Abstract
PURPOSE
Baseline metabolic tumor volume (MTV) is a promising biomarker in diffuse large B-cell lymphoma (DLBCL). Our aims were to determine the best statistical relationship between MTV and survival and to compare MTV with the International Prognostic Index (IPI) and its individual components to derive the best prognostic model.
METHODS
PET scans and clinical data were included from five published studies in newly diagnosed diffuse large B-cell lymphoma. Transformations of MTV were compared with the primary end points of 3-year progression-free survival (PFS) and overall survival (OS) to derive the best relationship for further analyses. MTV was compared with IPI categories and individual components to derive the best model. Patients were grouped into three groups for survival analysis using Kaplan-Meier analysis; 10% at highest risk, 30% intermediate risk, and 60% lowest risk, corresponding with expected clinical outcome. Validation of the best model was performed using four studies as a test set and the fifth study for validation and repeated five times.
RESULTS
The best relationship for MTV and survival was a linear spline model with one knot located at the median MTV value of 307.9 cm3. MTV was a better predictor than IPI for PFS and OS. The best model combined MTV with age as continuous variables and individual stage as I-IV. The MTV-age-stage model performed better than IPI and was also better at defining a high-risk group (3-year PFS 46.3% v 58.0% and 3-year OS 51.5% v 66.4% for the new model and IPI, respectively). A regression formula was derived to estimate individual patient survival probabilities.
CONCLUSION
A new prognostic index is proposed using MTV, age, and stage, which outperforms IPI and enables individualized estimates of patient outcome.
INTRODUCTION
The prognosis of diffuse large B-cell lymphoma (DLBCL) is assessed by the International Prognostic Index (IPI), which was introduced in 1993,1 and included age, performance status (PS), Ann Arbor stage, serum lactate dehydrogenase (LDH), and extranodal involvement. Since then, developments in diagnosis and therapy have improved the prognosis of DLBCL,2-5 especially for high-risk groups. Therefore, although IPI remains prognostic, its ability to estimate treatment failure has reduced. Adjustments to reduce the number of prognostic groups (R-IPI),6 increase the age cutoff from 60 to 70 years,7,8 and apply multiple scores for IPI components (National Comprehensive Cancer Network [NCCN]-IPI)9 have made modest improvements. A recent report showed that the NCCN-IPI performed best; however, the 5-year overall survival (OS) of the poorest prognostic group was still 49%.10
CONTEXT
Key Objective
The International Prognostic Index (IPI) has been used for estimating prognosis of diffuse large B-cell lymphoma since 1993. Metabolic tumor volume (MTV) also predicts outcome in diffuse large B-cell lymphoma but the optimal way to use MTV was unknown. Previous studies examined its role in a dichotomous fashion. This study examined the relationship between MTV as a continuous variable with survival and the best way to incorporate MTV with (components of) IPI
Knowledge Generated
A linear spline model was the best way to express the relationship between MTV and survival, with a larger effect of MTV increments at lower values. A new model (International Metabolic Prognostic Index = IMPI) combines MTV, age (as continuous variables), and stage, and predicts relapse and survival better than IPI.
Relevance (J.W. Friedberg)
-
The IMPI is a prognostic index that allows individualized estimates of probability of relapse and survival for patients with diffuse large B-cell lymphoma, and may assist with future clinical trial design.*
*Relevance section written by JCO Editor-in-Chief Jonathan W. Friedberg, MD.
Metabolic tumor volume (MTV) using 18-fluorine fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET-CT) is prognostic in several lymphoma subtypes11-18 including DLBCL, despite variations in measurement methods,16,19,20 statistical analyses used to evaluate the relationship between MTV and survival,12,16 and different cutoff points used to separate high from low MTV19-21 between studies. MTV was reported to be independent of IPI for prediction of progression-free survival (PFS)13 and OS,22 although in one report MTV was highly correlated with all IPI factors except age.16 Until now, MTV has been evaluated as a categorical variable, although biomarkers predict outcomes better as continuous variables.23 Currently, it is unknown how best to use MTV, either as a continuous or categorical variable using cutoff points, and either alone or in combination with IPI factors to estimate prognosis in DLBCL.
The aims of this study were to (1) determine the best expression of the relationship between baseline MTV and survival, (2) compare MTV with IPI categories for prediction of patient outcomes, (3) compare the combination of MTV with IPI categories and individual IPI components to decide the best model to predict survival, and (4) validate the best performing model.
