Abstract
BACKGROUND
Previous studies have reported that lung transplant recipients (LTR) develop a poor response to two doses of COVID-19 vaccine, but data regarding the third dose are lacking. We investigated the antibody response after three doses of mRNA vaccine in LTR and its predictive factors.
METHODS
A total of 136 LTR, including 10 LTR previously infected and 126 COVID-19-naive LTR, were followed during and after three doses of mRNA vaccine. We retrospectively measured anti-receptor-binding domain (RBD) IgG response and neutralizing antibodies. In a posthoc analysis, we used a multivariate logistic regression model to assess the association between vaccine response and patient characteristics, including viral DNA load (VL) of the ubiquitous Torque teno virus (TTV) (optimal cut-off set by ROC curve analysis), which reflects the overall immunosuppression.
RESULTS
After 3 doses, 47/126 (37.3%) COVID-19-naive LTR had positive anti-RBD IgG (responders) and 14/126 (11.1%) had antibody titers above 264 Binding Antibody Units/mL. None neutralized the omicron variant versus 7 of the 10 previously infected LTR. Nonresponse was associated with TTV VL ≥6.2 log10 copies/mL before vaccination (Odds Ratio (OR) = 17.87, 95% confidence interval (CI95) = 3.02-105.72), mycophenolate treatment (OR = 4.73, CI95 = 1.46-15.34) and BNT162b2 (n = 34; vs mRNA-1273, n = 101) vaccine (OR = 6.72, CI95 = 1.75-25.92). In second dose non-responders, TTV VL ≥6.2 or <3.2 log10 copies/mL before the third dose was associated with low (0/19) and high (9/10) rates of seroconversion.
CONCLUSION
COVID-19-naive LTR respond poorly to three doses of mRNA vaccine, especially those with high TTV VL. Future studies could further evaluate this biomarker as a guide for vaccine strategies.
Keywords: lung transplantation, COVID-19 vaccine, Torque teno virus, vaccine response, immunosuppression
Abbreviations: BAU, Binding Antibody Unit; ED50, half-maximal effective dilution; MMF/MPA, mycophenolate mofetil/mycophenolic acid; TTV, Torque teno virus
Introduction
Lung transplant recipients (LTR) are at high risk for severe COVID-19 due to their lung disease and the high doses of immunosuppressive drug therapy they receive to prevent allograft rejection.1 , 2 The highly transmissible omicron variant is resistant to several anti-SARS-CoV-2 monoclonal antibodies, and still represents a major issue in this population. Moreover, the combination of protease inhibitors nirmatrelvir/ritonavir (Paxlovid) is difficult to use in this population due to significant drug interactions. In this context, the COVID vaccine remains the safest strategy to protect LTR from severe disease.
By the end of 2020, solid organ transplant recipients were prioritized for COVID-19 mRNA vaccination. However, they have been shown to only develop a poor antibody response (34%-54%) to a 2-dose vaccine regimen compared to immunocompetent individuals,3 , 4 with lower response observed in LTR (0-40%) than in kidney or liver transplant recipients since they receive higher doses of immunosuppressive drugs.5, 6, 7, 8, 9, 10, 11 Previous studies showed that around half of solid organ recipients who were nonresponders to the second dose seroconverted after a third mRNA vaccine dose, given as booster.12, 13, 14, 15 However, only few studies have determined the effectiveness of a third vaccine dose in LTR,16 , 17 and markers predictive of vaccine response are still lacking. In order to quantify the impact of immunosuppression on vaccine response and hopefully to predict response or nonresponse, one should be able to quantify the level of immunosuppression. Previous studies have reported that the composition of the virome in plasma is affected by immunosuppressant drugs and may therefore predict the state of immunosuppression.18 In transplant recipients, the virome is mostly composed of Anelloviridae (68%) and Herpesviridae (13%).18 Torque teno virus (TTV) accounts for 97% of Anelloviridae fraction in the virome,18 and viral DNA loads (VL) in healthy individuals typically remain below 4 log10 cp/mL.19 In the case of immunosuppression, this virus replicates strongly, and the level of its burden has made it possible to stratify rejecting and non-rejecting recipients.18 This makes it a potential candidate for predicting vaccine response in transplant recipients. Among the Herpesviridae, increased VL of Epstein-Barr virus and Cytomegalovirus may also reflect immunosuppression status. However, antivirals used for prophylaxis after transplantation significantly decrease the load of these viruses and may prevent their use as effective markers of immunosuppression.
In this study, we characterized the antibody response in LTR after three doses of mRNA vaccine, by longitudinally analyzing anti-Receptor Binding Domain (RBD) IgG titers and neutralizing activity of sera against the ancestral strain D614G, B.1.617.2 (delta), and B.1.1.529 (omicron) variants. In posthoc analyses, we used logistic regression to assess for an association of demographic, clinical, and TTV VL variables with vaccine response.
Methods
Study design
We conducted a retrospective cohort study on LTR followed in the outpatient lung transplantation department of Strasbourg University Hospital. All LTR with serum samples available from 2 weeks to 2 months after the third booster dose of mRNA-based vaccine (administered between 1st April 2021 and 30th October 2021) were enrolled. To assess vaccine humoral responses and predictors in LTR without biases due to intercurrent events, patients who had received their first vaccine dose before lung transplantation and those infected between the first dose and the third dose, were excluded. Data including age, sex, body mass index, blood group, native lung disease, comorbidities, prior history of COVID-19, transplant type and date, and immunosuppressive drugs were collected just before vaccination, as well as information about COVID-19 vaccine types. The LTR cohort was divided into two groups according to previous SARS-CoV-2 infection status, as determined by serology performed in all patients before vaccination and by history of positive RT-PCR.
All patients provided informed written consent to the analysis of their samples included in the registered biobank n°DC2014-2222 for research purposes. This study was approved by the Institutional Review Board of the French learned Society for Respiratory Medicine: Société de Pneumologie de Langue Française (CEPRO 2022-009).
Antibody response
All available sera sampled within the three-month interval (median: 7 days, interquartile range [IQR]: 0-46) before vaccination and from 2 weeks to 2 months after each vaccine dose were retrospectively analyzed with the Abbott Architect SARS-CoV-2 IgG II Quant assay to assess the anti-receptor-binding domain (RBD) IgG response. All results were converted into Binding Antibody Units (BAU)/mL adapted to the World Health Organization standard for SARS-CoV-2 immunoglobulin by multiplication by a factor of 0.142 (quantification range: 1.0-11,360.0 BAU/mL, positivity threshold: 7.1 BAU/mL). Patients without history of COVID-19 who displayed positive IgG titers after a third vaccine dose were categorized as responders (vs seronegative patients who were classified as nonresponders). To exclude any humoral response following unidentified infection, the first seropositive sample of each COVID-19-naive vaccine responder was also examined for anti-nucleocapsid (N) IgG with the Abbott Architect SARS-CoV-2 IgG assay.
