Abstract
PURPOSE
The phase III KEYNOTE-048 (ClinicalTrials.gov identifier: NCT02358031) trial of pembrolizumab in recurrent or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC) included planned efficacy analyses in the total population and in participants with programmed death ligand-1 (PD-L1) combined positive score (CPS) ≥ 1 and CPS ≥ 20. To further characterize the predictive value of PD-L1 expression on outcome, we conducted efficacy analyses in the PD-L1 CPS < 1 and CPS 1-19 subgroups in KEYNOTE-048.
METHODS
Participants with R/M HNSCC and no prior systemic therapy for R/M disease were randomly assigned 1:1:1 to pembrolizumab, pembrolizumab-chemotherapy, or cetuximab-chemotherapy. Post hoc efficacy analyses of the PD-L1 CPS < 1 and CPS 1-19 subgroups were performed.
RESULTS
Of 882 participants enrolled, 128 had PD-L1 CPS < 1 and 373 had CPS 1-19. For pembrolizumab versus cetuximab-chemotherapy, the median overall survival was 7.9 versus 11.3 months in the PD-L1 CPS < 1 subgroup (hazard ratio [HR], 1.51 [95% CI, 0.96 to 2.37]) and 10.8 versus 10.1 months in the CPS 1-19 subgroup (HR, 0.86 [95% CI, 0.66 to 1.12]). For pembrolizumab-chemotherapy versus cetuximab-chemotherapy, the median overall survival was 11.3 versus 10.7 months in the PD-L1 CPS < 1 subgroup (HR, 1.21 [95% CI, 0.76 to 1.94]) and 12.7 versus 9.9 months in the CPS 1-19 subgroup (HR, 0.71 [95% CI, 0.54 to 0.94]).
CONCLUSION
Increased efficacy of pembrolizumab or pembrolizumab-chemotherapy was observed with increasing PD-L1 expression. PD-L1 CPS < 1 subgroup analysis was limited by small participant numbers. Results from the PD-L1 CPS 1-19 subgroup support previous findings of treatment benefit with pembrolizumab monotherapy and pembrolizumab-chemotherapy in patients with PD-L1 CPS ≥ 1 tumors. Although PD-L1 expression is informative, exploration of additional predictive biomarkers is needed for low PD-L1–expressing HNSCC.
INTRODUCTION
Programmed death ligand-1 (PD-L1) is frequently overexpressed in head and neck squamous cell carcinoma (HNSCC) and serves as a therapeutic target and predictive biomarker.1 PD-L1 overexpression activates the programmed death 1 (PD-1)/PD-L1 axis to promote immune evasion, permitting tumor growth.2,3 PD-1 is expressed on immune cells, including T cells, B cells, and activated monocytes, whereas PD-L1 is expressed by tumor cells, immune cells, and various nonhematopoietic cells.3,4 The anti–PD-1 antibodies pembrolizumab and nivolumab have demonstrated antitumor activity and acceptable safety in several cancers that overexpress PD-L1, including HNSCC.5-7 PD-L1 expression in pembrolizumab trials is described by tumor proportion score (TPS), defined as the percentage of viable tumor cells showing partial or complete membrane PD-L1 staining, or combined positive score (CPS), defined as the number of PD-L1–positive tumor cells, lymphocytes, and macrophages divided by the total number of tumor cells, multiplied by 100. Although expression of PD-L1 is common in HNSCC, some tumors have low or undetectable levels.8,9
CONTEXT
Key Objective
KEYNOTE-048 investigated pembrolizumab monotherapy and pembrolizumab plus chemotherapy in recurrent or metastatic head and neck squamous cell carcinoma. Efficacy was assessed in participants with a programmed death ligand-1 (PD-L1) combined positive score (CPS) ≥ 20 and CPS ≥ 1 and the total population. To further characterize the predictive value of PD-L1 expression, efficacy was analyzed in participants with PD-L1 CPS < 1 and CPS 1-19.
Knowledge Generated
Pembrolizumab and pembrolizumab-chemotherapy demonstrated antitumor activity in the PD-L1 CPS 1-19 subgroup, with pembrolizumab-chemotherapy leading to numerically longer overall survival than cetuximab-chemotherapy. In the PD-L1 CPS < 1 subgroup, neither pembrolizumab nor pembrolizumab-chemotherapy demonstrated improvement in overall survival compared with cetuximab-chemotherapy.
Relevance
These results support previous findings and demonstrate increased efficacy for pembrolizumab or pembrolizumab-chemotherapy with increasing PD-L1 expression. The results indicate that although PD-L1 expression is useful, additional predictive biomarkers are needed for informing treatment decisions in low PD-L1–expressing recurrent or metastatic head and neck squamous cell carcinoma.
Pembrolizumab produces durable responses and robust antitumor activity in recurrent or metastatic (R/M) HNSCC, with greater benefit observed in PD-L1–enriched populations.10,11 In the phase Ib KEYNOTE-012 study of pembrolizumab monotherapy in R/M HNSCC (N = 192), objective response rate (ORR) was higher in patients with PD-L1 CPS ≥ 1 than CPS < 1 (21% v 6%; one-sided P = .023) and median overall survival (OS) was longer in patients with CPS ≥ 1 than CPS < 1 (10 v 5 months; one-sided P = .008).6 In the phase III KEYNOTE-040 study, patients with platinum-refractory R/M HNSCC (N = 495) were randomly assigned to pembrolizumab or standard-of-care systemic therapy.10 In subgroup analysis, the ORR with pembrolizumab was higher in patients with PD-L1 CPS ≥ 1 (ORR, 17.3%) than CPS < 1 (ORR, 4.0%); PD-L1 expression did not affect response in patients receiving standard of care (ORR, 9.9% v 11.1%). Similar trends were observed for OS, with greater benefit observed for pembrolizumab in patients with PD-L1 CPS ≥ 1 than CPS < 1 and in patients with PD-L1 TPS ≥ 50% than TPS < 50%.
The phase III KEYNOTE-048 study investigated pembrolizumab monotherapy or pembrolizumab plus chemotherapy compared with cetuximab plus chemotherapy in previously untreated R/M HNSCC.11 Efficacy was assessed in PD-L1 CPS ≥ 20, CPS ≥ 1, and total populations. Pembrolizumab monotherapy significantly improved OS in the PD-L1 CPS ≥ 20 and CPS ≥ 1 populations and led to noninferior OS in the total population, with favorable safety v cetuximab-chemotherapy. Pembrolizumab-chemotherapy significantly improved OS in the PD-L1 CPS ≥ 20, CPS ≥ 1, and total populations compared with cetuximab-chemotherapy and demonstrated comparable safety. This trial established first-line pembrolizumab monotherapy or pembrolizumab-chemotherapy as the standard of care for most patients with R/M HNSCC. To further characterize the effect of PD-L1 expression, we present post hoc analysis of efficacy for participants in KEYNOTE-048 with PD-L1 CPS < 1 and CPS 1-19. These and previously presented data for the PD-L1 CPS ≥ 20 population11 provide insight into the efficacy of pembrolizumab monotherapy and pembrolizumab-chemotherapy over the complete spectrum of PD-L1 CPS from < 1 to ≥ 20.
METHODS
Study Design and Participants
The design of the randomized, open-label, phase III KEYNOTE-048 study (ClinicalTrials.gov identifier: NCT02358031) has been reported.11 Eligible participants had recurrent and/or metastatic squamous cell carcinoma of the oropharynx, oral cavity, hypopharynx, or larynx that was not curable by local therapy. Participants were randomly assigned 1:1:1 to pembrolizumab, pembrolizumab plus platinum and fluorouracil (pembrolizumab-chemotherapy), or EXTREME (cetuximab plus platinum plus fluorouracil; cetuximab-chemotherapy; Data Supplement, online only). PD-L1 expression was assessed at a central laboratory using the PD-L1 IHC 22C3 pharmDx (Agilent, Santa Clara, CA) and characterized by CPS.12
The study Protocol (online only) and amendments were approved by ethics committees at each center. The study was conducted in accordance with the Protocol and Good Clinical Practice. All participants provided written informed consent.