METHODS
Study Population
One thousand two hundred forty-one new patients with DLBCL from five published research studies with baseline 18F-FDG PET were included. Individual patient-level clinical information and PET scans were collated and harmonized by the PETRA consortium24. Individual studies were approved by institutional review boards and/or ethics committees, and all patients provided informed consent. Three studies were observational (GSTT15, NCRI, and SAKK)13,25,26 and two were randomized trials (HOVON-84 and PETAL)27,28 with no significant difference in progression and survival between treatment arms, allowing combined analysis of arms.
Quantitative PET Measurements
MTV was measured by including tumor with a standardized uptake value (SUV) ≥ 4.0 using ACCURATE29 (4 studies) or MIM software (version 6.7.10; SAKK study; MIM Software Inc, Cleveland, OH) by authors, on the basis of earlier work to determine the optimal measurement method.21 MTV was measured in 85 patients using both software programs. Delineations were performed by a nuclear medicine physician (GSTT15 and SAKK) or under supervision of a nuclear medicine physician by trained researchers (HOVON84, PETAL, and NCRI) blinded to patient outcome.
Statistical Analysis
The primary end point was 3-year PFS, defined as the time from baseline PET to progression, relapse, or death from any cause. After 3 years, patients were censored. Secondary end points were 3-year OS and 3-year time to progression (TTP). OS was defined as time from baseline PET to death. Patients alive at date of last contact or end of study were censored. TTP was defined as time from baseline PET to progression or relapse where patients dying within 3 years were censored.
The associations of survival end points with MTV and IPI and its components were examined in a stepwise fashion.
Step 1—To Determine the Best Expression of the Relationship Between MTV and Survival
The relationship between baseline MTV as a continuous variable and the end points was examined using Cox regression models. Transformations of the MTV variable tested were cubic root transformation, natural log transformation, squaring, restricted cubic spline, and linear spline (LSP) models. For the LSP model, we tested one knot located at the median MTV (50th percentile), two knots at the 33rd and 66th percentiles, and three knots at the 10th, 50th, and 90th percentiles. The same three knot locations were used for the restricted cubic spline model (the Data Supplement, online only, gives a detailed explanation of spline functions).
These transformations were compared with a linear model to determine the best shape and fit for MTV with survival and tested unadjusted and adjusted for IPI. The fit of the models was evaluated using the Akaike information criterion (AIC)30 and the cross-validated c-index. To test the robustness of the model, analyses were performed combining all five studies and repeated for the five separate study cohorts.
Step 2—To Compare MTV With IPI Categories for Prediction of Outcomes
The best fitting transformation for MTV from step 1 was compared with IPI risk categories (low, low-intermediate, high-intermediate, and high-risk) for prediction of survival end points.
Step 3—To Compare the Combination of MTV With IPI Categories or Individual IPI Components to Decide on the Best Predictive Model
Using the best fitting transformation of MTV as a comparator, we evaluated nine models combining MTV with IPI categories and combining MTV with individual IPI components (age, stage, LDH, PS, and extranodal involvement). We also tested variations of the models using age as a continuous variable versus dichotomous (< 60 and ≥ 60 years) and individual stages I-IV versus dichotomous (stages I-II and III-IV).
The best model was validated using a leave-one-out cross-validation approach.31 Patients from four studies were used as the test set, and then validated in the fifth independent data set. This was repeated five times using a different study each time as the external independent validation set. Within the same cross-validation loop, we determined overfitting in the regression coefficients of the best model by applying the train linear predictor (slope) in the test data sets. The slope value was used to correct the coefficients for overfitting.
Cox regression models were used to study the relationship between MTV and IPI variables with survival. AIC was used to decide the best model fit. To assess the strength of the relationships, hazard ratios (HRs) were calculated. The cross-validated c-index for discrimination was used to assess model performance.
For all relevant models, Kaplan-Meier curves were created. Statistical analysis was performed using R (version 4.1.0). A P value < .05 was considered statistically significant.
RESULTS
Table 1 shows patient characteristics. The clinical data and MTV measurements from the five studies were merged into a data set comprising 1,241 patients. Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone were given in 99.0% of patients. Three-year PFS was 74.5% (95% CI, 72.1 to 77.0), 3-year OS was 81.8% (95% CI, 79.7 to 84.0), and 3-year TTP was 79.7% (95% CI, 77.4 to 82.0) with a median follow-up of 55 months. Agreement between MTV measurements using ACCURATE and MIM software programs was excellent (R2 = 0.9997; limits of agreement 4.07 ± 26.04). The median MTV was 307.9 mL (interquartile range, 77.6-838.9 mL). The results for TTP were similar to PFS and OS and are given in the Data Supplement.
TABLE 1.