Pseudotyped virus-based neutralization assay
Neutralizing antibody titers were assessed by a pseudovirus-based assay in responders after the third vaccine dose against D614G, delta, and omicron variants, as described previously and in supplementary materials.20 The neutralization efficiency expressed as the log10 of the median half-maximal effective dilutions (ED50) was calculated using Prism 9.3.1. Sera were considered positive if they neutralized more than 50% SARS-CoV-2 pseudovirus at 1:40 dilution.
Viral genome amplification by real-time PCR
The TTV VL was retrospectively determined using the TTV R-GENE kit (bioMérieux) in the three months (median: 7 days, IQR: 0-46) preceding vaccination for all patients, as well as between the second and third dose if a blood sample was available.
Statistical analysis
We compared demographic, clinical and biological baseline characteristics between responders (patients displaying anti-RBD IgG ≥7.1 BAU/mL) and non-responder LTR to a three-dose vaccine regimen using the Fisher exact test and χ2 test for dichotomous variables and the Mann-Whitney U-test for continuous variables. Correlation analyses between continuous variables were performed using Spearman rank correlation test. Statistical tests were 2-tailed and significance was set at p < 0.05. Posthoc Receiver Operating Characteristic (ROC) curve analyses were conducted on TTV VL data, and values presenting sensitivity or specificity over 95% and the highest Youden index were selected as cut-offs. All analyses were performed using Prism 9.3.1. Multivariable logistic regression analysis was performed to identify independent predictors of antibody response after the third vaccine dose. Relevant parameters associated with nonresponse with a p-value <0.2 in the univariate analysis were included in the model. Statistical analyses were performed using SPSS 28.0 (IBM Statistics), and results were expressed as adjusted Odds Ratios (OR) with 95% confidence intervals (CI95).
Results
Participants
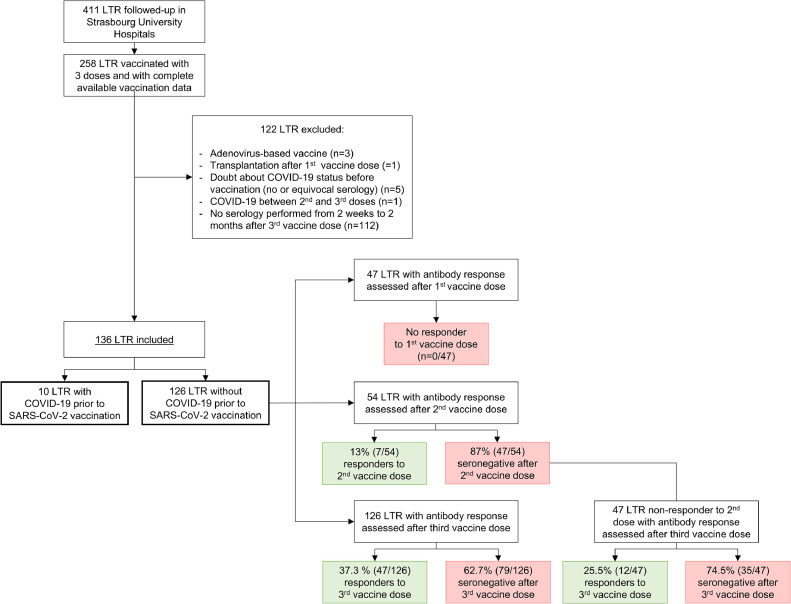
This study was conducted on 479 blood samples from 136 LTR, including 10 patients with and 126 without a history of COVID-19 (Figure 1 , Table S1). Median age was 62.5 years (IQR: 51.0-67.0), with 39% women in the cohort. Most patients had cardiovascular comorbidities, primarily hypertension (57.4%) and diabetes mellitus (49.3%). All received three doses of either BNT162b2 (Pfizer-BioNTech; n = 34, 25.0%) or mRNA-1273 (Moderna; n = 101, 74.3%) vaccines, except one patient who received a combination of both vaccines. The vaccination was carried out at a median of 5.5 years (IQR: 2.6-9.0) after lung transplantation. The first two doses were given 4 weeks apart, and the third dose was administered at a median interval of 59.0 days (IQR: 35.3-73.0) after the second dose. The maintenance immunosuppression regimen included glucocorticoids (94.1% of LTR), calcineurin inhibitors (97.0%; tacrolimus [86.0%] or cyclosporine [11.0%]), antimetabolites (91.9%) (mycophenolate mofetil/mycophenolic acid (MMF/MPA; 80.1%) or azathioprine (11.8%)), and everolimus (13.2%). Two patients received rituximab, 6.5 months before vaccination and three days after the first vaccine dose, respectively.
Figure 1.
Flow chart of lung transplant recipients (LTR) recruitment and antibody response to COVID-19 mRNA-based vaccination. The study was conducted on 136 LTR, including 10 and 126 patients with and without history of COVID-19 before vaccination, respectively. Sera sampled after each vaccine dose in COVID-19-naive patients were analyzed to assess the anti–receptor-binding domain (RBD) IgG response, with seropositive patents defined as responders.
Antibody response to the three-dose vaccine regimen in LTR with prior COVID-19
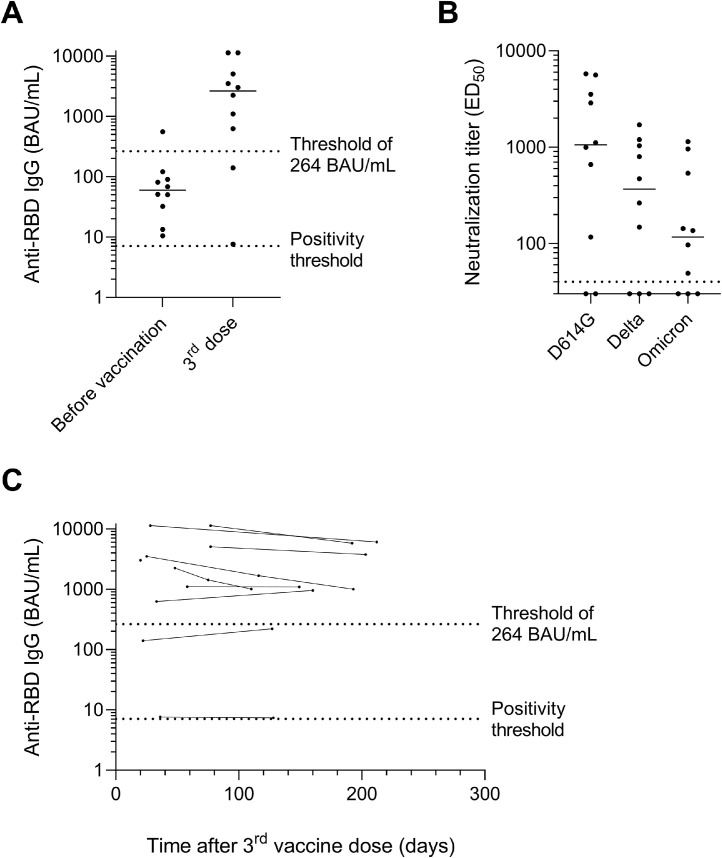
The 10 LTR with a history of COVID-19 before vaccination (proven by positive serology for anti-RBD IgG: n = 10/10 or by RT-PCR: 3/10) had asymptomatic (n = 5/10), mild (4) or severe (1) disease. All were seropositive before vaccination with anti-RBD IgG titers ranging from 10.5 to 554.8 BAU/mL (median: 68.2, IQR: 50.4-90.3). Unexpectedly, in one patient, anti-RBD IgG titer decreased from 13.3 to 7.6 BAU/mL despite 3-dose vaccination, whereas an antibody rebound was observed in the nine other patients (median increase: 2,633.1 BAU/mL, IQR: 742.8-4,658.7) (Figure 2 A). Among them, 8/10 patients harbored antibodies over 264 BAU/mL, which is the threshold now considered in France as a decision-making tool for the choice between prophylaxis with a fourth vaccine dose or with monoclonal antibodies therapy.21 , 22 Sera from these eight patients neutralized the D614G pseudovirus (log ED50: 2.07-3.76, median 3.02), seven of them also neutralizing the delta (log ED50: 2.17-3.23, median 2.55) and the omicron (log ED50: 1.69-3.06, median 2.06) variants (Figure 2B, Figure S1). Seven patients were followed up to seven months after the third dose without intercurrent antigenic stimulation or monoclonal antibody therapy, and all of them maintained anti-RBD IgG titers over 264 BAU/mL (Figure 2C).
