Abstract
PURPOSE
To assess the efficacy and safety of cabozantinib plus nivolumab in a phase II trial in patients with non–clear-cell renal cell carcinoma (RCC).
PATIENTS AND METHODS
Patients had advanced non–clear-cell renal carcinoma who underwent 0-1 prior systemic therapies excluding prior immune checkpoint inhibitors. Patients received cabozantinib 40 mg once daily plus nivolumab 240 mg once every 2 weeks or 480 mg once every 4 weeks. Cohort 1 enrolled patients with papillary, unclassified, or translocation-associated RCC; cohort 2 enrolled patients with chromophobe RCC. The primary end point was objective response rate (ORR) by RECIST 1.1; secondary end points included progression-free survival, overall survival, and safety. Next-generation sequencing results were correlated with response.
RESULTS
A total of 47 patients were treated with a median follow-up of 13.1 months. Objective response rate for cohort 1 (n = 40) was 47.5% (95% CI, 31.5 to 63.9), with median progression-free survival of 12.5 months (95% CI, 6.3 to 16.4) and median overall survival of 28 months (95% CI, 16.3 to not evaluable). In cohort 2 (n = 7), no responses were observed; one patient had stable disease > 1 year. Grade 3/4 treatment-related adverse events were observed in 32% treated patients. Cabozantinib and nivolumab were discontinued because of toxicity in 13% and 17% of patients, respectively. Common mutations included NF2 and FH in cohort 1 and TP53 and PTEN in cohort 2. Objective responses were seen in 10/12 patients with either NF2 or FH mutations.
CONCLUSION
Cabozantinib plus nivolumab showed promising efficacy in most non–clear-cell RCC variants tested in this trial, particularly those with prominent papillary features, whereas treatment effects were limited in chromophobe RCC. Genomic findings in non–clear-cell RCC variants warrant further study as predictors of response.
INTRODUCTION
Renal cell carcinoma (RCC) comprises 90% of kidney cancer cases with approximately 70% patients having the clear cell renal cell carcinoma (ccRCC) subtype.1 The remaining histologic variants are collectively classified as non–clear-cell renal cell carcinoma (nccRCC) and include genetically and histologically diverse tumors. Prominent examples include papillary, collecting duct, translocation-associated, chromophobe, and unclassified RCC.
CONTEXT
Key Objective
The combination of cabozantinib plus nivolumab has demonstrated improved objective response rates, progression-free survival, and overall survival in comparison to sunitinib in patients with clear-cell kidney cancer. Non–clear-cell kidney cancer represents a diverse mix of histologies and has been more resistant to treatment. This phase II study evaluates the efficacy cabozantinib plus nivolumab in these patients.
Knowledge Generated
In this phase II study, which analyzes non–clear-cell kidney cancer in two cohorts—unclassified/papillary/translocation-associated and chromophobe—the combination of cabozantinib and nivolumab demonstrated objective responses in the unclassified/papillary/translocation-associated cohort, but not in the chromophobe cohort.
Relevance
Cabozantinib plus nivolumab showed promising levels of activity in non–clear-cell histologies. Larger studies are necessary to see whether histologic subtypes or genomic factors may lead to differential responses to systemic therapy.
Although therapeutic approaches have evolved greatly over the past decade for metastatic ccRCC, treatment standards for nccRCC remain poorly defined. Clinical trials have explored vascular endothelial growth factor (VEGF)–, mammalian target of rapamycin (mTOR)–, and MET-directed therapies in patients with nccRCC histologies; however, objective response rates (ORRs) were lower than those reported for ccRCC.2-7 Cabozantinib, a small molecule receptor tyrosine kinase inhibitor that targets MET, AXL, and VEGFR2, has demonstrated superiority over sunitinib in patients with papillary RCC, which is often associated with MET activation.7
Recent studies have also shown the efficacy and safety of immune checkpoint inhibitors such as programmed death-1 (PD-1) inhibitors in advanced ccRCC and nccRCC. Monotherapy with nivolumab in advanced nccRCC has demonstrated an ORR of 14% in both HCRN GU16-260 Cohort B and CheckMate 374, and monotherapy with pembrolizumab showed an ORR of 27% in cohort B of KEYNOTE-427.8-10 In cohort 2 of CheckMate 920, the combination of ipilimumab and nivolumab demonstrated an ORR of 19.6%.11 There is also evidence that pairing anti-VEGF/VEGFR agents with immune checkpoint inhibitors improves efficacy in the treatment of advanced ccRCC. Cabozantinib and nivolumab, a human PD-1–blocking antibody, were studied in a phase III clinical trial to treat metastatic ccRCC (CheckMate 9ER).12 Cabozantinib plus nivolumab demonstrated longer progression-free survival (PFS) and higher ORR and overall survival (OS) compared with sunitinib, which led to regulatory approval of this combination for first-line treatment of metastatic ccRCC.12 The efficacy of cabozantinib plus nivolumab in nccRCC in unknown. We report the results of a single-center phase II study of cabozantinib plus nivolumab in patients with advanced or metastatic nccRCC.
PATIENTS AND METHODS
Eligibility
Patients age 18 years or older were required to have pathologic or histologically confirmed, unresectable advanced or metastatic nccRCC variants of interest as outlined below, per review by an expert GU pathologist at Memorial Sloan Kettering Cancer Center (MSK). Cohort 1 enrolled patients with papillary, unclassified, fumarate hydratase (FH)–deficient, and translocation-associated RCC, and cohort 2 enrolled patients with chromophobe RCC. To be eligible, patients were also required to have measurable disease as defined by Response Evaluation Criteria in Solid Tumors (RECIST) 1.1,13 zero or one prior systemic therapies, Karnofsky performance status ≥ 70%, and adequate organ function. Key exclusion criteria included prior therapy with an immunotherapy agent, including high-dose interleukin-2 (IL-2), anti–cytotoxic T-lymphocyte-associated protein-4 (CTLA-4), anti–PD-1, or anti–programmed death ligand-1 (PD-L1) agents; prior treatment with cabozantinib; and untreated brain metastases. The study was approved by the institutional review board at MSK; all patients provided written informed consent.
