their conductivity properties. However, the main problem with these materials is their high cost, which makes them difficult to use in large-scales MFCs systems. An alternative, already discussed, is the use of carbon-based electrodes, these materials are extensively used in MFCs because of their properties and affordable price.
Table 7 shows the cost of some of the materials used as electrodes and catalysts in MFCs. Several studies have been focused on development metal-base material electrodes to improve their performance and in that sense offset the acquisition value. Copper and stainless steel are examples of low-cost metal-base materials. Likewise, the studies focused their objectives on improving cathode catalysis, as mentioned in previous section 4.1, have succeeded in developing catalysts that are less expensive than those commonly used, such as platinum.
Table 7.
Cost and specifications of electrode materials.
| Electrodes | Dimensions | Price (US$ /g) | Reference | |
|---|---|---|---|---|
| Anode and Cathode materials | ||||
| 1 | 40% Pt on Carbon Paper, density 0.03 mg cm−2 | 5.00 x 5.00 cm, | 33,334.00 | [251] |
| 2 | 20% Pt on Carbon Cloth, density 0.03 mg cm−2 | 5.00 x 5.00 cm, | 20,000.00 | [251] |
| 3 | Reduced graphene oxide | 0.25 g | 947.20 | [252] |
| 4 | Graphene oxide | 1.00 g | 152.40 | [252] |
| 5 | Graphene paper, density 1.9 g cm−3 | 29.21 x 64.77 cm, | 100.00 | [252] |
| 6 | Graphite Felt, density 28.5 mg cm−2 | 20.00 x 20.00 cm, | 1.32 | [253] |
| 7 | Graphene nanoplatelets | 500.00 g | 0.58 | [252] |
| 8 | Graphite Plates, thickness 1.27 cm, density 0.066 lb in−3 | 15.24 x 15.24 cm, | 0.21 | [254] |
| 9 | Graphite Plates, thickness 1.27 cm, density 0.066 lb in−3 | 20.32 x 20.32 cm, | 0.18 | [255] |
| 10 | Stainless Steel sheet, thickness 0.091 cm, density 1.52 lb ft−2 | 30.48 x 60.96 cm, | 0.08 | [256] |
| Cathode materials | ||||
| 11 | Pt catalysts | 150.00 | [174] | |
| 12 | 10% Pt/C catalysts | 60.00 | [257] | |
| 13 | 10% Pt/C catalysts | 46.61 | [177] | |
| 14 | 10% Pt/C catalysts | 40.80 | [258] | |
| 15 | 10% Pt/C catalysts | 33.00 | [232], [259] | |
| 16 | 10% Pt/C catalysts | 27.00 | [181] | |
| 17 | Copper | 5.53 | [172] | |
| 18 | V2O5-NR/rGO | 4.80 | [259] | |
| 19 | Co/Cu@NC | 4.20 | [233] | |
| 20 | Fe-N-C | 3.50 | [174] | |
| 21 | NPOM | 3.00 | [178] | |
| 22 | CoO/MgO@NC | 1.80 | [232] | |
| 23 | N-G @CoNi/BCNT | 0.30 | [177] | |
| 24 | CuZn catalysts | 0.09 | [181] |
NC = Nitrogen doped carbon; V2O5-NR = Vanadium pentoxide nanorods; Fe-N-C = Iron-base Nitrogen-carbon
Das et al. [181] evaluated the use of CuZn microparticles as a cathode catalyst in a DC-MFC with a 150 mL cathode chamber. Their compared their results with those obtained for Pt/C catalyst in a similar system. One of the great advantages reported in this paper was the use of low-cost materials. They reported that the cost of 1 kg of CuZn catalyst was 300 times less expensive than 1 kg of 10% Pt/C catalyst as shown in Table 7. This suggests a great achievement using CuZn as a cathode catalyst on a large scale. However, different results were obtained when CuZn catalyst in a 25 L cathode chamber was used and low bioenergy production was obtained. Therefore, further research is needed with the aim of identifying the causes of the decrease in energy production when the MFC size is increased.
Similar works were conducted by Song et al., Hou et al., and Liang et al. [177], [178], [232], [233] Table 7 (for more details see sections of 4.1 Electrodes and 5 Electron transfer mechanism). They reported the use of the following alternative catalysts in which their low cost compared to platinum-based catalysts stands out. Song et al. [178] reported that the use of NPOMC as cathode catalyst represented less than 5% of the cost of Pt-based catalysts and, taking into account the cost of Pt/C, the estimated cost of NPMOC would be US$ 3.00 per gram. Hou et al. [177] evaluated the use of N-G@CoNi/BCNT catalyst and their cost was 200 times less expensive than Pt/C, which was estimated about US$ 0.30 per gram. And Liang et al. [232], [233] evaluated the use of CoO/MgO@NC catalyst and Co/Cu@NC catalyst and these catalysts represented US$ 1.8 and US$ 4.20 per gram, respectively.
Furthermore, another advantage of developing new catalysts compared to platinum catalyst is that the latter can easily be attacked by sulfur-based contaminants commonly present in wastewater [174], [184]. Even with the progress showed in these MFCs studies that evaluated new low-price catalysts, platinum-base catalysts reported higher energy efficiency, as well as higher prices. The cost of using 10% Pt/C catalysts is around US $ 60.00 per gram (7). This make large-scale systems difficult to be implemented.
The cost analysis of electrodes materials presented in this study Table 7 and Fig. 7 show a wide range of costs. The materials that can be used for both anode and cathode require particular evaluations in each MFC system in order to establish which electrode exerts the greatest economic weight in the system or, if it does not represent a transcendent effect due to its similarity in cost. According to literature review, there is evidence that highlight carbon-base materials as materials with great potential and affordable cost for electrodes. Among them, graphite is the most economical material followed by graphene, and both materials reported high rates of power density and organic matter degradation when they were used as electrodes in MFCs. Therefore, it is appropriate to continue the development of these materials to improve the performance of MFCs at the lowest cost.
Figure 7.
Cost of electrodes materials.
On the other hand, when MFCs systems use platinum-base catalyst materials for the cathode, they substantially increase their cost compared to the anode cost. Even though low-cost catalyst alternatives have been developed that would allow establishing an economic feasibility for scaling up MCS, the gap in energy production rates still continues. Some of the works that have evaluated these new catalytic materials has reported low energy production rates compared to their rates obtained using platinum-base catalyst. One material with great potential can be carbon-based, and it is also possible to include the use of nanotechnology and some alloys with low-cost metals such as Ni. Therefore, it is advisable to continue the development of these alternative catalytic materials with the objective of replacing platinum-based catalysts and favoring the balance in the cost/performance ratio of MFCs systems.
High membranes cost also affects the total cost of the MFCs, Table 2 shows examples of membrane materials typically used in DC-MFCs. As mentioned, the proton exchange membranes distributed by Nafion Dupont Inc., USA are the most widely used for their commercial availability and high proton exchange efficiency. However, the Nafion membrane tops the list of the materials with the highest price and also represents a high fraction of the overall cost of the MFCs systems [160] (Table 8), the price varies between US $ 9,000.00 and US $12,000.00 m−2 approximately. When the MFCs devices are built for laboratory-scale essay, the cost of the Nafion membrane does not generate a particular problem since the membrane dimensions in most devices do not exceed 0.5 m2. Nevertheless, when the project of MFCs is for larger scales the membrane becomes a big challenge, even though the power density generated is among 1,200.0 mW m−2 and 2,700.0 mW m−2 [148], [149], [154], thus the expensive membrane is a limitation that makes the MFCs an economically unfeasible technology nowadays.
Table 8.
Cost of PEM material.
| Membrane material | Thickness (cm) | Price (US$ m−2) | Reference |
|---|---|---|---|
| Nafion 117, DuPont | 0.01778 | 9,213.91 | [260] |
| Nafion 115, DuPont | 0.01778 | 10,290.30 | [260] |
| Nafion 112, DuPont | 0.01778 | *D | [260] |
| J-Cloth | 0.03 | 400.00 | [202] |
| Ultrex CMI 7,000 | 0.045 ± 0.0025 | 291.67 | [261] |
| Ultrex CMI 7,000 | 0.045 | 200.00 | [202] |
| SBC-600 | - | 77.00 | [160] |
| PVA sulfosuccinic acid | 0.016 - 0.018 | 1.88 | [262] |
| Dynamic membrane | 0.0225 | 0.30 | [202] |
*D= discontinue-product; PVA=polyvinyl alcohol
The scientific community has shown interest in implementing or innovating other membrane materials in MFCs. The main aims are to reduce the acquisition cost of the membrane, as well as to improve the proton exchange functionality. Ultrex membrane material distributed by Membrane International Inc. (Ringwood, New Jersey, USA) is one of the innovations with a price about 30 times lower than the Nafion on the order of US $291.67 per m2 (Table 8). The energy production in MFC with Ultrex membrane ranges between 1,287.0 mW m−2 and 3,600.0 mW m−2 which is higher than the systems that use Nafion membrane [131], [149], [153], [159] (Table 2). However, still few number of studies report the use of this membrane in MFC systems.
Another effort to reduce the membrane cost is presented by Chakraborty et al. [160], they used food waste (biochar) as part of the feedstock to make the SBC-600 membrane. The performance obtained with this SBC-600 membrane is slightly below than that obtained with the Nafion membrane. However, the advantage came from the cost of the SBC-600 membrane, which was 100 times cheaper than the Nafion membrane (Table 8). The dynamic membrane and PVA sulfosuccinic acid also stands out as a low-cost membranes with great potential to improve the performance of the MFCs. For these low-cost membranes, additional evaluations are recommended to complement the data and to obtain a better understanding of the behavior of the MFCs using different conditions, such as real domestic and industrial wastewater.
This cost analysis shows that Ultrex membrane is a commercial membrane with a more affordable purchase cost, compared to Nafion membrane. Besides, it was reported similar energy production in comparison with Nafion membranes in MFCs (2). However, it has been observed that the performance of the MFCs with Ultrex membrane and domestic wastewater as substrate is lower than those systems using Nafion membrane. Even though it is clear that the cost of Nafion membrane is the highest, and that makes it the main limitation to scale up MFCs. Their high protons permeability, resistance to different operating conditions, wide pH ranges and low gas crossover are some of the advantages for which it is still the most efficient and widely used proton exchange membrane in laboratory scale MFCs. Among the alternative PEM materials with the greatest potential, ceramic materials were identified and the main strengths of their use are their low manufacturing cost, high stability and resistance. Therefore, ceramic materials can be considered as a potential alternative to develop new membranes that can substantially reduce manufacturing costs, substitute the Nafion membrane and make the application of MFCs feasible in practical scenarios.
7. Conclusions
In this work, review and analysis of most recent scientific publications on MFCs technology have been performed in order to assess the contribution of configurations, electrode and membrane materials, electron transfer mechanisms, and cost of components on the current and future development of MFCs. Dual-chamber MFCs stand out for their excellent capability to adapt of the needs of the users. Likewise, the use of carbon-based electrode materials stands out as the ideal choice. In addition, it is possible to enhance their performance through the application of nanotechnology. The economic feasibility and potential for increasing power densities in MFCs are the main strengths of carbon-based material. Besides, the use of clay for membrane fabrication is suggested due to the low cost of both, the material itself and its manufacturing process. Also, this membrane produces power density similar to that obtained with the Nafion membranes. The use of a mixed consortium for biofilm formation at the anode, with domestic wastewater as substrate in the anodic chamber and HCl as catholyte, are the most favorable conditions, since they resemble those found in real scenarios. In this way, it is possible to consider wastewater as a potential source of energy and reusable water, rather than a hazardous waste. Therefore, it is strongly suggested that the main findings of this study be taken into account in order to continue adding efforts in the development and effective application of MFCs.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
This work was supported by the National Council of Science and Technology (CONACYT) of Mexico and the Tecnológico de Monterrey.
Contributor Information
Fátima Borja-Maldonado, Email: a00829683@tec.mx.
Miguel Ángel López Zavala, Email: miganloza@tec.mx.