Figure 2.
Antibody response after three vaccine doses in lung transplant recipients with history of COVID-19 (n = 10). (A) Anti-RBD IgG titers expressed in BAU/mL before vaccination and from two weeks to two months after the third dose. (B) Neutralizing antibody titers assessed by pseudovirus-based assay against D614G, delta and omicron variants. The dotted horizontal black line indicates the cutoff for positivity (1:40 dilution). (C) Dynamics of anti-RBD IgG titers expressed in BAU/mL over time after the third vaccine dose (n = 11 additional samples collected from 9 patients during follow-up). (A and C) The dotted lines indicate the positivity threshold (7.1 BAU/mL) and the threshold of 264 BAU/mL used in French recommendations to guide prophylactic strategy in immunosuppressed patients. BAU, Binding Antibody Units; ED50, half-maximal effective dilution; RBD, receptor-binding domain.
Antibody response to the 3-dose vaccine regimen in COVID-19-naive LTR
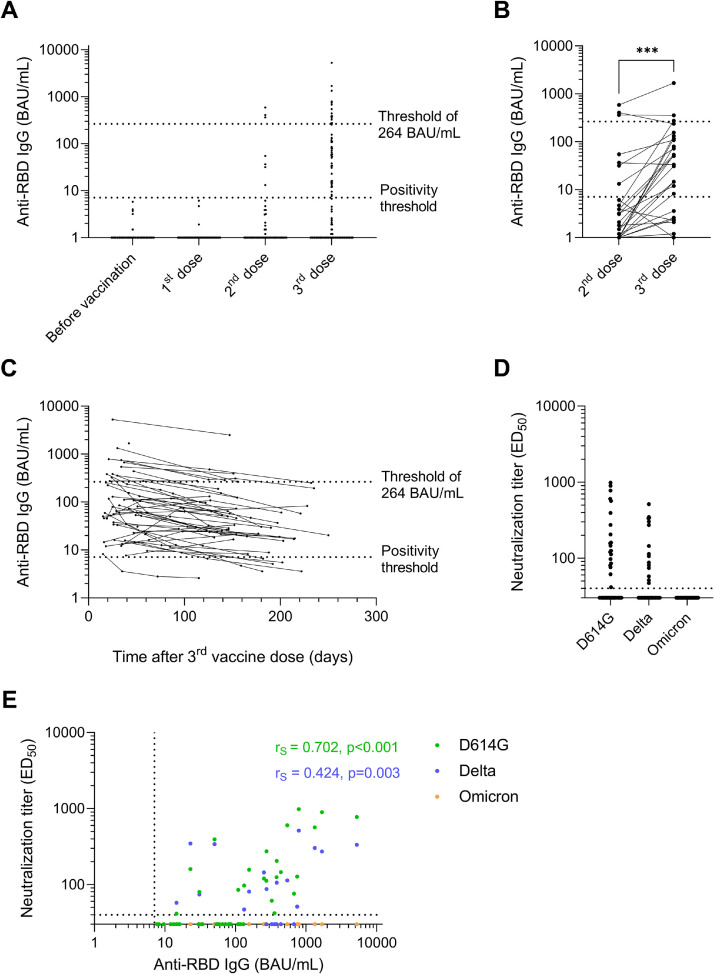
Anti-RBD IgG were available for 47, 54, and 126 COVID-19-naive LTR after the first, second, and third doses of vaccine, respectively. None of the LTR seroconverted after the first dose, and 13% (7/54) were anti-RBD IgG positive after the second dose, with titers ranging from 13.2 to 585.8 BAU/mL (median: 54.6, IQR: 34.1-383.5; Figure 3 A). After the third dose, 37.3% (47/126) LTR were seropositive with anti-RBD IgG ranging from 7.7 to 5,249.7 BAU/mL (median: 81.0, IQR: 32.0-274.1) including 14 patients with titers ≥264 BAU/mL (29.8% of responders and 11.1% of the whole cohort). Considering the 47 patients who tested seronegative after the second dose, the third dose led to a positive antibody response in 25.5% (12/47) patients, including one patient (representing 8.3% of these responders) with a titer over 264 BAU/mL (Figure 3B).
Figure 3.
Antibody response after three vaccine doses in COVID-19-naive lung transplant recipients (n = 126). (A) Anti-RBD IgG titers expressed in BAU/mL before vaccination (n = 126) and from two weeks to two months after the first (n = 47), the second (n = 54) and the third (n = 126) vaccine doses. (B) Anti-RBD IgG titers before and after the third vaccine doses in the 54 patients with sera available after the second dose. (C) Dynamics of anti-RBD IgG titers expressed in BAU/mL over time after the third vaccine dose (n = 95 additional samples collected from 47 patients during follow-up). (A, B and C) The dotted lines indicate the positivity threshold (7.1 BAU/mL) and the threshold of 264 BAU/mL used in French recommendations to guide prophylactic strategy in immunosuppressed patients. (D) Neutralizing antibody titers assessed by pseudovirus-based assay against D614G, delta and omicron variants in the 47 patients seropositive after the third dose (responders). 22 (46.8%) and 15 (31.9%) sera neutralized the D614G and the delta variants, respectively, but none neutralized the omicron variant. The dotted horizontal black line indicates the cutoff for positivity (1:40 dilution). (E) Spearman correlation between anti-RBD IgG titers and neutralizing antibody titers against D614G (green dots), delta (blue dots) and omicron (orange dots) variants. Green dots are overlapping blue and orange dots on the x axis, and blue dots are overlapping orange dots. The dotted horizontal and vertical black lines correspond to the positivity thresholds of neutralizing antibody titers and anti-RBD IgG titers, respectively. Spearman correlation coefficients (rS) and p-values related to the neutralization of the D614G mutant and the delta variant were calculated with the Graphpad Prism version 9.3.1. software and depicted in green and blue, respectively. BAU, Binding Antibody Units; ED50, half-maximal effective dilution; RBD, receptor-binding domain. ***p value <0.001.
