Summary
In this retrospective, we review the two research topics that formed the basis of the outstanding career of Dr. Paul S. Frenette. In the first part, we focus on sickle cell disease (SCD). The defining feature of SCD is polymerization of the deoxygenated mutant hemoglobin, which leads to a vicious cycle of hemolysis and vaso-occlusion. We survey important discoveries in SCD pathophysiology that have led to recent advances in treatment of SCD. The second part focuses on the hematopoietic stem cell (HSC) niche, the complex microenvironment within the bone marrow that controls HSC function and homeostasis. We detail the cells that constitute this niche, and the factors that these cells use to exert control over hematopoiesis. Here, we trace the scientific paths of Dr. Frenette, highlight key aspects of his research, and identify his most important scientific contributions in both fields.
Keywords: hematopoietic stem cell, niche, sickle cell disease
In this tribute article, Ito and colleagues provide a comprehensive summary of the main advances in the fields of sickle cell disease and hematopoietic stem cell biology while tracing the outstanding career of Dr. Paul S. Frenette. As a brilliant hematology-oncology researcher, Dr. Frenette has made valuable and unprecedent scientific contributions, many of which are highlighted in this review.
Sickle cell disease
Sickle cell disease (SCD), first described in 1910 (Herrick, 2014), is a frequently devastating monogenic disorder that causes chronic and acute pain, progressive multi-organ damage, and reduced life expectancy (Piel et al., 2017; Platt et al., 1994). The most common and severe form of SCD, sickle cell anemia, is the result of a homozygous mutation in the β-globin gene, and the consequent production of the abnormal tetramer hemoglobin S (α2βS2, HbS) (Bunn, 1997; Ingram, 1956, 1957; Pauling et al., 1949). Over 300,000 neonates are affected by SCD each year worldwide (Piel et al., 2013) and the number is expected to increase in the coming years (Piel et al., 2017). The pathophysiology of SCD is driven by hypoxia-induced polymerization of HbS in red blood cells (RBCs) leading to hemolysis, inflammation, and vaso-occlusion (Ballas et al., 2010; Steinberg, 2008; Sundd et al., 2019). In low-income countries, approximately 50% of individuals with SCD die in early childhood. In high-income countries, newborn screening programs and therapeutic interventions have increased the survival of pediatric SCD patients dramatically (Kato et al., 2018; Piel et al., 2017; Ware et al., 2017). However, life expectancy is still reduced by about 30 years compared with the average population, and quality of life is considerably reduced (Piel et al., 2017). In this section, we provide an overview of SCD pathophysiology and important discoveries, including those of Dr. Paul Frenette, that have led to the development of current and potential future therapies for management of this disease.
Pathophysiology of SCD
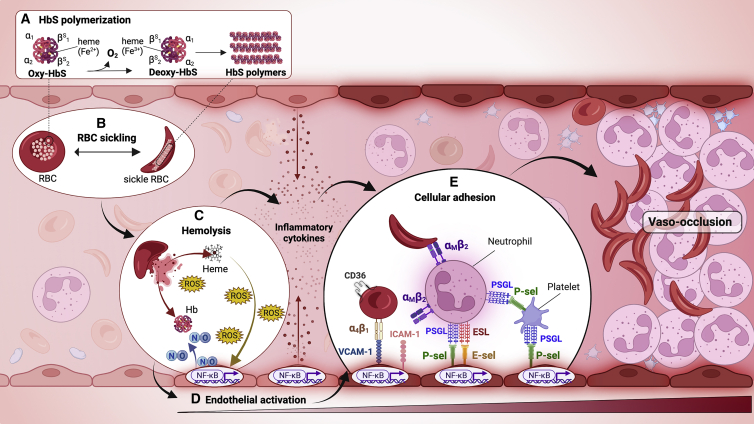
The pathophysiology of SCD is induced by hypoxia-induced polymerization of HbS in RBCs (Bunn, 1997; Kato et al., 2018; Rees et al., 2010; Sundd et al., 2019). HbS differs from normal adult hemoglobin (HbA) by a single amino acid substitution in the β-globin subunit tetramer (Ingram, 1957), caused by a missense mutation in the corresponding HBB gene. While the HbA tetramer (α2β2) is molecularly stable, HbS tetramers containing two mutated β-globin chains (α2βS2) have a high propensity to form polymers when deoxygenated (Figure 1A). The rate and extension of HbS polymerization, the main determinants of disease severity (Brittenham et al., 1985), depend mainly on the intracellular concentration of HbS, which is determined by numerous RBC properties, including volume, hydration, expression of fetal hemoglobin (HbF), and concomitant thalassemia mutations (Eaton and Bunn, 2017; Noguchi et al., 1988; Rees et al., 2010; Steinberg, 2005). Upon HbS polymerization, RBCs become stiff and sickle shaped (Figure 1B), leading to impaired rheology, oxidative stress, abnormal calcium homeostasis, dehydration, enhanced adhesive properties, vascular occlusion, and premature lysis (Bunn, 1997; Vekilov, 2007). In SCD, the RBC lifespan is approximately 10–20 days, with 120 days being normal (Ballas and Marcolina, 2006; de Ceulaer et al., 1983). Approximately 30% of SCD-related hemolysis is intravascular (Bensinger and Gillette, 1974; Hebbel, 2011), culminating in the daily release of up to 30 g of cell-free hemoglobin into the circulation (Figure 1C) (Bensinger and Gillette, 1974; Reiter et al., 2002). Non-compartmentalized oxygenated hemoglobin (Fe2+) degrades nitric oxide (NO), a key inhibitor of vascular contractility (Doherty et al., 1998; Reiter et al., 2002). This in turn generates reactive oxygen species (ROS) and oxidized (Fe3+) hemoglobin (methemoglobin), an unstable molecule that releases toxic cell-free heme, which perpetuates further oxidative stress and stimulates the innate immune system through toll-like receptor signaling, inflammasome formation, and the generation of neutrophil extra-cellular traps (Federico et al., 2022), causing deleterious sterile inflammation and endothelial damage (Figure 1D) (Kato et al., 2009, 2017; Repka and Hebbel, 1991). Endogenous inflammatory mediators that are released from damaged or dying cells, in this case sickled RBCs, are referred to as damage-associated molecular patterns (DAMPs) (Mendonca et al., 2016; Roh and Sohn, 2018).
Figure 1.
Vaso-occlusion in sickle cell disease
(A) During the passage of red blood cells (RBCs) from arterial to venous circulation, oxygen (O2) is released from oxygenated sickle hemoglobin (HbS), generating deoxygenated HbS (deoxy-HbS).
(B) The deoxy-HbS tetramer polymerizes within RBCs, creating a sickle shape and triggering a complex pathophysiology cascade.
(C) Membrane and cellular alterations in sickle RBCs cause their premature lysis, releasing cell-free hemoglobin (Hb) and heme that degrade nitric oxide (NO), generate reactive oxygen species (ROS), and promote inflammation, contributing to endothelial dysfunction and heterocellular adhesive interactions.
(D) Upregulation of nuclear factor κB (NF-κB) in activated endothelial cells induces the surface expression of adhesion molecules, including intercellular and vascular adhesion molecules-1 (ICAM-1 and VCAM-1, respectively) and endothelial E- and P-selectins. Activated endothelial cells also secrete inflammatory mediators that recruit and activate immune cells.
(E) SCD-associated inflammatory processes promote leukocyte activation, with neutrophil expression of αMβ2 (Mac-1) integrin and platelet activation and aggregation. Pathological cellular interactions that drive vaso-occlusion are mediated by various adhesion receptors and their ligands on activated blood and endothelial cells. Examples of adhesive interactions are shown for RBC-endothelium, sickle RBC-neutrophil, neutrophil-endothelium, platelet-neutrophil, and platelet-endothelium. ICAM-1, intercellular adhesion molecule-1; VCAM-1, vascular adhesion molecule-1; P-sel, P-selectin; E-sel, E-selectin; PSGL, P-selectin glycoprotein ligand; ESL, E-selectin ligand. Created with BioRender.com.
Early discoveries that sickle RBCs display impaired rheology and increased aggregation to endothelial cells (Hebbel et al., 1980; Hoover et al., 1979), and later neutrophils (Turhan et al., 2002), monocytes, and platelets (Wun et al., 2002), have further elucidated the complex process of microvascular occlusion in SCD, which causes ischemic tissue injury by interrupting blood supply. Subsequently, reperfusion with new oxygen delivery unleashes a second wave of inflammatory and oxidative damage. Repeated ischemia-reperfusion cycles induce progressive inflammation, endothelial dysfunction, and immune activation (Hebbel, 2014). This pathophysiology underlies SCD-associated pain crises, which are the leading cause of hospitalization (Ballas et al., 2012), and chronic organ damage that ultimately leads to premature mortality.
Vaso-occlusion in SCD is a multistep, multicellular process in which leukocytes, sickled RBCs, and platelets form sticky cellular aggregates that adhere to the microvasculature, obstructing blood flow (Frenette, 2002). Adhesive interactions driving vaso-occlusion are mediated by the induction of cell surface proteins and numerous inflammatory and pro-coagulant mediators. Augmented adherence of sickle RBCs to endothelial cells was demonstrated in vitro more than 40 years ago (Hebbel et al., 1980; Hoover et al., 1979). Subsequent studies of sickle RBCs (Gee and Platt, 1995; Joneckis et al., 1993; Setty et al., 2002; Swerlick et al., 1993) identified abnormal expression of cell surface adhesion molecules that bind to the endothelium, including intercellular adhesion molecule-4 (ICAM-4), Lutheran/basal cell adhesion molecule-1 (Lu/BCAM-1), and phosphatidylserine (PS). Integrin α4β1 and glycoprotein CD36 have also been identified, predominantly on SCD reticulocytes (Conran and Embury, 2021; Frenette and Atweh, 2007; Kaul et al., 2009).
The SCD endothelium is dysfunctional and abnormally activated (Figure 1D) (Hebbel et al., 2004), with increased expression of vascular adhesion molecule-1 (VCAM-1), ICAM-1, E-selectin, and P-selectin, which mediate adhesive interactions and activate innate immune cells (Figure 1E) (Conran and Embury, 2021; Frenette and Atweh, 2007; Kaul et al., 2009). Expression of P- and E-selectins on activated endothelial cells has been shown to mediate the recruitment and adhesion of leukocytes in vivo (Turhan et al., 2002) and adhesion to RBCs in vitro (Matsui et al., 2001). Activated endothelial cells upregulate the transcription factor nuclear factor B (NF-κB), which leads to the activation and recruitment of adherent leukocytes to venules (Belcher et al., 2006). The first in vivo evidence that leukocytes participate in SCD vaso-occlusion was demonstrated in a tumor necrosis factor alpha (TNFα)-stimulated SCD mouse model (Turhan et al., 2002). It was later shown that most leukocytes interacting with sickle RBCs and endothelial cells are neutrophils (Chiang et al., 2007). The capture of sickle RBCs by activated adherent neutrophils is dependent on their surface expression of αMβ2 integrin (Mac-1, or CD11b/CD18), which is induced by endothelial E- and P-selectins expressed by activated endothelial cells (Hidalgo et al., 2009). A prothrombotic state also occurs in SCD, where activated platelets generate RBC-platelet and leukocyte-platelet aggregates, mainly mediated by Mac-1 on the surface of neutrophils and P-selectin expressed by platelets (Hidalgo et al., 2009; Polanowska-Grabowska et al., 2010; Shet et al., 2020).
The chronic elevation of leukocytes, mainly activated neutrophils, is a hallmark of SCD that contributes to vaso-occlusion and has been associated with disease severity and increased risk of death (Platt, 2000; Platt et al., 1994). Circulating neutrophils are heterogeneous with respect to their activation state and pro-inflammatory potential in normal and pathological conditions, including SCD (Torres et al., 2021; Zhang et al., 2015). Neutrophil heterogeneity may result from their aging in circulation. In wild-type mice, the release of fresh neutrophils from the bone marrow and their clearance from the blood are rhythmically modulated during steady-state homeostasis (Casanova-Acebes et al., 2013). However, in SCD mice, the proportion of aged neutrophils in the blood, defined by low expression of L-selectin (CD62L) and high expression of CXCR4 (Casanova-Acebes et al., 2013; van Eeden et al., 1997), is increased (Zhang et al., 2015). Moreover, the aged population exhibits a highly pro-inflammatory phenotype, with elevated expression of Mac-1 and increased propensity to promote vaso-occlusion. Interestingly, neutrophil aging and vaso-occlusion were shown to be modulated by the gut microbiota, and the administration of broad-spectrum antibiotics protected SCD mice from aged neutrophil expansion, vaso-occlusion, and organ damage (Zhang et al., 2015).
