Abstract
Background & Aims
Liver transplant recipients (LTRs) show a decreased immune response after 2 severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) vaccinations compared with healthy controls (HCs). Here, we investigated the immunogenicity of additional vaccinations.
Methods
In this prospective study, humoral (anti-SARS-CoV-2 receptor-binding domain [anti-S RBD]) and cellular (interferon-gamma release assay) immune responses were determined after mRNA-based SARS-CoV-2 vaccination in 106 LTRs after a third vaccination and in 36 LTRs after a fourth vaccination. Patients with anti-S RBD antibody levels >0.8 arbitrary unit (AU)/mL after vaccination were defined as responders.
Results
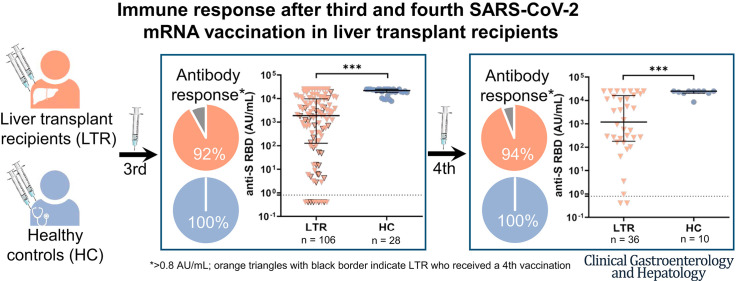
After 3 vaccinations, 92% (97/106) of LTRs compared with 100% (28/28) of HCs were responders. However, the antibody titer of LTRs was lower compared with HCs (1891.0 vs 21,857.0 AU/mL; P < .001). Between a second and third vaccination (n = 75), the median antibody level increased 67-fold in LTRs. In patients seronegative after 2 vaccinations, a third dose induced seroconversion in 76% (19/25), whereas all HCs were already seropositive after 2 vaccinations. A spike-specific T-cell response was detected in 72% (28/39) after a third vaccination compared with 32% (11/34) after a second vaccination. Independent risk factors for a low antibody response (anti-S RBD <100 AU/mL) were first vaccination within the first year after liver transplant (odds ratio [OR], 8.00; P = .023), estimated glomular filtration rate <45 mL/min (OR, 4.72; P = .006), and low lymphocyte counts (OR, 5.02; P = .008). A fourth vaccination induced a 9-fold increase in the median antibody level and seroconversion in 60% (3/5) of previous non-responders.
Conclusions
A third and fourth SARS-CoV-2 vaccination effectively increases the humoral and cellular immune response of LTRs, but to a lesser extent than in HCs. A fourth vaccination should be generally considered in LTRs.
Keywords: COVID-19, Immunosuppression, Liver Transplant Recipients, T Cells, Third and Fourth SARS-CoV-2 Vaccination
Abbreviations used in this paper: aHT, arterial hypertension; anti-S RBD, anti-SARS-CoV-2 receptor-binding domain; AU, arbitrary units; CI, confidence interval; CNI, calcineurin inhibitor; COVID-19, coronavirus disease 2019; eGFR, estimated glomerular filtration rate; HC, healthy controls; IGRA, interferon-gamma release assay; IFN-γ, interferon-gamma; IQR, interquartile range; LT, liver transplant; LTRs, liver transplant recipients; MMF, mycophenolate mofetil; NC, nucleocapsid; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus type 2; SOT, solid organ transplantation
Graphical abstract
What You Need to Know.
Background
Liver transplant recipients (LTRs) show a reduced immune response to severe acute respiratory syndrome coronavirus type 2 vaccination with a lower seroconversion rate and lower antibody levels compared with the healthy population.
Findings
Additional vaccinations increase seroconversion rates, antibody levels, and cellular immune response in LTRs, but to a lesser extent than in healthy controls. Many LTRs without humoral response develop memory T-cells.
Implications for patient care
Common comorbidities represent risk factors for a weak humoral response, but repeated vaccinations enhance the immune response. A fourth vaccination should be considered in LTRs.
Liver transplant recipients (LTRs) are at higher risk of developing more severe coronavirus disease 2019 (COVID-19) courses due to immunosuppression and the high prevalence of comorbidities.1 Therefore, adequate protection against COVID-19 is essential. Severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) vaccination of LTRs has been shown to reduce infection rates, disease severity, and death related to COVID-19.2 However, clinical studies demonstrated reduced seroconversion rates and low antibody titers in LTRs compared with healthy individuals after 2 vaccinations.3 , 4 In this ongoing pandemic with the emergence of novel SARS-CoV-2 variants, it has been shown that much higher vaccine-induced anti-spike antibody titers are required for viral neutralization,5 , 6 suggesting that indeed a large proportion of LTRs may be suboptimally protected against these novel strains. There is now ample data on the efficacy of a third vaccination in healthy individuals,7 and pilot studies also show promising results in solid organ transplant (SOT)recipients. 8, 9, 10, 11 However, these studies included only very few LTRs. In addition, a fourth vaccination has been suggested for immunosuppressed and older patients, but so far there is no data for LTRs.
Methods
Study Population and Data Collection
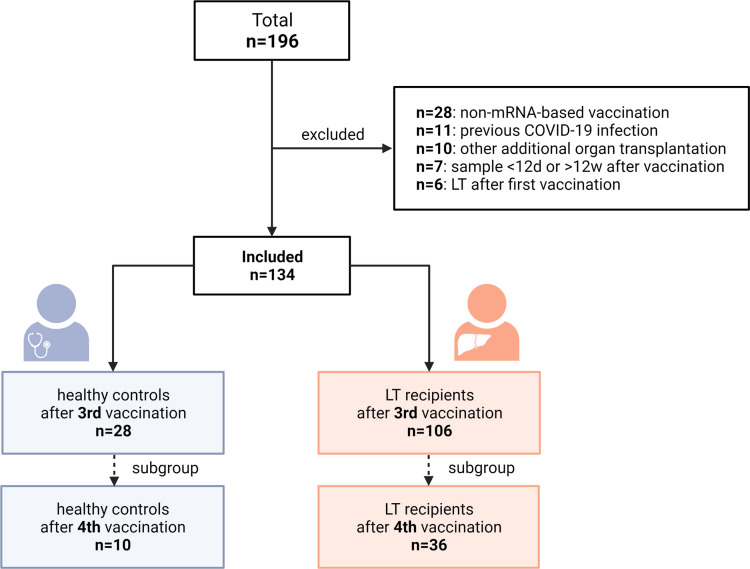
In this prospective observational cohort study, 106 LTRs who presented for follow-up after 2 or 3 SARS-CoV-2 vaccinations were enrolled at the University Medical Center Hamburg-Eppendorf over 6 months (Figure 1 ). Exclusion criteria were age <18 years, pregnancy, previous vaccination with a non-mRNA-based vaccine, positive antibodies against SARS-CoV-2 nucleocapsid (NC), liver transplant (LT) after the first vaccination, and combined organ transplantation. Patients with follow-up <12 or >85 days after a third vaccination were also excluded. Patients received mRNA-based (BNT162b2; BioNTech SE/Pfizer or mRNA-1273; Moderna [50 or 100 μg]) vaccinations. Clinical data were obtained from the patients’ electronic medical records. Glomerular filtration rate (eGFR) was estimated using the Chronic Kidney Disease Epidemiology Collaboration equation.12 Trough levels of immunsuppresive agents were determined at the time of the third vaccination or within 4 weeks before or after vaccination. In addition, 28 healthy controls (HCs) were included. The study was approved by the local Ethics Committee of Hamburg, Germany (Reg. number PV7103 and PV7298) and the Paul Ehrlich Institute, the German Federal Institute for Vaccines and Biomedicines (Reg. number NIS508). All participants signed written informed consent, and all authors had access to the study data and reviewed and approved the final manuscript.