Longitudinal serum samples collected from 6 to 8 months after the third dose were available for 21 responders. Anti-RBD IgG were still detectable for 16 of these responders (76.2%), but all titers were lower than 264 BAU/mL (Figure 3C). None of them had intercurrent infection, administration of a fourth vaccine dose, or monoclonal antibodies prophylaxis before the last serology performed during follow-up. Sera were not available for the remaining 26 responders at that time because their follow-up period after the third dose was shorter.
Analysis of the neutralizing activity of seropositive sera, collected at a median of 34 days post-third dose (IQR: 25.5-46.5 days), showed that 46.8% (22/47) neutralized the ancestral D614G strain with titers up to 2.99 log10 ED50 (median 2.14 log10 ED50) (Figure 3D). Only 31.9% (15/47) were able to neutralize the delta variant with 2.71 log10 ED50 as a maximal neutralizing antibody titer (median 2.06 log10 ED50; Figure 3D). Anti-RBD IgG titers over 132 BAU/mL and 748 BAU/mL were required to ensure neutralization of the D614G and the delta variants, respectively (Figure 3E). No serum was able to neutralize the omicron variant (Figure 3D, E).
During follow-up, seven (5.6%) patients including 5 nonresponders and 2 responders developed COVID-19 from 6.1 to 8.4 months after the third dose. These infections resulted in 3 asymptomatic, one mild and 3 severe diseases. The three severe cases were infected by the delta variant and were hospitalized in an intensive care unit, but with a good clinical outcome. Two of them were nonresponders and the third one displayed an antibody response lower than 264 BAU/mL 53 days after the third dose (132.5 BAU/mL), and a weak neutralizing activity against the delta variant (1.67 log10 ED50).
Predictors of vaccine response
Baseline demographic and clinical characteristics of the 126 LTR without a history of COVID-19 were compared between responders and nonresponders in Table 1 . Univariate analysis revealed that the 3-dose vaccine response is lower in LTR vaccinated with BNT162b2 (13.3%) compared to mRNA-1273 vaccine (44.2%, p = 0.004; Figure 4 A), and that the vaccine response rate increases with the time elapsed between transplantation and the first dose of vaccine. Nonresponse to vaccine was also associated with MMF/MPA treatment (p = 0.033), with high trough levels of MMF (p = 0.022) and tacrolimus (p < 0.001), and with high prednisone dosage (p = 0.039; Table 1, Table S2). Conversely, azathioprine treatment was associated with better vaccine response (p = 0.004). Of note, none of the 2 patients treated with rituximab seroconverted after the third dose.
Table 1.
Lung Transplant Recipient Characteristics Stratified by Antibody Response to a Three-Dose Regimen of COVID-19 Vaccine
| Variable | Nonresponders to three-dose vaccine regimen n = 79 | Responders to three-dose vaccine regimen n = 47 | p-value |
|---|---|---|---|
| Age, years, median (IQR) | 62.6 (53.7-65.9) | 63.0 (47.3-67.4) | 0.901 |
| Female, n (%) | 26 (32.9) | 21 (44.7) | 0.253 |
| Blood group | 0.788 | ||
| O, n (%) | 35 (44.3) | 22 (46.8) | |
| A, n (%) | 30 (38.0) | 18 (38.3) | |
| B, n (%) | 10 (12.7) | 3 (6.4) | |
| AB, n (%) | 3 (3.8) | 3 (6.4) | |
| Unknown, n (%) | 1 (1.3) | 1 (2.1) | |
| Primary disease | 0.609 | ||
| Chronic obstructive pulmonary disease, n (%) | 40 (50.6) | 20 (42.6) | |
| Cystic fibrosis, n (%) | 10 (12.7) | 8 (17.0) | |
| Interstitial lung disease, n (%) | 8 (10.1) | 3 (6.4) | |
| Other, n (%) | 21 (26.6) | 16 (34.0) | |
| BMI, kg/m², median (IQR) | 23.5 (20.3-26.7) | 23.9 (20.9-28.5) | 0.448 |
