Abstract
Laboratory model systems were developed for studying Shewanella putrefaciens adhesion and biofilm formation under batch and flow conditions. S. putrefaciens plays a major role in food spoilage and may cause microbially induced corrosion on steel surfaces. S. putrefaciens bacteria suspended in buffer adhered readily to stainless steel surfaces. Maximum numbers of adherent bacteria per square centimeter were reached in 8 h at 25°C and reflected the cell density in suspension. Numbers of adhering bacteria from a suspension containing 108 CFU/ml were much lower in a laminar flow system (modified Robbins device) (reaching 102 CFU/cm2) than in a batch system (reaching 107 CFU/cm2), and maximum numbers were reached after 24 h. When nutrients were supplied, S. putrefaciens grew in biofilms with layers of bacteria. The rate of biofilm formation and the thickness of the film were not dependent on the availability of carbohydrate (lactate or glucose) or on iron starvation. The number of S. putrefaciens bacteria on the surface was partly influenced by the presence of other bacteria (Pseudomonas fluorescens) which reduced the numbers of S. putrefaciens bacteria in the biofilm. Numbers of bacteria on the surface must be quantified to evaluate the influence of environmental factors on adhesion and biofilm formation. We used a combination of fluorescence microscopy (4′,6′-diamidino-2-phenylindole staining and in situ hybridization, for mixed-culture studies), ultrasonic removal of bacteria from surfaces, and indirect conductometry and found this combination sufficient to quantify bacteria on surfaces.
The behavior of adherent and growing bacteria on surfaces has received increasing attention during recent years. Examples of such behavior include fouling of ship hulls (5, 23) or contamination of medical devices (28, 36); also, the benefits of floc formation in sludge systems are being studied (3). Biofilm formation by sulfide-producing bacteria poses special problems, as corrosion of steel surfaces (microbially induced corrosion) can take place (6, 25).
The food industry has also realized that adhesion and colonization of bacteria may cause problems (22). Bacteria colonizing the processing equipment may be an important source of bacterial contamination, and studies have shown that both spoilage bacteria like Pseudomonas spp. (29) and pathogenic bacteria like Listeria monocytogenes may contaminate products directly from the processing environment (1, 30). Despite rigorous cleaning and disinfection procedures, pathogenic bacteria and spoilage microorganisms may be isolated from many types of surfaces in food processing plants (B. Fonnesbech Vogel, personal communication). This may at least partly be explained by an increased resistance of adherent bacteria to adverse conditions like disinfection (21, 32, 41).
Shewanella putrefaciens is a marine, gram-negative bacterium which is of importance in many areas. In the food industry, it plays a role as a spoilage bacterium of marine fish, some vacuum-packed meats, and chicken due to its ability to produce volatile sulfides, amines, and the fishy-smelling compound trimethylamine. In the environment, S. putrefaciens participates in the biogeochemical cycling of metals due to its ability to reduce a variety of compounds [e.g., Fe(III) and Mn(IV)] by anaerobic respiration (9, 31). S. putrefaciens is capable of adhering to and forming biofilms on different surfaces. Thus, Obuekwe et al. (33) showed that a thick fibrous biofilm was formed on stainless steel plates. Also, the adherence of S. putrefaciens to stainless steel surfaces coupled with its ability to produce sulfides and reduce iron explains its importance in microbially induced corrosion of steel surfaces (6, 7).
Despite the potential of this bacterium to adhere and grow on surfaces and the growing awareness of adherent bacteria in the food industry, little is known about the ability of this spoilage bacterium to adhere to different types of surfaces and its ability to persist in the seafood processing environment. The current study was undertaken to develop models to study the ability of the fish spoilage bacterium S. putrefaciens to adhere and form biofilms on food processing surfaces. Factors influencing biofilm formation by S. putrefaciens, such as nutrient concentration and accompanying microflora, were evaluated.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
S. putrefaciens strain A2 (35) and Pseudomonas fluorescens strain AH2 (15) were cultured on iron agar Lyngby (Oxoid CM964) (14) at 25°C.
Adhesion and biofilm formation in batch systems.
Stainless steel (AISI 316, unpolished) was cut into 10- by 20-mm disks with a thickness of 1 mm. Sterile steel disks were clamped vertically in a sterile steel circular rack placed in a beaker. The rack holds up to 20 disks in an arrangement which, when the rack is immersed in culture medium, allows the free circulation of liquid. For adhesion studies of S. putrefaciens, strain A2 was precultured in tryptone soya broth (TSB; Oxoid CM129) for 24 h with agitation at 25°C. The bacteria were harvested by centrifugation at 3,000 × g for 10 min and resuspended in phosphate-buffered saline (PBS; 0.8% NaCl, 0.02% KCl, 0.144% Na2HPO4, 0.024% NaH2PO4; pH 7.4). The sterile rack containing the disks was immersed in dilute (1:7) TSB for 30 min, allowing a conditioning film to be formed on the steel disks. The rack containing the disks was transferred to a new sterile beaker containing S. putrefaciens cells suspended in PBS at different concentrations. Adhesion was allowed to take place on both sides of the disks at room temperature under slow-stirring (250-rpm) conditions.
