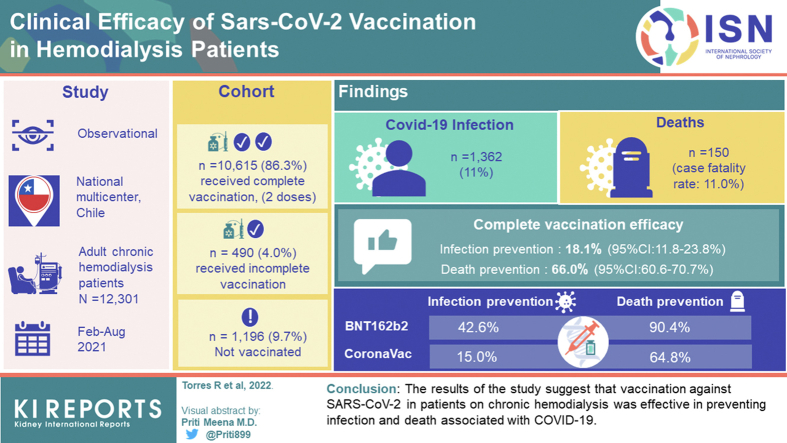
Abstract
Introduction
The COVID-19 pandemic is a global public health problem. Patients with end-stage renal disease on hemodialysis are at a higher risk of infection and mortality than the general population. Worldwide, a vaccination campaign has been developed that has been shown to reduce severe infections and deaths in the general population. However, there are currently limited data on the clinical efficacy of vaccinations in the hemodialysis population.
Methods
A national multicenter observational cohort was performed in Chile to evaluate the clinical efficacy of anti-SARS-CoV-2 vaccination in end-stage renal disease patients on chronic hemodialysis from February 2021 to August 2021. In addition, the BNT162b2 (Pfizer-BioNTech) and CoronaVac (Sinovac) vaccines were evaluated. The efficacy of vaccination in preventing SARS-CoV-2 infection, hospitalizations, and deaths associated with COVID-19 was determined.
Results
A total of 12,301 patients were evaluated; 10,615 (86.3%) received a complete vaccination (2 doses), 490 (4.0%) received incomplete vaccination, and 1196 (9.7%) were not vaccinated. During follow-up, 1362 (11.0%) patients developed COVID-19, and 150 died (case fatality rate: 11.0%). The efficacy of the complete vaccination in preventing infection was 18.1% (95% confidence interval [CI]:11.8–23.8%), and prevention of death was 66.0% (95% CI:60.6–70.7%). When comparing both vaccines, BNT162b2 and CoronaVac were effective in reducing infection and deaths associated with COVID-19. Nevertheless, the BNT162b2 vaccine had higher efficacy in preventing infection (42.6% vs. 15.0%) and deaths (90.4% vs. 64.8%) compared to CoronaVac.
Conclusion
The results of our study suggest that vaccination against SARS-CoV-2 in patients on chronic hemodialysis was effective in preventing infection and death associated with COVID-19.
Keywords: COVID-19, epidemiology, hemodialysis, mortality, renal dialysis, SARS-CoV-2
Graphical abstract
The COVID-19 pandemic has had a major global impact, with over 600 million people infected and over 6 million deaths as of August 2022.1 Among the highest risk groups are patients with end-stage renal disease undergoing renal replacement therapy, including hemodialysis, peritoneal dialysis, and kidney transplants, with a higher rate of infection and mortality compared with the general population.2, 3, 4
A recent study5 reported that the global prevalence of COVID-19 in patients on dialysis varies between 0% and 37%, with a hospitalization rate of between 35% and 88%, and with admissions to intensive care units ranging from 2% to 70%. In addition, these patients present a high case fatality rate of over 20%,6 which is more than 40% in hospitalized patients.5 These rates are much higher than those reported in the general population.2,3
Latin America is the area of the world with the highest mortality per capita from COVID-19, with over 1.7 million reported deaths as of August 2022.7,8 In Chile, the first case of COVID-19 was diagnosed on March 3, 2020, and the first case among patients on hemodialysis was detected on March 23, 2020. By December 2021, 4814 patients infected with COVID-19 in the hemodialysis population have been reported (cumulative infection rate: 22,900×100,000 people) and 879 deaths (case fatality rate: 18.3%). This equates to an infection rate of over 2.0 times and a case fatality rate 6.7 times higher than in the general population when adjusted for age and sex.9
Given the impact of the pandemic, major international efforts have been made to design anti-SARS-CoV-2 vaccines and distribute them around the world. To date, more than 10 vaccines have proven to be effective in clinical studies, primarily in the general population.10,11
It is highly likely that, as in the general population,12 the most effective prevention against severe illness or death from COVID-19 in hemodialysis patients is adequate vaccination. End-stage renal disease patients on hemodialysis with circulating anti-SARS-CoV-2 antibodies have a reduced risk of COVID-19.13 Nevertheless, in clinical studies where the postdevelopmental immune response of COVID-19 has been evaluated, anti-SARS-CoV-2 antibody titers have been shown to decline earlier in hemodialysis patients than in the general population.14 It has been recently reported that vaccinations with the BNT162b2 vaccine (Pfizer-BioNTech) allow the generation of anti-SARS-CoV-2 antibodies in hemodialysis patients, although at lower concentrations compared to healthy volunteers.15 This raises questions about whether vaccines are effective in this population. To date, there are limited data on the clinical efficacy of anti-SARS-CoV-2 vaccination in preventing infection and adverse outcomes in the chronic hemodialysis population.
In Chile, the vaccination campaign began on February 2, 2021, which included the inactivated virus vaccine CoronaVac (Sinovac) and the RNA messenger vaccine BNT162b2 (Pfizer-BioNTech). Clinical studies in the general population have shown that both CoronaVac16 and BNT162b210 decrease the infection and death rates associated with COVID-19. A recent study performed in Chile17 demonstrated that the CoronaVac vaccine prevented COVID-19 (mean effectiveness: 65.9%) and severe disease, including hospitalizations and death (mean effectiveness: 86.3%) in the general population.