| Cardiovascular comorbidities | |||
| Hypertension, n (%) | 46 (58.2) | 26 (55.3) | 0.853 |
| Diabetes mellitus, n (%) | 42 (53.2) | 22 (46.8) | 0.581 |
| Obesity, n (%) | 8 (10.1) | 6 (12.8) | 0.771 |
| Chronic heart failure, n (%) | 2 (2.5) | 2 (4.3) | 0.629 |
| History of transient ischemic attack or stroke, n (%) | 9 (11.4) | 5 (10.6) | >0.999 |
| History of heart attack, n (%) | 6 (7.6) | 6 (12.8) | 0.361 |
| Transplant type | 0.111 | ||
| Double lung transplant, n (%) | 65 (82.3) | 39 (83.0) | |
| Single lung transplant, n (%) | 6 (7.6) | 0 (0.0) | |
| Cardiopulmonary transplant, n (%) | 6 (7.6) | 3 (6.4) | |
| Lung and liver transplant, n (%) | 0 (0.0) | 1 (2.1) | |
| Lung and kidney transplant, n (%) | 2 (2.5) | 2 (4.3) | |
| Lung and islet transplant, n (%) | 0 (0.0) | 2 (4.3) | |
| History of treated allograft acute rejection, n (%) | 25 (31.6) | 9 (19.1) | 0.150 |
| Chronic lung allograft dysfunction, n (%) | 17 (21.5) | 14 (29.8) | 0.393 |
| Maintenance immunosuppression | |||
| Tacrolimus, n (%) | 71 (89.9) | 38 (80.9) | 0.182 |
| Dose, mg, median (IQR) | 4.0 (3.0-7.0) | 4.0 (3.0-4.6) | 0.086 |
| Trough level, µg/L, median (IQR) | 7.5 (6.4-8.8) | 6.1 (5.6-7.4) | <0.001 |
| Cyclosporine, n (%) | 8 (10.1) | 6 (12.8) | 0.771 |
| MMF/MPA, n (%) | 69 (87.3) | 33 (70.2) | 0.033 |
| Dose, mg, median (IQR) | 1,750 (1,000-2,000) | 1,080 (1,000-2,000) | 0.031 |
| Trough level, mg/L, median (IQR) | 2.8 (2.0-4.9) | 2.2 (1.7-2.9) | 0.022 |
| Azathioprine, n (%) | 3 (3.8) | 10 (21.3) | 0.004 |
| Prednisone, n (%) | 77 (97.4) | 43 (91.4) | 0.195 |
| Dose, mg, median (IQR, range) | 10 (10-10, 0-10) | 10 (10-10, 0-40) | 0.039 |
| Everolimus, n (%) | 9 (11.4) | 8 (17.0) | 0.424 |
| COVID-19 vaccine | 0.004 | ||
| mRNA-1273, n (%) | 53 (67.1) | 42 (89.4) | |
| BNT162b2, n (%) | 26 (32.9) | 4 (8.5) | |
| mRNA-1273 + BNT162b2, n (%) | 0 (0.0) | 1 (2.1) | |
| Biology | |||
| Creatinine, µmol/L, median (IQR) | 102.9 (80.2-136.4) | 109.9 (84.2-133.0) | 0.732 |
| Leucocytes, G/L, median (IQR) | 6.0 (4.7-8.3) | 6.5 (5.4-7.7) | 0.578 |
| Lymphocytes, G/L, median (IQR) | 1.4 (0.9-1.7) | 1.5 (1.1-1.9) | 0.506 |
| Monocytes, G/L, median (IQR) | 0.6 (0.5-0.8) | 0.6 (0.5-0.8) | 0.453 |
| CRP, mg/L, median (IQR) | <4.0 (<4.0-5.8) | <4.0 (<4.0-7.0) | 0.965 |
| Prevaccine TTV viral load, log10 cp/mL, median (IQR) | 4.9 (3.4-7.7) | 3.8 (2.5-5.4) | 0.004 |
| Time between transplantation and first vaccination, years, median (IQR) | 4.9 (1.7-8.3) | 5.9 (4.2-9.3) | 0.022 |
| Time of 2nd vaccine from 1st vaccine, days, median (IQR) | 28.0 (28.0-30.0) | 28.0 (28.0-28.0) | 0.145 |
| Time of 3rd vaccine from 2nd vaccine, days, median (IQR) | 56.0 (34.0-74.0) | 62.0 (39.0-71.0) | 0.945 |
| Time of 3rd vaccine to antibody testing, days, median (IQR) | 35.0 (24.0-47.0) | 32.0 (25.0-42.0) | 0.137 |
| Maximum anti-RBD IgG concentration after third dose, BAU/mL, median (IQR) | <1.0 (<1.0-1.2) | 108.0 (34.1-326.3) | <0.001 |
BAU, Binding Antibody Units; BMI, body mass index; CRP, C-reactive protein; IQR, interquartile range; MMF/MPA, mycophenolate mofetil/mycophenolic acid; TTV, Torque teno virus.
Age, biological parameters, and medication data (including dose and trough levels) were assessed just before the first vaccine dose. Patient characteristics were compared between responders (patients displaying anti-RBD IgG ≥7.1 BAU/mL) and nonresponders using the Fisher exact test and χ2 test for dichotomous variables and the Mann-Whitney U-test for continuous variables with Graphpad Prism version 9.3.1. software. Statistical significance was set at p < 0.05 (shown in bold).
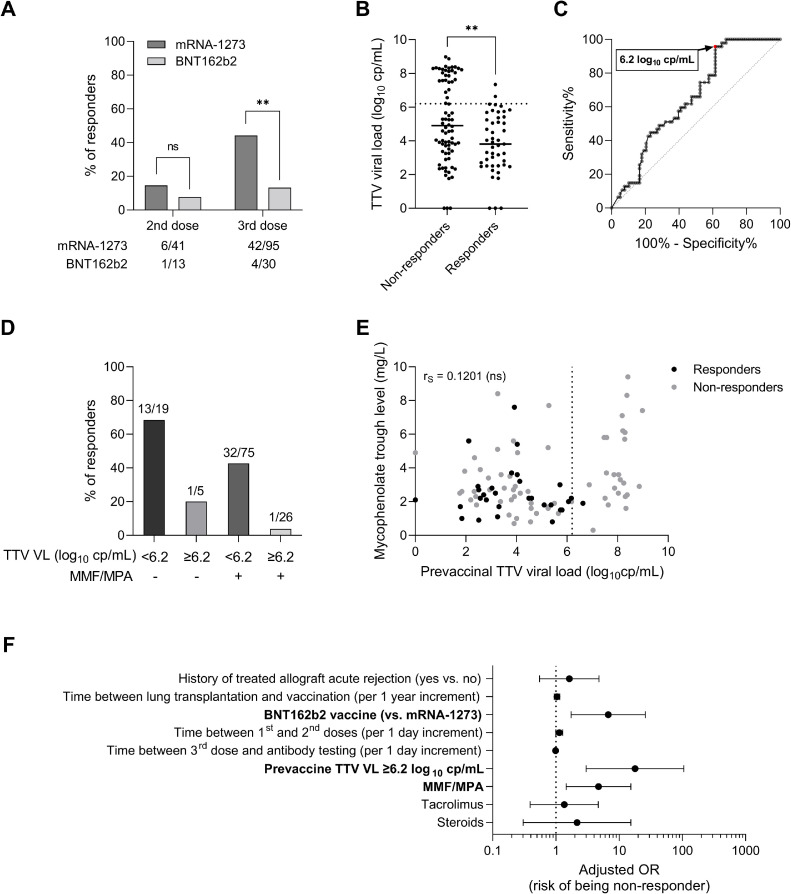
Figure 4.