The biofilm formation of S. putrefaciens on stainless steel was investigated in the batch system described above, except that growth medium (1:5 TSB or 1:7 TSB) was added instead of buffer, allowing the bacteria to proliferate. S. putrefaciens was inoculated at an initial level of 103 CFU/ml. A biofilm was allowed to develop on both sides of the stainless steel disks at room temperature under slow-stirring (250-rpm) conditions. The effect of iron excess or limitation was studied by addition of 100 μM FeCl3 or 200 μM ethylenediamine di(o-hydroxyphenylacetic acid) (EDDHA; Sigma E-4135), and the effect of extra carbohydrates was studied by the addition of 1% glucose. To study the effect of the presence of other bacteria, S. putrefaciens was coinoculated with a P. fluorescens strain, AH2, that was precultured in TSB at 25°C for 24 h. Different initial ratios of the two organisms were studied, covering starting concentrations of 103:103 and 105:103 of S. putrefaciens and P. fluorescens, respectively.
Adhesion and biofilm formation in flow systems.
The adhesion of S. putrefaciens to stainless steel disks was investigated in a flow model system using a modified Robbins device (MRD) (Tyler Research Corporation, Edmonton, Alberta, Canada). S. putrefaciens was precultured, and bacteria were harvested as described above. A suspension of 108 CFU of S. putrefaciens/ml of PBS (pH 7.4) was circulated in the MRD at a flow rate of 10 ml/min (equaling 0.0062 m/s). The formation of a biofilm by S. putrefaciens in the MRD was investigated with different food processing surfaces, stainless steel and polypropylene. S. putrefaciens was precultured in TSB for 24 h at 25°C and then inoculated in TSB diluted 1:7 with sterile water. The MRD was sterilized, and a conditioning film was allowed to develop by circulating TSB diluted 1:7 for 30 min, after which the MRD was inoculated for 3 h with a circulating suspension of approximately 106 CFU/ml in TSB diluted 1:7. Thereafter, sterile TSB diluted 1:7 was continuously supplied at a flow rate of 0.5 ml/min (equaling 0.00031 m/s).
Microscopic evaluation of adhesion and biofilm formation.
The disks were rinsed in 5 ml of sterile PBS, and nonadherent or poorly attached bacteria were removed by carefully placing the disk (both sides) on sterile absorbent paper. Care was taken not to swab or rub the disk at this stage. The amount of attached bacteria was estimated by fluorescence microscopy after staining with 2 μg of 4′,6′-diamidino-2-phenylindole (DAPI; Sigma D-9542) per ml for 5 min. The surface was examined by direct fluorescence microscopy (Olympus BH2 fluorescence microscope with a 320- to 400-nm excitation filter and a >420-nm barrier filter or a Zeiss Axioscope 20 microscope [Carl Zeiss, Brock & Michelsen, Birkerød, Denmark] using a Zeiss F31-600 filter set [excitation filter, D360/50; beam splitter, 400 dichroic long-pass emission; and barrier filter, D460/50]). Mixed biofilms of S. putrefaciens and P. fluorescens were developed, and numbers of each organism were estimated using a specific rRNA-targeted oligonucleotide. The disks were fixed in 4% paraformaldehyde and hybridized at 46°C with two rRNA-targeted oligonucleotides using 5 (and 6)-carboxytetramethyl rhodamine for S. putrefaciens and fluorescein isothiocyanate (FITC) for P. fluorescens (I. Huber, B. Spanggaard, K. F. Appel, L. Gram, and T. Nielsen, unpublished data). A minimum of 10 fields were examined for each plate under a Zeiss Axioscope 20 microscope using the following filter sets: red, HQ-Cy3; excitation filter, HQ545/30; beam splitter, Q565LP; and barrier filter, HQ610/75; green, excitation filter, HQ480/40; beam splitter, Q505LP; and barrier filter, HQ535/50 (for TAMRA and FITC, respectively).
Quantification of attached bacteria by indirect conductometry or removal by sonication.
Attached bacteria were also enumerated by indirect conductance measurements (18). The steel disks with the adherent bacteria were transferred to Malthus glass tubes containing 3 ml of TSB as a growth medium. Growth of the adherent bacteria causes development of CO2, which diffuses into an inner tube, containing 0.5 ml of sterile 0.1 M NaOH. Electrodes measure the conductance in the NaOH solution and thus the change in conductance as CO2 dissolves in the alkali. The time from the start of the measurement until a rapid change (decrease) in conductance occurs, the so-called detection time, is inversely related to the initial number of bacteria. The detection time can be related to the initial number of bacteria by use of a calibration curve constructed by using a 10-fold dilution series of bacteria. Calibration curves were constructed for each separate system, e.g., bacteria suspended in PBS to study attachment or bacteria from the liquid TSB used to study biofilm formation. Also, calibration curves were constructed at several time points during an experiment to evaluate the effect of growth phase on detection times. An estimate of numbers of adherent S. putrefaciens bacteria from a mixture also containing P. fluorescens was made using a direct conductometric method. The disks with a mixed biofilm were transferred to Malthus glass tubes containing 8 ml of a nutrient broth containing 1 g of trimethylamine oxide (TMAO) per liter (42). S. putrefaciens reduces the neutral TMAO to ionized trimethylamine by anaerobic respiration and causes an increase in conductance (11, 35). Pseudomonads, which do not respire using TMAO, cause no change in electrical properties of the medium.
