Abstract
Background/Objectives
To the best of our knowledge, there have been no previous studies conducted on the long-term effects of an exercise intervention on deficits in inhibitory control in obese individuals. The aim of this study was thus to examine the effect of 12 weeks of a combination of aerobic and resistance exercise on behavioral and cognitive electrophysiological performance involving cognitive interference inhibition in obese individuals.
Methods
Thirty-two qualified healthy obese women were randomly divided into either an exercise group (EG, age: 34.76 ± 5.52 years old; BMI: 29.35 ± 3.52 kg/m2) or a control group (CG, age: 33.84 ± 7.05 years old; BMI: 29.61 ± 4.31 kg/m2). All participants performed the Stroop task, with electrophysiological signals being collected simultaneously before and after a 12-week intervention. The estimated V̇O2max, muscular strength, and body fat percentage (measured with dual-energy X-ray absorptiometry) were also assessed within one week before and after the intervention. Participants in the EG group engaged in 30 min of moderate-intensity aerobic exercise combined with resistance exercise, 5 sessions per week for 12 weeks, while the participants in the CG group maintained their regular lifestyle without engaging in any type of exercise.
Results
The results revealed that although a 12-week exercise intervention did not enhance the behavioral indices [e.g., accuracy rates (ARs) and reaction times (RTs)] in the EG group, significantly shorter N2 and P3 latencies and greater P2 and P3 amplitudes were observed. Furthermore, the fat percentage distribution (e.g. total body fat %, trunk fat %, and leg fat %) and level of physical fitness (e.g. estimated V̇O2max and muscular strength) in the EG group were significantly improved. The changes prior to and after the intervention in the P3 amplitude and trunk fat percentage were significantly negatively correlated in the EG group (r = −0.521, p = 0.039).
Conclusions
These findings suggested that 12 weeks of aerobic exercise combined with resistance exercise in obese women affects cognitive function broadly, but not specifically in terms of inhibitory control. The percentage of decreased trunk fat may play a potential facilitating role in inhibition processing in obesity.
Keywords: Obesity, Inhibitory control, Event-related potential, Aerobic exercise, Resistance exercise
1. Introduction
The ability to suppress dominant responses and resist irrelevant stimuli is called inhibitory control1 and is believed to play a key role in the regulation of body weight.2 In comparison with normal-weight individuals, obese adults not only have reduced brain volume (e.g., frontal cortex and anterior cingulate cortex), but show greater activation when responding to food cues in brain regions involved in the regulation of food intake [e.g., the salience network3 and the hypothalamic network4 under both fasting or sated conditions.5 In addition, obese people exhibit lower dopamine D2 receptor density in the striatum, which is associated with higher metabolic activity in the prefrontal regions involved in inhibitory control and could be a potential mechanism contributing to overeating.6 Indeed, it has been well established that obese individuals exhibit deficits in various inhibitory controls.7, 8, 9 These previous findings thus suggest that impairment in inhibitory control is one of the core features associated with obesity and a potential therapy target for clinical interventions.
Exercise, which is an aspect of an active lifestyle, is effective in improving problems related to inhibitory control.10, 11, 12, 13 Obese individuals have been demonstrated to obtain advantages with regard to neural inhibitory processing in the attentional networks through engaging in regular exercise.14 Also, it has been well established that positive effects of acute exercise on inhibitory control have been observed among both healthy and obese adults.15, 16, 17 However, in terms of chronic exercise, it seems that at the moment, results on chronic exercise intervention and inhibitory control are somewhat equivocal. For example, although previous studies have shown that a long-term exercise intervention can facilitate inhibitory control among healthy young adults18 and patients with fibromyalgia,19 systematic reviews and meta-analysis reported only indicated modest benefits in the inhibitory control of healthy children and adolescents after they participated in various chronic exercise interventions.20 In additional, Kao et al. (2021) showed that 12 weeks of moderate-intensity aerobic exercise combined with resistance training did not change reaction times in women with systemic lupus erythematosus when performing the Stroop task.21 Therefore, it is worth exploring whether obese individuals experience improvement in their neurocognitive performance in terms of deficits in inhibitory control via a chronic exercise intervention.
The P2, N2, and P3 event-related potential (ERP) is ideal for capturing the rapid processes involved in attentional inhibition and inhibitory control.22, 23, 24 P2 is an ERP component of task-related target discrimination occurring around 170–270 ms post-stimulus, which is related to conscious activities and reflects initial semantic processing and attention distribution.25 N2, an early negative deflection occurring around 200–400 ms post-stimulus, is mainly associated with conflict monitoring processes and response inhibition.26 The following P3 wave, a positive component occurring around 300–600 ms post-stimulus, is associated with inhibition processing or motivation-related attentional engagement (e.g., P3 amplitude).7,27,28 The three ERP components have been demonstrated to effectually differentiate cognitive electrophysiological performance in obese and normal-weight individuals when performing cognitive tasks involving inhibitory control (e.g., Go/NoGo and Stroop tasks).29, 30, 31 However, Carbine et al. (2018) found that, when individuals performed the Go/NoGo task, there was no significant effect of BMI on inhibitory control.32
The Stroop task is considered to be an appropriate assessment of deficits in inhibitory control in obese individuals16 since it activates task-relevant fronto-cingulo-striatal neural networks comprising the left-hemispheric parieto-temporal and fronto-striatal regions.23 When an individual performs such a cognitive task, he/she has to be equipped with the ability to resist interference from stimuli in the external environment (e.g., interference control/attentional inhibition).33 Previous studies have used cognitive tasks to assess cognitive interference inhibition in overweight/obese children29 and obese women.9 Longer RTs29 and significantly longer ERP N2 latency and smaller P3 amplitude9 have been observed in obese groups relative to control groups. Beneficial effects of acute aerobic and resistance exercise on general wellbeing and inhibitory control in adolescents34 and adults15,35 when performing the Stroop task have also been reported. Most importantly, Wen and Tsai (2020a) also found that a combination of acute moderate-intensity aerobic and resistance exercise may improve neurophysiological inhibitory control (e.g., shorter N2 and P3 latencies, smaller N2 amplitudes, and greater P3 amplitudes) when performing the Stroop task among obese women in comparison to normal-weight controls.16 Therefore, this type of exercise modality in the form of a long-term intervention may be an effective strategy by which to remedy behavioral and, specifically, neurophysiological (e.g., ERP) deficits involving inhibitory control in obese individuals.
To summarize, body fat percentage begins to increase from the age of 30 to <40 years in women,36 and female adults suffering from obesity show problems in cognitive interference inhibition when performing the Stroop task.16 A combination of aerobic and resistance exercise with moderate intensity, as suggested by the American College of Sports Medicine (ACSM), has been proven to be beneficial to cognitive functions in overweight inactive adults.37 In addition, previous studies demonstrated that deficits in interference control in overweight/obese women can be improved through acute aerobic combined with resistance exercise.16 However, no studies have yet been conducted exploring whether deficits in inhibitory control in obese individuals can be remedied through a chronic exercise intervention. Accordingly, the main purpose of the present study was to investigate the effects of 12 weeks of combined aerobic and resistance exercise on neurocognitive function related to cognitive interference inhibition in obese women. Previous long-term exercise intervention studies proved there to be positive effects on behavioral performance in the Stroop task.38,39 We thus hypothesized that the behavioral performance (primary outcomes) and neuroelectrophysiological performance (secondary outcomes) would be improved after the 12-week intervention (e.g., improving AR; fasting RT, and N2, P2, and P3 amplitude enlarged, and latency shortened). In addition, chronic exercise can play an important role in helping overweight/obese individuals lose/manage their weight and improve their level of physical fitness. Up to the present, evidence of potential associations between changes in behavioral/neurophysiological indices and body fat percentage distribution/physical fitness after an exercise intervention in obese individuals have been limited. The second purpose of this study was thus to investigate the relationships between chronic exercise-induced neurocognitive changes and changes in body composition (e.g., fat percentage in upper, trunk, or lower body) and physical fitness (e.g., cardiorespiratory and muscular fitness) in obese women. Since better behavioral and neurophysiological (i.e., functional near infrared spectroscopy) performance was significantly correlated with greater weight loss over a 4-week combination of aerobic and anaerobic exercise intervention in overweight or obese individuals,40 it was hypothesized that significant correlations between the changes in neurocognitive performance and body composition and physical fitness would be observed in the obese women of interest in the present study.
2. Methods
2.1. Ethical approval
All participants gave informed consent prior to the study, and all protocols were approved by the Research Ethics Committee of Tzu Chi Hospital in Hualien, Taiwan (approved number: IRB106-32-A) and complied with the standards set out in clause 35 of the Declaration of Helsinki.
2.2. Participants
This study was advertised on a local radio station and also posted in a local newspaper in Hualien County, Taiwan. Based on the criteria for obesity established by the Western Pacific Regional Offce of the World Health Organization for Asian populations according to related mortality and morbidity risks,41,42 healthy obese women with a body mass index (BMI) > 25.0 kg/m2 were recruited as the participants in the present study. We only recruited female participants because the effects of exercise on cognitive degeneration may be gender-dependent.43,44 Participants were screened for their current diet by counting their energy expenditure calories, and physical activity level of all participants was evaluated to confirm the homogeneity of both groups. A participant was excluded if she self-reported attending regular physical activity/exercise over twice a week when interviewed. A flow diagram contouring the process of the participants and how the present trial was designed, analyzed, and elucidated in line with criteria established by the Consolidated Standards of Reporting Trials (CONSORT) is provided in Fig. 1. Additional inclusion criteria included (a) right hand dominant, as assessed by the Edinburgh Handedness Inventory using arbitrary cut-off points between 0 to 60,45 (b) non-smokers, (c) normal or corrected-to-normal vision, (d) no symptoms of depression as measured by the Beck depression inventory II (BDI-II; all scored below 13),46 and (e) cognitive integrity as measured by the Mini-Mental State Examination (MMSE, where all participants scored above 24).47 G∗Power software 3.1 was used to conduct a priori analysis of the achieved power level based on previous research.16 The test family was set to “F-tests,” and “ANOVA: Repeated measures” was selected for the statistical test. The required sample size was computed given α, power, and effect size (effect size f = 0.26, α err prob = 0.05, power (1-β err prob = 0.80), where the correlations among the repeated measures = 0.5, and the nonsphericity correction = 1, leading to a total sample size = 32. A minimum sample size of ∼16 participants for each group was thus required to target a power of 80% and moderate effect sizes.48
Fig. 1.
Schematic representation of the experimental procedure.
2.3. Experimental procedure
Fifty women from the community in Hualien City were interested in participating in the study. Eighteen were excluded because they failed to meet the criteria or declined to participate after hearing a detailed explanation of the protocol. Eligible participants were self-reported to be free of metabolic or cardiovascular diseases, neurological, or psychiatric disorders, attending regular physical activity/exercise less than twice a week, and professed to be absent from a history of brain injuries or medication intake that would influence central nervous system (CNS) functioning. Thirty-two eligible healthy obese women were then randomized to an exercise group (EG) or a control group (CG). The demographic characteristics data for the two groups are provided in Table 1.