Regulation of the immune system by the gut microbiota is crucial for host immunity, and perturbation of the microbiome can lead to immune pathologies (Postler and Ghosh, 2017). The gut microbiome in SCD has altered density, diversity, and composition (Brim et al., 2021; Lim et al., 2018; Stewart et al., 2021). The mechanisms involved in this dysbiosis, however, remain unclear. Intrinsic SCD pathologies, including vaso-occlusion injury to the gastrointestinal microvasculature, may cause increased gut permeability that alters the microbiome (Stewart et al., 2021). Environmental factors might also be involved, particularly the long-term use of prophylactic antibiotics that are administered to prevent potentially fatal bacterial sepsis in children with SCD (Brim et al., 2021; Stewart et al., 2021). Accumulating evidence suggests that psychological stress can affect the composition of the microbiota (Bailey et al., 2011; Mayer et al., 2014; Moloney et al., 2014). Psychological stress in SCD mice caused altered gut permeability and exacerbated TNFα-induced vaso-occlusion, inflammation, and aged neutrophil expansion. These processes were prevented by depletion of the microbiota (Xu et al., 2020).
Dr. Paul Frenette was a pioneer in elucidating the participation of neutrophils in SCD vaso-occlusion. His discovery that neutrophils interact directly with the endothelium through P- and E-selectin-mediated adhesion, and capture sickle RBC through αMβ2 integrin, placed neutrophils as a key component in the pathophysiology of SCD. These findings provided the basis for new therapeutic interventions targeting cellular interactions that drive vaso-occlusion, such as the development of a humanized P-selectin antibody, discussed later (Figure 2). The intimate connection between neutrophils and the gut microbiome, discovered by Dr. Frenette’s team, should lead to therapeutic improvements in the management of SCD.
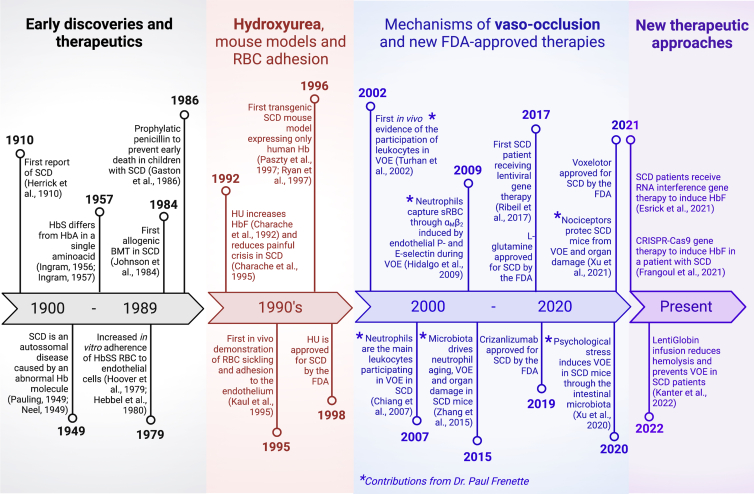
Figure 2.
Timeline of discovery and development of therapy for sickle cell disease
Major landmarks in the understanding of sickle cell disease (SCD) pathophysiology from its discovery to the current state of therapeutic development. SCD history has been arbitrarily divided into four distinct eras. Early discoveries and therapies (gray) cover the first 80 years from the first description of SCD. The 1990s frame (red) is marked mainly by studies in hydroxyurea (HU), the first drug approved by the US Food and Drug Administration (FDA) to prevent painful vaso-occlusive episodes (VOEs) in SCD. The elucidation of the mechanisms involved in VOE occurred in early 2000s (blue), when cells and molecules promoting adhesive interactions that lead to vaso-occlusion were discovered. Most relevant contributions from Dr. Paul Frenette to the field occurred in this era and are indicated by asterisks (∗). Recent studies and clinical trials (purple) may indicate the beginning of a new era of therapeutic advances, including gene therapy, mechanism-based drugs, and combination drug therapy. BM, bone marrow; BMT, bone marrow transplantation; RBC, red blood cells; sRBC, sickle red blood cells; VOE, vaso-occlusive episodes; HbSS, homozygous for hemoglobin sickle hemoglobin S; HbF, fetal hemoglobin. (Esrick et al., 2021; Gaston et al., 1986; Johnson et al., 1984; Larson et al., 2020; Neel, 1949; Paszty et al., 1997; Ribeil et al., 2017; Ryan et al., 1997). Created with BioRender.com.
Clinical complications of SCD
The spectrum of clinical complications in SCD is broad. Acute complications include recurrent vaso-occlusive pain crisis, stroke, splenic sequestration, and acute chest syndrome. Common chronic complications include persistent pain, avascular necrosis of bone, kidney disease, stroke, and pulmonary hypertension (Ballas et al., 2010; Kato et al., 2018; Steinberg, 2008). Acute vaso-occlusion is associated with unpredictable and excruciating episodes of pain, which lead to hospital admissions in more than 90% of cases (Ballas and Lusardi, 2005). Frequency of acute painful vaso-occlusive crisis is a measure of disease severity and correlates with early death (Platt et al., 1991). Damage resulting from acute pain episodes frequently leads to persistent pain and deterioration in the quality of life (Ballas et al., 2012).
Hyperalgesia has been demonstrated in a SCD mouse model and may be linked to systemic inflammation (Ballas et al., 2012; Kohli et al., 2010). In a mouse model of SCD, hypoxia-induced RBC sickling exacerbated hypersensitivity to mechanical and thermal stimuli, which was shown to be mediated through the transient receptor potential vanilloid 1 (TRPV1) (Hillery et al., 2011). TRPV1 has a pivotal role in transmitting noxious signals and can be activated by inflammatory stimuli, which are abundant in SCD. Surprisingly, although TRPV1 deficiency reduces hyperalgesia in SCD mice, a recent study from Dr. Frenette’s group showed that nociception-deficient SCD mice exhibited significant worsening in inflammation and vaso-occlusion parameters, and elevation of aged neutrophil numbers, suggesting that nociception protects SCD mice from vaso-occlusion (Xu et al., 2021). The authors demonstrated that the protective effects of nociception were mediated by the neuropeptide calcitonin gene-related peptide (CGRP), a neurotransmitter secreted by nociceptor neurons during the propagation of noxious signals. Administration of CGRP to SCD mice protected against vaso-occlusion and inflammation and inhibited long-term organ damage (Xu et al., 2021). These remarkable findings by Dr. Frenette’s team suggest that while SCD pain markedly worsens the patient’s quality of life, the underlying mechanism is somehow protective. Presumably, future therapeutic approaches will investigate the therapeutic potential of specific nociceptive mediators as a target for dampening SCD pathophysiology to inhibit end-organ damage.
Although pain is the major manifestation of SCD, acute chest syndrome is the leading cause of premature death (Ballas et al., 2012; Serjeant, 2013). This complication, defined as a new radiodensity on chest imaging, accompanied by fever and/or respiratory symptoms, frequently develops 2–3 days after the start of a vaso-occlusive crisis (Vichinsky et al., 2000). Lung lesions are accompanied by an exacerbated inflammatory response and pulmonary vasculature vaso-occlusion. In 30% of cases, acute chest syndrome is triggered by pulmonary infection, but other common causes include fat embolism from necrotic bone marrow and pulmonary infarction (Novelli and Gladwin, 2016). Management of acute chest syndrome includes antibiotics, supplemental oxygen, and RBC transfusion, aiming to reduce HbS polymerization, RBC sickling, and hemolysis by increasing tissue oxygen delivery (Novelli and Gladwin, 2016).
Phenotypic manifestations of SCD are highly variable and may be modulated by genetic and environmental factors. Fetal hemoglobin (HbF, α2γ2) expression is a major modifier of clinical severity of SCD and an important therapeutic target (Steinberg, 2020). Normally, expression of the γ-globin genes (HBG1 and HBG2), which encode a β-like hemoglobin subunit, is switched off postnatally, resulting in gradual replacement of HbF by HbA (or HbS in SCD) over the first year of life (Stamatoyannopoulos, 2005). Thus, individuals with SCD become symptomatic as pathological HbS replaces normal HbF. The levels of HbF in normal adults are usually less than 1%. However, HbF expression is commonly higher and more variable in SCD due to incompletely understood mechanisms including erythropoietic stress and genetic factors. The severity of SCD is reduced by high levels of HbF since they dilute HbS, do not incorporate into HbS polymers, and prevent HbS polymerization (Steinberg, 2009). Environmental modifiers of SCD severity include climate (e.g., temperature and humidity), air quality, altitude, socioeconomic features, and lifestyle (Tewari et al., 2015). Psychological health is also critical for disease development. Patients with SCD are at high risk for depression, due to the unpredictable impact of the disease on their personal and professional lives. Depression in SCD is associated with frequent acute vaso-occlusive pain, hospitalizations, and blood transfusions, and correlates with worse outcomes (Adam et al., 2017; Pecker and Darbari, 2019).
Therapeutic interventions for management and prevention of SCD complications
Hydroxyurea (or hydroxycarbamide), the first drug approved by the US Food and Drug Administration (FDA) for SCD (Figure 2) (Charache et al., 1992), promotes increased HbF production to inhibit HbS polymerization; reduces hemolysis; decreases leukocyte, platelet, and reticulocyte counts; reduces vascular cell adhesion; and enhances NO bioavailability to improve endothelial function (McGann and Ware, 2015). In turn, this reduces the incidence of vaso-occlusive crisis, hospitalizations, blood transfusion frequency, and mortality in patients with SCD (Charache et al., 1995). Hydroxyurea therapy reduces acute events, improves quality of life, and slows the accumulation of organ damage in SCD patients (Ballas et al., 2006; Rai and Ataga, 2020). Indeed, current management guidelines recommend that all children more than 9 months old with SCD be offered hydroxyurea therapy as a preventative measure. However, clinical responses to hydroxyurea vary between SCD patients, and its long-term benefits, including prevention of chronic complications, are not fully defined (Nevitt et al., 2017). The growing understanding of SCD pathophysiology and the regulation of globin gene transcription has stimulated the development of new therapies acting through a variety of mechanisms (Salinas Cisneros and Thein, 2021; Torres and Conran, 2019). Three drugs discussed below have been recently approved by the FDA for use in SCD (Figure 2).
Voxelotor (GBT440) is an allosteric effector of HbS that increases the affinity of HbS to oxygen (Metcalf et al., 2017). By maintaining HbS in the oxygenated state, voxelotor inhibits its polymerization (Metcalf et al., 2017) and prevents the sickling of RBCs in SCD patients (Oksenberg et al., 2016). Voxelotor also improves RBC deformability and reduces SCD blood viscosity (Dufu et al., 2018). In a phase-3 clinical trial, voxelotor reduced hemolysis and increased hemoglobin levels in SCD patients (Vichinsky et al., 2019). A theoretical concern is that pharmacological enhancement of HbS affinity for oxygen could impair its release in peripheral tissues, including the central nervous system (CNS) (Eaton and Bunn, 2017; Hebbel and Hedlund, 2018; Henry et al., 2021). Long-term post-marketing studies of voxelotor should resolve this issue. L-glutamine exerts an antioxidant activity to improve the redox potential of RBC, preventing oxidative damage (Niihara et al., 1998), RBC sickling, and RBC adherence to the endothelium (Niihara et al., 2005). Oral administration of L-glutamine to SCD patients has been shown to reduce the frequency of acute vaso-occlusive crisis, hospitalizations, and acute chest syndrome (Niihara et al., 2018). Crizanlizumab is a humanized monoclonal antibody that binds to P-selectin expressed on activated platelets and endothelial cells in SCD and blocks its interaction with P-selectin glycoprotein ligand-1 (PSGL-1) expressed on SCD RBC and leukocytes, thus preventing cellular adhesion that leads to vaso-occlusion (Ataga et al., 2017). Crizanlizumab has been shown to reduce the frequency of acute vaso-occlusive events in SCD (Ataga et al., 2017).
Although these different pharmacotherapeutic strategies are effective in preventing acute events in SCD, none are 100% effective, nor have they been proven to protect against chronic end-organ damage. Thus, in the future, medical therapy for SCD is likely to require a multi-drug approach that targets multiple aspects of disease pathophysiology. Improved new therapies may come from further studies of the relationships between microbiome, pain sensitization, and psychological health regulating inflammation, vaso-occlusion, and organ damage, and/or the identification of new, more effective HbF inducers.
Curative therapies for SCD and future perspectives
Currently, the standard curative therapy for SCD is allogenic hematopoietic stem cell transplantation (HSCT). The outcomes of this therapy are encouraging, with disease free survival occurring in more than 90% of patients who receive HSCT from an HLA-matched sibling donor (Bernaudin et al., 2007; Mahesri et al., 2021). However, these donors are available for less than 20% of SCD patients (Mentzer et al., 1994). Moreover, serious risks including severe graft-versus-host disease (GVHD), graft rejection, and transplantation-related death can occur in some patients (Bernaudin et al., 2007; Mentzer et al., 1994). Ongoing research seeks to reduce further the risks of treating SCD with allogeneic HSCT using HLA-matched siblings and alternative donors.
