Abstract
PURPOSE
Triple-negative breast cancer (TNBC) is considered aggressive, and therefore, virtually all young patients with TNBC receive (neo)adjuvant chemotherapy. Increased stromal tumor-infiltrating lymphocytes (sTILs) have been associated with a favorable prognosis in TNBC. However, whether this association holds for patients who are node-negative (N0), young (< 40 years), and chemotherapy-naïve, and thus can be used for chemotherapy de-escalation strategies, is unknown.
METHODS
We selected all patients with N0 TNBC diagnosed between 1989 and 2000 from a Dutch population–based registry. Patients were age < 40 years at diagnosis and had not received (neo)adjuvant systemic therapy, as was standard practice at the time. Formalin-fixed paraffin-embedded blocks were retrieved (PALGA: Dutch Pathology Registry), and a pathology review including sTILs was performed. Patients were categorized according to sTILs (< 30%, 30%-75%, and ≥ 75%). Multivariable Cox regression was performed for overall survival, with or without sTILs as a covariate. Cumulative incidence of distant metastasis or death was analyzed in a competing risk model, with second primary tumors as competing risk.
RESULTS
sTILs were scored for 441 patients. High sTILs (≥ 75%; 21%) translated into an excellent prognosis with a 15-year cumulative incidence of a distant metastasis or death of only 2.1% (95% CI, 0 to 5.0), whereas low sTILs (< 30%; 52%) had an unfavorable prognosis with a 15-year cumulative incidence of a distant metastasis or death of 38.4% (32.1 to 44.6). In addition, every 10% increment of sTILs decreased the risk of death by 19% (adjusted hazard ratio: 0.81; 95% CI, 0.76 to 0.87), which are an independent predictor adding prognostic information to standard clinicopathologic variables (χ2 = 46.7, P < .001).
CONCLUSION
Chemotherapy-naïve, young patients with N0 TNBC with high sTILs (≥ 75%) have an excellent long-term prognosis. Therefore, sTILs should be considered for prospective clinical trials investigating (neo)adjuvant chemotherapy de-escalation strategies.
INTRODUCTION
Approximately one in every 18 patients with breast cancer is under age 40 years at diagnosis. In the United States alone, breast cancer under age 40 years affects more than 11,000 women annually.1 Compared with older women, young women are more often diagnosed with triple-negative breast cancer (TNBC), a subtype with relatively high incidences of germline BRCA1 mutations.2 Because of the absence of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) on the cell surface of TNBC cells, commonly used treatments like tamoxifen, aromatase inhibitors, and trastuzumab, which target these receptors, are not effective in patients with TNBC. To improve survival, most early-stage TNBC patients are treated with (neo)adjuvant chemotherapy.3 Although systemic chemotherapy improves survival, it also induces age-related acute and chronic side effects, eg, premature ovarian failure and cognitive impairment.4,5 Given the heterogeneous biology of TNBC, it might be undesirable to treat all patients with (the same) chemotherapy.
CONTEXT
Key Objective
Triple-negative breast cancer (TNBC) in young women has a poor prognosis. Adjuvant systemic therapy is, therefore, indicated for most of these patients. Stromal tumor-infiltrating lymphocytes (sTILs) have shown prognostic and predictive value in patients with early TNBC; however, this has not been validated in young patients. This study examined whether sTILs are prognostic in young patients with node-negative (N0) TNBC who are systemic therapy–naïve.
Knowledge Generated
sTILs are a strong prognostic factor for 15-year overall survival and distant metastasis-free survival in young patients with N0 TNBC. In total, 21% (94 of 441) of N0 TNBC patients had high sTILs (≥ 75%). Patients with high sTILs have an excellent prognosis in the absence of systemic therapy, whereas patients with low sTILs (< 30%) have an unfavorable prognosis.
Relevance
sTILs are highly prognostic in young patients with N0 TNBC. Validation of this biomarker in prospective, chemotherapy de-escalation trials should strongly be considered.
Commonly used multigene prognostic tests for early-stage breast cancer, such as MammaPrint and Oncotype-DX, do not apply to patients with TNBC.6,7 Therefore, prognostic biomarkers are needed that tailor treatment strategies for (young) patients with TNBC. Compared with older patients, young patients are disproportionally affected by the chronic effects of chemotherapy on their welfare and well-being.8 One putative prognostic biomarker for TNBC is stromal tumor-infiltrating lymphocytes (sTILs). sTILs are a mix of mononuclear immune cells and may represent the systemic anticancer immune response.9 sTILs have been shown to be prognostic in early-stage patients treated with and without (neo)adjuvant chemotherapy.10-12
The prognostic importance of sTILs is, however, unexplored in patients diagnosed under age 40 years, let alone in the subgroup of systemic therapy–naïve patients. In this study, we aim to validate the prognostic value of sTILs in young patients with node-negative (N0) TNBC who did not receive adjuvant chemotherapy. Specifically, we aim to identify an ultralow-risk sTILs subgroup with such a favorable prognosis that, if confirmed in prospective clinical trials, may lead to de-escalation or even omission of chemotherapy in the future. In the Netherlands, before the year 2000, node negativity was considered a favorable prognostic factor. In addition, in that era, node-positive, premenopausal breast cancer patients had an indication for adjuvant chemotherapy, whereas node-positive postmenopausal breast cancer patients were advised adjuvant endocrine therapy. Hormone receptor status was not yet incorporated in guiding the choice of adjuvant systemic therapy. By selecting a population-based cohort of young patients who are N0 before 2000, risk of indication bias was minimized.
METHODS
Patient Selection and Follow-Up Collection
Patients were selected from the population-based PARADIGM cohort. The methods on patient selection, follow-up collection, and pathology review have been published previously.13 In short, PARADIGM includes women selected from the prospective Netherlands Cancer Registry (NCR), which has more than 95% nationwide coverage. Patients were under age 40 years when diagnosed with N0, primary invasive breast cancer between 1989 and 2000. They had undergone locoregional treatment only, including adequate axillary surgery, according to standard practice at the time of diagnosis, ie, they had not received any (neo)adjuvant systemic treatment. We excluded patients with a prior malignancy, no information on tumor size, or no tumor tissue available (Fig 1).
FIG 1.
