Abstract
PURPOSE
Resistance to immune checkpoint inhibition (ICI) in advanced non–small-cell lung cancer (NSCLC) represents a major unmet need. Combining ICI with vascular endothelial growth factor (VEGF)/VEGF receptor inhibition has yielded promising results in multiple tumor types.
METHODS
In this randomized phase II Lung-MAP nonmatch substudy (S1800A), patients ineligible for a biomarker-matched substudy with NSCLC previously treated with ICI and platinum-based chemotherapy and progressive disease at least 84 days after initiation of ICI were randomly assigned to receive ramucirumab plus pembrolizumab (RP) or investigator's choice standard of care (SOC: docetaxel/ramucirumab, docetaxel, gemcitabine, and pemetrexed). With a goal of 130 eligible patients, the primary objective was to compare overall survival (OS) using a one-sided 10% level using the better of a standard log-rank (SLR) and weighted log-rank (WLR; G[rho = 0, gamma = 1]) test. Secondary end points included objective response, duration of response, investigator-assessed progression-free survival, and toxicity.
RESULTS
Of 166 patients enrolled, 136 were eligible (69 RP; 67 SOC). OS was significantly improved with RP (hazard ratio [80% CI]: 0.69 [0.51 to 0.92]; SLR one-sided P = .05; WLR one-sided P = .15). The median (80% CI) OS was 14.5 (13.9 to 16.1) months for RP and 11.6 (9.9 to 13.0) months for SOC. OS benefit for RP was seen in most subgroups. Investigator-assessed progression-free survival (hazard ratio [80% CI]: 0.86 [0.66 to 1.14]; one-sided SLR, P = .25 and .14 for WLR) and response rates (22% RP v 28% SOC, one-sided P = .19) were similar between arms. Grade ≥ 3 treatment-related adverse events occurred in 42% of patients in the RP group and 60% on SOC.
CONCLUSION
This randomized phase II trial demonstrated significantly improved OS with RP compared with SOC in patients with advanced NSCLC previously treated with ICI and chemotherapy. The safety was consistent with known toxicities of both drugs. These data warrant further evaluation.
INTRODUCTION
First-line treatment of metastatic non–small-cell lung cancer (NSCLC) commonly includes inhibitors of programmed death 1 (PD-1), or its ligand, programmed death ligand 1 (PD-L1), alone or in combination with chemotherapy or cytotoxic T-lymphocyte–associated antigen 4 inhibition, for tumors with PD-L1 expression.1 However, tumor resistance ultimately develops and remains a major unmet need. Despite numerous clinical trials to date, no immune-oncology agent or combination has shown activity in this refractory setting.2 Combinations with immune checkpoint inhibitors are being evaluated in an attempt to restore sensitivity to immunotherapy.
CONTEXT
Key Objective
Resistance to immunotherapy develops in most advanced non–small-cell lung cancer (NSCLC) treated with immune checkpoint inhibition (ICI). Therapeutic strategies for these patients have been lacking. Vascular endothelial growth factor (VEGF) and its receptor modulate the tumor immune microenvironment, and combined ICI and VEGF/VEGF receptor therapy demonstrated benefit across multiple malignancies. This study evaluated ramucirumab and pembrolizumab, anti-vascular endothelial growth factor receptor 2, and anti–programmed death-1 therapy in advanced NSCLC after progression on prior ICI and platinum-based doublet chemotherapy using the Lung-MAP master protocol platform.
Knowledge Generated
Ramucirumab and pembrolizumab led to improved overall survival compared with standard of care in patients with advanced NSCLC previously treated with chemotherapy and immunotherapy with acquired resistance to prior ICI in this randomized phase II trial. Similar benefit was seen across subgroups.
Relevance
To our knowledge, this is the first trial in the ICI-acquired resistance setting to demonstrate potential survival benefit compared with standard of care including docetaxel and ramucirumab.
Vascular endothelial growth factor (VEGF) and VEGF receptor inhibitors are approved for multiple cancer indications.3 VEGF modulates the tumor immune microenvironment by enhancing tumor infiltration of immune cells and counteracting immunosuppression by myeloid-derived suppressor cells.4,5 Consequently, studies have evaluated immune checkpoint inhibitors combined with VEGF receptor inhibitors yielding significant clinical benefit in multiple tumor types,3 including advanced renal cell carcinoma (axitinib and pembrolizumab,6 axitinib and avelumab,7 cabozantinib and nivolumab,8 and lenvatinib and pembrolizumab9) compared with single-agent sunitinib, and lenvatinib and pembrolizumab in advanced endometrial cancer compared with chemotherapy.10 Additionally, bevacizumab and atezolizumab demonstrated clinical benefit in advanced hepatocellular carcinoma.11 A preliminary signal of activity with ramucirumab plus pembrolizumab (RP) was seen in a phase I study of untreated and previously treated NSCLC.12,13 IMPower150 provides additional support for immune checkpoint inhibition plus antiangiogenic therapies in NSCLC.14 It was the first trial to demonstrate improved progression-free survival (PFS) and overall survival (OS) with the combination of ICI and angiogenesis inhibition (bevacizumab) with chemotherapy for front-line advanced NSCLC.
S1800A, a substudy of Lung-MAP, evaluated RP versus standard of care in patients with stage IV or recurrent NSCLC after progression on prior ICI. Lung-MAP is a master protocol encompassing molecularly matched and nonmatched immunotherapy approaches for previously treated metastatic or recurrent NSCLC.15,16
METHODS
Lung-MAP Protocol and Biomarker Screening
Patients with pathologically proven stage IV or recurrent NSCLC were eligible to enroll in S1800A, a nonmatch substudy of Lung-MAP, if they had been screened by the original Lung-MAP screening protocol (S1400; ClinicalTrials.gov identifier: NCT03851445)15,16 or screened under the new Lung-MAP screening protocol (LUNGMAP; ClinicalTrials.gov identifier: NCT03971474) and were not eligible for any of the actively accruing biomarker-driven Lung-MAP substudies.
Patients
Patients must have received at least one line of anti–PD-1 or anti–PD-L1 (anti–PD-L1) therapy for stage III, IV, or recurrent disease and at most one line of anti–PD-L1 therapy for stage IV or recurrent disease, given sequentially or combined with platinum-based chemotherapy with disease progression at least 84 days after initiation of anti–PD-L1 therapy. Patients must have received platinum-based chemotherapy for stage IV/recurrent disease or for stage I-III with disease progression within 1 year from the last dose. Progression on prior therapy was based on investigator assessment. Exclusions included active autoimmune disease that required systemic treatment in the past 2 years, history of primary immunodeficiency, an immune-related adverse event, organ transplant that required use of immunosuppressives, and history of pneumonitis that required steroids or current pneumonitis/interstitial lung disease. Full eligibility criteria are given in the Protocol (online only).
Study Procedures and Treatment
The study was approved by an Independent Ethics Committee, and all patients provided written informed consent. Patients were randomly assigned to open label ramucirumab (10 mg/kg intravenous [IV]) plus pembrolizumab (200 mg IV) once every 21 days or investigator's choice standard-of-care (SOC) chemotherapy. Chemotherapy options were limited to docetaxel (75 mg/m2) IV; ramucirumab (10 mg/kg) plus docetaxel (75 mg/m2) IV once every 21 days; gemcitabine (1,000 mg/m2) IV on days 1 and 8 every 21 days; or for nonsquamous NSCLC patients only, pemetrexed (500 mg/m2) IV once every 21 days. Random assignment was done using a dynamic balancing algorithm stratifying by PD-L1 tumor status (< 1% v ≥1% or unknown), tumor histology (squamous v nonsquamous), and whether the planned treatment would include ramucirumab (yes v no) if randomly assigned to SOC. Treatment continued until disease progression as defined in RECIST 1.1, symptomatic deterioration, unacceptable toxicity, treatment delay for any reason > 84 days, or patient choice. Full information about guidance regarding treatment decisions is provided in the Protocol.
Tumor imaging was performed at baseline and every 6 weeks for the first year and then every 12 weeks until disease progression and discontinuation of protocol treatment. After off-protocol treatment following progression, laboratory tests and scans were required every 6 months for 2 years and then at the end of year 3. Adverse events were reported using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0.
