Highlights
-
•
Neutrophil-to-lymphocyte ratio was predictive of OS in cervical cancer patients treated with PD-1/PD-L1 inhibitors.
-
•
For those with a NLR < 8 the probability of survival at one year was 57% compared to 26% for those with NLR ≥ 8.
-
•
No significant association between NLR and progression free survival or response to treatment was noted.
-
•
Assessment of ORR was limited due to 5/14 patients in the NLR ≥ 8 group passing before assessment of disease response.
-
•
NLR holds promise as a predictive marker for survival in this population and warrants further evaluation.
Keywords: Cervical cancer, Neutrophil-to-lymphocyte ratio, Immunotherapy, Checkpoint inhibitors, Prognostic marker
Abstract
Objective
To evaluate the association between pre-treatment neutrophil-to-lymphocyte ratio (NLR) and survival outcomes among patients with recurrent/metastatic cervical cancer treated with PD-1/PD-L1 inhibitors.
Methods
A retrospective analysis of patients with recurrent/metastatic cervical cancer treated with PD-1/PD-L1 inhibitors from 2016 to 2021 was conducted. Progression free survival (PFS) and overall survival (OS) outcomes were assessed for patients stratified by NLR (<8 vs ≥ 8) utilizing Kaplan-Meier method. Univariable analysis was performed to compare baseline characteristics between the two groups.
Results
A total of 49 patients were included in analysis. A majority of patients had squamous cell histology (57%), were PD-L1 positive (55%), received ≤ 1 prior lines of systemic therapy (57%), and had distant metastatic disease at the time of treatment (69%). The groups were well-balanced with respect to age, race, histology, smoking status, PD-L1 positivity, prior lines of treatment (≤1 vs > 1), prior radiation therapy, ECOG performance status, and disease distribution for patients with a NLR < 8 (n = 35) compared to those with a NLR ≥ 8 (n = 14). A pre-treatment NLR of < 8 was associated with improved survival (p < 0.01), with 57% (95% CI: 41%, 78%) probability of survival at one year compared to 26% (95% CI: 10%, 66%) for those with NLR ≥ 8. No statistically significant differences in probability of PFS at 1 year were seen between NLR < 8 compared to those with NLR ≥ 8 (p = 0.70).
Conclusions
Pre-treatment NLR may hold prognostic value for patients with metastatic/recurrent cervical cancer treated with PD-1/PD-L1 inhibitors, with NLR < 8 associated with improved survival.
1. Introduction
Cervical cancer is the third most common gynecologic malignancy in the United States with an estimated 14,480 new cases of invasive cervical cancer diagnosed in 2020 (Siegel et al., 2021). While only 15% of patients present with distant disease at initial diagnosis, 15–61% of women will develop recurrent disease, typically within the first two years of completing primary treatment (Surveillance, Epidemiology, and End results, 2009-2018, Rose et al., 1999). In the setting of distant recurrence or metastatic disease, cervical cancer is largely considered incurable with a 5-year survival rate of only 17.6% (Surveillance, Epidemiology, and End results, 2009-2018). Historically, standard systemic therapy for primary or recurrent metastatic disease involved platinum-based therapy and bevacizumab (Tewari et al., 2017). More recent phase 3 data have resulted in FDA-approval of the incorporation of pembrolizumab, a PD-1 inhibitor, in the front-line setting for patients whose tumors exhibit positive PD-L1 expression with an improvement in overall survival observed (Colombo et al., 2021). Pembrolizumab was initially FDA-approved in 2018 for the treatment of PD-L1 positive recurrent/metastatic cervical cancer that had progressed after primary platinum-based therapy based on findings from KEYNOTE-158 which demonstrated a favorable objective response rate of 14.6% compared to other therapies in the second-line setting(Chung et al., 2019). Similar favorable response rates have since been observed in the second-line setting for other PD-1 inhibitors (Naumann et al., 2019, Tewari et al., O'Malley et al., 2021).