The sponsor collaborated with senior authors on study design, gathering, analyzing, and interpreting results. The authors had access to all study data, reviewed and edited the manuscript, and had final responsibility for the decision to submit. The sponsor funded medical writing and editorial assistance.
Outcomes
Primary end points of OS and progression-free survival (PFS) and secondary end points of ORR and safety for the primary analysis populations of KEYNOTE-048—the total, PD-L1 CPS ≥ 1, and CPS ≥ 20 populations—have been reported.11 Efficacy outcomes for the PD-L1 CPS < 1 and CPS 1-19 subgroups (OS, PFS, and ORR) were not prespecified. PFS and ORR were assessed by RECIST v1.1 per blinded independent central review. Time from random assignment to data cutoff (study follow-up) was assessed for each treatment group in the three PD-L1 CPS subgroups.
Statistical Analysis
This post hoc exploratory analysis included all participants with PD-L1 CPS < 1 or CPS 1-19 tumors. OS and PFS were estimated by the Kaplan-Meier method. Hazard ratios (HRs) and 95% CIs were based on a Cox regression model with the Efron method of tie handling with treatment as a covariate. Nominal one-sided P values were calculated using the log-rank test for the PD-L1 CPS < 1 and CPS 1-19 subgroups. P values are reported as a measure of the strength of association between end points (OS or PFS) and the treatment effect; no formal hypothesis testing was conducted. No adjustment for multiple analyses was performed. No interaction term was used because of limited patient numbers and the exploratory nature of the analysis. Analysis for the PD-L1 CPS ≥ 20 population was stratified by random assignment stratification factors. Analyses for the PD-L1 CPS < 1 and CPS 1-19 subgroups were unstratified. ORR and 95% CIs were calculated using the Clopper-Pearson exact binomial method, and point estimates of ORR were summarized by treatment. The data cutoff was February 25, 2019 (final analysis).
RESULTS
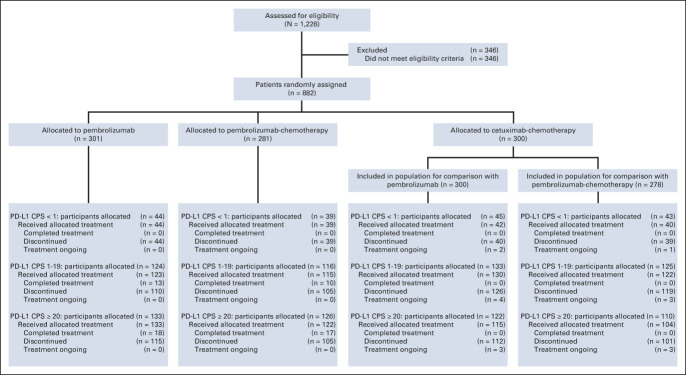
Of 882 participants enrolled, 301 were randomly assigned to pembrolizumab, 281 to pembrolizumab-chemotherapy, and 300 to cetuximab-chemotherapy.11 In total, 128 (14.5%) participants had PD-L1 CPS < 1, 373 (42.3%) had CPS 1-19, and 381 (43.2%) had CPS ≥ 20. Of 128 participants with PD-L1 CPS < 1 tumors, 44 were randomly assigned to pembrolizumab, 39 to pembrolizumab-chemotherapy, and 45 to cetuximab-chemotherapy (Fig 1 and the Data Supplement). Of 373 participants with PD-L1 CPS 1-19 tumors, 124 were randomly assigned to pembrolizumab, 116 to pembrolizumab-chemotherapy, and 133 to cetuximab-chemotherapy. Of 381 participants with PD-L1 CPS ≥ 20 tumors, 133 were randomly assigned to pembrolizumab, 126 to pembrolizumab-chemotherapy, and 122 to cetuximab-chemotherapy.11 Because random assignment of participants to pembrolizumab-chemotherapy was halted temporarily,11 the cetuximab-chemotherapy population for the pembrolizumab-chemotherapy versus cetuximab-chemotherapy comparison included only those randomly assigned to cetuximab-chemotherapy while pembrolizumab-chemotherapy enrollment was ongoing.11
FIG 1.
Trial profile. Analysis in participants with PD-L1 CPS ≥ 20 was prespecified, and the trial profile for these participants has been published previously.11 Reasons that patients discontinued treatment in each subgroup are presented in the Data Supplement. CPS, combined positive score; PD-L1, programmed death ligand-1.
The median study follow-up in the PD-L1 CPS < 1 subgroup was 33.8 months for pembrolizumab versus cetuximab-chemotherapy and 33.1 months for pembrolizumab-chemotherapy versus cetuximab-chemotherapy (Data Supplement) and was 33.2 and 32.4 months in the CPS 1-19 subgroup, respectively. The median follow-up for the PD-L1 CPS ≥ 20 population was 33.0 months for both pembrolizumab versus cetuximab-chemotherapy and pembrolizumab-chemotherapy versus cetuximab-chemotherapy.
Baseline characteristics for the PD-L1 CPS < 1 and CPS 1-19 subgroups and CPS ≥ 20 population were generally comparable between pembrolizumab monotherapy and cetuximab-chemotherapy. Exceptions were a lower proportion of participants with locoregionally recurrent-only disease in the pembrolizumab arms of the CPS < 1 and CPS 1-19 subgroups (n = 16 [36.4%] v n = 23 [51.1%]; n = 57 [46.0%] v n = 77 [57.9%]) and a higher proportion of participants with a primary tumor location of the larynx in the pembrolizumab arms of the CPS < 1 subgroup (n = 17 [38.6%] v n = 8 [17.8%]; Data Supplement). Characteristics were also comparable between the pembrolizumab-chemotherapy and cetuximab-chemotherapy arms, except for a higher proportion of participants with a primary tumor location of the hypopharynx (n = 11 [28.2%] v n = 6 [14.0%]) and a lower proportion with a primary tumor location of the oral cavity (n = 5 [12.8%] v n = 11 [25.6%]) in the pembrolizumab-chemotherapy arm of the PD-L1 CPS < 1 subgroup (Data Supplement). Baseline characteristics of participants in the PD-L1 CPS < 1 and CPS 1-19 subgroups and the CPS ≥ 20 population were generally comparable with those of the total population, except for a higher proportion of current or former smokers in the pembrolizumab and cetuximab-chemotherapy arms of the PD-L1 CPS < 1 subgroups.11
The use of subsequent therapy in the PD-L1 CPS < 1, CPS 1-19, and CPS ≥ 20 subgroups was generally comparable with the total population.11 The most common in all groups was chemotherapy, followed by epidermal growth factor receptor inhibitors in the pembrolizumab and pembrolizumab-chemotherapy arms and immune checkpoint inhibitors (ICIs) in the cetuximab-chemotherapy arm. In the cetuximab-chemotherapy arm, fewer participants with PD-L1 CPS < 1 received subsequent ICIs (n = 7 [15.6%]) compared with CPS 1-19 (n = 36 [27.1%]) or CPS ≥ 20 (n = 32 [26.2%]).
Efficacy
Pembrolizumab versus cetuximab-chemotherapy.