Statistical Methods
The primary end point was OS, defined as the duration from random assignment to death due to any cause. OS was chosen as the primary end point because neither response nor PFS has been demonstrated to be a robust and reliable end point in the immunotherapy relapsed setting. The primary analysis was based on a one-sided testing at the 10% level using a modified intention-to-treat analysis including only eligible patients. As many studies evaluating immunotherapy in NSCLC appear to have a delayed separation in time-to-event curves which can result in nonproportional hazards, testing was performed using a standard stratified log-rank test and a weighted log-rank test with weights equal to 1-S(t), where S(t) is the pooled survival estimate at time t (G[rho = 0, gamma = 1]).17 The weighted test weights later events over earlier events and has more power than the standard log-rank test under a delayed separation in the curves. If either P value from the two tests was < .0972, the study would be considered to have rejected the null at the one-sided 10% level. The study design had an accrual goal of 130 eligible patients with the analysis when at least 90 deaths occurred. The study had 90% power to detect the scenario with overlapping curves up to 3 months and a hazard ratio (HR) of 0.5 after 3 months, assuming exponentially distributed survival times (piece-wise for the investigational arm), a median OS of 10.5 months in the SOC arm, and uniform accrual over 21-24 months. The study included two interim analyses evaluating early closure of accrual for futility. The first interim analysis was based on a single-arm assessment of response and disease control at 12 weeks among patients randomly assigned to RP when the first 18 eligible patients reached at least 24 weeks of follow-up. The second futility analysis took place when 50% of expected events (45 deaths) with at least 30 events with 3 months after random assignment were reported. The study was monitored by the SWOG Data and Safety Monitoring Committee.
Nominal P values are reported for secondary analyses. Secondary end points included investigator-assessed progression-free survival (IA-PFS) defined as the time from random assignment to the date of first progression, symptomatic deterioration, or death due to any cause. IA-PFS for patients last known to be alive without a report of progression, symptomatic deterioration, or death was censored at the date of last disease assessment. Best objective response was defined as complete, partial, unconfirmed complete, or unconfirmed partial response by RECIST 1.1. Patients not known to achieve a response were coded as nonresponders.
Survival distributions were estimated using the method of Kaplan-Meier (OS, PFS, and duration of response [DOR]). IA-PFS was compared using both the standard and weighted log-rank tests as described for OS. Treatment effects for time-to-event outcomes were summarized using a Cox proportional hazards model including the stratification factors and 80% CIs. Binary proportions were compared using a chi-squared test at the one-sided 5% level. Subgroup analyses were performed comparing OS and IA-PFS between the arms within the stratification factors (PD-L1 and histology), tumor mutational burden (TMB), and performance status (PS) using a Cox proportional hazards model.
RESULTS
Patients and Treatments
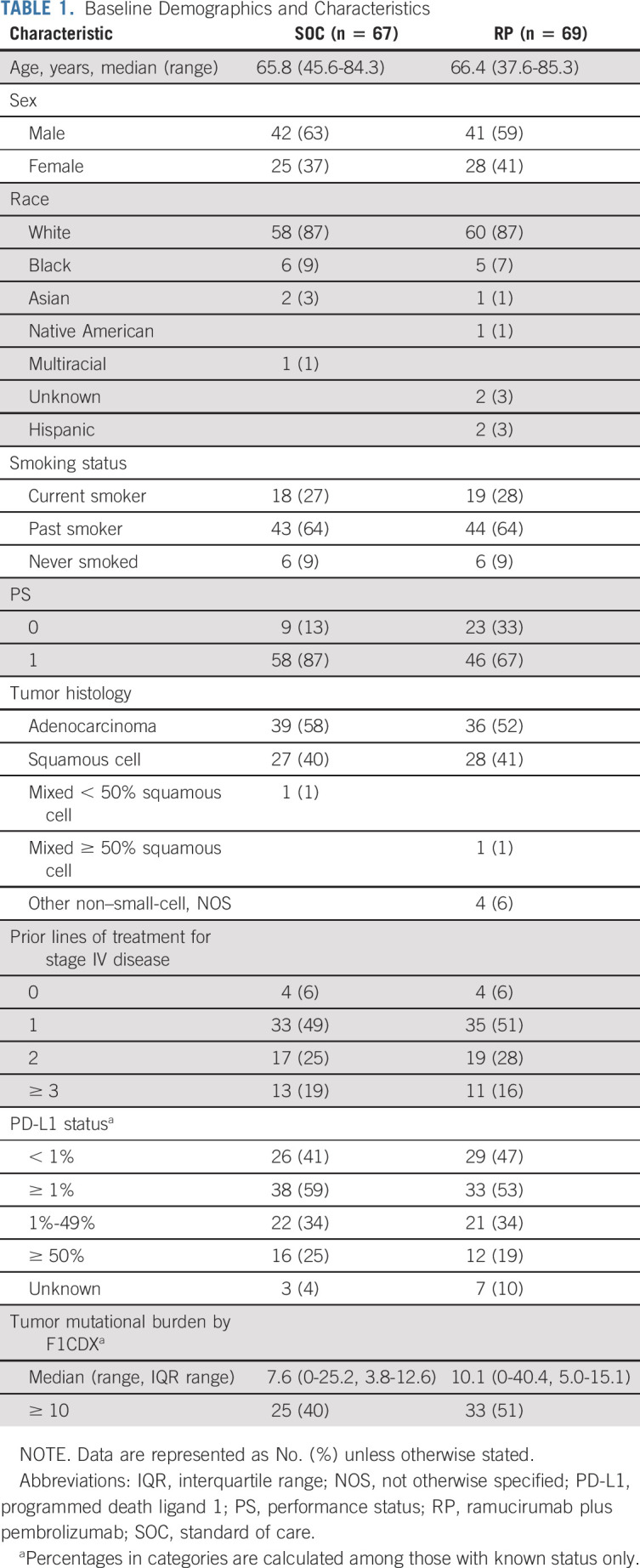
Between May 2019 and November 2020, 166 patients were randomly assigned to receive RP (n = 82) or SOC (n = 84) and 136 met eligibility (RP n = 69, SOC n = 67). The study CONSORT diagram is shown in Figure 1 and describes reasons for ineligibility in detail. Patient characteristics are described in Table 1. The median age of patients was 66 years (range, 38-85), and 61% were male. Most patients were current or former smokers (91%), and more patients with an Eastern Cooperative Oncology Group performance score 1 were in SOC versus RP arms (87% v 67%; Table 1). On the RP arm, of the 62 (90%) with known PD-L1 levels, 47%, 34%, and 19% had PD-L1 < 1%, 1%-49%, and ≥ 50%, respectively. For the SOC arm, of the 64 (96%) with known PD-L1 levels, 41%, 34%, and 25% had PD-L1 < 1%, 1%-49%, and ≥ 50%, respectively. Other patient baseline demographics and clinical characteristics were similar between the two treatment groups.
FIG 1.
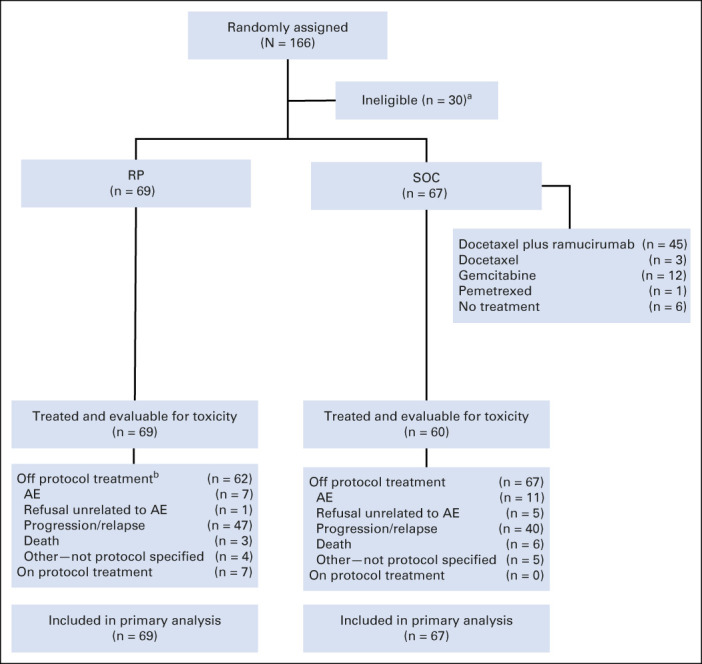
CONSORT diagram of patient disposition. aOf the 84 patients randomly assigned to the SOC arm, 17 patients were not eligible because of the following reasons: not progressing from platinum-based chemotherapy (four), not receiving or progressing from anti–PD-1/PD-L1 therapy per protocol-specified timeframe (two), permanent discontinuation of prior anti–PD-1/PD-L1 therapy because of toxicity (two), baseline scans for measurable disease not performed within the protocol timeframe (two), brain metastases requiring continued steroid treatment beyond the time of registration (two), not receiving and progressing on all SOC–targeted therapies for an oncogenic driver alteration, no measurable disease identified before registration, baseline blood pressure outside of protocol-specified range, receiving more than one line of anti–PD-1/PD-L1 therapy, and baseline scans for measurable disease not of diagnostic quality (one patient each). Of the 82 patients randomly assigned to the investigational arm, 13 patients were not eligible because of the following reasons: not receiving or progressing from anti–PD-1/PD-L1 therapy per protocol-specified timeframe (four), receiving more than one line of anti–PD-1/PD-L1 therapy (two), not progressing from platinum-based chemotherapy (two), no measurable disease identified before registration, receiving systemic therapy within 21 days before random assignment, not receiving platinum-based chemotherapy, receiving radiation therapy within 14 days before random assignmentand inadequate renal function, and receiving corticosteroids for brain metastasis within 7 days before random assignment (one patient each). bOf the 55 on the RP arm with reported progression, 41 (75%) went off-RP at the time of progression (PD), four (7%) discontinued treatment before PD, and 10 received treatment after PD. Of the 10, durations were four for < 1 month, two for 1-3 months, one for 3-6 months, and two 6-18 months, and one remains on treatment as of last follow-up at 2.1 months after PD. AE, adverse event; PD, progression of disease; PD-1, programmed death 1; PD-L1, programmed death ligand 1; RP, ramucirumab plus pembrolizumab; SOC, standard of care.