Table 1.
Demographic characteristics of the participants.
| Characteristics | Control group (n = 16) | Obese group (n = 16) | p value (CI) |
|---|---|---|---|
| Age (years) | 33.84 ± 7.05 | 33.95 ± 5.99 | 0.962 (−4.61, 4.83) |
| Height (cm) | 158.79 ± 5.68 | 159.88 ± 4.87 | 0.563 (−2.73, 4.91) |
| Weight (kg) | 74.94 ± 13.58 | 75.74 ± 11.11 | 0.855 (−8.15, 9.77) |
| BMI (kg/m2) | 29.61 ± 4.31 | 29.50 ± 3.57 | 0.942 (−2.96, 2.75) |
| SBP (mmHg) | 112.31 ± 13.86 | 113.56 ± 12.23 | 0.789 (−8.19, 10.69) |
| DBP (mmHg) | 69.63 ± 9.41 | 75.00 ± 8.24 | 0.096 (−1.01, 11.76) |
| Resting HR (bpm) | 76.13 ± 8.85 | 73.00 ± 4.15 | 0.211 (−8.11, 1.86) |
| BDI-II | 7.63 ± 3.12 | 6.23 ± 3.59 | 0.257 (−3.85, 1.07) |
| MMSE | 29.13 ± 0.84 | 29.67 ± 0.72 | 0.072 (−0.05, 1.12) |
| Physical activity (kcal) | 1980.98 ± 469.23 | 1953.21 ± 296.68 | 0.846 (−318.44, 262.90) |
| Diet (kcal/day) | 1905.10 ± 558.73 | 2018.59 ± 579.98 | 0.420 (−266.42, 619.41) |
| Waist circum. (cm) | 85.78 ± 9.76 | 87.81 ± 9.95 | 0.565 (−5.09, 9.14) |
| Hip circum. (cm) | 108.02 ± 9.66 | 110.71 ± 7.55 | 0.388 (−3.57, 8.95) |
| Waist-Hip Ratio | 0.79 ± 0.05 | 0.79 ± 0.05 | 0.909 (−0.04, 0.03) |
BMI, body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; HR: heart rate; bpm, beat per minute; BDI, Beck depression inventory; MMSE, mini-mental state examination; and PA, physical activity; circum.: Circumference. CI: Confidence intervals. Values are means ± SD. ∗p < 0.05.
All participants were scheduled for the body composition and cardiorespiratory/muscular fitness measurements using dual-energy X-ray absorptiometry (DXA) at Tzu Chi Hospital, and cardiorespiratory fitness measurements (2 km walk test) were carried out at Tzu Chi University, respectively. Within one week, each participant visited the cognitive neurophysiology laboratory at Tzu Chi University at approximately 7:50–08:30 a.m. to control for circadian influences and was asked to refrain from caffeine or alcohol intake and strenuous exercise for 24 h before the formal neurocognitive test. The research assistant explained the experimental procedure to each participant and then asked her to complete an informed consent form, a medical history, a handedness inventory, a demographic questionnaire, the MMSE, and the BDI-II. After completing all of the questionnaires, each participant sat comfortably 80 cm in front of a laptop screen in a semi-dark room with a suitable electrocap before the neurocognitive test. After a practice session to acquaint the participant with the experimental procedure, a formal cognitive task test with concomitant electroencephalogram recording was performed. The first exercise intervention session was carried out within one week after the neurocognitive function assessment. The participants in the EG group began to perform an aerobic-and-resistance exercise intervention (please see the detailed protocols in Section 2.4.), whereas the participants in the CG group were instructed to maintaine their regular lifestyle during the 12-week experiment period. After the intervention, the body composition, cardiorespiratory/muscular fitness, and neurocognitive tests were assessed again.
2.4. Exercise intervention
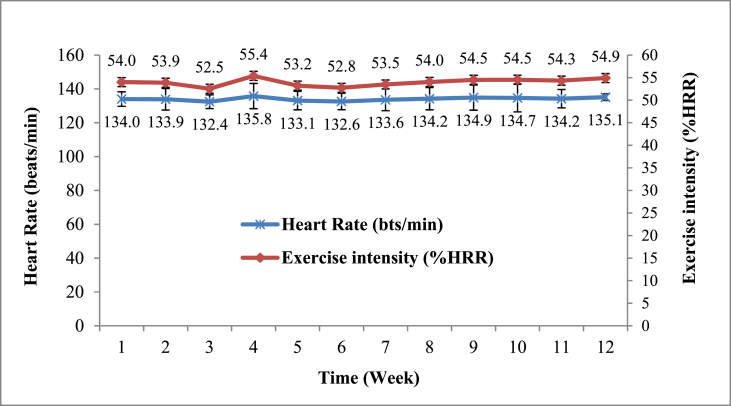
The resting heart rate was measured during ERP recording in the pretest. Each participant's target exercise HR was determined based on a 55% HR reserve, i.e., [(220-age)-HR rest] × 40–59% + HR rest in the beginning, gradually increasing to a ∼59% HR reserve.49 HR was monitored throughout the exercise session using a telemetry HR monitor (S810, Polar, Kempele, Finland). The mean exercise intensity (53.97 ± 4.27 %HR reserve) and HR (134.04 ± 7.11 beats per min) for each session during the 12 weeks of exercise intervention in the EG group is illustrated in Fig. 2.
Fig. 2.
The mean exercise intensity and heart rates for each session during the 12-week intervention in the exercise group.
The exercise program (see Fig. 3) consisted of a 3–5 min warm-up session, 30-min of supervised moderate-intensity aerobic dance alternately combined with dumbbell resistance exercises specifically designed for this study, followed by a 3–5 min cool-down session (see the details of the exercise modality in Wen & Tsai (2020a).16 Participants in the EG group were instructed to exercise a total of 5 sessions a week for 12 weeks during the experimental period. The exercise procedures were performed in a group setting (3–20 participants depending on their schedules) instructed by the researcher and/or a well-trained research assistant. The average attendance rate during the 12-week intervention in the EG group reached 82.72 ± 24.49% (attending 51.22 ± 11.45 sessions for a total of 60 sessions).
Fig. 3.
Exercise program.
2.5. Body composition and cardiorespiratory fitness and muscular assessments
2.5.1. Body composition
A dual-energy X-ray absorptiometer (DXA; Lunar Prodigy, GE Inc., USA), a tool used to measure and validate body composition,50 was used to measure body composition. Measurements were carried out by certified technicians in accordance with standard operating procedures. The scanning instructions and procedures for all participants were standardized. The torso included the area from the bottom of the neckline to the top of the pelvis, excluding the arms. The mass output of the DXA scanner was expressed in grams. The accuracy of the densitometry was calibrated using the manufacturer's spine model with the known density of hydroxyapatite on each day before beginning the test.
2.5.2. Cardiorespiratory fitness
Cardiorespiratory fitness was assessed in an indoor gymnasium using a 2-km Walk Test.51,52 Participants were encouraged to walk as fast and as steadily as possible throughout the test. HR was continuously monitored with a telemetry HR monitor during the walk. V̇O2max was calculated according to the established equation.51,52
2.5.3. Muscular assessments
2.5.3.1. Leg extension
Leg extension one repetition maximum (1RM) was determined according to the predictive equation proposed by Brzycki (1993). The participants performed a sub-max leg extension test using a leg extension machine (Paramount, FS-50, USA). They started with a warm-up series of 6–10 repetitions with approximately 50% of the workload established for this test. After a 2-min rest, the test was initiated. The participants were told to attempt to perform as many repetitions as possible until they were not able to lift the load. Participants who were not able to do 10 repetitions of the applied load had the load and the number of repetitions recorded, and this was then used to estimate the 1-RM values for the leg extension.53,54
2.5.3.2. Chair stand
The chair stand test was used to assess leg strength and endurance.55,56 The participants sat in the middle of a chair and placed their hands on the opposite shoulder crossed at the wrists while keeping their feet flat on the floor. After hearing the instructor say “Go,” the participants were asked to get up from the chair into a full standing position and then to sit down again as soon as possible for as many times possible over a 30-s period. During the test, the participants were asked to keep their back straight and their arms against their chest. The total number of stand-ups in 30 s was measured twice, and the best performance was recorded.
2.5.3.3. Sit-up
A one-minute half sit-up test was used to examine the abdominal muscular strength and endurance. Participants were instructed to lie on a mat with the knees bent, feet flat on the floor, with their hands crossed over the chest, where they were required to stay throughout the test. They were instructed to breathe out on the way up and perform as many sit-ups as they could in 1 min. The repetitions were recorded at 30 s and 60 s to determine the trunk muscular strength and endurance, respectively.57
2.6. Estimation of total energy expenditure and dietary assessment
2.6.1. 7-d physical activity recall (7-d PAR)
The 7-day Recall Physical Activity Questionnaire, designed by Sallis et al.58 was used to estimate the calories expended on all activities during the previous 7 days. The participants self-reported and filled in information consisting of the duration of highly strenuous activities (at least 7 metabolic equivalents), strenuous activities (5.1–6.9 METs), moderate activities (3–5 METs), and sleep time. Energy expenditure was calculated based on the average energy depletion for all reported activities. The intraclass correlation coefficient for test-retest reliability for the 7-day Recall Physical Activity Questionnaire was 0.89.59 The total energy expenditure was significantly correlated with the average activity counts per min, as measured with tri-axial accelerometers (r = 0.49).59 For details of the test-retest ICC, refer to.60
2.6.2. Dietary assessment
The participants were asked to complete a simple frequent food questionnaire (SFFQ)61 recorded over three consecutive days, and the calories were analyzed by a certified dietitian at pretest.
2.7. The Stroop task
The Stroop task9,16 was designed by E-prime (Psychology Software Tools, Sharpsburg, PA, USA). It involves a choice between colors and words arising from an interference dilemma in the stimulus-encoding stage or during response production. In addition, evidence has been found suggesting that semantic meaning significantly interferes with color naming, whereas color does not interfere with the reading of words, which has been attributed to highly automated processes that occur in heavily-trained adults.62 The stimuli were two color names in Chinese (“紅” (red) and ”綠” (green)). The participants were invited to sit comfortably and quietly in a dim room at an 80 cm distance from a computer screen. After 5 min, the participants received an explanation and responded to a 4.5 × 4.5 cm stimulus presented in the center of a 21-in. screen. Before the formal test, 10 practice trials were performed to ensure that the participants understood the instructions. The participants were instucted to use the index finger ("N" key) and middle finger ("M" key) of their right hand to press buttons on the computer to respond to the color of the word, while ignoring the word, and were asked respond as quickly and accurately as possible to only the meaning. A fixation period of 300 ms was presented across the interstimulus intervals. The timing of the stimuli, responses within 1500 ms, and the intertrial intervals in the Stroop task totaled 2000 ms. In the congruent condition, the word meaning and its color matched, whereas in the incongruent codition, the word meaning did not match its color. The test comprised a total of 240 trials (two sets), where the congruent and incongruent conditions were randomly represented 50% of the time, with a 2 min break between the two sets. Each stimulus appeared 1.5–2 s after the response. EEG data for the participants was recorded while they performed the Stroop task.