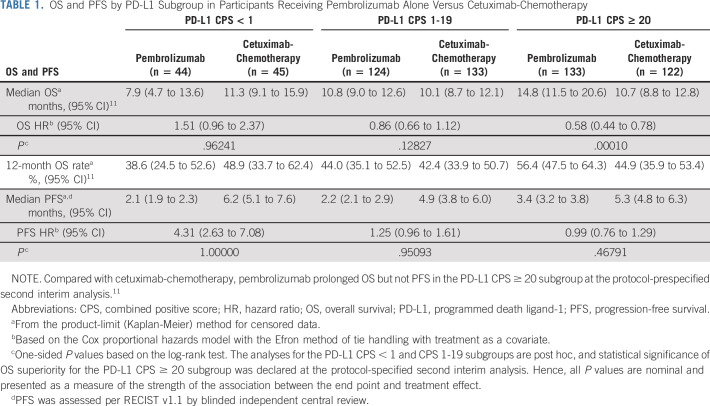
In the PD-L1 CPS < 1 subgroup, 40 participants (90.9%) in the pembrolizumab arm and 35 (77.8%) in the cetuximab-chemotherapy arm had died; the median OS was 7.9 versus 11.3 months (HR, 1.51; 95% CI, 0.96 to 2.37; P = .96241; Fig 2A, Table 1, and the Data Supplement). The 12-month OS rate was 39% with pembrolizumab and 49% with cetuximab-chemotherapy. Forty-four participants (100%) in the pembrolizumab arm and 40 (88.9%) in cetuximab-chemotherapy experienced disease progression or died; the median PFS was 2.1 versus 6.2 months (HR, 4.31; 95% CI, 2.63 to 7.08; P = 1.00000; Fig 3A and Table 1).
FIG 2.
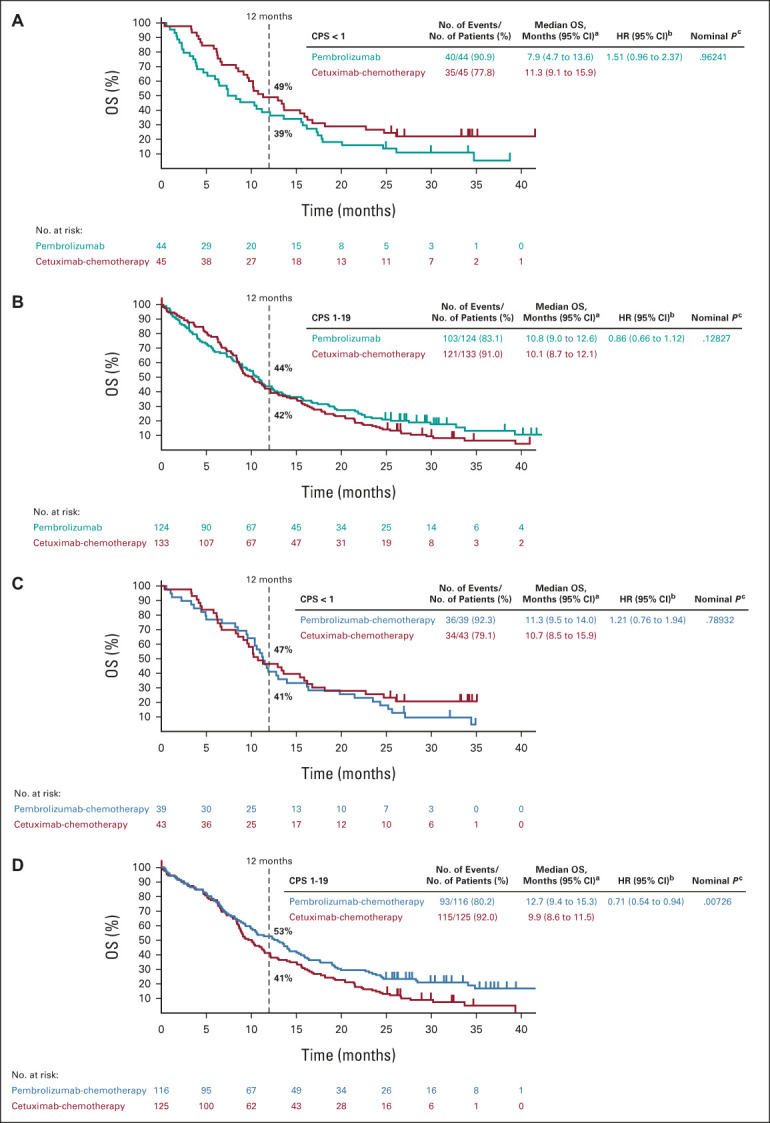
Kaplan-Meier estimates of OS. Tick marks show censoring of the data at the last time the participant was known to be alive. Pembrolizumab alone versus cetuximab-chemotherapy in the (A) PD-L1 CPS < 1 subgroup and (B) PD-L1 CPS 1-19 subgroup. Pembrolizumab-chemotherapy versus cetuximab-chemotherapy in the (C) PD-L1 CPS < 1 subgroup and (D) PD-L1 CPS 1-19 subgroup. Kaplan-Meier estimates of OS in the PD-L1 CPS ≥ 20 subgroup at final analysis have been published previously.11 aFrom the product-limit (Kaplan-Meier) method for censored data. bBased on a Cox proportional hazards model with the Efron method of tie handling with treatment as a covariate. cOne-sided P values based on the log-rank test. All P values for the PD-L1 CPS < 1 and CPS 1-19 subgroups are nominal and are presented as a measure of the strength of the association between the end point (OS) and the treatment effect. CPS, combined positive score; HR, hazard ratio; OS, overall survival; PD-L1, programmed death ligand-1.
TABLE 1.
OS and PFS by PD-L1 Subgroup in Participants Receiving Pembrolizumab Alone Versus Cetuximab-Chemotherapy
FIG 3.
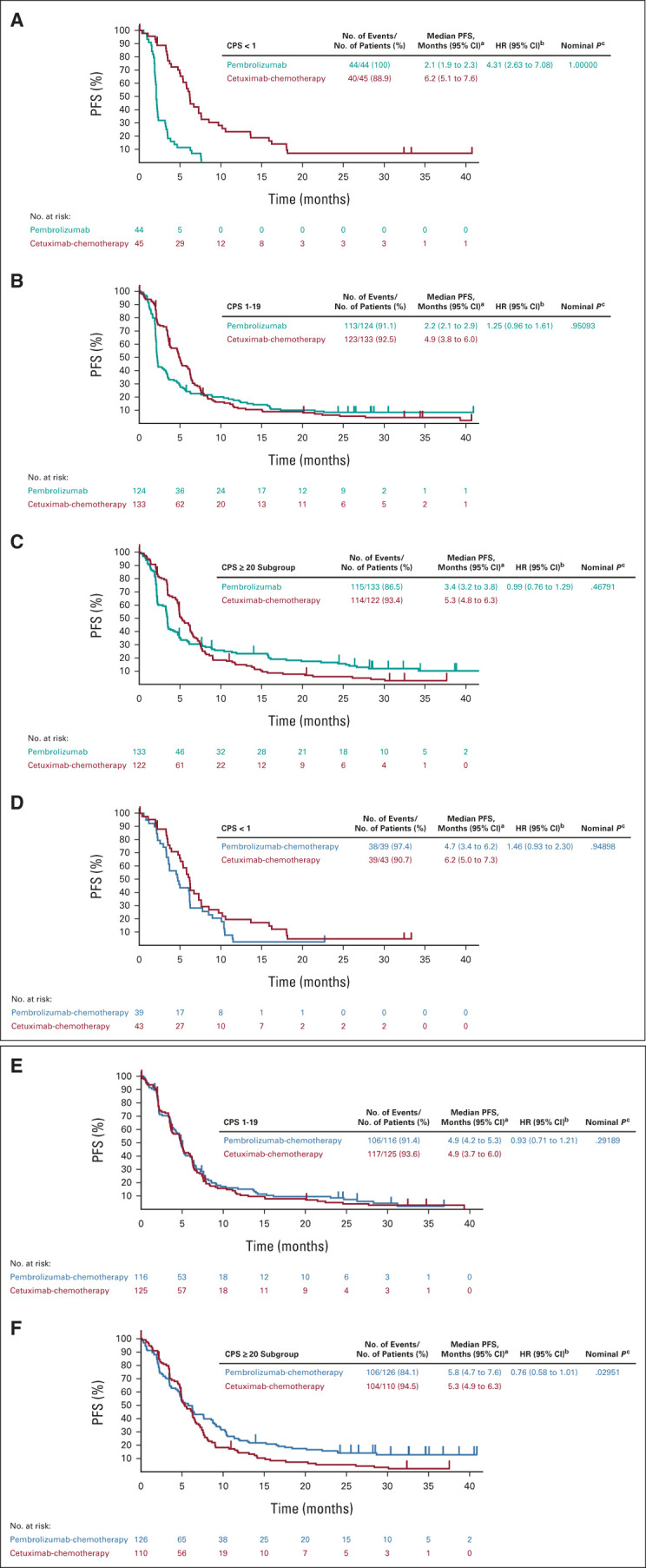
Kaplan-Meier estimates of PFS assessed per RECIST v1.1 by blinded independent central review. Tick marks show censoring of the data at the time of the last imaging assessment. Pembrolizumab alone versus cetuximab-chemotherapy in the (A) PD-L1 CPS < 1 subgroup, (B) PD-L1 CPS 1-19 subgroup, and (C) PD-L1 CPS ≥ 20 subgroup. Pembrolizumab-chemotherapy versus cetuximab-chemotherapy in the (D) PD-L1 CPS < 1 subgroup, (E) PD-L1 CPS 1-19 subgroup, and (F) PD-L1 CPS ≥ 20 subgroup. aFrom the product-limit (Kaplan-Meier) method for censored data. bBased on a Cox proportional hazards model with the Efron method of tie handling with treatment as a covariate. cOne-sided P values based on the log-rank test. All P values for the PD-L1 CPS < 1 and CPS 1-19 subgroups are nominal and are presented as a measure of the strength of the association between the end point (PFS) and the treatment effect. Definitive results in the PD-L1 CPS ≥ 20 population have been published previously.11 CPS, combined positive score; HR, hazard ratio; PD-L1, programmed death ligand-1; PFS, progression-free survival.