Considering the high impact of COVID-19 on the population of patients with chronic kidney disease in renal replacement therapy, on March 29, 2020, the Chilean Society of Nephrology created the anti-COVID-19 task force in chronic kidney disease (FUTAC Team) to develop strategies to reduce the transmission of COVID-19 among patients with end-stage renal disease (hemodialysis, peritoneal dialysis, and kidney transplantation) and among the health team responsible for their care. In addition, the incidence and death rates from COVID-19 in these patient groups have been determined at the national level by the creation of a national registry covering over 90% of the population undergoing chronic renal replacement therapy in the country. This registry was used by health authorities to develop COVID-19 prevention strategies in this population, including a priority vaccination for these high-risk groups independent of age, since February 2021.18 At the end of October 2021, over 90% of these patients received complete vaccination with 2 doses, and over 93% received at least 1 dose.
The objective of this study was to evaluate the clinical efficacy of vaccinations against SARS-CoV-2 in patients on hemodialysis, from a national multicenter observational cohort, especially in terms of infection, hospitalization, and mortality rates. A comparison was made between patients according to their vaccination status, and the efficacy of the CoronaVac and BNT162b2 vaccines was evaluated independently in this group of patients.
Methods
Study Design
The HDVAC study, conducted by the FUTAC Team of the Chilean Society of Nephrology, is a national multicenter observational cohort of patients on chronic hemodialysis in Chile conducted in 2021 to determine the efficacy of the anti-SARS-CoV-2 vaccination. Baseline demographic and clinical data were collected from patients. The anti-COVID-19 immunization rate in patients on chronic hemodialysis was determined, and the SARS-CoV-2 infection rate was evaluated according to vaccine use and the development of adverse outcomes, including hospitalization and death. This study was approved by the Institutional Ethics Committee.
Chile Immunization Program
On February 2, 2021, the national vaccination campaign began in Chile, which included patients with end-stage renal disease (hemodialysis, peritoneal dialysis, and kidney transplant) within the priority vaccination group, regardless of age. The campaign included the use of the inactivated virus anti-SARS-CoV-2 vaccine CoronaVac (Sinovac) and the mRNA vaccine BNT162b2 (Pfizer-BioNTech). The campaign included the administration of 2 doses of the vaccine to each patient (CoronaVac or BNT162b2), separated by a 4-week period. The decision about which vaccine would be administered was based on onsite availability of the vaccines; however, the same vaccine was administered for the 2 doses. The administration of the vaccines was funded by the government of Chile and was free for patients. A complete vaccination was defined as a patient who received 2 vaccine doses and completed a minimum of 14 days after the second vaccination.
Inclusion and Exclusion Criteria
To evaluate vaccination efficacy, patients older than 15 years with a diagnosis of end-stage renal disease on chronic hemodialysis were included. The following were excluded: (i) patients with SARS-CoV-2 infection prior to February 2, 2021, (ii) patients with a medical contraindication to the vaccination, (iii) patients with palliative management, (iv) pregnant or lactating women, and (v) patients with incomplete vaccination data.
Evaluation of Patients
The diagnosis of SARS-CoV-2 was confirmed by a polymerase chain reaction test, reported on the Epivigila platform (used by the Chilean Ministry of Health to follow-up with COVID-19 patients).19 Patients who had a COVID-19-related death with a laboratory confirmation (polymerase chain reaction test) were analyzed, which corresponds to code U07.1 in the International Classification of Diseases, tenth revision.20 The follow-up of patients was carried out between February 2, 2021 (start date of the national immunization program) and August 31, 2021. Patients were classified according to their immunization status as follows: (i) unvaccinated, (ii) incomplete vaccination, and (iii) complete vaccination. An unvaccinated patient was considered as the person who did not receive any vaccine during follow-up period. A patient with a complete vaccination was considered as a person who had received 2 doses and completed at least 14 days after the last dose. A patient with an incomplete vaccination was considered as a person who was vaccinated but did not fulfill the complete vaccination criteria (this group included patients with 1 dose only, and patients with 2 doses but developed COVID-19 before 14 days after the second dose). In addition, efficacy was compared between the CoronaVac and BNT162b2 vaccines in patients with complete vaccination. The outcomes assessed were the development of SARS-CoV-2 infection, hospitalization associated with COVID-19, and death associated with COVID-19. Crude and adjusted analyses for patient baseline demographic and clinical variables were performed.
Statistical Analysis
The vaccination efficacy analysis was based on methods previously used by Thompson et al.21 and Jara et al.17 Discrete variables were expressed as absolute values (percentages), and continuous variables were expressed as arithmetic mean ± SD. For comparisons of baseline data between groups, χ2 test for discrete variables was used. To evaluate the efficacy of the vaccines, the hazard ratio between vaccinated and unvaccinated patients was determined using a proportional Cox model, crude, and adjusted for baseline characteristics (age, sex, comorbidities, time on dialysis), considering the vaccination as a time-varying covariate, to prevent immortal time bias and Kaplan-Meier analysis. The vaccination efficacy was estimated as 1 minus the hazard ratio, obtained by the previous model, adjusted for potential covariates (expressed as percentages). A stratified subanalysis was performed according to the type of vaccine used (BNT162b2 and CoronaVac), patients over 60 years of age, and patients with diabetes. All analyses were 2-tailed. A P-value of less than 5% (P < 0.05) was considered statistically significant. The software Stata SE v.15.0 (StataCorp LLC, College Station, TX), and GraphPad Prism v.8.0 (GraphPad Software, San Diego, CA), were used for the analyses.