TABLE 1.
Baseline Demographics and Characteristics
Protocol Treatment
Among 67 eligible in the SOC arm, 45 (67%) received ramucirumab and docetaxel; 12 (18%) received gemcitabine; three (4%) received docetaxel; one (1%) received pemetrexed; and six (9%) did not receive therapy. Reasons patients did not receive therapy included withdrawal (2), symptomatic deterioration (2—hemorrhage from large occipital mass and dyspnea), disease status improvement, and death.
As of April 14, 2022, 129 patients (62 RP and 67 SOC) had gone off protocol treatment and seven patients on RP remained on study treatment. Treatment discontinuation reasons were progressive disease for 87 patients (47 RP; 40 SOC), adverse events for 18 (seven RP; 11 SOC), death for nine (three RP; six SOC), and not protocol specified for nine (four RP; five SOC). Three patients withdrew consent after treatment initiation (one RP, two SOC). No patients were lost to follow-up. Patients on RP received a median (range) of six (1-37) cycles of ramucirumab and six (0-35) cycles of pembrolizumab. Patients on SOC received a median (range) of five (1-27) cycles of ramucirumab, five (1-28) cycles of docetaxel (with or without ramucirumab), or 5.5 (1-19) cycles of gemcitabine. The one patient on pemetrexed received six cycles. Ten (14%) patients on the RP arm received study therapy beyond progression, with six for < 3 months and two for > 6 months.
Prior Treatment
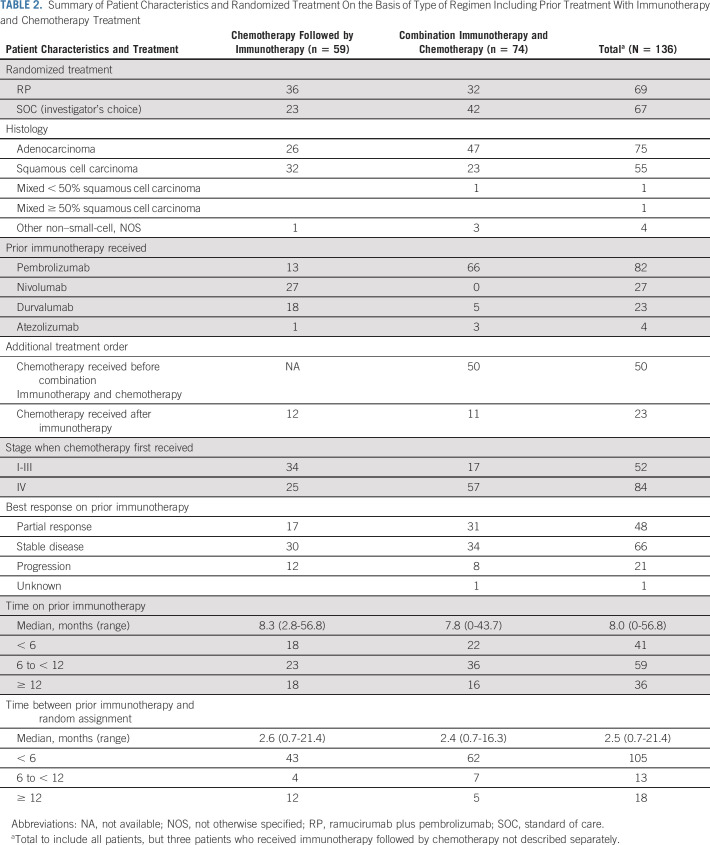
Of the 136 eligible patients, 74 (54%) previously received immunotherapy combined with platinum-based chemotherapy, 59 (43%) received platinum-based chemotherapy, followed by immunotherapy, and three (2%) received immunotherapy, followed by platinum-based chemotherapy (Table 2). Twenty-three patients received additional chemotherapy after their platinum-based chemotherapy and immunotherapy regimens; 50 patients received chemotherapy before combination immunotherapy and chemotherapy (16 for stage I-III disease and 34 for stage IV disease). Most patients received prior pembrolizumab (82, 60%), followed by nivolumab (27, 20%), durvalumab (23, 17%), and atezolizumab (four, 3%). Best response to prior immune checkpoint inhibitor-containing therapy was partial response for 48 (35%), stable disease for 66 (49%), progressive disease for 21 (15%), and unknown for one patient. The time between initiation of prior immunotherapy and progression for patients with progression as best response ranged between 3 and 14.7 months with a median (interquartile range) of 4.9 (3.8-7.1) months.
TABLE 2.
Summary of Patient Characteristics and Randomized Treatment On the Basis of Type of Regimen Including Prior Treatment With Immunotherapy and Chemotherapy Treatment
Toxicity
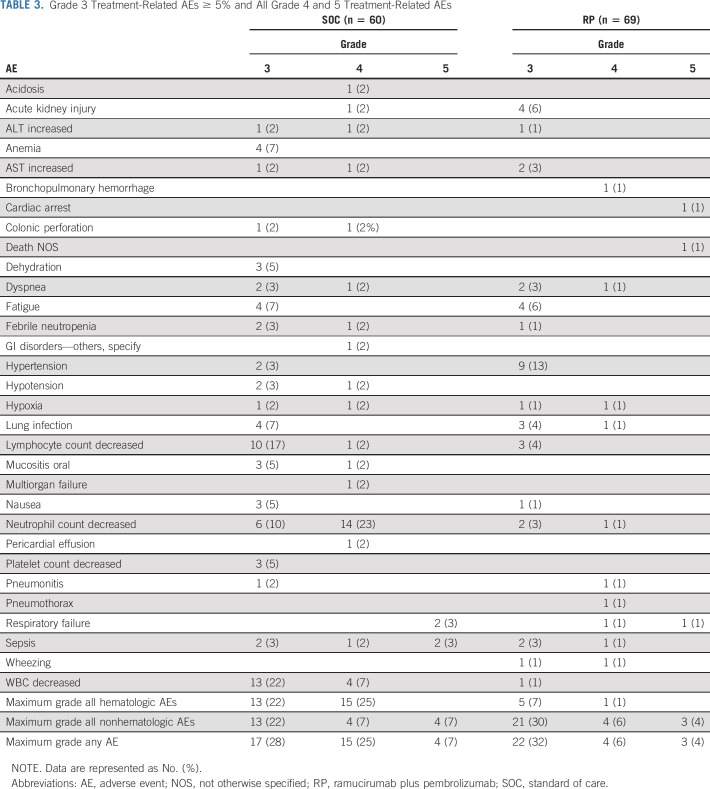
Grade 3-5 treatment-related adverse events for all grade 4 and 5 events and grade 3 events reported in at least 5% of patients are summarized in Table 3. Of 69 patients on RP assessed for adverse events, there were three treatment-related deaths: one due to cardiac arrest, one due to respiratory failure, and one where exact cause of death could not be determined. Additionally, four patients experienced treatment-related grade 4 events as the highest grade. Twenty-nine patients on RP experienced grade 3-5 adverse events, and nine (31%) were classified as immune-related adverse events (Appendix Table A1, online only) by the study chairs.