To validate the numbers of adherent bacteria derived from the conductometric measurements and conversion via the standard curve, parallel experiments were conducted in which four disks from an experiment were used for conductometric measurements and four disks were used to enumerate bacteria after removal by sonication. The rinsed disks were placed in 5 ml of PBS, and bacteria were removed from the surface by two treatments for 10 s each using an MSE Soniprep 150 ultrasonic disintegrator (Sanoy, Integrated Services, TCP Inc.) at 27 kHz. The disks were rinsed with 5 ml of PBS into the same tube. Colony counts were determined by 10-fold serial dilution and plating onto iron agar Lyngby (Oxoid CM964). The tubes were placed on ice before sonication, and the temperature did not exceed 22°C during treatment.
RESULTS
Calibration curves relating detection times to colony counts.
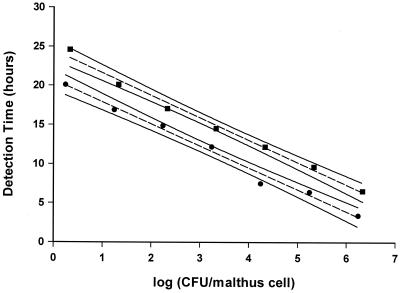
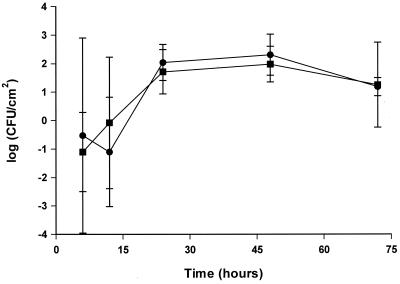
The detection times, as expected, decreased with increasing initial counts of bacteria. Comparing initial CFU per Malthus cell to detection times gave, for all systems studied, a linear relationship. The calibration curves were similar as S. putrefaciens in the batch system gave rise to statistically identical curves independently of being grown in 1:7 TSB or suspended in PBS (data not shown). Calibration curves made from bacteria released from the biofilm flow system had a significantly lower intersect with the y axis (Fig. 1), indicating a shorter lag phase than for bacteria taken from the batch system. Identical curves were obtained for the indirect measurement and the direct measurement (data not shown). All CFU per square centimeter reported below were derived from the appropriate calibration curve. As the curves cover a range of 1 × 101 to 5 × 106 CFU/Malthus cell, higher or lower counts are derived by extrapolation of the standard curve. The bacteria used to develop the calibration curves, although taken from suspensions in which surfaces and adhernt bacteria are immersed, are not surface-bound bacteria.
FIG. 1.
Comparison of CFU of S. putrefaciens per milliliter determined by colony counts with conductometric detection times (duplicate samples) determined by indirect measurements for adhesion (▪) and biofilm formation (●) in flow systems. Measurements were made at 25°C. Ninety-five percent confidence intervals are shown.
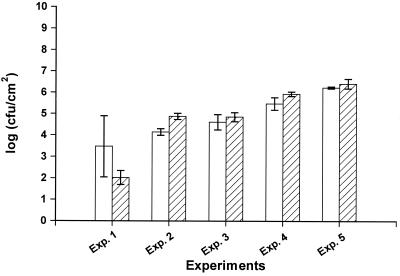
To verify that the conductance detection times did reflect the numbers of surface-associated bacteria, we compared CFU per square centimeter as derived from the calibration curve with the number of bacteria from a parallel set of stainless steel disks from which bacteria were removed by sonication and enumerated by plate counts. Excellent agreement was obtained between the numbers determined by the two methods (Fig. 2). We used two 10-s treatments, which had no effect on the viability of cells and were as effective as longer treatments (e.g., two 30-s treatments).
FIG. 2.
Colony counts per square centimeter of stainless steel disks as determined by use of conductance measurements and transformation by the standard curve (open bars) or by use of ultrasound removal of cells and subsequent enumeration by plate counts (hatched bars). Error bars are standard deviations of quadruplicate determinations.
Adhesion of S. putrefaciens in batch systems.
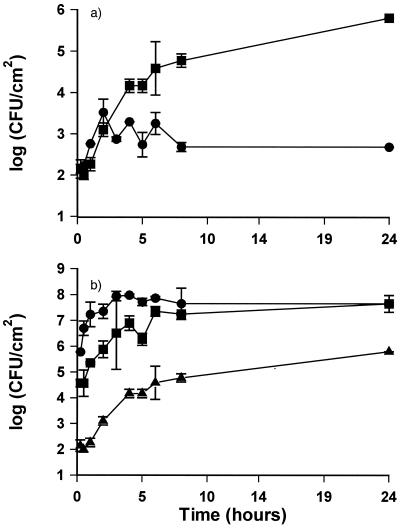
S. putrefaciens adhered readily to stainless steel disks, increasing from 102 CFU/cm2 immediately after immersion to 105 CFU/cm2 after 8 h of incubation (Fig. 3a). The adhesion was facilitated by the formation of an initial conditioning film of TSB, as a significantly lower level of bacteria adhered to disks on which no conditioning film had been formed (Fig. 3a). The number of bacteria adhering reflected the level of bacteria in suspension (Fig. 3b). Within 8 h, the adhesion reached a stationary state corresponding to the number of CFU per milliliter in the suspension. The number of bacteria in suspension did not increase during this 8-h period; however, growth did occur when the adhesion experiment was extended for 24 h (data not shown). The number of adherent S. putrefaciens bacteria was not systematically influenced by the presence of P. fluorescens (Fig. 4).