Results
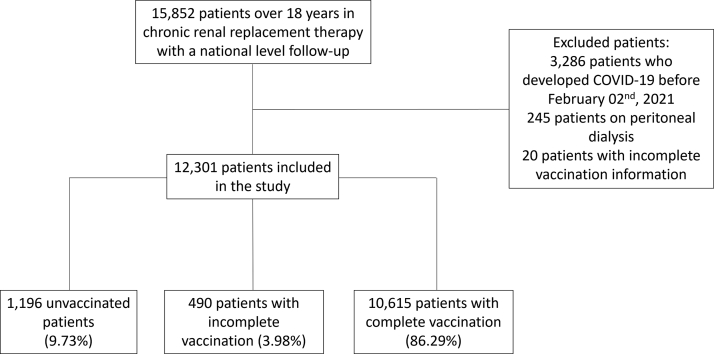
A total of 15,852 patients on chronic renal replacement therapy were evaluated between February 2, 2021, and August 31, 2021 (Figure 1). Of these, 12,301 hemodialysis patients fulfilled the study criteria. The baseline characteristics of the patients are presented in Table 1. Mean age was 60.6 ± 14 years. Females were 5280 (42.92%). Patients with diabetes were 2269 (18.45%), and the mean time on dialysis was 46 [range: 21–89] months. During the follow-up period, 10,615 (86.29%) patients received complete vaccination, 490 (3.98%) received incomplete vaccination, and 1196 (9.73%) were not vaccinated.
Figure 1.
Flowchart of patients evaluated in the study. Patients on chronic hemodialysis over 15 years were evaluated between February 2, 2021 and August 31, 2021. Patients who had had SARS-CoV-2 infection prior to February 2, 2021, patients on other renal replacement therapies, or with incomplete information on vaccination status were excluded.
Table 1.
Baseline characteristics of patients in the study
| Characteristics | Total cohort |
Patients without COVID-19 |
Patients with COVID-19 |
P-valuea |
Unvaccinated |
Incomplete vaccination |
Complete vaccination |
P-valueb |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | N | % | |||
| Total | 12,301 | 100% | 10,939 | 88.93% | 1362 | 11.07% | - | 1196 | 9.73% | 490 | 3.98% | 10,615 | 86.29% | - |
| Sex | ||||||||||||||
| Male | 7021 | 57.08% | 6245 | 57.09% | 776 | 56.98% | 0.936 | 642 | 53.68% | 273 | 55.71% | 6106 | 57.52% | 0.032 |
| Female | 5280 | 42.92% | 4694 | 42.91% | 586 | 43.02% | 554 | 46.32% | 217 | 44.29% | 4509 | 42.48% | ||
| Age group | ||||||||||||||
| 18-19 yrs | 32 | 0.26% | 26 | 0.24% | 6 | 0.44% | 0.129 | 8 | 0.67% | 5 | 1.02% | 19 | 0.18% | < 0.001 |
| 20-29 yrs | 396 | 3.22% | 348 | 3.18% | 48 | 3.52% | 63 | 5.27% | 29 | 5.92% | 304 | 2.86% | ||
| 30-39 yrs | 908 | 7.38% | 799 | 7.30% | 109 | 8.00% | 144 | 12.04% | 48 | 9.80% | 716 | 6.75% | ||
| 40-49 yrs | 1436 | 11.67% | 1259 | 11.51% | 177 | 13.00% | 168 | 14.05% | 73 | 14.90% | 1195 | 11.26% | ||
| 50-59 yrs | 2687 | 21.84% | 2375 | 21.71% | 312 | 22.91% | 261 | 21.82% | 113 | 23.06% | 2313 | 21.79% | ||
| 60-69 yrs | 3421 | 27.81% | 3054 | 27.92% | 367 | 26.95% | 306 | 25.59% | 107 | 21.84% | 3008 | 28.34% | ||
| 70-79 yrs | 2573 | 20.92% | 2322 | 21.23% | 251 | 18.43% | 176 | 14.72% | 86 | 17.55% | 2311 | 21.77% | ||
| ≥ 80 yrs | 848 | 6.89% | 756 | 6.91% | 92 | 6.75% | 70 | 5.85% | 29 | 5.92% | 749 | 7.06% | ||
| Diabetes | ||||||||||||||
| No | 10,032 | 81.55% | 9076 | 82.97% | 956 | 70.19% | < 0.001 | 958 | 80.10% | 406 | 82.86% | 8668 | 81.66% | 0.315 |
| Yes | 2269 | 18.45% | 1863 | 17.03% | 406 | 29.81% | 238 | 19.90% | 84 | 17.14% | 1947 | 18.34% | ||
| Dialysis vintage | ||||||||||||||
| Less than 1 yr | 2067 | 16.80% | 1889 | 17.27% | 178 | 13.07% | < 0.001 | 340 | 28.43% | 112 | 22.86% | 1615 | 15.21% | < 0.001 |
| 1-2 yrs | 2757 | 22.41% | 2432 | 22.23% | 325 | 23.86% | 197 | 16.47% | 103 | 21.02% | 2457 | 23.15% | ||
| 3-5 yrs | 2816 | 22.89% | 2486 | 22.73% | 330 | 24.23% | 287 | 24.00% | 94 | 19.18% | 2435 | 22.94% | ||
| 6-10 yrs | 2797 | 22.74% | 2457 | 22.46% | 340 | 24.96% | 214 | 17.89% | 115 | 23.47% | 2468 | 23.25% | ||
| More than 10 yrs | 1864 | 15.15% | 1675 | 15.31% | 189 | 13.88% | 158 | 13.21% | 66 | 13.47% | 1640 | 15.45% | ||
P-value patients without COVID-19 versus patients with COVID-19.
P-value unvaccinated patients versus vaccinated patients (complete vaccination and incomplete vaccination).