TABLE 3.
Grade 3 Treatment-Related AEs ≥ 5% and All Grade 4 and 5 Treatment-Related AEs
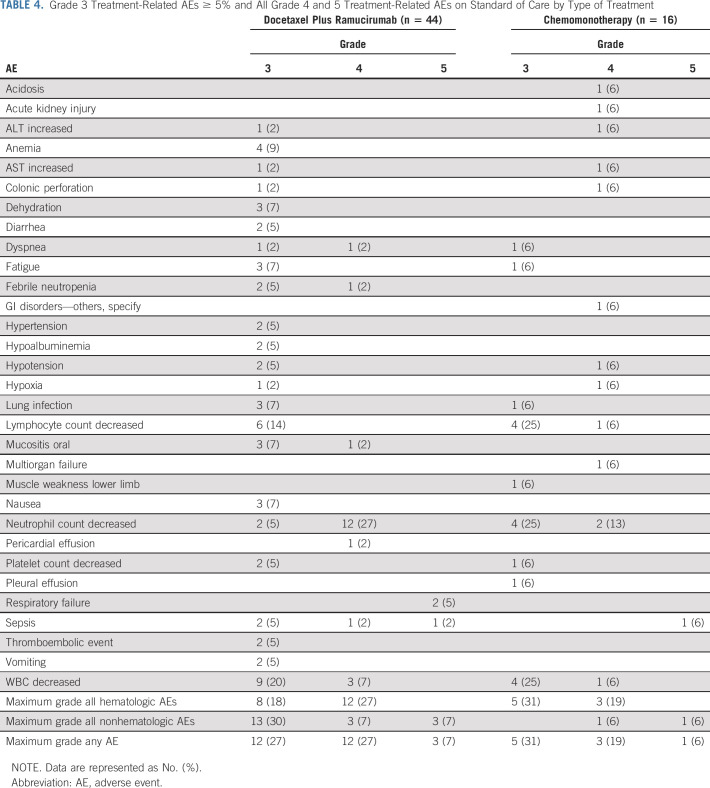
Of 60 patients on SOC assessed for adverse events (44 on docetaxel/ramucirumab and 16 on single-agent chemotherapy), there were four treatment-related deaths (three on docetaxel/ramucirumab and one single-agent chemotherapy): two due to sepsis (one docetaxel/ramucirumab) and two due to respiratory failure (both on docetaxel/ramucirumab). Additionally, 15 patients experienced treatment-related grade 4 events as their highest grade (12 of 15 on docetaxel/ramucirumab). The grade 4 adverse event listed as GI disorders–other was due to ischemic bowel. Table 4 describes the adverse events on SOC by type of treatment.
TABLE 4.
Grade 3 Treatment-Related AEs ≥ 5% and All Grade 4 and 5 Treatment-Related AEs on Standard of Care by Type of Treatment
OS
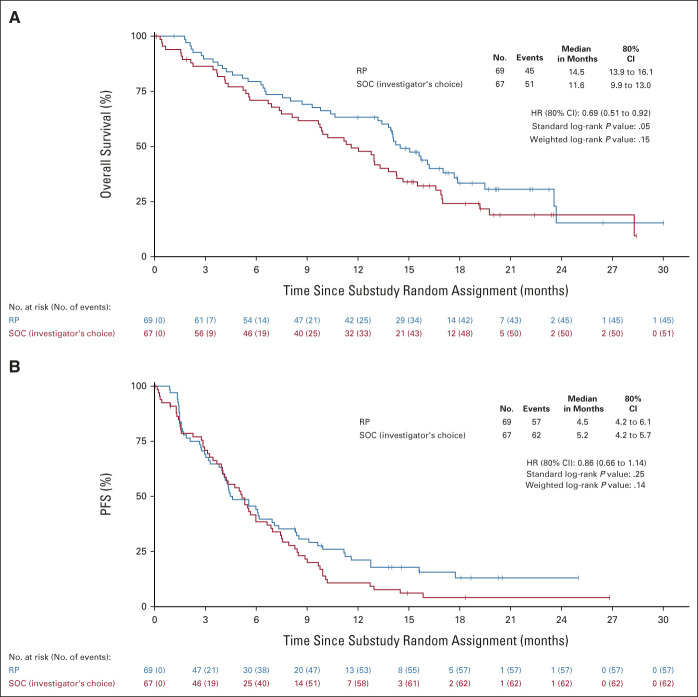
At the time of analysis, 96 deaths had been reported, and the median (range) of follow-up among those still alive (n = 40) was 17.9 months (1-30). OS was significantly longer with RP, with the one-sided P value from the standard log-rank test equal to .05 and .15 from the weighted log-rank test. RP reduced the risk of death by 31% (HR: 0.69 [80% CI, 0.51 to 0.92]; Fig 2A), and the median OS (80% CI) was 14.5 (13.9 to 16.1) months in this arm versus 11.6 (9.9 to 13.0) months in the SOC arm.
FIG 2.
(A) Overall survival and (B) PFS. P values from the standard log-rank test. HR, hazard ratio; PFS, progression-free survival; RP, ramucirumab plus pembrolizumab; SOC, standard of care.
Interpretation of subgroup analyses is limited by small sample sizes. The magnitude of OS benefit did not appear to differ by PD-L1 or TMB subgroups (Fig 3A). OS benefits were consistent across the majority of prespecified subgroups examined. Appendix Table A2 (online only) describes genomic alterations detected with next-generation sequencing as part of Lung-MAP screening.
FIG 3.
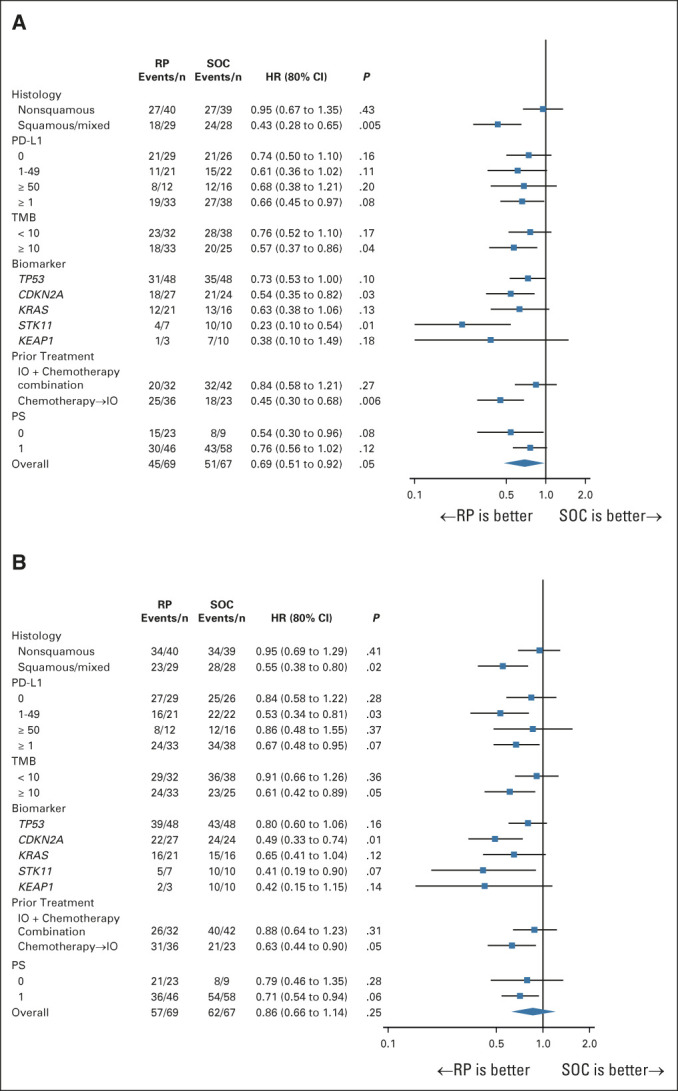
Subgroup analysis of (A) overall survival and (B) Progression-free survival. One-sided P values from the standard log-rank test. HR, hazard ratio; IO, immuno-oncology; PD-L1, programmed death ligand 1; PS, performance status; RP, ramucirumab plus pembrolizumab; SOC, standard of care; TMB, tumor mutational burden.