Study Design and Treatment
This is a single-institution, investigator-initiated phase II study (ClinicalTrials.gov identifier: NCT03635892). Study support and investigational agents (cabozantinib and nivolumab) were provided by Exelixis (Alameda, CA) and Bristol Myers Squibb (New York, NY). Since preclinical data have associated chromophobe histology with immune cell exclusion, which may correlate with resistance to immune checkpoint inhibitors, chromophobe RCC was evaluated as a separate cohort with different statistical design.8,14,15 Histopathology was reviewed at MSKCC by a dedicated GU pathologist (YC). Cohort 1 included patients with papillary, unclassified, FH-deficient, or translocation-associated RCC. Cohort 2 included patients with chromophobe RCC. Given the histologic overlaps among high-grade RCC with papillary architecture, cohort 1 was subdivided into three histologic subgroups: (1) papillary, including unclassified with papillary features, high-grade papillary, and FH-deficient RCC; (2) unclassified RCC without papillary features; and (3) translocation-associated RCC. All patients received concurrent therapy with cabozantinib (approved dose when used in combination of 40 mg by mouth once daily) and nivolumab (standard dose of 240 mg intravenously once every 2 weeks). The protocol was later amended to offer the option of nivolumab 480 mg intravenously once every 4 weeks per the updated US Food and Drug Administration label16 until disease progression, intolerable toxicity, or withdrawal of consent. To help prevent hand-foot syndrome from cabozantinib therapy, all patients applied clobetasol topically to their hands and feet twice per day for the first 12 weeks of therapy; topical steroids could be continued beyond 12 weeks at the investigator's discretion. Archival tumor tissue and serial peripheral bloods were prospectively banked to later develop biomarkers that correlate with treatment response.
Study Assessments
Cycle length was 28 days; cross-sectional imaging was repeated every 12 weeks for efficacy assessment per RECIST 1.1 by an independent RECIST radiologist (R.A.L.).13 Clinical and laboratory assessment, urinalysis, and blood draw were performed twice during every cycle. Toxicities were assessed and the protocol provided guidance on dose reductions for cabozantinib toxicity (20 mg once daily and 20 mg once every other day) and allowed for dose re-escalation up to 40 mg once daily in the event that adverse effects had improved after initial dose reduction and with implementation of supportive care measures. No dose reductions were allowed for nivolumab, but dosing could be delayed up to 8 weeks or permanently discontinued if held > 8 weeks. If either nivolumab or cabozantinib were discontinued, the remaining agent could be continued in the absence of disease progression.
Next-generation sequencing analysis was performed using the MSK-IMPACT (Integrated Mutation Profiling of Actionable Cancer Targets) platform as previously described.17 The assay achieves pull-down capture with target-specific probes and germline comparison (from peripheral blood leukocytes) for exons from 505 cancer-related genes, including oncogenes, tumor suppressor genes, and components of pathways deemed actionable by targeted therapies (for full list, see the Data Supplement, online only).
Statistical Analysis
The primary end point was the ORR according to RECIST v1.1, defined as the percentage of patients with measurable disease who had a complete response or partial response. Secondary end points included PFS, OS, ORR by immune-related RECIST (irRECIST), and assessment of treatment-related adverse events (AEs).
Cohort 1 was originally designed as a single-stage study with a total sample size of 20. This cohort was powered to detect an improvement in ORR from 10% to 35%. The regimen was considered promising if five or more patients achieved an objective response. After meeting the primary efficacy end point, cohort 1 was subsequently expanded to include an additional 20 patients for a total cohort size of n = 40 to more accurately estimate ORR (± 0.15) and clinical outcomes. Cohort 2 used a Simon optimal two-stage design with a sample size of up to 17. If no responses were seen in the first nine patients, the cohort would discontinue enrollment and the regimen would not be considered for further study in this RCC histology. The cohort 2 design discriminates between an ORR of 5% and a promising ORR of 25%. The cohort was closed early because of slow accrual and lack of efficacy in the initial seven patients. Both cohorts were designed to have a type I error of 5% and power of 80%.
PFS and OS were calculated using time-to-event methods to account for censoring. PFS was defined as time from treatment start to disease progression or death. Patients who did not progress or die during study follow-up were censored at the date of their last tumor imaging assessment. OS was defined as time from treatment start to death of any cause. Disease control rate was defined as patients with an objective response or stable disease (SD) at the first scheduled tumor assessment during the study. The clinical benefit rate was defined as the proportion of patients with disease control lasting at least 24 weeks. Statistical analysis was performed using SAS v9.4 (SAS Institute, Cary, NC).
RESULTS
Baseline Characteristics
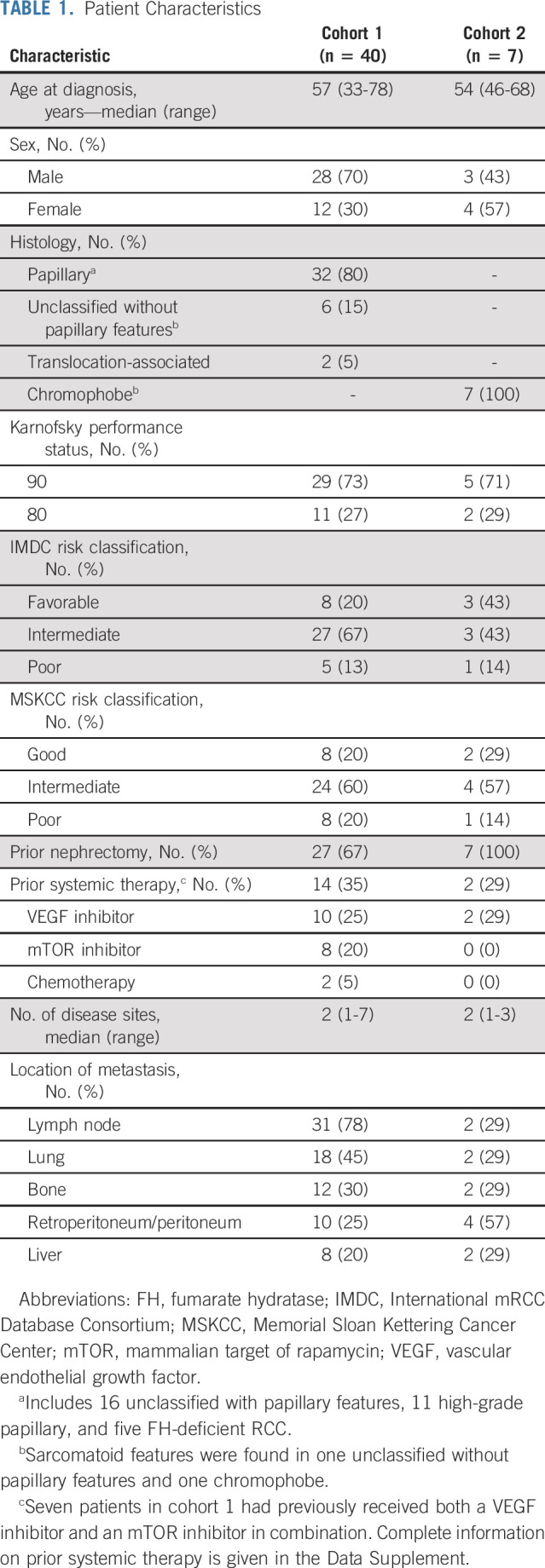
A total of 47 patients were enrolled onto the trial between August 28, 2018, and October 20, 2020. Median follow-up time was 13.1 months (range, 2.2-28.6 months) from enrollment until data collection cutoff (January 20, 2021). Patient characteristics are summarized in Table 1. Cohort 1 comprised 40 patients with papillary (32), unclassified without papillary features (six), and translocation-associated (two) RCC. Subclassification within papillary RCC included unclassified with papillary features (16), high-grade papillary (11), and FH-deficient (five) RCC. Cohort 2 comprised seven patients with chromophobe RCC. The median ages for cohorts 1 and 2 were 57 and 54, respectively, with men comprising 70% of cohort 1 and 43% of cohort 2. In cohort 1, patients were classified as favorable (20%), intermediate (67%), or poor risk (13%) using the International Metastatic RCC Database Consortium (IMDC) risk criteria.18 Fourteen patients (35%) in cohort 1 had received prior systemic therapy before study enrollment. Ten (25%) received VEGF-targeted therapy, 8 (20%) received mTOR-targeted therapy (seven patients received both), two patients received prior chemotherapy, one patient received prior crizotinib, and 26 patients were previously untreated (Data Supplement).