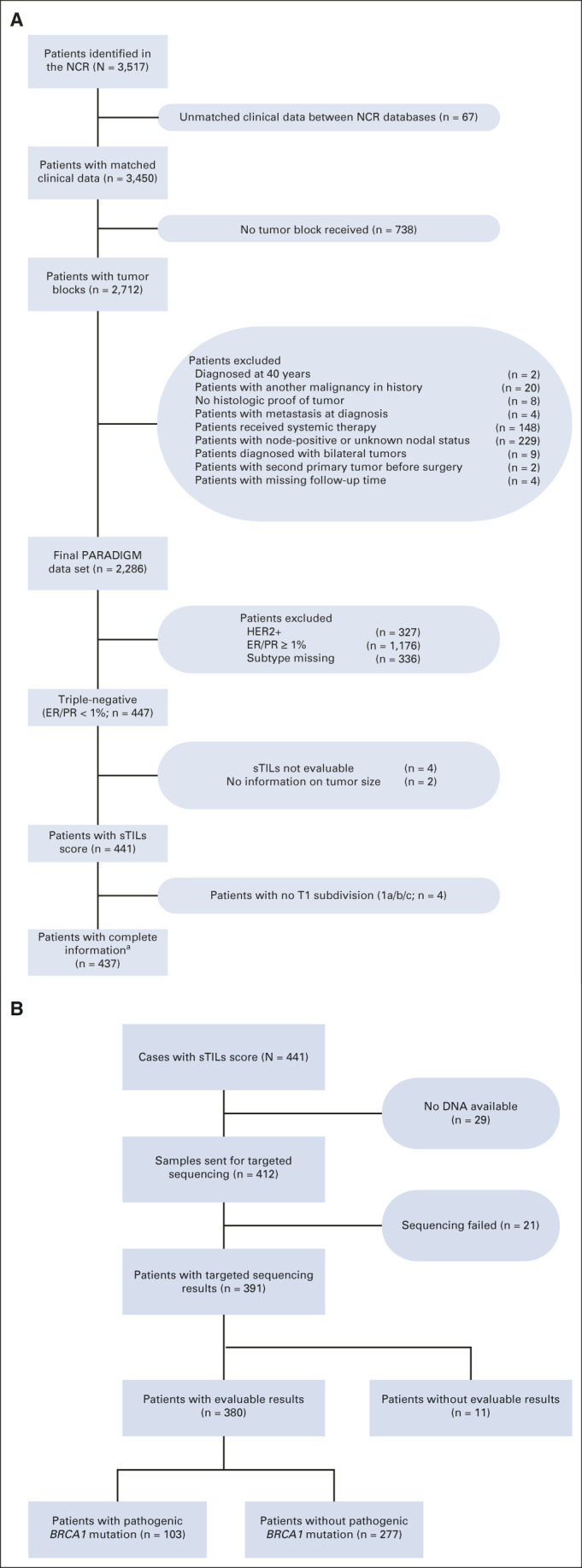
(A) CONSORT diagram of all patients included and excluded in the PARADIGM cohort, focusing on patients with sTILs information and tumor BRCA1 status. For 336 patients with a missing subtype, at least one of ER, PR, or HER2 scores was missing. For TNBC, ER-negative and PR-negative are defined with a < 1% expression. aFor all analyses where T stage was used, 437 patients were included. bFor the analyses with tumor BRCA1 mutation status, 380 patients were used. (B) CONSORT diagram for tumor BRCA1 testing. ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; NCR, Netherlands Cancer Registry; PR, progesterone receptor; sTILs, stromal tumor-infiltrating lymphocytes; T stage, tumor stage; TNBC, triple-negative breast cancer.
Information on (loco)regional recurrence, distant metastasis, and incidence of second primary malignancies was collected from individual hospital records (date of last follow-up: June 1, 2014). Survival data were collected through linkage with the municipality population register.13
This study was approved by the institutional review board of the Netherlands Cancer Institute.
Pathology Review and sTILs Evaluation
Tumor blocks with corresponding pathology reports were retrieved using PALGA (the nationwide network and registry of histo- and cytopathology in the Netherlands), and fresh slides were cut for hematoxylin and eosin (H&E) staining.14 These H&E slides were digitalized (Philips ultrafast scanner 1.6.1.3 RA [Philips, Amsterdam, the Netherlands] or NanoZoomer XR C12000-21/−22 [Hamamatsu photonics, Hamamatsu, Shizuoka, Japan]) and uploaded to the trait Enhanced Pathology Image Sharing platform.13 Breast cancer pathologists were blinded to clinicopathologic data and reassessed tumor characteristics (tumor cell percentage, morphology, histologic grade, and lymphovascular invasion).13 Tissue microarrays (TMAs) were constructed (TMA Grandmaster; 3DHistech, Budapest, Hungary), consisting of three 0.6-mm cores per patient. The TMAs were stained for ER, PR, and HER2 (Ventana BenchMark ULTRA; Ventana Medical Systems, Basel, Switzerland). In addition, for all patients, a HER2 silver in situ hybridization was performed. Tumors were characterized as HER2-negative when immunohistochemistry (IHC) 0/1+ or IHC 2+ and silver in situ hybridization–negative/equivocal. For the main analyses, patients with an ER/PR expression < 1% were considered ER-negative/PR-negative.
We evaluated sTILs on whole slides according to internationally established guidelines, by one trained pathologist.9 In brief, the relative proportion of stromal area to tumor area was determined from the pathology slide of a given tumor region. TILs were reported for the stromal compartment. The denominator used to determine the percent sTILs was the area of stromal tissue (the area occupied by mononuclear inflammatory cells over the total intratumoral stromal area) rather than the number of stromal cells (the fraction of total stromal nuclei that represent mononuclear inflammatory cell nuclei). This method has been demonstrated to be reproducible among trained pathologists.15,16 Scoring was performed in an online environment, blinded to clinical outcome data.17
sTILs were evaluated and grouped into three categories: low (< 30%), intermediate (30% to < 75%), and high (≥ 75%). We based these cutoffs on previous research on systemically untreated patients and a study that reports a high concordance between pathologists for the 30% and 75% cutoffs.11,15
Tumor BRCA1 Mutation Analysis
Tumor DNA was extracted according to our local protocol (Data Supplement, online only). Sequencing was performed at Agilent (Carpinteria, CA), using an Illumina NextSeq (Illumina, San Diego, CA). Samples with a (likely) pathogenic (class 4/class 5) variant were considered tumor BRCA1–mutated (tBRCA1m). All other samples were considered tumor BRCA1 wild-type (tBRCA1wt).
Statistical Analysis
Descriptive statistics were performed to summarize sTILs and clinicopathologic characteristics. Associations between continuous sTILs and clinicopathologic characteristics were investigated using Kruskal-Wallis, Wilcoxon rank-sum, or Jonckheere-Terpstra trend tests. The primary study end point was overall survival (OS), defined as the time from diagnosis to death from any cause. Kaplan-Meier curves were used to visualize OS by sTILs category, tumor stage (T stage), and tumor BRCA1 status. Multivariable Cox regression was used to estimate hazard ratios (HRs) for sTILs, adjusted for clinicopathologic characteristics. The prognostic value of sTILs was tested using a likelihood ratio test between a model with only clinicopathologic factors and a model with clinicopathologic factors plus sTILs. Schoenfeld residuals were used to test the proportionality assumption; no violations occurred. Using restricted cubic splines, we assessed the linearity of continuous sTILs. The secondary end point was distant metastasis-free survival (DMFS), analyzed with a competing risk model. Events of interest were distant metastasis or death from any cause, with second primary malignancies as competing events. Cumulative incidence functions were used to estimate incidences for distant metastasis or death by sTILs categories, T stage, and tumor BRCA1 status. The Fine and Gray method was used to estimate the subdistribution HRs (sHR) of sTILs adjusted for clinicopathologic characteristics. For all end points, patients at risk were censored at a 15-year follow-up. We defined ultralow risk, with the same bounds as in our funding request, as an OS ≥ 94% at a 10-year follow-up with the lower bound of the 95% CI ≥ 92%.