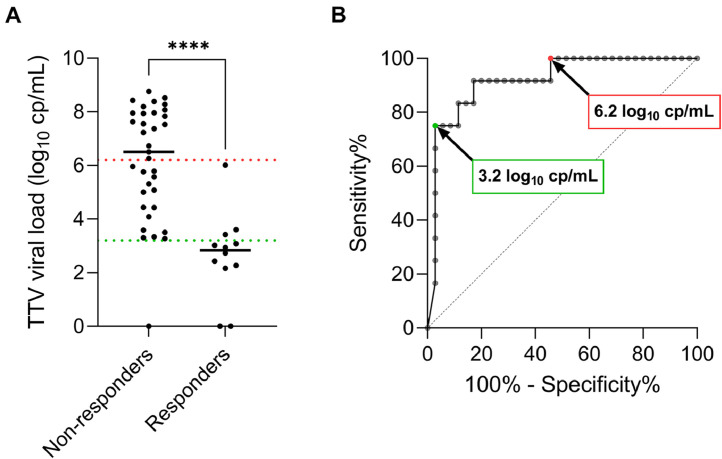
Investigation of predictors of vaccine response in COVID-19-naive lung transplant recipients (n = 125). (A) Vaccine response rates after the second and the third vaccine doses according to mRNA vaccine type. The number of responders to each vaccine dose is detailed below the bar chart. (B) Prevaccine TTV viral load in nonresponders (n = 78) and responders (n = 47) to a three-dose regimen of COVID-19 mRNA-based vaccine, with medians represented as solid horizontal lines. The dotted line indicates the predictive threshold of 6.2 log10 cp/mL. Comparison was computed with Mann-Whitney test using Graphpad Prism version 9.3.1. software. (C) Receiver operating characteristics (ROC) curve for prediction of vaccine response based on prevaccine TTV viral load. Area under the curve (AUC): 0.6533. The threshold of 6.2 log cp/mL is associated with a negative predictive value of 93.5% (in case of high TTV viral load) and a positive predictive value of 47.9% (in case of low TTV viral load) for vaccine response in this cohort. (D) Bar charts representing the percentage of responders after three vaccine doses according to the prevaccine TTV viral load and to MMF/MPA treatment at the time of the first vaccination. The number of LTR in each category of patients is mentioned above bars. (E) Spearman correlation between TTV viral load and MPA trough level measured at the same time before vaccination in responders (black dots) and nonresponders (grey dots) to three vaccine doses. The dotted line indicates the TTV viral load predictive threshold of 6.2 log10 cp/mL. Correlation coefficient (rS) was calculated using the Graphpad Prism version 9.3.1. software. (F) Forest plot showing Odds Ratios (OR) estimates (indicated by black dots) and 95% confidence intervals (indicated by whiskers) of association between patient characteristics and lack of vaccine response to a three-dose regimen of COVID-19 mRNA-based vaccine. Multivariable logistic regression analysis was performed using SPSS 28.0 (IBM Statistics). Factors independently associated with poor vaccine response are in bold. MMF/MPA: mycophenolate mofetil/mycophenolic acid; OR: Odds ratio; TTV: Torque teno virus; VL: viral load. **p value <0.010, ns: not significant.
To determine whether vaccine response varies with TTV replication, TTV plasma VL was assessed before the first vaccine dose administration in 125 LTR without a history of COVID-19. TTV VL ranged from 1.8 to 9.0 log10 cp/mL, with 6 patients displaying undetectable TTV DNA. Nonresponders displayed higher prevaccine TTV VL (n = 78, median: 4.9 log10 cp/mL, IQR: 3.4-7.7) than responders (n = 47, median: 3.8 log10 cp/mL, IQR: 2.5-5.4; p = 0.004; Table 1, Figure 4B). ROC curve analysis found the optimal TTV prevaccine VL threshold of 6.2 log10 cp/mL as predictive of overall lack of vaccine antibody response, with a sensitivity of 95.7% and a specificity of 37.2% (p<0.001; Figure 4C). LTR displaying TTV VL below this threshold before vaccination seroconverted in 47.9% of cases (45/94) after the third dose versus 6.5% (2/31) of LTR with higher VL (p < 0.001), corresponding to a negative predictive value (NPV) for seroconversion of 93.5%. Using TTV VL in conjunction with MMF/MPA treatment status further discriminated between responders and nonresponders, with 1/26 (3.8%) responders among LTR treated with MMF/MPA and harboring TTV VL ≥6.2 log10 cp/mL versus 13/19 (68.4%) in the opposite conditions of no MMF/MPA and TTV VL <6.2 log10 cp/mL (Figure 4D, E). A multivariable logistic regression analysis confirmed that LTR displaying TTV VL ≥6.2 log10 cp/mL before vaccination (OR = 17.87, CI95 = 3.02-105.72, p = 0.001) or treated with MMF/MPA (OR = 4.73, CI95 = 1.46-15.34, p = 0.010) have significantly lower odds of generating a positive antibody response after adjusting for other variables in the predictive model, as well as patients vaccinated with BNT162b2 compared to mRNA-1273 vaccine (OR = 6.72, CI95 = 1.75-25.92, p = 0.006; Figure 4F, Table 2 , Table S3).
Table 2.
Multivariable Analysis on Antibody Response in LTR to a Three-Dose Regimen of COVID-19 mRNA Vaccine
| Variable | B | SEB | eB (adjusted odds ratio) | 95% CI | p-value |
|---|---|---|---|---|---|
| Intercept | −8.758 | 2.599 | - | - | - |
| History of treated allograft acute rejection | 0.487 | 0.553 | 1.627 | 0.550-4.808 | 0.379 |
| Time between lung transplantation and vaccination (per 1 year increment) | 0.038 | 0.052 | 1.039 | 0.939-1.150 | 0.463 |
| BNT162b2 vaccine (vs mRNA-1273) | 1.906 | 0.688 | 6.724 | 1.745-25.916 | 0.006 |
| Time between first and second doses (per 1 day increment) | 0.123 | 0.064 | 1.131 | 0.998-1.283 | 0.055 |
| Time between third dose and antibody testing | |||||
| (per 1 day increment) | −0.011 | 0.017 | 0.989 | 0.957-1.022 | 0.516 |
| Prevaccine TTV viral load ≥6.2 log10 cp/mL | 2.883 | 0.907 | 17.866 | 3.019-105.716 | 0.001 |
| MMF/MPA | 1.554 | 0.600 | 4.730 | 1.458-15.343 | 0.010 |
| Tacrolimus | 0.303 | 0.633 | 1.354 | 0.391-4.684 | 0.632 |
| Steroids | 0.769 | 1.001 | 2.157 | 0.303-15.335 | 0.442 |
B, regression coefficient; CI, confidence interval; MMF/MPA, mycophenolate mofetil/mycophenolic acid; SE, standard error; TTV, Torque teno virus.
Multivariable logistic regression analysis of the risk to be a non-responder was performed using SPSS 28.0 (IBM Statistics). Factors independently associated with poor vaccine response (p < 0.05) are in bold. Overall model statistics: χ2 = 42.407, p-value <0.001.
Longitudinal follow-up indicated that TTV VL measured before the third vaccine dose could also be predictive of antibody response. Considering LTR who remained seronegative after 2 doses (n = 47), none of the 19 patients with TTV VL ≥6.2 log10 cp/mL before the third dose seroconverted after the booster (NPV of 100%), versus 42.9% (12/28) of LTR with VL lower than this cutoff (p < 0.001; Figure 5 A and B). Conversely, a TTV VL <3.2 log10 cp/mL was highly predictive of vaccine response (9/10 responders, versus 3/37 with TTV VL over this threshold, p < 0.001). This lower threshold was associated with a positive predictive value of 90.0% and a NPV of 91.9% for vaccine response in this cohort.
Figure 5.