Figure 1.
Flowchart of the study design.
Analysis of the SARS-CoV-2 Vaccine-specific Humoral Response
The vaccine-specific humoral immune response was quantitatively determined by the anti-SARS-CoV-2 receptor-binding domain (anti-S RBD) assay by Roche as described previously.3 A negative result or non-response was defined as <0.8 arbitrary units (AU)/mL, and a low positive response was defined from 0.8 to 100 AU/mL based on thresholds of validating studies and on cutoffs used in randomized trials.13 Antibody titers >100 AU/mL were defined as positive responses. Seroconversion was defined as a detectable response (≥0.8 AU/mL) after any vaccination in LTRs or HCs who previously showed no response (<0.8 AU/mL). To assess a previous SARS-CoV-2 infection, patients were tested for antibodies against SARS-CoV-2 nucleocapsid with the qualitative Elecsys anti-NC-SARS-CoV-2 Ig assay (Roche, Mannheim Germany; cutoff ≥1 COI/mL) at time of follow-up after third and fourth vaccination. NC-positive patients were excluded.
Analysis of Vaccine-induced Spike-specific T Cell Response
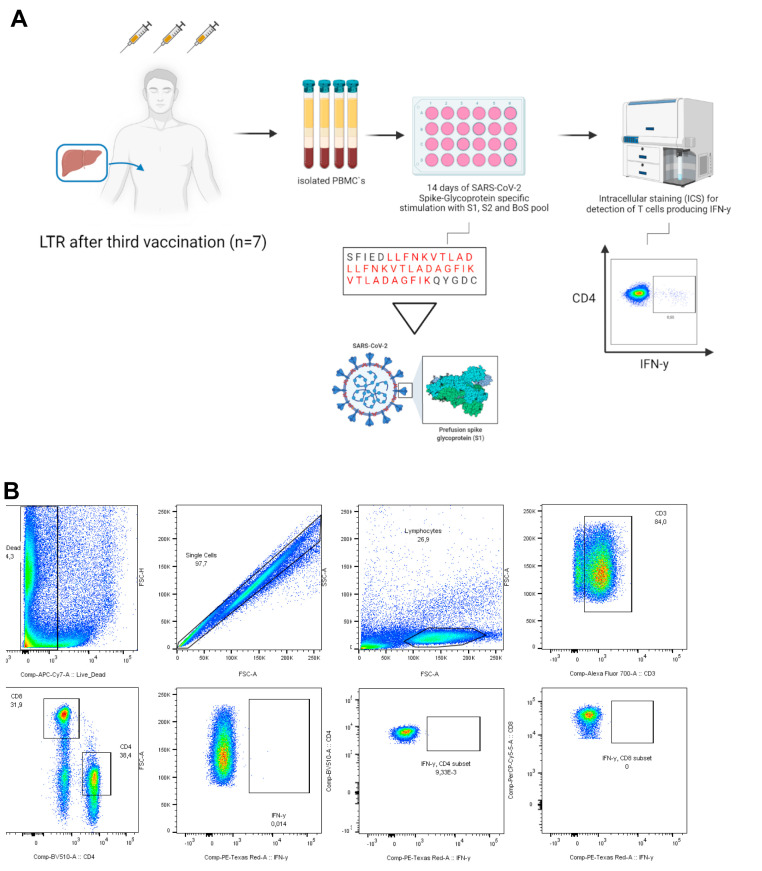
The vaccine-induced SARS-CoV-2 spike-specific T-cell response was assessed by a commercial, standardized interferon-gamma (IFN-γ) release assay (IGRA, EUROIMMUN) and a sensitive assay measuring cytokine production following the in vitro expansion of spike-specific T cells. The IGRA assay was performed as described previously.3 Values >100 mIU/mL and <200 mIU/mL were interpreted as low positive; values ≥200 mIU/mL as high positive. In vitro spike-specific T-cell expansion was induced by stimulation with a spike-glycoprotein peptide pool for 14 days, followed by an analysis of IFN-γ production after re-stimulation with the peptide pool by flow cytometry, described in detail in Supplementary Figure 1.14
Supplementary Figure 1.
A, Methods and gating strategy of the in vitro T cell assay. B, To further evaluate T-cell response, in vitro clonal T-cell expansion was induced by stimulation a pool of 12 previously determined, highly immunogenic 15-mer peptides stemming from the SARS-CoV-2 spike protein (Supplementary Table 2) and anti-CD28/anti-CD49d antibodies (BD Bioscience, Franklin Lakes, NJ), 50 U/mL rIL-2 (Miltenyi Biotec, Bergisch-Gladbach, Germany) for 14 days. The pre-cultured cells were restimulated with the peptides (10 μg/mL) for 16 hours at 37 °C and 5% CO2. After 1 hour, Brefeldin A (5 μg/mL) was added to inhibit cytokine secretion. The cells were stained with Zombie NIR fixable viability dye (BioLegend, San Diego, CA) and the following fluorochrome-conjugated monoclonal antibodies cocktail: anti-CD3, clone UCHT1 (AlexaFluor700, BioLegend), anti-CD4, clone SK3 (BV510, BioLegend), anti-CD8, clone RPA-T8 (PerCP-Cy5.5, BioLegend), anti-CD14, clone 63D3 (APC-Cy7, BioLegend) and anti-CD19, clone HIB19 (APC-Cy7, BioLegend). For intracellular staining of IFN-γ (clone 4S.B3, PE-Dazzle594, BioLegend), cells were fixated and permeabilized using the Foxp3 transcription factor staining buffer set (eBioscience, Thermo Fisher Scientific, Waltham, MA). Cells were acquired on a BD fluorescence activated cell sorting Canto II or LSRFortessa II cytometer (BD Biosciences), and FlowJo version 10.8.0 (BD Biosciences) or FACSDiva V8 was used for the flow cytometric analysis.
Statistical Analysis
SPSS Statistics Version 26 for Mac (IBM Corp, Armonk, NY) was used for statistical analyses, including significance tests according to the respective question (Pearson χ2 test, Fisher exact test, t test, Mann-Whitney U test, Kruskal-Wallis test, or Wilcoxon test), tests for correlation (Spearman rank test), and binary logistic regression analysis to identify risk factors for low immune response. GraphPad Prism version 8.0.0 for Mac (Graph-Pad Software, San Diego, CA) was used to create figures.