2.8. ERP recording and analysis
The participants’ electroencephalogram (EEG) information was recorded with the eego™ amplifier system (ANT Neuro, EE-211, revision Nr 1.2, Germany) using an elastic cap with 64 scalp sites (10–10 system) with Ag/AgCl electrodes (ANT Neuro Company, https://www.ant-neuro.com/products/eego_sports). CPz was used as an online and offline reference electrode, and the ground electrode was placed on AFz. All inter-electrode impedances were kept below 5 KΩ. Adhesive electrodes were placed below the left eye and connected to the system reference for vertical EOG (VEOG) eye movement activity. The raw EEG signal was acquired at an A/D rate of 512 Hz/channel. During the offline analysis, a 4th order Butterworth band-pass filter of 0.1–50 Hz and a 60-Hz notch filter were applied to the raw EEG data. We used a region-of-interest approach wherein the mean P2, N2 and P3 amplitudes were averaged across four frontocentral electrode sites63 chosen a priori. An offline electrooculographic correction performed using ASA software version 4.10.1 (ANT Neuro, Netherlands) was applied to the individual trials prior to averaging the ERP components. An offline eyeblink correction was performed based on a Partial Component Analysis (PCA) method, which models brain signals and artifact subspaces.64 Baseline offset was sampled from 100 ms data right before the stimulus presentation. Target-locked epochs used for averaging were created from −200 ms (prestimulus baseline) to 1000 ms around the stimuli. Following eyeblink correction and baseline correction, all trials with response errors and all trials with amplitudes below −100 μV or above 100 μV artifacts were excluded from further analysis, where the maximum rejected number of trials was 29 out of 200. All trials with response errors and the Stroop target RT < 200 ms or >1500 ms were deleted. The remaining effective ERP data were separately averaged offline and constructed from congruent and incongruent conditions in the Stroop task over a 1000 ms epoch beginning 200 ms before the onset of the target stimulus. The mean amplitudes and latencies of the P2, N2, and P3 components were measured at the Fz and Cz electrodes.65, 66, 67 The time windows for detection of the P2, N2, and P3 components were 170–270 ms,25,28,68 200–400 ms,69 and 300–600 ms,28,70 respectively. Latency was calculated as the time in milliseconds from the stimulus onset to the peak amplitude.
2.9. Data processing and statistical analyses
An independent t–test was used to examine the demographic backgrounds of the participants in both groups. For the behavioral [i.e., reaction time (RTs], and accuracy rate (AR)] analyses, all independent variables were analyzed using a 2 x 2 x 2 repeated measures analysis of variance (RM-ANOVA) [i.e., Group (EG vs. CG) × Time (pre-vs. post-test) × Condition (congruent vs. incongruent)]. For the electrophysiological (i.e., ERP P2, N2 and P3 latencies and amplitudes) analyses, all independent variables were analyzed using a 2 x 2 x 2 x 2 RM-ANOVA [i.e., Group (EG vs. CG) × Time (pre-vs. post-test) × Condition (congruent vs. incongruent) × Electrode (Fz and Cz)]. Body composition, cardiorespiratory fitness, and muscular strength were submitted separately to a 2 × 2 RM-ANOVA [i.e. (Group: EG vs. CG) x (Time: pre-test vs. post-test)]. Appropriate multiple comparisons were performed following any simple main effects. When a significant difference occurred, Bonferroni post hoc analyses were performed. Partial Eta squared (ηp2) was used to calculate effect sizes for significant main effects and interactions, with the following criteria used to determine the magnitude of the mean effect size: 0.01–0.079 (small effect size), which ranged between 0.08 and 0.14 (medium effect size), and >0.14 (large effect size). Pearson product-moment correlations and Spearman's rho correlations were used to examine changes in the fitness/parameters and neurocognitive variables. A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Demographic data
The basic characteristics of all participants are shown in Table 1. There were no statistically significant between-group differences in the demographic variables (ps > 0.05).
3.2. Behavioral performance in the stroop task
The accuracy rates (ARs) and reaction times (RTs) at pretest and posttest are shown in Fig. 4.
Fig. 4.
The mean ± SD of (a) ARs and (b) RTs of the EG and CG groups at pre- and post-test.
3.2.1. Accuracy rate (AR)
The RM-ANOVA for the ARs revealed significant main effects of Condition [F(1,30) = 47.54, p < 0.001, ηp2 = 0.62] and Group [F(1,30) = 9.87, p = 0.004, ηp2 = 0.25], with the ARs in the congruent condition (97.90 ± 2.49% [CVs = 2.54]) being significantly greater than those in the incongruent condition (95.13 ± 3.86% [CVs = 4.06], 95% CI: 0.44 [0.02, 0.04]) across both groups and the two time points, and with the ARs in the EG group (97.97 ± 1.19% [CVs = 1.21]) being significantly greater than those in the CG group (94.97 ± 3.62% [CVs = 3.81], 95% CI: 0.01, 0.06) across the two time points and under both conditions. No significant interactions of Time, Group, and Condition were found (ps > 0.05).
3.2.2. Reaction time (RT)
The RM-ANOVA for the RT revealed significant main effects of Condition [F(1,30) = 47.42, p < 0.001, ηp2 = 0.61], with the RT under the congruent condition (521.50 ± 49.79 ms [CVs = 9.55]) being significantly faster than that under the incongruent condition (568.44 ± 71.13 ms [CVs = 12.51], 95% CI: −76.53, −41.52) across both groups and the two time points. No significant interactions of Time, Group, and Condition were found (ps > 0.05).
3.3. Electrophysiological performance
Fig. 5 shows the grand-average ERP waveforms for the two midline electrodes pre- and post-test in the EG and CG groups when performing the Stroop task.
Fig. 5.
Grand averaged ERPs of P2, N2, and P3 waveforms under the congruent and incongruent conditions for two electrodes (Fz and Cz) for the exercise group (EG) and control group (CG) at pre- and post-test when performing the Stroop task.
3.3.1. P2 component
The RM-ANOVA for the P2 latencies revealed significant main effects of Electrode [F(1,30) = 1832.91, p = 0.005, ηp2 = 0.24], with the P2 latencies in the Fz site (233.25 ± 11.09 ms [CVs = 4.75]) being significantly slower than those in the Cz site (227.90 ± 9.450 ms [CVs = 4.15]) across both groups, conditions, and the two time points. No significant main effects or interactions of Time, Group, and Condition were found (ps > 0.05).
The RM-ANOVA for the P2 amplitudes revealed significant main effects of Electrode [F(1,30) = 18.72, p < 0.001, ηp2 = 0.38] and Group [F(1,30) = 13.50, p = 0.001, ηp2 = 0.31], with the P2 amplitudes in the Fz site (2.87 ± 1.26 μV [CVs = 43.90]) being significantly smaller than those in the Cz site (3.77 ± 1.42 μV [CVs = 37.67]) across both groups, conditions, and the two time points, and with the P2 amplitudes in the EG group (3.98 ± 1.09 μV [CVs = 27.39]) being significantly greater than those in the CG group (2.66 ± 0.94 μV [CVs = 35.34], 95% CI: 0.47, 1.55) across the two time points, under both conditions, and the two electrodes. The interactions of Group × Time [F(1,30) = 5.53, p = 0.025, ηp2 = 0.16] and Electrode × Group × Time [F(1,30) = 7.11, p = 0.012, ηp2 = 0.19] were also significant. The post hoc analysis showed that, when compared to the pre-test, the P2 amplitudes were significantly greater in the EG group at the Fz (pre vs. post: 3.79 ± 1.14 μV [CVs = 30.08] vs. 4.16 ± 1.06 μV [CVs = 25.48], p < 0.001) and Cz (pre vs. post: 4.04 ± 1.42 μV [CVs = 35.15] vs. 4.59 ± 1.41 μV [CVs = 30.72], p = 0.003) electrodes post-test across both conditions, while the P2 amplitudes at the Cz electrode decreased post-test in the CG group [t(15) = 2.10, p = 0.053] across both conditions (pre vs. post: 3.64 ± 1.76 μV [CVs = 48.35] vs. 2.82 ± 1.16 μV [CVs = 41.13], p = 0.053). No significant main effect of Condition [F(1,30) = 0.37, p = 0.548] was found.
3.3.2. N2 component
The RM-ANOVA for the N2 latencies revealed significant main effects of Electrode [F(1,30) = 123.24, p < 0.001, ηp2 = 0.80], Condition [F(1,30) = 35.02, p < 0.001, ηp2 = 0.54], and Group [F(1,30) = 4.90, p = 0.035, ηp2 = 0.14], with the N2 latencies in the Fz site (351.62 ± 17.48 ms [CVs = 4.97]) being significantly slower than those in the Cz site (312.30 ± 12.40 ms [CV = 3.97], p < 0.001) across both groups, conditions, and the two time points, with the N2 latencies under the congruent condition (326.20 ± 11.17 ms [CV = 3.42]) being significantly faster than those under the incongruent condition (337.72 ± 14.28 ms [CV = 4.23], p < 0.001) across both groups, electrodes, and the two time points, and with the N2 latencies in the EG group (336.16 ± 8.31 ms [CV = 2.47]) being significantly slower than those in the CG group (327.66 ± 0.94 μV [CV = 0.28], p = 0.001) across the two time points, both conditions, and the two electrodes. The interactions of Time × Condition [F(1,30) = 4.63, p = 0.040, ηp2 = 0.13], Group × Condition [F(1,30) = 5.43, p = 0.027, ηp2 = 0.15], Group × Time [F(1,30) = 17.25, p <0.001, ηp2 = 0.37], Electrode × Time [F(1,30) = 9.57, p = 0.004, ηp2 = 0.24], Group × Time × Condition [F(1,30) = 11.36, p = 0.002, ηp2 = 0.28], Electrode × Time × Condition [F(1,30) = 7.10, p = 0.012, ηp2 = 0.19], and Electrode × Group × Condition [F(1,30) = 8.34, p = 0.007, ηp2 = 0.22] were also significant. The post hoc analysis for the Group × Time × Condition interaction showed that under the incongruent condition, the N2 latencies in the EG group were significantly faster across both electrodes after 12 weeks (pre vs. post: 354.44 ± 15.55 ms [CV = 3.42] vs. 333.94 ± 13.50 ms [CV = 4.04], p < 0.001), while the N2 latencies were significantly slower in the CG group [t(15) = −2.76, p = 0.015] across both electrodes after 12 weeks (pre vs. post: 323.38 ± 19.74 ms [CV = 6.10] vs. 339.13 ± 16.70 ms [CV = 4.92], p = 0.015). However, the results showed no significant between-group differences 12 weeks after the intervention.