Patient Characteristics
Step 1—To Determine the Best Expression of the Relationship Between MTV and Survival
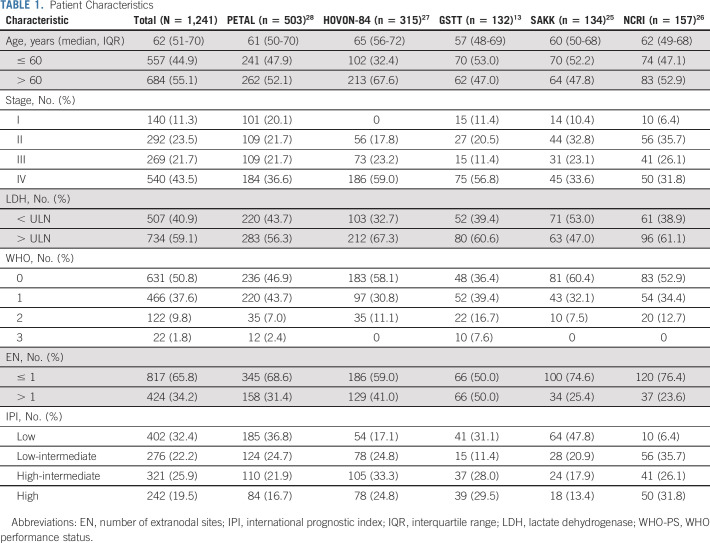
The best fit for the relationship between MTV with 3-year PFS and OS was obtained by expressing MTV as an LSP variable with one knot at the median MTV value, with and without adjustment for IPI (Data Supplement). Figure 1 illustrates MTV expressed as an LSP with the log HR for PFS. The log HR increased more rapidly for patients with MTV values below the median (log HR increased by 0.39095 per 100-mL increase in MTV) than for patients with values above the median (log HR increased by 0.03639 per 100-mL increase in MTV). This was also the best model considering each study cohort separately (Data Supplement). Therefore, in all further analyses, MTV was included using an LSP function with one knot at the median (Data Supplement).
FIG 1.
Relationship between the (log-transformed) HR for PFS with MTV. The relationship of MTV with outcome is described by two coefficients below and above the median value of 307.9 mL of 0.0039095 and 0.0003639, respectively, with 95% CIs shown by the dotted lines. HR, hazard ratio; MTV, metabolic tumor volume; PFS, progression-free survival.
Step 2—To Compare MTV With IPI Categories for Prediction of Patient Outcomes
Concordance was higher for MTV (c-index = 0.650 and 0.667 for PFS and OS, respectively) than for IPI (c-index = 0.619 and 0.646, respectively). This is supported by their AIC values, which was lowest for MTV. Hence, MTV was a better predictor (Data Supplement).
Step 3—To Compare the Combination of MTV With IPI Categories or Individual IPI Components to Decide on the Best Predictive Model
Models that combined MTV and IPI were better than models using MTV or IPI alone for predicting PFS and OS on the basis of AIC and the cross-validated c-index (Data Supplement). MTV was always the strongest predictor of PFS and OS in all the models examined. Subsequently, we examined whether we could obtain the same or better prediction by adding one or more individual IPI factors to MTV. Age and stage added to the prediction of PFS and OS, whether expressed as dichotomous or continuous variable (stage: early/advanced or I-IV and age: < / ≥ 60 or continuous) (Data Supplement). The models that included age as a continuous variable and individual stages improved the prediction of OS compared with models with dichotomous variables. PS, LDH, and extranodal sites did not add to the prediction of end points. Across the three outcomes and the two criteria (AIC and c-index), no model performed uniformly best (Data Supplement). We decided to regard the combination of MTV with age and stage as the best predicting model, as it was always among the three best models and in particular improved the prediction compared to the MTV + IPI model for both OS and TTP. Adding the individual study or treatment group as variables did not improve the model fit (data not shown).
We compared the new model of MTV, age, and stage with IPI, first dividing the study population into four groups with the same sizes as the IPI categories. Kaplan-Meier analysis (Fig 2, panels A-D and B-E curves) showed that 3-year PFS for the highest-risk group in the International Metabolic Prognostic Index (IMPI) was 55.0% and 3-year OS was 60.3% compared with 58.0% and 66.4%, respectively, for IPI highest-risk group.
FIG 2.
Kaplan-Meier survival curves according to the IPI and the MTV-age-stage (IMPI) prediction model. Three-year survival curves for PFS (A-C) stratified by (A) IPI categories, (B) IMPI with same group size as IPI categories, (C) IMPI with low-intermediate-high risk categories (60%-30%-10%); 3-year survival curves for OS (D-F) stratified by (D) IPI categories, (E) IMPI with same group size as IPI categories, and (F) IMPI with low-intermediate-high risk categories (60%-30%-10%). IMPI, International Metabolic Prognostic Index; IPI, International Prognostic Index; MTV, metabolic tumor volume; OS, overall survival; PFS, progression-free survival.