PFS
At the time of analysis, 119 PFS events had been reported. PFS was not significantly longer with RP, with the one-sided P value from the standard log-rank test equal to .25 and .14 from the weighted log-rank test (HR: 0.86 [80% CI, 0.66 to 1.14]; Fig 2B). The median PFS (80% CI) was 4.5 (4.2 to 6.1) months for RP and 5.2 (4.2 to 5.7) months in the SOC arm. Subgroup analyses were consistent with those for OS (Fig 3B).
Response and Disease Control
On RP, there were 12 confirmed partial responses and three unconfirmed partial responses for an objective response rate of 22% (15 of 69; 90% CI, 14 to 30). On SOC, there was one confirmed complete response, 13 confirmed partial responses, and five unconfirmed partial responses for an objective response rate of 28% (19 of 67; 90% CI, 19 to 37). Of the 19 responders on SOC, 18 received docetaxel and ramucirumab and one received gemcitabine. Thirty-seven patients on RP and 30 on SOC achieved stable disease as best response for a DCR of 75% (90% CI, 67 to 84) in the RP arm and 73% (90% CI, 64 to 82) in the SOC arm (P = .38). The median DOR (90% CI) was 12.9 (2.8 to not available) months for RP and 5.6 (4.6 to 7.8) months in the SOC arm. Eight and nine patients had a DOR ≥ 6 months on RP and SOC, respectively.
Postprotocol Treatment
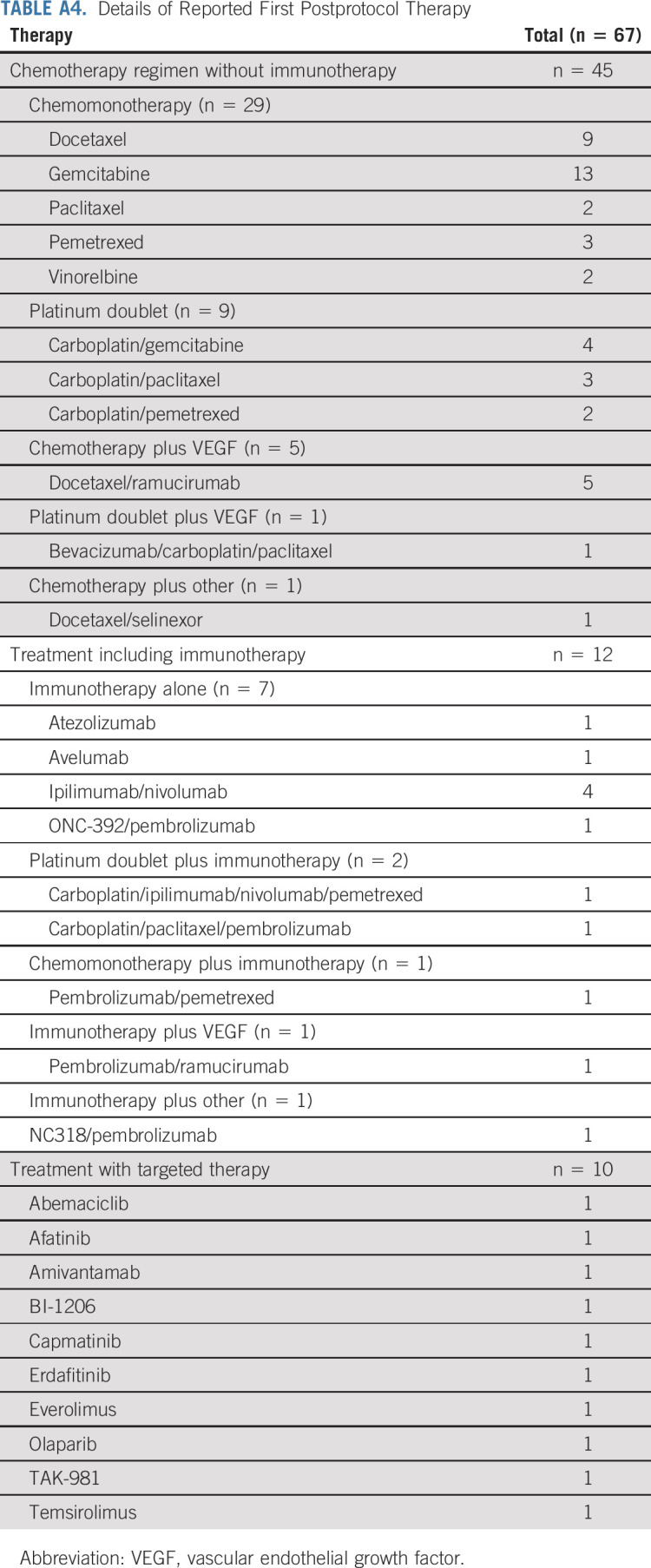
Sixty-seven patients had postprotocol systemic therapy reported with nine (five SOC, four RP) receiving treatment before progression and 58 (30 SOC, 28 RP) after progression on study. The type of postprotocol therapy and a description of the therapies are included in Appendix Table A3 (online only). Appendix Table A4 (online only) includes an extended description of post-treatment therapy.
DISCUSSION
S1800A represents a positive signal in immune checkpoint inhibitor-refractory cancers, arguably one of the greatest unmet needs in oncology. The rapid accrual of S1800A was facilitated by the unique Lung-MAP infrastructure. To our knowledge, this is the first trial for previously treated NSCLC without a chemotherapy backbone to demonstrate a potential survival benefit compared with SOC regimens including docetaxel and ramucirumab. The safety seen with RP was consistent with expected toxicities and fewer patients on RP versus SOC requiring treatment discontinuation because of adverse events.
Although SOC choice included single-agent chemotherapy, two-thirds of patients on SOC received docetaxel and ramucirumab. In the REVEL study, ramucirumab and docetaxel improved clinical outcomes compared with docetaxel alone in previous platinum-based doublet therapy treated, immunotherapy-naive, advanced NSCLC.18 A retrospective study evaluating docetaxel and ramucirumab after progressive disease on nivolumab suggested clinical benefit using a historical comparison.19 Together, this implies that most on SOC received the most active therapy available.
S1800A evaluated RP in patients who experienced disease progression at least 84 days after start of ICI, our definition of acquired resistance. Multiple trials are evaluating combination therapies in the acquired resistance setting, but a standardized definition has not been established.2 Definitions of acquired resistance are further complicated for combination ICI plus chemotherapy regimens in the frontline setting, where the component contributing to efficacy and resistance is not easily discerned.
Importantly, the OS hazard ratios for all subgroups were less than one and relatively consistent across PD-L1 expression and TMB levels. There was some variability, but suggested benefit, by mutations (notably STK11, Fig 3A), despite other studies suggesting reduced efficacy of single-agent ICI in these populations.20,21 Finally, of note was the effect size in squamous histology. ICI is beneficial in squamous NSCLC,22 and contrary to nonsquamous histologies, independent of PD-L1 status for second line.23,24 Thus, the squamous population should be evaluated further as ramucirumab is not restricted to nonsquamous histology.
Although this is a randomized phase II trial, we choose OS as the primary end point because response and PFS benefit are not always seen with ICI in advanced NSCLC potentially because of increased immune cell infiltration or prolonged time to tumor reduction, which is not seen with cytotoxic regimens.24,25 Lack of PFS benefit with RP is consistent with postprogression prolongation of survival seen in other studies with PD-1 and PD-L1 antibody therapy.25 The postprogression prolongation of survival phenomenon is likely to be responsible for the OS findings, especially since patients who were progressing immediately on ICI-achieved OS improvement similar to the overall population in the subgroup analysis.
The randomized phase II design and resulting smaller sample size imply that the study results should not be interpreted as definitive and limits interpretation of subgroup effects. Heterogeneity in type of prior immune checkpoint inhibitor-containing regimen is a potential limitation that reflects real-world therapy for advanced NSCLC. An imbalance in patients with PS 1 was seen in the SOC arm, and we analyzed the overall treatment effect adjusting for PS, which demonstrated that directionally the treatment effects remain in favor of RP. Additionally, the population was not completely unselected as S1800A excluded patients who had qualifying genomic alterations for Lung-MAP substudies S1900A (BRCA/LOH) and S1900C (STK11) and met the substudy eligibility criteria. Additionally, most next-generation sequencing and PD-L1 expression were based on archival tissue.
In summary, RP demonstrated improved OS over investigator's choice SOC, which largely consisted of docetaxel and ramucirumab, suggesting modulation of the immune microenvironment by an antiangiogenic agent, allowing resensitization to ICI. Further evaluation of this approach is warranted.