TABLE 1.
Patient Characteristics
Efficacy
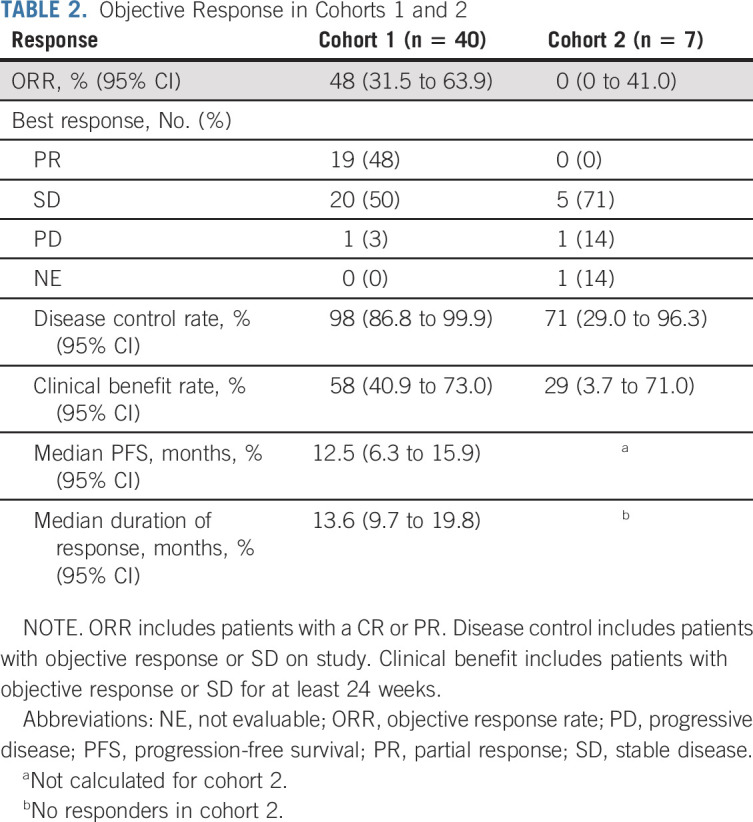
Efficacy outcomes for cohorts 1 and 2 are summarized in Table 2. ORR for cohort 1 was 48% (95% CI, 31.5 to 63.9), with 19 (48%) patients showing partial response, 20 (50%) with SD, and one (3%) with progressive disease as best response (Table 2). The disease control rate was 98% (95% CI, 86.8 to 99.9), and the clinical benefit rate was 75% (95% CI, 58.8 to 87.3). Similar outcomes were also seen when patients were stratified by prior therapy status (Data Supplement).
TABLE 2.
Objective Response in Cohorts 1 and 2
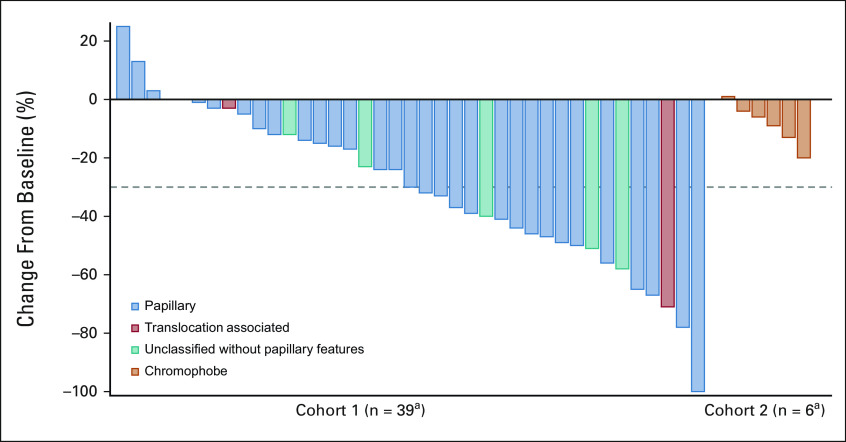
When stratified by histologies within cohort 1, objective responses were observed in 15 of 32 (47%, 95% CI, 29 to 65) patients with papillary histology. In unclassified RCC without papillary features, three of six patients had objective responses and one of two patients with translocation-associated RCC had objective responses (Fig 1). Because of the small sample sizes, there were no strong correlations between specific type of papillary histology (papillary, unclassified with papillary features, and FH-deficient) and response; however, all five patients with FH-deficient RCC achieved an objective response.
FIG 1.
Maximum change from baseline in target lesions in combined cohort. aTwo patients with rapid clinical progression had unevaluable lesions are excluded from the plot.