Results
Patient Characteristics
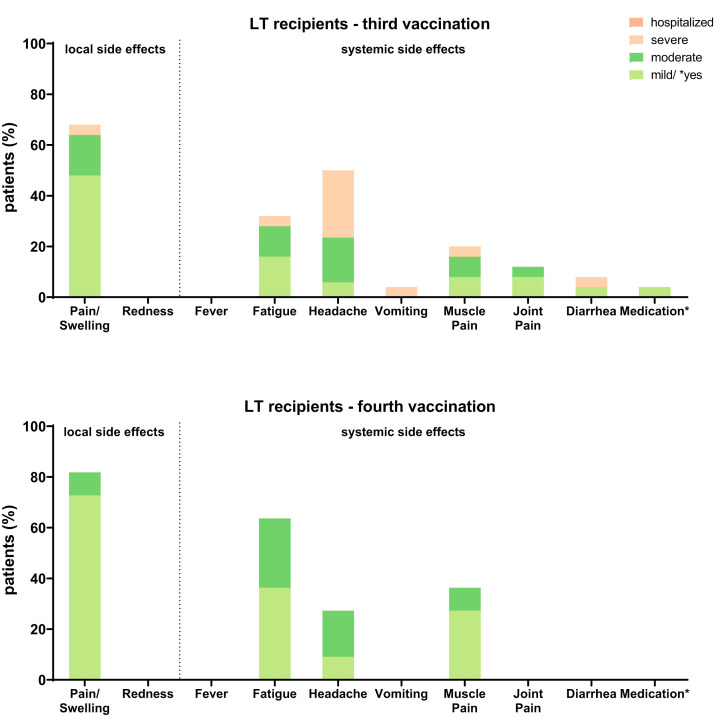
The clinical data of 106 LTRs and 28 HCs included in our analysis are given in Table 1 . Altogether, 36 LTRs also received a fourth vaccination. None of the patients or HCs included reported severe side effects after third or fourth vaccination. The frequency of mild side effects is displayed in Supplementary Figure 2.
Table 1.
Patient Characteristics
| LTRs (n = 106) | HCs (n = 28) | P value | LTRs 4th vaccination (n = 36) |
HCs (n = 10) | P value | |
|---|---|---|---|---|---|---|
| Age at 3rd vaccination, y | 59.0 (51.0–68.3) | 33.0 (25.5–43.8) | < .001 | 61.0 (52.5.–67.0) | 53.0 (32.3–60.0) | .092 |
| Females | 42 (39.6) | 17 (60.7) | 13 (36.1) | 5 (50.0) | ||
| Time 2nd to 3rd vaccination, d | 157.0 (127.0–188.0) | 214.0 (190.0–252.0) | < .001 | 130.0 (98.3–164.3) | 222.0 (216.5–228.0) | < .001 |
| Time 3rd to 4th vaccination, d | 125.0 (94.5–147.5) | 126.0 (93.0–148.0) | 123.5 (91.2–186.0) | .532 | ||
| BMI, kg/m2 | 25.3 (22.1–28.3) | 22.0 (19.2–25.6) | .0546 | 24.2 (22.7–27.9) | 24.5 (21.7–28.0) | .879 |
| Diabetes mellitus | 22 (20.8) | 0 (0.0) | 11 (30.6) | 0 (0.0) | ||
| Arterial hypertension | 80 (75.4) | 0 (0.0) | 28 (77.8) | 0 (0.0) | ||
| CKD | ||||||
| GFR 30‒59 mL/min | 46 (43.4) | 0 (0.0) | 16 (44.4) | 0 (0.0) | ||
| GFR <30 mL/min | 13 (12.3) | 0 (0.0) | 7 (19.4) | 0 (0.0) | ||
| Etiology of liver disease | ||||||
| ALD | 22 (20.8) | 4 (11.1) | ||||
| AILD | 20 (18.7) | 8 (22.2) | ||||
| NASH | 7 (6.6) | 1 (3.8) | ||||
| Viral | 19 (17.9) | 3 (8.3) | ||||
| ALF | 8 (7.5) | 3 (8.3) | ||||
| Pediatric | 6 (5.7) | 2 (5.6) | ||||
| Other | 28 (26.4) | 15 (41.7) | ||||
| HCC before LT | 23 (21.7) | 5 (13.9) | ||||
| Time from 1st LT, y | 8.8 (2.6–14.8) | 10.0 (2.6–21.3) | ||||
| Vaccination ≤1 y post LT | 9 (8.5) | 3 (8.3) | ||||
| Immunosuppression | ||||||
| Monotherapy | 24 (22.6) | 8 (22.2) | ||||
| Tacrolimus | 19 (17.9) | 6 (16.7) | ||||
| Cyclosporine | 2 (1.9) | 1 (3.8) | ||||
| mTORi | 2 (1.9) | 0 (0.0) | ||||
| MMF | 1 (0.9) | 1 (3.8) | ||||
| CNI + MMF | 44 (41.5) | 15 (41.7) | ||||
| CNI + azathioprine | 2 (1.9) | 1 (3.8) | ||||
| CNI + mTORi | 16 (15.1) | 2 (5.6) | ||||
| CNI + prednisone | 7 (6.6) | 5 (13.9) | ||||
| mTORi + MMF | 1 (0.9) | 0 (0.0) | ||||
| mTORi + azathioprine | 0 (0.0) | 0 (0.0) | ||||
| mTORi + prednisone | 4 (3.8) | 2 (5.6) | ||||
| ≥ 3 immunosuppressants | 8 (7.5) | 3 (8.3) | ||||
| Biologicals | 2 (1.9) | 2 (5.6) | ||||
| Laboratory | ||||||
| HbA1c, % (ref. 4.8-5.6) | 5.6 (5.2–6.5) | 5.6 (5.3–6.6) | ||||
| Creatinine, mg/dL | 1.2 (0.9–1.5) | 1.4 (1.1–2.0) | ||||
| eGFR (CKD-EPI), mL/min | 54.5 (41.0–76.0) | 49.0 (33.0–68.0) | ||||
| Lymphocytes, billion/μl | 1.3 (0.9–2.0) | 1.2 (0.8–1.7) |
Note: Data are presented as number (%), mean (standard deviation), or median (interquartile range).
Note: Boldface P values indicate statistical significance.
AILD, Autoimmune liver disease; ALD, alcoholic liver disease; ALF, acute liver failure; BMI, body mass index; CKD, chronic kidney disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CNI, calcineurin inhibitor; eGFR, estimated glomerular filtration rate; HC, healthy control; HCC, hepatocellular carcinoma; LTR, liver transplant recipient; MMF, mycophenolate mofetil; mTORi, mammalian target of rapamycin inhibitors; NASH, nonalcoholic steatohepatitis.
Supplementary Figure 2.
Adverse events in LTRs after third and fourth SARS-CoV-2 vaccinations.
Spike-specific Humoral Immune Response After a Third SARS-CoV-2 Vaccination in LTRs and HCs
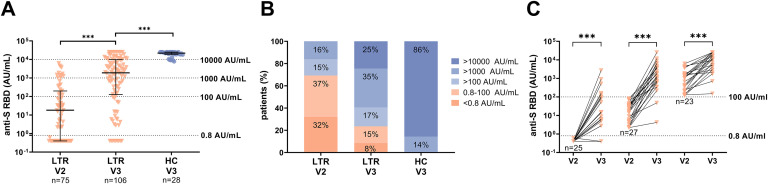
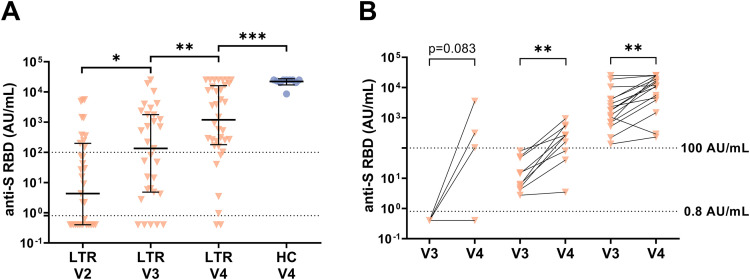
The anti-S RBD levels were analyzed in 106 LTRs and 28 HCs after a third vaccination (LTRs: median, 29.5 days; interquartile range [IQR], 23.3‒49.0 days; HCs: median, 20.0 days; IQR, 16.0‒23.0) (Figure 2 , A‒C). At that time point, 92% (97/106) of LTRs and 100% of HCs tested positive (Figure 2, B), but the overall median antibody level was significantly lower in LTRs compared with HCs (1891.0 vs 21,857.0 AU/mL; P < .001) (Figure 2, A). Looking at the 75 LTRs with available samples after a second vaccination (V2), the proportion of seroconverted LTRs increased from 67% (50/75) to 92% (69/75), and the median antibody titer increased 67-fold from 18.1 to 1214.0 AU/mL (P < .001). In the 25 LTRs with a previous non-response, a seroconversion was achieved in 76% (19/25), but with a lower median anti-S RBD level compared with patients with a previous low positive (0.8‒100 AU/mL) or positive (>100 AU/mL) humoral immune response (8.9 vs 1727.0 vs 10478.0 AU/mL, respectively; P < .001) (Figure 2, C).