While PD-L1 tumor positivity is currently the FDA-approved indication for use of PD-1 inhibitors in both the first- and second-line setting, it is an imperfect biomarker with responses observed irrespective of PD-L1 expression in some studies (Colombo et al., 2021, Tewari et al.). Thus, efforts have focused on identifying other potential predictive markers for response to treatment. These studies have examined tumor mutational burden, tumor infiltrating lymphocytes (CD8 + to CD4 + ratios) as well as pretreatment blood parameters as candidates (Otter et al., Dec 2019, Marabelle et al., 2020).
Pretreatment neutrophil-to-lymphocyte ratio (NLR) has been proposed as a potential prognostic marker for survival outcomes in cancer patients treated with immunotherapy. A significant association has been observed between high NLR and worse survival outcomes in melanoma, non-small cell lung cancer, gastrointestinal, breast, and gynecologic cancers treated with immunotherapy (Criscitiello et al., 2020, Kartolo et al., 2020, Valero et al., 2021). A well-established hallmark of cancer is its ability to evade the immune system. The NLR is an attractive potential biomarker not only because it is easily available in clinical practice, but also because it may provide a surrogate measure of the tumor microenvironment balance between inflammatory response and adaptive immune surveillance. The role of NLR as a possible predictive marker for response to immune checkpoint inhibitors (ICIs) is also of particular interest as ICIs target this particular mechanism of cancer immune evasion.
The objective of the current study is to determine the association of pre-treatment neutrophil-to-lymphocyte ratio with response to treatment, PFS, and OS for patients with recurrent/metastatic cervical cancer treated with PD-1/PD-L1 checkpoint inhibitors.
2. Methods
A single-institution retrospective study of all patients with advanced/recurrent cervical cancer treated with a PD-1 or PD-L1 inhibitor from June 2016 to August of 2021 was conducted. All data was collected after approval by the institutional review board (#2021C0152) with a waiver of consent. Baseline demographic data, smoking status, performance status, tumor histology, PD-L1 status, type of recurrence, previous radiation treatment, and previous lines of systemic therapy were obtained from the medical record.
Pre-treatment NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count obtained through routine blood work collected within one week of initiating immunotherapy. An ideal cutoff for NLR has not yet been established, thus, various NLR cutoffs were evaluated in this study. A standard cutoff of 6 was evaluated as well as a cutoff based on the top 20th percentile of the current cohort (cutoff of 8). Ultimately, a cutoff of 8 was utilized in final analysis with a high NLR characterized as ≥ 8 and a low NLR characterized as < 8. The rationale for this decision was based on previous published literature which has demonstrated relative heterogeneity among different cancer subtypes in regards to baseline NLR and given the relatively high value compared to other disease sites, it was felt this was most representative of the group which had the worst prognosis.
Univariable analysis was performed to assess differences in baseline characteristics between the two groups with respect to age, race, smoking status, ECOG performance status, histology, previous radiation treatment, PD-L1 positivity, recurrence type/location, number of metastatic sites, prior lines of treatment (≤1 vs > 1), immunotherapy agent utilized, and whether or not therapy was utilized as monotherapy or combination therapy. Pearson’s chi-squared test was performed to compare disease response by imaging and clinical assessment at the time of first assessment between those with a high versus low NLR. The Kaplan-Meier method was performed to assess progression free survival (PFS) probability and overall survival (OS) probability for those with a high NLR compared to those with a low NLR. P-values ≤ 0.05 were considered statistically significant and all confidence intervals are presented at their nominal levels. All analyses were performed in R version 4.1.2.
3. Results
A total of 49 patients with metastatic/recurrent cervical cancer treated with a PD-1/PD-L1 inhibitor were included. Most had squamous cell histology (57%), were PD-L1 positive (55%), had received ≤ 1 prior lines of systemic therapy (57%), had received prior radiation treatment (76%), and had distant metastatic disease at the time of treatment initiation (69%). Most patients were treated with pembrolizumab (63%) and received monotherapy (73%). Those with a NLR < 8 and those with a NLR ≥ 8 were well-balanced in regard to their baseline characteristics (Table 1).