Investigation of TTV viral load as potential predictive biomarker of response to a third vaccine dose in COVID-19-naive lung transplant recipients determined as nonresponders to the second dose. (A) TTV viral load measured after the second vaccine dose in nonresponders (n = 35) and responders (n = 12) to the third vaccine dose, with medians represented as solid horizontal lines. The dotted lines indicate the predictive thresholds of 6.2 log10 cp/mL and 3.2 log10 cp/mL associated with low and high rates of vaccine response, respectively. Comparison was computed with Mann-Whitney test using Graphpad Prism version 9.3.1. software. (B) Receiver operating characteristics (ROC) curve for prediction of vaccine response to the third dose based on TTV viral load measured in seronegative LTR after the second vaccine dose. Area under the curve (AUC): 0.9190. The upper threshold of 6.2 log copies/mL is associated with a negative predictive value of 100% (in case of high TTV viral load) and a positive predictive value of 40.0% (in case of low TTV viral load) for vaccine response. Conversely, the lower threshold of 3.2 log copies/mL is associated with a negative predictive value of 91.9% (in case of high TTV viral load) and a positive predictive value of 90.0% (in case of low TTV viral load) for vaccine response in this cohort. TTV: Torque teno virus. ****p value <0.0001.
Discussion
This study revealed a substantially weak humoral immune response among COVID-19-naive LTR after a three-dose regimen of COVID-19 mRNA-based vaccine. In contrast with the correlation observed between anti-RBD IgG levels and neutralizing antibody titers for the D614G mutant, and to a lesser extent for the delta variant, no patient displayed neutralizing activity against the omicron variant in this cohort, including the few patients with high anti-RBD titers over 1,000 BAU/mL. We showed that these anti-RBD IgG titers decreased over time but persisted up to 8 months after the third dose, and identified TTV VL as a potential predictive marker of vaccine response in LTR.
Our results confirmed previous data observed in LTR and other types of solid organ transplant recipients where vaccine nonresponse was associated with mycophenolate treatment.4 , 6 , 9 , 12 , 13 , 23 This may be due to a greater impairment of both T and B-cell functions with mycophenolate compared to azathioprine or no antimetabolites, which was already shown to impair the humoral response to influenza vaccine.24
A higher seroconversion rate was observed with mRNA-1273 compared to BNT162b2 vaccine in our cohort, similarly to findings reported after one or two vaccine doses.4 , 5 , 7 , 23 Both vaccines contain mRNA encoding the spike protein stabilized into the pre-fusion conformation, but the higher dose of 100 µg in the mRNA-1273 versus 30 µg in the BNT162b2 preparation could explain these differences in immunogenicity in LTR.25 Alter's group recently confirmed the superiority of mRNA-1273 vaccine over the BNT162b2 vaccine.26 They showed that mRNA-1273 vaccine elicits higher concentrations of RBD- and N-terminal domain-specific IgA and higher levels of antibodies eliciting neutrophil phagocytosis and natural killer cell activation than the BNT162b2 vaccine.
Our study revealed a strong association between high prevaccine TTV VL and lack of vaccine response, highlighting the potential of this virological marker to predict antibody response in the LTR population. This was particularly true when TTV VL was measured before the third dose for non-responders to the second vaccine dose: no vaccine response was observed in patients with VL ≥6.2 log10 cp/mL, whereas TTV VL <3.2 log10 cp/mL was highly predictive of seroconversion. This virological marker varies with the strength of humoral and cellular immunity and reflects the overall state of the immune system. TTV VL could therefore help to identify patients who may not respond to three vaccine doses.
Limitations of our study include the absence of a control group of healthy vaccinated adults for comparison and the lack of serological follow-up after the first and second vaccine doses for some patients, which could limit the accuracy of vaccine response rates determined at these timepoints. Furthermore, memory T-cell response could not be explored in this retrospective study, whereas cases of T cell response without detectable antibodies have been reported in vaccinated LTR.7 , 13 , 27 Finally, missing data about vaccine type and vaccination dates, and lack of sera available after third vaccine dose led us to exclude many LTR followed-up in our hospital. Despite these limitations, this study strongly suggests that COVID-19-naive LTR develop a poor antibody response to a 3-dose regimen of COVID-19 mRNA-based vaccine, in contrast to LTR infected with SARS-CoV-2 before vaccination. This study also revealed that BNT162b2 (vs mRNA-1273) vaccine, MMF/MPA treatment, and high TTV VL at the time of vaccination were associated with significantly reduced odds of seroconversion. This marker could help clinicians to decide which patients require additional vaccine doses. A very recent study conducted on LTR reported that prevaccine TTV VL over 6.5 log10 cp/mL was predictive of poor vaccine response to a second dose of mRNA-1273 vaccine, which strongly supports our findings.11 Future studies could assess the validity of our predictive model in other cohorts of LTR, evaluate this virological marker as a guide for vaccine strategies, and explore its potential as a predictive tool of vaccine response in other types of transplanted and immunocompromised patients, as well as against other pathogens.
Author contributions
FG, BRP, RK and SFK conceived and designed the study. FG and BRP collected clinical and biological data and organized the database. FG and ES performed the experiments. FG, BRP, MS, EL, SC and SFK contributed to data analysis and interpretation. FG, MS and SFK wrote the manuscript. SFK and RK supervised this study. All authors listed have critically reviewed the manuscript for important intellectual content and approved it for publication.
Disclosure Statement
TTV real-time PCR kits were provided free of charge by bioMérieux (Marcy l'Etoile, France). SFK lab received research grants from bioMérieux. MS received a travel grant from bioMérieux. SC participated as an expert to a board for AstraZeneca and received support for congress fees from Sanofi and Novartis. None of the other authors has any conflicts of interest to disclose. SFK lab is supported by Strasbourg University Hospital (SeroCoV-HUS; PRI 7782), the Agence Nationale de la Recherche (ANR-18-CE17-0028), Laboratoire d'Excellence TRANSPLANTEX (ANR-11-LABX-0070_TRANSPLANTEX), Strasbourg University and Institut National de la Santé et de la Recherche Médicale (UMR_S 1109).
Declaration of Competing Interest
None.
Acknowledgments
The authors are grateful to all participants of this study and would like to thank Olivier Schwartz (Institut Pasteur, Paris, France) who provided HEK293T-ACE2 cells, Paola Rossolillo (IGBMC, Strasbourg, France) who provided the plasmids for pseudovirus production, Eren Canpolat (HUS) who performed TTV real-time PCR, and Dr Kate Dunning (HUS) who provided manuscript edition and proofreading.
Footnotes
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.healun.2022.07.008.