Figure 2.
Humoral immune response after a second (V2) and third (V3) SARS-CoV-2 vaccination in LTRs (orange descending triangles) and HCs (blue dots). A, Anti-S RBD (AU/mL) levels. B, The bar graphs indicate the proportions of negative (<0.8 AU/mL), low positive (<100 AU/mL), positive (>100 AU/mL), high (>1000 AU/mL), and very high response (>10000 AU/mL). C, Humoral response after a third vaccination depending on the humoral response level after a second vaccination (<0.8, 0.8‒100, >100 AU/mL) in a subgroup of 75 LTRs. Statistical analysis was performed by the Mann-Whitney test (∗P < .05; ∗∗P < .01; ∗∗∗P < .001). Solid horizontal lines indicate medians and interquartile; dotted horizontal lines indicate cutoff values for no response, low positive, positive, high, and very high response.
Risk Factors for a Low Humoral Response After a Third SARS-CoV-2 Vaccination in LTRs
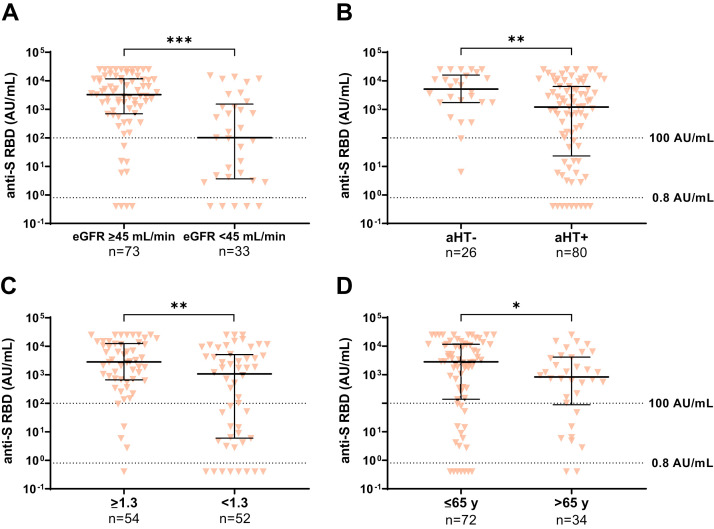
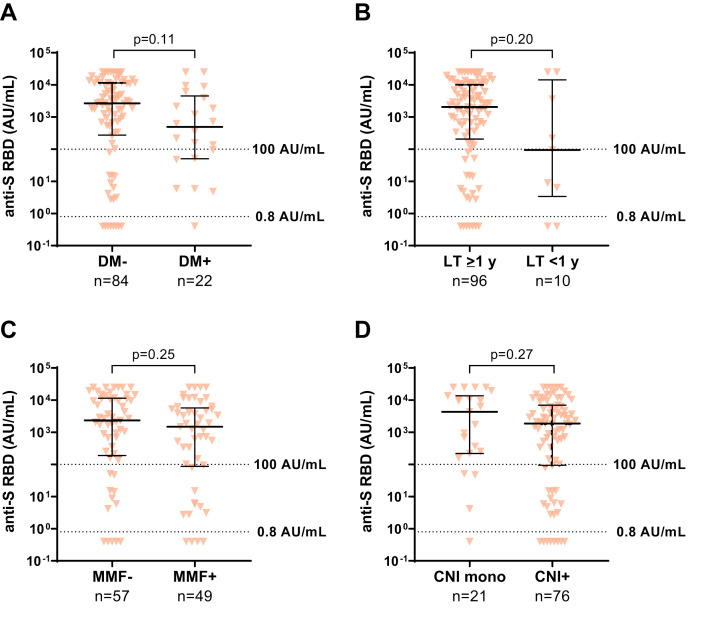
To analyze risk factors for low humoral response to a third vaccination, a univariate and multivariate regression analysis was done (Table 2 ). Factors associated with an increased risk for low antibody levels (<100 AU/mL) were: first vaccination within the first year after LT (odds ratio [OR], 8.00; 95% confidence interval [CI], 1.34‒47.77; P = .023), impaired kidney function with eGFR <45 mL/min (OR, 4.72; 95% CI, 1.56‒14.38; P = .006), and low lymphocyte counts (OR, 5.02; 95% CI, 1.53‒16.42; P = .008). A list of all tested variables is given with the Supplementary material (Supplementary Table 1). In addition, the median antibody levels were compared and found to be significantly lower in patients with an eGFR <45 mL/min, arterial hypertension (aHT), lymphocyte counts <1.3/nL, and age >65 years (Supplementary Figure 3). Other factors that showed a trend to be associated with lower antibody titers but did not reach significance were diabetes mellitus, first vaccination within the first year after LT, immunosuppressive therapy including mycophenolate mofetil (MMF), or combination therapy including calcineurin inhibitors (CNIs) compared with CNI monotherapy (Supplementary Figure 4). When comparing the median antibody titers of various immunosuppression regimens with each other, there was a significant difference between patients on CNI monotherapy (n = 21) and patients on MMF with a daily dosage >1 g (n = 11) (4312.0 vs 340.5 AU/mL; P < .05). No difference was found between patients with low (tacrolimus <4 μg/L, cyclosporine <70 μg/L; n = 29) and high (n = 64) CNI trough levels (1215 [IQR, 28‒10,228] vs 2352 [IQR, 346‒10,244] AU/mL; P = .332) or with low (tacrolimus <2 mg/d, cyclosporine <130 mg/d; n = 23) and high (n = 73) CNI dosages (1214 [IQR, 363‒3755] vs 2909 [IQR, 125‒10,635] AU/mL; P = .458). Also, in the subgroup of patients on tacrolimus (n = 72), the trough level neither showed a significant effect on the humoral response (OR, 1.06; P = .768) nor a strong correlation with antibody levels (r = 0.034; P = .777).
Table 2.
Variables With Significance in the Univariate Logistic Regression Analysis for a Low Humoral Response (<100 AU/mL) in LTRs After a Third SARS-CoV-2 Vaccination Based on the Anti-S RBD Immunoassay
| Univariate OR (95% CI) | P | Multivariate OR (95% CI) | P | |
|---|---|---|---|---|
| Vaccination <1 y post LT (n = 10/106) | 3.80 (1.01–14.42) | .049 | 8.00 (1.34–47.77) | .023 |
| Arterial hypertension (n = 80/106) | 4.84 (1.06–22.17) | .042 | 5.93 (0.90–39.22) | .065 |
| eGFR, <45 mL/min (n = 33/106) | 6.69 (2.52–17.77) | < .001 | 4.72 (1.56–14.38) | .006 |
| Lymphocytes, <1.3/nla (n = 52/106) | 6.13 (2.09–17.97) | .001 | 5.02 (1.53–16.42) | .008 |
Note: Boldface P values indicate statistical significance.