In cohort 1, the median PFS by RECIST was 12.5 months (95% CI, 6.3 to 15.9) and PFS estimate at 12 months was 52.8% (95% CI, 34.1 to 68.5; Fig 2). Median PFS by irRECIST was 12.5 months (95% CI, 6.3 to 16.4) and the PFS estimate at 12 months was 52.6% (95% CI, 33.8 to 68.3; Data Supplement). The median duration of response was 13.6 months (95% CI, 9.7 to 19.8; Data Supplement). Of the 19 responders, 10 experienced subsequent disease progression and nine remained on treatment with continued response at the time of the data cutoff.
FIG 2.
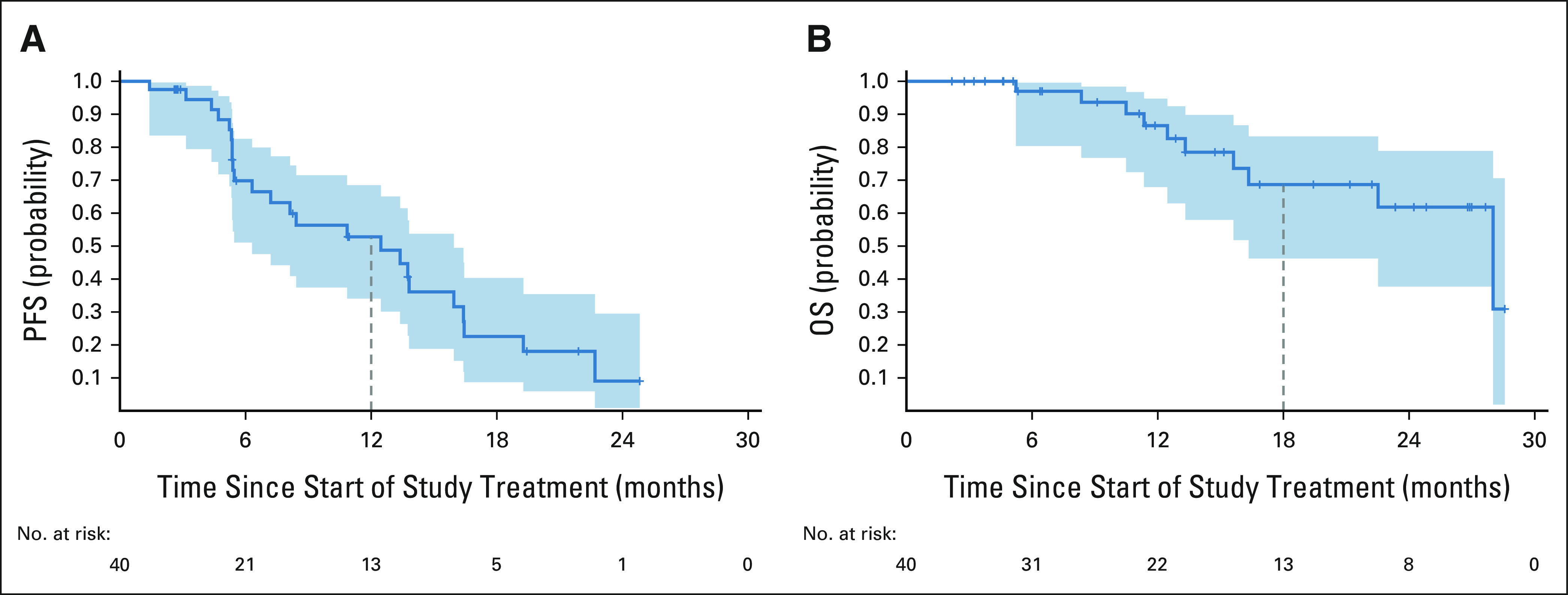
PFS and OS in Cohort 1. (A) PFS, RECIST 1.1. There were 22 PFS events (20 progressions and two deaths with no progression). Median PFS is 12.5 months (95% CI, 6.3 to 15.9). The PFS estimate is 52.8% (95% CI, 34.1 to 68.5) at 12 months. (B) OS. There were 10 deaths in the 40-patient cohort. Median OS is 28.0 months (95% CI, 16.3 to NE). The OS estimate is 68.7% (95% CI, 46.3 to 83.3) at 18 months. Median follow-up time for survivors is 13.1 months (range, 2.2-28.6 months). NE, not evaluable; OS, overall survival; PFS, progression-free survival.
Median follow-up time for survival was 13.1 months (range, 2.2-28.6 months). Median OS for cohort 1 was 28 months (95% CI, 16.3 to NE) and the OS estimate at 18 months was 68.7% (95% CI, 46.3 to 83.3; Fig 2).
None of the seven patients with chromophobe RCC treated in cohort 2 achieved an objective response, and further enrollment was subsequently discontinued on the basis of lack of efficacy and slow accrual. The disease control rate was 71% (95% CI, 29.0 to 96.3) and clinical benefit rate was 29% (95% CI, 3.7 to 71.0) for cohort 2. Because of the small cohort size, the median PFS was not calculated. In the two patients with PFS > 24 weeks, one patient remains on treatment with prolonged SD, and one patient withdrew from protocol to undergo ablation.
Treatment Exposure and AEs
Treatment exposure and tolerance were analyzed in aggregate for all 47 patients receiving protocol therapy (Data Supplement). At the time of data cutoff, the median duration of study treatment was 11.0 months (95% CI, 7.8 to 21.1) with 20 patients remaining on treatment with one or both therapies, 27 patients discontinuing cabozantinib, and 28 discontinuing nivolumab. Twenty patients discontinued treatment because of progressive disease. A total of 10 (21%) patients discontinued one or both drugs because of treatment-related AEs.
The median duration of treatment for cabozantinib was 9.1 months (95% CI, 7.4 to 21.1). Cabozantinib dosage was reduced in 37 (79%) patients, and six patients (13%) discontinued cabozantinib for AEs. The most common AE leading to cabozantinib discontinuation was palmar-plantar erythrodysesthesia in two patients. The median duration of treatment for nivolumab 10.6 months (95% CI, 6.0 to 18.8), and eight (17%) patients discontinued nivolumab for AEs. High-dose steroids (≥ 40 mg prednisone equivalent) were required in 8/47 (17%) patients. The most common AE leading to nivolumab discontinuation was grade 3 hepatitis in three patients, of which two also had pancreatitis.