Only P values for analyses concerning sTILs were reported with two-sided values < .05 considered as statistically significant. Statistical analyses were performed using R version 4.0 (R Core Team, Vienna, Austria).18
RESULTS
Study Population
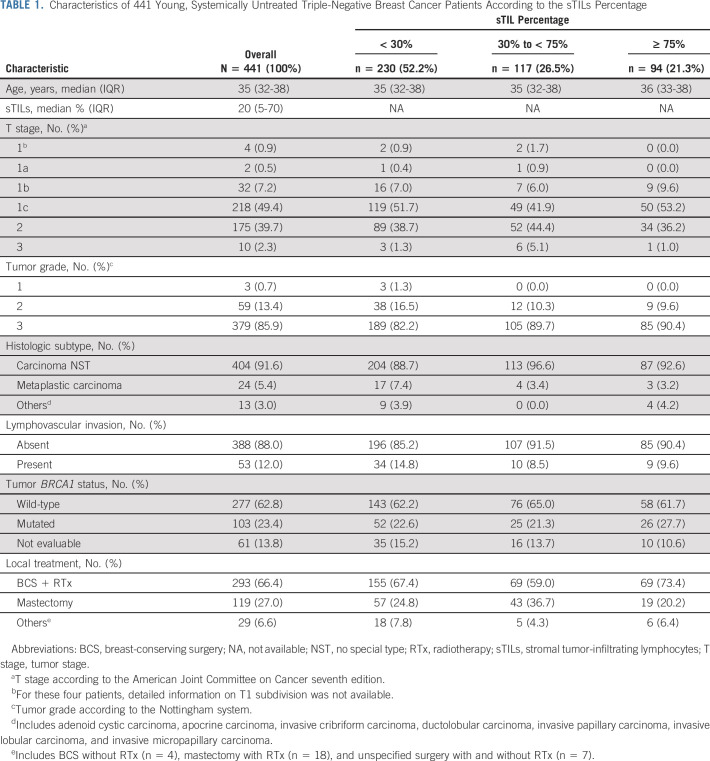
We identified 441 TNBC patients with ER/PR< 1% and known sTILs status (Fig 1). Patient characteristics according to the sTILs percentage are shown in Table 1. The median age at diagnosis was 35 years, 49.4% of tumors were T1c, 85.9% were histologic grade 3, and 66.4% of the patients underwent breast-conserving therapy with radiotherapy (Table 1).
TABLE 1.
Characteristics of 441 Young, Systemically Untreated Triple-Negative Breast Cancer Patients According to the sTILs Percentage
Tumor BRCA1 Mutation
DNA was extracted from the tumor tissue of 412 patients. For 380 of 412 patients (92.2%), DNA was of sufficient quality to generate targeted sequencing results. Of the 380 patients, 27.1% were tBRCA1m (Fig 1). Patients with BRCA1m tumors did not differ substantially from patients with tBRCA1wt tumors regarding standard clinicopathologic factors (data not shown).
sTILs
sTIL results were available for 441 patients with a median score of 20%. About half of the patients had low sTILs (sTILs < 30%), 27% intermediate (sTILs 30%-75%), and 21% high sTILs (sTILs ≥ 75%). Increased sTILs percentages were associated with grade 3 tumors (P < .001), but not with the T stage (P = .82), histologic subtype (P = .06), age (P = .35), or tumor BRCA1 mutation status (P = .51; Data Supplement).
OS
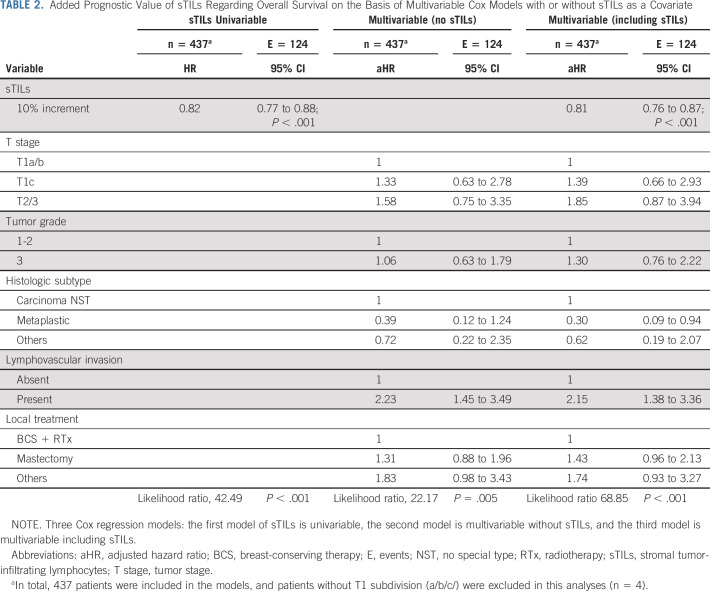
In total, 126 patients died during follow-up; eight patients were lost to follow-up and therefore censored. Figure 2 shows the Kaplan-Meier curves and 3-year, 10-year, and 15-year OS according to sTILs categories of all patients and split by T stage. Patients with ≥ 75% sTILs had a better prognosis compared with patients with < 30% sTILs (Fig 2A and Data Supplement). In the univariable Cox model, patients had a relative reduction of 18% in risk of death (HR, 0.82; 95% CI, 0.77 to 0.88) per 10% sTILs increment (Table 2). After adjustment for clinicopathologic variables, the relative reduction was 19% (adjusted HR, 0.81; 95% CI, 0.76 to 0.87). Adding sTILs to a Cox regression model with only clinicopathologic factors significantly increased the prognostic capabilities of the model (χ2 = 46.7, P < .001). There was no evidence of nonlinearity of the univariable sTILs model (P = .45; Data Supplement).
FIG 2.