In the PD-L1 CPS 1-19 subgroup, 103 participants (83.1%) in the pembrolizumab arm and 121 (91.0%) in cetuximab-chemotherapy arm had died; the median OS was 10.8 versus 10.1 months (HR, 0.86; 95% CI, 0.66 to 1.12; P = .12827; Fig 2B, Table 1, and the Data Supplement). The 12-month OS rate was 44% with pembrolizumab and 42% with cetuximab-chemotherapy. In total, 113 participants (91.1%) in the pembrolizumab arm and 123 (92.5%) in the cetuximab-chemotherapy arm experienced disease progression or died; the median PFS was 2.2 versus 4.9 months (HR, 1.25; 95% CI, 0.96 to 1.61; P = .95093; Fig 3B and Table 1).
OS but not PFS results for the PD-L1 CPS ≥ 20 subgroup at final analysis have been published.11 In the PD-L1 CPS ≥ 20 subgroup, 115 participants (86.5%) in the pembrolizumab arm and 114 (93.4%) in the cetuximab-chemotherapy arm experienced disease progression or died; the median PFS was 3.4 versus 5.3 months (HR, 0.99; 95% CI, 0.76 to 1.29; P = .46791; Fig 3C and Table 1).
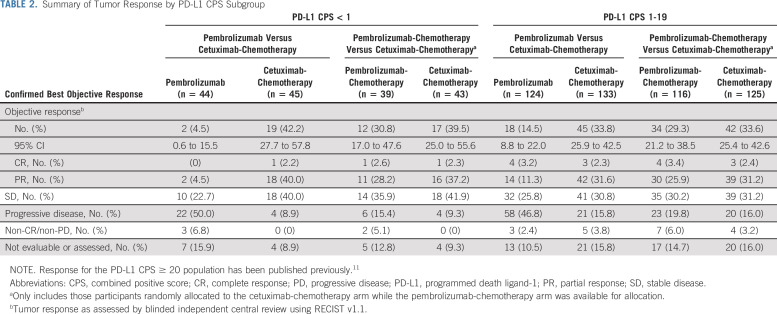
In the PD-L1 CPS < 1 subgroup, the ORR was 4.5% (n = 2; 95% CI, 0.6 to 15.5) for pembrolizumab versus 42.2% (n = 19; 95% CI, 27.7 to 57.8) for cetuximab-chemotherapy (Table 2). No participants in the pembrolizumab arm had complete response (CR), 2 (4.5%) had partial response (PR), and 10 (22.7%) had stable disease (SD); 1 (2.2%) participant in the cetuximab-chemotherapy arm had CR, 18 (40.0%) had PR, and 18 (40.0%) had SD. The median time to response (TTR) was 1.9 (range, 1.7-2.1) months for pembrolizumab versus 2.1 (range, 1.9-4.9) months for cetuximab-chemotherapy.
TABLE 2.
Summary of Tumor Response by PD-L1 CPS Subgroup
In the PD-L1 CPS 1-19 subgroup, the ORR was 14.5% (n = 18; 95% CI, 8.8 to 22.0) for pembrolizumab versus 33.8% (n = 45; 95% CI, 25.9 to 42.5) for cetuximab-chemotherapy (Table 2). Four (3.2%) participants in the pembrolizumab arm had CR, 14 (11.3%) had PR, and 32 (25.8%) had SD; 3 (2.3%) in the cetuximab-chemotherapy arm had CR, 42 (31.6%) had PR, and 41 (30.8%) had SD. The median TTR was 2.2 (range, 2.0-7.6) months for pembrolizumab and 2.1 (range, 1.3-10.4) months for cetuximab-chemotherapy.
Pembrolizumab-chemotherapy versus cetuximab-chemotherapy.
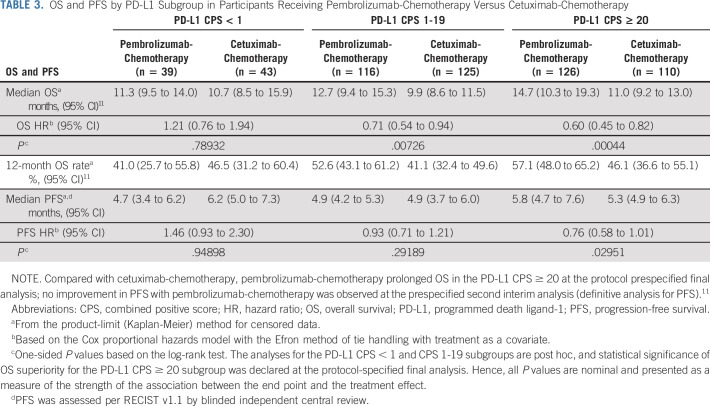
Among the PD-L1 CPS < 1 subgroup, 36 participants (92.3%) in the pembrolizumab-chemotherapy arm and 34 (79.1%) in the cetuximab-chemotherapy arm had died; the median OS was 11.3 versus 10.7 months (HR, 1.21; 95% CI, 0.76 to 1.94; P = .78932; Fig 2C, Table 3, and the Data Supplement). The 12-month OS rate was 41% with pembrolizumab-chemotherapy and 47% with cetuximab-chemotherapy. Thirty-eight participants (97.4%) in the pembrolizumab-chemotherapy arm and 39 (90.7%) in the cetuximab-chemotherapy arm experienced disease progression or died; the median PFS was 4.7 versus 6.2 months (HR, 1.46; 95% CI, 0.93 to 2.30; P = .94898; Fig 3D and Table 3).
TABLE 3.
OS and PFS by PD-L1 Subgroup in Participants Receiving Pembrolizumab-Chemotherapy Versus Cetuximab-Chemotherapy
In the PD-L1 CPS 1-19 subgroup, 93 participants (80.2%) in the pembrolizumab-chemotherapy arm and 115 (92.0%) in the cetuximab-chemotherapy arm had died; the median OS was 12.7 and 9.9 months (HR, 0.71; 95% CI, 0.54 to 0.94; P = .00726; Fig 2D, Table 3, Data Supplement). The 12-month OS rate was 53% with pembrolizumab-chemotherapy and 41% with cetuximab-chemotherapy. There were 106 participants (91.4%) in the pembrolizumab-chemotherapy arm and 117 (93.6%) in the cetuximab-chemotherapy arm who experienced disease progression or died; the median PFS was 4.9 months for both (HR, 0.93; 95% CI, 0.71 to 1.21; P = .29189; Fig 3E and Table 3).