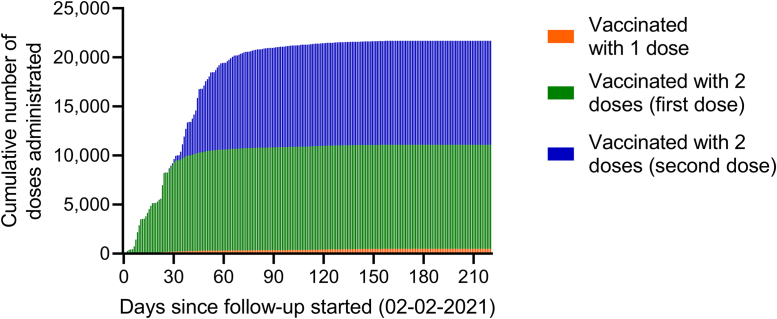
The temporal evolution of the vaccination program in the population of patients undergoing hemodialysis is shown in Figure 2. It was observed that, of the total number of patients with complete immunization, 50% completed 2 doses in the first 45 days of the start of the vaccination campaign, and over 90% completed the vaccination in the first 90 days. Of the total number of vaccinated people, 2963 (26.7%) received the BNT162b2 vaccine and 8142 (73.3%) received the CoronaVac vaccine.
Figure 2.
Cumulative number of vaccine doses administrated during follow-up period. The number of vaccine doses administrated between February 2, 2021 and August 31, 2021, in patients who received the complete vaccination (green bar = first dose, blue bar = second dose) or only 1 dose (yellow bar) during follow-up period are presented.
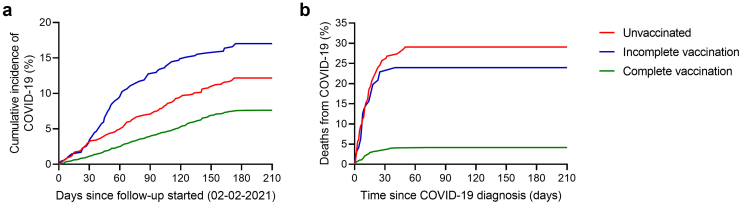
During the follow-up period, 1362 patients became infected with COVID-19 (11.1% of the total evaluated population), 364 patients were hospitalized for COVID-19 (2.9% of the total evaluated population, 26.7% of those infected), and 150 patients died (1.2% of the total evaluated population, 11.0% of those infected). In Figure 3, the cumulative incidence of COVID-19 infection (Figure 3A) and death (Figure 3B) stratified by patient vaccination status, are shown.
Figure 3.
COVID-19 infection rate and case fatality rate during follow-up, according to vaccination status. Hemodialysis patients were classified according to their vaccination status: unvaccinated (n = 1196 – red line), incomplete vaccination (n = 490 – blue line) and complete vaccination (n = 10,615 – green line). (A) Cumulative incidence rate of COVID-19 infection. (B) Case fatality rate in patients who developed COVID-19.
The infection, hospitalization, and death incidence rates of COVID-19 among unvaccinated patients and those with complete vaccination are shown in Table 2. The infection rate among unvaccinated patients was 0.870×1000 person-days versus 0.515×1000 person-days among patients with complete vaccination. Regarding hospitalizations, unvaccinated patients had a rate of 0.363×1000 person-days versus 0.104×1000 person-days in patients with complete vaccination. Regarding death associated with COVID-19, the unvaccinated rate was 0.279×1000 person-days versus 0.025×1000 person-days in those with complete vaccination. This showed that the efficacy of a complete vaccination in hemodialysis patients was 18.1% (95% CI: 11.8–23.8%) in preventing COVID-19 infection, 42.8% (95% CI: 35.6–49.1%) in preventing hospitalizations, and 66.0% (95% CI: 60.6–70.7%) in preventing deaths associated with COVID-19. To assess the efficacy of each vaccine (BNT162b2 and CoronaVac), patients with a complete vaccination (2860 patients with the BNT162b2 vaccine and 7857 patients with the CoronaVac vaccine) versus unvaccinated patients were evaluated. The infection incidence rates and adverse outcomes (hospitalization and death rates) associated with COVID-19 for unvaccinated patients and those with complete vaccination with BNT162b2 and CoronaVac, respectively, are shown in Table 3. It was observed that both the BNT162b2 vaccine and CoronaVac vaccine decreased infection, hospitalization, and death incidence associated with COVID-19, compared with the unvaccinated population. When comparing both vaccines, patients vaccinated with BNT162b2 compared to those vaccinated with CoronaVac had a lower infection incidence (BNT162b2: 0.421×1000 person-days, CoronaVac: 0.550×1000 person-days), hospitalization (BNT162b2: 0.083×1000 person-days, CoronaVac: 0.116×1000 person-days), and death associated with COVID-19 (BNT162b2: 0.008×1000 person-days, CoronaVac: 0.031×1000 person-days). When comparing the efficacy of the 2 vaccines, BNT162b2 showed greater efficacy than CoronaVac in decreasing the infection rate (BNT162b2: 42.6%, CoronaVac: 15.0%), hospitalization rate (BNT162b2: 68.6%, CoronaVac: 40.1%), and death rate (BNT162b2: 90.4%, CoronaVac: 64.8%). A subanalysis was performed, including the population aged more than 60 years (Supplementary Table S1 and S2) and patients with diabetes (Supplementary Table S3 and S4), which showed that vaccinations with BNT162b2 or CoronaVac prevented infection, hospitalization, and death from COVID-19 in these groups. Nevertheless, BNT162b2 had superior efficacy compared to CoronaVac in both elderly and diabetic patients (Supplementary Table S2 and S4).
Table 2.