References
- 1.United Nations World Water Assessment Programme . The United Nations World Water; Paris: 2014. UNESCO, The United Nations World Water Development Report 2014: Water and Energy.https://www.un.org/waterforlifedecade/water_and_energy.shtml [Google Scholar]
- 2.U. N. W. W. A. Programme . The United Nations World Water; Francia: 2016. UNESCO, The United Nations World Water Development Report 2017. Water and Jobs.http://www.unwater.org Tech. Rep. Report 2017. [Google Scholar]
- 3.United Nations Educational, Scientific and Cultural Organization, International Centre for Water Security and Sustainable Management, Water reuse within a circular economy context, United Nations Educational, 2020, CC bY-SA 3.0 IGO [7886].
- 4.Health World Organization Water . World Health Organization; Geneva: 2018. Water, Sanitation and Hygiene. STRATEGY 2018-2025.http://apps.who.int/iris Tech. Rep., WHO/CED/PHE/WSH/18.03. [Google Scholar]
- 5.Corcoran E., Nellemann C., Baker E., Bos R., Osborn D., Savelli H. 2010. The Central Role of Wastewater Management in Sustainable Development. Tech. Rep., Environment Programme/United Nations Human Settlements Programme/GRID-Arendal (UNEP/UNHabitat) [Google Scholar]
- 6.BP p.l.c., BP statistical review of world energy 2020, Review, BP Statistical Review of World Energy. 2020. https://www.bp.com/
- 7.United Nations Climate change. jan 2016. https://www.un.org/en/sections/issues-depth/climate-change/index.html
- 8.Zhao W., Ci S. In: Nanomaterials for the Removal of Pollutants and Resource Reutilization, Micro and Nano Technologies. Luo X., Deng F., editors. Elsevier; 2019. 7 - Nanomaterials as electrode materials of microbial electrolysis cells for hydrogen generation; pp. 213–242.http://www.sciencedirect.com/science/article/pii/B978012814837200007X [Google Scholar]
- 9.The United Nations World Water; París, Francia: 2017. S. a. C. O. United Nations World Water Assessment Programme, Ryder, Guy, UNESCO, The United Nations World Water Development Report 2017. Wastewater: The Untapped Resource.www.unwater.org [Google Scholar]
- 10.Venkata Mohan S., Chiranjeevi P., Chandrasekhar K., Babu P.S., Sarkar O. Biohydrogen. Elsevier; 2019. Acidogenic biohydrogen production from wastewater; pp. 279–320.https://linkinghub.elsevier.com/retrieve/pii/B9780444642035000113 [Google Scholar]
- 11.Yang Y., Liu T., Zhu X., Zhang F., Ye D., Liao Q., Li Y. Boosting power density of microbial fuel cells with 3D nitrogen-doped graphene aerogel electrode. Adv. Sci. 2016;3(8) doi: 10.1002/advs.201600097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai W., Xu X., Yang F. High-rate contact stabilization process-coupled membrane bioreactor for maximal recovery of organics from municipal wastewater. Water. 2018;10(7):878. http://www.mdpi.com/2073-4441/10/7/878 [Google Scholar]
- 13.McCarty P.L., Bae J., Kim J. Domestic wastewater treatment as a net energy producer–can this be achieved? Environ. Sci. Technol. 2011;45(17):7100–7106. doi: 10.1021/es2014264. [DOI] [PubMed] [Google Scholar]
- 14.Capodaglio A., Cecconet D., Molognoni D. An integrated mathematical model of microbial fuel cell processes: bioelectrochemical and microbiologic aspects. Processes. 2017;5(4):73. http://www.mdpi.com/2227-9717/5/4/73 [Google Scholar]
- 15.Logan B.E., Hamelers B., Rozendal R., Schröder U., Keller J., Freguia S., Aelterman P., Verstraete W., Rabaey K. Microbial fuel cells: methodology and technology †. Environ. Sci. Technol. 2006;40(17):5181–5192. doi: 10.1021/es0605016. [DOI] [PubMed] [Google Scholar]
- 16.Min B., Logan B.E. Continuous electricity generation from domestic wastewater and organic substrates in a flat plate microbial fuel cell. Environ. Sci. Technol. 2004;38(21):5809–5814. doi: 10.1021/es0491026. [DOI] [PubMed] [Google Scholar]
- 17.Tang C., Zhao Y., Kang C., Yang Y., Morgan D., Xu L. Towards concurrent pollutants removal and high energy harvesting in a pilot-scale CW-MFC: insight into the cathode conditions and electrodes connection. Chem. Eng. J. 2019;373:150–160. https://linkinghub.elsevier.com/retrieve/pii/S1385894719310496 [Google Scholar]
- 18.Ge Z., He Z. Long-term performance of a 200 liter modularized microbial fuel cell system treating municipal wastewater: treatment, energy, and cost. Environ. Sci., Water Res. Technol. 2016;2(2):274–281. [Google Scholar]
- 19.Hiegemann H., Littfinski T., Krimmler S., Lübken M., Klein D., Schmelz K.-G., Ooms K., Pant D., Wichern M. Performance and inorganic fouling of a submergible 255 l prototype microbial fuel cell module during continuous long-term operation with real municipal wastewater under practical conditions. Bioresour. Technol. 2019;294 doi: 10.1016/j.biortech.2019.122227. https://www.sciencedirect.com/science/article/pii/S0960852419314579 [DOI] [PubMed] [Google Scholar]
- 20.Blatter M., Delabays L., Furrer C., Huguenin G., Cachelin C.P., Fischer F. Stretched 1000-l microbial fuel cell. J. Power Sources. 2021;483 https://linkinghub.elsevier.com/retrieve/pii/S0378775320314245 [Google Scholar]
- 21.Potter M.C. Electrical effects accompanying the decomposition of organic compounds. Proc. R. Soc. Lond. B, Biol. Sci. 1911;84(571):260–276. [Google Scholar]
- 22.Davis J., Yarbrough H., Jr. Preliminary experiments on a microbial fuel cell. Science. 1962;137(3530):615–616. doi: 10.1126/science.137.3530.615. [DOI] [PubMed] [Google Scholar]
- 23.Bennetto H.P., Stirling J.L., Tanaka K., Vega C.A. Anodic reactions in microbial fuel cells. Biotechnol. Bioeng. 1983;25(2):559–568. doi: 10.1002/bit.260250219. [DOI] [PubMed] [Google Scholar]
- 24.Elsevier B.V Scopus - analyze search results | microbial fuel cells. 2022. https://www.scopus.com
- 25.Kim H., Hyun M., Chang I., Kim B. A microbial fuel cell type lactate biosensor using a metal-reducing bacterium, Shewanella putrefaciens. J. Microbiol. Biotechnol. 1999;9(3):365–367. https://www.scopus.com/inward/record.uri?eid=2-s2.0-0344780799&partnerID=40&md5=ccb9c75154bc8693f136ce76a38e87c8 [Google Scholar]
- 26.Logan B.E. Wiley-Interscience; United States of America: 2008. Microbial Fuel Cells.http://0-search-ebscohost-com.biblioteca-ils.tec.mx/login.aspx?direct=true&db=cat03431a&AN=bdis.b1167887&lang=es&site=eds-live&scope=site [Google Scholar]
- 27.Santoro C., Arbizzani C., Erable B., Ieropoulos I. Microbial fuel cells: from fundamentals to applications. A review. J. Power Sources. 2017;356:225–244. doi: 10.1016/j.jpowsour.2017.03.109. https://linkinghub.elsevier.com/retrieve/pii/S0378775317304159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verma M., Verma M., Singh V., Singh J., Singh V., Mishra V. Advancements in applicability of microbial fuel cell for energy recovery from human waste. Bioresour. Technol. Rep. 2022;17 [Google Scholar]
- 29.Elhenawy S., Khraisheh M., Almomani F., Al-Ghouti M., Hassan M. From waste to Watts: updates on key applications of microbial fuel cells in wastewater treatment and energy production. Sustainability (Switzerland) 2022;14(2):955. [Google Scholar]
- 30.Pandit S., Savla N., Sonawane J.M., Sani A.M., Gupta P.K., Mathuriya A.S., Rai A.K., Jadhav D.A., Jung S.P., Prasad R. Agricultural waste and wastewater as feedstock for bioelectricity generation using microbial fuel cells: recent advances. Fermentation. 2021;7(3):169. https://www.mdpi.com/2311-5637/7/3/169 [Google Scholar]
- 31.Dutta A., Jacob C.A., Das P., Corton E., Stom D., Barbora L., Goswami P. A review on power management systems: an electronic tool to enable microbial fuel cells for powering range of electronic appliances. J. Power Sources. 2022;517 https://linkinghub.elsevier.com/retrieve/pii/S0378775321011836 [Google Scholar]
- 32.Olias L.G., Di Lorenzo M. Microbial fuel cells for in-field water quality monitoring. RSC Adv. 2021;11(27):16307–16317. doi: 10.1039/d1ra01138c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaturvedi A., Kundu P. Nanostructured graphene utilization in microbial fuel cells for green energy and wastewater treatment: recent developments and future perspectives. J. Hazardous Toxic Radioact. Waste. 2022;26(2) [Google Scholar]
- 34.Boas J., Oliveira V., Simões M., Pinto A. Review on microbial fuel cells applications, developments and costs. J. Environ. Manag. 2022;307 doi: 10.1016/j.jenvman.2022.114525. [DOI] [PubMed] [Google Scholar]
- 35.Selvasembian R., Mal J., Rani R., Sinha R., Agrahari R., Joshua I., Santhiagu A., Pradhan N. Recent progress in microbial fuel cells for industrial effluent treatment and energy generation: fundamentals to scale-up application and challenges. Bioresour. Technol. 2022;346 doi: 10.1016/j.biortech.2021.126462. [DOI] [PubMed] [Google Scholar]
- 36.Tan W.H., Chong S., Fang H.-W., Pan K.-L., Mohamad M., Lim J.W., Tiong T.J., Chan Y.J., Huang C.-M., Yang T.C.-K. Microbial fuel cell technology—a critical review on scale-up issues. Processes. 2021;9(6):985. https://www.mdpi.com/2227-9717/9/6/985 [Google Scholar]
- 37.Jadhav D.A., Mungray A.K., Arkatkar A., Kumar S.S. Recent advancement in scaling-up applications of microbial fuel cells: from reality to practicability. Sustain. Energy Technol. Assess. 2021;45 https://linkinghub.elsevier.com/retrieve/pii/S2213138821002368 [Google Scholar]
- 38.Zhou E., Lekbach Y., Gu T., Xu D. Bioenergetics and extracellular electron transfer in microbial fuel cells and microbial corrosion. Current Opinion Electrochem. 2022;31 [Google Scholar]
- 39.Andriukonis E., Celiesiute-Germaniene R., Ramanavicius S., Viter R., Ramanavicius A. From microorganism-based amperometric biosensors towards microbial fuel cells. Sensors. 2021;21(7):2442. doi: 10.3390/s21072442. https://www.mdpi.com/1424-8220/21/7/2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aiyer K.S. How does electron transfer occur in microbial fuel cells? World J. Microbiol. Biotechnol. 2020;36(2):19. doi: 10.1007/s11274-020-2801-z. [DOI] [PubMed] [Google Scholar]
- 41.Umar M.F., Abbas S.Z., Mohamad Ibrahim M.N., Ismail N., Rafatullah M. Insights into advancements and electrons transfer mechanisms of electrogens in benthic microbial fuel cells. Membranes. 2020;10(9):205. doi: 10.3390/membranes10090205. https://www.mdpi.com/2077-0375/10/9/205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heydorn R.L., Engel C., Krull R., Dohnt K. Strategies for the targeted improvement of anodic electron transfer in microbial fuel cells. Chem. Bio. Eng. Rev. 2020;7(1):4–17. [Google Scholar]