Anti-S RBD, Anti-SARS-CoV-2 receptor-binding domain; CI, confidence interval; eGFR, estimated glomerular filtration rate; IS, immunosuppression; LT, liver transplantation; LTR, liver transplant recipient; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus type 2.
Median of overall LT cohort.
Supplementary Figure 3.
Risk factors for a low humoral response after third SARS-CoV-2 vaccination. Anti-S RBD (AU/mL) after the third vaccination in LTRs. A, eGFR <45 mL/min vs ≥45 mL/min. B, Normal blood pressure vs aHT. C, Lymphocyte count ≥1.3/nL vs <1.3/nL. D, Age groups ≤65 and >65 years. Statistical analysis was performed by the Mann-Whitney test (∗P < .05; ∗∗P < .01; ∗∗∗P < .001). Solid horizontal lines indicate medians and interquartile ranges; dotted horizontal lines indicate cutoff values for no response (<0.8 AU/mL), low positive (0.8‒100 AU/mL), and positive (>100 AU/mL).
Supplementary Figure 4.
Risk factors with no significance for a low humoral response after third SARS-CoV-2 vaccination. Anti-S RBD (AU/mL) after the third vaccination in LTRs. A, No diabetes mellitus vs diabetes mellitus. B, First vaccination ≥1 year after LT vs first vaccination within the 1 year after LT. C, Immunosuppression without MMF vs including MMF. D, Immunosuppression with CNI monotherapy vs CNI plus any other immunosuppressive medication. Statistical analysis was performed by the Mann-Whitney test. Solid horizontal lines indicate medians and interquartile ranges; dotted horizontal lines indicate cutoff values for no response (<0.8 AU/mL), low positive (0.8‒100 AU/mL), and positive (>100 AU/mL). DM, Diabetes mellitus.
Spike-specific Cellular Immune Response After a Third SARS-CoV-2 Vaccination in LTRs and HCs
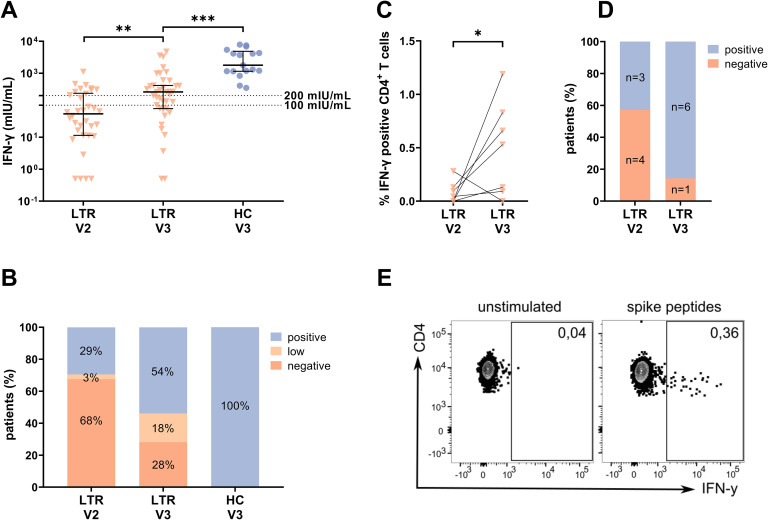
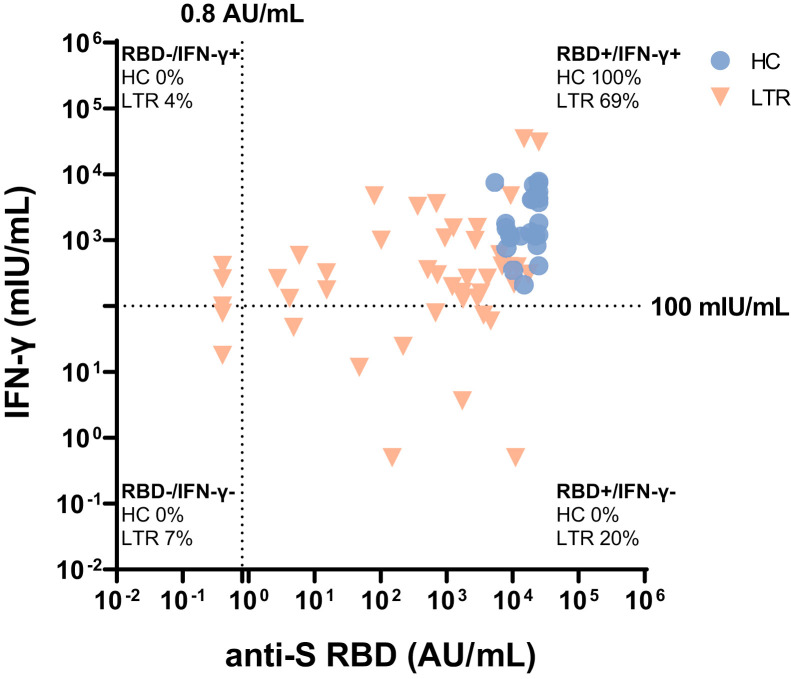
The spike-specific T-cell response was assessed by an IGRA in 39 unselected LTRs and 17 HCs as previously described. After a third vaccination, the median response level increased from 53.7 to 260.2 mIU/mL (P < .01), and the proportion of patients with a positive response increased from 32% (11/34) to 72% (28/39) (Figure 3 , A‒B). However, the median level of response in the semiquantitative IGRA was lower in LTRs compared with HCs (260.2 vs 1792 mIU/mL; P < .001) (Figure 3, A). Overall, more patients showed a positive humoral response than a positive T-cell response (positive anti-S RBD: 92% vs positive IGRA: 72%). Altogether, only 7% (3/45) of LTR had neither a detectable antibody nor IGRA response after a third (Supplementary Figure 5) as compared with 24% (8/34) after a second vaccination.
Figure 3.
T-cell response using IGRA (A‒B) and in vitro (C‒E) assay after a third vaccination. A, IFN-γ level (mIU/mL) of LTRs and HCs. Solid horizontal lines indicate medians and interquartile ranges; dotted horizontal lines indicate cutoff values for no response (<100 mIU/mL), low positive (100‒200 mIU/mL), and positive (>200 mIU/mL). B, The bar graphs indicate the proportions of negative (<100 mIU/mL), low positive (100‒200 mIU/mL), and positive (>200 mIU/mL). C, Percentage of IFN-γ+ cells of all CD4+ T cells before and after a third vaccination. D, Proportions of negative and positive cellular response before and after third vaccination detected following spike-specific T-cell expansion. E, Representative fluorescence activated cell sorting plots for C. Statistical analysis was performed by Mann-Whitney test (∗P < .05; ∗∗P < .01; ∗∗∗P < .001).
Supplementary Figure 5.
Correlation between humoral and T-cell immune response after third vaccination. Correlation between humoral and T-cell immune response for LTR (red descending triangles) and healthy controls (blue dots) after third vaccination. Humoral response measured with anti-S RBD (AU/mL); T-cell response measured with IFN-γ release (mIU/mL). Dotted lines indicate cutoff values. Percentages indicate proportions of values for every patient group.