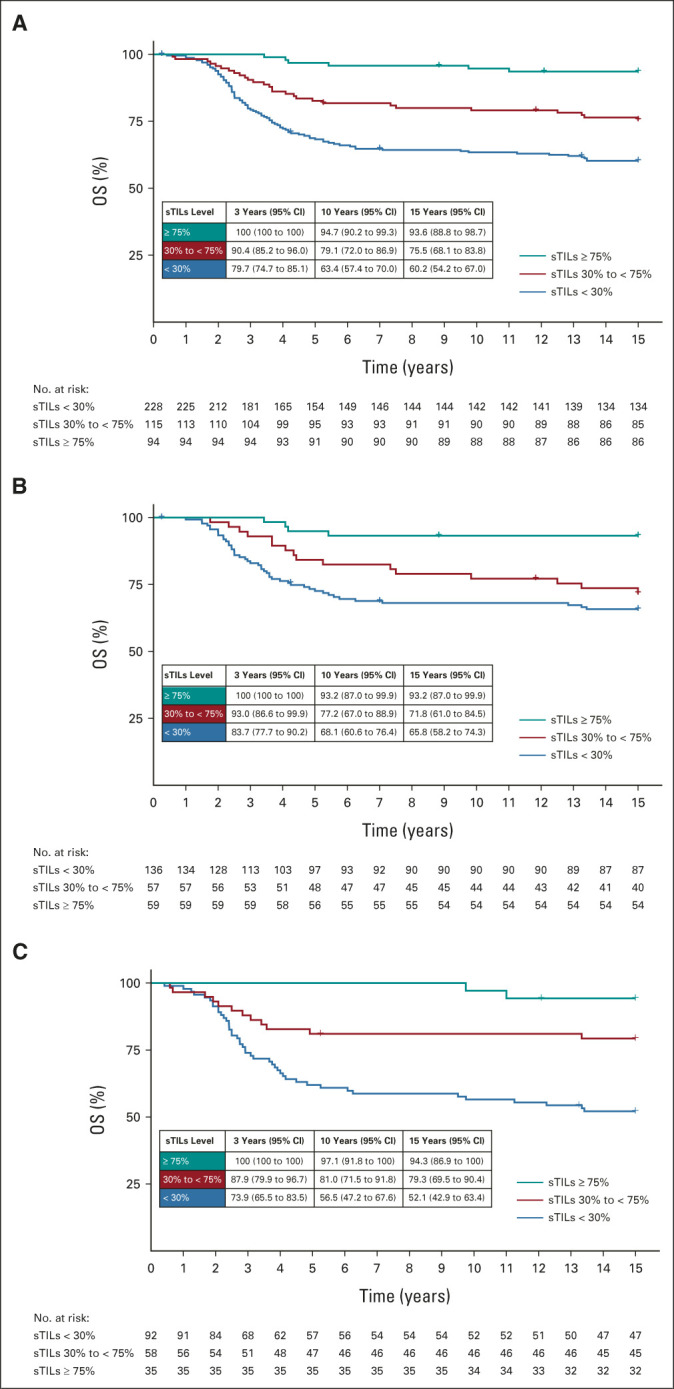
Kaplan-Meier curves for OS according to sTILs categories and T stage: (A) all patients, (B) patients with T1a/b/c tumors, and (C) patients with T2/3 tumors. OS, overall survival; sTILs, stromal tumor-infiltrating lymphocytes; T stage, tumor stage.
TABLE 2.
Added Prognostic Value of sTILs Regarding Overall Survival on the Basis of Multivariable Cox Models with or without sTILs as a Covariate
DMFS
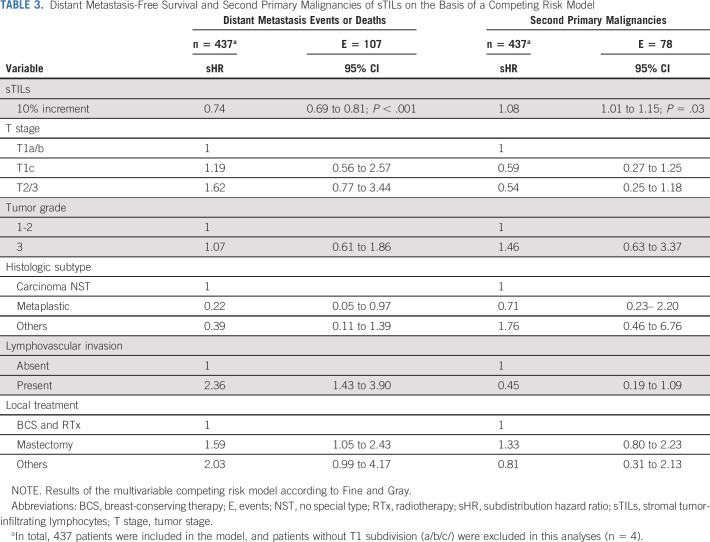
During a median follow-up of 15 years, 107 patients developed distant metastases or death, and 78 patients a second primary malignancy as the first event. Most second primaries concerned contralateral breast cancers (n = 57; Data Supplement). Figure 3 shows the cumulative incidence functions and 3-year, 10-year, and 15-year cumulative incidences of distant metastasis or death and of second primary malignancies according to sTILs categories for all patients and split by T stage. At 10 years, patients with high sTILs had lower cumulative incidence of distant metastasis or death (2.1%; 95% CI, 0 to 5.0) compared with patients with low sTILs (37.0%; 95% CI, 30.7 to 43.3; Fig 3A and Data Supplement). The 10-year cumulative incidence of second primary malignancy for patients with high sTILs was 16.0% (95% CI, 8.5 to 23.4), compared with 9.7% (95% CI, 5.8 to 13.6) for patients with low sTILs (Fig 3A). Results were similar for patients with T1 and T2/3 tumors (Figs 3B and 3C and Data Supplement). In a multivariable competing risk analysis, per 10% sTILs increment was associated with a decreased incidence of distant metastasis or death (sHR, 0.74; 95% CI, 0.69 to 0.81). On the other hand, per 10% sTILs increment was associated with a significantly increased incidence of second primary malignancies (sHR, 1.08; 95% CI, 1.01 to 1.15; Table 3).
FIG 3.
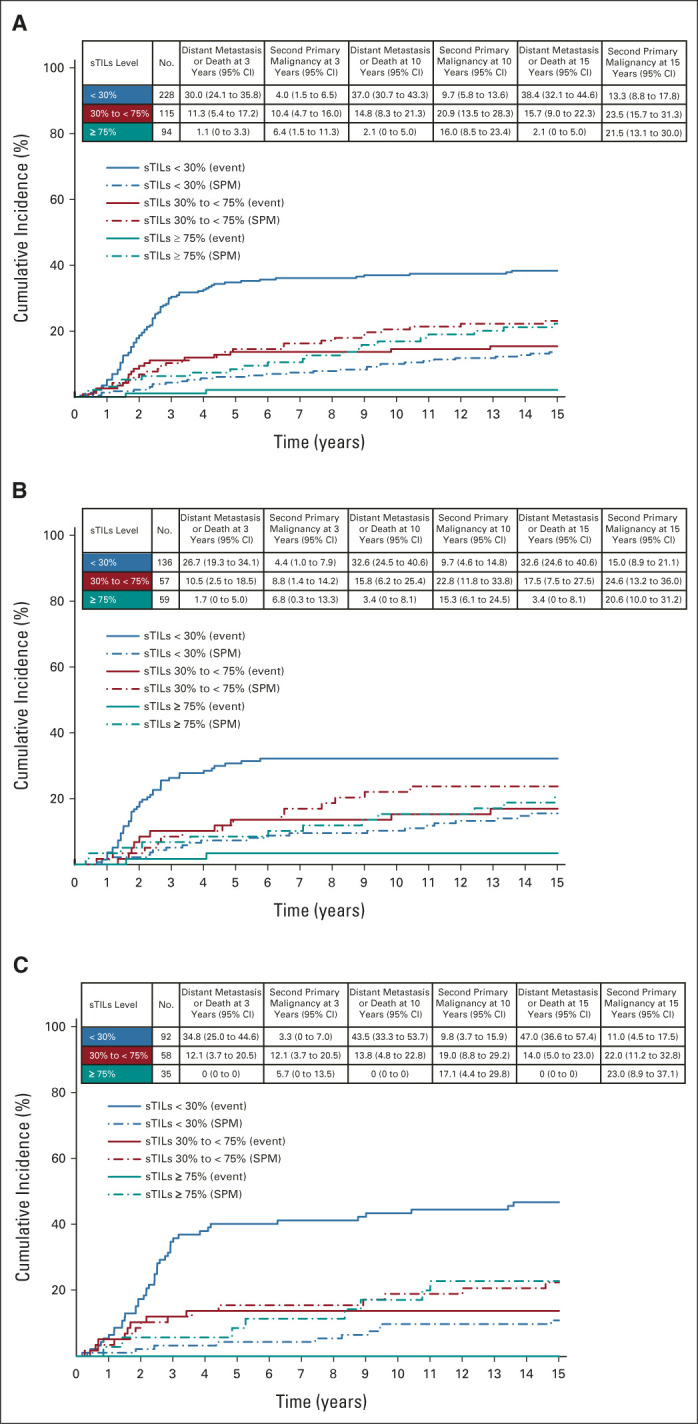
Cumulative incidence functions for distant metastasis or death and second primary malignancy according to sTILs categories and T stage: (A) all patients, (B) patients with T1a/b/c tumors, and (C) patients with T2/3 tumors. Solid lines represent incidence of distant metastasis or death, and dashed lines represent the incidence of competing event. Event, distant metastasis or death; SPM, second primary malignancy; sTILs, stromal tumor-infiltrating lymphocytes; T stage, tumor stage.