HSCT for SCD using genetically modified autologous HSC donor cells is a promising experimental approach that circumvents some toxicities of allogeneic HSCT (Doerfler et al., 2021; Drysdale et al., 2021; Rosanwo and Bauer, 2021). One approach for HSC modification is transduction with a lentiviral vector encoding a modified β-globin gene with anti-sickling properties (LentiGlobin), which results in the production of HbA (Figure 2). In a phase 1–2 study, patients who received a single LentiGlobin infusion experienced a sustained increase of total hemoglobin, reduction of HbS levels, reduction of hemolysis markers, and complete resolution of severe vaso-occlusive events (Kanter et al., 2022). Another approach involves genetic reactivation of the γ-globin gene expression to induce the production of HbF. A patient infused with autologous CRISPR-Cas9-edited HSCs (genetically modified to restore the production of HbF), showed high levels of HbF, resolution of vaso-occlusive episodes, and transfusion independence (Frangoul et al., 2021). Other approaches under study include genetic correction of the SCD mutation with Cas9-mediated homology directed repair (Pattabhi et al., 2019), and the use of base editor proteins that convert the SCD mutation to a benign variant (Newby et al., 2021) or induce HbF (Li et al., 2021; Zeng et al., 2020). Such therapies are highly promising, but their long-term effects are as yet unknown. One emerging concern is that SCD itself may predispose to the development of premalignant somatic mutations that increase risk for developing myelodysplastic syndrome or leukemia following autologous HSCT (Jones and DeBaun, 2021).
With the continued advance in gene therapy and bone marrow transplantation, an important aspect of SCD to be considered is its potential impact on the bone marrow homeostasis. The bone marrow niche in SCD mice is highly disturbed due to hemolysis, hypoxia, and vaso-occlusion in the bone marrow vasculature (Park et al., 2020). Tang et al. (2021) demonstrated that cell-free heme contributed to a highly oxidative microenvironment in the bone marrow of SCD mice, triggering mesenchymal stem cell (MSC) defects and oxidative stress in hematopoietic stem cells (HSCs). These alterations in the bone marrow niche are expected to reduce HSC homing and engraftment efficiency in SCD patients, impairing the success of current and future curative strategies. Fortunately, such abnormalities may be manageable. Park et al. (2020), for example, showed that bone marrow vascular disorganization observed in SCD mice can be alleviated by RBC transfusion. Overall, these findings support the need for a deeper understanding of the effects of SCD on the bone marrow microenvironment.
The concept of bone marrow niche
Living organisms cannot survive in complete isolation but depend on their surrounding environment to provide them with critical input needed to carry out their activities. The same is true for the cells that make up our bodies, which are not able to function normally without support from the surrounding environment, and in fact their function can be directly controlled by microenvironmental or long-range cues. Blood cells such as white blood cells, RBCs, and platelets control immune response and tissue repair and are essential for maintaining vital activities. The bone marrow produces blood cells throughout life, and, under steady-state conditions, generates 10 billion RBCs and up to 1 billion white blood cells per hour. This constantly replaces short-lived blood cells and thus maintains homeostasis. The hematopoietic system has an efficient and rational hierarchical structure, and the HSC, ancestor of all blood cells, reigns at the top of the hierarchy. HSCs reside in a special microenvironment in the bone marrow called the HSC niche (or, more simply, the niche) and maintain hematopoietic homeostasis through their specific capacities of self-renewal, differentiation, and proliferation. The concept of the niche was first proposed by Dr. Schofield in 1978 (Schofield, 1978). Recent studies have revealed that various types of hematopoietic and non-hematopoietic cells in the bone marrow function as niche cells and regulate HSC functions through complex mechanisms (Figure 3) (Gao et al., 2018). We will discuss the latest findings on the roles of the bone marrow microenvironment in niche cell populations and review the elaborate mechanisms that operate within the immediate neighborhood of HSCs that regulate their state and activities.
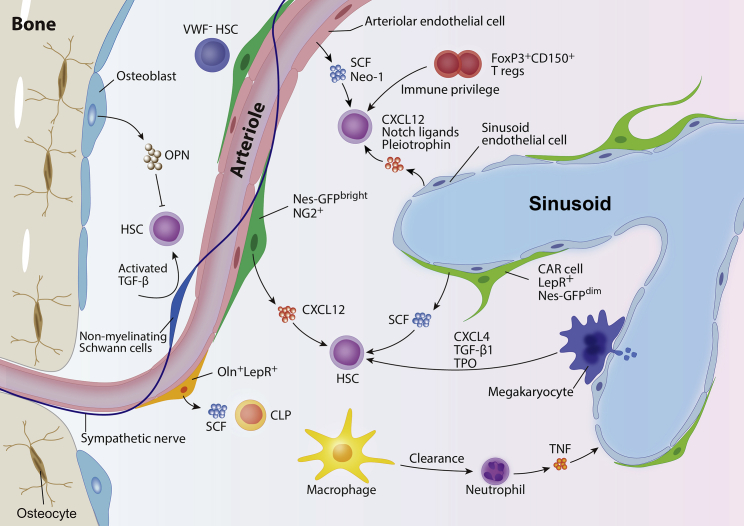
Figure 3.
Niche players for HSC homeostasis
Various cell types contribute to HSC regulation via direct or indirect mechanisms in the bone marrow. Osteoblasts negatively regulate the HSC pool by secreting osteopontin. Nes-GFPbrightNG2+ peri-arteriolar cells and LepR+ stromal cells reside in the perivascular area and differentially regulate HSCs. Non-myelinating Schwann cells that are closely associated with arterioles maintain HSC quiescence through activating TGF-β. Lymphoid-biased von Willebrand factor (vWF)-GFP− HSCs are enriched in the vicinity of, and selectively regulated by, Ng2+ peri-arteriolar cells. Oln+LepR+ stromal cells, found mainly around arteries, may regulate CLPs. SCF or Neo-1 secreted from arteriolar endothelial cells contributes to HSC maintenance. Sinusoidal endothelial cells regulate HSCs through CXCL12, Notch ligands, and pleiotrophin. Various progenies of HSCs also participate in the orchestration of HSC function. Clearance of aged neutrophils by macrophages alters CAR cell function to retain HSCs in the bone marrow. Neutrophils enhance endothelial regeneration after transplantation through TNF secretion, leading to accelerated hematopoietic recovery. FoxP3+CD150+ Tregs provide immune privilege to HSCs. Megakaryocytes maintained quiescence of HSCs through secreting CXCL4, TGF-β, and TPO. HSC, hematopoietic stem cell; OPN, osteopontin; TGF-β, transforming growth factor-β; SCF, stem cell factor; Neo-1, neogenin-1; CXCL12; C-X-C motif chemokine ligand 12; Nes-GFP, nestin-GFP; NG2, nerve/glial antigen 2; Oln, osteolectin; LepR, leptin receptor; CLP, common lymphoid progenitor; CXCL4, C-X-C motif chemokine ligand 4; TPO, thrombopoietin; CAR cell, CXCL12-abundant reticular cell; TNF, tumor necrosis factor.
Niche players in the bone marrow
Osteolineage cells
At the dawn of research into the HSC niche, osteoblasts, which produce bone at the interface between bone marrow and bone tissue, attracted attention as niche cells. Bone is the closest tissue to the bone marrow, which is literally the marrow of the bone. Osteoblastic cells exhibit the ability to maintain hematopoietic progenitors in vitro, and when in vitro-labeled hematopoietic progenitor cells were infused intravenously into mice, they were abundantly distributed around the endosteum in the bone marrow (Nilsson et al., 2001), supporting the existence of niche cells in the endosteum. In 2003, two independent groups simultaneously reported that expansion of osteolineage cells in vivo by genetic modifications led to a substantial increase of HSCs, suggesting that bone forming mesenchymal cells regulate the size of the HSC pool and are therefore niche components (Calvi et al., 2003; Zhang et al., 2003). However, the role of mature osteoblasts for HSC niche function remains highly debated. In mice with osteoblast-specific reduction of C-X-C motif chemokine ligand 12 (CXCL12) or stem cell factor (SCF), both of which are confirmed niche factors essential for HSC maintenance, little change in the number of HSCs in the bone marrow was found, suggesting that osteoblasts do not contribute to HSC maintenance, at least through these niche factors (Greenbaum et al., 2013). However, osteoblasts may regulate HSCs in the setting of bone marrow transplant conditioning where imaging studies have demonstrated close physical proximity (Lo Celso et al., 2009; Silberstein et al., 2016; Xie et al., 2009) or through indirect effect of secreted factors such as osteopontin that negatively regulates the HSC pool in both adult and fetal bone marrow (Cao et al., 2019; Nilsson et al., 2005). Tie2, encoded by the Tek gene, is a receptor tyrosine kinase expressed on vascular endothelial cells and a small fraction of HSCs, and Tie2-expressing HSCs possess the ability to maintain long-term hematopoiesis over the long term (Arai et al., 2004; Ito et al., 2016). Osteoblasts express angiopoietin-1 (Ang-1), a ligand for Tie2, and augmentation of Ang-1 signals both in vitro and in vivo increased HSCs in the G0 cell cycle phase. These results support osteoblasts as cells capable of regulating HSC quiescence via the Tie2/Ang-1 pathway (Arai et al., 2004); however, another study deleted Angpt1 in osteoblasts and noted little effect on HSC (Zhou et al., 2015). An earlier study focused on the importance of N-cadherin (N-cad), an adhesion molecule on the surface of osteoblasts, in hematopoietic homeostasis (Zhang et al., 2003), while subsequent studies have suggested that the phenotypic HSCs defined by specific markers, including the signaling lymphocytic activation molecule (SLAM) family, are not affected by the removal of N-cad molecule in osteoblasts (Bromberg et al., 2012; Greenbaum et al., 2013; Kiel et al., 2007). A recent study identified that N-cad+ bone and marrow stromal progenitor cells (BMSPCs) in the endosteum serve as a niche functionally supporting HSCs. In this study, the authors showed that a subpopulation of long-term HSCs marked by CD49b−CD48−CD34−CD135− LSK (Lineage−Sca-1+c-Kit+) are resistant to chemotherapy. These long-term HSCs predominantly localize adjacent to the N-cad+ stromal cells, which display MSC characteristics. Conditional deletion of SCF from N-cad+ stromal cells resulted in a decrease in HSCs both at a steady state and under chemotherapy stress (Zhao et al., 2019). Further examination will be needed to better understand osteoblasts as a niche constituent and their roles in proper HSC maintenance. This is made complex by the fact that many studies have used Cre recombinase driven constitutively by promoters that are active at a time within the development program of osteolineage cells. All descendant cells will therefore bear the genetic modification making it difficult to know when the modified gene was playing a role in HSC regulation.
Vascular endothelial cells
HSCs have a close relationship with vascular endothelial cells during development. HSCs arise from cells with the characteristics of vascular endothelial cells in the hematogenic endothelium of the dorsal aorta in the aorta-gonad-mesonephros (AGM) region during fetal life and establish definitive hematopoiesis in the bone marrow after initial hematopoiesis in the fetal liver (Medvinsky and Dzierzak, 1996; Muller et al., 1994). Early in vitro studies have shown that vascular endothelial cells isolated from bone marrow provide long-term support for human cord blood-derived hematopoietic progenitor cells or peripheral blood mononuclear cells (Rafii et al., 1995). A decade later, Kiel et al. (2005) showed that HSCs can be identified with high probability by combining a simple set of antibodies, LSK CD48−CD41−CD150+. Endogenous HSCs stained with these markers reside near the vascular endothelium in the bone marrow (Kiel et al., 2005), bringing the perivascular area into the limelight as a niche for HSCs. Concurrently, it was shown that intravascularly injected hematopoietic stem and progenitor cell (HSPC) traffic to CXCL12-expressing endothelial regions lodges perivascularly and proliferates, demonstrating a functional niche (Sipkins et al., 2005). However, it should be noted that the bone marrow is richly endowed with blood vessels and, by sheer density of the vascularity, no cell can be more than 25 μm from blood vessels (mean diameter of HSPC ∼15 μm). Therefore, vascular and perivascular cells provide key elements of the hematopoietic microenvironment. Specifically regarding endothelium, it was shown in vivo that recovery of vascular endothelial cells is essential for hematopoietic regeneration after myelosuppression (Hooper et al., 2009), and detailed mechanisms of HSC regulation by vascular endothelial cells have since been reported (Rafii et al., 2016). Further, human umbilical vein endothelial cells with enforced activation of the Akt signaling pathway support the proliferation of hematopoietic progenitor cells in vitro, and an increased number of HSCs in bone marrow was observed in mice with the vascular endothelial cell-specific enhancement of Akt signaling (Kobayashi et al., 2010). A subsequent study provided in vivo evidence that vascular endothelial cells stimulate Notch signaling in HSPCs through Jagged1 (Jag1), one of the angiocrine factors, and regulate the maintenance of the HSC pool and quiescence in steady state, as well as lineage-specific differentiation of HSPCs during hematopoietic recovery from myelosuppression (Poulos et al., 2013). Pleiotrophin (PTN), a heparin-binding growth factor that promotes HSC expansion in vitro and HSC regeneration in vivo (Himburg et al., 2010, 2012), was derived from vascular endothelial cells facilitating HSC regeneration following irradiation (Himburg et al., 2018). Furthermore, genetically modified mice in which CXCL12 or SCF was specifically removed from vascular endothelial cells in vivo showed reduced HSCs (Ding and Morrison, 2013; Ding et al., 2012; Greenbaum et al., 2013). The results of these studies indicate that vascular endothelial cells play an essential role in HSC regulation, but these studies did not analyze vascular endothelial cells separately by vessel subtype (arterial and sinusoid). More recent studies have shown that vascular endothelial cells in the bone marrow are not a uniform population. For example, Sipkins et al. (2005) showed that some endothelial cells expressed higher levels of CXCL12 and E-selectin. Further, Kusumbe et al. (2014) demonstrated that vascular endothelial cells in the bone marrow can be divided into type H endothelial cells, which are abundantly distributed near the growth plates and highly express both CD31 and endomucin, and type L endothelial cells, which are widely distributed in sinusoids and weakly positive for both CD31 and endomucin. Type H blood vessels highly express the niche factor SCF, and, in mice with increased type H blood vessels due to enhanced Notch signaling, the number of HSCs increased in accordance with the elevation of SCF (Kusumbe et al., 2016). Xu et al. (2018) analyzed vascular endothelial cells in bone marrow in more detail and demonstrated that these cells can be prospectively divided into arterial endothelial cells (AECs) and sinusoid endothelial cells (SECs) according to the expression patterns of Sca-1 and podoplanin (PDPN). Intriguingly, AECs (Sca-1bright, PDPN–) express higher level of canonical niche factors, such as SCF, CXCL12, and Jagged1, than SECs (Sca-1dim, PDPN+) in the bone marrow. Indeed, specific depletion of SCF in AECs targeted by Bmx-CreERT2, but not SECs, led to a decrease of HSCs both in the steady state and during hematopoietic recovery after myelosuppression (Xu et al., 2018). AECs also secrete neogenin-1, which has been identified as a regulator of axon guidance. Neogenin-1 has a variety of functions in cell survival and angiogenesis and activates the NF-κB pathway in HSCs via netrin-1, a receptor expressed on HSCs, to regulate HSC quiescence, differentiation, and maintenance (Renders et al., 2021). A recent study has further shown that a subset of endothelial cells with low-affinity nerve growth factor receptor (CD271) expression in human bone marrow exhibits characteristics of both vascular endothelial cells and mesenchymal cells. These cells differentiate into mesenchymal cells, the major niche cells in bone marrow, by endothelial-mesenchymal transition (Kenswil et al., 2021).