To also uncover potentially low-level spike-specific CD4+ T cells, a sensitive in vitro approach was performed in 7 LTRs with a negative humoral and IGRA response before a third vaccination. Spike-specific T cells were cultured for 2 weeks, and IFN-γ production was measured after spike-specific re-stimulation (Supplementary Figure 1). With this approach, 3 of 7 LTRs (43%) showed a positive response before a third vaccination despite a negative IGRA. The response rate increased to 86% (6/7) after a third vaccination, and the mean proportion of IFN-γ+ CD4+ T cells increased from 0.08% to 0.50% (P < .05) (Figure 3, C‒D). Two of the 7 patients remained negative in the IGRA after a third vaccination, but tested positive by this in vitro approach.
Humoral and Cellular Immune Response After a Fourth SARS-CoV-2 Vaccination in LTRs and HCs
Altogether, 36 LTRs received a fourth vaccine dose after a median of 123 days (IQR, 91‒186 days). The seroconversion rate of patients with a previous non-response was 60% (3/5), and the median anti-S RBD titer increased 9-fold from 134.6 to 1196.0 AU/mL (P < .01) (Figure 4 , A‒B). However, in LTRs, the median antibody level remained lower than in HCs (1196 vs 25,000 AU/mL; P < .001). Furthemore, 2 of 4 previously IGRA-negative patients developed a new spike-specific T-cell response, but quantitatively, the cellular immune response assessed by IGRA did not increase after a fourth dose (176.3 vs 78.2 mIU/mL; P = .518). Overall the cellular immune response was significantly higher in HCs as compared with LTRs (987.9 vs 78.2 mIU/mL; P < .01).
Figure 4.
Humoral immune response in LTRs and HCs after fourth vaccination (V4). A, Anti-S RBD levels (AU/mL). B, Humoral response after a fourth vaccination depending on the humoral response level after a third vaccination (<0.8, 0.8‒100, >100 AU/mL). Solid horizontal lines indicate medians and interquartile ranges; dotted horizontal lines indicate cutoff values for no response (<0.8 AU/mL), low positive (0.8‒100 AU/mL), and positive (>100 AU/mL). Statistical analysis was performed by the Mann-Whitney test (∗P < .05; ∗∗P < .01; ∗∗∗P < .001).
Discussion
This is the first study to investigate the humoral and cellular immune response after third and fourth SARS-CoV-2 vaccinations in a large cohort of LTRs. After a third vaccination, an overall seroconversion rate of 92% was found. In LTRs with a previous non-response after 2 vaccinations, a seroconversion rate of 76% was achieved. These response rates are somewhat higher than those reported for other SOT recipients,8, 9, 10, 11 but still well below that of HCs in the current study. However, it is important to highlight the strong 67-fold boost of antibody levels after a third vaccination in LTR in comparison to the 7-fold increase in a health care worker cohort with a predominantly strong response after a second vaccination.15
Although the exact antibody level required to protect against infection with variants of concern is unknown, the degree of protection against infections and severe disease courses increases with rising antibody levels.16 It is assumed that evolving variants of concern, like the delta and omicron variants, require much (10‒50-fold) higher antibody levels in the approved assays compared with the wild-type variant.5 , 6 Very high antibody levels (>10,000 AU/mL) were only present in a minority of LTRs after a third vaccination (25%). Therefore, in these patients, insufficient humoral protection against SARS-CoV-2 infection in combination with a weaker spike-specific T-cell response is still a relevant concern.
The results of the IGRA also showed an effect of a third vaccination on the detection and magnitude of the spike-specific T-cell response, with an increase in the response rate from 32% to 72% of LTRs. To assess the cellular immune response to vaccination more comprehensively, we investigated whether low-level spike-specific CD4+ T cells were detectable in LTRs who seemed to were non-responsive to vaccination with a highly sensitive approach with prior in vitro cultivation of T cells in a subgroup of 7 patients with negative humoral and IGRA response. Indeed, using this method, a positive cellular immune response was detected in 3 of 7 LTRs, demonstrating that spike-specific CD4+ memory T cells were primed by vaccination at least in some LTRs with no detectable response in the commercially available assays. It is interesting to hypothesize whether LTRs with no or low antibody levels are still protected from severe COVID-19 by this low-level T-cell response. Notably, our results show that these T-cell responses can be further enhanced by additional vaccinations. Here, 86% (6/7) of patients showed a specific T-cell response after a third dose.
In a multivariate regression analysis, we identified liver transplantation within the year of the first vaccination, impaired kidney function, and lower lymphocytes to be independently associated with lower antibody levels after a third vaccination. This is in line with our previous results in LTRs after 2 doses of SARS-CoV-2 vaccines3 and with those reported for other patient cohorts receiving immunosuppression.4 , 17, 18, 19 Moreover, a low eGFR was also demonstrated to be a negative predictor for response to a third SARS-CoV-2 vaccination.20
In contrast to previous studies after 2 vaccinations, we could not detect a significantly lower median antibody level in patients on more than 1 immunosuppressive agent.21 Also, several previous studies investigating the immune response of patients under immunosuppression after 2 vaccinations19 , 22 and a recent study looking at the effect of a third vaccination in LTRs23 identified MMF as an important risk factor for a low vaccine-induced humoral immune response. Furthermore, a dose-dependent effect of MMF on the seroconversion rate and the level of antibody titers was described for COVID-19-naïve patients.22 , 24 However, in a small cohort of LTRs who recovered from COVID-19, MMF treatment did not seem to attenuate the humoral response after vaccination.24
In our patient cohort, patients on MMF had lower median antibody levels compared with patients without MMF, but the difference did not reach statistical significance (Supplementary Figure 4, C). This may be due to the low average daily dose of MMF in our cohort of only 1 g. Indeed, we found significantly lower antibody levels in the subgroup of patients on MMF with a daily dosage >1 g compared with patients on CNI monotherapy.
Also in contrast to previous studies, we could not detect lower antibody levels after a third vaccination in patients with higher trough levels or higher daily dosages of CNI.25 , 26 This may be due to the small number of patients on CNI monotherapy (n = 21) in this study, which does not allow for adequate investigation of this issue. Until more data are available, we would currently recommend not generally reducing CNIs or discontinuing MMF before additional booster vaccination, but rather checking whether lower MMF doses are temporarily acceptable without the risk of rejection.
However, in real life, not only single but combined risk factors are probably most important for the vaccine-induced immune response. For example, when we looked at patients with aHT, an eGFR <45 mL/min, and lymphocytes <1.3/nl (n = 21) and compared them with patients without these comorbidities (n = 12), the median antibody levels were strikingly different (47.0 AU/mL vs 13,486.0 AU/mL; P < .001).
A small number of LTRs was analyzed after a fourth vaccination. In line with the results of pilot studies in other SOT recipients, we achieved a seroconversion rate of 60% in the LTRs with a previous non-response.26 , 27 Also, the median antibody level increased 9-fold after a fourth vaccination, although it remained relatively low (1196.0 AU/mL). Notably, this patient cohort does not represent the vaccination efficacy of a fourth dose in the overall LTR cohort, since mainly LTRs with previous low vaccination efficacy were selected for a fourth dose. So, after a third vaccination, the median antibody titer in this subgroup was much lower than in the overall population (134.6 vs. 1891.0 AU/mL; P < .001).
Nonetheless, as a prospective real-world study, the current study has several limitations. First, our analysis did not comprise the determination of neutralizing antibodies. However, a previous study showed that the anti-RBD assays overall correlate well with the results of neutralization assays.28 Another limitation is the different doses of the Moderna booster (50 μg or 100 μg) due to vaccine availability, as well as the doctor’s and patient’s preference. Therefore, further studies are needed to investigate a possible dose-dependent effect of a third and fourth vaccination. Also, the cohort of 106 LTRs was too small to comprehensively evaluate combinations of different risk factors. Therefore, larger cohorts are urgently needed to quantify the risk of vaccination failure for the different combinations of comorbidities and, thereby, adjust the vaccination guidelines for LTRs.