- 43.Abbas S., Yong Y.-C., Chang F.-X. Anode materials for soil microbial fuel cells: recent advances and future perspectives. Int. J. Energy Res. 2022;46(2):712–725. [Google Scholar]
- 44.Yaqoob A.A., Mohamad Ibrahim M.N., Rafatullah M., Chua Y.S., Ahmad A., Umar K. Recent advances in anodes for microbial fuel cells: an overview. Materials. 2020;13(9):2078. doi: 10.3390/ma13092078. https://www.mdpi.com/1996-1944/13/9/2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Banerjee A., Calay R., Eregno F. Role and important properties of a membrane with its recent advancement in a microbial fuel cell. Energies. 2022;15(2):444. [Google Scholar]
- 46.Ramirez-Nava J., Martínez-Castrejón M., García-Mesino R.L., López-Díaz J.A., Talavera-Mendoza O., Sarmiento-Villagrana A., Rojano F., Hernández-Flores G. The implications of membranes used as separators in microbial fuel cells. Membranes. 2021;11(10):738. doi: 10.3390/membranes11100738. https://www.mdpi.com/2077-0375/11/10/738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shabani M., Younesi H., Pontié M., Rahimpour A., Rahimnejad M., Zinatizadeh A.A. A critical review on recent proton exchange membranes applied in microbial fuel cells for renewable energy recovery. J. Clean. Prod. 2020;264 https://linkinghub.elsevier.com/retrieve/pii/S0959652620314931 [Google Scholar]
- 48.Das S., Dutta K., Rana D. Polymer electrolyte membranes for microbial fuel cells: a review. Polym. Rev. 2018;58(4):610–629. [Google Scholar]
- 49.Pandey R.P., Shukla G., Manohar M., Shahi V.K. Graphene oxide based nanohybrid proton exchange membranes for fuel cell applications: an overview. Adv. Colloid Interface Sci. 2017;240:15–30. doi: 10.1016/j.cis.2016.12.003. https://linkinghub.elsevier.com/retrieve/pii/S0001868616301695 [DOI] [PubMed] [Google Scholar]
- 50.Dhilllon S., Kundu P., Jain R. Catalytic advancements in carbonaceous materials for bio-energy generation in microbial fuel cells: a review. Environ. Sci. Pollut. Res. Int. 2022 doi: 10.1007/s11356-021-17529-9. [DOI] [PubMed] [Google Scholar]
- 51.Nandikes G., Peera S., Singh L. Perovskite-based nanocomposite electrocatalysts: an alternative to platinum ORR catalyst in microbial fuel cell cathodes. Energies. 2021;15(1):272. [Google Scholar]
- 52.Li S., Ho S.-H., Hua T., Zhou Q., Li F., Tang J. Sustainable biochar as an electrocatalysts for the oxygen reduction reaction in microbial fuel cells. Green Energy Environ. 2021;6(5):644–659. https://linkinghub.elsevier.com/retrieve/pii/S246802572030203X [Google Scholar]
- 53.Xu Z., Chen S., Guo S., Wan D., Xu H., Yan W., Jin X., Feng J. New insights in light-assisted microbial fuel cells for wastewater treatment and power generation: a win-win cooperation. J. Power Sources. 2021;501 https://linkinghub.elsevier.com/retrieve/pii/S0378775321005280 [Google Scholar]
- 54.Verma J., Kumar D., Singh N., Katti S.S., Shah Y.T. Electricigens and microbial fuel cells for bioremediation and bioenergy production: a review. Environ. Chem. Lett. 2021;19(3):2091–2126. [Google Scholar]
- 55.Zhang M., Ma Z., Song H. Carbon supports on preparing iron-nitrogen dual-doped carbon (Fe-N/C) electrocatalysts for microbial fuel cells: mini-review. Chemosphere. 2021;273 doi: 10.1016/j.chemosphere.2020.128570. https://linkinghub.elsevier.com/retrieve/pii/S004565352032765X [DOI] [PubMed] [Google Scholar]
- 56.Priyadarshini M., Ahmad A., Das S., Ghangrekar M.M. Metal organic frameworks as emergent oxygen-reducing cathode catalysts for microbial fuel cells: a review. Int. J. Environ. Sci. Technol. 2021 [Google Scholar]
- 57.Qiu S., Guo Z., Naz F., Yang Z., Yu C. An overview in the development of cathode materials for the improvement in power generation of microbial fuel cells. Bioelectrochemistry. 2021;141 doi: 10.1016/j.bioelechem.2021.107834. https://linkinghub.elsevier.com/retrieve/pii/S1567539421000979 [DOI] [PubMed] [Google Scholar]
- 58.Anjum A., Ali Mazari S., Hashmi Z., Sattar Jatoi A., Abro R. A review of role of cathodes in the performance of microbial fuel cells. J. Electroanal. Chem. 2021;899 https://linkinghub.elsevier.com/retrieve/pii/S1572665721006998 [Google Scholar]
- 59.Maddalwar S., Kumar Nayak K., Kumar M., Singh L. Plant microbial fuel cell: opportunities, challenges, and prospects. Bioresour. Technol. 2021;341 doi: 10.1016/j.biortech.2021.125772. https://linkinghub.elsevier.com/retrieve/pii/S0960852421011135 [DOI] [PubMed] [Google Scholar]
- 60.Rusyn I. Role of microbial community and plant species in performance of plant microbial fuel cells. Renew. Sustain. Energy Rev. 2021;152 https://linkinghub.elsevier.com/retrieve/pii/S1364032121009710 [Google Scholar]
- 61.Khandaker S., Das S., Hossain M.T., Islam A., Miah M.R., Awual M.R. Sustainable approach for wastewater treatment using microbial fuel cells and green energy generation – a comprehensive review. J. Mol. Liq. 2021;344 https://linkinghub.elsevier.com/retrieve/pii/S0167732221025204 [Google Scholar]
- 62.Yang X., Chen S. Microorganisms in sediment microbial fuel cells: ecological niche, microbial response, and environmental function. Sci. Total Environ. 2021;756 doi: 10.1016/j.scitotenv.2020.144145. https://linkinghub.elsevier.com/retrieve/pii/S0048969720376762 [DOI] [PubMed] [Google Scholar]
- 63.Sharif H.M.A., Farooq M., Hussain I., Ali M., Mujtaba M., Sultan M., Yang B. Recent innovations for scaling up microbial fuel cell systems: significance of physicochemical factors for electrodes and membranes materials. J. Taiwan Inst. Chem. Eng. 2021;129:207–226. https://linkinghub.elsevier.com/retrieve/pii/S1876107021005277 [Google Scholar]
- 64.Mukherjee A., Patel V., Shah M.T., Jadhav D.A., Munshi N.S., Chendake A.D., Pant D. Effective power management system in stacked microbial fuel cells for onsite applications. J. Power Sources. 2022;517 https://linkinghub.elsevier.com/retrieve/pii/S0378775321011794 [Google Scholar]
- 65.Huang X., Duan C., Duan W., Sun F., Cui H., Zhang S., Chen X. Role of electrode materials on performance and microbial characteristics in the constructed wetland coupled microbial fuel cell (CW-MFC): a review. J. Clean. Prod. 2021;301 https://linkinghub.elsevier.com/retrieve/pii/S0959652621011707 [Google Scholar]
- 66.Vishwanathan A.S. Microbial fuel cells: a comprehensive review for beginners. 3 Biotech. 2021;11(5):248. doi: 10.1007/s13205-021-02802-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jatoi A.S., Akhter F., Mazari S.A., Sabzoi N., Aziz S., Soomro S.A., Mubarak N.M., Baloch H., Memon A.Q., Ahmed S. Advanced microbial fuel cell for waste water treatment—a review. Environ. Sci. Pollut. Res. Int. 2021;28(5):5005–5019. doi: 10.1007/s11356-020-11691-2. [DOI] [PubMed] [Google Scholar]
- 68.Munoz-Cupa C., Hu Y., Xu C., Bassi A. An overview of microbial fuel cell usage in wastewater treatment, resource recovery and energy production. Sci. Total Environ. 2021;754 doi: 10.1016/j.scitotenv.2020.142429. https://linkinghub.elsevier.com/retrieve/pii/S0048969720359581 [DOI] [PubMed] [Google Scholar]
- 69.Serra P.M.D., Espírito-Sanot A Sourcing power with microbial fuel cells: a timeline. J. Power Sources. 2021;482 https://linkinghub.elsevier.com/retrieve/pii/S0378775320312222 [Google Scholar]
- 70.Gul H., Raza W., Lee J., Azam M., Ashraf M., Kim K.-H. Progress in microbial fuel cell technology for wastewater treatment and energy harvesting. Chemosphere. 2021;281 doi: 10.1016/j.chemosphere.2021.130828. https://www.sciencedirect.com/science/article/pii/S0045653521012996 [DOI] [PubMed] [Google Scholar]
- 71.Deb D., Patel R., Balas V.E. A review of control-oriented bioelectrochemical mathematical models of microbial fuel cells. Processes. 2020;8(5):583. https://www.mdpi.com/2227-9717/8/5/583 [Google Scholar]
- 72.Xia C., Zhang D., Pedrycz W., Zhu Y., Guo Y. Models for microbial fuel cells: a critical review. J. Power Sources. 2018;373:119–131. https://linkinghub.elsevier.com/retrieve/pii/S037877531731460X [Google Scholar]
- 73.Saravanan A., Karishma S., Kumar P.S., Yaashikaa P.R., Jeevanantham S., Gayathri B. Microbial electrolysis cells and microbial fuel cells for biohydrogen production: current advances and emerging challenges. Biomass Convers. Biorefin. 2020 [Google Scholar]
- 74.Koók L., Nemestóthy N., Bélafi-Bakó K., Bakonyi P. Treatment of dark fermentative H2 production effluents by microbial fuel cells: a tutorial review on promising operational strategies and practices. Int. J. Hydrog. Energy. 2021;46(7):5556–5569. https://linkinghub.elsevier.com/retrieve/pii/S0360319920342865 [Google Scholar]
- 75.Savla N., Anand R., Pandit S., Prasad R. Utilization of nanomaterials as anode modifiers for improving microbial FuelCells performance. J. Renew. Mater. 2020;8(12):1581–1605. https://www.techscience.com/jrm/v8n12/40551 [Google Scholar]
- 76.Sonawane J.M., Yadav A., Ghosh P.C., Adeloju S.B. Recent advances in the development and utilization of modern anode materials for high performance microbial fuel cells. Biosens. Bioelectron. 2017;90:558–576. doi: 10.1016/j.bios.2016.10.014. http://www.sciencedirect.com/science/article/pii/S0956566316310107 [DOI] [PubMed] [Google Scholar]
- 77.Tang R.C.O., Jang J.-H., Lan T.-H., Wu J.-C., Yan W.-M., Sangeetha T., Wang C.-T., Ong H.C., Ong Z.C. Review on design factors of microbial fuel cells using Buckingham's Pi Theorem. Renew. Sustain. Energy Rev. 2020;130 https://linkinghub.elsevier.com/retrieve/pii/S1364032120301714 [Google Scholar]
- 78.Cai T., Meng L., Chen G., Xi Y., Jiang N., Song J., Zheng S., Liu Y., Zhen G., Huang M. Application of advanced anodes in microbial fuel cells for power generation: a review. Chemosphere. 2020;248 doi: 10.1016/j.chemosphere.2020.125985. https://linkinghub.elsevier.com/retrieve/pii/S0045653520301776 [DOI] [PubMed] [Google Scholar]
- 79.Yaqoob A.A., Ibrahim M.N.M., Rodríguez-Couto S. Development and modification of materials to build cost-effective anodes for microbial fuel cells (MFCs): an overview. Biochem. Eng. J. 2020;164 https://linkinghub.elsevier.com/retrieve/pii/S1369703X20303338 [Google Scholar]
- 80.Kaur R., Marwaha A., Chhabra V.A., Kim K.-H., Tripathi S. Recent developments on functional nanomaterial-based electrodes for microbial fuel cells. Renew. Sustain. Energy Rev. 2020;119 https://linkinghub.elsevier.com/retrieve/pii/S1364032119307592 [Google Scholar]