Efficacy of the anti-SARS-Co-2 vaccination to prevent COVID-19, hospitalizations, and death
| Outcome and immunization status | Total cohort |
Patients with COVID-19 |
Vaccine efficacy |
||
|---|---|---|---|---|---|
| Number of person-ds | No. of persons | Incidence rate (number of events/1000 person-ds) | Age-adjusted and sex-adjusted analysis | Analysis adjusted for all variables | |
| COVID-19 diagnosis | |||||
| Unvaccinated | 221,873 | 193 | 0.870 | - | - |
| (0.755–1.001) | |||||
| Complete vaccination | 2,075,679 | 1069 | 0.515 | 18.4% | 18.1% |
| (0.485–0.546) | (12.2%–24.1%) | (11.8%–23.8%) | |||
| Hospitalizations | |||||
| Unvaccinated | 236,976 | 86 | 0.363 | - | - |
| (0.294–0.448) | |||||
| Complete vaccination | 2,187,193 | 227 | 0.104 | 42.8% | 42.8% |
| (0.091–0.118) | (35.7%–49.1%) | (35.6%–49.1%) | |||
| Deaths | |||||
| Unvaccinated | 240,169 | 67 | 0.279 | - | - |
| (0.219–0.354) | |||||
| Complete vaccination | 2,223,586 | 56 | 0.025 | 65.8% | 66.0% |
| (0.019–0.032) | (60.4%–70.5%) | (60.6%–70.7%) | |||
The incidence rate (number of events per 1000 person-days) from infection, hospitalization, and death from COVID-19 in unvaccinated patients (n =1196) and in patients with complete vaccination (n = 10,215) is shown.
Patients with complete vaccination were considered as those who had greater than or equal to 14 days after receipt of the second dose. Efficacy of vaccination (estimated as 1 minus the hazard ratio adjusted for covariates, expressed as percentage) to reduce risk of infection, hospitalization, or death with its 95% CI are presented.
Table 3.
Efficacy of the BNT162b2 and CoronaVac vaccines to prevent COVID-19, hospitalizations, and death
| Outcome and immunization status | Total cohort |
Patients with COVID-19 |
Vaccine efficacy |
||
|---|---|---|---|---|---|
| Number of person-ds | No. of persons | Incidence rate (number of events/1000 person-ds) | Age-adjusted and sex-adjusted analysis | Analysis adjusted for all variables | |
| COVID-19 diagnosis | |||||
| Unvaccinated | 221,873 | 193 | 0.870 | - | - |
| (0.755–1.001) | |||||
| Complete vaccination (BNT162b2 vaccine) | 556,267 | 234 | 0.421 | 42.6% | 42.6% |
| (0.370–0.478) | (32.2%–51.3%) | (32.1%–51.3%) | |||
| Complete vaccination (CoronaVac vaccine) | 1,519,412 | 835 | 0.550 | 15.3% | 15.0% |
| (0.513–0.588) | (8.6%–21.7%) | (8.3%–21.4%) | |||
| Hospitalizations | |||||
| Unvaccinated | 226,976 | 86 | 0.379 | - | - |
| (0.306–0.468) | |||||
| Complete vaccination (BNT162b2 vaccine) | 567,392 | 47 | 0.083 | 67.6% | 68.6% |
| (0.062–0.110) | (56.8%–75.7%) | (57.6%–76.6%) | |||
| Complete vaccination (CoronaVac vaccine) | 1,549,800 | 180 | 0.111 | 42.8% | 40.1% |
| (0.096–0.129) | (35.0%–50.5%) | (32.0%–48.3%) | |||
| Deaths | |||||
| Unvaccinated | 240,169 | 67 | 0.279 | - | - |
| (0.219–0.354) | |||||
| Complete vaccination (BNT162b2 vaccine) | 592,493 | 5 | 0.008 | 90.2% | 90.4% |
| (0.004–0.020) | (84.9%–92.3%) | (85.3%–92.4%) | |||
| Complete vaccination (CoronaVac vaccine) | 1,631,093 | 51 | 0.031 | 64.7% | 64.8% |
| (0.024–0.041) | (58.5%–70.0%) | (58.6%–70.0%) | |||
The incidence rate (number of events per 1000 person-days) from infection, hospitalization, and death from COVID-19 in unvaccinated patients (n = 1196) and patients with complete vaccination with BNT162b2 (n = 2860) or CoronaVac (n = 7857) is shown. Patients with complete vaccination were considered as those who had greater than or equal to 14 days after receipt of the second dose. Efficacy of vaccination (estimated as 1 minus the hazard ratio adjusted for covariates, expressed as percentage) to reduce risk of infection, hospitalization, or death with its 95% CI are presented.
Discussion
The results from this national multicenter observational study showed that BNT162b2 and CoronaVac vaccines prevented COVID-19 infection, and reduced hospitalization and death rates associated with infection among patients with end-stage renal disease on chronic hemodialysis who were vaccinated against SARS-CoV-2. In addition, greater efficacy was observed with BNT162b2 than with CoronaVac in preventing infection and death from COVID-19. This is one of the first studies to demonstrate the clinical benefits of vaccinations in patients undergoing chronic hemodialysis. Furthermore, this is the first clinical study that has evaluated the efficacy of both BNT162b2 and CoronaVac vaccines in this population. To date, most of the literature related to the effects of anti-SARS-CoV-2 vaccination in hemodialysis patients have dealt with the effects of laboratory immunological parameters, specifically anti-SARS-CoV-2 antibody titers.22, 23, 24 These studies showed that vaccinations in hemodialysis patients increased antibody titers, but by a lower proportion than in the healthy population.
In this study, data are provided on the efficacy of the administration of the CoronaVac and BNT162b2 vaccines in a population of 12,301 people on hemodialysis in Chile, following a massive vaccination campaign throughout the country. Among the hemodialysis patients with a complete vaccination, the effectiveness of the vaccination was 18.1% for COVID-19 development, 42.8% for hospitalization, and 66.0% for COVID-19-associated deaths. Nevertheless, the efficacy of the BNT162b2 vaccine was higher than that of CoronaVac in preventing infections and deaths associated with COVID-19. This result is in line with work that has shown that RNA platforms provide a greater immune response in this group of patients.22
Whereas this study was not designed to compare the efficacy of vaccinations between the general population and patients on hemodialysis, our results suggest that the clinical efficacy of vaccinations among patients on hemodialysis is lower than that in the general population. In the study by Jara et al.17 the CoronaVac vaccine was evaluated in the Chilean general population. It was observed that, among patients with complete vaccination, the efficacy to prevent COVID-19 was 65.9% (95% CI: 65.2–66.6%) and efficacy to prevent deaths associated with COVID-19 was 86.3% (95% CI: 84.5–87.9%). This result is higher than the efficacy found in our group of patients receiving CoronaVac, which was 15.0% (95% CI: 8.3–21.4%) and 64.8% (95% CI: 58.6–70.0%) in preventing infection and deaths associated with COVID-19, respectively.