Table 1.
Baseline characteristics.
| Characteristic | Total (n = 49) |
Pre-treatment NLR1 < 8 (n = 35) |
Pre-treatment NLR ≥ 8 (n = 14) |
P-value2 |
|---|---|---|---|---|
| Age in years, mean (SD) | 51 (12) | 51 (13) | 50 (11) | >0.9 |
| Race | ||||
| Non-Hispanic white | 41 (84) | 31 (88) | 10 (72) | 0.2 |
| Non-Hispanic Black | 3 (6) | 2 (6) | 1 (7) | |
| Hispanic | 2 (4) | 1 (3) | 1 (7) | |
| Asian | 1 (2) | 0 (0) | 1 (7) | |
| More than one race | 1 (2) | 0 (0) | 1 (7) | |
| Unknown/not reported | 1 (2) | 1 (3) | 0 (0) | |
| Smoking status | 0.6 | |||
| Never | 23 (47) | 15 (43) | 8 (57) | |
| Former | 12 (24) | 10 (29) | 2 (14) | |
| Current | 14 (29) | 10 (29) | 4 (29) | |
| ECOG performance status | 0.9 | |||
| 0 | 24 (49) | 17 (49) | 7 (50) | |
| 1 | 20 (41) | 15 (43) | 5 (36) | |
| 2 | 2 (4) | 1 (3) | 1 (7) | |
| Unknown | 3 (6) | 2 (6) | 1 (7) | |
| Histology | 0.7 | |||
| Squamous cell carcinoma | 28 (57) | 18 (51) | 10 (71) | |
| Adenocarcinoma | 15 (31) | 12 (34) | 3 (21) | |
| Adenosquamous carcinoma | 2 (4) | 2 (6) | 0 (0) | |
| Other | 4 (8) | 3 (9) | 1 (7) | |
| Previous radiation | 0.5 | |||
| Yes | 37 (76) | 25 (71) | 12 (86) | |
| No | 12 (24) | 10 (29) | 2 (14) | |
| PD-L1 status | >0.9 | |||
| Positive | 27 (55) | 19 (54) | 8 (57) | |
| Negative | 10 (20) | 7 (20) | 3 (21) | |
| Unknown | 12 (24) | 9 (26) | 3 (21) | |
| Recurrence type/location | 0.9 | |||
| Pelvic confined | 5 (10) | 3 (9) | 2 (14) | |
| Retroperitoneal lymph nodes | 4 (8) | 3 (9) | 1 (7) | |
| Peritoneal disease | 6 (12) | 4 (11) | 2 (14) | |
| Distant metastases | 34 (69) | 25 (71) | 9 (64) | |
| Number of metastatic sites | 0.3 | |||
| <5 | 11 (22) | 6 (17) | 5 (36) | |
| ≥5 | 38 (78) | 29 (83) | 9 (64) | |
| Number of previous lines of systemic therapy | 0.5 | |||
| ≤1 | 28 (57) | 21 (60) | 7 (50) | |
| >1 | 21 (43) | 14 (40) | 7 (50) | |
| Immunotherapy agent | 0.4 | |||
| Pembrolizumab | 31 (63) | 22 (63) | 9 (64) | |
| Nivolumab | 13 (27) | 8 (23) | 5 (36) | |
| Atezolizumab | 5 (10) | 5 (14) | 0 (0) | |
| Therapy type | >0.9 | |||
| Monotherapy | 36 (73) | 26 (74) | 10 (71) | |
| Combination | 13 (27) | 9 (26) | 4 (29) |
All variables displayed as n (%) unless otherwise noted.
NLR = neutrophil to lymphocyte ratio.
Using Fisher’s exact test for categorical variables and two-sample t-test for continuous variables.
Patients were assessed for response per provider discretion or as dictated by clinical trial with imaging (CT/MRI/PET). Best response was determined per RECIST criteria for those patients enrolled in clinical trial or clinician assessment of imaging for those not enrolled on clinical trial.