- 81.Dey N., Samuel G., Raj D., Gajalakshmi B. Nanomaterials as potential high performing electrode materials for microbial fuel cells. App. Nanosci. (Switzerland) 2022 [Google Scholar]
- 82.Li T., Cai Y., Yang X.-L., Wu Y., Yang Y.-L., Song H.-L. Microbial fuel cell-membrane bioreactor integrated system for wastewater treatment and bioelectricity production: overview. J. Environ. Eng. 2020;146(1) [Google Scholar]
- 83.Palanisamy G., Jung H.-Y., Sadhasivam T., Kurkuri M.D., Kim S.C., Roh S.-H. A comprehensive review on microbial fuel cell technologies: processes, utilization, and advanced developments in electrodes and membranes. J. Clean. Prod. 2019;221:598–621. https://linkinghub.elsevier.com/retrieve/pii/S0959652619305712 [Google Scholar]
- 84.Al Lawati M.J., Jafary T., Baawain M.S., Al-Mamun A. A mini review on biofouling on air cathode of single chamber microbial fuel cell; prevention and mitigation strategies. Biocatal. Agricult. Biotechnol. 2019;22 https://linkinghub.elsevier.com/retrieve/pii/S1878818119311028 [Google Scholar]
- 85.Noori M., Ghangrekar M., Mukherjee C., Min B. Biofouling effects on the performance of microbial fuel cells and recent advances in biotechnological and chemical strategies for mitigation. Biotechnol. Adv. 2019;37(8) doi: 10.1016/j.biotechadv.2019.107420. https://linkinghub.elsevier.com/retrieve/pii/S073497501930120X [DOI] [PubMed] [Google Scholar]
- 86.Wang J., Zhao S., Kakade A., Kulshreshtha S., Liu P., Li X. A review on microbial electrocatalysis systems coupled with membrane bioreactor to improve wastewater treatment. Microorganisms. 2019;7(10):372. doi: 10.3390/microorganisms7100372. https://www.mdpi.com/2076-2607/7/10/372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mian M.M., Liu G., Fu B. Conversion of sewage sludge into environmental catalyst and microbial fuel cell electrode material: a review. Sci. Total Environ. 2019;666:525–539. doi: 10.1016/j.scitotenv.2019.02.200. https://linkinghub.elsevier.com/retrieve/pii/S0048969719306886 [DOI] [PubMed] [Google Scholar]
- 88.Aarthy M., Rajesh T., Thirunavoukkarasu M. Critical review on microbial fuel cells for concomitant reduction of hexavalent chromium and bioelectricity generation. J. Chem. Technol. Biotechnol. 2019;95(5) [Google Scholar]
- 89.Cao Y., Mu H., Liu W., Zhang R., Guo J., Xian M., Liu H. Electricigens in the anode of microbial fuel cells: pure cultures versus mixed communities. Microb. Cell Fact. 2019;18(1):39. doi: 10.1186/s12934-019-1087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leung D.H.L., Lim Y.S., Uma K., Pan G.-T., Lin J.-H., Chong S., Yang T.C.-K. Engineering S. oneidensis for performance improvement of microbial fuel cell—a mini review. Appl. Biochem. Biotechnol. 2021;193(4):1170–1186. doi: 10.1007/s12010-020-03469-6. [DOI] [PubMed] [Google Scholar]
- 91.Mouhib M., Antonucci A., Reggente M., Amirjani A., Gillen A.J., Boghossian A.A. Enhancing bioelectricity generation in microbial fuel cells and biophotovoltaics using nanomaterials. Nano Res. 2019;12(9):2184–2199. [Google Scholar]
- 92.Leininger A., Ko W.-M., Ramirez M., Kjellerup B.V. Implementation of upscaled microbial fuel cells for optimized net energy benefit in wastewater treatment systems. J. Environ. Eng. 2019;145(5) [Google Scholar]
- 93.Hu J., Zhang Q., Lee D.-J., Ngo H.H. Feasible use of microbial fuel cells for pollution treatment. Renew. Energy. 2018;129:824–829. https://linkinghub.elsevier.com/retrieve/pii/S0960148117300794 [Google Scholar]
- 94.Zhang Y., Liu M., Zhou M., Yang H., Liang L., Gu T. Microbial fuel cell hybrid systems for wastewater treatment and bioenergy production: synergistic effects, mechanisms and challenges. Renew. Sustain. Energy Rev. 2019;103:13–29. https://linkinghub.elsevier.com/retrieve/pii/S1364032118308220 [Google Scholar]
- 95.Cui Y., Lai B., Tang X. Microbial fuel cell-based biosensors. Biosensors. 2019;9(3):92. doi: 10.3390/bios9030092. https://www.mdpi.com/2079-6374/9/3/92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kumar S.S., Kumar V., Malyan S.K., Sharma J., Mathimani T., Maskarenj M.S., Ghosh P.C., Pugazhendhi A. Microbial fuel cells (MFCs) for bioelectrochemical treatment of different wastewater streams. Fuel. 2019;254 http://www.sciencedirect.com/science/article/pii/S0016236119308579 [Google Scholar]
- 97.Singh H.M., Pathak A.K., Chopra K., Tyagi V., Anand S., Kothari R. Microbial fuel cells: a sustainable solution for bioelectricity generation and wastewater treatment. Biofuels. 2019;10(1):11–31. [Google Scholar]
- 98.Slate A.J., Whitehead K.A., Brownson D.A., Banks C.E. Microbial fuel cells: an overview of current technology. Renew. Sustain. Energy Rev. 2019;101:60–81. https://linkinghub.elsevier.com/retrieve/pii/S1364032118306920 [Google Scholar]
- 99.Flimban S.G.A., Ismail I.M.I., Kim T., Oh S.-E. Overview of recent advancements in the microbial fuel cell from fundamentals to applications: design, major elements, and scalability. Energies. 2019;12(17):3390. https://www.mdpi.com/1996-1073/12/17/3390 [Google Scholar]
- 100.Nagendranatha Reddy C., Nguyen H.T., Noori M.T., Min B. Potential applications of algae in the cathode of microbial fuel cells for enhanced electricity generation with simultaneous nutrient removal and algae biorefinery: current status and future perspectives. Bioresour. Technol. 2019;292 doi: 10.1016/j.biortech.2019.122010. https://linkinghub.elsevier.com/retrieve/pii/S0960852419312404 [DOI] [PubMed] [Google Scholar]
- 101.Guadarrama-Pérez O., Gutiérrez-Macías T., García-Sánchez L., Guadarrama-Pérez V.H., Estrada-Arriaga E.B. Recent advances in constructed wetland-microbial fuel cells for simultaneous bioelectricity production and wastewater treatment: a review. Int. J. Energy Res. 2019;43(10):5106–5127. [Google Scholar]
- 102.Rusli S.F.N., Abu Bakar M.H., Loh K.S., Mastar M.S. Review of high-performance biocathode using stainless steel and carbon-based materials in microbial fuel cell for electricity and water treatment. Int. J. Hydrog. Energy. 2019;44(58):30772–30787. https://linkinghub.elsevier.com/retrieve/pii/S0360319918337509 [Google Scholar]
- 103.Chen S., Patil S.A., Brown R.K., Schröder U. Strategies for optimizing the power output of microbial fuel cells: transitioning from fundamental studies to practical implementation. Appl. Energy. 2019;233–234:15–28. https://linkinghub.elsevier.com/retrieve/pii/S0306261918315721 [Google Scholar]
- 104.Maksimova Y.G. Microorganisms and carbon nanotubes: interaction and applications (review) Appl. Biochem. Microbiol. 2019;55(1):1–12. [Google Scholar]
- 105.Yasri N., Roberts E.P., Gunasekaran S. The electrochemical perspective of bioelectrocatalytic activities in microbial electrolysis and microbial fuel cells. Energy Rep. 2019;5:1116–1136. https://linkinghub.elsevier.com/retrieve/pii/S2352484719301544 [Google Scholar]
- 106.Fang C., Achal V. The potential of microbial fuel cells for remediation of heavy metals from soil and water—review of application. Microorganisms. 2019;7(12):697. doi: 10.3390/microorganisms7120697. https://www.mdpi.com/2076-2607/7/12/697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Goswami R., Mishra V.K. A review of design, operational conditions and applications of microbial fuel cells. Biofuels. 2018;9(2):203–220. [Google Scholar]
- 108.Du Z., Li H., Gu T. A state of the art review on microbial fuel cells: a promising technology for wastewater treatment and bioenergy. Biotechnol. Adv. 2007;25(5):464–482. doi: 10.1016/j.biotechadv.2007.05.004. https://linkinghub.elsevier.com/retrieve/pii/S0734975007000547 [DOI] [PubMed] [Google Scholar]
- 109.Mathuriya A.S., Jadhav D.A., Ghangrekar M.M. Architectural adaptations of microbial fuel cells. Appl. Microbiol. Biotechnol. 2018;102(22):9419–9432. doi: 10.1007/s00253-018-9339-0. [DOI] [PubMed] [Google Scholar]
- 110.Do M., Ngo H., Guo W., Liu Y., Chang S., Nguyen D., Nghiem L., Ni B. Challenges in the application of microbial fuel cells to wastewater treatment and energy production: a mini review. Sci. Total Environ. 2018;639:910–920. doi: 10.1016/j.scitotenv.2018.05.136. https://linkinghub.elsevier.com/retrieve/pii/S0048969718317777 [DOI] [PubMed] [Google Scholar]
- 111.Li M., Zhou M., Tian X., Tan C., McDaniel C.T., Hassett D.J., Gu T. Microbial fuel cell (MFC) power performance improvement through enhanced microbial electrogenicity. Biotechnol. Adv. 2018;36(4):1316–1327. doi: 10.1016/j.biotechadv.2018.04.010. https://linkinghub.elsevier.com/retrieve/pii/S073497501830082X [DOI] [PubMed] [Google Scholar]
- 112.Kumar R., Singh L., Zularisam A.W., Hai F.I. Microbial fuel cell is emerging as a versatile technology: a review on its possible applications, challenges and strategies to improve the performances: microbial fuel cell is emerging as a versatile technology. Int. J. Energy Res. 2018;42(2):369–394. [Google Scholar]
- 113.Javed M.M., Nisar M.A., Ahmad M.U., Yasmeen N., Zahoor S. Microbial fuel cells as an alternative energy source: current status. Biotechnol. Genet. Eng. Rev. 2018;34(2):216–242. doi: 10.1080/02648725.2018.1482108. [DOI] [PubMed] [Google Scholar]
- 114.Bhargavi G., Venu V., Renganathan S. Microbial fuel cells: recent developments in design and materials. IOP Conf. Ser., Mater. Sci. Eng. 2018;330 [Google Scholar]
- 115.Gajda I., Greenman J., Ieropoulos I.A. Recent advancements in real-world microbial fuel cell applications. Current Opinion Electrochem. 2018;11:78–83. doi: 10.1016/j.coelec.2018.09.006. https://linkinghub.elsevier.com/retrieve/pii/S2451910318301820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ma F., Fu C., Rong X., Wang H. 2017 Chinese Automation Congress (CAC) IEEE; Jinan: 2017. Advanced energy harvesting in microbial fuel cells for environmental monitoring; pp. 7357–7361.http://ieeexplore.ieee.org/document/8244107/ [Google Scholar]
- 117.He L., Du P., Chen Y., Lu H., Cheng X., Chang B., Wang Z. Advances in microbial fuel cells for wastewater treatment. Renew. Sustain. Energy Rev. 2017;71:388–403. https://linkinghub.elsevier.com/retrieve/pii/S1364032116311248 [Google Scholar]