Taking advantage of IMPI being continuous, we divided the population into three groups on the basis of expected clinical outcomes32; highest risk (highest 10%) corresponding to primary refractory disease incidence, intermediate risk (middle 30%) corresponding to relapse after initial response, and lowest risk (lowest 60%) corresponding to long-term remission. Survival analysis showed excellent performance for IMPI, with significant separation of three groups and a better performance than IPI for the highest-risk group in particular. Three-year PFS for the highest-risk group in the IMPI was 46.3% and 3-year OS was 51.5% compared with 58.0% and 66.4%, respectively.
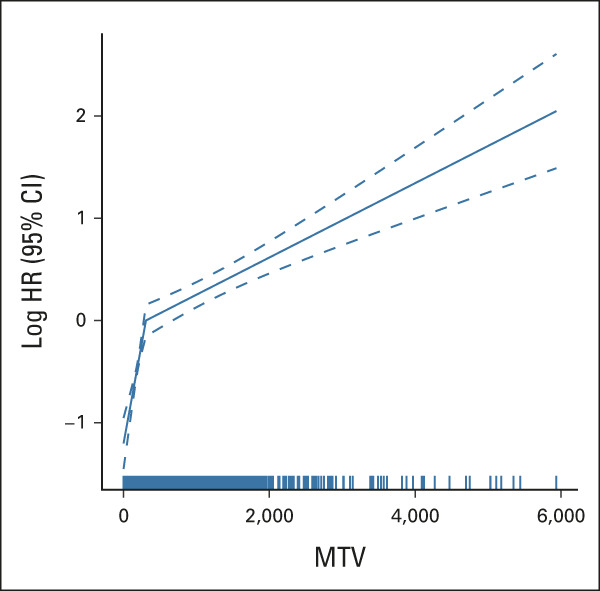
The IMPI model can be used to estimate individual patient risk probabilities using a regression formula and PFS calculator given in the Data Supplement, with patient examples in Figure 3.
FIG 3.
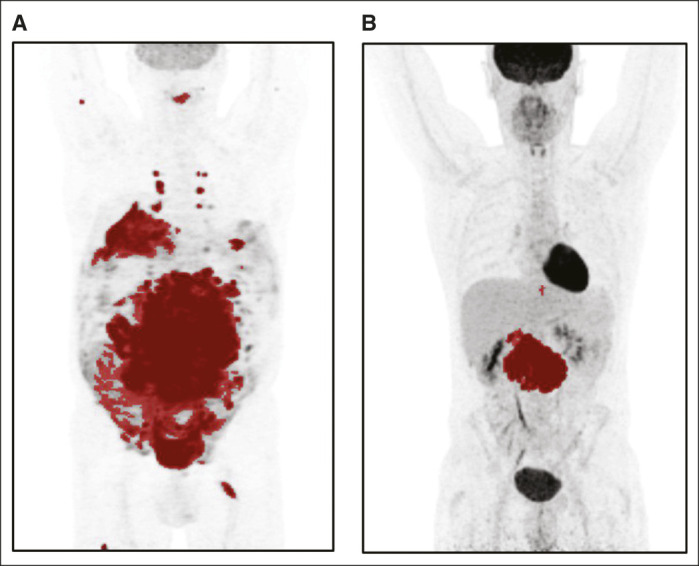
Maximum-intensity projections using SUV0-10 scale of (A) a high-risk patient according to the MTV model and (B) a low-risk patient according to the MTV model. Images are scaled using an SUV0-10 scale. The risk of progression, relapse, or death is for patient A with MTV 4,091 mL, age 61 years, and stage IV disease is 68.51%, and for patient B with MTV 274 mL, age 40 years, and stage II disease is 15.76%. Details of how to implement the regression formula 1 – (exp(–(exp(lp_risk – 1.241946) × 0.208042380))) are given in the Data Supplement. MTV, metabolic tumor volume; SUV, standardized uptake value.
Validation of the MTV-age-stage model confirmed that it consistently outperformed the other models for predicting PFS and OS (Data Supplement).
DISCUSSION
We present a simple and robust prognostic index that predicts outcomes for DLBCL better than IPI. The IMPI uses three factors: MTV as representative of total disease burden, stage as a measure of disease dissemination, and age reflecting the biologic reserve of the patient.