ACKNOWLEDGMENT
We wish to acknowledge Dr Jhanelle Gray, SWOG Lung Committee Chair; Dr Thomas Stinchcombe, Alliance Lung Committee Chair; Dr Julie Brahmer, ECOG-ACRIN Lung Committee Chair; and Dr Jeffrey Bradley, NRG Lung Committee Chair, for their support and important critical review of the manuscript.
APPENDIX
TABLE A1.
Grade 3-5 irAEs on RP
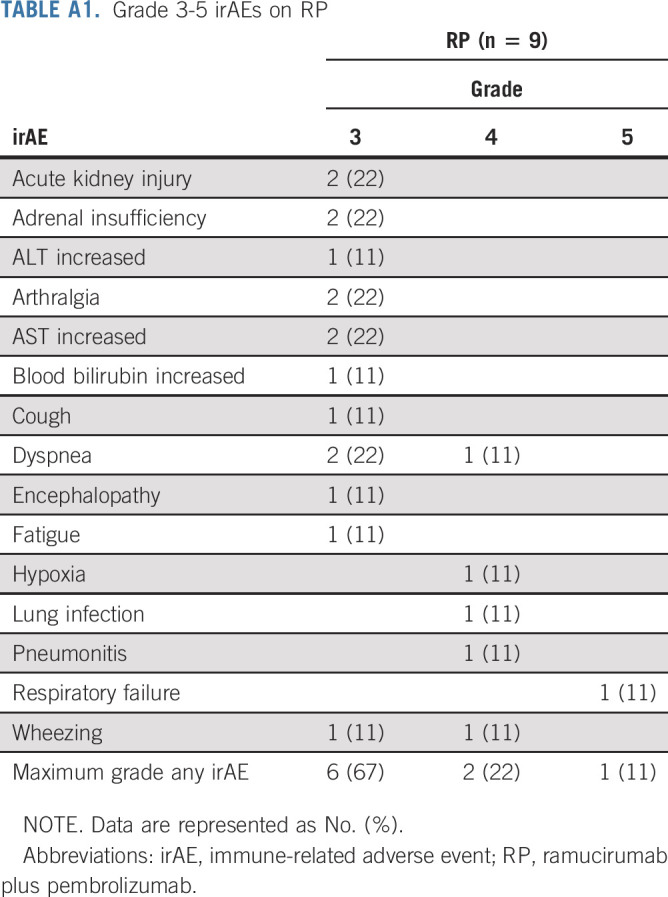
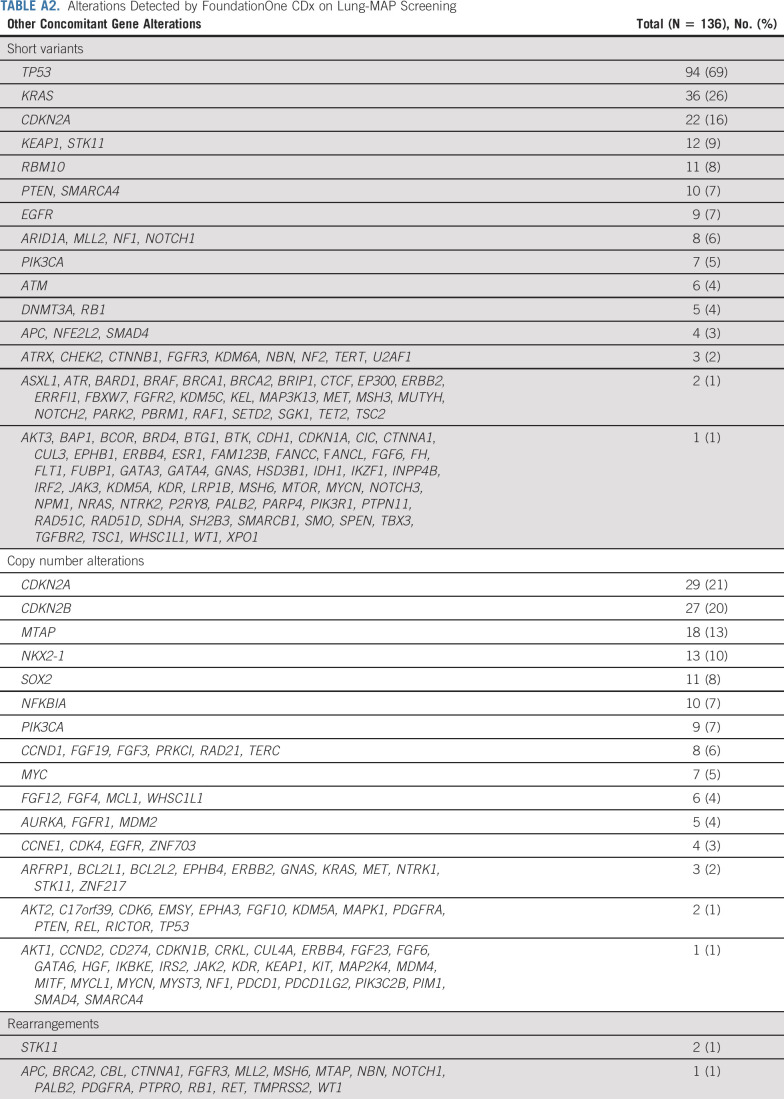
TABLE A2.
Alterations Detected by FoundationOne CDx on Lung-MAP Screening
TABLE A3.
Reported First Postprotocol Therapy by the Randomized Treatment Arm
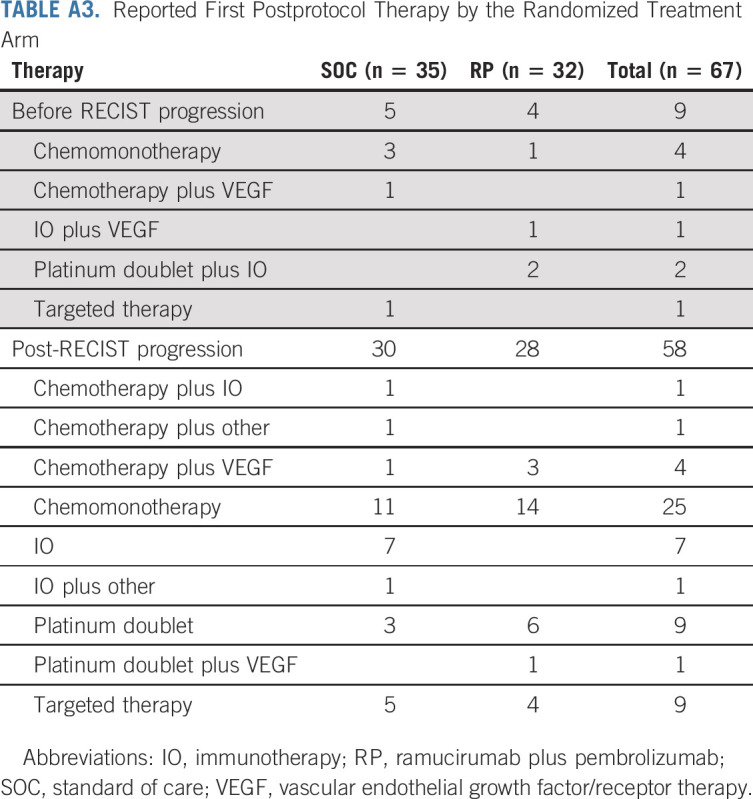
TABLE A4.