- 118.Ucar D., Zhang Y., Angelidaki I. An overview of electron acceptors in microbial fuel cells. Front. Microbiol. 2017;8:643. doi: 10.3389/fmicb.2017.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Choudhury P., Uday U.S.P., Mahata N., Nath Tiwari O., Narayan Ray R., Kanti Bandyopadhyay T., Bhunia B. Performance improvement of microbial fuel cells for waste water treatment along with value addition: a review on past achievements and recent perspectives. Renew. Sustain. Energy Rev. 2017;79:372–389. https://linkinghub.elsevier.com/retrieve/pii/S1364032117307360 [Google Scholar]
- 120.Uddin S.S., Shatil A.H.M., Roni K.S., Walid A.B. 2016 4th International Conference on the Development in the in Renewable Energy Technology (ICDRET) IEEE; Dhaka, Bangladesh: 2016. Prototype design and overview of a low price microbial fuel cell; pp. 1–6.http://ieeexplore.ieee.org/document/7421526/ [Google Scholar]
- 121.Song H.-L., Zhu Y., Li J. Electron transfer mechanisms, characteristics and applications of biological cathode microbial fuel cells – a mini review. Arab. J. Chem. 2015 https://linkinghub.elsevier.com/retrieve/pii/S187853521500026X [Google Scholar]
- 122.Hernández-Fernández F., Pérez de los Ríos A., Salar-García M., Ortiz-Martínez V., Lozano-Blanco L., Godínez C., Tomás-Alonso F., Quesada-Medina J. Recent progress and perspectives in microbial fuel cells for bioenergy generation and wastewater treatment. Fuel Process. Technol. 2015;138:284–297. https://linkinghub.elsevier.com/retrieve/pii/S0378382015300163 [Google Scholar]
- 123.Venkata Mohan S., Velvizhi G., Modestra J. Annie, Srikanth S. Microbial fuel cell: critical factors regulating bio-catalyzed electrochemical process and recent advancements. Renew. Sustain. Energy Rev. 2014;40:779–797. https://linkinghub.elsevier.com/retrieve/pii/S1364032114005619 [Google Scholar]
- 124.Wei J., Liang P., Huang X. Recent progress in electrodes for microbial fuel cells. Bioresour. Technol. 2011;102(20):9335–9344. doi: 10.1016/j.biortech.2011.07.019. https://linkinghub.elsevier.com/retrieve/pii/S096085241100945X [DOI] [PubMed] [Google Scholar]
- 125.Franks A.E., Nevin K.P. Microbial fuel cells, a current review. Energies. 2010;3(5):899–919. http://www.mdpi.com/1996-1073/3/5/899 [Google Scholar]
- 126.Liu H., Cheng S., Logan B.E. Production of electricity from acetate or butyrate using a single-chamber microbial fuel cell. Environ. Sci. Technol. 2005;39(2):658–662. doi: 10.1021/es048927c. [DOI] [PubMed] [Google Scholar]
- 127.Chandrasekhar K., Ahn Y.-H. Effectiveness of piggery waste treatment using microbial fuel cells coupled with elutriated-phased acid fermentation. Bioresour. Technol. 2017;244:650–657. doi: 10.1016/j.biortech.2017.08.021. https://linkinghub.elsevier.com/retrieve/pii/S0960852417313263 [DOI] [PubMed] [Google Scholar]
- 128.Rossi R., Yang W., Zikmund E., Pant D., Logan B.E. In situ biofilm removal from air cathodes in microbial fuel cells treating domestic wastewater. Bioresour. Technol. 2018;265:200–206. doi: 10.1016/j.biortech.2018.06.008. https://linkinghub.elsevier.com/retrieve/pii/S0960852418307879 [DOI] [PubMed] [Google Scholar]
- 129.Noori M., Tiwari B., Mukherjee C., Ghangrekar M. Enhancing the performance of microbial fuel cell using Ag Pt bimetallic alloy as cathode catalyst and anti-biofouling agent. Int. J. Hydrog. Energy. 2018;43(42):19650–19660. https://linkinghub.elsevier.com/retrieve/pii/S0360319918326934 [Google Scholar]
- 130.Das I., Das S., Das S., Ghangrekar M. Proficient sanitary wastewater treatment in laboratory and field-scale microbial fuel cell with anti-biofouling Cu0.5Mn0.5Fe2O4as cathode catalyst. J. Electrochem. Soc. 2021;168(5) [Google Scholar]
- 131.Mehravanfar H., Mahdavi M.A., Gheshlaghi R. Economic optimization of stacked microbial fuel cells to maximize power generation and treatment of wastewater with minimal operating costs. Int. J. Hydrog. Energy. 2019;44(36):20355–20367. https://linkinghub.elsevier.com/retrieve/pii/S0360319919322219 [Google Scholar]
- 132.Zhang G., Zhou Y., Yang F. Hydrogen production from microbial fuel cells-ammonia electrolysis cell coupled system fed with landfill leachate using Mo2C/N-doped graphene nanocomposite as HER catalyst. Electrochim. Acta. 2019;299:672–681. https://linkinghub.elsevier.com/retrieve/pii/S0013468619300684 [Google Scholar]
- 133.Ren L., Ahn Y., Logan B.E. A two-stage microbial fuel cell and anaerobic fluidized bed membrane bioreactor (MFC-AFMBR) system for effective domestic wastewater treatment. Environ. Sci. Technol. 2014;48(7):4199–4206. doi: 10.1021/es500737m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Florio C., Nastro R.A., Flagiello F., Minutillo M., Pirozzi D., Pasquale V., Ausiello A., Toscano G., Jannelli E., Dumontet S. Biohydrogen production from solid phase-microbial fuel cell spent substrate: a preliminary study. J. Clean. Prod. 2019;227:506–511. https://linkinghub.elsevier.com/retrieve/pii/S0959652619310327 [Google Scholar]
- 135.Sun M., Mu Z.-X., Sheng G.-P., Shen N., Tong Z.-H., Wang H.-L., Yu H.-Q. Hydrogen production from propionate in a biocatalyzed system with in-situ utilization of the electricity generated from a microbial fuel cell. Int. Biodeterior. Biodegrad. 2010;64(5):378–382. https://linkinghub.elsevier.com/retrieve/pii/S0964830510000594 [Google Scholar]
- 136.Wang Y., Lin Z., Su X., Zhao P., Zhou J., He Q., Ai H. Cost-effective domestic wastewater treatment and bioenergy recovery in an immobilized microalgal-based photoautotrophic microbial fuel cell (PMFC) Chem. Eng. J. 2019;372:956–965. https://linkinghub.elsevier.com/retrieve/pii/S1385894719310071 [Google Scholar]
- 137.Yadav G., Sharma I., Ghangrekar M., Sen R. A live bio-cathode to enhance power output steered by bacteria-microalgae synergistic metabolism in microbial fuel cell. J. Power Sources. 2020;449 https://linkinghub.elsevier.com/retrieve/pii/S0378775319315538 [Google Scholar]
- 138.Pushkar P., Mungray A.K. Exploring the use of 3 dimensional low-cost sugar-urea carbon foam electrode in the benthic microbial fuel cell. Renew. Energy. 2020;147:2032–2042. https://linkinghub.elsevier.com/retrieve/pii/S0960148119314867 [Google Scholar]
- 139.Peixoto L., Parpot P., Martins G. Assessment of electron transfer mechanisms during a long-term sediment microbial fuel cell operation. Energies. 2019;12(3):481. http://www.mdpi.com/1996-1073/12/3/481 [Google Scholar]
- 140.Ren H., Pyo S., Lee J.-I., Park T.-J., Gittleson F.S., Leung F.C., Kim J., Taylor A.D., Lee H.-S., Chae J. A high power density miniaturized microbial fuel cell having carbon nanotube anodes. J. Power Sources. 2015;273:823–830. https://linkinghub.elsevier.com/retrieve/pii/S0378775314015845 [Google Scholar]
- 141.Araneda I., Tapia N., Lizama K., Vargas I. Constructed wetland-microbial fuel cells for sustainable greywater treatment. Water. 2018;10:940. [Google Scholar]
- 142.Chen Z., Niu Y., Zhao S., Khan A., Ling Z., Chen Y., Liu P., Li X. A novel biosensor for p-nitrophenol based on an aerobic anode microbial fuel cell. Biosens. Bioelectron. 2016;85:860–868. doi: 10.1016/j.bios.2016.06.007. https://linkinghub.elsevier.com/retrieve/pii/S0956566316305395 [DOI] [PubMed] [Google Scholar]
- 143.Di Lorenzo M., Thomson A.R., Schneider K., Cameron P.J., Ieropoulos I. A small-scale air-cathode microbial fuel cell for on-line monitoring of water quality. Biosens. Bioelectron. 2014;62:182–188. doi: 10.1016/j.bios.2014.06.050. https://linkinghub.elsevier.com/retrieve/pii/S0956566314004710 [DOI] [PubMed] [Google Scholar]
- 144.Catal T., Kul A., Atalay V.E., Bermek H., Ozilhan S., Tarhan N. Efficacy of microbial fuel cells for sensing of cocaine metabolites in urine-based wastewater. J. Power Sources. 2019;414:1–7. https://linkinghub.elsevier.com/retrieve/pii/S0378775318314290 [Google Scholar]
- 145.Ayyaru S., Dharmalingam S. Enhanced response of microbial fuel cell using sulfonated poly ether ether ketone membrane as a biochemical oxygen demand sensor. Anal. Chim. Acta. 2014;818:15–22. doi: 10.1016/j.aca.2014.01.059. https://linkinghub.elsevier.com/retrieve/pii/S0003267014001457 [DOI] [PubMed] [Google Scholar]
- 146.Rossi R., Evans P.J., Logan B.E. Impact of flow recirculation and anode dimensions on performance of a large scale microbial fuel cell. J. Power Sources. 2019;412:294–300. https://linkinghub.elsevier.com/retrieve/pii/S0378775318313016 [Google Scholar]
- 147.Fan Gao, Tian L. Applications of nanoscale polypyrrole proton exchange membrane in microbial fuel cells. Int. J. Electrochem. Sci. 2019:470–480. http://www.electrochemsci.org/abstracts/vol14/140100470.pdf [Google Scholar]
- 148.López Zavala M., Torres Delenne P., González Peña O. Improvement of wastewater treatment performance and power generation in microbial fuel cells by enhancing hydrolysis and acidogenesis, and by reducing internal losses. Energies. 2018;11(9):2309. http://www.mdpi.com/1996-1073/11/9/2309 [Google Scholar]
- 149.Hou J., Liu Z., Li Y., Yang S., Zhou Y. A comparative study of graphene-coated stainless steel fiber felt and carbon cloth as anodes in MFCs. Bioprocess Biosyst. Eng. 2015;38(5):881–888. doi: 10.1007/s00449-014-1332-0. [DOI] [PubMed] [Google Scholar]
- 150.Chou T.-Y., Whiteley C.G., Lee D.-J. Anodic potential on dual-chambered microbial fuel cell with sulphate reducing bacteria biofilm. Int. J. Hydrog. Energy. 2014;39(33):19225–19231. https://linkinghub.elsevier.com/retrieve/pii/S0360319914009641 [Google Scholar]
- 151.Hou J., Liu Z., Zhang P. A new method for fabrication of graphene/polyaniline nanocomplex modified microbial fuel cell anodes. J. Power Sources. 2013;224:139–144. https://linkinghub.elsevier.com/retrieve/pii/S0378775312015224 [Google Scholar]
- 152.He Z., Liu J., Qiao Y., Li C.M., Tan T.T.Y. Architecture engineering of hierarchically porous chitosan/vacuum-stripped graphene scaffold as bioanode for high performance microbial fuel cell. Nano Lett. 2012;12(9):4738–4741. doi: 10.1021/nl302175j. [DOI] [PubMed] [Google Scholar]
- 153.Xiao L., Damien J., Luo J., Jang H.D., Huang J., He Z. Crumpled graphene particles for microbial fuel cell electrodes. J. Power Sources. 2012;208:187–192. https://linkinghub.elsevier.com/retrieve/pii/S0378775312003965 [Google Scholar]
- 154.Zhang Y., Mo G., Li X., Zhang W., Zhang J., Ye J., Huang X., Yu C. A graphene modified anode to improve the performance of microbial fuel cells. J. Power Sources. 2011;196(13):5402–5407. https://linkinghub.elsevier.com/retrieve/pii/S0378775311004940 [Google Scholar]