Among the PD-L1 CPS ≥ 20 subgroup, 106 participants (84.1%) in the pembrolizumab-chemotherapy arm and 104 (94.5%) in the cetuximab-chemotherapy arm experienced disease progression or died; the median PFS was 5.8 versus 5.3 months (HR, 0.76; 95% CI, 0.58 to 1.01; Fig 3F and Table 3).
In the PD-L1 CPS < 1 subgroup, the ORR was 30.8% (n = 12; 95% CI, 17.0 to 47.6) for pembrolizumab-chemotherapy versus 39.5% (n = 17; 95% CI, 25.0 to 55.6) for cetuximab-chemotherapy (Table 2). One (2.6%) participant in the pembrolizumab-chemotherapy arm had CR, 11 (28.2%) had PR, and 14 (35.9%) had SD; 1 (2.3%) participant in the cetuximab-chemotherapy arm had CR, 16 (37.2%) had PR, and 18 (41.9%) had SD. The median TTR was 2.2 (range, 2.1-3.4) months for pembrolizumab-chemotherapy versus 2.1 (range, 1.9-4.9) months for cetuximab-chemotherapy.
In the PD-L1 CPS 1-19 subgroup, the ORR was 29.3% (n = 34; 95% CI, 21.2 to 38.5) for pembrolizumab-chemotherapy versus 33.6% (n = 42; 95% CI, 25.4 to 42.6) for cetuximab-chemotherapy (Table 2). Four (3.4%) participants in the pembrolizumab-chemotherapy arm had CR, 30 (25.9%) had PR, and 35 (30.2%) had SD; 3 (2.4%) in the cetuximab-chemotherapy arm had CR, 39 (31.2%) had PR, and 39 (31.2%) had SD. The median TTR was 2.1 (range, 1.9-6.1) months for pembrolizumab-chemotherapy and 2.1 (range, 1.3-10.4) months for cetuximab-chemotherapy.
DISCUSSION
First-line pembrolizumab monotherapy resulted in a statistically significant and clinically meaningful improvement in OS over cetuximab-chemotherapy in the PD-L1 CPS ≥ 20 and CPS ≥ 1 populations in the primary analysis of KEYNOTE-048, as did pembrolizumab-chemotherapy in the overall, CPS ≥ 20, and CPS ≥ 1 populations.11 Results from the PD-L1 CPS 1-19 subgroup (n = 373) in the current analysis were generally consistent with the previously reported results of KEYNOTE-048,11 with pembrolizumab monotherapy compared with cetuximab-chemotherapy associated with a HR for death of 0.86 (95% CI, 0.66 to 1.12; P = .12827). The 12-month OS rates in the PD-L1 CPS 1-19 subgroup were similar between arms (44% v 42%). Pembrolizumab-chemotherapy showed an OS benefit compared with cetuximab-chemotherapy in the PD-L1 CPS 1-19 subgroup (HR, 0.71; 95% CI, 0.54 to 0.94; P = .00726), which was also reflected in the 12-month OS rate (53% v 41%). In the PD-L1 CPS < 1 subgroup (n = 128), neither pembrolizumab monotherapy (HR, 1.51; 95% CI, 0.96 to 2.37; P = .96241) nor pembrolizumab-chemotherapy (HR, 1.21; 95% CI, 0.76 to 1.94; P = .78932) demonstrated OS benefit over cetuximab-chemotherapy.
Although definitive results for prespecified analysis of OS and PFS in the PD-L1 CPS ≥ 20, CPS ≥ 1, and total populations have been published,11 the current analysis of the CPS < 1 and CPS 1-19 subgroups was not prespecified and no hypothesis testing was conducted. The P values reported here are therefore indicative of the strength of association between end points (OS or PFS) and the treatment effect, but no definitive conclusions can be drawn.
No improvement in PFS was observed with pembrolizumab monotherapy in the PD-L1 CPS ≥ 20 population or with pembrolizumab-chemotherapy in the CPS ≥ 20 or total populations at second interim analysis, which was definitive for PFS in KEYNOTE-048.11 Similarly, no improvement in PFS was observed with pembrolizumab monotherapy or pembrolizumab-chemotherapy in the PD-L1 CPS 1-19 or CPS < 1 subgroups at final analysis. Although the results of the current analysis shed light on the efficacy of pembrolizumab-based therapy, clinicians will need to personalize treatment to best fit the characteristics and needs of their individual patients.
Overall, this analysis showed a trend toward increasing pembrolizumab efficacy with increasing PD-L1 expression. However, these were unplanned subgroup analyses. Treatment comparisons in the PD-L1 CPS < 1 subgroup should be interpreted cautiously given the small sample size (n = 89, pembrolizumab monotherapy v cetuximab-chemotherapy; n = 82, pembrolizumab-chemotherapy v cetuximab-chemotherapy), whereas the PD-L1 CPS 1-19 subgroup was similar in size to the CPS ≥ 20 subgroup (n ≈ 250 per treatment comparison). In addition, baseline characteristics between arms in the PD-L1 CPS < 1 subgroup had imbalances, likely because of random variation in small subgroups, resulting in imbalances in PD-L1–associated characteristics, such as subsite, disease stage, and smoking status. Even noting these limitations, the results of the current analysis are consistent with those of the primary analysis, demonstrating the treatment effect to be most pronounced when PD-L1 expression is highest.
Although these findings support the utility of PD-L1 expression as a predictive biomarker for ICIs in HNSCC, there may be patients with low or no PD-L1 expression who derive benefits from ICIs. In prior trials of pembrolizumab, ORR for patients with PD-L1 CPS < 1 of 4% and 6% and median OS of 5 and 6.3 months were observed.6,10 Further work is required to explore other biomarkers to identify responders within the CPS < 1 population. Candidates under investigation include tumor mutational burden and the T-cell–inflamed gene expression profile.13,14 Biomarker analysis in KEYNOTE-012 indicated that inflammatory markers (gene expression profile and PD-L1) and tumor mutational burden may serve as distinct and complementary biomarkers predictive of response to pembrolizumab.15 However, validation in larger studies is required. Future trials in HNSCC should examine novel biomarkers to identify patients likely to benefit from PD-1 axis inhibitors and to select patients for anti–PD-1 monotherapy. Another potential explanation for lack of correlation of PD-L1 staining with treatment benefit is intratumoral heterogeneity in PD-L1 expression.
Among participants with PD-L1 CPS 1-19 tumors, both pembrolizumab monotherapy and pembrolizumab-chemotherapy demonstrated activity. Pembrolizumab-chemotherapy led to numerically superior OS compared with cetuximab-chemotherapy in the PD-L1 CPS 1-19 subgroup, with CI for the HR excluding 1. Among participants with PD-L1 CPS < 1, neither pembrolizumab monotherapy nor pembrolizumab-chemotherapy demonstrated benefit over cetuximab-chemotherapy. Although these results should be interpreted cautiously given the post hoc nature of the analysis and the small PD-L1 CPS < 1 subgroup, they remain consistent with the US Food and Drug Administration approval of pembrolizumab as monotherapy for first-line treatment of patients with R/M HNSCC with PD-L1 CPS ≥ 1 and of pembrolizumab plus chemotherapy for first-line treatment irrespective of PD-L1 status.16 These results suggest that PD-L1 expression may be useful in informing treatment decisions for some subgroups; however, additional biomarkers are needed to further select patients who will benefit from PD-1 inhibition.