The overall response rate was 11.1% (1/9 evaluable patients) for those with a NLR ≥ 8 compared to 28.6% (10/35) for those with a NLR < 8. The clinical benefit rate was 44.4% (4/9 evaluable patients) and 60% (21/35), respectively. Of those patients with a NLR ≥ 8, 35.7% (5/14) died within 1–4 months after initiating therapy, before disease response could be assessed (Table 2). In regards to receipt of combination therapy, 28.6% (4/14) of the patients with NLR ≥ 8 received combination therapy compared to 23% (8/35) of the patients with NLR < 8. With an ORR of 0% and 33.3% observed in these subgroups, respectively.
Table 2.
Response rates by NLR group.
| NLR < 8 (n = 21)1 |
NLR ≥ 8 (n = 9) |
|
|---|---|---|
| Best Response Category | ||
| Complete response | 2 (9.5%) | 0 (0%) |
| Partial response | 8 (38.1%) | 1 (11.1%) |
| Stable disease | 11 (52.4%) | 3 (33.3%) |
| Died prior to first disease assessment | 0 (0%) | 5 (35.7%)2 |
Displayed as n (%).
This group included those without disease assessment available (all 14 patients).
Survival probability was assessed at various NLR values based on a previously established cutoff of 6 as well as a cutoff of 8 based on the top 20th percentile of the current cohort. Fig. 1 demonstrates survival probability utilizing three different groups (NLR < 6, NLR 6–8, and NLR ≥ 8). Ultimately a cutoff of 8 was utilized for final survival analyses based on the top 20th percentile of the cohort.
Fig. 1.
Survival probability based on various NLR cutoffs. NLR = neutrophil to lymphocyte ratio.
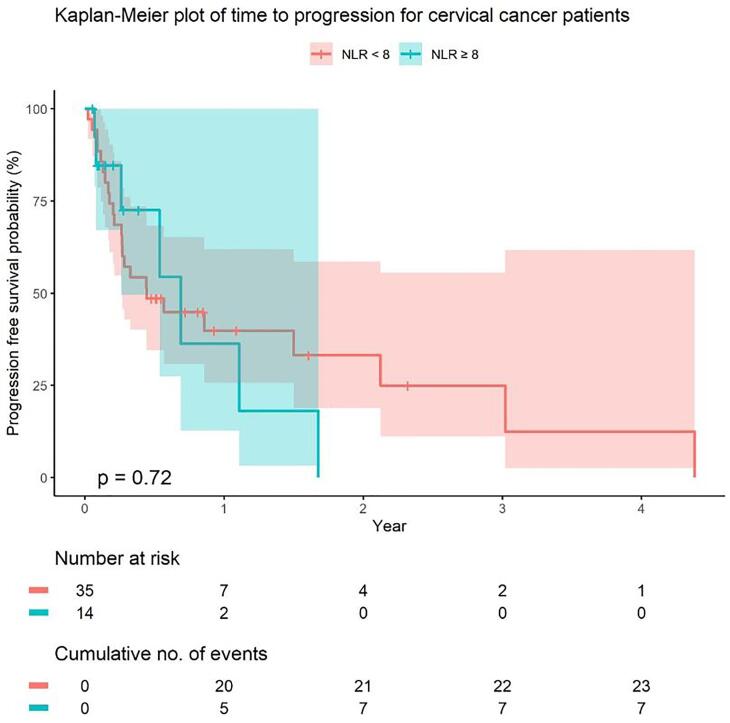
PFS at one year did not differ significantly between those with a NLR ≥ 8 and those with a NLR < 8, with 1-year PFS probability of 36% (95% CI: 15%, 100%) and 40% (95% CI: 26%, 62%), respectively (Fig. 2).
Fig. 2.
Progression free survival probability. NLR = neutrophil to lymphocyte ratio.
A significant improvement in the probability of survival at one year was observed for patients with a NLR < 8 (57% [95% CI: 41%, 78%]) compared to a NLR ≥ 8 (26% [10%, 66%]) (Fig. 3).