The RM-ANOVA for the N2 amplitude showed no significant main effects or interactions of Group, Time, and Condition (ps > 0.05).
3.3.3. P3 component
The RM-ANOVA for the P3 latency revealed significant main effects of Time [F(1,30) = 6.73, p = 0.015, ηp2 = 0.18] and Electrode [F(1,30) = 101.91, p < 0.001, ηp2 = 0.77], with the P3 latency being significantly faster in the post-test (456.72 ± 16.58 ms [CV = 3.63]) than in the pre-test (465.70 ± 21.79 ms [CV = 4.68]) across both conditions, both groups, and the two electrodes, and with the P3 latency being significantly shorter at the Cz site (442.94 ± 16.13 ms [CV = 3.64]) as compared to at the Fz site (479.48 ± 21.12 ms [CV = 4.40]) across the two time points, both groups, and the two conditions. The interactions of Group × Time [F(1,30) = 11.21, p = 0.002, ηp2 = 0.27] were also significant. The post hoc analysis revealed that the P3 latency in the EG group was significantly faster across both conditions and both electrodes after 12 weeks (pre vs. post: 466.34 ± 12.03 ms [CV = 2.58] vs. 445.78 ± 9.94 ms [CV = 2.23]).
The RM-ANOVA for the P3 amplitude revealed significant main effects of Group [F(1,30) = 4.44, p = 0.043, ηp2 = 0.13] and Electrode [F(1,30) = 90.27, p < 0.001, ηp2 = 0.75], with the P3 amplitude in the EG group (2.26 ± 0.80 μV [CV = 35.40]) being significantly greater than that in the CG group (1.53 ± 1.13 μV [CV = 73.86]), and with the P3 amplitude in the Cz electrode (2.75 ± 1.45 μV [CV = 52.73]) being significantly greater than that in the Fz electrode (1.05 ± 0.74 μV [CV = 70.48], p < 0.001) across both groups, two time points, and the two conditions. The interaction of Group × Time [F(1,30) = 11.80, p = 0.002, ηp2 = 0.28] was also significant. The post hoc analysis revealed that the P3 amplitude in the EG group was significantly greater across both conditions and both electrodes after the intervention (pre-test vs. post-test: 1.73 ± 0.71 [CV = 41.04] vs. 2.79 ± 1.10 μV [CV = 39.43], p < 0.001).
Since the Stroop task conditions and the ERP electrodes potentially were not the factors influencing the pre-post outcomes, a one-way ANCOVA with pre-test values used as the covariates to adjust for the baseline effect was implemented for the P3 amplitude.71,72 As shown in Table 2, the mean between-group difference in the change from the P3 amplitude baseline at 12 weeks for the EG and CG groups was 1.48 and reached statistical significance (p < 0.001) after adjusting for the effect of the baseline.
Table 2.
The P3 amplitude of the participants at pre- and post-test.
| P3 amplitude |
Difference between means (95% CI) | p value | ||
|---|---|---|---|---|
| EG (n = 16) | CG (n = 16) | |||
| Pre-test | 2.59 ± 0.87 | 2.06 ± 1.15 | 0.52 (−0.21, 1.26) | 0.156 |
| Analysis | ||||
| Post-test | 3.49 ± 1.01 | 1.68 ± 1.09 | 1.82 (1.05, 2.58) | <0.001 |
| Change scorea | 0.91 ± 0.63 | −0.39 ± 1.12 | 1.29 (0.64, 1.95) | <0.001 |
| ANCOVA | 1.48 (0.85, 2.11) | <0.001 | ||
| (partial η2 = 0.87) | ||||
Change score = Post-test - Pre-test.
3.4. Body composition, cardiorespiratory fitness, and muscular strength assessment
The circumferences of the waist, abdomen, and hip, the whole and regional body compositions (e.g., total body fat%, arm fat%, trunk fat%, and leg fat%), and the estimated V̇O2max and lower-extremity muscular fitness at pre-test and post-test are shown in Table 3. Only the exercise groups showed a decreased fat% on the total body, upper limb, trunk, and lower limb regions with no change in body weight, and cardiorespiratory fitness and lower-extremity muscular fitness was significantly improved.
Table 3.
The body composition, cardiorespiratory fitness, and muscular strength of the participants at pre- and post-test.
| Time point | Groups |
|||||
|---|---|---|---|---|---|---|
| Exercise group |
Mean difference within group (95% CI) | Control group |
Mean difference within group (95% CI) | |||
| Pre-test | Post-test | Pre-test | Post-test | |||
| Body weight (kg) | 75.74 ± 11.11 | 74.79 ± 11.03 | 0.106 (−0.23, 2.14) | 74.94 ± 13.58 | 75.08 ± 14.04 | 0.748 (−1.03, 0.76) |
| Total body fat percentage (%) | 41.01 ± 3.09 | 37.78 ± 2.60 | <0.001 (2.23, 4.22)a | 43.24 ± 4.47 | 43.23 ± 4.35 | 0.973 (−0.38, 0.40) |
| Upper limbs fat percentage (%) | 48.25 ± 3.59 | 45.34 ± 4.55 | <0.001 (1.76, 4.05)a | 45.94 ± 3.87 | 46.14 ± 3.38 | 0.582 (−0.97, 0.57) |
| Trunk fat percentage (%) | 41.53 ± 3.75 | 37.59 ± 3.32 | <0.001 (2.36, 5.52)a | 44.67 ± 5.39 | 44.73 ± 5.20 | 0.880 (−0.90, 0.78) |
| Lower limbs fat percentage (%) | 41.55 ± 4.45 | 38.59 ± 3.59 | <0.001 (1.90, 4.04)a | 42.52 ± 4.11 | 42.50 ± 4.22 | 0.950 (−0.60, 0.64) |
| Percentage lean mass (%) | 56.08 ± 2.96 | 59.22 ± 2.44 | <0.001 (−4.14, −2.14)a | 55.02 ± 4.14 | 55.04 ± 4.05 | 0.868 (−0.39, 0.33) |
| Total lean body mass (kg) | 42.05 ± 5.44 | 43.24 ± 5.64 | 0.014 (−0.21, −0.28)a | 40.91 ± 5.71 | 41.17 ± 5.94 | 0.321 (−0.80, 0.28) |
| Total fat mass (kg) | 31.08 ± 6.46 | 27.80 ± 5.47 | <0.001 (2.15, 4.41)a | 31.80 ± 8.75 | 31.94 ± 8.65 | 0.484 (−0.57, 0.28) |
| Estimated V̇O2max (ml/kg/min) | 23.66 ± 3.03 | 28.59 ± 4.92a | <0.001 (−7.31, −2.56)a | 23.83 ± 6.37 | 23.30 ± 6.99 | 0.504 (−1.11, 2.16) |
| Leg extension 1 repetition maximum (kg) | 32.14 ± 7.96 | 36.88 ± 8.04 | <0.001 (−6.89, −2.59)a | 35.73 ± 8.95 | 32.13 ± 8.58 | 0.050 (0.01, 7.19) |
| Chair stand (time) | 22.13 ± 4.35 | 27.63 ± 5.03 | <0.001 (−7.29, −3.71)a | 16.06 ± 4.78 | 17.25 ± 3.45 | 0.299 (−3.54, 1.17) |
| 60-s sit-up (time) | 22.00 ± 5.61 | 26.50 ± 6.31 | 0.002 (−7.01, −1.99)a | 17.94 ± 7.72 | 18.88 ± 7.67 | 0.472 (−3.64, 1.77) |
Significant within-group difference. Values are means ± SD. CI: Confidence intervals.
3.4.1. Cardiorespiratory fitness
The RM-ANOVA for the estimated V̇O2max revealed significant main effects of Time [F(1,30) = 10.62, p = 0.003, ηp2 = 0.26], with the V̇O2max in the pre-test (23.74 ± 4.90 ml/kg/min) being significantly smaller than in the post-test (25.95 ± 6.52 ml/kg/min, p = 0.012) across both groups. The interaction of Group × Time [F(1,30) = 16.29, p < 0.001, ηp2 = 0.35] was significant. The post-hoc test revealed that the V̇O2max in EG was significantly improved after the 12-week exercise intervention (pre vs. post: 23.66 ± 3.03 vs. 28.59 ± 4.92 ml/kg/min, p < 0.001), while the V̇O2max in CG showed no significant change during the 12-week experimental period (pre vs. post: 23.83 ± 6.37 vs. 23.30 ± 6.99 ml/kg/min, p = 0.504).
3.4.2. Muscular strength assessment
The RM-ANOVA for the 1RM of knee extension revealed a significant interaction of Group × Time [F(1,30) = 18.73, p <0.001, ηp2 = 0.39]. The post-hoc test revealed that the 1RM knee extension in EG was significantly improved after the 12-week exercise intervention (pre vs. post: 32.14 ± 7.96 vs. 236.88 ± 8.04 kg, p < 0.001), while the V̇O2max in CG was significantly decreased during the 12-week experimental period (pre vs. post: 35.73 ± 8.95 kg vs. 32.13 ± 8.58, p = 0.050).
The RM-ANOVA for the chair stand revealed significant main effects of Time [F(1,30) = 23.21, p < 0.001, ηp2 = 0.44], and Group [F(1,30) = 33.98, p < 0.001, ηp2 = 0.53], with the chair stand values being significantly smaller in the pre-test (19.09 ± 5.45 times) than in the post-test (22.44 ± 6.77 times, p < 0.001) across both groups, with the chair stand values in the EG group (24.88 ± 4.39 times) being significantly greater than in the CG group (16.66 ± 3.54 times, p < 0.001) across the two time points. The interaction of Group × Time [F(1,30) = 9.65, p = 0.004, ηp2 = 0.24] was significant. The post-hoc test revealed that the chair stand in EG was significantly improved after the 12-week exercise intervention (pre vs. post: 22.13 ± 4.35 vs. 27.63 ± 5.03 times, p < 0.001), while the V̇O2max in CG was significantly decreased during the 12-week experimental period (pre vs. post: 16.07 ± 4.78 vs. 17.25 ± 3.45 times, p = 0.299).
The RM-ANOVA for the sit-up 60 s revealed significant main effects of Time [F(1,30) = 9.87, p = 0.004, ηp2 = 0.25], and Group [F(1,30) = 6.59, p = 0.015, ηp2 = 0.18], with the 60-s sit-up values being significantly fewer in the pre-test (19.97 ± 6.95 times) than in the post-test (22.69 ± 7.92 times, p = 0.004) across both groups, with the 60-s sit-up exercise in the EG group (24.25 ± 5.49 times) being significantly shorter than in the CG group (18.41 ± 7.26 times, p = 0.015) across the two time points. The interaction of Group × Time [F(1,30) = 4.24, p = 0.048, ηp2 = 0.12] was significant. The post-hoc test revealed that the 60-s sit-up exercise in EG was significantly improved after the 12-week exercise intervention (pre vs. post: 22.00 ± 5.61 vs. 26.50 ± 6.31 times, p < 0.001), while the 60-s sit-up exercise in CG did not change during the 12-week experimental period (pre vs. post: 17.94 ± 7.72 vs. 18.88 ± 7.67 times, p = 0.472).