ACKNOWLEDGMENT
The authors thank the participants and their families and all investigators and site personnel. Medical writing and editorial assistance were provided by Holly C. Cappelli, PhD, CMPP; Jemimah Walker, PhD; and Doyel Mitra, PhD, CMPP, of ApotheCom (Yardley, PA).
Barbara Burtness
Consulting or Advisory Role: Merck, Debiopharm Group, CUE Biopharma, Maverick Therapeutics, Rakuten Medical, Nanobiotix, MacroGenics, ALX Oncology, IO Biotech, Ipsen, Genentech/Roche, Kura Oncology, Exelixis, Merck KGaA, PPD Global
Speakers' Bureau: Cancer Education Alliance, Oncology Education
Research Funding: Merck (Inst), Exelixis (Inst), CUE Biopharma (Inst), Kura Oncology, Nektar, Eisai, Genentech, AstraZeneca, Merck, Rakuten Medical
Travel, Accommodations, Expenses: Merck, Debiopharm Group
Danny Rischin
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Research Funding: Genentech/Roche (Inst), Merck (Inst), Regeneron (Inst), Bristol Myers Squibb (Inst), GlaxoSmithKline (Inst), Sanofi (Inst), Kura Oncology (Inst), Merck KGaA (Inst)
Uncompensated Relationships: Regeneron, Merck, GlaxoSmithKline, Sanofi
Richard Greil
Honoraria: Celgene, Roche, Merck, Takeda, AstraZeneca, Novartis, Amgen, Bristol Myers Squibb, MSD, Sandoz, AbbVie, Gilead Sciences, Daiichi Sankyo
Consulting or Advisory Role: Celgene, Novartis, Roche, Bristol Myers Squibb, Takeda, AbbVie, AstraZeneca, Janssen, MSD, Merck, Gilead Sciences, Daiichi Sankyo
Research Funding: Celgene (Inst), Merck (Inst), Takeda (Inst), AstraZeneca (Inst), Novartis (Inst), Amgen (Inst), Bristol Myers Squibb (Inst), MSD (Inst), Sandoz (Inst), Gilead Sciences (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Roche, Amgen, Janssen-Cilag, AstraZeneca, Novartis, MSD, Celgene, Gilead Sciences, Bristol Myers Squibb, AbbVie, Daiichi Sankyo
Denis Soulières
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Merck, Novartis, Pfizer, AstraZeneca, Ipsen, Bristol Myers Squibb, Eisai, Adlai Nortye
Consulting or Advisory Role: Merck, Pfizer, Ipsen, Adlai Nortye, Eisai
Research Funding: Novartis (Inst), Pfizer (Inst), Merck (Inst), Roche/Genentech (Inst), Bristol Myers Squibb (Inst), Lilly (Inst), Adlai Nortye (Inst)
Makoto Tahara
Honoraria: Merck Serono, Bristol Myers Squibb, Eisai, Ono Pharmaceutical, MSD
Consulting or Advisory Role: Ono Pharmaceutical, MSD, Pfizer, Bristol Myers Squibb, Rakuten Medical, Bayer, Lilly
Research Funding: Merck Sharp & Dohme (Inst), AstraZeneca (Inst), Ono Pharmaceutical (Inst), Novartis (Inst), Pfizer (Inst), Bristol Myers Squibb (Inst), Rakuten Medical (Inst), Bayer (Inst), GlaxoSmithKline (Inst), Lilly (Inst)
Gilberto de Castro Junior
Honoraria: AstraZeneca, Pfizer, Merck Sharp & Dohme, Bristol Myers Squibb, Novartis, Roche, Amgen, Janssen
Consulting or Advisory Role: Boehringer Ingelheim, Pfizer, Bayer, Roche, Merck Sharp & Dohme, Bristol Myers Squibb, AstraZeneca, Yuhan, Merck Serono, Janssen, Libbs, Sanofi, Novartis
Speakers' Bureau: AstraZeneca, Bayer, Novartis, Roche, Merck Serono, Bristol Myers Squibb, Merck Sharp & Dohme, Boehringer Ingelheim, Pfizer, Janssen, Amgen
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Novartis, Pfizer, Roche, AstraZeneca, Boehringer Ingelheim, Bayer, Bristol Myers Squibb, Merck Serono
Amanda Psyrri
Honoraria: Merck Serono, Roche, BMS, MSD Oncology, Genesis Pharmaceuticals, Bayer, Rakuten Medical, AstraZeneca, Pfizer
Consulting or Advisory Role: AstraZeneca, MSD Oncology, Pfizer, Bristol Myers Squibb, Amgen, Rakuten Medical, eTheRNA Immunotherapies
Research Funding: Kura Oncology, Bristol Myers Squibb, Roche, Amgen, Boehringer Ingelheim, Pfizer, Demo Pharmaceutical, Pharmathen
Travel, Accommodations, Expenses: Roche, MSD Oncology, Ipsen, Bristol Myers Squibb
Uncompensated Relationships: AstraZeneca
Irene Brana
Consulting or Advisory Role: MSD, Sanofi, Achilles Therapeutics, eTheRNA Immunotherapies, Cancer Expert Now
Speakers' Bureau: Bristol Myers Squibb, Merck Serono, Roche, MSD
Research Funding: AstraZeneca (Inst), Bristol Myers Squibb (Inst), Celgene (Inst), Gliknik (Inst), GlaxoSmithKline (Inst), Janssen Oncology (Inst), Kura Oncology (Inst), Merck Sharp & Dohme (Inst), Novartis (Inst), Pfizer (Inst), Roche (Inst), Shattuck Labs (Inst), Nanobiotix (Inst), Seattle Genetics (Inst), Immutep (Inst), Debiopharm Group (Inst), Regeneron (Inst), Boehringer Ingelheim (Inst), ISA Pharmaceuticals (Inst), Merck Serono (Inst), Seattle Genetics (Inst), Northern Biologics (Inst), VCN Biosciences (Inst), Instituto Salud Carlos III Research funding: personal grant - Rio Hortega Contract - CM15/00255
Travel, Accommodations, Expenses: MSD Oncology
Neus Basté
Consulting or Advisory Role: Roche, Merck Serono, Bristol Myers Squibb, Eisai, Bayer, MSD Oncology, BioNTech, ISA Pharmaceuticals, Novartis, Amgen
Research Funding: Merck Serono
Prakash Neupane
Research Funding: Merck Sharp & Dohme
Åse Bratland
Honoraria: MSD Oncology, Bristol Myers Squibb, Sanofi, Merck Serono
Research Funding: Bristol Myers Squibb (Inst), MSD Oncology (Inst)
Travel, Accommodations, Expenses: MSD Oncology
Thorsten Fuereder
Honoraria: MSD, Bristol Myers Squibb/Celgene, Roche, Merck KGaA, Pfizer, Boehringer Ingelheim, Amgen
Consulting or Advisory Role: Merck KGaA, MSD, Amgen, Boehringer Ingelheim, Janssen
Research Funding (Inst): MSD, Merck KGaA, Bristol-Myers Squibb/Celgene, Kura Oncology
Travel, Accommodations, Expenses: MSD, Bristol Myers Squibb/Celgene, Merck KGaA
Brett G.M. Hughes
Consulting or Advisory Role: MSD Oncology, Bristol Myers Squibb, Roche, Pfizer, Boehringer Ingelheim, AstraZeneca, Eisai, Sanofi/Regeneron
Research Funding: Amgen (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Bristol Myers Squibb
Ricard Mesia
Consulting or Advisory Role: Bristol Myers Squibb, MSD, Merck KGaA, Roche, AstraZeneca, Amgen, Bayer, Nanobiotix, Segean
Speakers' Bureau: Bristol Myers Squibb, Merck KGaA, MSD
Travel, Accommodations, Expenses: Bristol Myers Squibb
Nuttapong Ngamphaiboon
Consulting or Advisory Role: MSD, Novartis, Amgen, Eisai, Merck
Speakers' Bureau: Roche, Eisai, MSD, Novartis
Research Funding: MSD (Inst), Pfizer (Inst), Roche (Inst), Exelixis (Inst), RAPT Therapeutics (Inst), BeiGene (Inst)
Travel, Accommodations, Expenses: Roche
Wan Zamaniah Wan Ishak
Honoraria: Roche/Genentech, Amgen, Eisai, Merck Sharp & Dohme, Pfizer, Merck Serono, Novartis
Consulting or Advisory Role: Eisai, Merck Sharp & Dohme, Novartis
Speakers' Bureau: Merck Sharp & Dohme, Amgen, Eisai
Research Funding: Merck Sharp & Dohme, Roche/Genentech, Novartis
Joy Ge
Employment: Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ
Stock and Other Ownership Interests: Merck & Co, Inc, Kenilworth, NJ
Ramona F. Swaby
Employment: Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ (at the time of the conduct of the study)
Burak Gumuscu
Employment: Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ
Stock and Other Ownership Interests: Merck & Co, Inc, Kenilworth, NJ
Travel, Accommodations, Expenses: MSD
Kevin Harrington
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Arch Oncology (Inst), AstraZeneca (Inst), BMS (Inst), Boehringer Ingelheim (Inst), Merck Serono (Inst), MSD (Inst), Oncolys BioPharma (Inst), Pfizer (Inst), Replimune (Inst), Inzen Therapeutics (Inst), Codiak Biosciences (Inst)
Consulting or Advisory Role: Arch Oncology (Inst), AstraZeneca (Inst), BMS (Inst), Boehringer Ingelheim (Inst), Merck Serono (Inst), MSD (Inst), Oncolys BioPharma (Inst), Replimune (Inst), Inzen Therapeutics (Inst)
Speakers' Bureau: BMS (Inst), Merck Serono (Inst), MSD (Inst)
Research Funding: AstraZeneca (Inst), Merck Sharp & Dohme (Inst), Replimune (Inst), Boehringer Ingelheim (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the American Association for Cancer Research Virtual Meeting, April 27-28, 2020.