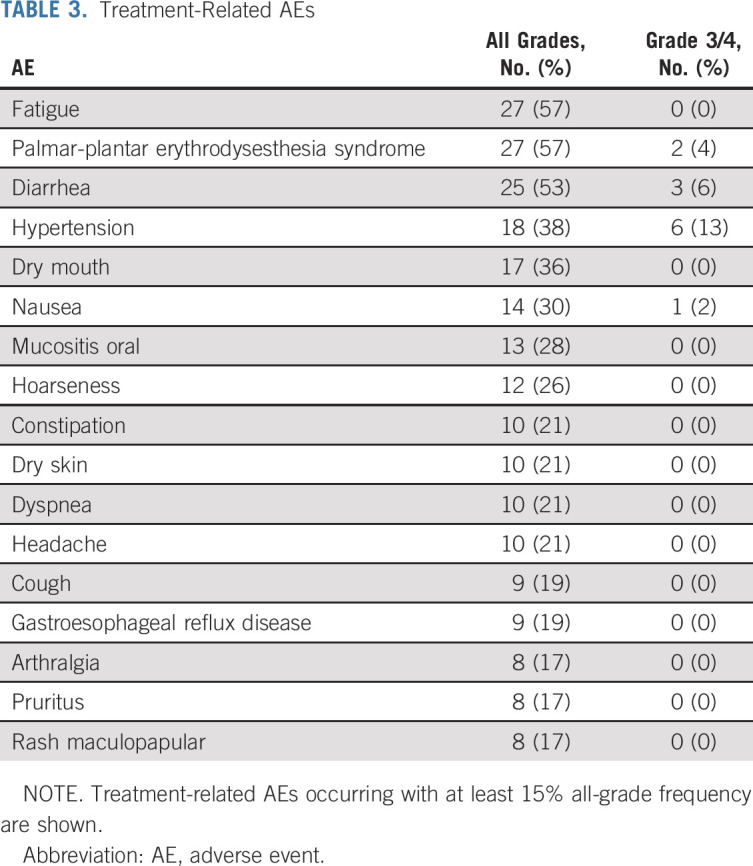
A total of 41 (87%) patients experienced treatment-related AEs of any grade, and 15 (32%) experienced treatment-related AEs of grade 3-4 (Table 3 and Data Supplement). The most common treatment-related AEs of any grade were fatigue (57%), palmar-plantar erythrodysesthesia syndrome (57%), and diarrhea (53%). The most common grade 3-4 treatment-related AEs were hypertension (13%), diarrhea (6%), and palmar-plantar erythrodysesthesia syndrome (4%). High-grade (≥ CTCAE grade 3) treatment-related laboratory abnormalities were infrequent except for hypophosphatemia (30%), elevated amylase (15%), elevated lipase (13%), elevated ALT (13%), and elevated AST (11%; Table 3). No treatment-related grade 5 AEs were seen. Treatment-related AEs were similar regardless of prior therapy status (Data Supplement).
TABLE 3.
Treatment-Related AEs
Correlative Analysis
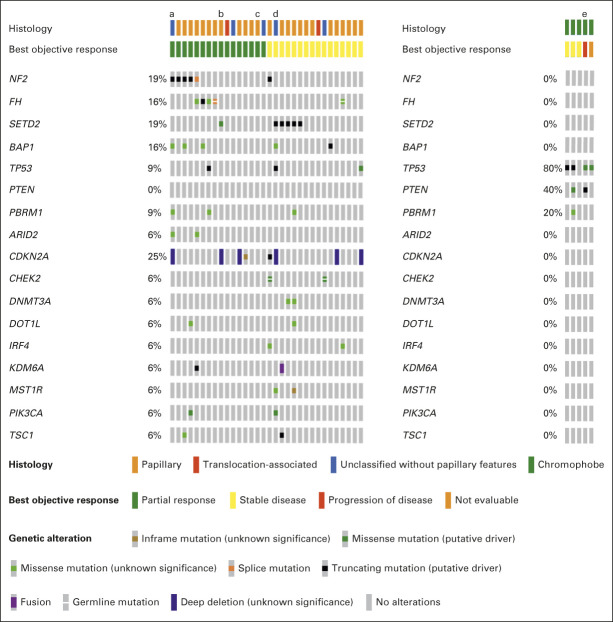
Targeted exome sequencing by MSK-IMPACT was performed on 37 patients (n = 32 in cohort 1 and n = 5 in cohort 2; Fig 3). As expected for non–clear-cell histologies, no alterations in VHL were seen, and cohorts 1 and 2 demonstrated distinct mutation profiles. In cohort 1, the most common alterations were in CDKN2a (25%), NF2 (19%), SETD2 (19%), FH (16%), and BAP1 (16%). In cohort 2, the most common mutations were in TP53 (80%) and PTEN (40%). Objective responses were seen in 5/6 patients with NF2 mutations, and 5/6 patients with FH mutations; however, only 1/6 patients with SETD2 mutations had an objective response. Of note, one patient with FH mutation had targeted exome sequencing performed by ampliseq and was not included in Figure 3.
FIG 3.
Genomic analysis by targeted exome sequencing. Genomic analysis oncoprint of patients with tissue available for targeted exome sequencing by MSK-IMPACT. aSarcomatoid; bmultiple samples; cTFEB-amplified; dcollecting duct; multiple samples; emultiple samples.
DISCUSSION
This phase II trial assessed the combination of cabozantinib plus nivolumab in patients with advanced non–clear-cell renal carcinoma, a heterogeneous group of diseases with poorly defined standards of care.19 Our trial separated the diverse histologies into 2 cohorts and demonstrated differing efficacy results. Cohort 1 (predominantly papillary) met its primary end point with promising efficacy and was subsequently expanded, whereas cohort 2 (chromophobe) closed to accrual early for lack of objective responses and slow accrual. Among 40 patients in cohort 1 who had papillary, unclassified, or translocation-associated RCC and had previously received up to 1 prior line of systemic therapy, the ORR was 47.5%, median PFS 12.5 months, and clinical benefit rate 71%. Furthermore, primary progression on this regimen was extremely rare with 39/40 (98.5%) patients achieving at least SD at 12 weeks, the time of the first imaging assessment, and nearly all patients achieved some shrinkage in tumor burden. The observed AEs in non–clear-cell RCC were consistent with the experience in clear-cell RCC, and no new safety signals were observed. Increased AST/ALT could be seen from either cabozantinib or nivolumab. For cabozantinib-related increases in AST/ALT, dose hold followed by dose reduction improved these toxicities without the need for systemic steroids. Compared with the phase III trial of cabozantinib and nivolumab in ccRCC (CheckMate 9ER), the rate of discontinuation of either drug was similar (21% v 20%, this study and Checkmate 9ER, respectively); however, the rate of dose reduction for cabozantinib was higher (79% v 56.3%, this study and Checkmate 9ER, respectively). This may be due to multiple factors including the relatively small sample size, the ability to dose escalate after dose reduction, differences in histology on tolerability, and treatment of both first- and second-line patients on this study.