3.5. Correlation analysis
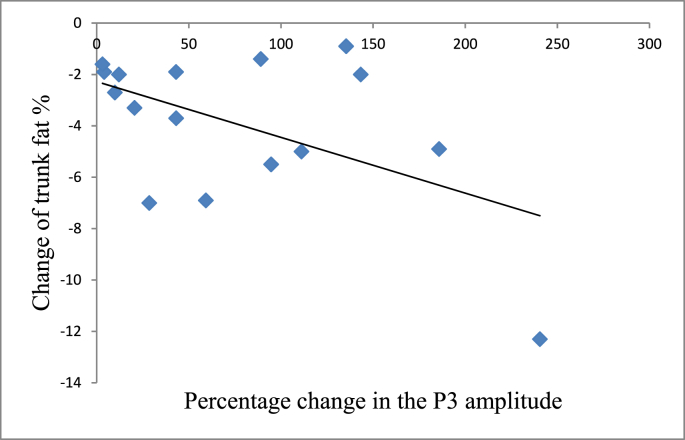
The correlations between all of the changes in the body composition variables (e.g., fat percentage in the upper and lower body) and physical fitness (e.g., cardiorespiratory and muscular fitness) did not achieve significance. Only the change prior to and after the intervention between the P3 amplitude and trunk fat percentage was significantly and negatively correlated in the EG group (r = −0.521, p = 0.039) based on the Person's correlation (Fig. 6). However, after deleting one outlier, no significant correlation was found when using a Spearman's rho.
Fig. 6.
Correlations between the changes in ERP P3 amplitudes and trunk fat percentage in the EG group.
4. Discussion
The purpose of the current study was to investigate the effects of a 12-week moderate-intensity aerobic exercise program combined with resistance exercise on neurocognitive inhibitory control among obese women and to examine the correlations between the changes in body fat percentage/physical fitness and neurocognitive indicators. Our findings highlight the fact that, although the behavioral performance was not improved in the obese women through a 12-week intervention period of aerobic exercise combined with resistance exercise, the exercise modality resulted in significantly positive effects on neurophysiological indices (e.g., shorter N2 and P3 latencies and greater P2 and P3 amplitudes) during the Stroop task, as well as on the body composition parameters (whole body fat% and regional fat %), cardiorespiratory fitness (estimated V̇O2max), and muscular fitness. In addition, changes in the trunk fat percentage could be a body composition mediator in the beneficial effects on neurophysiological (i.e., ERP P3 amplitude) performance after a chronic exercise intervention. However, correlations between changes in whole/other regional body fat% or physical fitness and changes in other neurocognitive performance indices did not reach significant levels in the EG group in the present study.
Combined aerobic and resistance training has been recommended to be the most efficacious exercise modality to reduce anthropometric outcomes and should be recommended in the prevention and treatment of overweight and obesity.73 However, in the present study, a 12-week intervention comprising a combination of aerobic and resistance exercise did not generate significant improvements in the ARs and RTs in obese women when performing the Stroop task, suggesting that this type of chronic exercise intervention cannot promote specific behavioral effects on response inhibition/interference. These findings are compatible with previous findings of a significant behavioral improvement in a switching condition but not in an inhibition condition (Stroop task) after 3–5.7 months of aerobic/contemporary dance training.39,74 Indeed, Wen & Tsai (2020a) also found that a single bout of combined aerobic and resistance exercise did not produce significant behavioral improvements (e.g., ARs and RTs) in the Stroop test performance in the obese women under consideration.16 The possible explanation for the null effect in the behavioral indices is that conflict processing improves from childhood to adulthood and declines from adulthood to old age.75 Therefore, although significant behavioral improvements in the Stroop task after an 8 to 12-week exercise intervention in obese adolescents has been observed,76,77 it may be that conflict management occurs during developmental stages. However, the participants in the present study were middle-aged and elderly individuals in which the conflict inhibition function is gradually degrading, and obesity also leads to obstacles in cognitive functions involving cognitive interference inhibition.9 Another possibility explaining the lack of improvement in accuracy might be a ceiling effect since the participants in both groups in this study responded correctly to over 90% of all trials at pre-test, indicating a small reserve for exercise-induced facilitation of accuracy. Since a significant behavioral improvement in the Stroop interference task was observed after 12 months of resistance training in community-dwelling women,78 further research is warranted in this area, possibly examining the possible neuropsychological benefits via aerobic activities combined with resistance exercise for longer durations in obese women. What's more, in the future, significant improvements in behavioral performance could be obtained by adjusting the design of the Stroop task protocols, such as increasing the difficulty40 or using different stimuli (e.g., a food-related Stroop task)28 to further explore the interventional effects of long-term aerobic combined with resistance exercise in obese individuals.
In spite of the null effect in behavioral performance in the present study, the chronic exercise intervention facilitated the cognitive electrophysiological indices (e.g., shortening the ERP N2 and P3 latencies and increasing the ERP P2 and P3 amplitudes) in the obese women under consideration when performing the Stroop task. Since when cognitive control involves a linguistic component, such as the Stroop task, interference occurs after the stimulus-encoding stage,24,79 the present findings regarding the exercise-intervention-related modulation of brain electrocortical activity at baseline suggest that 12 weeks of a combined aerobic and resistance exercise program is still an effective strategy by which to remedy deficits in cognitive interference inhibition related to obesity. A larger P2 amplitude after the exercise intervention was observed in this study. As previously mentioned, feedback P2 is not only sensitive to reward signals,25 but also is thought to reflect processing associated with attention allocation and salience detection.25 The modulation of the P2 amplitude after the intervention likely reflects an increase in attention-related cerebral information processing during relatively early, perceptual stages of information-processing, indicating that more automatic, exogenous brain cortical activity80 could be facilitated through a chronic aerobic-and-resistance exercise intervention in obese women.
Previous studies have demonstrated that obese women relative to normal-weight controls exhibit a significantly longer Stroop N2 latency,9 reflecting a dysfunction within the anterior cingulate or prefrontal cortex.81,82 Importantly, the N2 latencies were significantly shortened under the incongruent condition after the 12-week exercise intervention in the obese women in the present study. When individuals perform the Stroop task, the control-related N2 is thought to mirror cognitive control processes involved in conflict detection and monitoring.83 Additionally, the anterior N2 in cognitive control is linked to stimulus distinction and identification cognitive processes and is typically evoked before motor responses.84 The shortened N2 latency under the incongruent condition after the intervention in the present study suggests that a 12-week moderate-intensity aerobic exercise program combined with a resistance exercise intervention can effectively attenuate the deficits in early modality-specific inhibition as those are the only trials that require inhibition of non-relevant information (e.g., conflict monitoring processes and response inhibition)85 in obese women because incongruence is the most important situation for measuring inhibition and control, and N2 represents the ability of inhibiting conflict detection. Therefore, this result shows that exercise intervention can indeed improve neural processing related to inhibiting conflict detection in obese women. This result aligns with another study that reported moderate-intensity dance exercise and brisk walking three times per week for 3 months could shorten N2 latency in healthy elderly females leading a sedentary lifestyle when performing the Flanker task.86 In contrast, there was no change in the N2 latency following 10 weeks of soccer training in children with a developmental coordination disorder when performing a visuospatial attention orienting task.13 These conflicting results might be because of variations in the exercise modes87 and the different ages in the population studied while performing various cognitive tasks. Accordingly, future research efforts should continue to address this uncertainty regarding chronic-exercise-induced effects on the ERP N2 component because of its potential as a cognitive electrophysiological biomarker that can be used as an index of the effects of chronic exercise on conflict-related processing.88
The ERP P3 component reflects late general inhibition.13,85 Compatible with an earlier study investigating the effects of a series of 50-min exercise sessions conducted five times a week for 10 weeks on inhibitory control in children with developmental coordination disorder when performing a visuospatial attention task that reported beneficial effects in the strength of ERP P3 latency,13 in the present study, the P3 components (amplitude and latency) significantly improved after 12 weeks of moderate-intensity aerobic exercise combined with a resistance exercise intervention in these obese women. Similarly, a shorter P3 latency was observed following a structured exercise program.89 In addition, since previous studies have found that obese women relative to normal weight individuals exhibit a smaller P3 amplitude in the Stroop task,9 and the long-term exercise intervention in the present study increased the P3 amplitude, this suggests that neurological deficits related to late general inhibition were also improved, like the early modality specific inhibition (e.g., the ERP N2 component), as mentioned above.85 This finding is partially in line with previous research.76,89 Ludyga et al. investigated the effects of an exercise intervention during a school break period in adolescents aged 12–15 years. It was found that 8 weeks of aerobic and coordinative exercise performed 20 min per school day increased the P3 amplitude, which was most pronounced in the parieto-occipital region when performing the Stroop task.76 It is worth noting that the amplitude of the ERP P3 component has also been suggested to be proportional to the resources allocated towards the suppression of superfluous neuronal activity.90 The increase in the P3 amplitude demonstrated in the present study highlights how the effects of chronic exercise influence the brain, which is considered to reflect a facilitation of inhibitory control processes and decreases in difficulty related to processing stimuli due to improved inhibition of extraneous neuronal activity. In previous studies, such benefits in cognitive control have only been observed after an acute exercise aerobic exercise program in school-aged children, young adults,91,92 obese women,16 and older adults.92 The present results extend the current state of knowledge on neurophysiological processes and suggest that a chronic exercise intervention affects cognitive function more broadly than has been thought, but not specifically inhibitory control in obese women.
An elevated risk for cardiovascular diseases (CVDs) and mortality is associated with high amounts of abdominal, and especially visceral fat, accumulation. Visceral adipose tissue mass and volume increases with age throughout the lifespan.36 In particular, the body fat percentage begins to increase from the age of 30 to <40 years in women.36 In the present study, the body weight of the participants was not significantly reduced, but the estimated V̇O2max and muscular strength were significantly improved after 12 weeks of aerobic training combined with a resistance exercise intervention in the obese women under consideration. In addition, this exercise program significantly decreased not only upper-and-lower-extremity adiposity but also the central adiposity in these obese women. Since central adiposity has been proven to be associated with non-communicable diseases (e.g., diabetes mellitus, CVDs, chronic obstructive pulmonary diseases, cancers, etc.),93 the present finding implies that a chronic exercise modality could effectively decrease the risk of non-communicable diseases.