MTV is a good predictor of outcome in DLBCL and other lymphoma subtypes, regardless of the measurement method.11-16,20,33,34 However previous reports analyzed MTV in a dichotomous manner, dividing patients into low and high MTV groups, using different cutoff values, losing valuable prognostic information.23 Therefore, the first aim of this study was to examine the relationship between MTV as a continuous variable with survival, which was not a simple linear relationship. At lower values of MTV, incremental increases had a larger adverse effect on survival than the same increments at the higher MTV range. The best model was the LSP model with one knot at the median MTV both for the merged data set and individual studies, adjusted and unadjusted for IPI. The advantage of an LSP model is that it uses continuous MTV data and allows individual patient risk prediction using two coefficients: above and below the median (of 308 mL). Moreover, the discussion about the optimal cutoff point for low risk and high risk almost disappears, ie, the weakness of a dichotomous risk prediction giving two different survival estimates for values close to the cutoff (eg, 300 and 320 mL), when the actual survival is similar and is more accurately predicted with an LSP model.
MTV was a better predictor of outcomes compared with IPI using AIC and cross validated c-index. However, models combining MTV and IPI (four risk groups) were better than either MTV or IPI alone for predicting PFS and OS. The prediction of the model improved when stage was used as I-IV and age as a continuous variable compared with using these variables as dichotomous like the original IPI, confirming the importance of using all prognostic information available.
The factors in IPI represent disease burden and biology (LDH, stage, and extranodal involvement) and host factors (age and PS). As MTV measures disease burden, we hypothesized that the addition of MTV to IPI in a prognostic model could replace some of the factors that reflect disease burden. Interestingly, this analysis showed that only age and stage added to the predictive performance of MTV within a combined MTV/IPI model, suggesting MTV reflects disease burden better than the surrogate measures in the IPI. It is conceivable that stage, however, also represents disease dissemination and therefore adds useful prognostic information, which is independent of MTV. Recent work showed that combining MTV with tumor dissemination measured by the furthest distance between lesions in the body improved DLBCL risk stratification at staging.35 Members of our group also reported that distance between the bulkiest lesion and the lesion furthest away (Dmax-bulk) and the peak SUV were independent predictors of TTP from MTV36; however, Dmax is not currently routinely measurable in clinical practice. Similarly, it is intuitive that age as a continuous variable is an independent prognostic factor reflecting the host biologic reserve and that a single age cutoff will underestimate its effects on health and life expectancy. Previous reports increasing the age cutoff from 60 to 70 years7,8 or allocating ascending risk scores by age9 improved IPI performance.
We used a highly robust method for cross-validation of the MTV-age-stage model. We used a leave-one-study-out cross-validation approach that allowed us to train and externally test the models using patients with DLBCL from different populations.
To make the best use of the continuous nature of IMPI, we divided the population into three groups on the basis of anticipated clinical outcomes:32 highest, intermediate, and lowest risk (10%, 30%, and 60%, respectively) corresponding to incidence of primary refractory disease, relapse after initial response, and long-term remission (Figs 2C and 2F). There were clinically meaningful differences with 3-year OS of 90.0%, 75.5%, and 51.5%, respectively, for low-, intermediate-, and high-risk groups. IMPI was also better at defining a high-risk group than IPI (3-year PFS 46.3% v 58.0% and OS 51.5% v 66.4% for IMPI and IPI, respectively).
Most importantly, the new IMPI enables clinicians to estimate personalized prognosis on the basis of a patient's MTV, age, and stage using a simple regression formula (Data Supplement). This individualized level of prediction is more accurate than the traditional four IPI categories. IMPI can be applied in clinical practice and clinical trials in a similar fashion to the standard IPI, eg, to stratify patients for treatment comparisons or select patient groups with a defined prognosis to test new treatment approaches.
There are several advantages for the new index, which is simple and uses three factors with clinical rationale. Age and stage are readily available and are the most robust factors in IPI, unlike PS, which is subjective and can fluctuate or LDH level, which might progressively increase and depends on the measurement time point. MTV measurement is becoming increasingly automated, and modern software programs make it feasible for routine clinical reporting. Our group has tested reproducibility and ease of MTV measurements using different methods.19,21 The method used here can be replicated using commercially available software programs with close agreement between platforms as we have demonstrated. We recommend SUV4 for clinical implementation currently, acknowledging that artificial intelligence and mathematical modeling methods may evolve. Perhaps, the greatest advantage of the new model is incorporating MTV and age as continuous variables. This avoids loss of valuable information and reliance on optimal cutoffs that may be heavily influenced by the data distribution in the study population and disproportionately underestimate or overestimate risk with values close to these arbitrary cutoffs. Finally, the model provides a simple formula to estimate individual patient outcome.
Other groups have tested a combination of MTV with IPI factors. In patients age > 60 years in the REMARC study,17 IPI was not predictive; so, investigators selected three factors: MTV, PS, and treatment arm, which were significant in univariate analysis and tested a model combining MTV (dichotomous) and PS, which was predictive of prognosis. Our study used a different approach by testing variables as both dichotomous and continuous, evaluating the best statistical method for the association of MTV with survival and by comparing nine models comprising several possible combinations of factors. However, both studies concur that MTV can replace many of the factors in IPI.