Historically, both VEGF-targeted and mTOR-targeted monotherapy have been studied and were the standard of care for non–clear-cell RCC. In a randomized protocol, ESPN (N = 68) and ASPEN (N = 108) demonstrated superiority of sunitinib over everolimus for non–clear-cell RCC; however, the activity sunitinib in this setting remains modest (ESPN sunitinib ORR = 9%, median PFS 6.1 months; ASPEN sunitinib ORR = 18%, median PFS 8.3 months).5,6
Although early, the results with the combination of cabozantinib plus nivolumab are promising, even in the context of contemporary monotherapy and combination data in this space. CheckMate 374 investigated nivolumab monotherapy in patients with non–clear-cell RCC with up to three prior lines of systemic therapy (N = 44), which included 55% of patients with papillary histology. CheckMate 374 showed a 13.6% ORR, and a median PFS of 2.2 months (95% CI, 1.8 to 5.4).9 HCRN GU16-260 cohort B investigated nivolumab monotherapy followed by nivolumab and ipilimumab salvage therapy in patients with treatment-naive non–clear-cell RCC (N = 35), which included 54% of patients with papillary histology.10 HCRN GU16-260 demonstrated an ORR of 14.3% and a median PFS of 4.0 months (95% CI, 2.7 to 4.3). Of the 16 patients eligible for salvage ipilimumab and nivolumab, only 1 patient had an objective response. The combination of ipilimumab and nivolumab as initial therapy was also studied in treatment-naive nccRCC patients in CheckMate 920 (N = 52), which included 35% of patients with papillary RCC.11 In CheckMate 920, the ORR was 19.6% and the median PFS was 3.7 months (95% CI, 2.7 to 4.6). KeyNote-427 cohort B investigated pembrolizumab monotherapy in patients with treatment-naive nccRCC (N = 165), which included 71.5% of patients with papillary histology.8 Across the entire nccRCC cohort, the ORR was 26.7% and the median PFS was 4.2 months (95% CI, 2.9 to 5.6). In patients with papillary histology, the ORR was 28.8%.8 Together, these studies demonstrate that immune checkpoint inhibitors have activity in non–clear-cell RCC.
VEGF-targeted therapy either as monotherapy or combination therapy has also demonstrated activity in nccRCC. The multiarm randomized PAPMET trial (SWOG S1500) enrolled patients with VEGF-targeted therapy-naive papillary RCC, and demonstrated an ORR of 23% and median PFS of 9.0 months (95% CI, 6 to 12) on cabozantinib monotherapy (N = 44).7 Combination therapy with a VEGF-targeted therapy in combination with a programmed death ligand-1 inhibitor has also been investigated for nccRCC with both the combination of bevacizumab plus atezolizumab and cabozantinib plus atezolizumab.20 The phase II study of bevacizumab plus atezolizumab included patients with non–clear-cell and clear-cell with sarcomatoid features (N = 60) with any number of prior lines of therapy excluding prior immune checkpoint inhibitors. Bevacizumab plus atezolizumab demonstrated an ORR of 33% and a median PFS of 8.3 months in non–clear-cell and clear-cell with sarcomatoid features (N = 60), but when limited to nccRCC (n = 42), the ORR was 26%.20 In cohort 10 of COSMIC-21, cabozantinib plus atezolizumab (n = 30) demonstrated an ORR of 33% and a median PFS of 9.5 months (95% CI, 5.5 to NE) in patients with up to 1 prior VEGF-targeted therapy.21 Other combinations of VEGF-targeted therapy with non–immune checkpoint inhibitors have also been studied. The combination of lenvatinib plus everolimus demonstrated an ORR of 26% and a median PFS of 9.2 months in patients with nccRCC.22 In select non–clear-cell histologies, previous studies with the combination of bevacizumab plus either the mTOR inhibitor, everolimus, or the EGFR inhibitor, erlotinib, have also demonstrated efficacy rates superior to historic results with single agents, although activity was largely limited to papillary variants (ORR = 35%; median PFS = 13.7 months) and FH-deficient subtypes (ORR = 54%, median PFS = 14.3 months), respectively.23-25
These studies, in addition to ours, support the use of combination therapy with immune checkpoint inhibitors and VEGF-targeted therapy in patients with several variants of nccRCC including papillary, unclassified, and translocation-associated RCC subtypes. Several efforts are ongoing to corroborate these data including the combination of cabozantinib plus atezolizumab in checkpoint inhibitor–refractory patients (ClinicalTrials.gov identifier: NCT04338269) and lenvatinib plus pembrolizumab in treatment-naive patients (ClinicalTrials.gov identifier: NCT04704219).
Although the current study enrolled patients with specific attention to histologic classification, cohort 1 remains a diverse collection of currently recognized histologic and/or molecular subtypes, partially because of our limited understanding of the pathogenesis of high-grade RCC with papillary architecture. Our targeted exome sequencing data suggest that mutations in NF2, FH, and SETD2 are frequent in non–clear-cell kidney cancer and collectively comprise more than half of the tumors in cohort 1. Of interest, 10 of 12 patients with mutations in NF2 or FH achieved an objective response to cabozantinib plus nivolumab, whereas only one of six patients with mutations in SETD2 had an objective response. Although conclusions are limited by the small sample size, these findings are hypothesis-generating that genomic alterations may be associated with differential treatment responses in nccRCC. In addition, historical retrospective studies evaluating outcomes to single-agent treatment in RCC observed worse outcomes for NF2-mutant or FH-deficient RCC compared with nccRCC harboring other mutations.26 Mutations in NF2 and FH are both associated with lower tumor mutational burden, but high fraction of genome altered.27 In retrospective analysis of patients with FH-deficient RCC, VEGF monotherapy was associated with an ORR of 20% and checkpoint inhibitor therapy was associated with an ORR of 0%.27 Our data suggest that these historically more aggressive and treatment-resistant mutations may be more responsive broadly to the combination of dual immune checkpoint and VEGF pathway inhibition, and specifically to cabozantinib plus nivolumab. However, these numbers remain small, and this study will be expanded to validate these initial observations.