Perivascular stromal cells
Perivascular stromal cells, along with vascular endothelial cells, have attracted attention as niche cells. These cells include CXCL12-abundant reticular (CAR) cells, which express high levels of CXCL12 and represent a reticular morphology (Sugiyama et al., 2006), leptin receptor (LepR)-expressing stromal cells (Ding and Morrison, 2013; Ding et al., 2012), CD51+CD140a+ mesenchymal stromal progenitors (Pinho et al., 2013), and stromal cells labeled by green fluorescent protein (GFP) expressed downstream of the nestin promoter (Nes-GFP+) (Mendez-Ferrer et al., 2010). All these cell types exhibit common characteristics: they locate in perivascular spaces, have MSC characteristics, and secrete high levels of niche factors. CXCL12 (also known as stromal cell-derived factor-1 [SDF-1]), one of the most well-recognized niche factors, was identified as a growth factor for B cell progenitors (Nagasawa et al., 1994). Subsequent studies revealed that CXCL12 interacts with its cognate receptor CXC chemokine receptor 4 (CXCR4) on HSCs to maintain the HSC pool or anchor HSCs in the bone marrow. CXCL12-GFP knockin mice have been established to visualize the endogenous gene expression of CXCL12. In these mice, GFP-positive cells were mainly distributed around sinusoid in the bone marrow as cells with long protrusions that formed a tangled network and were named CAR cells (Sugiyama et al., 2006). CAR cells express high levels of SCF and are adipo-osteogenic progenitors with the specific transcriptional factors forkhead box C1 (FOXC1) and early B cell factor 3 (EBF3) (Omatsu et al., 2014; Seike et al., 2018). The depletion of CAR cells in vivo results in a decrease of HSCs in the bone marrow (Omatsu et al., 2010, 2014). The human counterpart of CAR cells was also identified by EBF3 staining in a human bone marrow biopsy sample (Aoki et al., 2021). A study using Scfgfp knockin mice with a knockin of Egfp (GFP) into the endogenous Scf locus demonstrated that stroma cells expressing LepR were the main source of SCF in the bone marrow (Ding et al., 2012). LepR-Cre+ stromal cells express high levels of both SCF and CXCL12, and in fact the majority of these cells overlap with CAR cells (Asada et al., 2017; Zhou et al., 2014). Deletion of Scf from LepR-Cre+ cells markedly reduced HSCs in the bone marrow, whereas Cxcl12 deletion had little impact on HSC number in the bone marrow and rather resulted in HSC mobilization to the peripheral blood and spleen (Asada et al., 2017; Ding and Morrison, 2013). Much like CAR cells, LepR-Cre+ cells are an important source of adipocytes and all osteolineage cells (Zhou et al., 2014). They are the most abundant non-hematopoietic cells in the bone marrow (Baryawno et al., 2019). They contribute to most osteoblastic cells in the bone in either normal development or after injury (Zhou et al., 2014). The majority of LepR+ stromal cells are around sinusoids in the bone marrow; however, some LepR+ cells reside in peri-arteriolar locations, and these cells have been shown to express osteolectin (Oln), an osteogenic growth factor (Shen et al., 2021). Peri-arteriolar LepR+Oln+ cells are osteoblast progenitors and express CXCL12 and SCF comparable with other LepR+ stromal cells. However, deletion of Scf from peri-arteriolar Oln-iCreER targeted cells led to a decrease in common lymphoid progenitors (CLPs), but no change in the number of HSCs in the bone marrow. Similar to prior studies showing that mature osteoblastic cell depletion affects CLPs but not HSCs (Ding and Morrison, 2013; Greenbaum et al., 2013; Yu et al., 2015), the Oln+ cells contribute to a lymphoid niche in the bone marrow (Shen et al., 2021). These data do not exclude the possibility of other cell types (e.g., cells at early stages along osteogenesis) in endosteal regions supporting HSCs. Indeed, N-cad+ bone-lining cells, giving rise to osteoblasts, adipocytes, and chondrocytes, maintain long-term HSCs during homeostasis and regeneration (Zhao et al., 2019).
HSCs are constantly moving in and out of the bone marrow and peripheral blood in a steady state, and adrenergic signaling through the β3-adrenergic receptor downregulates CXCL12 levels in the bone marrow and facilitates HSC release from the bone marrow (Mendez-Ferrer et al., 2008). This was the first time that neural contributions to a stem cell niche had been defined, and, in so doing, Dr. Frenette expanded the concept of a niche to include systemic inputs. In a subsequent study, Mendez-Ferrer et al. (2010) showed that stromal cells with Gfp expression under the control of nestin promotor (Nes-GFP+) have MSC potential and modulate HSC release from bone marrow through β3-adrenergic signals. Nes-GFP+ cells in the bone marrow are classified according to the intensity of GFP expression into Nes-GFPbright cells, which are distributed around arterioles and strongly express GFP, and Nes-GFPdim cells, which are distributed mainly around sinusoids and show low GFP expression (Kunisaki et al., 2013). Dr. Frenette and his group developed bone marrow three-dimensional (3D) image analysis using mouse whole-mount sternum to evaluate the relationships between and within bone marrow structures. Using these imaging systems, endogenous HSCs were reported to be predominantly distributed in the vicinity of Nes-GFPbright cells around arterioles, while regenerative HSCs during the recovery process after fluorouracil (5-FU) administration were further from Nes-GFPbright cells. Nes-GFPbright cells express the pericyte marker nerve/glial antigen 2 (Ng2), and the deletion of cells targeted by NG2-CreERTM in vivo resulted in a reduction of HSCs, loss of quiescence, and relocation of HSCs further away from arterioles, suggesting that Nes-GFPbrightNg2+ peri-arteriolar stromal cells function as an HSC niche (Kunisaki et al., 2013). Others have shown that Tie2-expressing HSCs are preferentially distributed around arterioles (Asada et al., 2017; Ito et al., 2016; Kunisaki et al., 2013). In contrast, studies analyzing the distribution of HSCs defined by Ctnna1-GFP expression and c-kit immunostaining in optically cleared bone marrow reported that most of these HSCs are in contact with the very abundant LepR+ cells (Acar et al., 2015). Another study used homeobox B5 (Hoxb5)-GFP mice to label LT-HSCs and demonstrated that >94% of Hoxb5-GFP+ HSCs are in contact with VE-cadherin+ vascular endothelial cells in optically cleared mice tibia, although the roles of arterioles and sinusoids were not separately assessed (Chen et al., 2016). Another study using optically cleared bone marrow sections explored the special relationship between various niche cells and Ctnna1-GFP+ HSCs, Mds1-GFP+ HSCs, or label-retaining HSCs. Interestingly, the authors failed to see an enrichment of HSCs in any particular location and concluded that the distribution of HSCs is random (Kokkaliaris et al., 2020). Indeed, prior studies on Lpr+ cells as niche cells have also shown that HSCs are distributed equivalent to random dots (Kiel et al., 2005). These varying results have yet to be reconciled, but the concept that multiple non-hematopoietic cell types contribute to HSC regulation is undisputed and it may occur without requiring direct cell-cell interaction.
While both types of Nes-GFP-positive cells express niche factors, most Nes-GFPdimLepR+ cells overlap with CAR cells and LepR+ cells, and exhibit higher levels of expression of Cxcl12 and Scf than Nes-GFPbrightNg2+ peri-arteriolar cells (Baccin et al., 2020; Baryawno et al., 2019; Kunisaki et al., 2013). However, when Cxcl12 was deleted from peri-arteriolar Nes-GFPbright cells using NG2-CreERTM or Myh11-CreERT2, these conditional knockout mice exhibited a reduced number of HSCs in the bone marrow (Asada et al., 2017). The conditional deletion of Cxcl12 in Nes-GFPdimLepR+ cells had little impact on HSC numbers in the bone marrow, while mobilization of HSCs to the peripheral blood and spleen was enhanced (Asada et al., 2017; Ding and Morrison, 2013). Imaging of endogenous HSCs in bone marrow revealed that Cxcl12 deletion in peri-arteriolar Nes-GFPbright cells, but not from Nes-GFPdimLepR+ cells, identified HSCs far from arterioles (Asada et al., 2017). In contrast, deletion of Scf in Nes-GFPbright cells had no effect on HSC numbers, but mice with Scf removal from Nes-GFPdimLepR+ cells showed a marked decrease in the number of HSCs (Asada et al., 2017; Ding et al., 2012). These results support that identical niche factors from distinct niche cells may have different functions in HSCs. A similar phenomenon has been reported for other niche factors. For instance, pleiotrophin (PTN) is an important niche factor, secreted by both perivascular LepR+ cells and vascular endothelial cells. PTN from vascular endothelial cells facilitates the regeneration of HSCs after irradiation, while PTN from LepR+ cells is indispensable for HSC maintenance (Himburg et al., 2010, 2018).
It remains a matter of debate whether cellular components of the bone marrow microenvironment (e.g., peri-arteriolar, peri-sinusoidal, or endosteal areas) provide distinctive functional HSC niches (Comazzetto et al., 2019; Pinho and Frenette, 2019). Vascular-forming endothelial cells compose the bone marrow vascular network, and blood vessel sub-types may be associated with specific micro-anatomical regions and distinct properties to regulate HSC maintenance and cellular trafficking. Indeed, direct measurements of pO2 in the bone marrow of live animals has revealed a unique hypoxic landscape (Spencer et al., 2014) in which quiescent HSCs with low levels of ROS were localized around arterioles, while HSCs with high ROS levels were distributed among the sinusoids (Itkin et al., 2016). Lymphoid-biased von Willebrand factor (vWF)-GFP− HSCs are selectively regulated by Ng2+ peri-arteriolar cells and enriched in their vicinity, while myeloid-biased vWF-GFP+ HSCs (Sanjuan-Pla et al., 2013) are located adjacent to megakaryocytes, which regulate their reconstitution potential (Pinho et al., 2018).
Megakaryocytes
One of the descendants of HSCs, the megakaryocytes (MKs), are primarily responsible for platelet production, and play indispensable roles in HSC regulation. In vitro co-culture with MKs has led to slight increases in the repopulation potential of HSCs (Heazlewood et al., 2013). Several groups independently reported that ∼20% of phenotypic HSCs in the bone marrow are in contact with MKs, which maintain HSC quiescence through secretion of CXCL4 (also known as platelet factor 4), transforming growth factor β1 (TGF-β1), and thrombopoietin (TPO) in steady-state conditions (Bruns et al., 2014; Nakamura-Ishizu et al., 2014, 2015, 2020; Zhao et al., 2014). Interestingly, in contrast to steady state, MKs accelerate the recovery of HSCs after chemotherapeutic stress by producing fibroblast growth factor 1 (Zhao et al., 2014). MKs also increase the number of osteoblasts by secreting platelet-derived growth factor-BB during hematopoietic recovery to support HSC engraftment (Olson et al., 2013). Recent studies from Dr. Frenette’s laboratory separately analyzed two distinct sub-types of HSCs based on their vWF expression (vWF-GFP+ and vWF-GFP− HSCs) and showed that MKs specifically confer on vWF-GFP+ HSCs a differentiation potential toward a myeloid-biased output (Pinho et al., 2018).