Unfortunately, we cannot compare the new model to NCCN-IPI because of the lack of exact LDH levels. Another limitation of our study is that the comparison of the MTV-based prediction with the IPI may be somewhat biased, as we selected for MTV the best fitting model from a set of models. However, as this model (LSP with one knot) is a parsimonious model that was corrected for overfitting, we regarded this bias as negligible.
Strengths of this study are inclusion of a large population with quality-assured PET scans from the PETRA consortium with patient-level clinical and imaging information. All MTV measurements were done the same way, including tumor with SUV4 or greater.21 This is the first study, to our knowledge, to incorporate MTV as a continuous variable and to show that LSP is the best function to express the relationship between MTV and survival rather than a linear relationship and provide individualized risk estimates. We also accounted for the competing risks of progression and death by analyzing the data using Cox models and three different outcomes, and found consistent results in model fit and performance.
In conclusion, we present a simple robust new prognostic index that can be used in clinical practice and clinical trials for adults with newly diagnosed DLBCL on the basis of MTV (measured with SUV4), age, and stage, used as continuous variables, which allows individualized estimates of patient outcome.
Ulrich Dührsen
Honoraria: Amgen, CPT Cellex Patient Treatment
Research Funding: Celgene (Inst)
Christine Schmitz
Research Funding: Novartis (Inst)
Travel, Accommodations, Expenses: AbbVie
Andreas Hüttmann
Honoraria: Takeda
Consulting or Advisory Role: Takeda
Travel, Accommodations, Expenses: Takeda, Roche Pharma AG
Pieternella J. Lugtenburg
Consulting or Advisory Role: Takeda, Servier, Roche/Genentech, Genmab, Celgene, Regeneron, Incyte, AbbVie
Research Funding: Takeda (Inst), Servier (Inst)
Travel, Accommodations, Expenses: Celgene
Emanuele Zucca
Consulting or Advisory Role: BeiGene, Incyte, Janssen Biotech, Merck, Roche, Miltenyi Biomedicine
Research Funding: AstraZeneca (Inst), Celgene (Inst), Incyte (Inst), Janssen (Inst), Roche (Inst), BeiGene (Inst)
Travel, Accommodations, Expenses: Roche (Inst), Janssen (Inst)
Sally F. Barrington
Research Funding: Bristol Myers Squibb (Inst), Amgen (Inst), Pfizer (Inst), Novartis Pharmaceuticals UK Ltd (Inst)
No other potential conflicts of interest were reported.
DISCLAIMER
The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
SUPPORT
The PETRA project is supported by the Alpe d’HuZes/KWF fund, provided by the Dutch Cancer Society (VU 2012-5848). King's College London and UCL Comprehensive Cancer Imaging Centre is funded by the CRUK and EPSRC in association with the MRC and Department of Health and Social Care (England). This work was also supported by core funding from the Wellcome/EPSRC Centre for Medical Engineering at King's College London [WT203148/Z/16/Z] and the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London and/or the NIHR Clinical Research Facility. The PETAL trial was supported by grants from Deutsche Krebshilfe (107592 and 110515 to U.D.). This work is supported by KWF Dutch Cancer Society (project 11648; Radiomics for better prediction of outcome in DLBCL patients). S.F.B. acknowledges support from the National Institute for Health Research and Social Care (NIHR) [RP-2-16-07-001].