TABLE 3.
Distant Metastasis-Free Survival and Second Primary Malignancies of sTILs on the Basis of a Competing Risk Model
Tumor BRCA1 Status, sTILs, and Outcomes
Patients with tBRCA1m and high sTILs had better OS compared with those with low sTILs (10-year OS: 88.9%; 95% CI, 77.8 to 100 and 46.2%, 95% CI, 34.4 to 61.9, respectively; Data Supplement). Patients with high sTILs had a lower incidence of distant metastasis or death at 10 years compared with those with low sTILs (3.7%; 95% CI, 0 to 11.0; and 53.8%; 95% CI, 40.1 to 67.5, respectively). The 10-year cumulative incidence of second primary malignancy for patients with tBRCA1m and high sTILs was 44.4% (95% CI, 25.2 to 63.6), compared with 17.3% (95% CI, 6.7 to 27.9) for patients with tBRCA1m and low sTILs (Data Supplement). At 10 years, patients with tBRCA1wt and high sTILs had an excellent OS of 96.6% (95% CI, 92.0 to 100) and a 3.4% (95% CI, 0 to 8.1) incidence of second primary malignancy. However, for tBRCA1wt patients with low sTILs, 10-year OS was low (68.7%; 95% CI, 61.5 to 76.7) and cumulative incidence of distant metastasis or death was relatively high (31.3%; 95% CI, 23.7 to 38.9; Data Supplement).
Effect of sTILs on Staging
We investigated whether sTILs scoring influenced the prognosis according to the stage (American Joint Committee on Cancer [AJCC] 8th edition). Stage II patients with sTILs > 75% appeared to have a better prognosis than stage IB with sTILs < 30% (10-year OS, 97.1%; 95% CI, 91.5 to 100) versus 66.6% (95% CI, 58.5 to 75.7; Data Supplement).
DISCUSSION
We confirm the prognostic value of sTILs in young patients with early-stage N0 TNBC who are systemic therapy-naïve by taking advantage of a prospectively collected population-based cohort. Increasing sTILs are significantly associated with improved OS and DMFS. Patients with high sTILs (≥ 75%) had an excellent 10-year OS and a very low 10-year incidence of distant metastasis or death.
Our findings are consistent with previous reports showing improved outcomes in TNBC patients with high sTILs.10-12,19-22 Most studies, however, were performed in women treated with chemotherapy and included only a few young women with N0 disease.10,12,19-22 Of note, our study population consists solely of N0, systemic treatment–naïve women age < 40 years at diagnosis. Our OS results, however, are comparable with a study in chemotherapy-naïve patients, which included predominantly postmenopausal women.11 Distribution of sTILs between the two studies, however, differed considerably as we identified 52% of patients with TNBC with low (< 30%) sTILs compared with 71% in the study by Park et al.11 The difference in sTILs distribution is also observed when compared with other publications.10,12,19-22 Fewer patients with low sTILs in our study could be due to younger age at diagnosis and no involved axillary lymph nodes. Recent studies showed that younger patients with TNBC more often have high sTILs tumors when compared with older patients and that there is an inverse correlation between sTILs levels and the number of positive lymph nodes.12,23 Moreover, the sTILs distribution in our study was in line with the sTILs distribution in the young patient subgroup of the study reported by Aine et al.23 One explanation might be the changing composition and function of immune cells with age.24 Further research is needed to increase our understanding of the interaction between the immune system, the hormonal system, age, and breast cancer. Another explanation may be the difference in tumor grade between younger and older patients. Younger patients tend to have higher-grade tumors, and these higher-grade tumors are associated with more sTILs.11,12,23,25
In our study, patients with high sTILs and tBRCA1wt had a 10-year OS of 96.6% and were considered ultralow risk according to the predefined end point. In patients diagnosed age < 50 years with ER-poor tumors, The Early Breast Cancer Trialists' Collaborative Group (EBCTCG) found a 25% reduction in death rate at 10 years for polychemotherapy compared with nil.26 Therefore, the added benefit of (neo)adjuvant chemotherapy in the high sTILs group may be limited and should be balanced against treatment-related morbidity.4,5,27 Of note, the EBCTCG did not evaluate whether adjuvant chemotherapy benefits differ between sTILs categories. Another unresolved question is whether the link between high sTILs and improved chemotherapy response is true regardless of the type of chemotherapeutic agent.22,25
Conversely, we identified clinically relevant high-risk patients on the basis of low sTILs (< 30%) independent of the tumor size. According to our data, patients with T1a/b tumors and low sTILs have a high cumulative incidence of distant metastasis or death. On the basis of current guidelines, patients with T1a/b tumors are considered low-risk and may forego adjuvant chemotherapy. Our data, however, suggest that these patients may not be low risk and should be considered candidates for (neo)adjuvant systemic therapy, and because of the small sample size, additional evidence from other studies is needed to confirm these findings.