One of the strengths of this work is the large amount of data obtained, which covers over 80% of the national population on hemodialysis. These data include information on demographic and clinical baseline variables, and further information on COVID-19 infection, including hospitalization and associated deaths, that identify the efficacy of vaccinations during the first 7 months of the vaccination program. The information was obtained during a fast national vaccination campaign, which has had high acceptance in the population. In addition, this period included the months with the highest rate of community transmission and number of deaths during the pandemic, allowing the effectiveness of vaccinations to prevent infection and death to be evaluated in a relatively short period. In addition, Chile has one of the highest rates of polymerase chain reaction testing for COVID-19 in Latin America, which helps to reduce the number of undetected cases in the general population. This process was aided by the activity of the FUTAC Team, which has provided integrated collaborative action between the different nationwide dialysis teams, including updated information on patients attending the respective hemodialysis centers, which have facilitated a high detection of cases and information obtained during hospitalization and death.
The large population sample allowed us to estimate vaccine effectiveness for the full 2-dose vaccination program, as well as to evaluate the individual efficacy of the BNT162b2 and CoronaVac vaccines. It also provided information for a subgroup analysis of adults aged 60 years and older and patients with diabetes. These groups of patients have an increased risk of severe disease and are frequently underrepresented in vaccine clinical trials.25 The vaccine efficacy analysis was calculated by adjusting for baseline comorbidities (including diabetes), age, sex, and hemodialysis vintage, which are risk factors associated with worse outcomes among these patients;6,26 therefore, they could have confounding effects when analyzing the efficacy of vaccines.
Regarding the limitations of this study, its observational nature has intrinsic biases, because some influencing patient variables are difficult to isolate. Compared to those with complete doses, unvaccinated patients had a mildly increased percentage of women, younger age, and lower hemodialysis vintage. These variables are associated with a reduced risk of SARS-CoV-2 infection and death.6,26 Therefore, these differences do not explain the worse outcomes observed in this group, thereby supporting the role of vaccination. Additional characteristics in the unvaccinated population not evaluated in the study could make them refuse the vaccination, such as beliefs about vaccines, conspiracy theories, fear of social control or by others, decrease the use of vaccines and their consultation at hospital centers, and may be associated with increased infection and death rates. To address this, the FUTAC Team conducted a national educational campaign on vaccinations at dialysis centers, with the participation of the dialysis center directors, attending doctors and nurses to provide accurate information on the risks of COVID-19, infection prevention measures, and the benefits of the vaccination. This allowed all patients (vaccinated or unvaccinated) to have access to accurate information and may contribute to increasing adherence to vaccinations and medical controls.
An additional limitation to the study is that a follow-up of 7 months does not allow us to conclude that vaccine protection will be maintained in the long-term. Therefore, there is a potential risk of an increase in infection and death rates in the vaccinated population. This risk is also supported by the literature, which indicates that anti-SARS-CoV-2 antibody titers decay early in hemodialysis patients compared with the general population.14 This has led to the introduction of a third dose of the vaccine among vulnerable patient groups, including our patients. This is supported by recent data showing that hemodialysis patients have an increase in antibody titers after a third dose of anti-SARS-CoV-2 vaccine.27 Given this, long-term follow-up of the effect of this vaccination remains to be evaluated. Finally, no information is available regarding the different SARS-CoV-2 lineages involved. A change in the virological spectrum of the variants of the virus has also been described worldwide, which is currently dominating other lineages different from those with which the BNT162b2 and CoronaVac vaccines were designed that may be able to influence their efficacy.28,29 In Chile, an increase in lineage P.1 (Gamma variant) and lineage B.1.617.2 (Delta variant) has been reported during the second half of 2021.30 Further studies with increased patient follow-up are required to determine whether vaccine efficacy is maintained with the change in viral lineages in patients undergoing chronic renal replacement therapy.
In conclusion, the results of our study suggest that COVID-19 vaccination in patients on chronic hemodialysis is highly effective in preventing SARS-CoV-2 infection and reducing the case fatality rate associated with COVID-19, with greater efficacy for BNT162b2 compared to CoronaVac. These results support the importance of conducting national vaccination campaigns aimed at this high-risk population to prevent the development of adverse outcomes in this vulnerable group.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This research was supported by grants of the Chilean Society of Nephrology, plus FONDECYT de Iniciacion 11171141 (LT) and FONDECYT Regular 1221571 (LT) grants of the Agencia Nacional de Investigacion y Desarrollo (ANID).
We thank all the nursing staff who helped with the recording of this data. Hugo Arias Troncoso, Victor Huenchulaf Vasquez, Elena Segovia Vega, Hugo Lazo Rejano, Pamela Cortez, Alejandra Chelme, Emperatriz Guevara Pizarro, Natalie Araya Cortés, Johanna Maita Hernandez, Ana Payeros Rivera, Camila Serrano Barrera, Angélica Orrego Lazo, Maria Jose Fernandez, Gloria Araya Aburto, Carolina Parra, Gladys Araya Vergara, Karina Rodriguez, Andrea Salgado Avila, Mónica Arteaga, Ruth Bolados Rubio, Bella Velis Jaque, Veronica Campos Osses, Viviana Alfaro Castro, and Karina Ponce Torres; P. Villavicencio, Pamela; Doris de la Fuente, Soledad Gallardo, Deissy Escamilla, Bernardita Leigh, Ronald Gonzalez, Jessica Valdes, Olga Artunduaga, Isabel Alvarez, Amelia Cabello, Pamela Barahona, Carla Borquez, Rossana Maulen, Erica Segura, Mariela Gonzalez, Pamela Yañez, Cristina Gallardo, Emperatriz Guevara, Catalina Cruz, Jorge Aguilar, Carolina Parra, María José Fernandez, Veronica Campos, Paulina Araya, Karina Rodriguez, Monica Arteaga, Marcela Vargas, Gloria Araya, Ivonne Gaete, Daniela Ubilla, Barbara Salazar, Cecilia Vivallo, Carolina Perez, Rosymery Valencia, and Daniela Parada.