In summary, this study demonstrates a robust efficacy of a third and fourth SARS-CoV-2 vaccination in LTRs in terms of seroconversion, increase in antibody level, and priming of a cellular immune response. However, antibody levels remained significantly lower than in HCs after a third shot, and therefore, we propose a fourth vaccination for LTR, in particular for those with a previously low humoral response. Sensitive T-cell assays could help to further assess vulnerability to COVID-19 of LTRs with a weak humoral response. LTRs with frequent comorbidities represent a highly vulnerable population, in whom protection by vaccination may not be achievable. So, alternative strategies for prophylaxis and early treatment may be required in case of infection with SARS-CoV-2.
Acknowledgment
The authors thank all study participants and contributing departments of the University Medical Center Hamburg-Eppendorf for their active participation in the study.
CRediT Authorship Contributions
Aenne Harberts (Conceptualization: Equal; Formal analysis: Equal; Supervision: Equal; Visualization: Equal; Writing – original draft: Lead; Writing – review & editing: Lead)
Golda Meline Schaub (Data curation: Lead; Formal analysis: Equal; Visualization: Lead; Writing – original draft: Equal; Writing – review & editing: Equal)
Darius Ferenc Ruether (Formal analysis: Equal; Software: Equal; Visualization: Supporting; Writing – original draft: Supporting)
Paul-Maria Duengelhoef (Data curation: Equal; Formal analysis: Supporting; Visualization: Supporting; Writing – original draft: Supporting)
Thomas Theo Brehm (Data curation: Supporting; Writing – original draft: Supporting)
Hendrik Karsten (Data curation: Supporting; Visualization: Supporting)
Anahita Fathi (Writing – original draft: Supporting; Writing – review & editing: Supporting)
Jacqueline Jahnke-Triankowski (Data curation: Equal; Project administration: Equal)
Lutz Fischer (Funding acquisition: Supporting; Resources: Supporting)
Marylyn Martina Addo (Funding acquisition: Supporting; Resources: Equal; Writing – original draft: Supporting)
Friedrich Haag (Funding acquisition: Supporting; Methodology: Supporting; Supervision: Supporting; Writing – original draft: Supporting)
Marc Luetgehetmann (Conceptualization: Supporting; Funding acquisition: Supporting; Methodology: Supporting; Resources: Lead)
Ansgar Wilhelm Lohse (Supervision: Supporting; Writing – original draft: Supporting)
Julian Schulze zur Wiesch (Conceptualization: Lead; Funding acquisition: Lead; Methodology: Equal; Supervision: Lead; Visualization: Supporting; Writing – original draft: Equal; Writing – review & editing: Equal)
Martina Sterneck (Conceptualization: Lead; Funding acquisition: Lead; Methodology: Equal; Supervision: Lead; Visualization: Supporting; Writing – original draft: Equal; Writing – review & editing: Equal)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Friedrich Haag, Julian Schulze zur Wiesch, and Hendrik Karsten receive research funding from SFB1328, project A12; Golda Meline Schaub was supported by a German Center for Infection Research (DZIF) MD Stipend through the DZIF Academy. Anahita Fathi and Marylyn Martina Addo report funding from the DZIFTTU 01709. No further funding was used for the research reported.
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2022.06.028.
Supplementary Material
Supplementary Table 1.
Univariate and Multivariate Logistic Regression Analysis for a Low Humoral Response (<100 AU/mL) in LTRs After a Third SARS-CoV-2 Vaccination Based on the Anti-S RBD Immunoassay
| Univariate OR (95% CI) | P | Multivariate OR (95% CI) | P | |
|---|---|---|---|---|
| Age, y | 1.01 (0.98–1.05) | .419 | ||
| Age >65 years (n = 34/106) | 0.99 (0.38–2.60) | .993 | ||
| Female sex (n = 42/106) | 0.82 (0.32–2.07) | .672 | ||
| Time from 1st LT, y | 0.97 (0.91–1.02) | .239 | ||
| Vaccination <1 y post LT (n = 10/106) | 3.80 (1.01–14.42) | .049 | 8.00 (1.34–47.77) | .023 |
| BMI, kg/m2 | 0.99 (0.88–1.11) | .829 | ||
| BMI ≥30 kg/m2 (n = 13/99) | 0.99 (0.25–3.95) | .989 | ||
| Arterial hypertension (n = 80/106) | 4.84 (1.06–22.17) | .042 | 5.93 (0.90–39.22) | .065 |
| Diabetes mellitus (n = 22/106) | 1.71 (0.61–4.83) | .310 | ||
| eGFR, mL/min | 0.96 (0.94–0.99) | .001 | ||
| eGFR <45 mL/min (n = 33/106) | 6.69 (2.52–17.77) | < .001 | 4.72 (1.56–14.38) | .006 |
| HbA1c, % | 1.01 (0.57–1.79) | .978 | ||
| HbA1c >5.6%a (n = 42/88) | 0.89 (0.34–2.33) | .805 | ||
| Lymphocytes, billion/μL | 0.16 (0.06–0.46) | .001 | ||
| Lymphocytes <1.3/nLa (n = 52/106) | 6.13 (2.09–17.97) | .001 | 5.02 (1.53–16.42) | .008 |
| Immunosuppression | ||||
| CNI monotherapy (n = 21/106) | 0.48 (0.13–1.78) | .270 | ||
| CNI + prednisone (n = 7/106) | 1.32 (0.24–7.27) | .748 | ||
| CNI + MMF (n = 44/106) | 0.97 (0.39–2.42) | .947 | ||
| CNI + mTORi (n = 16/106) | 1.21 (0.35–4.21) | .762 | ||
| Tac trough level, μg/L (n = 71) | 1.06 (0.74–1.52) | .768 | ||
| CSA trough level, μg/L (n = 22) | 0.87 (0.73–1.04) | .127 | ||
| IS including MMF (n = 49/106) | 1.51 (0.61–3.73) | .368 | ||
| MMF >1 g/d (n = 11/106) | 2.01 (0.54–7.54) | .299 | ||
| IS including mTOR, μg/L (n = 25) | 1.46 (0.53–4.08) | .466 | ||
| mTORi trough level (continuous, n = 25) | 1.12 (0.64–1.98) | .693 | ||
| IS including prednisone (n = 20/106) | 1.08 (0.35–3.35) | .890 | ||
| IS inclusing MMF or mTORi (n = 74/106) | 2.00 (0.68–5.10) | .210 | ||
Note: Long version listing all the tested variables with a group size of at least 10% each of LTRs (n = 10) in the case of categorial variables.
Note: Boldface P values indicate statistical significance.
Anti-S RBD, Anti-SARS-CoV-2 receptor-binding domain; BMI, body mass index; CI, confidence interval; CSA, cyclosporin; CNI, calcineurin inhibitor; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; IS, immunosuppression; LD, liver disease; LT, liver transplantation; LTR, liver transplant recipient; MMF, mycophenolate mofetil; mTORi, mammalian target of rapamycin inhibitor; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus type 2; Tac, tacrolimus.
Median of overall LT cohort.
Supplementary Table 2.