SUPPORT
Funding for this research was provided by Merck Sharp & Dohme Corp, a subsidiary of Merck & Co Inc, Kenilworth, NJ.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company's clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the United States and European Union or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country-specific or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
AUTHOR CONTRIBUTIONS
Conception and design: Barbara Burtness, Denis Soulières, Amanda Psyrri, Joy Ge, Kevin Harrington
Financial support: Denis Soulières
Administrative support: Denis Soulières
Provision of study materials or patients: Richard Greil, Makoto Tahara, Gilberto de Castro Junior, Amanda Psyrri, Irene Brana, Neus Basté, Prakash Neupane, Åse Bratland, Brett G.M. Hughes, Ricard Mesia, Kevin Harrington
Collection and assembly of data: Barbara Burtness, Danny Rischin, Richard Greil, Denis Soulières, Gilberto de Castro Junior, Irene Brana, Prakash Neupane, Åse Bratland, Thorsten Fuereder, Ricard Mesia, Nuttapong Ngamphaiboon, Tamara Rordorf, Joy Ge, Ramona F. Swaby, Burak Gumuscu, Kevin Harrington
Data analysis and interpretation: Barbara Burtness, Danny Rischin, Richard Greil, Denis Soulières, Makoto Tahara, Gilberto de Castro Junior, Amanda Psyrri, Irene Brana, Neus Basté, Prakash Neupane, Åse Bratland, Thorsten Fuereder, Brett G.M. Hughes, Ricard Mesia, Nuttapong Ngamphaiboon, Wan Zamaniah Wan Ishak, Joy Ge, Ramona F. Swaby, Burak Gumuscu, Kevin Harrington
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Pembrolizumab Alone or With Chemotherapy for Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma in KEYNOTE-048: Subgroup Analysis by Programmed Death Ligand-1 Combined Positive Score
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Barbara Burtness
Consulting or Advisory Role: Merck, Debiopharm Group, CUE Biopharma, Maverick Therapeutics, Rakuten Medical, Nanobiotix, MacroGenics, ALX Oncology, IO Biotech, Ipsen, Genentech/Roche, Kura Oncology, Exelixis, Merck KGaA, PPD Global
Speakers' Bureau: Cancer Education Alliance, Oncology Education
Research Funding: Merck (Inst), Exelixis (Inst), CUE Biopharma (Inst), Kura Oncology, Nektar, Eisai, Genentech, AstraZeneca, Merck, Rakuten Medical
Travel, Accommodations, Expenses: Merck, Debiopharm Group
Danny Rischin
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Research Funding: Genentech/Roche (Inst), Merck (Inst), Regeneron (Inst), Bristol Myers Squibb (Inst), GlaxoSmithKline (Inst), Sanofi (Inst), Kura Oncology (Inst), Merck KGaA (Inst)
Uncompensated Relationships: Regeneron, Merck, GlaxoSmithKline, Sanofi
Richard Greil
Honoraria: Celgene, Roche, Merck, Takeda, AstraZeneca, Novartis, Amgen, Bristol Myers Squibb, MSD, Sandoz, AbbVie, Gilead Sciences, Daiichi Sankyo
Consulting or Advisory Role: Celgene, Novartis, Roche, Bristol Myers Squibb, Takeda, AbbVie, AstraZeneca, Janssen, MSD, Merck, Gilead Sciences, Daiichi Sankyo
Research Funding: Celgene (Inst), Merck (Inst), Takeda (Inst), AstraZeneca (Inst), Novartis (Inst), Amgen (Inst), Bristol Myers Squibb (Inst), MSD (Inst), Sandoz (Inst), Gilead Sciences (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Roche, Amgen, Janssen-Cilag, AstraZeneca, Novartis, MSD, Celgene, Gilead Sciences, Bristol Myers Squibb, AbbVie, Daiichi Sankyo
Denis Soulières
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Merck, Novartis, Pfizer, AstraZeneca, Ipsen, Bristol Myers Squibb, Eisai, Adlai Nortye
Consulting or Advisory Role: Merck, Pfizer, Ipsen, Adlai Nortye, Eisai
Research Funding: Novartis (Inst), Pfizer (Inst), Merck (Inst), Roche/Genentech (Inst), Bristol Myers Squibb (Inst), Lilly (Inst), Adlai Nortye (Inst)
Makoto Tahara
Honoraria: Merck Serono, Bristol Myers Squibb, Eisai, Ono Pharmaceutical, MSD
Consulting or Advisory Role: Ono Pharmaceutical, MSD, Pfizer, Bristol Myers Squibb, Rakuten Medical, Bayer, Lilly
Research Funding: Merck Sharp & Dohme (Inst), AstraZeneca (Inst), Ono Pharmaceutical (Inst), Novartis (Inst), Pfizer (Inst), Bristol Myers Squibb (Inst), Rakuten Medical (Inst), Bayer (Inst), GlaxoSmithKline (Inst), Lilly (Inst)
Gilberto de Castro Junior
Honoraria: AstraZeneca, Pfizer, Merck Sharp & Dohme, Bristol Myers Squibb, Novartis, Roche, Amgen, Janssen
Consulting or Advisory Role: Boehringer Ingelheim, Pfizer, Bayer, Roche, Merck Sharp & Dohme, Bristol Myers Squibb, AstraZeneca, Yuhan, Merck Serono, Janssen, Libbs, Sanofi, Novartis
Speakers' Bureau: AstraZeneca, Bayer, Novartis, Roche, Merck Serono, Bristol Myers Squibb, Merck Sharp & Dohme, Boehringer Ingelheim, Pfizer, Janssen, Amgen
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Novartis, Pfizer, Roche, AstraZeneca, Boehringer Ingelheim, Bayer, Bristol Myers Squibb, Merck Serono
Amanda Psyrri
Honoraria: Merck Serono, Roche, BMS, MSD Oncology, Genesis Pharmaceuticals, Bayer, Rakuten Medical, AstraZeneca, Pfizer
Consulting or Advisory Role: AstraZeneca, MSD Oncology, Pfizer, Bristol Myers Squibb, Amgen, Rakuten Medical, eTheRNA Immunotherapies
Research Funding: Kura Oncology, Bristol Myers Squibb, Roche, Amgen, Boehringer Ingelheim, Pfizer, Demo Pharmaceutical, Pharmathen
Travel, Accommodations, Expenses: Roche, MSD Oncology, Ipsen, Bristol Myers Squibb
Uncompensated Relationships: AstraZeneca
Irene Brana
Consulting or Advisory Role: MSD, Sanofi, Achilles Therapeutics, eTheRNA Immunotherapies, Cancer Expert Now
Speakers' Bureau: Bristol Myers Squibb, Merck Serono, Roche, MSD
Research Funding: AstraZeneca (Inst), Bristol Myers Squibb (Inst), Celgene (Inst), Gliknik (Inst), GlaxoSmithKline (Inst), Janssen Oncology (Inst), Kura Oncology (Inst), Merck Sharp & Dohme (Inst), Novartis (Inst), Pfizer (Inst), Roche (Inst), Shattuck Labs (Inst), Nanobiotix (Inst), Seattle Genetics (Inst), Immutep (Inst), Debiopharm Group (Inst), Regeneron (Inst), Boehringer Ingelheim (Inst), ISA Pharmaceuticals (Inst), Merck Serono (Inst), Seattle Genetics (Inst), Northern Biologics (Inst), VCN Biosciences (Inst), Instituto Salud Carlos III Research funding: personal grant - Rio Hortega Contract - CM15/00255
Travel, Accommodations, Expenses: MSD Oncology
Neus Basté
Consulting or Advisory Role: Roche, Merck Serono, Bristol Myers Squibb, Eisai, Bayer, MSD Oncology, BioNTech, ISA Pharmaceuticals, Novartis, Amgen