In addition, each of the public and private dialysis centers that participated in the registry of the FUTAC Team in Chile. Complejo Asistencial Sótero del Río, Clinica Alemana Santiago, diálisis Antares Talagante, Dialisis Calama, Centro médico y de Diálisis Ltda. Sucursal Carmen Mena, Centro Médico y de Dialisis Ltda. Unidad Celia Zegers, CID Servicio integral de salud S.A, Centro de dialisis Diamar, Hospital Militar, Centro de Dialisis de Mendoza Ltda, diálisis Maiquén, Dialisis Municipal La Granja, dialisis Ñuñoa, sucursal Quinta Normal, Diálisis Ñuñoa, Dialisis Ñuñoa Chacabuco, Dialisis Padre Hurtado de Cerrillos, dialisis Antares Peñaflor, Dialisis Peñaflor, dialisis San Juan de Dios, Centro de Dialisis San Lucas, Dialisis San Ramon, Centro de Diálisis Vespucio, dialisis La Reina, Fresenius Chile, Diaverum Chile, Reddialisis, Centro Renal SPA, diálisis Lampa, Clínica Indisa, Clinica Santa Maria, Unidial S.A., Hospital Puerto Natales, Hospital Puerto Montt, Unidad Dialisis Modular Hospital Comunitario 21 de Mayo Taltal, Hospital Magallanes, centro dialisis Clínica Croacia Punta Arenas, Unidad de dialisis Hospital Carlos Cisternas de Calama, Dialisis Nordial Ltda, Hospital Marcos Macuada Tocopilla, Diálisis Bupa Antofagasta, centro dialisis Ehrlich Ltda, Centro de Dialisis Hospital Comunitario de Mejillones, Dialisis Tecdial, Unidad de diálisis Hospital Regional de Antofagasta, Centro de diálisis Renacer, Centro hemodiálisis crónica Hospital Clínico Universidad de Chile, Centro hemodiálisis crónica UC-Christus, Hospital Regional Coyhaique, Hospital Puerto Aysen, Urodial, Nefrodial Molina, Diálisis del Libertador Rengo, Hospital regional Libertador Bernardo O´Higgins, Nefrodial Linares, Diálisis Aguamarina, Serdial Ltda, Centro de diálisis Curicó Ltda, Nefrodial San Javier, Rancagua Dial Ltda, Diálisis San Isidro, Diálisis Gran Avenida, Premio Nobel, Vidadial Paillaco, and Vidadial Lanco.
Data Sharing Statement
The data that support the findings of this study are available upon reasonable request to the corresponding author, R.T. The data are not publicly available because they contain information that could compromise the privacy of research participants and third-party restrictions.
In Memoriam
This publication is dedicated to Dr. Andres Boltansky, MD, who died in December 2020 from a COVID-19 infection. Dr. Boltansky was an expert nephrologist dedicated to kidney transplantation, a Chilean Society of Nephrology directive, and a cofounder of the FUTAC Team.
Author Contributions
RT, LT, MES, EL, MO, JP, RC, and EM designed the study. FG, PH, AM, AF, ER, CM, CA, EQ, JL, ML, ER, AM, EP, AF, RM, GS, MG, CM, EB, EG, SM, WB, XR, OE, EZ, HA, MB, MV, LE, DZ, IF, BT, TB, and PH collected the data. LT and GC analyzed the data. RT, LT, and AP created the figures. RT, LT, MES, and EM drafted the initial manuscript, which was revised by all authors. All authors approved the final version of the manuscript.
Footnotes
Table S1. Efficacy of the anti-SARS-Co-2 vaccination to prevent COVID-19 and death in patients over 60 years.
Table S2. Efficacy of the BNT162b2 and CoronaVac vaccines to prevent COVID-19 and death in patients over 60 years of age.
Table S3. Efficacy of the anti-SARS-Co-2 vaccination to prevent COVID-19 and death in patients with diabetes.
Table S4. Efficacy of the BNT162b2 and CoronaVac vaccines to prevent COVID-19 and death in patients with diabetes.
STROBE Statement.
Contributor Information
Rubén Torres, Email: rtorres@med.uchile.cl.
Luis Toro, Email: ltoro@med.uchile.cl.
Supplementary Material
Table S1. Efficacy of the anti-SARS-Co-2 vaccination to prevent COVID-19 and death in patients over 60 years.
Table S2. Efficacy of the BNT162b2 and CoronaVac vaccines to prevent COVID-19 and death in patients over 60 years of age.
Table S3. Efficacy of the anti-SARS-Co-2 vaccination to prevent COVID-19 and death in patients with diabetes.
Table S4. Efficacy of the BNT162b2 and CoronaVac vaccines to prevent COVID-19 and death in patients with diabetes.
STROBE Statement.