One of the primary functions of adipose tissue is the storage of triglycerides during positive calorie balance and the release of free fatty acids in periods of energy demand. Most studies have proven the long-term positive effects of exercise on body composition and cardio-metabolic risk factors.94,95 For example, Park et al. reported that 12 weeks of combined exercises, comprising elastic-band resistance training and walking/running on a treadmill, and riding a bicycle at 60–70% of the maximum HR, significantly lowered body fat.94 However, in contrast, Zhang et al. investigated the effects of 12-week exercise interventions consisting of either all-out sprint interval training, supramaximal sprint interval training, high-intensity interval training, or moderate-intensity continuous training (MICT). The exercise training-induced reductions in the abdominal visceral fat area resulting from the three interval training interventions were greater in comparison with MICT in obese young women.96 These previous and present findings suggest that the exercise modality and degree of intensity may affect exercise-induced visceral fat loss in obese women. In addition, total exercise time is also an important factor. Based on the ACSM, at least 150 min of moderate-intensity aerobic exercise combined with resistance exercise per week for overweight and obese adults is recommended to obtain a modest weight loss (∼2–3 kg) intended to improve overall health.97 The exercise prescription designed in this study significantly reduced the total body fat mass (3.28 kg)/trunk body fat percentage (3.94%) and increased the total lean body mass (1.19 kg) in the EG group after 12 weeks of aerobic combined with resistance training. The beneficial effects on these physiological indices were much higher than those obtained with Zhang and colleague's obesity-and-exercise experiment during 12 weeks of high-intensity interval aerobic exercise (30 min/session) and moderate-intensity continuous aerobic training (60 min/session), 3 days per week,98 thus supporting the premise that aerobic combined with resistance training and a higher volume of exercise appears to be a more efficient strategy for improving total lean body mass and decreasing total/trunk body fat in obese individuals.99
Previous studies have indicated that obesity may be associated with slower basic information processing and fitness.100 Since improvements in physical fitness are related to increases in cognitive processing speed related to complex attention, verbal memory, and visual memory in obese individuals,101 it is worth noting that, although improvements in the fitness parameters (e.g., cardiorespiratory fitness and muscular endurance) in the EG group after a 12-week moderate intensity aerobic combined with a resistance exercise intervention were observed which is in line with previous studies,102 no significant correlations with the changes in the neurocognitive parameters being considered were found in the present study. A previous cross-sectional study mentioned that older adults with high visceral fat regions had significantly lower cognitive functions than those with low visceral fat regions.103 However, importantly, the findings of the current study revealed a significant negative correlation between changes in the percentage of trunk fat and the P3 amplitude during the Stroop task in the exercise group, suggesting that a decreased percentage of trunk fat, but not leg and arm fat appear to enhance inhibition processing or motivation-related attentional engagement, and thus enabled these women to better distinguish the cognitive interference inhibition stimulus in the Stroop task. Also, the percentage of trunk fat appears to be associated with a particular aspect of inhibition control attention; specifically, the percentage of trunk fat may mediate the neural network involved in the top-down allocation of attentional resources. Indeed, greater improvements in the VAT/trunk fat percentage lead to greater enhancement of inhibition processing and motivation-related attentional engagement (e.g., P3 amplitude).7,27,28 Therefore, decreases in the percentage of trunk fat may serve as a potential mediator of the beneficial effects on neurophysiological (i.e., ERP P3 amplitude) performance after a chronic exercise intervention in obese individuals. However, no significant results were found after deleting the outlier, so this result should be interpreted with caution.104 In terms of the only significant correlation when so many correlations were being examined, it is quite possible that this significant finding could be the result of a type I error.105
There are some limitations of the present study, which must be considered. First, sex differences could be a potential modulator of chronic exercise-induced benefits on cognitive control and the biological drive to regain weight and increase food intake.106 To control for sex, only healthy obese women were recruited in the present study. Thus, the results may not be generalizable to obese men. Second, a confounding factor (e.g. the social context of the training setting, peak oxygen consumption) that may have contributed to the observed effects could be in the present study, which may have enhanced some cognitive functions.87 However, it is still worth noting that a previous study reported that the influence of social context on executive functions by including a social control group did not reveal any differences between no-contact and social control groups.107 Also, a previous study mentioned that a 6-month period of aerobic exercise combined with calorie restrictions reduced body weight and improved peak oxygen consumption, but the cognitive and brain activity remained unchanged in overweight and obese women.108 Thirdly, a normal-weight control group is warranted in the future to further interpret and generalize the present findings. Fourth, an artifact of any behavioral trial is the inability to implement a double-blind placebo-controlled design, due to the fact that participants know if they are exercising or not. Therefore, an adequate control condition that matches as many aspects of the treatment arm as possible is best to use to improve the rigor of such studies. Fourthly, it is worth noting that although almost all of the participants had a high attendance rate (over 85%), low attendance rates (about 60%) were observed in three participants in the EG group. After checking the cardiorespiratory fitness, muscle strength, and body fat pre- and post-intervention, the three indices were obviously improved in the three participants as compared to the rest of the EG group, indicating that they still achieved the effects of a 12-week exercise intervention intended to improve physical fitness and weight status. Therefore, they were still included in the analysis of the study outcomes, which could have potentially biased the effect measurement. Fifth, the original sample size calculation may have been overestimated based on a previous study.20 However, the high post-hoc power, 0.99, for the change of P3 amplitude pre- and post-intervention based on the observed effect (partial η2 = 0.87) suggested that the current sample size ensures a significant effect of experimental intervention. Finally, the participants were instructed to maintain their regular lifestyle by self-monitoring, including dietary and physical activity during the experimental period, which may be another limitation of this study. Collectively, an avenue for future research would be to examine the potential effects of chronic exercise intervention, social interaction, sex, calorie restrictions, and neurocognition interactions.
5. Conclusions
In the present study, the 12-week aerobic exercise program combined with a resistance exercise intervention was effective in terms of not only improving the fat percentage distribution (e.g., total body fat %, trunk fat %, and leg fat %) and the physical fitness levels of the obese subjects (e.g., estimated V̇O2max and muscular fitness) but also affecting cognitive function broadly rather than specifically affecting inhibition control in these participants although the exercise modality did not enhance the behavioral indices. In addition, since the changes prior to and after the intervention between the ERP P3 amplitude and the percentage of trunk fat revealed a significantly negative correlation in the exercise group, this suggested that the percentage of trunk fat could serve as a mediator between physical exercise and improvements in neural inhibitory processes.
Author statement
Huei-Jhen Wen: Conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing—original draft preparation, review and editing, visualization, supervision, project administration, and funding acquisition. Chia-Liang Tsai: Conceptualization, writing—review and editing. Shu-Hsin Liu: Methodology and software. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Technology [MOST 107-2410-H-320-005]; and the Buddhist Tzu Chi Medical Foundation, Taiwan [TCRD110-64].
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgments
We wish to express our appreciation for the cooperation of all participants and the facility support provided by Tzu Chi University. We also wish to acknowledge graduate student Pei-Qing Lee, Ke-Hsin Chen, and research assistant Wan-Tien Ji for assisting with the data collection and the technicians, Mr. Lu-Tan Wang and the Department of Nuclear Medicine at Tzu Chi Hospital, for assisting with the DXA measurements.
Contributor Information
Huei-Jhen Wen, Email: win@gms.tcu.edu.tw.
Shu-Hsin Liu, Email: shuhsin0816@tzuchi.com.tw.
Chia-Liang Tsai, Email: andytsai@mail.ncku.edu.tw.
References
- 1.Nigg J.T. On inhibition/disinhibition in developmental psychopathology: views from cognitive and personality psychology and a working inhibition taxonomy. Psychol Bull. 2000;126:220–246. doi: 10.1037/0033-2909.126.2.220. [DOI] [PubMed] [Google Scholar]
- 2.Lavagnino L., Arnone D., Cao B., Soares J.C., Selvaraj S. Inhibitory control in obesity and binge eating disorder: a systematic review and meta-analysis of neurocognitive and neuroimaging studies. Neurosci Biobehav Rev. 2016;68:714–726. doi: 10.1016/j.neubiorev.2016.06.041. [DOI] [PubMed] [Google Scholar]
- 3.García-García I., Jurado M., Garolera M., et al. Alterations of the salience network in obesity: a resting-state fMRI study. Hum Brain Mapp. 2013;34:2786–2797. doi: 10.1002/hbm.22104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kullmann S., Heni M., Linder K., et al. Resting-state functional connectivity of the human hypothalamus. Hum Brain Mapp. 2014;35:6088–6096. doi: 10.1002/hbm.22607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hogenkamp P.S., Zhou W., Dahlberg L.S., et al. Higher resting-state activity in reward-related brain circuits in obese versus normal-weight females independent of food intake. Int J Obes. 2016;40:1687–1692. doi: 10.1038/ijo.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volkow N.D., Wang G.J., Telang F., et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage. 2008;42:1537–1543. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsai C.L., Chen F.C., Pan C.Y., Tseng Y.T. The neurocognitive performance of visuospatial attention in children with obesity. Front Psychol. 2016;7 doi: 10.3389/fpsyg.2016.01033. 1033-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai C.L., Huang T.H., Tsai M.C. Neurocognitive performances of visuospatial attention and the correlations with metabolic and inflammatory biomarkers in adults with obesity. Exp Physiol. 2017;102:1683–1699. doi: 10.1113/EP086624. [DOI] [PubMed] [Google Scholar]