In our study, T stage showed limited prognostic power for OS or DMFS, which may be explained by a relatively small sample size. In our cohort, the limited prognostic value shown for tumor size suggests that sTILs can upgrade or downgrade clinicopathologic staging in TNBC; patients with stage II disease (AJCC 8th edition) and high sTILs have a better outcome than patients with stage Ib with low sTILs. When sTILs were added to the multivariable regression model, the following variables seemed to retain some independent prognostic value: the presence of lymphovascular invasion and the histologic subtype. The presence of lymphovascular invasion suggested a poorer prognosis, as has been described before.28 Although not formally tested, our analyses indicated that young, patients with early-stage TNBC with a metaplastic carcinoma had a more favorable prognosis compared with women with carcinoma no special type. Previous studies have described a favorable prognosis for low-grade metaplastic carcinomas, but not for high-grade metaplastic carcinomas.29,30 However, since lymphovascular invasion and histologic subtype were not variables of interest in our multivariable regression models, the effects of these covariates might have been biased by some unmeasured confounders.31
We did not observe an association between tumor BRCA1 mutation status and sTILs quantity. We did, however, observe a difference in second primary malignancy incidence between the tBRCA1m and tBRCAwt groups. The cumulative incidence of contralateral breast cancer in the tBRCA1m group ranged between 19.2% and 60.0% at 15 years, depending on the sTILs category, and these findings are in line with earlier reports.32,33 We also identified a remarkable difference in second primary tumor incidence between patients with high and low sTILs, especially in the tBRCA1m group. We hypothesize that tBRCA1m patients with high sTILs have more second primary tumors because of their better survival outcomes and hence longer at-risk time.
The strength of our analyses is the unique population-based cohort of systemically untreated, young, early-stage breast cancer patients with high-quality clinical data, collected in a standardized manner. Since guidelines at the time of diagnosis exempted patients who were N0 from systemic therapy, indication bias is virtually absent in this study. This cohort is, therefore, very suitable to investigate prognostic biomarkers. Another strength is that a standardized sTILs scoring method has been used with a high concordance between pathologists, similar to the one reported for HER2-negative and hormone receptor scoring.15,34 Moreover, sTILs scoring in our study was performed blinded to clinical outcomes.
However, our study has some limitations. First, we used tumor BRCA1 mutation status instead of germline status. Nonetheless, on the basis of the literature, we expect at least 80% of the tumor BRCA1 mutations to be germline although no studies were published specifically for women under age 40 years.35,36 Second, in the Netherlands, BRCA1 germline testing was not regularly performed in young patients with breast cancer during the 1990s. As a result, mutation carriers might have gone unnoticed and therefore did not receive prophylactic surgery.37,38 Survival of patients with tBRCA1m-associated tumors in our study may consequently be worse than it would be nowadays with screening programs and preventive strategies available for germline BRCA1 mutation carriers.39 Since information on mode of detection was lacking, we cannot answer whether sTILs carry differential prognostic information in screen-detected versus symptomatic TNBC. Finally, (neo)adjuvant systemic therapy for a second (breast) cancer or locoregional recurrence might have affected OS. Unfortunately, consistent information on subsequent treatments was unavailable.40,41
sTILs as a prognostic biomarker have some clear advantages above other (new) biomarkers. sTIL scoring is highly reproducible, with concordance rates of more than 0.90 for the 75% cutoff and more than 0.80 for the 30% cutoff.15 In addition, pathologists can be trained to score sTILs easily (freely available educational resources are available on International TILS Working Group),42 and it is inexpensive as the diagnostic H&E slide is used. The assessment of genomic biomarkers and PDL1-IHC is expensive, laborious, and not always easily implementable in low-to-middle income countries.
In conclusion, we found that young (< 40 years) patients with N0 TNBC with high sTILs (≥ 75%) have an excellent prognosis. These data could be used as a starting point for designing a randomized controlled chemotherapy de-escalation trial. The current study confirms the importance of sTILs as a valuable addition to the set of standard prognostic factors in patients with TNBC.
ACKNOWLEDGMENT
The authors would like to acknowledge our deceased colleague Prof Dr Jan G. van den Tweel for his contribution to this project. The authors thank the registration team of the Netherlands Comprehensive Cancer Organisation (IKNL) for the collection of data for the Netherlands Cancer Registry. Furthermore, the authors would like to thank the Dutch Pathology Registry and PALGA for providing the histopathological data and the FFPE tissues and the NKI-AVL Core Facility Molecular Pathology & Biobanking for the help with biobanking and laboratory support. Finally, the authors would like to thank CTMM-TraIT and Slide Score for their IT infrastructure.
Nikolas Stathonikos
Research Funding: Pfizer (Inst)
Zsuzsanna Varga
Consulting or Advisory Role: Roche
Carolien H.M. van Deurzen
Research Funding: AstraZeneca/Daiichi Sankyo (Inst)
Stefan M. Willems
Consulting or Advisory Role: Roche (Inst)
Speakers' Bureau: Roche (Inst)
Research Funding: Roche (Inst), Pfizer (Inst), Bayer (Inst), MSD (Inst), AstraZeneca/Merck (Inst), Amgen (Inst), Amgen (Inst)
Jelle Wesseling
Research Funding: Cancer Research UK (Inst), KWF Dutch Cancer Society (Inst)
Ales Ryska
Consulting or Advisory Role: MSD Oncology, Amgen, Roche, AstraZeneca/Daiichi Sankyo, Bristol Myers Squibb/Pfizer
Speakers' Bureau: Amgen, Gilead Sciences, Roche, Bristol Myers Squibb/Celgene
Sherene Loi
Consulting or Advisory Role: Roche/Genentech (Inst), Aduro Biotech (Inst), Novartis (Inst), G1 Therapeutics (Inst), PUMA Biotechnology (Inst), GlaxoSmithKline (Inst), AstraZeneca (Inst), Seattle Genetics (Inst), BMS (Inst), Silverback Therapeutics (Inst), Pfizer (Inst), Gilead Sciences (Inst), Gilead Sciences (Inst), Daiichi Sankyo/Lilly (Inst), Tallac Therapeutics (Inst)
Research Funding: Roche/Genentech (Inst), Novartis (Inst), Merck (Inst), Puma Biotechnology (Inst), Bristol Myers Squibb (Inst), Seattle Genetics (Inst), AstraZeneca (Inst), Nektar (Inst), Lilly (Inst)
Other Relationship: Roche Medical writing support
Stefan Michiels
Consulting or Advisory Role: IDDI, Sensorion, Biophytis, Servier, Yuhan, Amaris Consulting, Roche
Gabe S. Sonke
Consulting or Advisory Role: Novartis (Inst), Seattle Genetics (Inst), Biovica (Inst)
Research Funding: Merck Sharp & Dohme (Inst), Agendia (Inst), AstraZeneca/Merck (Inst), Roche (Inst), Novartis (Inst)
Paul J. van Diest
Patents, Royalties, Other Intellectual Property: DDX3 as a biomarker for cancer and methods related thereto (Inst)
Marleen Kok
Consulting or Advisory Role: Bristol Myers Squibb/Medarex (Inst), Roche (Inst), MSD (Inst), AZ/Daiichi (Inst)
Research Funding: Bristol Myers Squibb (Inst), Roche (Inst), AstraZeneca/MedImmune (Inst)
Roberto Salgado
Consulting or Advisory Role: Roche, AstraZeneca, BMS
Research Funding: Merck, Puma Biotechnology, Roche
Travel, Accommodations, Expenses: Roche, Merck
Sabine C. Linn
Consulting or Advisory Role: Daiichi Sankyo (Inst)
Research Funding: Genentech/Roche (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst), Tesaro (Inst), Merck (Inst), Immunomedics (Inst), Eurocept Pharmaceuticals (Inst), Agendia (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Daiichi Sankyo Europe GmbH (Inst)
No other potential conflicts of interest were reported.