Fig. 3.
Overall survival probability. NLR = neutrophil to lymphocyte ratio.
4. Discussion
The current study demonstrated a significant association between a low pre-treatment NLR (<8) and improved overall survival for patients with metastatic/recurrent cervical cancer treated with PD-1/PD-L1 inhibitors. This finding is consistent with previously published data in other disease sites such as melanoma and non-small cell lung carcinoma (NSCLC) which demonstrated improved PFS and OS with a low NLR (<5) (Kartolo et al., 2020). An additional study by Criscitiello et al similarly demonstrated improved PFS and OS with a low derived NLR (<3) in multiple disease sites including gynecologic cancers (Criscitiello et al., 2020).
The question of what constitutes a ‘high’ NLR versus a ‘low’ NLR has not been extensively explored. Several different established cutoffs have been utilized previously in the literature, with a median cutoff of 4 utilized in solid tumors (Coleman et al., 2021). Other studies have utilized cutoffs based on percentiles with the top 20th percentile being characterized as a ‘high’ NLR within each cancer subtype (Kartolo et al., 2020). When assessing the NLR based on percentiles, it has been demonstrated that there is relative heterogeneity among different cancer sites as to what would constitute a ‘high’ NLR (Criscitiello et al., 2020, Kartolo et al., 2020, Valero et al., 2021). One strength of the current study was assessing the NLR specifically in patients with cervical cancer and evaluating survival in three different groups based on a standard cutoff of 6 as well as a cutoff of ≥ 8, which in our cohort was consistent with the top 20th percentile.
The role of NLR as a predictive marker for response to treatment with immunotherapy remains less clear. While Valero et al demonstrated significantly worse response rates and clinical benefit rates among those with high NLR, other studies, including our own, did not demonstrate a significant association (Criscitiello et al., 2020, Kartolo et al., 2020, Valero et al., 2021). While the ORR was not significantly different between the two groups, it is worth noting that 5 of the patients in the group with a NLR ≥ 8 died prior to obtaining any assessment for disease response (within 1–4 months of initiating treatment). This left only 9 patients who were evaluable for response. Of those 9 patients, only one had a partial response. This brings up another limitation of the current study which is the small sample size making it difficult to draw conclusions about the utility of NLR as a predictive marker at this time.
Although this study demonstrated an association between NLR and survival in patients with metastatic/recurrent cervical cancer treated with PD-1/PD-L1 inhibitors, more data is required to determine the optimal use for this information. Additional studies are necessary to determine whether or not a high pre-treatment NLR is reflective of worse survival outcomes in patients treated with immunotherapy alone, or holds prognostic value for all patients, regardless of the treatment utilized. Assessing the prognostic value of pre-treatment NLR across multiple therapies is of particular interest as it has the potential to impact treatment decisions. For example, with the recent FDA approval of tisotumab vedotin (TV) in the second-line setting, it could help guide decisions regarding patients who have progressed after standard cytotoxic therapy, as to whether or not to recommend pembrolizumab with a response rate of 15% and the possibility of a durable response versus TV which has a response rate of 24% but perhaps a less durable response (median DOR of 8.3 months) (Chung et al., 2019, Coleman et al., 2021). Not only do these decisions have clinical implications in regards to toxicity profiles but are also important as it relates to cost of therapies as well.
Limitations of the current study include the small sample size and retrospective nature of the study. Due to the small sample size, additional comorbidities that may confound the effect of NLR could not be controlled for in this study.
Despite these limitations, the current study provides further support that pre-treatment NLR holds prognostic value in regards to survival in patients with metastatic/recurrent cervical cancer treated with immunotherapy. With the recent approval of pembrolizumab in the front-line setting in combination with cytotoxic chemotherapy, we may soon have greater opportunity to discern the potential predictive value of NLR in this setting as well.
These findings warrant further evaluation and validation of the prognostic value and possible predictive value of NLR in larger studies both in the front-line treatment setting, as well as in the second-line setting compared to other cytotoxic therapies.