Nervous system
Anatomical studies using laboratory animals have reported that autonomic nerves and sensory nerves are predominantly distributed in the bone marrow. Sympathetic nerves enter the bone marrow along the nutrient arteries, and most of them wrap around the arteries and travel, but some leave the arteries and stretch their nerve endings to the bone marrow parenchyma (Mach et al., 2002). The roles of sympathetic nerves distributed in the bone marrow were largely unknown until 2002 when Takeda et al. reported that sympathetic nerve stimulation inhibits osteoblast function via β-adrenergic receptors (ARs) on osteoblasts, which are responsible for osteogenesis on the endosteum (Takeda et al., 2002). HSCs reside in niches in the bone marrow, but administration of granulocyte colony-stimulating factor (G-CSF) mobilizes them into the blood circulation. Although the mechanism of G-CSF-induced HSC mobilization has not been fully elucidated, Katayama et al. (2006) demonstrated that mice lacking galactocerebroside, a major glycolipid component of the neuromyelin sheath, have impaired osteoblast function and lack G-CSF-induced HSC mobilization. Osteoblasts were functionally suppressed by G-CSF administration. In mice lacking catecholamine signaling due to blocked adrenergic neurotransmission, osteoblasts were not suppressed by G-CSF administration, while HSC mobilization was impaired. These results suggest the indispensable roles of the sympathetic nervous system (SNS) in HSC niche regulation (Katayama et al., 2006) (Figure 4). Adrenergic signals triggered by G-CSF also suppress osteocytes, terminally differentiated osteolineage cells, through β2-AR, and serve as another suppressor for the HSC niche and its mobilization (Asada et al., 2013). Sympathetic nerves express G-CSF receptors, and Lucas et al. (2012) have shown that direct stimulation by G-CSF enhances local sympathetic signaling by reducing the efficiency of norepinephrine reuptake at nerve endings. Administration of desipramine, a norepinephrine reuptake inhibitor, enhanced the efficiency of G-CSF-induced mobilization.
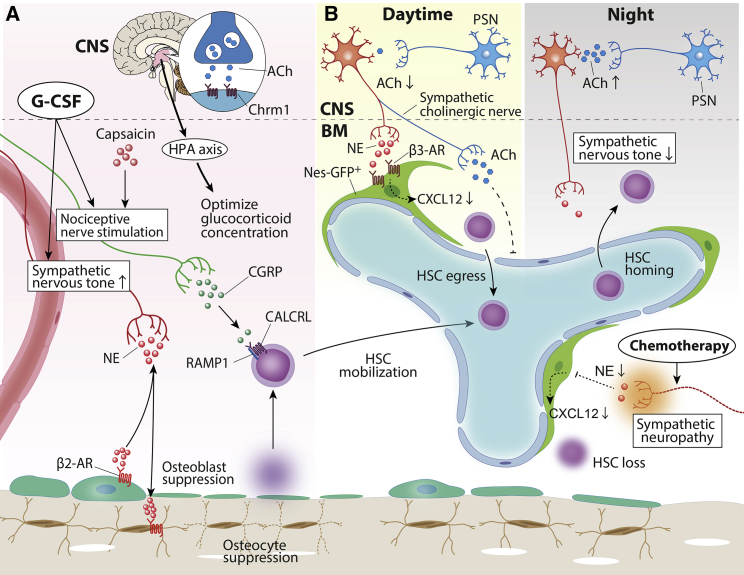
Figure 4.
Neural regulation of HSC and their niche
(A and B) Increased sympathetic nervous tone induced by granulocyte colony-stimulating factor (G-CSF) treatment suppressed osteolineage cells via β2-ARs, leading to HSC mobilization into the circulation. CGRP released from nociceptive nerves drove G-CSF-induced HSC mobilization. Cholinergic signals mediated by the ACh-Chrm1 axis in the CNS is required to optimize glucocorticoid levels in the bone marrow for optimal HSC mobilization (A). Diurnal alterations in bone marrow microenvironment controlled by the autonomic nervous system promote the circadian fluctuation of HSC mobilization. In the daytime, sympathetic adrenergic/cholinergic signals promote HSC egress by the reduction of C-X-C motif chemokine ligand 12 (CXCL12) expression from Nes-GFP+ bone marrow niche cells (red) and the suppression of adhesion molecules on endothelial cells (blue) (B, left). At night, parasympathetic cholinergic signals in CNS diminish sympathetic noradrenergic tone and decrease HSC egress into the blood (B, right). Anti-cancer drugs cause sympathetic neuropathy, leading to niche cell dysfunction and delayed hematopoietic recovery. HSC, hematopoietic stem cell; ACh, acetylcholine; Chrm1, cholinergic receptor muscarinic 1; HPA, hypothalamus-pituitary-adrenal; CGRP, calcitonin gene-related peptide; NE, norepinephrine; CALCRL, calcitonin receptor-like; RAMP1, receptor activity modifying protein 1; β2-AR, beta 2 adrenergic receptor; PSN, parasympathetic neuron.
SNS signals modulate HSC niche function in steady state as well as under stress conditions. Non-myelinating Schwann cells are the glial cells of the sympathetic nerves, which travel along arteries in the bone marrow, and maintain HSC quiescence by activating latent TGF-β and SMAD signaling (Yamazaki et al., 2011). In this study, surgical denervation of sympathetic nerves led to reduced HSC numbers along with a decrease in non-myelinating Schwann cells. However, the resulting outcomes were dependent on the method of sympathetic denervation. Systemic chemical denervation of sympathetic nerves by treatment with 6-hydroxydopamine had no effect on HSCs in bone marrow (Lucas et al., 2013; Mendez-Ferrer et al., 2008), while local surgical sympathectomy showed a decrease in functional HSCs only in the denervated bone marrow (Maryanovich et al., 2018). Chemotherapeutic agents are still the mainstay of anti-cancer treatment. It is well known that repeated anti-cancer drug therapy delays the recovery of hematopoiesis. Neurotoxic drugs, cisplatin and/or vincristine, cause sympathetic neuropathy in the bone marrow and sensory neuropathy. Loss of niche protection by SNS signaling leads to exit from quiescence by Nes-GFP+ HSC niche cells, resulting in increased susceptibility to chemotherapy and delayed hematopoietic recovery (Lucas et al., 2013). These niche impairments are alleviated by the administration of either the neuroprotective agent 4-methylcatechol or glial-derived neurotrophic factor prior to chemotherapy, which potentially promotes hematopoietic recovery after chemotherapy (Lucas et al., 2013).
As in homeostatic tissue regulation, the function of the autonomic nervous system in tumor formation, growth, progression, and metastasis mechanisms in various malignant tumors has recently received much attention (Zahalka and Frenette, 2020). Hanoun et al. (2014) reported that, in mice with transduced MLL-AF9, aggressive acute myeloid leukemia (Wei et al., 2020) cells, abnormal expansion of functionally impaired Nes-GFPdim stromal cells, and reduction of Nes-GFPbrightNG2+ peri-arteriolar niche cells were observed along with a decrease in healthy HSCs in the bone marrow. These alterations in niche components induced by AML were mediated by the disruption of SNS signaling, and treatment with β2-AR agonist led to both a reduction in leukemic stem cells in the bone marrow and prolonged survival of leukemic mice (Hanoun et al., 2014). In a mouse model of myeloproliferative neoplasm caused by mutations in Janus kinase 2, interleukin (IL)-1β secreted by abnormal HSCs caused damage to sympathetic nerves and Schwann cells in the bone marrow, resulting in a reduction of healthy HSCs due to impaired function of Nes-GFP+ stromal cells. The administration of β3-AR agonist prevented tumor progression by restoring Nes-GFP+ stromal cells and reducing LSCs (Arranz et al., 2014).
The role of the parasympathetic nervous system (PNS) in the regulation of hematopoiesis is less understood than the role of the SNS. Cholinergic signals via hypothalamic muscarinic receptors induces corticosterone production in the adrenal gland via the hypothalamic-pituitary-adrenal axis toward an optimal level for maintenance of HSC migration potential during HSC mobilization induced by G-CSF (Pierce et al., 2017). Garcia-Garcia et al. (2019) explored the function of the PNS in the mechanism of HSC transit between peripheral blood and bone marrow. SNS signals guide HSCs to the bone marrow at night by upregulating adhesion molecules on bone marrow vascular endothelial cells via β2-AR, and, during the day, promote HSC egress into the peripheral blood by downregulating niche factors via β3-AR. PNS signals in the CNS suppressed sympathetic nerve activity at night and reduced HSC egress from the bone marrow (Garcia-Garcia et al., 2019). The rhythmic movement of HSCs following circadian rhythms is finely tuned through skillful manipulation of the balance between SNS and PNS.
Sensory fibers are also distributed in the bone marrow and express neuropeptides CGRP and substance P (Bjurholm et al., 1988; Chartier et al., 2018; Mach et al., 2002). Notably, CGRP+ nociceptive fibers and tyrosine hydroxylate (TH)-positive sympathetic fibers exhibit similar nerve lengths in the bone marrow. Denervation of either sympathetic or nociceptive nerves in vivo had little effect on HSCs in the bone marrow; however, dual denervation of both nerves led to an expansion of HSCs and increased the myeloid bias of hematopoiesis (Gao et al., 2021). Depletion of nociceptive nerves using resiniferatoxin (RTX) or Nav1.8-Cre transgenic mice blunted G-CSF-induced HSC mobilization, and these mobilization defects were ameliorated by CGRP supplementation. CGRP acts on its heterodimeric receptor RAMP1/CALCRL on HSCs and promotes HSC mobilization by activating downstream Gαs-adenyl cyclase-cAMP signaling (Gao et al., 2021). CGRP treatment also alleviates the mobilization defect via G-CSF in mice with cisplatin-induced injury of SNS signaling in the bone marrow (Lucas et al., 2013). More interestingly, feeding mice with spicy food containing capsaicin, which stimulates nociceptors, increased the efficiency of mobilization via activation of the nociceptive nerve (Gao et al., 2021), suggesting that nociceptor stimulation may be a new pathway for improving the efficiency of HSC mobilization by G-CSF.
Progeny of HSCs that regulate niche cells
Matured hematopoietic cells participate in the regulation of the HSC niche in the bone marrow. For example, in vivo depletion of macrophages, as found in macrophage Fas-induced apoptosis transgenic mice or produced by clodronate-containing liposome administration to wild-type mice, induced disruption of niche function and mobilization of HSPCs into the blood (Winkler et al., 2010). Chow et al. (2011) independently revealed that removal of CD169 (SIGLEC1)-expressing macrophages results in a decrease of CXCL12 in Nes-GFP+ perivascular niche cells and HSPC mobilization. The Nes-GFP+ niche requires support signals from macrophages and sympathetic signals via β3-ARs to maintain niche function (Mendez-Ferrer et al., 2010). G-CSF administration cancels these supportive signals but increases sympathetic tone to inhibit niche function of Nes-GFP+ cells, promoting HSC mobilization.
Neutrophils, the most abundant cell types among leukocytes in circulation in mammals, are also involved in regulating the HSC niche. The number of neutrophils in the circulating blood oscillates in a circadian manner. Aged neutrophils circulate back to the bone marrow and are cleared by macrophages, and the clearance of aged neutrophils by macrophages alters CAR cell function to retain HSPCs through activation of liver X receptors (LXRα and LXRβ) in macrophages, eventually leading to a release of HSPCs into the circulation (Casanova-Acebes et al., 2013). Bone marrow-derived neutrophils, but not peripheral blood neutrophils, enhance endothelial cell regeneration after transplantation, leading to accelerated hematopoietic recovery. In this recovery process, neutrophil-derived TNF-α acts on TNFR1 on endothelial cells to promote vessel growth (Bowers et al., 2018). As discussed before, aged neutrophils are abundant in blood from SCD patients and SCD mice and have been associated with severe vaso-occlusive processes in vivo. However, the impact of this imbalance on the bone marrow microenvironment remains unknown. It would not be surprising if abnormal oscillations in bone marrow release or clearance of neutrophils could negatively affect bone marrow homeostasis or recovery after transplantation in these patients.
Lymphoid descendants of HSCs are also involved in HSC regulation. HSCs in the bone marrow are resistant to various cytotoxic stresses, and FoxP3+ regulatory T cells (Tregs), which suppress the function of effector T cells, play crucial roles in providing immune-privileged sites to HSCs in the bone marrow (Fujisaki et al., 2011; Hirata et al., 2018). Allogeneic HSCs transplanted in non-irradiated mice survived for 1 month without immunosuppressive drugs, and most of them colocalized with Tregs in the bone marrow (Fujisaki et al., 2011). Depletion of Tregs, as observed in vivo in FoxP3-GFP diphtheria toxin receptor (DTR) mice or after injection of anti-CD25 antibody, led to a robust reduction of surviving donor HSCs in the bone marrow, suggesting that Tregs enable HSCs to escape from the attack by allogeneic immune cells (Fujisaki et al., 2011). A distinct subpopulation of Tregs that express high levels of CD150 maintained HSC quiescence and provided immune privilege through adenosine (Hirata et al., 2018).