FIG. 3.
Adhesion of S. putrefaciens suspended in PBS to stainless steel. (a) 105 CFU/ml adhering to conditioned (▪) or nonconditioned surfaces (●). (b) Adhesion to conditioned surfaces from suspensions with 105 (▴), 107 (▪), or 109 (●) CFU/ml. All experiments were performed at 25°C with slow stirring. Counts are derived from standard curves. Error bars are standard deviations of duplicate samples.
FIG. 4.
Adhesion of S. putrefaciens to stainless steel as monoculture or mixed culture with P. fluorescens. Bacteria were suspended in PBS at 25°C. Symbols: ▪, S. putrefaciens (106 CFU/ml); ●, S. putrefaciens plus P. fluorescens (106:106 CFU/ml); ▴, S. putrefaciens plus P. fluorescens (106:104 CFU/ml). Counts are derived from standard curves in TMAO broth. Error bars are standard deviations of duplicate samples.
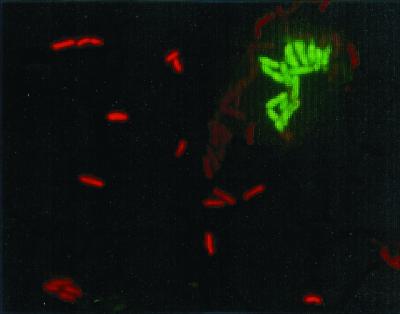
Microscopic examination of the mixed adhered cultures using in situ hybridization showed an organized colonization of the surface. Cells of S. putrefaciens were evenly distributed, whereas cells of P. fluorescens clustered together (Fig. 5).
FIG. 5.
Fluorescence microscopy of mixed adhesion of S. putrefaciens and P. fluorescens to stainless steel. The bacteria are visualized by 16S rRNA oligonucleotide probes with TAMRA (red) for S. putrefaciens and FITC (green) for P. fluorescens. Bacteria were suspended in PBS at 25°C.
Adhesion of S. putrefaciens in flow systems.
The kinetics of adhesion was very different in the flow system from that in the batch system. Despite a high number of bacteria (108 CFU/ml) in the circulating suspension, bacteria adhered slowly, reaching approximately 102 CFU/cm2 after 30 h and remaining at this level for the rest of the experimental period. No difference was seen in numbers of adherent bacteria, depending on preconditioning of the steel surface (Fig. 6). Initially, when low numbers adhered, the standard deviation was large. The low number is based on extrapolation from the calibration curve, and this could, in part, be a reason for the large deviation.
FIG. 6.
Adhesion of S. putrefaciens to stainless steel in a flow system (MRD). With (▪) or without (●) a conditioning film, bacteria were suspended in PBS at 108 CFU/ml, and the suspension was recirculated at 25°C. Counts are derived from standard curves. Error bars are standard deviations of triplicate samples.
Formation of S. putrefaciens biofilms in batch systems.
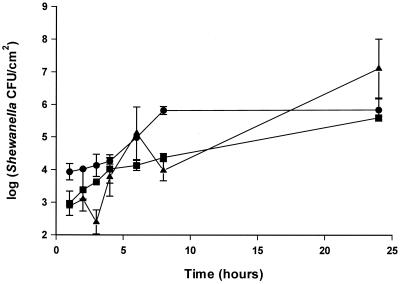
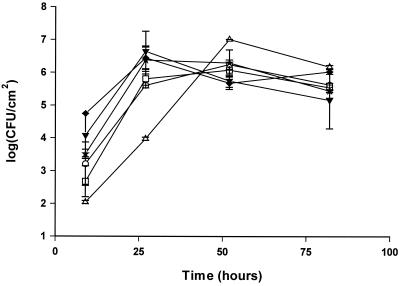
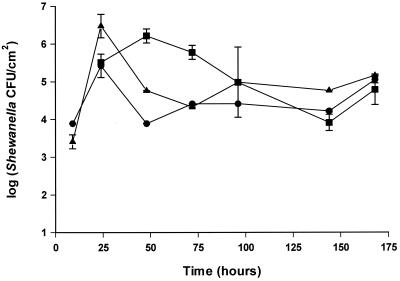
The effects of nutrient and iron limitation, excess glucose or iron, and an associated microflora on biofilm formation were investigated by culturing the bacteria in 1:7 diluted TSB. S. putrefaciens strain A2 formed a multilayered biofilm on the stainless steel disks, reaching 106 to 107 CFU/cm2 in 1 to 2 days (Fig. 7 and 8). The addition of glucose or iron had no effect on biofilm formation in the batch system. Addition of the iron chelator EDDHA caused slower growth; however, the biofilm formation as a function of cell growth in the medium was similar to iron-rich culture conditions (Fig. 7). The addition of a competing organism (P. fluorescens) decreased the number of S. putrefaciens bacteria on the surface between 25 and 100 h compared to the numbers adhering from a monoculture of S. putrefaciens (Fig. 9). As in the adhesion experiment, P. fluorescens clustered whereas S. putrefaciens bacteria were evenly distributed.
FIG. 7.