Author contributions
-
•
Corinne Calo, DO: Project design, writing IRB, building data collection tools, data collection, data analysis, writing manuscript
-
•
David Barrington, MD: data collection and analysis, editing manuscript
-
•
Morgan Brown, MD: data collection
-
•
Lynette Gonzalez: data collection
-
•
Jae Baek: data collection
-
•
Allison Huffman: data collection
-
•
Jason Benedict, MS: Project design, Statistical analysis
-
•
Floor Backes, MD: Project design, manuscript editing
-
•
Laura Chambers, DO: Project design, manuscript editing
-
•
David Cohn, MD: Project design, manuscript editing
-
•
Casey Cosgrove, MD: Project design, manuscript editing
-
•
Larry Copeland, MD: Project design, manuscript editing
-
•
Christa Nagel, MD: Project design, manuscript editing
-
•
David O’Malley, MD: Project design, manuscript editing
-
•
Kristin Bixel, MD: Study conceptualization, principal investigator, data analysis, editing manuscript
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chung H.C., Ros W., Delord J.-P., Perets R., Italiano A., Shapira-Frommer R., Manzuk L., Piha-Paul S.A., Xu L., Zeigenfuss S., Pruitt S.K., Leary A. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2019;37(17):1470–1478. doi: 10.1200/JCO.18.01265. [DOI] [PubMed] [Google Scholar]
- Coleman R.L., Lorusso D., Gennigens C., González-Martín A., Randall L., Cibula D., Lund B., Woelber L., Pignata S., Forget F., Redondo A., Vindeløv S.D., Chen M., Harris J.R., Smith M., Nicacio L.V., Teng M.S.L., Laenen A., Rangwala R., Manso L., Mirza M., Monk B.J., Vergote I., Raspagliesi F., Melichar B., Gaba Garcia L., Jackson A., Henry S., Kral Z., Harter P., De Giorgi U., Bjurberg M., Gold M., O'Malley D., Honhon B., Vulsteke C., De Cuypere E., Denys H., Baurain J.-F., Zamagni C., Tenney M., Gordinier M., Bradley W., Schlumbrecht M., Spirtos N., Concin N., Mahner S., Scambia G., Leath C., Farias-Eisner R., Cohen J., Muller C., Bhatia S. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): a multicentre, open-label, single-arm, phase 2 study. LancetOncol. 2021;22(5):609–619. doi: 10.1016/S1470-2045(21)00056-5. [DOI] [PubMed] [Google Scholar]
- Colombo N., Dubot C., Lorusso D., Caceres M.V., Hasegawa K., Shapira-Frommer R., Tewari K.S., Salman P., Hoyos Usta E., Yañez E., Gümüş M., Olivera Hurtado de Mendoza M., Samouëlian V., Castonguay V., Arkhipov A., Toker S., Li K., Keefe S.M., Monk B.J. Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. N. Engl. J. Med. 2021;385(20):1856–1867. doi: 10.1056/NEJMoa2112435. [DOI] [PubMed] [Google Scholar]
- Criscitiello C., Marra A., Morganti S., Zagami P., Viale G., Esposito A., Curigliano G. Pretreatment Blood Parameters Predict Efficacy from Immunotherapy Agents in Early Phase Clinical Trials. Oncologist. 2020;25(11):e1732–e1742. doi: 10.1634/theoncologist.2020-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartolo A., Holstead R., Khalid S., Emack J., Hopman W., Robinson A., Baetz T. Serum neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in prognosticating immunotherapy efficacy. Immunotherapy. 2020;12(11):785–798. doi: 10.2217/imt-2020-0105. [DOI] [PubMed] [Google Scholar]