HSC niche in the dawn and dusk of hematopoiesis
In mammals, definitive hematopoiesis emerges through endothelial-to-hematopoietic transitions in the AGM region of the fetus (Medvinsky and Dzierzak, 1996; Muller et al., 1994). HSCs migrate to the fetal liver at E11.5, where HSCs progressively proliferate until E16 before migrating to the bone marrow at E17.5 (Bowie et al., 2006; Morrison et al., 1995). Specific stromal cell lines established from the fetal liver support HSC expansion for up to 5–7 weeks in vitro, suggesting that HSCs are regulated by extrinsic signals in the fetal liver as well as the bone marrow (Moore et al., 1997; Wineman et al., 1996). Although various extrinsic signals have been identified in the microenvironment that maintain and expand fetal liver HSCs in vitro (Lewis et al., 2021), our knowledge of niche cells in vivo is limited. In mouse fetal livers, endothelial cells, hepatoblasts, and mesenchymal stromal cells have been proposed as HSC niche cells. Endothelial protein C receptor (EPCR)+ HSCs reside in close proximity to Lyve-1+ endothelial cells in fetal livers. Co-culturing of EPCR+ HSPCs with Lyve-1+ endothelial cells restored their repopulation capacity, suggesting the pivotal roles of endothelial cells for niche cells in the fetal liver (Iwasaki et al., 2010).
A line of evidence indicates that the cells expressing SCF in the fetal liver are hepatoblasts, which have the potential to differentiate into hepatocyte and biliary epithelial cells. SCF+ DLK (delta-like leucine zipper kinase)+ hepatoblasts highly express cytokines that expand HSCs in vitro, including angiopoietin-like 3 (Angptl3), TPO, and CXCL12. These cells are able to support the repopulating activity of fetal liver HSCs in a co-culture system (Chou et al., 2013; Chou and Lodish, 2010; Zhang et al., 2006).
Nes-GFP+ stromal cells, the key component of the HSC niche in adult bone marrow, also conduct HSC expansion in the fetal liver. Khan et al. (2016) have revealed that HSCs exhibit close association with Nes-GFP+Ng2+ pericytes located around portal vessels in fetal liver. Nes-GFP+Ng2+ cells express high levels of HSC niche genes, including Cxcl12, Scf, Angptl2, and Igf2 (encoding insulin-like growth factor 2). Depletion of Ng2+ cells in vivo using transgenic Ng2-Cre mice crossed with Cre-inducible diphtheria toxin A (iDTA) mice resulted in a significant reduction of fetal liver HSCs at E14.5, while no difference was observed in HSCs at E12 to E12.5. 3D imaging analyses of consecutive cryosections of Nes-GFP+ fetal liver from E12 to E14.5 demonstrated that portal vessels and Nes-GFP+ niche cells expanded at the same scale as HSCs, according to fractal geometries (Khan et al., 2016). These results indicate that rapid growth of the portal vascular tree during the fetal liver stage boosts the expansion of HSCs before their movement toward the bone marrow.
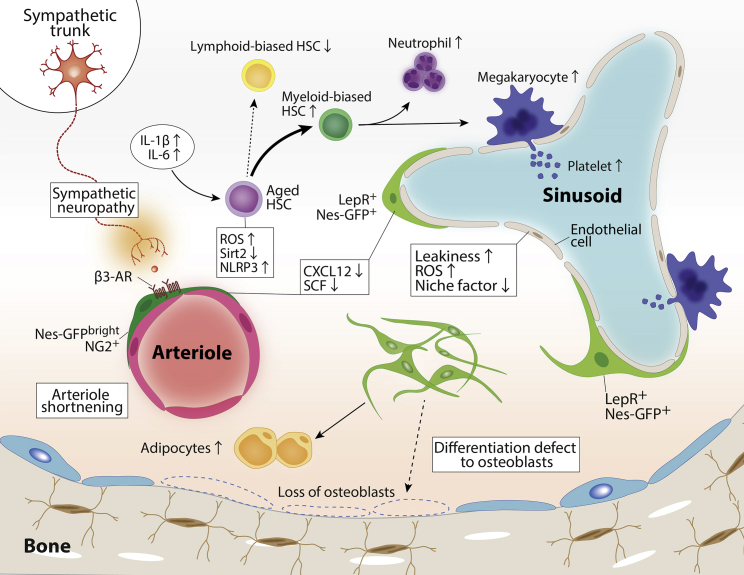
Aging is inevitable for all living organisms. Aged HSCs display increased numbers, impaired homing and repopulating ability, and loss of polarity, and are skewed to myeloid differentiation at the expense of lymphoid cell production. Aging in HSCs depends primarily on intrinsic cellular changes, including differential transcriptional and epigenetic profiles, increased ROS levels, and DNA damage (Geiger et al., 2013; Ito and Suda, 2014; Nakamura-Ishizu et al., 2020). A growing body of evidence indicates that alterations in niche cells are also considerably involved in HSC aging (Figure 5). Upon physiological aging, the bone marrow microenvironment undergoes a variety of alterations. MSCs in aged mice exhibit a reduced ability to differentiate into osteoblasts and a bias toward differentiating into adipocytes (Singh et al., 2016), leading to an accumulation of adipocytes in the bone marrow and bone loss. Bone marrow vasculature is remodeled upon aging and exhibits increased overall vascular density (Ho et al., 2019; Maryanovich et al., 2018) but it is functionally impaired, and exhibits increased vascular permeability, elevated ROS levels, and decreased expression of niche factors (Poulos et al., 2017). Type H endothelial cells, a distinct subtype among endothelial cells, decrease in number in the bone marrow of aged mice, which also exhibit decreases in arterioles and artery-associated PDGFRβ+/NG2+ perivascular cells along with declining SCF levels. Activation of Notch signaling in endothelial cells restores these alterations of the microenvironment but does not rescue the function of senescent HSCs (Kusumbe et al., 2016). Consistent with these findings, Nes-GFPbright arterioles and peri-arteriolar stromal cells are disrupted in aged mice, while Nes-GFPdimLepR+ stromal cells remain unchanged (Maryanovich et al., 2018; Sacma et al., 2019). Age-related changes in the bone marrow niche include higher levels of niche-derived soluble factors, such as pro-inflammatory cytokines (e.g., IL-1β, IL-6) (Pietras et al., 2016). Pro-inflammatory cytokines drive myeloid-biased differentiation and potentially contribute to the development of human age-related clonal hematopoiesis (Jaiswal et al., 2014; Meyer et al., 2018). ROS are known to activate an innate immune sensor, the NLR family pyrin domain containing 3 (NLRP3) inflammasome, and, once triggered, the NLRP3 inflammasome induces pro-inflammatory cytokine secretion. NLRP3 is highly expressed in neutrophils and has been studied as a source of DAMPs in the pathogenesis of SCD, but also functions in HSCs (He et al., 2020; Luo et al., 2019). NLRP3 is a substrate of Sirtuin 2 (Sirt2). The reduction of Sirt2 in aged HSCs activates the NLRP3 inflammasome, which enhances mitochondrial stress-induced HSC deterioration. Further studies will clarify whether inflammation is the cause or the consequence of aging in neutrophils and HSCs. Notably, however, the HSC aged phenotype is not affected by parabiosis or transfusion, or even transplant into a young environment, and therefore appears not to be reversible (Ho et al., 2021).
Figure 6.
Paul S Frenette, MD (1965–2021)
(A) Dr. Frenette welcomed attendees to the Stem Cell Symposium at Einstein in 2017.
(B) Group photo of the second Einstein Stem Cell Institute Retreat at Mohonk Mountain House, NY, in 2018.
(C) Dr. Frenette (right) with invited speakers, Drs. Andreas Trumpp and Toshio Suda (center left and right, respectively), and Einstein faculty Dr. Keisuke Ito (left), in the reception of an inaugural Einstein Stem Cell Symposium at a rooftop bar in 2012.
Figure 5.
Alterations in the microenvironment in the aged bone marrow
The changes of microenvironment during aging include the diminished β3-adrenergic receptor (AR) signals due to neuropathy of sympathetic nerves, arteriole shortening accompanied by decreased Nes-GFPbrightNG2+ stromal cells, differentiation skewing of mesenchymal stem and progenitor cells toward adipogenesis at the expense of osteogenesis, alterations in adrenergic signaling and inflammation, and endothelial cell dysfunction. These functional changes in the HSC niches upon aging contribute to HSC aging with myeloid-biased hematopoiesis and their downstream progeny. HSC, hematopoietic stem cell; Nes-GFP, nestin-GFP; NG2, nerve/glial antigen 2; ROS, reactive oxygen species; Sirt2, Sirtuin 2; NLRP3, NLR family pyrin domain containing 3; LepR, leptin receptor; SCF, stem cell factor; IL-1β, interleukin 1 beta; IL-6, interleukin 6.
Alteration of SNS signals has been proposed as a contributor to HSC aging. Maryanovich et al. (2018) demonstrated that the density of adrenergic nerve fibers was reduced in aged mice, and this reduction was accompanied by numerical expansion of Nes-GFPbright cells with deteriorated HSC niche function. Surgical denervation of the unilateral hindlimb in young mice induced premature aging-like HSC phenotypes, such as myeloid-biased skewing, reduced repopulating capacity, loss of polarity, and increased DNA damage. These phenotypes were observed in the denervated bone but not the sham-operated bone. Remarkably, β3-AR agonist but not β2-AR agonist rejuvenated the repopulating capacity of aged HSCs, with partial restoration of arteriolar structure and α-SMA+ peri-arteriolar cell density, in both old mice and denervated bone marrow. Conversely, young mice in which β3-ARs were deleted in the microenvironment showed premature HSC aging, suggesting that loss of β3-AR signaling in niche cells drives HSC aging (Maryanovich et al., 2018). In sharp contrast to these findings, Ho et al. (2019) reported that sympathetic nerve fibers are increased in the aged bone marrow and enhanced β2 adrenergic signaling promotes MK differentiation through IL-6 from the microenvironment, while β3-AR signaling contributes to normalizes myeloid-biased skewing of HSCs during aging. Future studies will clarify the roles of SNS signaling in the aging process of the bone marrow microenvironment and HSCs.
Concluding remarks
In the last few decades, to formally identify niche cells, crucial regulatory factors have been conditionally deleted in vivo from candidate cell types in mouse models. Advances in imaging techniques of the bone marrow have contributed to remarkable progress in the research field. These efforts have revealed that various cell types, both hematopoietic and non-hematopoietic, serve as HSC niches in the bone marrow. The diversity of these niches appears to be an essential mechanism for the lifelong persistence of HSCs, which are critical for maintaining life. However, caution should be exercised in interpreting the results of knockout studies, which have proposed multiple constituent cell types on the basis of cell-by-cell or signal-by-signal assays, because depletion of a single factor in genetically engineered animals can induce compensatory mechanisms by other pathways or unanticipated indirect effects. Critically, as the niche is not composed of a single cell type, a comprehensive understanding of how signaling pathways from multiple niche cells cooperate to regulate HSC fate decisions is critical. Similarly, attempts to define the precise anatomical localization of HSCs in 3D have been unsatisfactory using the standard method of labeling one or two niche components at a time, and, more importantly, most studies so far have used fixed samples for analysis. In future research, it would be highly advantageous to develop a system that allows us to precisely visualize the molecular activities between HSCs and the bone marrow microenvironment in real time in live animals. Additionally, recent advances in cellular analyses of the bone marrow microenvironment at single-cell resolution have enabled us to achieve a deeper understanding of the interaction between various niche cells in steady-state, aged, and pathogenic bone marrow (Baccin et al., 2020; Baryawno et al., 2019; Helbling et al., 2019; Tikhonova et al., 2019). Further elucidation of the molecular mechanism of HSC regulation by niche cells will enable the development of novel therapies that promote the recovery of hematopoiesis after radiation therapy, chemotherapy, targeted therapies such as Venetoclax, or HSC transplantation. Our current understanding of the crucial role of HSCs and the bone marrow microenvironment in the genesis and homeostasis of hematopoiesis, in combination with recent advances in HSC-based gene therapy, represent a promising curative opportunity for numerous inherited hematological disorders, including SCD.
Tribute
Dr. Paul S. Frenette (Figure 6) was an outstanding scientist, both deeply insightful and extraordinarily innovative, and was respected by his colleagues around the world for his remarkable contributions to hematology. Paul passed away on July 26th, 2021, at the age of 56. The news of his passing stunned all those who loved him, knew him, or were aware of his work. Paul was a prominent investigator who made key discoveries in diverse areas of research, including the HSC niche, SCD vaso-occlusion, and the role of the nervous system in cancer. His death at an early age was tragic but his legacy will persist.