Biofilm formation of S. putrefaciens on stainless steel. S. putrefaciens was grown in 1:7 TSB with no addition □, 100 μM FeCl3 ○, 200 μM EDDHA ▵, 1% glucose ★, 1% lactate ▾, or 1% lactate plus 100 μM FeCl3 ♦. All experiments were carried out at 25°C. Counts are derived from standard curves. Error bars are standard deviations of duplicate samples.
FIG. 9.
Biofilm formation of S. putrefaciens on stainless steel as monoculture or mixed culture with P. fluorescens. Bacteria were grown in 1:7 TSB at 25°C. Symbols: ▪, S. putrefaciens (105 CFU/ml); ●, S. putrefaciens plus P. fluorescens (105:105 CFU/ml); ▴, S. putrefaciens plus P. fluorescens (105:103 CFU/ml). Counts are derived from standard curves. Error bars are standard deviations of duplicate samples.
Formation of S. putrefaciens biofilms in flow systems.
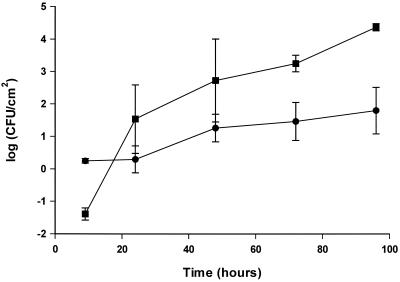
S. putrefaciens also formed biofilms on stainless steel in a flow system. As for the adhesion process, a longer time was required to reach a steady state (Fig. 10). Surface type influenced the biofilm formation, as there was a lower number of bacteria adhering to polypropylene than to stainless steel (Fig. 10).
FIG. 10.
Biofilm formation of S. putrefaciens in flow systems on different surfaces, stainless steel (▪) and polypropylene (●). Bacteria were grown in continuously freshened 1:7 TSB at 25°C. Counts are derived from standard curves. Error bars are standard deviations of triplicate samples.
DISCUSSION
S. putrefaciens colonizes food and is an important food spoilage bacterium. Its biofilm formation has been investigated due to its role in microbially induced corrosion caused by production of hydrogen sulfide and reduction of iron(III) (6, 33). We demonstrated in this study that S. putrefaciens readily adheres to inert food processing surfaces (stainless steel and polypropylene), and if nutrients are supplied, multilayered biofilms are formed (Fig. 8). The adhesion of S. putrefaciens to surfaces occurred rapidly. Similar kinetics has been reported for the attachment of L. monocytogenes to stainless steel and buna-N-rubber (39). L. monocytogenes reached maximum attached numbers (104 CFU/cm2 from a suspension of 107 CFU/ml) in approximately 3 h, whereas the number of adherent S. putrefaciens bacteria increased for almost 8 h. Thus, during a normal shift (8 to 12 h) in a food processing plant, there is ample time for the organism to attach to surfaces.
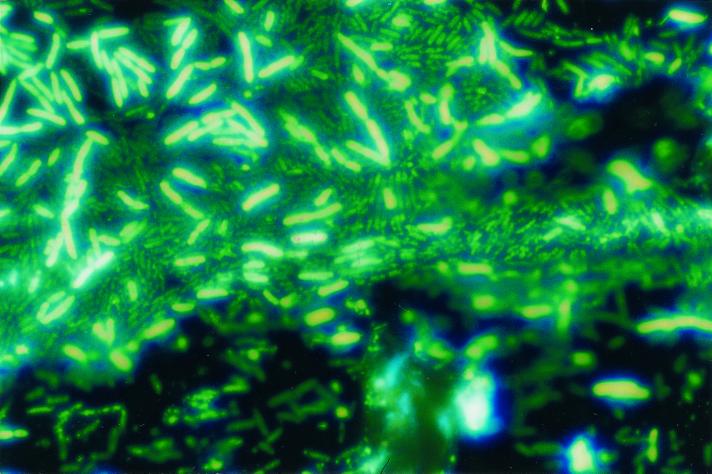
FIG. 8.
DAPI staining of a biofilm of S. putrefaciens on stainless steel. The bacteria were grown in 1:7 TSB at 25°C.
In most environments, including the food processing industry, a layer of organic material (a conditioning film) will rapidly be formed on surfaces. We found that the adhesion of S. putrefaciens to stainless steel was facilitated by a conditioning film of TSB (Fig. 3a). Hood and Zottola (17) found that, for some bacteria like Salmonella enterica serovar Typhimurium, adhesion to stainless steel was similarly facilitated by an initial organic layer, whereas higher numbers of both Pseudomonas fragi and L. monocytogenes bacteria were found on nonconditioned surfaces. Some food components, e.g., milk proteins, may actually decrease attachment of bacteria (2). In theory, the thin layer of organic material on the surface may also have allowed the bacteria to multiply; however, application of rRNA probes revealed a very low signal after 2 days, indicating that no growth took place (unpublished data).
Increasing the flow across the surface dramatically reduced the number of S. putrefaciens bacteria adhering to stainless steel (Fig. 6) even at the very low flow (0.0062 m/s) used. Also, Duddridge et al. (10) reported that increasing the shear stress significantly reduced the number of P. fluorescens bacteria attaching to a stainless steel surface. Reaching the maximum number of S. putrefaciens bacteria under flow conditions took longer (30 h) than under batch conditions. Harkes et al. (16) found that Escherichia coli already after 3 h under flow conditions had reached a maximum level of approximately 8 × 105 CFU/cm2. Formation of a conditioning film had no effect on the adhesion; however, this could be due to a washout of the film before adhesion commenced.