References
- 1.Hopkins University and Medicine John. John Hopkins University School of Medicine; 2022. Coronavirus Resource Center. COVID-19 map.https://coronavirus.jhu.edu/map.html [Google Scholar]
- 2.Yi Y., Lagniton P.N.P., Ye S., et al. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci. 2020;16:1753–1766. doi: 10.7150/ijbs.45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torres Díaz R., Lorca Herrera E. Covid-19 in chronic kidney patients: a warning. Rev Med Chil. 2020;148:711–712. doi: 10.4067/S0034-98872020000500711. [DOI] [PubMed] [Google Scholar]
- 4.Ng J.H., Hirsch J.S., Wanchoo R., et al. Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney Int. 2020;98:1530–1539. doi: 10.1016/j.kint.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alfano G., Ferrari A., Magistroni R., et al. The frail world of haemodialysis patients in the COVID-19 pandemic era: a systematic scoping review. J Nephrol. 2021;34:1387–1403. doi: 10.1007/s40620-021-01136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu C.M., Weiner D.E., Aweh G., et al. COVID-19 among US dialysis patients: risk factors and outcomes from a national dialysis provider. Am J Kidney Dis. 2021;77:748–756.e1. doi: 10.1053/j.ajkd.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boadle A. COVID-19 deaths in Latin America surpass 1 mln as outbreak worsens. 2021. Reutrs. https://www.reuters.com/world/americas/covid-19-deaths-latin-america-set-surpass-1-mln-outbreak-worsens-2021-05-21/
- 8.Number Statista. of deaths due to the novel coronavirus (COVID-19) in Latin America and the Caribbean as of August 15, 2022, by country. 2022. https://www.statista.com/statistics/1103965/latin-america-caribbean-coronavirus-deaths/
- 9.Chilean Society of Nephrology Results of the national survey of COVID-19 infection in end-stage renal disease patients on hemodialysis. 2021. https://www.nefro.cl/covid/img/encuestas/Registro_Encuesta_infeccion_31-12-2021.pdf
- 10.Thomas S.J., Moreira E.D., Jr., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med. 2021;385:1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tavilani A., Abbasi E., Kian Ara F., Darini A., Asefy Z. COVID-19 vaccines: current evidence and considerations. Metabol Open. 2021;12 doi: 10.1016/j.metop.2021.100124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Occupational Safety and Health Administration . United States Department of Labor; 2021. Protecting workers: guidance on mitigating and preventing the spread of COVID-19 in the workplace.https://www.osha.gov/coronavirus/safework#about-covid-19 [Google Scholar]
- 13.Clarke C.L., Prendecki M., Dhutia A., et al. Longevity of SARS-CoV-2 immune responses in hemodialysis patients and protection against reinfection. Kidney Int. 2021;99:1470–1477. doi: 10.1016/j.kint.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alcázar-Arroyo R., Portolés J., López-Sánchez P., et al. Rapid decline of anti-SARS-CoV-2 antibodies in patients on haemodialysis: the COVID-FRIAT study. Clin Kidney J. 2021;14:1835–1844. doi: 10.1093/ckj/sfab048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simon B., Rubey H., Treipl A., et al. Haemodialysis patients show a highly diminished antibody response after COVID-19 mRNA vaccination compared with healthy controls. Nephrol Dial Transplant. 2021;36:1709–1716. doi: 10.1093/ndt/gfab179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanriover M.D., Doğanay H.L., Akova M., et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398:213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jara A., Undurraga E.A., González C., et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385:875–884. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chilean Ministry of Health . Chilean Ministry of Health; 2021. Mass vaccination schedule against COVID-19.https://www.minsal.cl/calendario-de-vacunacion-masiva-contra-covid-19/ [Google Scholar]
- 19.Chilean Ministry of Health Epidemiological surveillance system EPIVIGILA. 2021. http://epi.minsal.cl/sistema-de-vigilancia-epidemiologica-epivigila-antecedentes/
- 20.World Health Organization Emergency use ICD codes for COVID-19 disease outbreak. https://www.who.int/standards/classifications/classification-of-diseases/emergency-use-icd-codes-for-covid-19-disease-outbreak
- 21.Thompson M.G., Burgess J.L., Naleway A.L., et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers - eight U.S. Morb Mortal Wkly Rep. 2021;70:495–500. doi: 10.15585/mmwr.mm7013e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia P., Anand S., Han J., et al. 2021.08.02.21261516. Published 2021 Aug 4; 2021. COVID19 vaccine type and humoral immune response in patients receiving dialysis. Preprint. medRxiv. 2021. [DOI] [Google Scholar]
- 23.Kolb T., Fischer S., Müller L., et al. Impaired immune response to SARS-CoV-2 vaccination in dialysis patients and in kidney transplant recipients. Kidney360. 2021;2:1491–1498. doi: 10.34067/KID.0003512021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Espi M., Charmetant X., Barba T., et al. The Romanov study found impaired humoral and cellular immune responses to SARS-CoV-2 mRNA vaccine in virus-unexposed patients receiving maintenance hemodialysis. Kidney Int. 2021;100:928–936. doi: 10.1016/j.kint.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flores L.E., Frontera W.R., Andrasik M.P., et al. Assessment of the inclusion of racial/ethnic minority, female, and older individuals in vaccine clinical trials. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.37640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kakkanattu T.J., Sankarasubbaiyan S., Yadav A.K., et al. Outcome and determinants of outcome of COVID-19 infection among hemodialysis patients: findings from a national dialysis network program in India. Kidney Int Rep. 2021;6:1429–1432. doi: 10.1016/j.ekir.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ducloux D., Colladant M., Chabannes M., et al. Humoral response after 3 doses of the BNT162b2 mRNA COVID-19 vaccine in patients on hemodialysis. Kidney Int. 2021;100:702–704. doi: 10.1016/j.kint.2021.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hacisuleyman E., Hale C., Saito Y., et al. Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med. 2021;384:2212–2218. doi: 10.1056/NEJMoa2105000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chau N.V.V., Ngoc N.M., Nguyet L.A., et al. An observational study of breakthrough SARS-CoV-2 Delta variant infections among vaccinated healthcare workers in Vietnam. EClinicalMedicine. 2021;41 doi: 10.1016/j.eclinm.2021.101143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Informe epidemiologico N°13 vigilancia genomica de SARS-CoV-2 (COVID-19). Departamento de Epidemiología. https://www.minsal.cl/wp-content/uploads/2021/09/Informe-Variantes-N13-05-09-2021.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.