AUTHOR CONTRIBUTIONS
Conception and design: N. George Mikhaeel, Martijn W. Heymans, Jakoba J. Eertink, Henrica C.W. de Vet, Luca Ceriani, Otto S. Hoekstra, Sally F. Barrington
Collection and assembly of data: N. George Mikhaeel, Jakoba J. Eertink, Henrica C.W. de Vet, Ronald Boellaard, Ulrich Dührsen, Luca Ceriani, Christine Schmitz, Sanne E. Wiegers, Andreas Hüttmann, Emanuele Zucca, Gerben J.C. Zwezerijnen, Otto S. Hoekstra, Josée M. Zijlstra, Sally F. Barrington
Provision of study materials or patients: N. George Mikhaeel, Ulrich Dührsen, Luca Ceriani, Andreas Huttmann, Pieternella J. Lugtenburg, Emanuele Zucca, Josée M. Zijlstra, Sally F. Barrington
Data analysis and interpretation: N. George Mikhaeel, Martijn W. Heymans, Jakoba J. Eertink, Henrica C.W. de Vet, Ronald Boellaard, Ulrich Dührsen, Christine Schmitz, Pieternella J. Lugtenburg, Emanuele Zucca, Josée M. Zijlstra, Sally F. Barrington
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Proposed New Dynamic Prognostic Index for Diffuse Large B-Cell Lymphoma: International Metabolic Prognostic Index
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ulrich Dührsen
Honoraria: Amgen, CPT Cellex Patient Treatment
Research Funding: Celgene (Inst)
Christine Schmitz
Research Funding: Novartis (Inst)
Travel, Accommodations, Expenses: AbbVie
Andreas Hüttmann
Honoraria: Takeda
Consulting or Advisory Role: Takeda
Travel, Accommodations, Expenses: Takeda, Roche Pharma AG
Pieternella J. Lugtenburg
Consulting or Advisory Role: Takeda, Servier, Roche/Genentech, Genmab, Celgene, Regeneron, Incyte, AbbVie
Research Funding: Takeda (Inst), Servier (Inst)
Travel, Accommodations, Expenses: Celgene
Emanuele Zucca
Consulting or Advisory Role: BeiGene, Incyte, Janssen Biotech, Merck, Roche, Miltenyi Biomedicine
Research Funding: AstraZeneca (Inst), Celgene (Inst), Incyte (Inst), Janssen (Inst), Roche (Inst), BeiGene (Inst)
Travel, Accommodations, Expenses: Roche (Inst), Janssen (Inst)
Sally F. Barrington
Research Funding: Bristol Myers Squibb (Inst), Amgen (Inst), Pfizer (Inst), Novartis Pharmaceuticals UK Ltd (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.International Non-Hodgkin's Lymphoma Prognostic Factors Project : A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med 329:987-994, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Sehn LH, Donaldson J, Chhanabhai M, et al. : Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol 23:5027-5033, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Coiffier B, Lepage E, Briere J, et al. : CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 346:235-242, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Cheson BD, Fisher RI, Barrington SF, et al. : Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol 32:3059-3068, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metser U, Prica A, Hodgson DC, et al. : Effect of PET/CT on the management and outcomes of participants with Hodgkin and aggressive non-Hodgkin lymphoma: A multicenter registry. Radiology 290:488-495, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Sehn LH, Berry B, Chhanabhai M, et al. : The revised international prognostic index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood 109:1857-1861, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Gang AO, Pedersen M, d'Amore F, et al. : A clinically based prognostic index for diffuse large B-cell lymphoma with a cut-off at 70 years of age significantly improves prognostic stratification: Population-based analysis from the Danish lymphoma registry. Leuk Lymphoma 56:2556-2562, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Mikhaeel NG: Is 70 the new 60? New international prognostic index with an older age cut-off for diffuse large B-cell lymphoma. Leuk Lymphoma 56:2487-2488, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Zhou Z, Sehn LH, Rademaker AW, et al. : An enhanced international prognostic index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 123:837-842, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruppert AS, Dixon JG, Salles G, et al. : International prognostic indices in diffuse large B-cell lymphoma: A comparison of IPI, R-IPI, and NCCN-IPI. Blood 135:2041-2048, 2020 [DOI] [PubMed] [Google Scholar]
- 11.Ceriani L, Gritti G, Cascione L, et al. : SAKK38/07 study: Integration of baseline metabolic heterogeneity and metabolic tumor volume in DLBCL prognostic model. Blood Adv 4:1082-1092, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meignan M, Cottereau AS, Versari A, et al. : Baseline metabolic tumor volume predicts outcome in high-tumor-burden follicular lymphoma: A pooled analysis of three multicenter studies. J Clin Oncol 34:3618-3626, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Mikhaeel NG, Smith D, Dunn JT, et al. : Combination of baseline metabolic tumour volume and early response on PET/CT improves progression-free survival prediction in DLBCL. Eur J Nucl Med Mol Imaging 43:1209-1219, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tout M, Casasnovas O, Meignan M, et al. : Rituximab exposure is influenced by baseline metabolic tumor volume and predicts outcome of DLBCL patients: A lymphoma study association report. Blood 129:2616-2623, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Cottereau AS, El-Galaly TC, Becker S, et al. : Predictive value of PET response combined with baseline metabolic tumor volume in peripheral T-cell lymphoma patients. J Nucl Med 59:589-595, 2018 [DOI] [PubMed] [Google Scholar]