- 155.Chae K.-J., Choi M.-J., Lee J.-W., Kim K.-Y., Kim I.S. Effect of different substrates on the performance, bacterial diversity, and bacterial viability in microbial fuel cells. Bioresour. Technol. 2009;100(14):3518–3525. doi: 10.1016/j.biortech.2009.02.065. https://linkinghub.elsevier.com/retrieve/pii/S0960852409002314 [DOI] [PubMed] [Google Scholar]
- 156.Yang W., Chata G., Zhang Y., Peng Y., Lu J.E., Wang N., Mercado R., Li J., Chen S. Graphene oxide-supported zinc cobalt oxides as effective cathode catalysts for microbial fuel cell: high catalytic activity and inhibition of biofilm formation. Nano Energy. 2019;57:811–819. https://linkinghub.elsevier.com/retrieve/pii/S2211285518310024 [Google Scholar]
- 157.Vilas Boas J., Oliveira V., Marcon L., Simões M., Pinto A. Optimization of a single chamber microbial fuel cell using Lactobacillus pentosus: influence of design and operating parameters. Sci. Total Environ. 2019;648:263–270. doi: 10.1016/j.scitotenv.2018.08.061. https://linkinghub.elsevier.com/retrieve/pii/S0048969718330274 [DOI] [PubMed] [Google Scholar]
- 158.Montpart N., Rago L., Baeza J.A., Guisasola A. Hydrogen production in single chamber microbial electrolysis cells with different complex substrates. Water Res. 2015;68:601–615. doi: 10.1016/j.watres.2014.10.026. https://linkinghub.elsevier.com/retrieve/pii/S0043135414007246 [DOI] [PubMed] [Google Scholar]
- 159.Rabaey K., Lissens G., Siciliano S.D., Verstraete W. A microbial fuel cell capable of converting glucose to electricity at high rate and efficiency. Biotechnol. Lett. 2003;25(18):1531–1535. doi: 10.1023/a:1025484009367. [DOI] [PubMed] [Google Scholar]
- 160.Chakraborty I., Das S., Dubey B., Ghangrekar M. Novel low cost proton exchange membrane made from sulphonated biochar for application in microbial fuel cells. Mater. Chem. Phys. 2020;239 https://linkinghub.elsevier.com/retrieve/pii/S0254058419308223 [Google Scholar]
- 161.Walter X.A., Santoro C., Greenman J., Ieropoulos I.A. Scalability of self-stratifying microbial fuel cell: towards height miniaturisation. Bioelectrochemistry. 2019;127:68–75. doi: 10.1016/j.bioelechem.2019.01.004. https://linkinghub.elsevier.com/retrieve/pii/S1567539418305474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ye Z., Hou J., Ellis M.W., Behkam B. Volume 6: Energy, Parts A and B. ASME; Houston, Texas, USA: 2012. Effect of anode surface roughness on power generation in microbial fuel cells; p. 1409. [Google Scholar]
- 163.Mohamed H.O., Sayed E.T., Obaid M., Choi Y.-J., Park S.-G., Al-Qaradawi S., Chae K.-J. Transition metal nanoparticles doped carbon paper as a cost-effective anode in a microbial fuel cell powered by pure and mixed biocatalyst cultures. Int. J. Hydrog. Energy. 2018;43(46):21560–21571. https://linkinghub.elsevier.com/retrieve/pii/S0360319918331288 [Google Scholar]
- 164.Masoudi M., Rahimnejad M., Mashkour M. Fabrication of anode electrode by a novel acrylic based graphite paint on stainless steel mesh and investigating biofilm effect on electrochemical behavior of anode in a single chamber microbial fuel cell. Electrochim. Acta. 2020;344 https://linkinghub.elsevier.com/retrieve/pii/S0013468620305600 [Google Scholar]
- 165.Pant D., Van Bogaert G., Porto-Carrero C., Diels L., Vanbroekhoven K. Anode and cathode materials characterization for a microbial fuel cell in half cell configuration. Water Sci. Technol. 2011;63(10):2457–2461. doi: 10.2166/wst.2011.217. https://iwaponline.com/wst/article/63/10/2457/31595/Anode-and-cathode-materials-characterization-for-a [DOI] [PubMed] [Google Scholar]
- 166.Aquino Neto S., Reginatto V., De Andrade A.R. Electrochemical Water and Wastewater Treatment. Elsevier; 2018. Microbial fuel cells and wastewater treatment; pp. 305–331.https://linkinghub.elsevier.com/retrieve/pii/B9780128131602000122 [Google Scholar]
- 167.Raccichini R., Varzi A., Passerini S., Scrosati B. The role of graphene for electrochemical energy storage. Nat. Mater. 2015;14(3):271–279. doi: 10.1038/nmat4170. http://www.nature.com/articles/nmat4170 [DOI] [PubMed] [Google Scholar]
- 168.ElMekawy A., Hegab H.M., Losic D., Saint C.P., Pant D. Applications of graphene in microbial fuel cells: the gap between promise and reality. Renew. Sustain. Energy Rev. 2017;72:1389–1403. https://linkinghub.elsevier.com/retrieve/pii/S1364032116306992 [Google Scholar]
- 169.Choi Y.-J., Mohamed H.O., Park S.-G., Al Mayyahi R.B., Al-Dhaifallah M., Rezk H., Ren X., Yu H., Chae K.-J. Electrophoretically fabricated nickel/nickel oxides as cost effective nanocatalysts for the oxygen reduction reaction in air-cathode microbial fuel cell. Int. J. Hydrog. Energy. 2020;45(10):5960–5970. https://linkinghub.elsevier.com/retrieve/pii/S0360319919319470 [Google Scholar]
- 170.Feng H., Liang Y., Guo K., Chen W., Shen D., Huang L., Zhou Y., Wang M., Long Y. TiO nanotube arrays modified titanium: a stable, scalable, and cost-effective bioanode for microbial fuel cells. Environ. Sci. Technol. Lett. 2016;3(12):420–424. [Google Scholar]
- 171.Sabirov I., Enikeev N., Murashkin M., Valiev R. Springer International Publishing; 2015. Bulk Nanostructured Materials with Multifunctional Properties.https://books.google.com.mx/books?id=Wlp4CgAAQBAJ (SpringerBriefs in Materials). [Google Scholar]
- 172.Baudler A., Schmidt I., Langner M., Greiner A., Schröder U. Does it have to be carbon? Metal anodes in microbial fuel cells and related bioelectrochemical systems. Energy Environ. Sci. 2015;8(7):2048–2055. [Google Scholar]
- 173.Papiya F., Das S., Pattanayak P., Kundu P.P. The fabrication of silane modified graphene oxide supported Ni–Co bimetallic electrocatalysts: a catalytic system for superior oxygen reduction in microbial fuel cells. Int. J. Hydrog. Energy. 2019;44(47):25874–25893. https://linkinghub.elsevier.com/retrieve/pii/S0360319919329362 [Google Scholar]
- 174.Santoro C., Serov A., Stariha L., Kodali M., Gordon J., Babanova S., Bretschger O., Artyushkova K., Atanassov P. Iron based catalysts from novel low-cost organic precursors for enhanced oxygen reduction reaction in neutral media microbial fuel cells. Energy Environ. Sci. 2016;9(7):2346–2353. [Google Scholar]
- 175.Rajesh P.P., Noori M.T., Ghangrekar M.M. Improving performance of microbial fuel cell by using polyaniline-coated carbon–felt anode. J. Hazardous Toxic Radioact. Waste. 2020;24(3) [Google Scholar]
- 176.Hu M., Li X., Xiong J., Zeng L., Huang Y., Wu Y., Cao G., Li W. Nano-Fe3C@PGC as a novel low-cost anode electrocatalyst for superior performance microbial fuel cells. Biosens. Bioelectron. 2019;142 doi: 10.1016/j.bios.2019.111594. https://linkinghub.elsevier.com/retrieve/pii/S0956566319306736 [DOI] [PubMed] [Google Scholar]
- 177.Hou Y., Yuan H., Wen Z., Cui S., Guo X., He Z., Chen J. Nitrogen-doped graphene/CoNi alloy encased within bamboo-like carbon nanotube hybrids as cathode catalysts in microbial fuel cells. J. Power Sources. 2016;307:561–568. https://linkinghub.elsevier.com/retrieve/pii/S0378775316300180 [Google Scholar]
- 178.Song Y.E., Lee S., Kim M., Na J.-G., Lee J., Lee J., Kim J.R. Metal-free cathodic catalyst with nitrogen- and phosphorus-doped ordered mesoporous carbon (NPOMC) for microbial fuel cells. J. Power Sources. 2020;451 https://linkinghub.elsevier.com/retrieve/pii/S0378775320301191 [Google Scholar]
- 179.Shengke Y., Yanhua W., Yang Z., Huihui L., Wenke W. Using graphene/polyaniline-modified electrodes enhance the performance of two-chambered microbial fuel cells. Pol. J. Environ. Stud. 2017;26(3):1233–1243. [Google Scholar]
- 180.Liu S.-H., Lai C.-Y., Chang P.-H., Lin C.-W., Chen Y.-H. Enhancing copper recovery and electricity generation from wastewater using low-cost membrane-less microbial fuel cell with a carbonized clay cup as cathode. J. Clean. Prod. 2020;247 https://linkinghub.elsevier.com/retrieve/pii/S0959652619339885 [Google Scholar]
- 181.Das I., Das S., Ghangrekar M. Application of bimetallic low-cost CuZn as oxygen reduction cathode catalyst in lab-scale and field-scale microbial fuel cell. Chem. Phys. Lett. 2020;751 https://linkinghub.elsevier.com/retrieve/pii/S0009261420304516 [Google Scholar]
- 182.Nourbakhsh F., Mohsennia M., Pazouki M. Highly efficient cathode for the microbial fuel cell using LaXO3 (X = [Co, Mn, Co0.5Mn0.5]) perovskite nanoparticles as electrocatalysts. SN Appl. Sci. 2020;2(3):391. [Google Scholar]
- 183.Santoro C., Kodali M., Herrera S., Serov A., Ieropoulos I., Atanassov P. Power generation in microbial fuel cells using platinum group metal-free cathode catalyst: effect of the catalyst loading on performance and costs. J. Power Sources. 2018;378:169–175. doi: 10.1016/j.jpowsour.2017.12.017. https://linkinghub.elsevier.com/retrieve/pii/S0378775317316191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mecheri B., Gokhale R., Santoro C., Costa de Oliveira M.A., D'Epifanio A., Licoccia S., Serov A., Artyushkova K., Atanassov P. Oxygen reduction reaction electrocatalysts derived from iron salt and benzimidazole and aminobenzimidazole precursors and their application in microbial fuel cell cathodes. ACS App. Energy Mater. 2018;1(10):5755–5765. doi: 10.1021/acsaem.8b01360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yang W., Li J., Lan L., Li Z., Wei W., Lu J., Chen S. Facile synthesis of Fe/N/S-doped hollow carbon tubes as high-performance cathode and anode for microbial fuel cells. ChemCatChem. 2019;11(24) [Google Scholar]
- 186.Wang Y., Chen Y., Wen Q., Zheng H., Xu H., Qi L. Electricity generation, energy storage, and microbial-community analysis in microbial fuel cells with multilayer capacitive anodes. Energy. 2019 [Google Scholar]
- 187.Santoro C., Winfield J., Theodosiou P., Ieropoulos I. Supercapacitive paper based microbial fuel cell: high current/power production within a low cost design. Bioresour. Technol. Rep. 2019;7 doi: 10.1016/j.biteb.2019.100297. https://linkinghub.elsevier.com/retrieve/pii/S2589014X19301872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Su L., Yin T., Du H., Zhang W., Fu D. Synergistic improvement of Shewanella loihica PV-4 extracellular electron transfer using a TiO2@TiN nanocomposite. Bioelectrochemistry. 2020;134 doi: 10.1016/j.bioelechem.2020.107519. https://linkinghub.elsevier.com/retrieve/pii/S1567539419306541 [DOI] [PubMed] [Google Scholar]