- 9.Wen H.J., Tsai C.L. Neurocognitive inhibitory control ability performance and correlations with biochemical markers in obese women. Int J Environ Res Publ Health. 2020;17:2726. doi: 10.3390/ijerph17082726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joyce J., Graydon J., McMorris T., Davranche K. The time course effect of moderate intensity exercise on response execution and response inhibition. Brain Cognit. 2009;71:14–19. doi: 10.1016/j.bandc.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Tsai C.L., Wang C.H., Pan C.Y., Chen F.C., Huang S.Y., Tseng Y.T. The effects of different exercise types on visuospatial attention in the elderly. Psychol Sport Exerc. 2016;26:130–138. [Google Scholar]
- 12.Tsai C.L. The effectiveness of exercise intervention on inhibitory control in children with developmental coordination disorder: using a visuospatial attention paradigm as a model. Res Dev Disabil. 2009;30:1268–1280. doi: 10.1016/j.ridd.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Tsai C.L., Wang C.H., Tseng Y.T. Effects of exercise intervention on event-related potential and task performance indices of attention networks in children with developmental coordination disorder. Brain Cognit. 2012;79:12–22. doi: 10.1016/j.bandc.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Tsai C.L., Pan C.Y., Chen F.C., Huang T.H., Tsai M.C., Chuang C.Y. Differences in neurocognitive performance and metabolic and inflammatory indices in male adults with obesity as a function of regular exercise. Exp Physiol. 2019;104:1650–1660. doi: 10.1113/EP087862. [DOI] [PubMed] [Google Scholar]
- 15.Dora K., Suga T., Tomoo K., et al. Effect of very low-intensity resistance exercise with slow movement and tonic force generation on post-exercise inhibitory control. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wen H.J., Tsai C.L. Effects of acute aerobic exercise combined with resistance exercise on neurocognitive performance in obese women. Brain Sci. 2020;10:767. doi: 10.3390/brainsci10110767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilke J., Giesche F., Klier K., Vogt L., Herrmann E., Banzer W. Acute effects of resistance exercise on cognitive function in healthy adults: a systematic review with multilevel meta-analysis. Sports Med. 2019;49:905–916. doi: 10.1007/s40279-019-01085-x. [DOI] [PubMed] [Google Scholar]
- 18.Zimmer P., Stritt C., Bloch W., et al. The effects of different aerobic exercise intensities on serum serotonin concentrations and their association with Stroop task performance: a randomized controlled trial. Eur J Appl Physiol. 2016;116:2025–2034. doi: 10.1007/s00421-016-3456-1. [DOI] [PubMed] [Google Scholar]
- 19.Martinsen S., Flodin P., Berrebi J., et al. The role of long-term physical exercise on performance and brain activation during the Stroop colour word task in fibromyalgia patients. Clin Physiol Funct Imag. 2018;38:508–516. doi: 10.1111/cpf.12449. [DOI] [PubMed] [Google Scholar]
- 20.Amatriain-Fernández S., Ezquerro García-Noblejas M., Budde H. Effects of chronic exercise on the inhibitory control of children and adolescents: a systematic review and meta-analysis. Scand J Med Sci Sports. 2021;31:1196–1208. doi: 10.1111/sms.13934. [DOI] [PubMed] [Google Scholar]
- 21.Kao V.P., Wen H.J., Pan Y.J., Pai C.S., Tsai S.T., Su K.Y. Combined aerobic and resistance training improves physical and executive functions in women with systemic lupus erythematosus. Lupus. 2021;30:946–955. doi: 10.1177/0961203321998749. [DOI] [PubMed] [Google Scholar]
- 22.Frings C., Groh-Bordin C. Electrophysiological correlates of visual identity negative priming. Brain Res. 2007;1176:82–91. doi: 10.1016/j.brainres.2007.07.093. [DOI] [PubMed] [Google Scholar]
- 23.Rubia K., Smith A.B., Woolley J., et al. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp. 2006;27:973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahinoglu B., Dogan G. Event-related potentials and the stroop effect. Eurasian J Med. 2016;48:53–57. doi: 10.5152/eurasianjmed.2016.16012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potts G.F., Tucker D.M. Frontal evaluation and posterior representation in target detection. Brain Res Cogn Brain Res. 2001;11:147–156. doi: 10.1016/s0926-6410(00)00075-6. [DOI] [PubMed] [Google Scholar]
- 26.Ridderinkhof K.R., Ullsperger M., Crone E.A., Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 27.Band G.P., van Boxtel G.J. Inhibitory motor control in stop paradigms: review and reinterpretation of neural mechanisms. Acta Psychol. 1999;101:179–211. doi: 10.1016/s0001-6918(99)00005-0. [DOI] [PubMed] [Google Scholar]
- 28.Nijs I.M., Franken I.H., Muris P. Food-related Stroop interference in obese and normal-weight individuals: behavioral and electrophysiological indices. Eat Behav. 2010;11:258–265. doi: 10.1016/j.eatbeh.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Reyes S., Peirano P., Peigneux P., Lozoff B., Algarin C. Inhibitory control in otherwise healthy overweight 10-year-old children. Int J Obes. 2015;39:1230–1235. doi: 10.1038/ijo.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bauer L.O., Kaplan R.F., Hesselbrock V.M. P300 and the stroop effect in overweight minority adolescents. Neuropsychobiology. 2010;61:180–187. doi: 10.1159/000297735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamijo K., Pontifex M.B., Khan N.A., et al. The association of childhood obesity to neuroelectric indices of inhibition. Psychophysiology. 2012;49:1361–1371. doi: 10.1111/j.1469-8986.2012.01459.x. [DOI] [PubMed] [Google Scholar]
- 32.Carbine K.A., Duraccio K.M., Kirwan C.B., Muncy N.M., LeCheminant J.D., Larson M.J. A direct comparison between ERP and fMRI measurements of food-related inhibitory control: implications for BMI status and dietary intake. Neuroimage. 2018;166:335–348. doi: 10.1016/j.neuroimage.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Garon N., Bryson S.E., Smith I.M. Executive function in preschoolers: a review using an integrative framework. Psychol Bull. 2008;134:31–60. doi: 10.1037/0033-2909.134.1.31. [DOI] [PubMed] [Google Scholar]
- 34.Browne R.A., Costa E.C., Sales M.M., Fonteles A.I., Moraes J.F., Barros J.F. Acute effect of vigorous aerobic exercise on the inhibitory control in adolescents. Rev Paul Pediatr. 2016;34:154–161. doi: 10.1016/j.rppede.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsukamoto H., Suga T., Takenaka S., et al. An acute bout of localized resistance exercise can rapidly improve inhibitory control. PLoS One. 2017;12 doi: 10.1371/journal.pone.0184075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ofenheimer A., Breyer-Kohansal R., Hartl S., et al. Reference values of body composition parameters and visceral adipose tissue (VAT) by DXA in adults aged 18–81 years—results from the LEAD cohort. Eur J Clin Nutr. 2020;74:1181–1191. doi: 10.1038/s41430-020-0596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quintero A.P., Bonilla-Vargas K.J., Correa-Bautista J.E., et al. Acute effect of three different exercise training modalities on executive function in overweight inactive men: a secondary analysis of the BrainFit study. Physiol Behav. 2018;197:22–28. doi: 10.1016/j.physbeh.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Gajewski P.D., Falkenstein M. Long-term habitual physical activity is associated with lower distractibility in a Stroop interference task in aging: behavioral and ERP evidence. Brain Cognit. 2015;98:87–101. doi: 10.1016/j.bandc.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Predovan D., Fraser S.A., Renaud M., Bherer L. The effect of three months of aerobic training on stroop performance in older adults. J Aging Health. 2012;2012 doi: 10.1155/2012/269815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu X., Deng Z.Y., Huang Q., Zhang W.X., Qi C.Z., Huang J.A. Prefrontal cortex-mediated executive function as assessed by Stroop task performance associates with weight loss among overweight and obese adolescents and young adults. Behav Brain Res. 2017;321:240–248. doi: 10.1016/j.bbr.2016.12.040. [DOI] [PubMed] [Google Scholar]
- 41.Wen C.P., Cheng T.Y., Tsai S.P., et al. Are Asians at greater mortality risks for being overweight than Caucasians? redefining obesity for Asians. Publ Health Nutr. 2009;12:497–506. doi: 10.1017/S1368980008002802. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization . Health Communications; Sydney, Australia: 2000. The Asia-Pacific Perspective: Redefining Obesity and its Treatment. [Google Scholar]
- 43.Levine D.A., Gross A.L., Briceño E.M., et al. Sex differences in cognitive decline among US adults. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li C., Zheng J., Wang J., Gui L., Li C. An fMRI stroop task study of prefrontal cortical function in normal aging, mild cognitive impairment, and Alzheimer's disease. Curr Alzheimer Res. 2009;6:525–530. doi: 10.2174/156720509790147142. [DOI] [PubMed] [Google Scholar]
- 45.Dragovic M. Categorization and validation of handedness using latent class analysis. Acta Neuropsychiatr. 2004;16:212–218. doi: 10.1111/j.0924-2708.2004.00087.x. [DOI] [PubMed] [Google Scholar]
- 46.Beck A.T., Steer R.A., Brown G.K., BDI-II . second ed. The Psychological Corporation.; San Antonio, TX, USA: 1996. Manual. [Google Scholar]
- 47.Folstein M.F., Folstein S.E., McHugh P.R. Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 48.Cohen J.E. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 49.Garber C.E., Blissmer B., Deschenes M.R., et al. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 50.Prior B.M., Cureton K.J., Modlesky C.M., et al. In vivo validation of whole body composition estimates from dual-energy X-ray absorptiometry. J Appl Physiol. 1997;83:623–630. doi: 10.1152/jappl.1997.83.2.623. [DOI] [PubMed] [Google Scholar]
- 51.Laukkanen R., Oja P., Pasanen M., Vuori I. Validity of a two kilometre walking test for estimating maximal aerobic power in overweight adults. Int J Obes Relat Metab Disord. 1992;16:263–268. [PubMed] [Google Scholar]
- 52.Laukkanen R.M., Kukkonen-Harjula T.K., Oja P., Pasanen M.E., Vuori I.M. Prediction of change in maximal aerobic power by the 2-km walk test after walking training in middle-aged adults. Int J Sports Med. 2000;21:113–116. doi: 10.1055/s-2000-8872. [DOI] [PubMed] [Google Scholar]
- 53.do Nascimento M.A., Cyrino E.S., Nakamura F.Y., Romanzini M., Cardoso Pianca H.J., Queiróga M.R. Validation of the Brzycki equation for the estimation of 1-RM in the bench press. Rev Bras de Medicina do Esporte. 2007;13:40e–42e. [Google Scholar]
- 54.Brzycki M. Strength testing-predicting a one-rep max from reps-to-fatigue. J Phys Educ Recreat Dance. 1993;64:88–90. [Google Scholar]
- 55.Sherwood J.J., Inouye C., Webb S.L., O J. Reliability and validity of the sit-to-stand as a muscular power measure in older adults. J Aging Phys Activ. 2019;28(3):455–466. doi: 10.1123/japa.2019-0133. [DOI] [PubMed] [Google Scholar]
- 56.Rikli R.E., Jones C.J. Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. Gerontol. 2013;53:255–267. doi: 10.1093/geront/gns071. [DOI] [PubMed] [Google Scholar]
- 57.Diener M.H., Golding L.A., Diener D. Validity and reliability of a one-minute half sit-up test of abdominal strength and endurance. Sports Med Train Rehabil. 1995;6:105–119. [Google Scholar]
- 58.Sallis J.F., Haskell W.L., Wood P.D., et al. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;121:91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 59.Dubbert P.M., Vander Weg M.W., Kirchner K.A., Shaw B. Evaluation of the 7-day physical activity recall in urban and rural men. Med Sci Sports Exerc. 2004;36:1646–1654. doi: 10.1249/01.mss.0000139893.65189.f2. [DOI] [PubMed] [Google Scholar]