DISCLAIMER
None of the funders had any influence on study design; data collection; and/or project management; data analysis and interpretation; or manuscript preparation, review, or approval.
PRIOR PRESENTATION
Presented at the virtual European Society for Medical Oncology Annual Meeting, September 20, 2020.
SUPPORT
Supported by grants from The Netherlands Organization for Health Research and Development (Project number 836021019), A Sister's Hope, De Vrienden van UMC Utrecht, Agilent Technologies Inc, and the Dutch Cancer Society (KWF) 11655/2018-1. Supported by [Z]aan de Wandel. Roberto Salgado was supported by the Breast Cancer Research Foundation (BCRF, grant No. 17-194).
V.M.T.d.J. and Y.W. contributed equally to this work as first author; G.M.H.E.D., R.S., and S.C.L. contributed equally to this work as last author.
DATA SHARING STATEMENT
The data that support the findings of this study are available from the Netherlands Cancer Registry, hosted by the Netherlands Comprehensive Cancer Center (IKNL), but restrictions apply to the availability of these data, which were used under license for the current study. Data are available from the authors upon reasonable request and with permission of The Netherlands Comprehensive Cancer Center (IKNL).
AUTHOR CONTRIBUTIONS
Conception and design: Vincent M.T. de Jong, Mark Opdam, Michael Hauptmann, Annegien Broeks, Sherene Loi, Paul J. van Diest, Marjanka K. Schmidt, Marleen Kok, Gwen M.H.E. Dackus, Roberto Salgado, Sabine C. Linn
Financial support: Marjanka K. Schmidt, Sabine C. Linn
Administrative support: Nikolas Stathonikos, Sabine C. Linn
Provision of study materials or patients: Nikolas Stathonikos, Antien L. Mooyaart, Alicia Córdoba, Stefan M. Willems, Annegien Broeks, Elsken van der Wall, Gwen M.H.E. Dackus, Roberto Salgado
Collection and assembly of data: Vincent M.T. de Jong, Natalie D. ter Hoeve, Mark Opdam, Nikolas Stathonikos, Sten Cornelissen, Willem Vreuls, Efraim H. Rosenberg, Esther A. Koop, Carolien H.M. van Deurzen, Antien L. Mooyaart, Emma J. Groen, Joost Bart, Jelle Wesseling, Anna Sapino, Ewa Chmielik, Ales Ryska, Annegien Broeks, Sabine Siesling, Gwen M.H.E. Dackus
Data analysis and interpretation: Vincent M.T. de Jong, Yuwei Wang, Mark Opdam, Katarzyna Jóźwiak, Michael Hauptmann, Efraim H. Rosenberg, Zsuzsanna Varga, Carolien H.M. van Deurzen, Antien L. Mooyaart, Alicia Córdoba, Joost Bart, Stefan M. Willems, Jelle Wesseling, Ales Ryska, Adri C. Voogd, Sherene Loi, Stefan Michiels, Gabe S. Sonke, Elsken van der Wall, Sabine Siesling, Paul J. van Diest, Marjanka K. Schmidt, Marleen Kok, Gwen M.H.E. Dackus, Roberto Salgado, Sabine C. Linn
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Prognostic Value of Stromal Tumor-Infiltrating Lymphocytes in Young, Node-Negative, Triple-Negative Breast Cancer Patients Who Did Not Receive (neo)Adjuvant Systemic Therapy
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Nikolas Stathonikos
Research Funding: Pfizer (Inst)
Zsuzsanna Varga
Consulting or Advisory Role: Roche
Carolien H.M. van Deurzen
Research Funding: AstraZeneca/Daiichi Sankyo (Inst)
Stefan M. Willems
Consulting or Advisory Role: Roche (Inst)
Speakers' Bureau: Roche (Inst)
Research Funding: Roche (Inst), Pfizer (Inst), Bayer (Inst), MSD (Inst), AstraZeneca/Merck (Inst), Amgen (Inst), Amgen (Inst)
Jelle Wesseling
Research Funding: Cancer Research UK (Inst), KWF Dutch Cancer Society (Inst)
Ales Ryska
Consulting or Advisory Role: MSD Oncology, Amgen, Roche, AstraZeneca/Daiichi Sankyo, Bristol Myers Squibb/Pfizer
Speakers' Bureau: Amgen, Gilead Sciences, Roche, Bristol Myers Squibb/Celgene
Sherene Loi
Consulting or Advisory Role: Roche/Genentech (Inst), Aduro Biotech (Inst), Novartis (Inst), G1 Therapeutics (Inst), PUMA Biotechnology (Inst), GlaxoSmithKline (Inst), AstraZeneca (Inst), Seattle Genetics (Inst), BMS (Inst), Silverback Therapeutics (Inst), Pfizer (Inst), Gilead Sciences (Inst), Gilead Sciences (Inst), Daiichi Sankyo/Lilly (Inst), Tallac Therapeutics (Inst)
Research Funding: Roche/Genentech (Inst), Novartis (Inst), Merck (Inst), Puma Biotechnology (Inst), Bristol Myers Squibb (Inst), Seattle Genetics (Inst), AstraZeneca (Inst), Nektar (Inst), Lilly (Inst)
Other Relationship: Roche Medical writing support
Stefan Michiels
Consulting or Advisory Role: IDDI, Sensorion, Biophytis, Servier, Yuhan, Amaris Consulting, Roche
Gabe S. Sonke
Consulting or Advisory Role: Novartis (Inst), Seattle Genetics (Inst), Biovica (Inst)
Research Funding: Merck Sharp & Dohme (Inst), Agendia (Inst), AstraZeneca/Merck (Inst), Roche (Inst), Novartis (Inst)
Paul J. van Diest
Patents, Royalties, Other Intellectual Property: DDX3 as a biomarker for cancer and methods related thereto (Inst)
Marleen Kok
Consulting or Advisory Role: Bristol Myers Squibb/Medarex (Inst), Roche (Inst), MSD (Inst), AZ/Daiichi (Inst)
Research Funding: Bristol Myers Squibb (Inst), Roche (Inst), AstraZeneca/MedImmune (Inst)
Roberto Salgado
Consulting or Advisory Role: Roche, AstraZeneca, BMS
Research Funding: Merck, Puma Biotechnology, Roche
Travel, Accommodations, Expenses: Roche, Merck
Sabine C. Linn
Consulting or Advisory Role: Daiichi Sankyo (Inst)
Research Funding: Genentech/Roche (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst), Tesaro (Inst), Merck (Inst), Immunomedics (Inst), Eurocept Pharmaceuticals (Inst), Agendia (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Daiichi Sankyo Europe GmbH (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Johnson RH, Anders CK, Litton JK, et al. : Breast cancer in adolescents and young adults. Pediatr Blood Cancer 65:e27397, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tung N, Lin NU, Kidd J, et al. : Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J Clin Oncol 34:1460-1468, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists' Collaborative Group : Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: A patient-level meta-analysis of 37 298 women with early breast cancer in 26 randomised trials. Lancet 393:1440-1452, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poorvu PD, Frazier AL, Feraco AM, et al. : Cancer treatment-related infertility: A critical review of the evidence. JNCI Cancer Spectr 3:pkz008, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menning S, de Ruiter MB, Kieffer JM, et al. : Cognitive impairment in a subset of breast cancer patients after systemic therapy-results from a longitudinal study. J Pain Symptom Manage 52:560-569 e1, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Piccart M, van 't Veer LJ, Poncet C, et al. : 70-gene signature as an aid for treatment decisions in early breast cancer: Updated results of the phase 3 randomised MINDACT trial with an exploratory analysis by age. Lancet Oncol 22:476-488, 2021 [DOI] [PubMed] [Google Scholar]