- 189.Tahernia M., Mohammadifar M., Gao Y., Panmanee W., Hassett D.J., Choi S. A 96-well high-throughput, rapid-screening platform of extracellular electron transfer in microbial fuel cells. Biosens. Bioelectron. 2020;162 doi: 10.1016/j.bios.2020.112259. https://linkinghub.elsevier.com/retrieve/pii/S0956566320302542 [DOI] [PubMed] [Google Scholar]
- 190.Patel R., Deb D., Dey R., Balas V.E. vol. 161. Springer International Publishing; Cham: 2020. Adaptive and Intelligent Control of Microbial Fuel Cells. (Intelligent Systems Reference Library). [Google Scholar]
- 191.Teng H.C., Kok B.C., Uttraphan C., Yee M.H. Performance enhancement of single-chamber sediment-microbial fuel cell with variation in cathode surface area. Renew. Energy Res. 2020:8. [Google Scholar]
- 192.Liew K.B., Wan Daud W.R., Ghasemi M., Loh K.S., Ismail M., Lim S.S., Leong J.X. Manganese oxide/functionalised carbon nanotubes nanocomposite as catalyst for oxygen reduction reaction in microbial fuel cell. Int. J. Hydrog. Energy. 2015;40(35):11625–11632. https://linkinghub.elsevier.com/retrieve/pii/S0360319915008952 [Google Scholar]
- 193.Ray A., Mukhopadhyay I., Pati R. Electrocatalysts for fuel cells and hydrogen evolution: theory to design, IntechOpen. 2018. https://books.google.com.mx/books?id=ki-RDwAAQBAJ
- 194.Daud S.M., Daud W.R.W., Bakar M.H.A., Kim B.H., Somalu M.R., Muchtar A., Jahim J.M., Muhammed Ali S.A. Low-cost novel clay earthenware as separator in microbial electrochemical technology for power output improvement. Bioprocess Biosyst. Eng. 2020;43(8):1369–1379. doi: 10.1007/s00449-020-02331-7. [DOI] [PubMed] [Google Scholar]
- 195.Hasani-Sadrabadi M.M., Dashtimoghadam E., Majedi F.S., Kabiri K., Solati-Hashjin M., Moaddel H. Novel nanocomposite proton exchange membranes based on Nafion® and AMPS-modified montmorillonite for fuel cell applications. J. Membr. Sci. 2010;365(1–2):286–293. doi: 10.1039/c0cc01125h. https://linkinghub.elsevier.com/retrieve/pii/S0376738810007118 [DOI] [PubMed] [Google Scholar]
- 196.Chae K.J., Choi M., Ajayi F.F., Park W., Chang I.S., Kim I.S. Mass transport through a proton exchange membrane (nafion) in microbial fuel cells †. Energy Fuels. 2008;22(1):169–176. [Google Scholar]
- 197.Pujiastuti S., Onggo H. vol. 1711. 2016. Effect of Various Concentration of Sulfuric Acid for Nafion Membrane Activation on the Performance of Fuel Cell; p. 060006. (AIP Conference Proceedings). cited By 8. [Google Scholar]
- 198.Napoli L., Lavorante M., Franco J., Sanguinetti A., Fasoli H. Effects on Nafion® 117 Membrane using different strong acids in various concentrations. J. New Mater. Electrochem.l Syst. 2013;16(3):151–156. http://new-mat.org/ejournal/index.php/jnmes/article/view/4 [Google Scholar]
- 199.Hernández-Flores G., Poggi-Varaldo H., Romero-Castañón T., Solorza-Feria O., Rinderknecht-Seijas N. Harvesting energy from leachates in microbial fuel cells using an anion exchange membrane. Int. J. Hydrog. Energy. 2017;42(51):30374–30382. [Google Scholar]
- 200.Sevda S., Dominguez-Benetton X., Vanbroekhoven K., Sreekrishnan T., Pant D. Characterization and comparison of the performance of two different separator types in air-cathode microbial fuel cell treating synthetic wastewater. Chem. Eng. J. 2013;228:1–11. [Google Scholar]
- 201.Fan Y., Hu H., Liu H. Enhanced Coulombic efficiency and power density of air-cathode microbial fuel cells with an improved cell configuration. J. Power Sources. 2007;171(2):348–354. https://linkinghub.elsevier.com/retrieve/pii/S0378775307013419 [Google Scholar]
- 202.Li X., Liu G., Sun S., Ma F., Zhou S., Lee J., Yao H. Power generation in dual chamber microbial fuel cells using dynamic membranes as separators. Energy Convers. Manag. 2018;165:488–494. [Google Scholar]
- 203.Jafary T., Ghasemi M., Alam J., Aljlil S.A., Yusup S. Carbon-Based Polymer Nanocomposites for Environmental and Energy Applications. Elsevier; 2018. Carbon-based polymer nanocomposites as electrodes for microbial fuel cells; pp. 361–390.https://linkinghub.elsevier.com/retrieve/pii/B9780128135747000150 [Google Scholar]
- 204.Das I., Das S., Sharma S., Ghangrekar M. Ameliorated performance of a microbial fuel cell operated with an alkali pre-treated clayware ceramic membrane. Int. J. Hydrog. Energy. 2020;45(33):16787–16798. https://linkinghub.elsevier.com/retrieve/pii/S0360319920315603 [Google Scholar]
- 205.Cheraghipoor M., Mohebbi-Kalhori D., Noroozifar M., Maghsoodlou M.T. Production of greener energy in microbial fuel cell with ceramic separator fabricated using native soils: effect of lattice and porous SiO2. Fuel. 2021;284 https://linkinghub.elsevier.com/retrieve/pii/S0016236120319347 [Google Scholar]
- 206.Raychaudhuri A., Sahoo R.N., Behera M. Application of clayware ceramic separator modified with silica in microbial fuel cell for bioelectricity generation during rice mill wastewater treatment. Water Sci. Technol. 2021;84(1):66–76. doi: 10.2166/wst.2021.213. https://iwaponline.com/wst/article/84/1/66/82403/Application-of-clayware-ceramic-separator-modified [DOI] [PubMed] [Google Scholar]
- 207.Kracke F., Vassilev I., Krömer J.O. Microbial electron transport and energy conservation – the foundation for optimizing bioelectrochemical systems. Front. Microbiol. 2015;6:575. doi: 10.3389/fmicb.2015.00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Das I., Noori M.T., Bhowmick G.D., Ghangrekar M. Synthesis of bimetallic iron ferrite Co0.5Zn0.5Fe2O4 as a superior catalyst for oxygen reduction reaction to replace noble metal catalysts in microbial fuel cell. Int. J. Hydrog. Energy. 2018;43(41):19196–19205. https://linkinghub.elsevier.com/retrieve/pii/S0360319918326570 [Google Scholar]
- 209.I. Metcalf & Eddy. Tchobanoglous G., Burton F., Stensel H., Burton F. McGraw-Hill Higher Education, McGraw-Hill Education; 2003. Wastewater Engineering: Treatment and Reuse.https://books.google.com.mx/books?id=L1MAXTAkL-QC [Google Scholar]
- 210.Satar I., Daud W.R.W., Kim B.H., Somalu M.R., Ghasemi M., Bakar M.H.A., Jafary T., Timmiati S.N. Performance of titanium–nickel (ti/ni) and graphite felt-nickel (GF/ni) electrodeposited by ni as alternative cathodes for microbial fuel cells. J. Taiwan Inst. Chem. Eng. 2018;89:67–76. https://linkinghub.elsevier.com/retrieve/pii/S1876107018302219 [Google Scholar]
- 211.Pankratova G., Hederstedt L., Gorton L. Extracellular electron transfer features of Gram-positive bacteria. Anal. Chim. Acta. 2019;1076:32–47. doi: 10.1016/j.aca.2019.05.007. https://linkinghub.elsevier.com/retrieve/pii/S0003267019305513 [DOI] [PubMed] [Google Scholar]
- 212.Reguera G., McCarthy K.D., Mehta T., Nicoll J.S., Tuominen M.T., Lovley D.R. Extracellular electron transfer via microbial nanowires. Nature. 2005;435(7045):1098–1101. doi: 10.1038/nature03661. http://www.nature.com/articles/nature03661 [DOI] [PubMed] [Google Scholar]
- 213.Li C., Lesnik K.L., Fan Y., Liu H. Millimeter scale electron conduction through exoelectrogenic mixed species biofilms. FEMS Microbiol. Lett. 2016;363(15) doi: 10.1093/femsle/fnw153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Malvankar N.S., Tuominen M.T., Lovley D.R. Biofilm conductivity is a decisive variable for high-current-density geobacter sulfurreducens microbial fuel cells. Energy Environ. Sci. 2012;5(2):5790. [Google Scholar]
- 215.Li C., Lesnik K.L., Fan Y., Liu H. Redox conductivity of current-producing mixed species biofilms. PLoS ONE. 2016;11(5) doi: 10.1371/journal.pone.0155247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhang K., Wu X., Luo H., Li X., Chen W., Chen J., Mo Y., Wang W. CH4 control and associated microbial process from constructed wetland (CW) by microbial fuel cells (MFC) J. Environ. Manag. 2020;260 doi: 10.1016/j.jenvman.2020.110071. https://linkinghub.elsevier.com/retrieve/pii/S0301479720300098 [DOI] [PubMed] [Google Scholar]
- 217.Zhao Z., Li Y., Zhang Y., Lovley D.R. Sparking anaerobic digestion: promoting direct interspecies electron transfer to enhance methane production. iScience. 2020;23(12) doi: 10.1016/j.isci.2020.101794. https://linkinghub.elsevier.com/retrieve/pii/S2589004220309913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wang R., Wang X., Zhou X., Yao J. Effect of anolytic nitrite concentration on electricity generation and electron transfer in a dual-chamber microbial fuel cell. Environ. Sci. Pollut. Res. Int. 2020;27(9):9910–9918. doi: 10.1007/s11356-019-07323-z. [DOI] [PubMed] [Google Scholar]
- 219.Feng Y., Kayode O., Harper W.F. Using microbial fuel cell output metrics and nonlinear modeling techniques for smart biosensing. Sci. Total Environ. 2013;449:223–228. doi: 10.1016/j.scitotenv.2013.01.004. https://linkinghub.elsevier.com/retrieve/pii/S0048969713000090 [DOI] [PubMed] [Google Scholar]
- 220.Franks A.E., Malvankar N., Nevin K.P. Bacterial biofilms: the powerhouse of a microbial fuel cell. Biofuels. 2010;1(4):589–604. [Google Scholar]
- 221.Estevez-Canales M., Kuzume A., Borjas Z., Füeg M., Lovley D., Wandlowski T., Esteve-Núñez A. A severe reduction in the cytochrome c content of G eobacter sulfurreducens eliminates its capacity for extracellular electron transfer: haem-free Geobacter strain. Environ. Microbiol. Rep. 2015;7(2):219–226. doi: 10.1111/1758-2229.12230. [DOI] [PubMed] [Google Scholar]
- 222.Gu Y., Srikanth V., Salazar-Morales A.I., Jain R., O'Brien J.P., Yi S.M., Soni R.K., Samatey F.A., Yalcin S.E., Malvankar N.S. Structure of Geobacter pili reveals secretory rather than nanowire behaviour. Nature. 2021;597:430–434. doi: 10.1038/s41586-021-03857-w. https://www.nature.com/articles/s41586-021-03857-w number: 7876 Publisher: Nature Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Liu X., Walker D.J.F., Nonnenmann S.S., Sun D., Lovley D.R. Direct observation of electrically conductive pili emanating from Geobacter sulfurreducens. mBio. 2021;12(4) doi: 10.1128/mBio.02209-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Yalcin S.E., O'Brien J.P., Gu Y., Reiss K., Yi S.M., Jain R., Srikanth V., Dahl P.J., Huynh W., Vu D., Acharya A., Chaudhuri S., Varga T., Batista V.S., Malvankar N.S. Electric field stimulates production of highly conductive microbial OmcZ nanowires. Nat. Chem. Biol. 2020;16(10):1136–1142. doi: 10.1038/s41589-020-0623-9. https://www.nature.com/articles/s41589-020-0623-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Picioreanu C., Katuri K.P., Head I.M., van Loosdrecht M.C.M., Scott K. Mathematical model for microbial fuel cells with anodic biofilms and anaerobic digestion. Water Sci. Technol. 2008;57(7):965–971. doi: 10.2166/wst.2008.095. https://iwaponline.com/wst/article/57/7/965/12533/Mathematical-model-for-microbial-fuel-cells-with [DOI] [PubMed] [Google Scholar]