Research Funding: Merck Serono
Prakash Neupane
Research Funding: Merck Sharp & Dohme
Åse Bratland
Honoraria: MSD Oncology, Bristol Myers Squibb, Sanofi, Merck Serono
Research Funding: Bristol Myers Squibb (Inst), MSD Oncology (Inst)
Travel, Accommodations, Expenses: MSD Oncology
Thorsten Fuereder
Honoraria: MSD, Bristol Myers Squibb/Celgene, Roche, Merck KGaA, Pfizer, Boehringer Ingelheim, Amgen
Consulting or Advisory Role: Merck KGaA, MSD, Amgen, Boehringer Ingelheim, Janssen
Research Funding (Inst): MSD, Merck KGaA, Bristol-Myers Squibb/Celgene, Kura Oncology
Travel, Accommodations, Expenses: MSD, Bristol Myers Squibb/Celgene, Merck KGaA
Brett G.M. Hughes
Consulting or Advisory Role: MSD Oncology, Bristol Myers Squibb, Roche, Pfizer, Boehringer Ingelheim, AstraZeneca, Eisai, Sanofi/Regeneron
Research Funding: Amgen (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Bristol Myers Squibb
Ricard Mesia
Consulting or Advisory Role: Bristol Myers Squibb, MSD, Merck KGaA, Roche, AstraZeneca, Amgen, Bayer, Nanobiotix, Segean
Speakers' Bureau: Bristol Myers Squibb, Merck KGaA, MSD
Travel, Accommodations, Expenses: Bristol Myers Squibb
Nuttapong Ngamphaiboon
Consulting or Advisory Role: MSD, Novartis, Amgen, Eisai, Merck
Speakers' Bureau: Roche, Eisai, MSD, Novartis
Research Funding: MSD (Inst), Pfizer (Inst), Roche (Inst), Exelixis (Inst), RAPT Therapeutics (Inst), BeiGene (Inst)
Travel, Accommodations, Expenses: Roche
Wan Zamaniah Wan Ishak
Honoraria: Roche/Genentech, Amgen, Eisai, Merck Sharp & Dohme, Pfizer, Merck Serono, Novartis
Consulting or Advisory Role: Eisai, Merck Sharp & Dohme, Novartis
Speakers' Bureau: Merck Sharp & Dohme, Amgen, Eisai
Research Funding: Merck Sharp & Dohme, Roche/Genentech, Novartis
Joy Ge
Employment: Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ
Stock and Other Ownership Interests: Merck & Co, Inc, Kenilworth, NJ
Ramona F. Swaby
Employment: Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ (at the time of the conduct of the study)
Burak Gumuscu
Employment: Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ
Stock and Other Ownership Interests: Merck & Co, Inc, Kenilworth, NJ
Travel, Accommodations, Expenses: MSD
Kevin Harrington
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Arch Oncology (Inst), AstraZeneca (Inst), BMS (Inst), Boehringer Ingelheim (Inst), Merck Serono (Inst), MSD (Inst), Oncolys BioPharma (Inst), Pfizer (Inst), Replimune (Inst), Inzen Therapeutics (Inst), Codiak Biosciences (Inst)
Consulting or Advisory Role: Arch Oncology (Inst), AstraZeneca (Inst), BMS (Inst), Boehringer Ingelheim (Inst), Merck Serono (Inst), MSD (Inst), Oncolys BioPharma (Inst), Replimune (Inst), Inzen Therapeutics (Inst)
Speakers' Bureau: BMS (Inst), Merck Serono (Inst), MSD (Inst)
Research Funding: AstraZeneca (Inst), Merck Sharp & Dohme (Inst), Replimune (Inst), Boehringer Ingelheim (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Seiwert TY, Burtness B, Mehra R, et al. : Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet Oncol 17:956-965, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Patel SP, Kurzrock R: PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther 14:847-856, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Sharpe AH, Wherry EJ, Ahmed R, et al. : The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol 8:239-245, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Alsaab HO, Sau S, Alzhrani R, et al. : PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: Mechanism, combinations, and clinical outcome. Front Pharmacol 8:561, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferris RL, Blumenschein G, Jr, Fayette J, et al. : Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 375:1856-1867, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehra R, Seiwert TY, Gupta S, et al. : Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: Pooled analyses after long-term follow-up in KEYNOTE-012. Br J Cancer 119:153-159, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grigg C, Rizvi NA: PD-L1 biomarker testing for non-small cell lung cancer: Truth or fiction? J Immunother Cancer 4:48, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang WF, Wong MCM, Thomson PJ, et al. : The prognostic role of PD-L1 expression for survival in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral Oncol 86:81-90, 2018 [DOI] [PubMed] [Google Scholar]
- 9.Schneider S, Kadletz L, Wiebringhaus R, et al. : PD-1 and PD-L1 expression in HNSCC primary cancer and related lymph node metastasis: Impact on clinical outcome. Histopathology 73:573-584, 2018 [DOI] [PubMed] [Google Scholar]
- 10.Cohen EEW, Soulieres D, Le Tourneau C, et al. : Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet 393:156-167, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Burtness B, Harrington KJ, Greil R, et al. : Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 394:1915-1928, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Emancipator K, Huang L, Aurora-Garg D, et al. : Comparing programmed death ligand 1 scores for predicting pembrolizumab efficacy in head and neck cancer. Mod Pathol 34:532-541, 2021 [DOI] [PubMed] [Google Scholar]
- 13.Ayers M, Lunceford J, Nebozhyn M, et al. : IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest 127:2930-2940, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodman AM, Kato S, Bazhenova L, et al. : Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther 16:2598-2608, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haddad RI, Seiwert TY, Man Chow LQ, et al. : Influence of tumor mutational burden, inflammatory gene expression profile, and PD-L1 expression on response to pembrolizumab in head and neck squamous cell carcinoma. J Immunother Cancer 10:e003026, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.KEYTRUDA® (pembrolizumab) injection, for intravenous use, 02/2022. Merck Sharp & Dohme Corp: Whitehouse Station, NJ, 2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company's clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the United States and European Union or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country-specific or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.