- 60.Soundy A., Taylor A., Faulkner G., Rowlands A. Psychometric properties of the 7-Day Physical Activity Recall questionnaire in individuals with severe mental illness. Arch Psychiatr Nurs. 2007;21:309–316. doi: 10.1016/j.apnu.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 61.Cheng Z., Shuai P., Qiao Q., Li T. Validity and reliability of a simplified food frequency questionnaire: a cross sectional study among physical health examination adults in southwest region of China. Nutr J. 2020;19 doi: 10.1186/s12937-020-00630-z. 114-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stroop J.R. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- 63.Larson M.J., Farrer T.J., Clayson P.E. Cognitive control in mild traumatic brain injury: conflict monitoring and conflict adaptation. Int J Psychophysiol. 2011;82:69–78. doi: 10.1016/j.ijpsycho.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 64.Ille N., Berg P., Scherg M. Artifact correction of the ongoing EEG using spatial filters based on artifact and brain signal topographies. J Clin Neurophysiol. 2002;19:113–124. doi: 10.1097/00004691-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 65.Carter C.S., Braver T.S., Barch D.M., Botvinick M.M., Noll D., Cohen J.D. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 66.Rebai M., Bernard C., Lannou J. The Stroop's test evokes a negative brain potential, the N400. Int J Neurosci. 1997;91:85–94. doi: 10.3109/00207459708986367. [DOI] [PubMed] [Google Scholar]
- 67.West R., Alain C. Event-related neural activity associated with the Stroop task. Brain Res Cogn Brain Res. 1999;8:157–164. doi: 10.1016/s0926-6410(99)00017-8. [DOI] [PubMed] [Google Scholar]
- 68.Thomas S.J., Johnstone S.J., Gonsalvez C.J. Event-related potentials during an emotional Stroop task. Int J Psychophysiol. 2007;63:221–231. doi: 10.1016/j.ijpsycho.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 69.Bruin K.J., Wijers A.A., van Staveren A.S. Response priming in a go/nogo task: do we have to explain the go/nogo N2 effect in terms of response activation instead of inhibition? Clin Neurophysiol. 2001;112:1660–1671. doi: 10.1016/s1388-2457(01)00601-0. [DOI] [PubMed] [Google Scholar]
- 70.Jonkman L.M., Lansbergen M., Stauder J.E. Developmental differences in behavioral and event-related brain responses associated with response preparation and inhibition in a go/nogo task. Psychophysiology. 2003;40:752–761. doi: 10.1111/1469-8986.00075. [DOI] [PubMed] [Google Scholar]
- 71.Dimitrov D.M., Rumrill P.D., Jr. Pretest-posttest designs and measurement of change. Work. 2003;20:159–165. [PubMed] [Google Scholar]
- 72.Vickers A.J., Altman D.G. Statistics notes: analysing controlled trials with baseline and follow up measurements. BMJ. 2001;323:1123–1124. doi: 10.1136/bmj.323.7321.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schwingshackl L., Dias S., Strasser B., Hoffmann G. Impact of different training modalities on anthropometric and metabolic characteristics in overweight/obese subjects: a systematic review and network meta-analysis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0082853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coubard O.A., Duretz S., Lefebvre V., Lapalus P., Ferrufino L. Practice of contemporary dance improves cognitive flexibility in aging. Front Aging Neurosci. 2011;3:13. doi: 10.3389/fnagi.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Killikelly C., Szűcs D. Asymmetry in stimulus and response conflict processing across the adult lifespan: ERP and EMG evidence. Cortex. 2013;49:2888–2903. doi: 10.1016/j.cortex.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ludyga S., Gerber M., Herrmann C., Brand S., Pühse U. Chronic effects of exercise implemented during school-break time on neurophysiological indices of inhibitory control in adolescents. Trends Neurosci Educ. 2018;10:1–7. [Google Scholar]
- 77.Liu J.H., Alderman B.L., Song T.F., Chen F.T., Hung T.M., Chang Y.K. A randomized controlled trial of coordination exercise on cognitive function in obese adolescents. Psychol Sport Exerc. 2018;34:29–38. doi: 10.1016/j.psychsport.2019.101604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu-Ambrose T., Donaldson M.G. Exercise and cognition in older adults: is there a role for resistance training programmes? Br J Sports Med. 2009;43:25–27. doi: 10.1136/bjsm.2008.055616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heidlmayr K., Kihlstedt M., Isel F. A review on the electroencephalography markers of stroop executive control processes. Brain Cognit. 2020;146 doi: 10.1016/j.bandc.2020.105637. [DOI] [PubMed] [Google Scholar]
- 80.Näätänen R., Simpson M., Loveless N.E. Stimulus deviance and evoked potentials. Biol Psychol. 1982;14:53–98. doi: 10.1016/0301-0511(82)90017-5. [DOI] [PubMed] [Google Scholar]
- 81.Huttenlocher P.R., Dabholkar A.S. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 82.Pardo J.V., Pardo P.J., Janer K.W., Raichle M.E. The anterior cingulate cortex mediates processing selection in the stroop attentional conflict paradigm. Proc Natl Acad Sci USA. 1990;87:256–259. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boenke L.T., Ohl F.W., Nikolaev A.R., Lachmann T., van Leeuwen C. Different time courses of stroop and garner effects in perception-an event-related potentials study. Neuroimage. 2009;45:1272–1288. doi: 10.1016/j.neuroimage.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 84.Folstein J.R., Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45:152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jonkman L.M., Lansbergen M., Stauder J.E. Developmental differences in behavioral and event-related brain responses associated with response preparation and inhibition in a go/nogo task. Psychophysiology. 2003;40:752–761. doi: 10.1111/1469-8986.00075. [DOI] [PubMed] [Google Scholar]
- 86.Chuang L.Y., Hung H.Y., Huang C.J., Chang Y.K., Hung T.M. A 3-month intervention of Dance Dance Revolution improves interference control in elderly females: a preliminary investigation. Exp Brain Res. 2015;233:1181–1188. doi: 10.1007/s00221-015-4196-x. [DOI] [PubMed] [Google Scholar]
- 87.Tsai C.L., Pan C.Y., Chen F.C., Tseng Y.T. Open- and closed-skill exercise interventions produce different neurocognitive effects on executive functions in the elderly: a 6-month randomized, controlled trial. Front Aging Neurosci. 2017;9:294. doi: 10.3389/fnagi.2017.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alderman B.L., Olson R.L., Brush C.J. Using event-related potentials to study the effects of chronic exercise on cognitive function. Int J Sport Psychol. 2019;17:106–116. [Google Scholar]
- 89.Chang Y.K., Tsai Y.J., Chen T.T., Hung T.M. The impacts of coordinative exercise on executive function in kindergarten children: an ERP study. Exp Brain Res. 2013;225:187–196. doi: 10.1007/s00221-012-3360-9. [DOI] [PubMed] [Google Scholar]
- 90.Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chang Y.K., Alderman B.L., Chu C.H., Wang C.C., Song T.F., Chen F.T. Acute exercise has a general facilitative effect on cognitive function: a combined ERP temporal dynamics and BDNF study. Psychophysiology. 2017;54:289–300. doi: 10.1111/psyp.12784. [DOI] [PubMed] [Google Scholar]
- 92.Hsieh S.S., Huang C.J., Wu C.T., Chang Y.K., Hung T.M. Acute exercise facilitates the N450 Inhibition marker and P3 attention marker during stroop test in young and older adults. J Clin Med. 2018;7 doi: 10.3390/jcm7110391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nono Nankam P.A., Nguelefack T.B., Goedecke J.H., Blüher M. Contribution of adipose tissue oxidative stress to obesity-associated diabetes risk and ethnic differences: focus on women of african ancestry. Antioxidants. 2021;10:622. doi: 10.3390/antiox10040622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Park W., Jung W.S., Hong K., Kim Y.Y., Kim S.W., Park H.Y. Effects of moderate combined resistance- and aerobic-exercise for 12 weeks on body composition, cardiometabolic risk factors, blood pressure, arterial stiffness, and physical functions, among obese older men: a pilot study. Int J Environ Res Publ Health. 2020;17(19):7233. doi: 10.3390/ijerph17197233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Son W.M., Sung K.D., Bharath L.P., Choi K.J., Park S.Y. Combined exercise training reduces blood pressure, arterial stiffness, and insulin resistance in obese prehypertensive adolescent girls. Clin Exp Hypertens. 2017;39:546–552. doi: 10.1080/10641963.2017.1288742. [DOI] [PubMed] [Google Scholar]
- 96.Zhang H., Tong T.K., Kong Z., Shi Q., Liu Y., Nie J. Exercise training-induced visceral fat loss in obese women: the role of training intensity and modality. Scand J Med Sci Sports. 2021;31:30–43. doi: 10.1111/sms.13803. [DOI] [PubMed] [Google Scholar]
- 97.Donnelly J.E., Blair S.N., Jakicic J.M., Manore M.M., Rankin J.W., Smith B.K. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 98.Zhang H., Tong T.K., Qiu W., et al. Comparable effects of high-Intensity interval training and prolonged continuous exercise training on abdominal visceral fat reduction in obese young women. J Diabetes Res. 2017;2017 doi: 10.1155/2017/5071740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dupuit M., Rance M., Morel C., et al. Moderate-intensity continuous training or hgh-intensity interval training with or without resistance training for altering body composition in postmenopausal women. Med Sci Sports Exerc. 2020;52:736–745. doi: 10.1249/MSS.0000000000002162. [DOI] [PubMed] [Google Scholar]
- 100.Song T.F., Chi L., Chu C.H., Chen F.T., Zhou C., Chang Y.K. Obesity, cardiovascular fitness, and inhibition function: an electrophysiological study. Front Psychol. 2016;7:1124. doi: 10.3389/fpsyg.2016.01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Prickett C., Brennan L., Stolwyk R. Examining the relationship between obesity and cognitive function: a systematic literature review. Obes Res Clin Pract. 2015;9:93–113. doi: 10.1016/j.orcp.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 102.Ho S.S., Dhaliwal S.S., Hills A.P., Pal S. The effect of 12 weeks of aerobic, resistance or combination exercise training on cardiovascular risk factors in the overweight and obese in a randomized trial. BMC Publ Health. 2012;12:704. doi: 10.1186/1471-2458-12-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ozato N., Saitou S., Yamaguchi T., et al. Association between visceral fat and brain structural changes or cognitive function. Brain Sci. 2021;11:1036. doi: 10.3390/brainsci11081036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pernet C.R., Wilcox R., Rousselet G.A. Robust correlation analyses: false positive and power validation using a new open source matlab toolbox. Front Psychol. 2012;3:606. doi: 10.3389/fpsyg.2012.00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schwarzkopf D.S., De Haas B., Rees G. Better ways to improve standards in brain-behavior correlation analysis. Front Hum Neurosci. 2012;6:200. doi: 10.3389/fnhum.2012.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Foright R.M., Johnson G.C., Kahn D., et al. Compensatory eating behaviors in male and female rats in response to exercise training. Am J Physiol Regul Integr Comp Physiol. 2020;319:R171–r183. doi: 10.1152/ajpregu.00259.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gajewski P.D., Falkenstein M. Training-induced improvement of response selection and error detection in aging assessed by task switching: effects of cognitive, physical, and relaxation training. Front Hum Neurosci. 2012;6:130. doi: 10.3389/fnhum.2012.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Žlibinaitė L., Solianik R., Vizbaraitė D., Mickevičienė D., Skurvydas A. The effect of combined aerobic exercise and calorie restriction on mood, cognition, and motor behavior in overweight and obese women. J Phys Activ Health. 2020;17:204–210. doi: 10.1123/jpah.2019-0373. [DOI] [PubMed] [Google Scholar]