The simultaneous attachment of P. fluorescens had no effect on the adhesion of S. putrefaciens (Fig. 4). In a study determining the simultaneous attachment of a range of bacteria, McEldowney and Fletcher (27) found that, in most cases, the bacteria had no effect on each other. However, in some cases bacteria decreased the attachment of others, whereas in one case, the attachment of a Staphylocccus sp. strain was increased by the simultaneous presence of an Acinetobacter strain.
Numbers of S. putrefaciens reached their maximum in the biofilm stage after approximately 1 to 2 days in 1:7 TSB. Dewanti and Wong (8) found that, depending on the nutrient medium, the biofilm formation of E. coli reached a stationary level in 2, 8, and 5 days for TSB, 1:5 TSB, and Bacto Peptone, respectively. Further, they found that biofilm development occurred faster in Bacto Peptone and reached a higher level of attached bacteria. Similarly, Kim and Frank (20) showed that the attachment of L. monocytogenes was higher in D10 (low-nutrient medium) than in TSB, and only a modification of D10, either by increase of ammonium chloride or by reduction of iron, affected the attachment of L. monocytogenes. Both modifications showed a decrease in the attachment. McEldowney and Fletcher (26) showed that changing the carbon source or the level of nitrogen resulted in variations in the level of attachment of different bacteria. Therefore, for some bacteria the nutrient level has been shown to affect the adhesion of bacteria as well as the biofilm formation
The biofilm formation of S. putrefaciens was not affected by the concentration of glucose. Similarly, Kim and Frank (20) found that glucose had no consistent effect on biofilm formation of L. monocytogenes. In contrast, the structure and thickness of a mixed-species biofilm were influenced by addition of glucose (40). We speculated that lactate, which is readily metabolized by S. putrefaciens, would be a better carbohydrate source and thus would result in exopolysaccharide production and thicken the biofilm; however, adding lactate had no effect on biofilm formation.
Caccavo et al. (3) reported that flocs and/or biofilms of Shewanella algae where influenced by iron(III) as the addition of Fe(III) resulted in deflocculation. As S. putrefaciens also is a prominent Fe(III) reducer (9, 31), we evaluated the effect on biofilms of limiting iron or adding a surplus. Neither had, in our study, any effect on the number of bacteria adhering or on the kinetics of biofilm formation.
A bacterium will only in very selective niches exist as a pure culture. Most commonly, the organism will be part of a community containing several species. The fluorescent pseudomonads and S. putrefaciens will be the most common members of the microbial community in a range of chilled, proteinaceous foods (4, 13, 14), and we therefore monitored the adhesion and biofilm formation of S. putrefaciens when coinoculated with P. fluorescens. The lower numbers of S. putrefaciens bacteria adhering when grown with P. fluorescens could be caused by the antagonistic capability of P. fluorescens (15). In contrast, numbers of L. monocytogenes in a biofilm became higher and persisted for longer time when P. fragi was also present (37). This was believed to be due to the extracellular matrix produced by P. fragi, which embedded and protected the listeriae.
Many approaches have been used for quantifying bacteria on nontransparent surfaces. If cell numbers are high, fluorescence microscopy is useful, although a detailed quantification is difficult when bacteria are arranged in clusters or layers. Some authors detach bacteria from the surface by ultrasound, by mixing, or by swabbing (38) and quantify these by standard plate counts thereafter. Ultrasonic removal of cells has been used by several authors (24, 34), and we found that this procedure for quantification was in excellent agreement with counts obtained using Malthus detection times converted via a standard curve. Similarly, Flint et al. (12) used the Malthus method to determine the number of thermophilic streptococci on stainless steel, and Johnston and Jones (19) used impedance measurements to examine the number of adherent Proteus mirabilis, Staphylococcus aureus, and P. aeruginosa bacteria in a disinfection experiment. Also, the conductometric method may, by substrate manipulations, be used for indicative (e.g., TMAO-reducing) or selective quantification of specific bacteria.
In conclusion, we have found that the fish spoilage bacterium S. putrefaciens is able to attach and form biofilms on food processing surfaces. In contrast to our expectations, nutrient conditions did not affect biofilm formation, whereas the presence of P. fluorescens reduced numbers of adhering S. putrefaciens bacteria.
ACKNOWLEDGMENT
The study was supported by a grant from The Danish Agency for Trading and Industry of the Danish Ministry of Commerce.