- 16.Schmitz C, Huttmann A, Muller SP, et al. : Dynamic risk assessment based on positron emission tomography scanning in diffuse large B-cell lymphoma: Post-hoc analysis from the PETAL trial. Eur J Cancer 124:25-36, 2020 [DOI] [PubMed] [Google Scholar]
- 17.Vercellino L, Cottereau A, Casasnovas O, et al. : High total metabolic tumor volume at baseline predicts survival independent of response to therapy. Blood 135:1396-1405, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cottereau AS, Versari A, Loft A, et al. : Prognostic value of baseline metabolic tumor volume in early-stage Hodgkin lymphoma in the standard arm of the H10 trial. Blood 131:1456-1463, 2018 [DOI] [PubMed] [Google Scholar]
- 19.Ilyas H, Mikhaeel NG, Dunn JT, et al. : Defining the optimal method for measuring baseline metabolic tumour volume in diffuse large B cell lymphoma. Eur J Nucl Med Mol Imaging 45:1142-1154, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrington SF, Meignan M: Time to prepare for risk adaptation in lymphoma by standardizing measurement of metabolic tumor burden. J Nucl Med 60:1096-1102, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barrington SF, Zwezerijnen BG, de Vet HC, et al. : Automated segmentation of baseline metabolic total tumor burden in diffuse large B-cell lymphoma: Which method is most successful. J Nucl Med 62:332-337, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sasanelli M, Meignan M, Haioun C, et al. : Pretherapy metabolic tumour volume is an independent predictor of outcome in patients with diffuse large B-cell lymphoma. Eur J Nucl Med Mol Imaging 41:2017-2022, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Royston P, Altman DG, Sauerbrei W: Dichotomizing continuous predictors in multiple regression: A bad idea. Stat Med 25:127-141, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Eertink JJ, Burggraaff CN, Heymans MW, et al. : Optimal timing and criteria of interim PET in DLBCL: A comparative study of 1692 patients. Blood Adv 5:2375-2384, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mamot C, Klingbiel D, Hitz F, et al. : Final results of a prospective evaluation of the predictive value of interim positron emission tomography in patients with diffuse large B-cell lymphoma treated with R-CHOP-14 (SAKK 38/07). J Clin Oncol 33:2523-2529, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Mikhaeel NG, Cunningham D, Counsell N, et al. : FDG-PET/CT after two cycles of R-CHOP in DLBCL predicts complete remission but has limited value in identifying patients with poor outcome—Final result of a UK national cancer research institute prospective study. Br J Haematol 192:504-513, 2021 [DOI] [PubMed] [Google Scholar]
- 27.Lugtenburg PJ, de Nully Brown P, van der Holt B, et al. : Rituximab-CHOP with early rituximab intensification for diffuse large B-cell lymphoma: A randomized phase III trial of the HOVON and the nordic lymphoma group (HOVON-84). J Clin Oncol 38:3377-3387, 2020 [DOI] [PubMed] [Google Scholar]
- 28.Duhrsen U, Muller S, Hertenstein B, et al. : Positron emission tomography-guided therapy of aggressive non-Hodgkin lymphomas (PETAL): A multicenter, randomized phase III trial. J Clin Oncol 36:2024-2034, 2018 [DOI] [PubMed] [Google Scholar]
- 29.Boellaard R: Quantitative oncology molecular analysis suite: ACCURATE. J Nucl Med 59:1753, 2018 [Google Scholar]
- 30.Harrell F: Chapter 9, section 9.8.1, in Regression Modeling Strategies with Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis (ed 2). New York, NY, Springer, 2015. p 204 [Google Scholar]
- 31.Debray TP, Moons KG, Ahmed I, et al. : A framework for developing, implementing, and evaluating clinical prediction models in an individual participant data meta-analysis. Stat Med 32:3158-3180, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Sehn LH, Gascoyne RD: Diffuse large B-cell lymphoma: Optimizing outcome in the context of clinical and biologic heterogeneity. Blood 125:22-32, 2015 [DOI] [PubMed] [Google Scholar]
- 33.Song MK, Chung JS, Shin HJ, et al. : Clinical significance of metabolic tumor volume by PET/CT in stages II and III of diffuse large B cell lymphoma without extranodal site involvement. Ann Hematol 91:697-703, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cottereau AS, Becker S, Broussais F, et al. : Prognostic value of baseline total metabolic tumor volume (TMTV0) measured on FDG-PET/CT in patients with peripheral T-cell lymphoma (PTCL). Ann Oncol 27:719-724, 2016 [DOI] [PubMed] [Google Scholar]
- 35.Cottereau AS, Nioche C, Dirand AS, et al. : 18F-FDG PET dissemination features in diffuse large B-cell lymphoma are predictive of outcome. J Nucl Med 61:40-45, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eertink JJ, van de Brug T, Wiegers SE, et al. : 18F-FDG PET baseline radiomics features improve the prediction of treatment outcome in diffuse large B-cell lymphoma. Eur J Nucl Med Mol Imaging 49:932-942, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]