- 7.Sparano JA, Gray RJ, Makower DF, et al. : Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med 379:111-121, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Champion VL, Wagner LI, Monahan PO, et al. : Comparison of younger and older breast cancer survivors and age-matched controls on specific and overall quality of life domains. Cancer 120:2237-2246, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salgado R, Denkert C, Demaria S, et al. : The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann Oncol 26:259-271, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams S, Gray RJ, Demaria S, et al. : Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 32:2959-2966, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JH, Jonas SF, Bataillon G, et al. : Prognostic value of tumor-infiltrating lymphocytes in patients with early-stage triple-negative breast cancers (TNBC) who did not receive adjuvant chemotherapy. Ann Oncol 30:1941-1949, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Loi S, Drubay D, Adams S, et al. : Tumor-infiltrating lymphocytes and prognosis: A pooled individual patient analysis of early-stage triple-negative breast cancers. J Clin Oncol 37:559-569, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dackus GM, Ter Hoeve ND, Opdam M, et al. : Long-term prognosis of young breast cancer patients (≤40 years) who did not receive adjuvant systemic treatment: Protocol for the PARADIGM initiative cohort study. BMJ Open 7:e017842, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casparie M, Tiebosch AT, Burger G, et al. : Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol 29:19-24, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kos Z, Roblin E, Kim RS, et al. : Pitfalls in assessing stromal tumor infiltrating lymphocytes (sTILs) in breast cancer. NPJ Breast Cancer 6:17, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denkert C, Wienert S, Poterie A, et al. : Standardized evaluation of tumor-infiltrating lymphocytes in breast cancer: Results of the ring studies of the international immuno-oncology biomarker working group. Mod Pathol 29:1155-1164, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Slide Score. www.slidescore.com
- 18.The R Foundation: The R Project for Statistical Computing. https://www.R-project.org/
- 19.Loi S, Michiels S, Salgado R, et al. : Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: Results from the FinHER trial. Ann Oncol 25:1544-1550, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Loi S, Sirtaine N, Piette F, et al. : Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 31:860-867, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Leon-Ferre RA, Polley MY, Liu H, et al. : Impact of histopathology, tumor-infiltrating lymphocytes, and adjuvant chemotherapy on prognosis of triple-negative breast cancer. Breast Cancer Res Treat 167:89-99, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denkert C, von Minckwitz G, Brase JC, et al. : Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol 33:983-991, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Aine M, Boyaci C, Hartman J, et al. : Molecular analyses of triple-negative breast cancer in the young and elderly. Breast Cancer Res 23:20, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fane M, Weeraratna AT: How the ageing microenvironment influences tumour progression. Nat Rev Cancer 20:89-106, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Boo L, Cimino-Mathews A, Lubeck Y, et al. : Tumour-infiltrating lymphocytes (TILs) and BRCA-like status in stage III breast cancer patients randomised to adjuvant intensified platinum-based chemotherapy versus conventional chemotherapy. Eur J Cancer 127:240-250, 2020 [DOI] [PubMed] [Google Scholar]
- 26.Early Breast Cancer Trialists' Collaborative Group : Adjuvant chemotherapy in oestrogen-receptor-poor breast cancer: Patient-level meta-analysis of randomised trials. Lancet 371:29-40, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Tachi T, Teramachi H, Tanaka K, et al. : The impact of outpatient chemotherapy-related adverse events on the quality of life of breast cancer patients. PLoS One 10:e0124169, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rakha EA, Martin S, Lee AH, et al. : The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer 118:3670-3680, 2012 [DOI] [PubMed] [Google Scholar]
- 29.WHO Classification of Tumours Editorial Board : Breast Tumours. Lyon, France, International Agency for Research on Cancer, 2019. pp 104-105 [Google Scholar]
- 30.Nelson RA, Guye ML, Luu T, et al. : Survival outcomes of metaplastic breast cancer patients: Results from a US population-based analysis. Ann Surg Oncol 22:24-31, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Westreich D, Greenland S: The table 2 fallacy: Presenting and interpreting confounder and modifier coefficients. Am J Epidemiol 177:292-298, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graeser MK, Engel C, Rhiem K, et al. : Contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol 27:5887-5892, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Metcalfe K, Lynch HT, Ghadirian P, et al. : Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J Clin Oncol 22:2328-2335, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Bueno-de-Mesquita JM, Nuyten DS, Wesseling J, et al. : The impact of inter-observer variation in pathological assessment of node-negative breast cancer on clinical risk assessment and patient selection for adjuvant systemic treatment. Ann Oncol 21:40-47, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Timms KM, Abkevich V, Hughes E, et al. : Association of BRCA1/2 defects with genomic scores predictive of DNA damage repair deficiency among breast cancer subtypes. Breast Cancer Res 16:475, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nik-Zainal S, Davies H, Staaf J, et al. : Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature 534:47-54, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van den Broek AJ, de Ruiter K, van 't Veer LJ, et al. : Evaluation of the Dutch BRCA1/2 clinical genetic center referral criteria in an unselected early breast cancer population. Eur J Hum Genet 23:588-595, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Copson ER, Maishman TC, Tapper WJ, et al. : Germline BRCA mutation and outcome in young-onset breast cancer (POSH): A prospective cohort study. Lancet Oncol 19:169-180, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baretta Z, Mocellin S, Goldin E, et al. : Effect of BRCA germline mutations on breast cancer prognosis: A systematic review and meta-analysis. Medicine (Baltimore) 95:e4975, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chao C, Bhatia S, Xu L, et al. : Incidence, risk factors, and mortality associated with second malignant neoplasms among survivors of adolescent and young adult cancer. JAMA Netw Open 2:e195536, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kassam F, Enright K, Dent R, et al. : Survival outcomes for patients with metastatic triple-negative breast cancer: Implications for clinical practice and trial design. Clin Breast Cancer 9:29-33, 2009 [DOI] [PubMed] [Google Scholar]
- 42.TILs Working Group. www.tilsinbreastcancer.org
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the Netherlands Cancer Registry, hosted by the Netherlands Comprehensive Cancer Center (IKNL), but restrictions apply to the availability of these data, which were used under license for the current study. Data are available from the authors upon reasonable request and with permission of The Netherlands Comprehensive Cancer Center (IKNL).