In summary, the combination of cabozantinib plus nivolumab showed promising efficacy in metastatic nccRCC patients with papillary, unclassified, and translocation-associated histologies but not chromophobe RCC. The observed AEs in nccRCC were consistent with the adverse-event profile of this combination in ccRCC. Genomic studies highlight the heterogeneity of nccRCC and warrant further study as predictors of response to systemic therapy.
Chung-Han Lee
Honoraria: Intellisphere, Research to Practice, American Institute of Continuing Medical Education
Consulting or Advisory Role: Eisai, Bristol Myers Squibb, Merck, Pfizer/EMD Serono, Exelixis
Research Funding: Eisai (Inst), Bristol Myers Squibb (Inst), Calithera Biosciences (Inst), Exelixis (Inst), Merck (Inst)
Martin H. Voss
Stock and Other Ownership Interests: CellGenix
Consulting or Advisory Role: Exelixis, Pfizer, Eisai, Merck, Calithera Biosciences, AVEO, OnQuality Pharmaceuticals, Genentech
Research Funding: Pfizer
Other Relationship: BMS
Uncompensated Relationships: Genentech/Roche, Aravive, BMS
Maria Isabel Carlo
Other Relationship: Prostate Cancer Foundation, Robert Wood Johnson Foundation
Andrea Knezevic
Consulting or Advisory Role: ByHeart
Robert A. Lefkowitz
Honoraria: SpringWorks Therapeutics
Neil J. Shah
Consulting or Advisory Role: Merck Sharp & Dohme Corp
Research Funding: Aravive (Inst)
Deaglan Joseph McHugh
Consulting or Advisory Role: Progenics
David Henry Aggen
Consulting or Advisory Role: Boehringer Ingelheim, Seattle Genetics, Astellas Pharma
Patents, Royalties, Other Intellectual Property: University of Illinois - Urbana Champaign
Open Payments Link: https://openpaymentsdata.cms.gov/physician/4226107
Ritesh Kotecha
Consulting or Advisory Role: Eisai
Research Funding: Pfizer (Inst), Takeda (Inst)
Darren R. Feldman
Consulting or Advisory Role: Cigna
Research Funding: Seattle Genetics, Decibel Therapeutics (Inst), Astellas Pharma
Other Relationship: UpToDate
Robert J. Motzer
Consulting or Advisory Role: Novartis, Eisai, Exelixis, Merck, Genentech/Roche, Incyte, Lilly, Pfizer, AstraZeneca, EMD Serono, Calithera Biosciences, Aveo
Research Funding: Pfizer (Inst), Bristol Myers Squibb (Inst), Eisai (Inst), Novartis (Inst), Genentech/Roche (Inst), Exelixis (Inst), Merck (Inst), Aveo (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb
No other potential conflicts of interest were reported.
SUPPORT
Study Support for this investigator-initiated trial was provided by BMS and Exelixis. Patient support also provided by the Cancer Center Support Core Grant (P30 CA008748). Also supported in part by the Academy of Kidney Cancer Investigators of the CDMRP/DOD (KC200127) (R.K.).
CLINICAL TRIAL INFORMATION
D.R.F. and R.J.M. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Chung-Han Lee, Ying-Bei Chen, Andrea Knezevic, Darren R. Feldman, Robert J. Motzer
Provision of study materials or patients: Martin H. Voss, Maria Isabel Carlo, Ying-Bei Chen, Colette Ngozi Owens, Deaglan Joseph McHugh, David Henry Aggen, Andrew Leonard Laccetti, Darren R. Feldman, Robert J. Motzer
Collection and assembly of data: Chung-Han Lee, Martin H. Voss, Maria Isabel Carlo, Ying-Bei Chen, Robert A. Lefkowitz, Natalie Shapnik, Chloe Dadoun, Colette Ngozi Owens, David Henry Aggen, Andrew Leonard Laccetti, Ritesh Kotecha, Darren R. Feldman, Robert J. Motzer
Data analysis and interpretation: Chung-Han Lee, Martin H. Voss, Maria Isabel Carlo, Ying-Bei Chen, Mark Zucker, Andrea Knezevic, Ed Reznik, Neil J. Shah, Colette Ngozi Owens, Deaglan Joseph McHugh, Ritesh Kotecha, Darren R. Feldman, Robert J. Motzer
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase II Trial of Cabozantinib Plus Nivolumab in Patients With Non–Clear-Cell Renal Cell Carcinoma and Genomic Correlates
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Chung-Han Lee
Honoraria: Intellisphere, Research to Practice, American Institute of Continuing Medical Education
Consulting or Advisory Role: Eisai, Bristol Myers Squibb, Merck, Pfizer/EMD Serono, Exelixis
Research Funding: Eisai (Inst), Bristol Myers Squibb (Inst), Calithera Biosciences (Inst), Exelixis (Inst), Merck (Inst)
Martin H. Voss
Stock and Other Ownership Interests: CellGenix
Consulting or Advisory Role: Exelixis, Pfizer, Eisai, Merck, Calithera Biosciences, AVEO, OnQuality Pharmaceuticals, Genentech
Research Funding: Pfizer
Other Relationship: BMS
Uncompensated Relationships: Genentech/Roche, Aravive, BMS
Maria Isabel Carlo
Other Relationship: Prostate Cancer Foundation, Robert Wood Johnson Foundation
Andrea Knezevic
Consulting or Advisory Role: ByHeart
Robert A. Lefkowitz
Honoraria: SpringWorks Therapeutics
Neil J. Shah
Consulting or Advisory Role: Merck Sharp & Dohme Corp
Research Funding: Aravive (Inst)
Deaglan Joseph McHugh
Consulting or Advisory Role: Progenics
David Henry Aggen
Consulting or Advisory Role: Boehringer Ingelheim, Seattle Genetics, Astellas Pharma
Patents, Royalties, Other Intellectual Property: University of Illinois - Urbana Champaign
Open Payments Link: https://openpaymentsdata.cms.gov/physician/4226107
Ritesh Kotecha
Consulting or Advisory Role: Eisai
Research Funding: Pfizer (Inst), Takeda (Inst)
Darren R. Feldman
Consulting or Advisory Role: Cigna
Research Funding: Seattle Genetics, Decibel Therapeutics (Inst), Astellas Pharma
Other Relationship: UpToDate
Robert J. Motzer
Consulting or Advisory Role: Novartis, Eisai, Exelixis, Merck, Genentech/Roche, Incyte, Lilly, Pfizer, AstraZeneca, EMD Serono, Calithera Biosciences, Aveo
Research Funding: Pfizer (Inst), Bristol Myers Squibb (Inst), Eisai (Inst), Novartis (Inst), Genentech/Roche (Inst), Exelixis (Inst), Merck (Inst), Aveo (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb
No other potential conflicts of interest were reported.