Fiften-mer Peptides Stemming From the SARS-CoV-2 Spike Protein
| Most frequently detected peptides of the Spike glycoprotein | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptide | aa position | Sequence | ||||||||||||||
| 27 | 131–145 | C | E | F | Q | F | C | N | D | P | F | L | G | V | Y | Y |
| 34 | 166–180 | C | T | F | E | Y | V | S | Q | P | F | L | M | D | L | E |
| 41 | 201–216 | F | K | I | Y | S | K | H | T | P | I | N | L | V | R | D |
| 48 | 236–250 | T | R | F | Q | T | L | L | A | L | H | R | S | Y | L | T |
| 63 | 311–325 | G | I | Y | Q | T | S | N | F | R | V | Q | P | T | E | S |
| 69 | 341–355 | V | F | N | A | T | R | F | A | S | V | Y | A | W | N | R |
| 70 | 346–360 | R | F | A | S | V | Y | A | W | N | R | K | R | I | S | N |
| 75 | 371–385 | S | A | S | F | S | T | F | K | C | Y | G | V | S | P | T |
| 90 | 446–460 | G | G | N | Y | N | Y | L | Y | R | L | F | R | K | S | N |
| 163 | 811–825 | K | P | S | K | R | S | F | I | E | D | L | L | F | N | K |
| 164 | 816–830 | S | F | I | E | D | L | L | F | N | K | V | T | L | A | D |
| 167 | 831–845 | A | G | F | I | K | Q | Y | G | D | C | L | G | D | I | A |
| 180 | 896–910 | I | P | F | A | M | Q | M | A | Y | R | F | N | G | I | G |
SARS-CoV-2, Severe acute respiratory syndrome coronavirus type 2.
References
- 1.Webb G.J., Marjot T., Cook J.A., et al. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol. 2020;5:1008–1016. doi: 10.1016/S2468-1253(20)30271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.John B.V., Deng Y., Khakoo N.S., et al. COVID-19 vaccination is associated with reduced SARS CoV2 infection and death in liver transplant recipients. Gastroenterology. 2022;162:645–647.e2. doi: 10.1053/j.gastro.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruether D.F., Schaub G.M., Duengelhoef P.M., et al. SARS-CoV2-specific humoral and T-cell immune response after second vaccination in liver cirrhosis and transplant patients. Clin Gastroenterol Hepatol. 2022;20:162–172.e9. doi: 10.1016/j.cgh.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabinowich L., Grupper A., Baruch R., et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75:435–438. doi: 10.1016/j.jhep.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffmann M., Krüger N., Schulz S., et al. The Omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic. Cell. 2022;185:447–456.e11. doi: 10.1016/j.cell.2021.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Planas D., Veyer D., Baidaliuk A., et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 7.Barda N., Dagan N., Cohen C., et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398:2093–2100. doi: 10.1016/S0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schrezenmeier E., Rincon-arevalo H., Stefanski A., et al. B and T cell responses after a third dose of SARS-CoV-2 vaccine in kidney transplant recipients. J Am Soc Nephrol. 2021;32:3027–3033. doi: 10.1681/ASN.2021070966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall V.G., Ferreira V.H., Ku T., et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385:1244–1246. doi: 10.1056/NEJMc2111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stumpf J., Tonnus W., Paliege A., et al. Cellular and humoral immune responses after 3 doses of BNT162b2 mRNA SARS-CoV-2 vaccine in kidney transplant. Transplantation. 2021;105:E267–E269. doi: 10.1097/TP.0000000000003903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westhoff T.H., Seibert F.S., Anft M., et al. A third vaccine dose substantially improves humoral and cellular SARS-CoV-2 immunity in renal transplant recipients with primary humoral nonresponse. Kidney Int. 2021;100:106–107. doi: 10.1016/j.kint.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levey A.S., Stevens L.A., Schmid C.H., et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Resman Rus K., Korva M., Knap N., et al. Performance of the rapid high-throughput automated electrochemiluminescence immunoassay targeting total antibodies to the SARS-CoV-2 spike protein receptor binding domain in comparison to the neutralization assay. J Clin Virol. 2021;139 doi: 10.1016/j.jcv.2021.104820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heide J., Schulte S., Kohsar M., et al. Broadly directed SARS-CoV-2-specific CD4+ T cell response includes frequently detected peptide specificities within the membrane and nucleoprotein in patients with acute and resolved COVID-19. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brehm TT, Ullrich F, Thompson M, et al. Three separate spike antigen exposures by COVID-19 vaccination or SARS-CoV-2 infection elicit strong humoral immune responses in healthcare workers. medRxiv 2022.03.06.22271718. [DOI] [PMC free article] [PubMed]
- 16.Khoury D.S., Cromer D., Reynaldi A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 17.Cuadrado A., Barrio M., Fortea J.I., et al. Antibody response to the messenger RNA-1273 vaccine (Moderna) in liver transplant recipients. Hepatol Commun. 2022;6:1673–1679. doi: 10.1002/hep4.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guarino M., Cossiga V., Esposito I., et al. Effectiveness of SARS-CoV-2 vaccination in liver transplanted patients: the debate is open. J Hepatol. 2022;76:237–239. doi: 10.1016/j.jhep.2021.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davidov Y., Indenbaum V., Tsaraf K., et al. A third dose of the BNT162b2 mRNA vaccine significantly improves immune responses among liver transplant recipients. J Hepatol. 2022 doi: 10.1016/j.jhep.2022.03.042. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamar N., Abravanel F., Marion O., et al. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thuluvath P.J., Robarts P., Chauhan M. Analysis of antibody responses after COVID-19 vaccination in liver transplant recipients and those with chronic liver diseases. J Hepatol. 2021;75:1434–1439. doi: 10.1016/j.jhep.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kantauskaite M., Müller L., Kolb T., et al. Intensity of mycophenolate mofetil treatment is associated with an impaired immune response to SARS-CoV-2 vaccination in kidney transplant recipients. Am J Transplant. 2022;22:634–639. doi: 10.1111/ajt.16851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meunier L., Sanavio M., Dumortier J., et al. Mycophenolate mofetil decreases humoral responses to three doses of SARS-CoV -2 vaccine in liver transplant recipients. Liver Int. 2022;42:1872–1878. doi: 10.1111/liv.15258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toniutto P., Falleti E., Cmet S., et al. Past COVID-19 and immunosuppressive regimens affect the long-term response to anti-SARS-CoV-2 vaccination in liver transplant recipients. J Hepatol. 2022;77:152–162. doi: 10.1016/j.jhep.2022.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rozen-Zvi B., Yahav D., Agur T., et al. Antibody response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: a prospective cohort study. Clin Microbiol Infect. 2021;27:1173.e1–1173.e4. doi: 10.1016/j.cmi.2021.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caillard S., Thaunat O., Benotmane I., Masset C., Blancho G. Antibody response to a fourth messenger RNA COVID-19 vaccine dose in kidney transplant recipients: a case series. Ann Intern Med. 2022;175:455–456. doi: 10.7326/L21-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamar N., Abravanel F., Marion O., et al. Assessment of 4 doses of SARS-CoV-2 messenger RNA-based vaccine in recipients of a solid organ transplant. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benning L., Morath C., Bartenschlager M., et al. Neutralizing antibody response against the B.1.617.2 (delta) and the B.1.1.529 (omicron) variant after a third mRNA SARS-CoV-2 vaccine dose in kidney transplant recipients. Am J Transplant. 2022;22:1873–1883. doi: 10.1111/ajt.17054. [DOI] [PMC free article] [PubMed] [Google Scholar]