Appendix. Supplementary materials
References
- 1.Messika J, Eloy P, Roux A, Hirschi S, Nieves A, Le Pavec J, et al. COVID-19 in Lung Transplant Recipients. Transplantation. 2021;105:177–186. doi: 10.1097/TP.0000000000003508. [DOI] [PubMed] [Google Scholar]
- 2.Mohanka MR, Mahan LD, Joerns J, Lawrence A, Bollineni S, Kaza V, et al. Clinical characteristics, management practices, and outcomes among lung transplant patients with COVID-19. J Heart Lung Transplant. 2021;40:936–947. doi: 10.1016/j.healun.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marion O, Del Bello A, Abravanel F, Faguer S, Esposito L, Hebral AL, et al. Predictive factors for humoral response after 2-dose SARS-CoV-2 vaccine in solid organ transplant patients. Transplant Direct. 2021;8:e1248. doi: 10.1097/TXD.0000000000001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Narasimhan M, Mahimainathan L, Clark AE, Usmani A, Cao J, Araj E, et al. Serological response in lung transplant recipients after two doses of SARS-CoV-2 mRNA vaccines. Vaccines. 2021;9:708. doi: 10.3390/vaccines9070708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hallett AM, Greenberg RS, Boyarsky BJ, Shah PD, Ou MT, Teles AT, et al. SARS-CoV-2 messenger RNA vaccine antibody response and reactogenicity in heart and lung transplant recipients. J Heart Lung Transplant. 2021;40:1579–1588. doi: 10.1016/j.healun.2021.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Havlin J, Svorcova M, Dvorackova E, Lastovicka J, Lischke R, Kalina T, et al. Immunogenicity of BNT162b2 mRNA COVID-19 vaccine and SARS-CoV-2 infection in lung transplant recipients. J Heart Lung Transplant. 2021;40:754–758. doi: 10.1016/j.healun.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffman TW, Meek B, Rijkers GT, van Kessel DA. Poor serologic response to two doses of an mRNA-Based SARS-CoV-2 vaccine in lung transplant recipients. Transplantation. 2021 doi: 10.1097/TP.0000000000003966. Published online October 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shostak Y, Shafran N, Heching M, Rosengarten D, Shtraichman O, Shitenberg D, et al. Early humoral response among lung transplant recipients vaccinated with BNT162b2 vaccine. Lancet Respir Med. 2021;9:e52–e53. doi: 10.1016/S2213-2600(21)00184-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aslam S, Danziger-Isakov L, Mehra MR. COVID-19 vaccination immune paresis in heart and lung transplantation. J Heart Lung Transplant. 2021;40:763–766. doi: 10.1016/j.healun.2021.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoek RA, Verschuuren EA, de Vries RD, Vonk JM, van Baarle D, van der Heiden M, et al. High Torque tenovirus (TTV) load before first vaccine dose is associated with poor serological response to COVID-19 vaccination in lung transplant recipients. J Heart Lung Transplant. 2022;16 doi: 10.1016/j.healun.2022.03.006. Published online March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benotmane I, Gautier G, Perrin P, Olagne J, Cognard N, Fafi-Kremer S, et al. Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA. 2021;326:1063–1065. doi: 10.1001/jama.2021.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peled Y, Ram E, Lavee J, Segev A, Matezki S, Wieder-Finesod A, et al. Third dose of the BNT162b2 vaccine in heart transplant recipients: immunogenicity and clinical experience. J Heart Lung Transplant. 2021;41:148–157. doi: 10.1016/j.healun.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Bello A, Abravanel F, Marion O, Couat C, Esposito L, Lavayssière L, et al. Efficiency of a boost with a third dose of anti-SARS-CoV-2 messenger RNA-based vaccines in solid organ transplant recipients. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2022;22:322–323. doi: 10.1111/ajt.16775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karaba AH, Zhu X, Liang T, Wang KH, Rittenhouse AG, Akinde O, et al. A third dose of SARS-CoV-2 vaccine increases neutralizing antibodies against variants of concern in solid organ transplant recipients. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2021;24 doi: 10.1111/ajt.16933. Published online December. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Havlin J, Skotnicova A, Dvorackova E, Hubacek P, Svorcova M, Lastovicka J, et al. Impaired humoral response to third dose of BNT162b2 mRNA COVID-19 vaccine despite detectable spike protein-specific T cells in lung transplant recipients. Transplantation. 2022;106:e183–e184. doi: 10.1097/TP.0000000000004021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman TW, Meek B, Rijkers GT, van Kessel DA. Serologic response to a third dose of an mRNA-based SARS-CoV-2 vaccine in lung transplant recipients. Transpl Immunol. 2022;72 doi: 10.1016/j.trim.2022.101599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Vlaminck I, Khush KK, Strehl C, Kohli B, Luikart H, Neff NF, et al. Temporal response of the human virome to immunosuppression and antiviral therapy. Cell. 2013;155:1178–1187. doi: 10.1016/j.cell.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulifaj D, Durgueil-Lariviere B, Meynier F, Munteanu E, Pichon N, Dubé M, et al. Development of a standardized real time PCR for Torque teno viruses (TTV) viral load detection and quantification: a new tool for immune monitoring. J Clin Virol. 2018;105:118–127. doi: 10.1016/j.jcv.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Charmetant X, Espi M, Benotmane I, Barateau V, Heibel F, Buron F, et al. Infection or a third dose of mRNA vaccine elicit neutralizing antibody responses against SARS-CoV-2 in kidney transplant recipients. Sci Transl Med. 2022;14:eabl6141. doi: 10.1126/scitranslmed.abl6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng C, Shi J, Fan Q, Wang Y, Huang H, Chen F, et al. Protective humoral and cellular immune responses to SARS-CoV-2 persist up to 1 year after recovery. Nat Commun. 2021;12:4984. doi: 10.1038/s41467-021-25312-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DGS-Urgent N°2022_16. Vaccination Contre La COVID-19 Des Personnes Sévèrement Immunodéprimées et de l'entourage Des Personnes à Risque de Forme Grave de La Maladie. Direction Générale de la Santé; 2022.
- 23.Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325:1784–1786. doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Natori Y, Shiotsuka M, Slomovic J, Hoschler K, Ferreira V, Ashton P, et al. A double-blind, randomized trial of high-dose vs standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin Infect Dis Off Publ Infect Dis Soc Am. 2018;66:1698–1704. doi: 10.1093/cid/cix1082. [DOI] [PubMed] [Google Scholar]
- 25.Heinz FX, Stiasny K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. Npj Vaccines. 2021;6:1–13. doi: 10.1038/s41541-021-00369-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaplonek P, Cizmeci D, Fischinger S, Collier AR, Suscovich T, Linde C, et al. mRNA-1273 and BNT162b2 COVID-19 vaccines elicit antibodies with differences in Fc-mediated effector functions. Sci Transl Med. 2022;29:eabm2311. doi: 10.1126/scitranslmed.abm2311. Published online March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schramm R, Costard-Jäckle A, Rivinius R, Fischer B, Müller B, Boeken U, et al. Poor humoral and T-cell response to two-dose SARS-CoV-2 messenger RNA vaccine BNT162b2 in cardiothoracic transplant recipients. Clin Res Cardiol Off J Ger Card Soc. 2021;110:1142–1149. doi: 10.1007/s00392-021-01880-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.