- Marabelle A., Fakih M., Lopez J., Shah M., Shapira-Frommer R., Nakagawa K., Chung H.C., Kindler H.L., Lopez-Martin J.A., Miller W.H., Italiano A., Kao S., Piha-Paul S.A., Delord J.-P., McWilliams R.R., Fabrizio D.A., Aurora-Garg D., Xu L., Jin F., Norwood K., Bang Y.-J. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. LancetOncol. 2020;21(10):1353–1365. doi: 10.1016/S1470-2045(20)30445-9. [DOI] [PubMed] [Google Scholar]
- Naumann R.W., Hollebecque A., Meyer T., Devlin M.-J., Oaknin A., Kerger J., López-Picazo J.M., Machiels J.-P., Delord J.-P., Evans T.R.J., Boni V., Calvo E., Topalian S.L., Chen T., Soumaoro I., Li B., Gu J., Zwirtes R., Moore K.N. Safety and Efficacy of Nivolumab Monotherapy in Recurrent or Metastatic Cervical, Vaginal, or Vulvar Carcinoma: Results From the Phase I/II CheckMate 358 Trial. J. Clin. Oncol. 2019;37(31):2825–2834. doi: 10.1200/JCO.19.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley D.M., Oaknin A., Monk B.J., Selle F., Rojas C., Gladieff L., Berton D., Leary A., Moore K.N., Estevez-Diz M.D.P., Hardy-Bessard A.-C., Alexandre J., Opperman C.P., de Azevedo C.R.A.S., Randall L.M., Feliu W.O., Ancukiewicz M., Ray-Coquard I. Phase II study of the safety and efficacy of the anti-PD-1 antibody balstilimab in patients with recurrent and/or metastatic cervical cancer. Gynecol. Oncol. 2021;163(2):274–280. doi: 10.1016/j.ygyno.2021.08.018. [DOI] [PubMed] [Google Scholar]
- Otter S.J., Chatterjee J., Stewart A.J., Michael A. The Role of Biomarkers for the Prediction of Response to Checkpoint Immunotherapy and the Rationale for the Use of Checkpoint Immunotherapy in Cervical Cancer. Clin Oncol (R Coll Radiol). Dec 2019;31(12):834–843. doi: 10.1016/j.clon.2019.07.003. [DOI] [PubMed] [Google Scholar]
- Rose P.G., Bundy B.N., Watkins E.B., Thigpen J.T., Deppe G., Maiman M.A., Clarke-Pearson D.L., Insalaco S. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N. Engl. J. Med. 1999;340(15):1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA Cancer J. Clin. Jan 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- Surveillance, Epidemiology, and End results. SEER registry data. Cervix uteri stage distribution of SEER incidence cases, 2009-2018. seer.cancer.gov/ (Accessed 11/12/2021).
- Tewari K, Monk B, Vergote I, et al. EMPOWER-Cervical 1/GOG-3016/ENGOT-CX9: Results of phase 3 trial of cemiplimab vs investigator's choice chemotherapy in recurrent/metastatic cervical carcinoma. Presented at IGCS 20212021.
- Tewari K.S., Sill M.W., Penson R.T., Huang H., Ramondetta L.M., Landrum L.M., Oaknin A., Reid T.J., Leitao M.M., Michael H.E., DiSaia P.J., Copeland L.J., Creasman W.T., Stehman F.B., Brady M.F., Burger R.A., Thigpen J.T., Birrer M.J., Waggoner S.E., Moore D.H., Look K.Y., Koh W.-J., Monk B.J. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240) Lancet. 2017;390(10103):1654–1663. doi: 10.1016/S0140-6736(17)31607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero C., Lee M., Hoen D., Weiss K., Kelly D.W., Adusumilli P.S., Paik P.K., Plitas G., Ladanyi M., Postow M.A., Ariyan C.E., Shoushtari A.N., Balachandran V.P., Hakimi A.A., Crago A.M., Long Roche K.C., Smith J.J., Ganly I., Wong R.J., Patel S.G., Shah J.P., Lee N.Y., Riaz N., Wang J., Zehir A., Berger M.F., Chan T.A., Seshan V.E., Morris L.G.T. Pretreatment neutrophil-to-lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors. Nat. Commun. 2021;12(1) doi: 10.1038/s41467-021-20935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]