Details of Reported First Postprotocol Therapy
Karen L. Reckamp
Consulting or Advisory Role: Amgen, Tesaro, Takeda, AstraZeneca, Seattle Genetics, Genentech, Blueprint Medicines, Daiichi Sankyo/Lilly, EMD Serono, Janssen Oncology, Lilly, Merck KGaA, GlaxoSmithKline, Mirati Therapeutics
Research Funding: Genentech/Roche (Inst), Janssen Oncology (Inst), Calithera Biosciences (Inst), Elevation Oncology (Inst), Daiichi Sankyo/AstraZeneca (Inst), Blueprint Medicines
Konstantin H. Dragnev
Research Funding: G1 Therapeutics (Inst), Lilly (Inst), Merck (Inst), Roche/Genentech (Inst), Novartis (Inst), PharmaMar (Inst), Io Therapeutics (Inst)
Liza Villaruz
Consulting or Advisory Role: Achilles Therapeutics, Daiichi Sankyo/AstraZeneca, Takeda, Bristol Myers Squibb/Celgene, Janssen Oncology, Jazz Pharmaceuticals
Research Funding: Merck (Inst), Celgene (Inst), Lilly (Inst), Genentech (Inst), AstraZeneca (Inst), Incyte (Inst), Rain Therapeutics (Inst), Exelixis (Inst), GlaxoSmithKline (Inst), BMS (Inst), Regeneron (Inst), Black Diamond Therapeutics (Inst), BioAtla (Inst)
Bryan Faller
Consulting or Advisory Role: LEK
Travel, Accommodations, Expenses: Genentech, Novartis, EB SQUIBB, Celgene, Boehringer Ingelheim, Eisai, AstraZeneca, Lilly, Amgen, Merck, Takeda
Open Payments Link: https://openpaymentsdata.cms.gov/physician/127090
Tareq Al Baghdadi
Stock and Other Ownership Interests: AstraZeneca, Bristol Myers Squibb, Spectrum Pharmaceuticals, Sunesis Pharmaceuticals, Epizyme, HERON
Honoraria: Cardinal Health
Consulting or Advisory Role: Bristol Myers Squibb, AbbVie/Genentech, MorphoSys, Karyopharm Therapeutics
Vassiliki Papadimitrakopoulou
Employment: Pfizer
Honoraria: Roche
Consulting or Advisory Role: Clovis Oncology, Genentech, Merck, Biothera, Lilly, Janssen, AstraZeneca, ARIAD, Nektar, Loxo, Araxes Pharma, AbbVie, Bristol Myers Squibb, Exelixis, Pfizer, Novartis, Takeda, TRM Oncology, Tesaro, Arrys Therapeutics, Gritstone Bio, Leads Biolabs, Bolt Biotherapeutics, G2 Innovation
Research Funding: Merck (Inst), Novartis (Inst), Celgene (Inst), Clovis Oncology (Inst), Bayer (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Pfizer (Inst), Janssen Oncology (Inst), ACEA Biosciences (Inst), Nektar (Inst), Roche (Inst), Lilly (Inst), Checkmate Pharmaceuticals (Inst), Incyte (Inst), Guardant Health (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Roche
Other Relationship: Roche
David R. Gandara
Honoraria: Merck, Amgen
Consulting or Advisory Role: AstraZeneca (Inst), Guardant Health (Inst), OncoCyte (Inst), IO Biotech (Inst), Roche/Genentech (Inst), Lilly, Novartis, Adagene (Inst), OncoHost (Inst), Ocean Genomics (Inst), Daiichi Sankyo Alliance, Sanofi
Research Funding: Merck (Inst), Amgen (Inst), Genentech (Inst), AstraZeneca (Inst)
Karen Kelly
Consulting or Advisory Role: AstraZeneca, Regeneron, Novartis, Takeda, Lilly, Amgen, EMD Serono, Genmab, Targeted Oncology, Genentech, Debiopharm Group, AbbVie, Daiichi Sanko, Janssen, Eisai, Sanofi
Research Funding: EMD Serono (Inst), Genentech (Inst), AbbVie (Inst), Regeneron (Inst), Astellas Pharma (Inst), Tizona Therapeutics, Inc (Inst), Lilly (Inst), Novartis (Inst), Amgen (Inst), Bristol Myers Squibb (Inst), Five Prime Therapeutics (Inst), Jounce Therapeutics (Inst), Seattle Genetics (Inst)
Patents, Royalties, Other Intellectual Property: Author Royalties for UpToDate, an evidence based, peer reviewed information resource, available via the web, desktop, and PDA
Travel, Accommodations, Expenses: Lilly, EMD Serono, Novartis, Takeda
Roy S. Herbst
Leadership: Junshi Pharmaceuticals, Immunocore
Consulting or Advisory Role: AstraZeneca, Genentech/Roche, Merck, Pfizer, AbbVie, Biodesix, Bristol-Myers Squibb, Lilly, EMD Serono, Heat Biologics, Junshi Pharmaceuticals, Loxo, Nektar, NextCure, Novartis, Sanofi, Seattle Genetics, Shire, Spectrum Pharmaceuticals, Symphogen, TESARO, Neon Therapeutics, Infinity Pharmaceuticals, ARMO Biosciences, Genmab, Halozyme, Tocagen, Bolt Biotherapeutics, I-Mab, Mirati Therapeutics, Takeda, Cybrexa Therapeutics, eFFECTOR Therapeutics, Candel Therapeutics, Oncternal Therapeutics, STCube Pharmaceuticals Inc, WindMIL, Xencor, Bayer, Checkpoint Therapeutics, DynamiCure Biotechnology, Foundation Medicine, Gilead/Forty Seven, HiberCell, Immune-Onc Therapeutics, Johnson and Johnson, Ocean Biomedical, OncoCyte, Refactor Health, Ribon Therapeutics, Ventana Medical Systems
Research Funding: AstraZeneca, Merck, Lilly, Genentech/Roche
No other potential conflicts of interest were reported.
See accompanying editorial on page 2285
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, Eli Lilly and Company, and Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ.
PRIOR PRESENTATION
Presented in part at the ASCO Virtual Annual Meeting, June 4-8, 2021; and ASCO Annual Meeting, Chicago, IL, June 3-7, 2022.
SUPPORT
Supported by National Institutes of Health/National Cancer Institute grant awards U10CA180888, U10CA180819, U10CA180821, UG1CA233323, UG1CA189830, UG1CA189971, UG1CA189858, and UG1CA233340; Foundation for the National Institutes of Health; and in part by Eli Lilly and Company and Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ.
CLINICAL TRIAL INFORMATION
Lung-MAP Sub-Study S1800A: NCT03971474
AUTHOR CONTRIBUTIONS
Conception and design: Karen L. Reckamp, Mary W. Redman, Konstantin H. Dragnev, Vassiliki Papadimitrakopoulou, David R. Gandara, Karen Kelly, Roy S. Herbst
Administrative support: Karen L. Reckamp, Leah Everhart, Louise Highleyman, Roy S. Herbst
Provision of study materials or patients: Karen L. Reckamp, Mary W. Redman, Konstantin H. Dragnev, David R. Gandara, Roy S. Herbst
Collection and assembly of data: Karen L. Reckamp, Mary W. Redman, Konstantin H. Dragnev, Katherine Minichiello, Liza Villaruz, Bryan Faller, Tareq Al Baghdadi, Susan Hines, Leah Everhart, Louise Highleyman, Vassiliki Papadimitrakopoulou, Karen Kelly, Roy S. Herbst
Data analysis and interpretation: Karen L. Reckamp, Mary W. Redman, Konstantin H. Dragnev, Katherine Minichiello, Liza Villaruz, Bryan Faller, Tareq Al Baghdadi, Vassiliki Papadimitrakopoulou, David R. Gandara, Karen Kelly, Roy S. Herbst
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase II Randomized Study of Ramucirumab and Pembrolizumab Versus Standard of Care in Advanced Non–Small-Cell Lung Cancer Previously Treated With Immunotherapy—Lung-MAP S1800A
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Karen L. Reckamp
Consulting or Advisory Role: Amgen, Tesaro, Takeda, AstraZeneca, Seattle Genetics, Genentech, Blueprint Medicines, Daiichi Sankyo/Lilly, EMD Serono, Janssen Oncology, Lilly, Merck KGaA, GlaxoSmithKline, Mirati Therapeutics
Research Funding: Genentech/Roche (Inst), Janssen Oncology (Inst), Calithera Biosciences (Inst), Elevation Oncology (Inst), Daiichi Sankyo/AstraZeneca (Inst), Blueprint Medicines
Konstantin H. Dragnev
Research Funding: G1 Therapeutics (Inst), Lilly (Inst), Merck (Inst), Roche/Genentech (Inst), Novartis (Inst), PharmaMar (Inst), Io Therapeutics (Inst)
Liza Villaruz
Consulting or Advisory Role: Achilles Therapeutics, Daiichi Sankyo/AstraZeneca, Takeda, Bristol Myers Squibb/Celgene, Janssen Oncology, Jazz Pharmaceuticals
Research Funding: Merck (Inst), Celgene (Inst), Lilly (Inst), Genentech (Inst), AstraZeneca (Inst), Incyte (Inst), Rain Therapeutics (Inst), Exelixis (Inst), GlaxoSmithKline (Inst), BMS (Inst), Regeneron (Inst), Black Diamond Therapeutics (Inst), BioAtla (Inst)
Bryan Faller
Consulting or Advisory Role: LEK
Travel, Accommodations, Expenses: Genentech, Novartis, EB SQUIBB, Celgene, Boehringer Ingelheim, Eisai, AstraZeneca, Lilly, Amgen, Merck, Takeda
Open Payments Link: https://openpaymentsdata.cms.gov/physician/127090
Tareq Al Baghdadi
Stock and Other Ownership Interests: AstraZeneca, Bristol Myers Squibb, Spectrum Pharmaceuticals, Sunesis Pharmaceuticals, Epizyme, HERON