Paul was born in Québec City, Canada, where he earned his MD degree, at the Université Laval, in 1988. He moved to the United States in 1991, as a clinical fellow in hematology-oncology at New England Medical Center, Tufts University (Boston, MA). From 1994 to 1997 he was a research fellow at the Center for Blood Research, Harvard Medical School, and Massachusetts Institute of Technology (Cambridge, MA). In 1998, Paul started his own laboratory at the Mount Sinai School of Medicine (New York, NY), where he joined the faculty as an Assistant Professor of Medicine; he would become a Professor of the Department of Gene and Cell Medicine just a few years later. Then in 2010, Paul joined the Albert Einstein College of Medicine (Bronx, NY) as a Professor of the Departments of Medicine and Cell Biology and became the first Director and Chair of the Ruth L. and David S. Gottesman Institute for Stem Cell and Regenerative Research, where he continued his excellent work.
Paul and his team made revolutionary discoveries in several fields. His group discovered that leukocytes play a fundamental role in SCD vaso-occlusion (Turhan et al., 2002), and demonstrated that endothelial selectins mediate the adhesive interactions of neutrophils and RBCs with the vasculature (Chiang et al., 2007; Hidalgo et al., 2009). These early discoveries culminated in the recent approval of an anti-P-selectin antibody to prevent acute vaso-occlusive pain in SCD. In subsequent studies, Paul’s group showed that the gut microbiome drives neutrophil aging and activation, promotes vaso-occlusion and organ damage (Zhang et al., 2015), and regulates psychological stress-induced inflammation (Xu et al., 2020). More recently, Paul’s team demonstrated that pain sensation protects against SCD outcomes through the release of CGRP by sensory nerves, and that oral capsaicin reduced vaso-occlusion and prevented organ damage in SCD mice (Xu et al., 2021).
In the bone marrow stem cell niche, Paul soon became a scientific authority as his laboratory built a track record of remarkable discovery. Together with his team, Paul demonstrated that (1) signals from the SNS regulate the attraction of HSCs to their niche (Katayama et al., 2006), and (2) circadian oscillations regulate the trafficking of HSCs through ARs (Mendez-Ferrer et al., 2008). These findings were followed by several other outstanding studies that had a tremendous impact on the field. Paul’s lab discovered that adrenergic nerve degeneration in the bone marrow leads to HSC niche aging (Maryanovich et al., 2018) and that nerve signals regulate cancer progression (Hanoun et al., 2014). More recently, his group showed that nociceptive nerves regulate HSC mobilization (Gao et al., 2021) and that HSC fate decisions under stress are controlled by the microbiota through iron availability in the bone marrow (Zhang et al., 2022).
However, the Frenette lab has always had simply the best when it comes to a scientific team. Paul not only knew who to recruit, he knew how to help them grow, reach independence, and become successful researchers, mentors, and leaders. “Show me the data!” was his mantra, because, to him, talking science meant talking data. He never came to us with answers, but instead posed questions, posited challenges. He never showed us the right path but taught us how to find it on our own. He believed in us and wanted us to go far. Behind the “tough Paul” was a very passionate researcher, a compassionate, deeply humble mentor, and a born leader who considered his team his family. And of course, his intelligence was extraordinary—he could look far down the road to see things no one else could—but he also had an incredible sense of humor; he was as funny as he was smart. He will be remembered with affection and respect by everyone who had the honor of knowing him; indeed, already he is profoundly missed. But good memories never go away, and to those of us who knew him, that’s a comfort. Paul inspired many during his life and will inspire many more in the years ahead. In a way, nothing has changed; he will always be an inspiration.
Author contributions
L.S.T. and N.A. drafted the manuscript. M.J.W. and K.I. reviewed and edited the first draft. M.J.W., A.T., T.S., D.T.S., and K.I. reviewed and edited the final version. All authors have read and agreed to the published version of the manuscript.
Conflicts of interest
D.T.S. is a co-founder and equity holder in Fate Therapeutics, Clear Creek Bio, and LifeVault Bio; he is a director, co-founder, and equity holder in Magenta Therapeutics; a director and equity holder in Agios Pharmaceuticals and Editas Medicine; a consultant for FOG Pharma and VCanBio; a DSMB member for Alexion; and a sponsored research recipient from Novartis.
Acknowledgments
We are deeply saddened by the passing of Dr. Paul S. Frenette on July 26th, 2021. He was a visionary scientist, inspiring leader, devoted educator, and a kind friend. Paul’s legacy will live on through his legacy at the Albert Einstein School of Medicine, including the myriad of trainees and faculty who he guided and mentored. The authors are grateful to members of the Einstein Stem Cell Institute for their comments on the topics of SCD and stem cell biology. N.A. and T.S. are supported by Grants-in-aid for Scientific Research (KAKENHI), T.S. is supported by the National Medical Research Council grant of Singapore Translational Research Investigator Award, and A.T. is supported by the Dietmar Hopp Foundation. M.J.W., D.T.S., and K. I. are supported by grants from the National Institutes of Health (P01HL053749 and R01HL156647 to M.J.W.; U19HL129903 to D.T.S.; and R01HL148852, R01DK098263, R01HL069438, and R01DK115577 to K.I.). L.S.T. is supported by the Paul S. Frenette Scholar Awards Program of the Ruth L. and David S. Gottesman Institute for Stem Cell Research and Regenerative Medicine, and K.I. is a research scholar of the Leukemia & Lymphoma Society (#1360–19).
References
- Acar M., Kocherlakota K.S., Murphy M.M., Peyer J.G., Oguro H., Inra C.N., Jaiyeola C., Zhao Z., Luby-Phelps K., Morrison S.J. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature. 2015;526:126–130. doi: 10.1038/nature15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam S.S., Flahiff C.M., Kamble S., Telen M.J., Reed S.D., De Castro L.M. Depression, quality of life, and medical resource utilization in sickle cell disease. Blood Adv. 2017;1:1983–1992. doi: 10.1182/bloodadvances.2017006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki K., Kurashige M., Ichii M., Higaki K., Sugiyama T., Kaito T., Ando W., Sugano N., Sakai T., Shibayama H., et al. Identification of CXCL12-abundant reticular cells in human adult bone marrow. Br. J. Haematol. 2021;193:659–668. doi: 10.1111/bjh.17396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai F., Hirao A., Ohmura M., Sato H., Matsuoka S., Takubo K., Ito K., Koh G.Y., Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Arranz L., Sánchez-Aguilera A., Martín-Pérez D., Isern J., Langa X., Tzankov A., Lundberg P., Muntión S., Tzeng Y.S., Lai D.M., et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature. 2014;512:78–81. doi: 10.1038/nature13383. [DOI] [PubMed] [Google Scholar]
- Asada N., Katayama Y., Sato M., Minagawa K., Wakahashi K., Kawano H., Kawano Y., Sada A., Ikeda K., Matsui T., Tanimoto M. Matrix-embedded osteocytes regulate mobilization of hematopoietic stem/progenitor cells. Cell Stem Cell. 2013;12:737–747. doi: 10.1016/j.stem.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Asada N., Kunisaki Y., Pierce H., Wang Z., Fernandez N.F., Birbrair A., Ma'ayan A., Frenette P.S. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat. Cell Biol. 2017;19:214–223. doi: 10.1038/ncb3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataga K.I., Kutlar A., Kanter J., Liles D., Cancado R., Friedrisch J., Guthrie T.H., Knight-Madden J., Alvarez O.A., Gordeuk V.R., et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N. Engl. J. Med. 2017;376:429–439. doi: 10.1056/NEJMoa1611770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccin C., Al-Sabah J., Velten L., Helbling P.M., Grünschläger F., Hernández-Malmierca P., Nombela-Arrieta C., Steinmetz L.M., Trumpp A., Haas S. Combined single-cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organization. Nat. Cell Biol. 2020;22:38–48. doi: 10.1038/s41556-019-0439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey M.T., Dowd S.E., Galley J.D., Hufnagle A.R., Allen R.G., Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav. Immun. 2011;25:397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballas S.K., Lusardi M. Hospital readmission for adult acute sickle cell painful episodes: frequency, etiology, and prognostic significance. Am. J. Hematol. 2005;79:17–25. doi: 10.1002/ajh.20336. [DOI] [PubMed] [Google Scholar]
- Ballas S.K., Marcolina M.J. Hyperhemolysis during the evolution of uncomplicated acute painful episodes in patients with sickle cell anemia. Transfusion. 2006;46:105–110. doi: 10.1111/j.1537-2995.2006.00679.x. [DOI] [PubMed] [Google Scholar]
- Ballas S.K., Barton F.B., Waclawiw M.A., Swerdlow P., Eckman J.R., Pegelow C.H., Koshy M., Barton B.A., Bonds D.R. Hydroxyurea and sickle cell anemia: effect on quality of life. Health Qual. Life Outcome. 2006;4:59. doi: 10.1186/1477-7525-4-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballas S.K., Gupta K., Adams-Graves P. Sickle cell pain: a critical reappraisal. Blood. 2012;120:3647–3656. doi: 10.1182/blood-2012-04-383430. [DOI] [PubMed] [Google Scholar]
- Ballas S.K., Lieff S., Benjamin L.J., Dampier C.D., Heeney M.M., Hoppe C., Johnson C.S., Rogers Z.R., Smith-Whitley K., Wang W.C., Telen M.J. Definitions of the phenotypic manifestations of sickle cell disease. Am. J. Hematol. 2010;85:6–13. doi: 10.1002/ajh.21550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baryawno N., Przybylski D., Kowalczyk M.S., Kfoury Y., Severe N., Gustafsson K., Kokkaliaris K.D., Mercier F., Tabaka M., Hofree M., et al. A cellular taxonomy of the bone marrow stroma in homeostasis and leukemia. Cell. 2019;177:1915–1932.e16. doi: 10.1016/j.cell.2019.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher J.D., Mahaseth H., Welch T.E., Otterbein L.E., Hebbel R.P., Vercellotti G.M. Heme oxygenase-1 is a modulator of inflammation and vaso-occlusion in transgenic sickle mice. J. Clin. Invest. 2006;116:808–816. doi: 10.1172/JCI26857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensinger T.A., Gillette P.N. Hemolysis in sickle cell disease. Arch. Intern. Med. 1974;133:624–631. doi: 10.1001/archinte.133.4.624. [DOI] [PubMed] [Google Scholar]
- Bernaudin F., Socie G., Kuentz M., Chevret S., Duval M., Bertrand Y., Vannier J.P., Yakouben K., Thuret I., Bordigoni P., et al. Long-term results of related myeloablative stem-cell transplantation to cure sickle cell disease. Blood. 2007;110:2749–2756. doi: 10.1182/blood-2007-03-079665. [DOI] [PubMed] [Google Scholar]
- Bjurholm A., Kreicbergs A., Terenius L., Goldstein M., Schultzberg M. Neuropeptide Y-tyrosine hydroxylase- and vasoactive intestinal polypeptide-immunoreactive nerves in bone and surrounding tissues. J. Auton. Nerv. Syst. 1988;25:119–125. doi: 10.1016/0165-1838(88)90016-1. [DOI] [PubMed] [Google Scholar]
- Bowers E., Slaughter A., Frenette P.S., Kuick R., Pello O.M., Lucas D. Granulocyte-derived TNFα promotes vascular and hematopoietic regeneration in the bone marrow. Nat. Med. 2018;24:95–102. doi: 10.1038/nm.4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie M.B., McKnight K.D., Kent D.G., McCaffrey L., Hoodless P.A., Eaves C.J. Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. J. Clin. Invest. 2006;116:2808–2816. doi: 10.1172/JCI28310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brim H., Taylor J., Abbas M., Vilmenay K., Daremipouran M., Varma S., Lee E., Pace B., Song-Naba W.L., Gupta K., et al. The gut microbiome in sickle cell disease: characterization and potential implications. PLoS One. 2021;16:e0255956. doi: 10.1371/journal.pone.0255956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittenham G.M., Schechter A.N., Noguchi C.T. Hemoglobin S polymerization: primary determinant of the hemolytic and clinical severity of the sickling syndromes. Blood. 1985;65:183–189. doi: 10.1182/blood.v65.1.183.bloodjournal651183. [DOI] [PubMed] [Google Scholar]