- 226.Choi Y., Jung E., Kim S., Jung S. Membrane fluidity sensoring microbial fuel cell. Bioelectrochemistry. 2003;59(1–2):121–127. doi: 10.1016/s1567-5394(03)00018-5. https://linkinghub.elsevier.com/retrieve/pii/S1567539403000185 [DOI] [PubMed] [Google Scholar]
- 227.Atkins P., de Paula J. OUP; Oxford: 2011. Physical Chemistry for the Life Sciences.https://books.google.com.mx/books?id=HXOcAQAAQBAJ [Google Scholar]
- 228.Yang Y., Choi C., Xie G., Park J.-D., Ke S., Yu J.-S., Zhou J., Lim B. Electron transfer interpretation of the biofilm-coated anode of a microbial fuel cell and the cathode modification effects on its power. Bioelectrochemistry. 2019;127:94–103. doi: 10.1016/j.bioelechem.2019.02.004. https://linkinghub.elsevier.com/retrieve/pii/S1567539419300738 [DOI] [PubMed] [Google Scholar]
- 229.Zhu W., Yao M., Gao H., Wen H., Zhao X., Zhang J., Bai H. Enhanced extracellular electron transfer between Shewanella putrefaciens and carbon felt electrode modified by bio-reduced graphene oxide. Sci. Total Environ. 2019;691:1089–1097. doi: 10.1016/j.scitotenv.2019.07.104. https://linkinghub.elsevier.com/retrieve/pii/S0048969719332176 [DOI] [PubMed] [Google Scholar]
- 230.Li Y., Liu J., Chen X., Yuan X., Li N., He W., Feng Y. Enhanced electricity generation and extracellular electron transfer by polydopamine–reduced graphene oxide (PDA–rGO) modification for high-performance anode in microbial fuel cell. Chem. Eng. J. 2020;387 https://linkinghub.elsevier.com/retrieve/pii/S1385894719328219 [Google Scholar]
- 231.Hemalatha M., Sravan J.S., Min B., Venkata Mohan S. Concomitant use of Azolla derived bioelectrode as anode and hydrolysate as substrate for microbial fuel cell and electro-fermentation applications. Sci. Total Environ. 2020;707 doi: 10.1016/j.scitotenv.2019.135851. https://linkinghub.elsevier.com/retrieve/pii/S0048969719358462 [DOI] [PubMed] [Google Scholar]
- 232.Liang B., Zhao Y., Zong M., Huo S., Khan I.U., Li K., Lv C. Hierarchically porous N-doped carbon encapsulating CoO/MgO as superior cathode catalyst for microbial fuel cell. Chem. Eng. J. 2020;385 https://linkinghub.elsevier.com/retrieve/pii/S1385894719332760 [Google Scholar]
- 233.Liang B., Ren C., Zhao Y., Li K., Lv C. Nitrogenous mesoporous carbon coated with Co/Cu nanoparticles modified activated carbon as air cathode catalyst for microbial fuel cell. J. Electroanal. Chem. 2020;860 https://linkinghub.elsevier.com/retrieve/pii/S1572665720300874 [Google Scholar]
- 234.Dong H., Yu H., Wang X., Zhou Q., Feng J. A novel structure of scalable air-cathode without nafion and pt by rolling activated carbon and PTFE as catalyst layer in microbial fuel cells. Water Res. 2012;46(17):5777–5787. doi: 10.1016/j.watres.2012.08.005. https://linkinghub.elsevier.com/retrieve/pii/S0043135412005611 [DOI] [PubMed] [Google Scholar]
- 235.Wu D., Sun F., Chua F.J.D., Zhou Y. Enhanced power generation in microbial fuel cell by an agonist of electroactive biofilm – Sulfamethoxazole. Chem. Eng. J. 2020;384 https://linkinghub.elsevier.com/retrieve/pii/S1385894719326506 [Google Scholar]
- 236.Li Y., Li Q., Wang H., Zhang L., Wilkinson D.P., Zhang J. Recent progresses in oxygen reduction reaction electrocatalysts for electrochemical energy applications. Electrochem. Energy Rev. 2019;2(4):518–538. [Google Scholar]
- 237.Wang Z., Deng H., Chen L., Xiao Y., Zhao F. In situ measurements of dissolved oxygen, pH and redox potential of biocathode microenvironments using microelectrodes. Bioresour. Technol. 2013-03;132:387–390. doi: 10.1016/j.biortech.2012.11.026. https://linkinghub.elsevier.com/retrieve/pii/S0960852412017002 [DOI] [PubMed] [Google Scholar]
- 238.Wang D.-W., Su D. Heterogeneous nanocarbon materials for oxygen reduction reaction. Energy Environ. Sci. 2014;7(2):576. [Google Scholar]
- 239.Mo Y., Du M., Yuan T., Liu M., Wang H., He B., Li J., Zhao X. Enhanced anodic oxidation and energy saving for dye removal by integrating O2-reducing biocathode into electrocatalytic reactor. Chemosphere. 2020;252 doi: 10.1016/j.chemosphere.2020.126460. https://linkinghub.elsevier.com/retrieve/pii/S0045653520306536 [DOI] [PubMed] [Google Scholar]
- 240.Freguia S., Rabaey K., Yuan Z., Keller J. Electron and carbon balances in microbial fuel cells reveal temporary bacterial storage behavior during electricity generation. Environ. Sci. Technol. 2007;41(8):2915–2921. doi: 10.1021/es062611i. [DOI] [PubMed] [Google Scholar]
- 241.Jadhav D., Ghadge A., Mondal D., Ghangrekar M. Comparison of oxygen and hypochlorite as cathodic electron acceptor in microbial fuel cells. Bioresour. Technol. 2014;154:330–335. doi: 10.1016/j.biortech.2013.12.069. https://linkinghub.elsevier.com/retrieve/pii/S0960852413019093 [DOI] [PubMed] [Google Scholar]
- 242.Shi X., Liu Y., Ma W., Liu C., Zhang J., Dang X., Huang T. Nitric oxide and oxygen: a promising binary electron acceptor for accelerating power output of microbial fuel cell. J. Power Sources. 2019;423:211–217. https://linkinghub.elsevier.com/retrieve/pii/S0378775319303167 [Google Scholar]
- 243.Xie G., Choi C. High-performance microbial fuel cell using metal ion complexes as electron acceptors. Bull. Korean Chem. Soc. 2020;41(3):348–357. [Google Scholar]
- 244.Wang S., Tian S., Zhang P., Ye J., Tao X., Li F., Zhou Z., Nabi M. Enhancement of biological oxygen demand detection with a microbial fuel cell using potassium permanganate as cathodic electron acceptor. J. Environ. Manag. 2019;252 doi: 10.1016/j.jenvman.2019.109682. https://linkinghub.elsevier.com/retrieve/pii/S0301479719314008 [DOI] [PubMed] [Google Scholar]
- 245.Tiwari A.K., Jain S., Mungray A.A., Mungray A.K. SnO2:PANI modified cathode for performance enhancement of air-cathode microbial fuel cell. J. Environ. Chem. Eng. 2020;8(1) https://linkinghub.elsevier.com/retrieve/pii/S2213343719307134 [Google Scholar]
- 246.Li M., Zhou S., Xu M. Graphene oxide supported magnesium oxide as an efficient cathode catalyst for power generation and wastewater treatment in single chamber microbial fuel cells. Chem. Eng. J. 2017;328:106–116. https://linkinghub.elsevier.com/retrieve/pii/S1385894717311750 [Google Scholar]
- 247.Dai H., Yang H., Liu X., Zhao Y., Liang Z. Performance of sodium bromate as cathodic electron acceptor in microbial fuel cell. Bioresour. Technol. 2016;202:220–225. doi: 10.1016/j.biortech.2015.12.008. https://linkinghub.elsevier.com/retrieve/pii/S0960852415016387 [DOI] [PubMed] [Google Scholar]
- 248.Lawson K., Rossi R., Regan J.M., Logan B.E. Impact of cathodic electron acceptor on microbial fuel cell internal resistance. Bioresour. Technol. 2020;316 doi: 10.1016/j.biortech.2020.123919. https://linkinghub.elsevier.com/retrieve/pii/S0960852420311913 [DOI] [PubMed] [Google Scholar]
- 249.Kim C., Lee C.R., Song Y.E., Heo J., Choi S.M., Lim D.-H., Cho J., Park C., Jang M., Kim J.R. Hexavalent chromium as a cathodic electron acceptor in a bipolar membrane microbial fuel cell with the simultaneous treatment of electroplating wastewater. Chem. Eng. J. 2017;328:703–707. https://linkinghub.elsevier.com/retrieve/pii/S138589471731224X [Google Scholar]
- 250.Rozendal R.A., Hamelers H.V., Rabaey K., Keller J., Buisman C.J. Towards practical implementation of bioelectrochemical wastewater treatment. Trends Biotechnol. 2008;26(8):450–459. doi: 10.1016/j.tibtech.2008.04.008. https://linkinghub.elsevier.com/retrieve/pii/S0167779908001595 [DOI] [PubMed] [Google Scholar]
- 251.The fuel cell store. 2022. https://www.fuelcellstore.com/fuel-cell-components/gas-diffusion-electrode 1902 Pinon Drive, Suite B, College Station, Texas 77845, phone 979-703-1925, sales@fuelcellstore.com, International.
- 252.Sigma-aldrich. 2022. https://www.sigmaaldrich.com/catalog/search?term=graphite+oxide*&interface=All&N=0+9539469&mode=mode20matchpartialmax&lang=es®ion=MX&focus=product PO Box 14508 St. Louis, MO 68178, Missouri, United States, phone (800) 325-3010.
- 253.Fuel cell earth. 2022. https://www.fuelcellearth.com/fuel-cell-products/g200/ Woburn, Massachusetts, phone: 781-935-2707, sales@fuelcellearth.com.
- 254.Fuel cell earth. 2022. https://www.fuelcellearth.com/fuel-cell-products/isomolded-graphite-plate-0-125h-x-6w-x-6l/ Woburn, Massachusetts, phone: 781-935-2707, sales@fuelcellearth.com.
- 255.Fuel cell earth. 2022. https://www.fuelcellearth.com/fuel-cell-products/isomolded-graphite-plate-8w-x-8l/ Woburn, Massachusetts, phone: 781-935-2707, sales@fuelcellearth.com.
- 256.2022. https://www.metalsdepot.com/stainless-steel-products/stainless-plate-304 Metals depot international, 4200 Revilo Road, Winchester, KY 40391 USA, 1-859-745-2650.
- 257.Fuel cell earth. 2022. https://www.fuelcellearth.com/catalyst/ Woburn, Massachusetts, phone: 781-935-2707, sales@fuelcellearth.com.
- 258.Sigma-aldrich. 2022. https://www.sigmaaldrich.com/catalog/search?term=platinum+on+carbon*&interface=All&N=0&mode=match20partialmax&lang=en®ion=US&focus=product PO Box 14508 St. Louis, MO 68178, Missouri, United States, phone (800) 325-3010.
- 259.Noori M.T., Mukherjee C., Ghangrekar M. Enhancing performance of microbial fuel cell by using graphene supported V2O5-nanorod catalytic cathode. Electrochim. Acta. 2017;228:513–521. https://linkinghub.elsevier.com/retrieve/pii/S0013468617300166 [Google Scholar]
- 260.Sigma-aldrich. 2022. https://www.sigmaaldrich.com/catalog/search?term=nafion+perfluorinated+membrane&interface=All&N=0&mode=match20partialmax&lang=en®ion=US&focus=product PO Box 14508 St. Louis, MO 68178, Missouri, United States, phone (800) 325-3010.
- 261.Membranes internatioanl inc. 2022. https://ionexchangemembranes.com/price-list/ 219 Margaret King Avenue, Ringwood, NJ 07456 * USA phone: 973-998-5530 / Fax: 973-998-5529, customerservice@membranesinternational.com.
- 262.Hou Y., Li K., Luo H., Liu G., Zhang R., Qin B., Chen S. Using crosslinked polyvinyl alcohol polymer membrane as a separator in the microbial fuel cell. Front. Environ. Sci. Eng. 2014;8(1):137–143. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.