Honoraria: Cardinal Health
Consulting or Advisory Role: Bristol Myers Squibb, AbbVie/Genentech, MorphoSys, Karyopharm Therapeutics
Vassiliki Papadimitrakopoulou
Employment: Pfizer
Honoraria: Roche
Consulting or Advisory Role: Clovis Oncology, Genentech, Merck, Biothera, Lilly, Janssen, AstraZeneca, ARIAD, Nektar, Loxo, Araxes Pharma, AbbVie, Bristol Myers Squibb, Exelixis, Pfizer, Novartis, Takeda, TRM Oncology, Tesaro, Arrys Therapeutics, Gritstone Bio, Leads Biolabs, Bolt Biotherapeutics, G2 Innovation
Research Funding: Merck (Inst), Novartis (Inst), Celgene (Inst), Clovis Oncology (Inst), Bayer (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Pfizer (Inst), Janssen Oncology (Inst), ACEA Biosciences (Inst), Nektar (Inst), Roche (Inst), Lilly (Inst), Checkmate Pharmaceuticals (Inst), Incyte (Inst), Guardant Health (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Roche
Other Relationship: Roche
David R. Gandara
Honoraria: Merck, Amgen
Consulting or Advisory Role: AstraZeneca (Inst), Guardant Health (Inst), OncoCyte (Inst), IO Biotech (Inst), Roche/Genentech (Inst), Lilly, Novartis, Adagene (Inst), OncoHost (Inst), Ocean Genomics (Inst), Daiichi Sankyo Alliance, Sanofi
Research Funding: Merck (Inst), Amgen (Inst), Genentech (Inst), AstraZeneca (Inst)
Karen Kelly
Consulting or Advisory Role: AstraZeneca, Regeneron, Novartis, Takeda, Lilly, Amgen, EMD Serono, Genmab, Targeted Oncology, Genentech, Debiopharm Group, AbbVie, Daiichi Sanko, Janssen, Eisai, Sanofi
Research Funding: EMD Serono (Inst), Genentech (Inst), AbbVie (Inst), Regeneron (Inst), Astellas Pharma (Inst), Tizona Therapeutics, Inc (Inst), Lilly (Inst), Novartis (Inst), Amgen (Inst), Bristol Myers Squibb (Inst), Five Prime Therapeutics (Inst), Jounce Therapeutics (Inst), Seattle Genetics (Inst)
Patents, Royalties, Other Intellectual Property: Author Royalties for UpToDate, an evidence based, peer reviewed information resource, available via the web, desktop, and PDA
Travel, Accommodations, Expenses: Lilly, EMD Serono, Novartis, Takeda
Roy S. Herbst
Leadership: Junshi Pharmaceuticals, Immunocore
Consulting or Advisory Role: AstraZeneca, Genentech/Roche, Merck, Pfizer, AbbVie, Biodesix, Bristol-Myers Squibb, Lilly, EMD Serono, Heat Biologics, Junshi Pharmaceuticals, Loxo, Nektar, NextCure, Novartis, Sanofi, Seattle Genetics, Shire, Spectrum Pharmaceuticals, Symphogen, TESARO, Neon Therapeutics, Infinity Pharmaceuticals, ARMO Biosciences, Genmab, Halozyme, Tocagen, Bolt Biotherapeutics, I-Mab, Mirati Therapeutics, Takeda, Cybrexa Therapeutics, eFFECTOR Therapeutics, Candel Therapeutics, Oncternal Therapeutics, STCube Pharmaceuticals Inc, WindMIL, Xencor, Bayer, Checkpoint Therapeutics, DynamiCure Biotechnology, Foundation Medicine, Gilead/Forty Seven, HiberCell, Immune-Onc Therapeutics, Johnson and Johnson, Ocean Biomedical, OncoCyte, Refactor Health, Ribon Therapeutics, Ventana Medical Systems
Research Funding: AstraZeneca, Merck, Lilly, Genentech/Roche
No other potential conflicts of interest were reported.
REFERENCES
- 1.Planchard D, Popat S, Kerr K, et al. : Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 29:iv192-iv237, 2018. (suppl 4) [DOI] [PubMed] [Google Scholar]
- 2.Schoenfeld AJ, Antonia SJ, Awad MM, et al. : Clinical definition of acquired resistance to immunotherapy in patients with metastatic non-small-cell lung cancer. Ann Oncol 32:1597-1607, 2021 [DOI] [PubMed] [Google Scholar]
- 3.Lee WS, Yang H, Chon HJ, et al. : Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med 52:1475-1485, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roland CL, Lynn KD, Toombs JE, et al. : Cytokine levels correlate with immune cell infiltration after anti-VEGF therapy in preclinical mouse models of breast cancer. PLoS One 4:e7669, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Cicco P, Ercolano G, Ianaro A: The new era of cancer immunotherapy: Targeting myeloid-derived suppressor cells to overcome immune evasion. Front Immunol 11:1680, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rini BI, Plimack ER, Stus V, et al. : Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380:1116-1127, 2019 [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Penkov K, Haanen J, et al. : Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380:1103-1115, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choueiri TK, Powles T, Burotto M, et al. : Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 384:829-841, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motzer R, Alekseev B, Rha SY, et al. : Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med 384:1289-1300, 2021 [DOI] [PubMed] [Google Scholar]
- 10.Makker V, Colombo N, Casado Herráez A, et al. : Lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med 386:437-448, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finn RS, Qin S, Ikeda M, et al. : Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 382:1894-1905, 2020 [DOI] [PubMed] [Google Scholar]
- 12.Herbst RS, Arkenau HT, Bendell J, et al. : Phase 1 expansion cohort of ramucirumab plus pembrolizumab in advanced treatment-naive NSCLC. J Thorac Oncol 16:289-298, 2021 [DOI] [PubMed] [Google Scholar]
- 13.Herbst RS, Arkenau HT, Santana-Davila R, et al. : Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): A multicohort, non-randomised, open-label, phase 1a/b trial. Lancet Oncol 20:1109-1123, 2019 [DOI] [PubMed] [Google Scholar]
- 14.Socinski MA, Jotte RM, Cappuzzo F, et al. : Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378:2288-2301, 2018 [DOI] [PubMed] [Google Scholar]
- 15.Redman MW, Papadimitrakopoulou VA, Minichiello K, et al. : Biomarker-driven therapies for previously treated squamous non-small-cell lung cancer (Lung-MAP SWOG S1400): A biomarker-driven master protocol. Lancet Oncol 21:1589-1601, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herbst RS, Gandara DR, Hirsch FR, et al. : Lung Master Protocol (Lung-MAP)-A biomarker-driven protocol for accelerating development of therapies for squamous cell lung cancer: SWOG S1400. Clin Cancer Res 21:1514-1524, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleming TR, Harrington DP, O'Sullivan M: Supremum versions of the log-rank and generalized Wilcoxon statistics. J Am Stat Assoc 82:312-320, 1987 [Google Scholar]
- 18.Garon EB, Ciuleanu TE, Arrieta O, et al. : Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): A multicentre, double-blind, randomised phase 3 trial. Lancet 384:665-673, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Shiono A, Kaira K, Mouri A, et al. : Improved efficacy of ramucirumab plus docetaxel after nivolumab failure in previously treated non-small cell lung cancer patients. Thorac Cancer 10:775-781, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skoulidis F, Goldberg ME, Greenawalt DM, et al. : STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov 8:822-835, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun L, Hsu M, Cohen RB, et al. : Association between KRAS variant status and outcomes with first-line immune checkpoint inhibitor-based therapy in patients with advanced non-small-cell lung cancer. JAMA Oncol 7:937-939, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sezer A, Kilickap S, Gümüş M, et al. : Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: A multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet 397:592-604, 2021 [DOI] [PubMed] [Google Scholar]
- 23.Brahmer J, Reckamp KL, Baas P, et al. : Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373:123-135, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borghaei H, Paz-Ares L, Horn L, et al. : Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627-1639, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rittmeyer A, Barlesi F, Waterkamp D, et al. : Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 389:255-265, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]