REFERENCES
- 1.Autio T, Hielm S, Miettinen M, Sjöberg A-M, Aarnisalo K, Björkroth J, Mattila-Sandholm T, Korkeala H. Sources of Listeria monocytogenes contamination in a cold-smoked rainbow trout processing plant detected by pulsed-field gel electrophoresis typing. Appl Environ Microbiol. 1999;65:150–155. doi: 10.1128/aem.65.1.150-155.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes L-M, Lo M F, Adams M R, Chamberlain A H L. Effect of milk proteins on adhesion of bacteria to stainless steel surfaces. Appl Environ Microbiol. 1999;65:4543–4548. doi: 10.1128/aem.65.10.4543-4548.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caccavo F, Jr, Frølund B, Van Ommen Kloeke F, Halkjær Nielsen P. Deflocculation of activated sludge by the dissimilatory Fe(III)-reducing bacterium Shewanella alga BrY. Appl Environ Microbiol. 1996;62:1487–1490. doi: 10.1128/aem.62.4.1487-1490.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chai T, Chen C, Rosen A, Levin R E. Detection and incidence of specific species of spoilage bacteria on fish. Appl Microbiol. 1968;16:1738–1741. doi: 10.1128/am.16.11.1738-1741.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Characklis W G. Fouling biofilm development: a process analysis. Biotechnol Bioeng. 1981;XXIII:1923–1960. doi: 10.1002/bit.22227. [DOI] [PubMed] [Google Scholar]
- 6.Dawood Z, Brözel V S. Corrosion-enhancing potential of Shewanella putrefaciens isolated from industrial cooling waters. J Appl Microbiol. 1998;84:929–936. [Google Scholar]
- 7.de Franca F P, Lutterbach M T S. Variation in sessile microflora during biofilm formation on AISI-304 steel coupons. J Ind Microbiol. 1996;17:6–10. doi: 10.1007/BF01570140. [DOI] [PubMed] [Google Scholar]
- 8.Dewanti R, Wong A C L. Influence of culture conditions on biofilm formation by Escherichia coli O157:H7. Int J Food Microbiol. 1995;26:147–164. doi: 10.1016/0168-1605(94)00103-d. [DOI] [PubMed] [Google Scholar]
- 9.DiChristina T J, Delong E F. Design and application of rRNA-targeted oligonucleotide probes for the dissimilatory iron- and manganese-reducing bacterium Shewanella putrefaciens. Appl Environ Microbiol. 1993;59:4152–4160. doi: 10.1128/aem.59.12.4152-4160.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duddridge J E, Kent C A, Laws J F. Effect of surface shear stress on attachment of Pseudomonas fluorescens to stainless steel under defined flow conditions. Biotechnol Bioeng. 1982;XXIV:153–164. doi: 10.1002/bit.260240113. [DOI] [PubMed] [Google Scholar]
- 11.Easter M C, Gibson D M, Ward F B. A conductance method for the assay and study of bacterial trimethylamine oxide reduction. J Appl Bacteriol. 1982;52:357–365. [Google Scholar]
- 12.Flint S H, Brooks J D, Bremer P J. Use of Malthus conductance growth analyser to determine numbers of thermophilic streptococci on stainless steel. J Appl Microbiol. 1997;83:335–339. doi: 10.1046/j.1365-2672.1997.00233.x. [DOI] [PubMed] [Google Scholar]
- 13.Gennari M, Tomaselli S, Cotrona V. The microflora of fresh and spoiled sardines (Sardina pilchardus) caught in Adriatic (Mediterranean) sea and stored in ice. Food Microbiol. 1999;16:15–28. [Google Scholar]
- 14.Gram L, Trolle G, Huss H H. Detection of specific spoilage bacteria from fish stored at low (0°) and high (20°) temperatures. Int J Food Microbiol. 1987;4:65–72. [Google Scholar]
- 15.Gram L. Inhibitory effect against pathogenic and spoilage bacteria of Pseudomonas strains isolated from spoiled and fresh fish. Appl Environ Microbiol. 1993;59:2197–2203. doi: 10.1128/aem.59.7.2197-2203.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harkes G, Feijen J, Dankert J. Adhesion of Escherichia coli on to a series of poly(methacrylates) differing in charge and hydrophobicity. Biomaterials. 1991;12:853–860. doi: 10.1016/0142-9612(91)90074-k. [DOI] [PubMed] [Google Scholar]
- 17.Hood S K, Zottola E A. Growth media and surface conditioning influence the adherence of Pseudomonas fragi, Salmonella typhimurium, and Listeria monocytogenes bacteria to stainless steel. J Food Prot. 1997;60:1034–1037. doi: 10.4315/0362-028X-60.9.1034. [DOI] [PubMed] [Google Scholar]
- 18.Johansen C, Falholt P, Gram L. Enzymatic removal and disinfection of bacterial biofilms. Appl Environ Microbiol. 1997;63:3724–3728. doi: 10.1128/aem.63.9.3724-3728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnston M D, Jones M V. Disinfection tests with intact biofilms: combined use of Modified Robbins Device with impedance detection. J Microbiol Methods. 1995;21:15–26. [Google Scholar]
- 20.Kim K Y, Frank J F. Effect of nutrients on biofilm formation by Listeria monocytogenes on stainless steel. J Food Prot. 1994;58:24–28. doi: 10.4315/0362-028X-58.1.24. [DOI] [PubMed] [Google Scholar]