REFERENCES
- 1.American Cancer Society Key statistics about kidney cancer. https://www.cancer.org/cancer/kidney-cancer/about/key-statistics.html
- 2.Molina AM, Feldman DR, Ginsberg MS, et al. Phase II trial of sunitinib in patients with metastatic non-clear cell renal cell carcinoma Invest New Drugs 30335–3402012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tannir NM, Plimack E, Ng C, et al. A phase 2 trial of sunitinib in patients with advanced non-clear cell renal cell carcinoma Eur Urol 621013–10192012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koh Y, Lim HY, Ahn JH, et al. Phase II trial of everolimus for the treatment of nonclear-cell renal cell carcinoma Ann Oncol 241026–10312013 [DOI] [PubMed] [Google Scholar]
- 5.Tannir NM, Jonasch E, Albiges L, et al. Everolimus versus sunitinib prospective evaluation in metastatic non-clear cell renal cell carcinoma (ESPN): A randomized multicenter phase 2 trial Eur Urol 69866–8742016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong AJ, Halabi S, Eisen T, et al. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): A multicentre, open-label, randomised phase 2 trial Lancet Oncol 17378–3882016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pal SK, Tangen C, Thompson IM, et al. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: A randomised, open-label, phase 2 trial Lancet 397695–7032021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDermott DF, Lee JL, Ziobro M, et al. Open-label, single-arm, phase II study of pembrolizumab monotherapy as first-line therapy in patients with advanced non-clear cell renal cell carcinoma J Clin Oncol 391029–10392021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogelzang NJ, Olsen MR, McFarlane JJ, et al. Safety and efficacy of nivolumab in patients with advanced non-clear cell renal cell carcinoma: Results from the phase IIIb/IV CheckMate 374 study Clin Genitourin Cancer 18461–468.e32020 [DOI] [PubMed] [Google Scholar]
- 10. Atkins MB, Jegede O, Haas NB, et al. Phase II study of nivolumab and salvage nivolumab + ipilimumab in treatment-naïve patients (pts) with advanced non-clear cell renal cell carcinoma (nccRCC) (HCRN GU16-260-Cohort B) J Clin Oncol. 2021;39 doi: 10.1200/JCO.21.02938. suppl 15; abstr 4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tykodi SS, Gordan LN, Alter RS, et al. Nivolumab plus ipilimumab in patients with advanced non-clear cell renal cell carcinoma (nccRCC): Safety and efficacy from CheckMate 920. J Clin Oncol. 2021;39:309. suppl 6; abstr 309. [Google Scholar]
- 12.Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma N Engl J Med 384829–8412021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer 45228–2472009 [DOI] [PubMed] [Google Scholar]
- 14. Koshkin VS, Barata PC, Zhang T, et al. Clinical activity of nivolumab in patients with non-clear cell renal cell carcinoma. J Immunother Cancer. 2018;6:9. doi: 10.1186/s40425-018-0319-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ricketts CJ, De Cubas AA, Fan H, et al. The Cancer Genome Atlas comprehensive molecular characterization of renal cell carcinoma Cell Rep 23313–326.e52018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Opdivo (nivolumab) [package insert] Princeton, NJ: Bristol Myers Squibb Company; 2021. [Google Scholar]
- 17.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology J Mol Diagn 17251–2642015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Motzer RJ, Bacik J, Murphy BA, et al. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma J Clin Oncol 20289–2962002 [DOI] [PubMed] [Google Scholar]
- 19.Albiges L, Escudier B.Current and future strategies in nonclear-cell metastatic renal cell carcinoma Curr Opin Urol 25367–3732015 [DOI] [PubMed] [Google Scholar]
- 20.McGregor BA, McKay RR, Braun DA, et al. Results of a multicenter phase II study of atezolizumab and bevacizumab for patients with metastatic renal cell carcinoma with variant histology and/or sarcomatoid features J Clin Oncol 3863–702020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McGregor BA, Agarwal N, Suarez C, et al. 709P Cabozantinib (C) in combination with atezolizumab (A) in non-clear cell renal cell carcinoma (nccRCC): Results from cohort 10 of the COSMIC-021 study. Ann Oncol. 2020;31:S558. [Google Scholar]
- 22.Hutson TE, Michaelson MD, Kuzel TM, et al. A single-arm, multicenter, phase 2 study of lenvatinib plus everolimus in patients with advanced non-clear cell renal cell carcinoma Eur Urol 80162–1702021 [DOI] [PubMed] [Google Scholar]
- 23.Feldman DR, Ged Y, Lee CH, et al. Everolimus plus bevacizumab is an effective first-line treatment for patients with advanced papillary variant renal cell carcinoma: Final results from a phase II trial Cancer 1265247–52552020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voss MH, Molina AM, Chen YB, et al. Phase II trial and correlative genomic analysis of everolimus plus bevacizumab in advanced non-clear cell renal cell carcinoma J Clin Oncol 343846–38532016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Srinivasan R, Gurram S, Al Harthy M, et al. Results from a phase II study of bevacizumab and erlotinib in subjects with advanced hereditary leiomyomatosis and renal cell cancer (HLRCC) or sporadic papillary renal cell cancer. J Clin Oncol. 2020;38 suppl 15; abstr 5004. [Google Scholar]
- 26. Chen YB, Xu J, Skanderup AJ, et al. Molecular analysis of aggressive renal cell carcinoma with unclassified histology reveals distinct subsets. Nat Commun. 2016;7:13131. doi: 10.1038/ncomms13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gleeson JP, Nikolovski I, Dinatale R, et al. Comprehensive molecular characterization and response to therapy in fumarate hydratase-deficient renal cell carcinoma Clin Cancer Res 272910–29192021 [DOI] [PMC free article] [PubMed] [Google Scholar]