- 21.Krysinski E P, Brown L J, Marchisello T J. Effects of cleaners and sanitizers on Listeria monocytogenes attached to product contact surfaces. J Food Prot. 1992;55:246–251. doi: 10.4315/0362-028X-55.4.246. [DOI] [PubMed] [Google Scholar]
- 22.Kumar C G, Anand S K. Significance of microbial biofilms in food industry: a review. Int J Food Microbiol. 1998;42:9–27. doi: 10.1016/s0168-1605(98)00060-9. [DOI] [PubMed] [Google Scholar]
- 23.Lewin R. Microbial adhesion is a sticky problem. Science. 1984;224:375–377. doi: 10.1126/science.6143401. [DOI] [PubMed] [Google Scholar]
- 24.Lindsay D, von Holy A. Evaluation of dislodging methods for laboratory-grown bacterial biofilms. Food Microbiol. 1997;14:383–390. [Google Scholar]
- 25.Little B, Wagner P, Hart K, Ray R, Lavoie D, Nealson K, Aguilar C. The role of biomineralization in microbiologically influenced corrosion. Biodegradation. 1998;9:1–10. doi: 10.1023/a:1008264313065. [DOI] [PubMed] [Google Scholar]
- 26.McEldowney S, Fletcher M. Effect of growth conditions and surface characteristics of aquatic bacteria on their attachment to solid surfaces. J Gen Microbiol. 1986;132:513–523. [Google Scholar]
- 27.McEldowney S, Fletcher M. Adhesion of bacteria from mixed cell suspension to solid surfaces. Arch Microbiol. 1987;148:57–62. doi: 10.1007/BF00429648. [DOI] [PubMed] [Google Scholar]
- 28.McLean R J C, Nickel J C, Olson M E. Biofilm associated urinary tract infections. In: Lappin-Scott H M, Costerton J W, editors. Microbial biofilms. Cambridge, United Kingdom: Cambridge University Press; 1995. pp. 261–273. [Google Scholar]
- 29.Michiels C W, Schellekens M, Soontjens C C F, Hauben K J A. Molecular and metabolic typing of resident and transient fluorescent pseudomonad flora from a meat mincer. J Food Prot. 1997;60:1515–1519. doi: 10.4315/0362-028X-60.12.1515. [DOI] [PubMed] [Google Scholar]
- 30.Miettinen M, Björkroth K J, Korkeala H. Characterization of Listeria monocytogenes from an ice cream plant by serotyping and pulsed-field gel electrophoresis. Int J Food Microbiol. 1999;46:187–192. doi: 10.1016/s0168-1605(98)00185-8. [DOI] [PubMed] [Google Scholar]
- 31.Nealson K, Myers C R. Microbial reduction of manganese and iron: new approaches to carbon cycling. Appl Environ Microbiol. 1992;58:439–443. doi: 10.1128/aem.58.2.439-443.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norwood D E, Gilmour A. The growth and resistance to sodium hypochlorite of Listeria monocytogenes in a steady-state multispecies biofilm. J Appl Microbiol. 2000;88:512–520. doi: 10.1046/j.1365-2672.2000.00990.x. [DOI] [PubMed] [Google Scholar]
- 33.Obuekwe C O, Westlake D W S, Cook F D, Costerton J W. Surface changes in mild steel coupons from the action of corrosion-causing bacteria. Appl Environ Microbiol. 1981;41:766–774. doi: 10.1128/aem.41.3.766-774.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oulahal-Lagsir N, Marial-Gros A, Bonneau M, Blum L J. Ultrasonic methodology coupled to ATP bioluminescence for the non-invasive detection of fouling in food processing equipment—validation and application to a dairy factory. J Appl Microbiol. 2000;89:433–441. doi: 10.1046/j.1365-2672.2000.01132.x. [DOI] [PubMed] [Google Scholar]
- 35.Ravn Jørgensen B, Gibson D M, Huss H H. Microbiological quality and shelf life prediction of chilled fish. Int J Food Microbiol. 1988;6:295–307. doi: 10.1016/0168-1605(88)90023-2. [DOI] [PubMed] [Google Scholar]
- 36.Reid G, Lam D, Policova Z, Neumann A W. Adhesion of two uropathogens to silicone and lubricious catheters: influence of pH, urea and creatine. J Mater Sci Mater Med. 1993;4:17–22. [Google Scholar]
- 37.Sasahara K C, Zottola E A. Biofilm formation by Listeria monocytogenes utilizes a primary colonizing microorganism in flowing systems. J Food Prot. 1993;56:1022–1028. doi: 10.4315/0362-028X-56.12.1022. [DOI] [PubMed] [Google Scholar]
- 38.Smoot L M, Pierson M D. Effect of environmental stress on the ability of Listeria monocytogenes Scott A to attach to food contact surfaces. J Food Prot. 1998;61:1293–1298. doi: 10.4315/0362-028x-61.10.1293. [DOI] [PubMed] [Google Scholar]
- 39.Smoot L M, Pierson M D. Influence of environmental stress on the kinetics and strength of attachment of Listeria monocytogenes Scott A to buna-N rubber and stainless steel. J Food Prot. 1998;61:1286–1292. doi: 10.4315/0362-028x-61.10.1286. [DOI] [PubMed] [Google Scholar]
- 40.Stoodley P, Dodds I, Boyle J D, Lappin-Scott H M. Influence of hydrodynamics and nutrients on biofilm structure. J Appl Microbiol Symp Suppl. 1999;85:19S–28S. doi: 10.1111/j.1365-2672.1998.tb05279.x. [DOI] [PubMed] [Google Scholar]
- 41.Wirtanen G, Mattila-Sandholm T. Removal of foodborne biofilms—comparison of surface and suspension tests. Part 1. Lebensm-Wiss Technol. 1992;25:43–49. [Google Scholar]
- 42.Wood A J, Baird E A. Reduction of trimethylamine oxide by bacteria. 1. The Enterobacteriaceae J Fish Res Board Can. 1943;6:194–201. [Google Scholar]