- Bromberg O., Frisch B.J., Weber J.M., Porter R.L., Civitelli R., Calvi L.M. Osteoblastic N-cadherin is not required for microenvironmental support and regulation of hematopoietic stem and progenitor cells. Blood. 2012;120:303–313. doi: 10.1182/blood-2011-09-377853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns I., Lucas D., Pinho S., Ahmed J., Lambert M.P., Kunisaki Y., Scheiermann C., Schiff L., Poncz M., Bergman A., Frenette P.S. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat. Med. 2014;20:1315–1320. doi: 10.1038/nm.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn H.F. Pathogenesis and treatment of sickle cell disease. N. Engl. J. Med. 1997;337:762–769. doi: 10.1056/NEJM199709113371107. [DOI] [PubMed] [Google Scholar]
- Calvi L.M., Adams G.B., Weibrecht K.W., Weber J.M., Olson D.P., Knight M.C., Martin R.P., Schipani E., Divieti P., Bringhurst F.R., et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Cao H., Cao B., Heazlewood C.K., Domingues M., Sun X., Debele E., McGregor N.E., Sims N.A., Heazlewood S.Y., Nilsson S.K. Osteopontin is an important regulative component of the fetal bone marrow hematopoietic stem cell niche. Cells. 2019;8:985. doi: 10.3390/cells8090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova-Acebes M., Pitaval C., Weiss L.A., Nombela-Arrieta C., Chèvre R., A-González N., Kunisaki Y., Zhang D., van Rooijen N., Silberstein L.E., et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 2013;153:1025–1035. doi: 10.1016/j.cell.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charache S., Dover G.J., Moore R.D., Eckert S., Ballas S.K., Koshy M., Milner P.F., Orringer E.P., Phillips G., Jr., Platt O.S., et al. Hydroxyurea: effects on hemoglobin F production in patients with sickle cell anemia. Blood. 1992;79:2555–2565. doi: 10.1182/blood.v79.10.2555.bloodjournal79102555. [DOI] [PubMed] [Google Scholar]
- Charache S., Terrin M.L., Moore R.D., Dover G.J., Barton F.B., Eckert S.V., McMahon R.P., Bonds D.R. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N. Engl. J. Med. 1995;332:1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- Chartier S.R., Mitchell S.A.T., Majuta L.A., Mantyh P.W. The changing sensory and sympathetic innervation of the young, adult and aging mouse femur. Neuroscience. 2018;387:178–190. doi: 10.1016/j.neuroscience.2018.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.Y., Miyanishi M., Wang S.K., Yamazaki S., Sinha R., Kao K.S., Seita J., Sahoo D., Nakauchi H., Weissman I.L. Hoxb5 marks long-term haematopoietic stem cells and reveals a homogenous perivascular niche. Nature. 2016;530:223–227. doi: 10.1038/nature16943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang E.Y., Hidalgo A., Chang J., Frenette P.S. Imaging receptor microdomains on leukocyte subsets in live mice. Nat. Methods. 2007;4:219–222. doi: 10.1038/nmeth1018. [DOI] [PubMed] [Google Scholar]
- Chou S., Lodish H.F. Fetal liver hepatic progenitors are supportive stromal cells for hematopoietic stem cells. Proc. Natl. Acad. Sci. U S A. 2010;107:7799–7804. doi: 10.1073/pnas.1003586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S., Flygare J., Lodish H.F. Fetal hepatic progenitors support long-term expansion of hematopoietic stem cells. Exp. Hematol. 2013;41:479–490.e4. doi: 10.1016/j.exphem.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A., Lucas D., Hidalgo A., Méndez-Ferrer S., Hashimoto D., Scheiermann C., Battista M., Leboeuf M., Prophete C., van Rooijen N., et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J. Exp. Med. 2011;208:261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comazzetto S., Murphy M.M., Berto S., Jeffery E., Zhao Z., Morrison S.J. Restricted hematopoietic progenitors and erythropoiesis require SCF from leptin Receptor+ niche cells in the bone marrow. Cell Stem Cell. 2019;24:477–486.e6. doi: 10.1016/j.stem.2018.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conran N., Embury S.H. Sickle cell vaso-occlusion: the dialectic between red cells and white cells. Exp. Biol. Med. 2021;246:1458–1472. doi: 10.1177/15353702211005392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ceulaer K., Higgs D.R., Weatherall D.J., Hayes R.J., Serjeant B.E., Serjeant G.R. alpha-Thalassemia reduces the hemolytic rate in homozygous sickle-cell disease. N. Engl. J. Med. 1983;309:189–190. doi: 10.1056/NEJM198307213090320. [DOI] [PubMed] [Google Scholar]
- Ding L., Morrison S.J. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L., Saunders T.L., Enikolopov G., Morrison S.J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler P.A., Sharma A., Porter J.S., Zheng Y., Tisdale J.F., Weiss M.J. Genetic therapies for the first molecular disease. J. Clin. Invest. 2021;131:146394. doi: 10.1172/JCI146394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty D.H., Doyle M.P., Curry S.R., Vali R.J., Fattor T.J., Olson J.S., Lemon D.D. Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nat. Biotechnol. 1998;16:672–676. doi: 10.1038/nbt0798-672. [DOI] [PubMed] [Google Scholar]
- Drysdale C.M., Nassehi T., Gamer J., Yapundich M., Tisdale J.F., Uchida N. Hematopoietic-stem-cell-targeted gene-addition and gene-editing strategies for beta-hemoglobinopathies. Cell Stem Cell. 2021;28:191–208. doi: 10.1016/j.stem.2021.01.001. [DOI] [PubMed] [Google Scholar]
- Dufu K., Patel M., Oksenberg D., Cabrales P. GBT440 improves red blood cell deformability and reduces viscosity of sickle cell blood under deoxygenated conditions. Clin. Hemorheol. Microcirc. 2018;70:95–105. doi: 10.3233/CH-170340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton W.A., Bunn H.F. Treating sickle cell disease by targeting HbS polymerization. Blood. 2017;129:2719–2726. doi: 10.1182/blood-2017-02-765891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esrick E.B., Lehmann L.E., Biffi A., Achebe M., Brendel C., Ciuculescu M.F., Daley H., MacKinnon B., Morris E., Federico A., et al. Post-transcriptional genetic silencing of BCL11A to treat sickle cell disease. N. Engl. J. Med. 2021;384:205–215. doi: 10.1056/NEJMoa2029392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federico L., McGrail D.J., Bentebibel S.E., Haymaker C., Ravelli A., Forget M.A., Karpinets T., Jiang P., Reuben A., Negrao M.V., et al. Distinct tumor-infiltrating lymphocyte landscapes are associated with clinical outcomes in localized non-small-cell lung cancer. Ann. Oncol. 2022;33:42–56. doi: 10.1016/j.annonc.2021.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangoul H., Altshuler D., Cappellini M.D., Chen Y.S., Domm J., Eustace B.K., Foell J., de la Fuente J., Grupp S., Handgretinger R., et al. CRISPR-Cas9 gene editing for sickle cell disease and beta-thalassemia. N. Engl. J. Med. 2021;384:252–260. doi: 10.1056/NEJMoa2031054. [DOI] [PubMed] [Google Scholar]
- Frenette P.S. Sickle cell vaso-occlusion: multistep and multicellular paradigm. Curr. Opin. Hematol. 2002;9:101–106. doi: 10.1097/00062752-200203000-00003. [DOI] [PubMed] [Google Scholar]
- Frenette P.S., Atweh G.F. Sickle cell disease: old discoveries, new concepts, and future promise. J. Clin. Invest. 2007;117:850–858. doi: 10.1172/JCI30920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaki J., Wu J., Carlson A.L., Silberstein L., Putheti P., Larocca R., Gao W., Saito T.I., Celso C.L., Tsuyuzaki H., et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474:216–219. doi: 10.1038/nature10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Xu C., Asada N., Frenette P.S. The hematopoietic stem cell niche: from embryo to adult. Development. 2018;145:dev139691. doi: 10.1242/dev.139691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Zhang D., Xu C., Li H., Caron K.M., Frenette P.S. Nociceptive nerves regulate haematopoietic stem cell mobilization. Nature. 2021;589:591–596. doi: 10.1038/s41586-020-03057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-García A., Korn C., García-Fernández M., Domingues O., Villadiego J., Martín-Pérez D., Isern J., Bejarano-García J.A., Zimmer J., Pérez-Simón J.A., et al. Dual cholinergic signals regulate daily migration of hematopoietic stem cells and leukocytes. Blood. 2019;133:224–236. doi: 10.1182/blood-2018-08-867648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston M.H., Verter J.I., Woods G., Pegelow C., Kelleher J., Presbury G., Zarkowsky H., Vichinsky E., Iyer R., Lobel J.S., et al. Prophylaxis with oral penicillin in children with sickle cell anemia. N. Engl. J. Med. 1986;314:1593–1599. doi: 10.1056/NEJM198606193142501. [DOI] [PubMed] [Google Scholar]
- Gee B.E., Platt O.S. Sickle reticulocytes adhere to VCAM-1. Blood. 1995;85:268–274. doi: 10.1182/blood.v85.1.268.bloodjournal851268. [DOI] [PubMed] [Google Scholar]
- Geiger H., de Haan G., Florian M.C. The ageing haematopoietic stem cell compartment. Nat. Rev. Immunol. 2013;13:376–389. doi: 10.1038/nri3433. [DOI] [PubMed] [Google Scholar]
- Greenbaum A., Hsu Y.M.S., Day R.B., Schuettpelz L.G., Christopher M.J., Borgerding J.N., Nagasawa T., Link D.C. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanoun M., Zhang D., Mizoguchi T., Pinho S., Pierce H., Kunisaki Y., Lacombe J., Armstrong S.A., Dührsen U., Frenette P.S. Acute myelogenous leukemia-induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell niche. Cell Stem Cell. 2014;15:365–375. doi: 10.1016/j.stem.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M., Chiang H.H., Luo H., Zheng Z., Qiao Q., Wang L., Tan M., Ohkubo R., Mu W.C., Zhao S., et al. An acetylation switch of the NLRP3 inflammasome regulates aging-associated chronic inflammation and insulin resistance. Cell Metabol. 2020;31:580–591.e5. doi: 10.1016/j.cmet.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heazlewood S.Y., Neaves R.J., Williams B., Haylock D.N., Adams T.E., Nilsson S.K. Megakaryocytes co-localise with hemopoietic stem cells and release cytokines that up-regulate stem cell proliferation. Stem Cell Res. 2013;11:782–792. doi: 10.1016/j.scr.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Hebbel R.P. Reconstructing sickle cell disease: a data-based analysis of the "hyperhemolysis paradigm" for pulmonary hypertension from the perspective of evidence-based medicine. Am. J. Hematol. 2011;86:123–154. doi: 10.1002/ajh.21952. [DOI] [PubMed] [Google Scholar]
- Hebbel R.P. Ischemia-reperfusion injury in sickle cell anemia: relationship to acute chest syndrome, endothelial dysfunction, arterial vasculopathy, and inflammatory pain. Hematol. Oncol. Clin. N. Am. 2014;28:181–198. doi: 10.1016/j.hoc.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Hebbel R.P., Hedlund B.E. Sickle hemoglobin oxygen affinity-shifting strategies have unequal cerebrovascular risks. Am. J. Hematol. 2018;93:321–325. doi: 10.1002/ajh.24975. [DOI] [PubMed] [Google Scholar]
- Hebbel R.P., Boogaerts M.A.B., Eaton J.W., Steinberg M.H. Erythrocyte adherence to endothelium in sickle-cell anemia. A possible determinant of disease severity. N. Engl. J. Med. 1980;302:992–995. doi: 10.1056/NEJM198005013021803. [DOI] [PubMed] [Google Scholar]
- Hebbel∗ R.P., Osarogiagbon R., Kaul D. The endothelial biology of sickle cell disease: inflammation and a chronic vasculopathy. Microcirculation. 2004;11:129–151. doi: 10.1080/10739680490278402. [DOI] [PubMed] [Google Scholar]
- Helbling P.M., Piñeiro-Yáñez E., Gerosa R., Boettcher S., Al-Shahrour F., Manz M.G., Nombela-Arrieta C. Global transcriptomic profiling of the bone marrow stromal microenvironment during postnatal development, aging, and inflammation. Cell Rep. 2019;29:3313–3330.e4. doi: 10.1016/j.celrep.2019.11.004. [DOI] [PubMed] [Google Scholar]
- Henry E.R., Metaferia B., Li Q., Harper J., Best R.B., Glass K.E., Cellmer T., Dunkelberger E.B., Conrey A., Thein S.L., et al. Treatment of sickle cell disease by increasing oxygen affinity of hemoglobin. Blood. 2021;138:1172–1181. doi: 10.1182/blood.2021012070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick J.B. Peculiar elongated and sickle-shaped red blood corpuscles in a case of severe anemia. JAMA. 2014;312:1063. doi: 10.1001/jama.2014.11011. [DOI] [PubMed] [Google Scholar]
- Hidalgo A., Chang J., Jang J.E., Peired A.J., Chiang E.Y., Frenette P.S. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nat. Med. 2009;15:384–391. doi: 10.1038/nm.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillery C.A., Kerstein P.C., Vilceanu D., Barabas M.E., Retherford D., Brandow A.M., Wandersee N.J., Stucky C.L. Transient receptor potential vanilloid 1 mediates pain in mice with severe sickle cell disease. Blood. 2011;118:3376–3383. doi: 10.1182/blood-2010-12-327429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himburg H.A., Harris J.R., Ito T., Daher P., Russell J.L., Quarmyne M., Doan P.L., Helms K., Nakamura M., Fixsen E., et al. Pleiotrophin regulates the retention and self-renewal of hematopoietic stem cells in the bone marrow vascular niche. Cell Rep. 2012;2:964–975. doi: 10.1016/j.celrep.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himburg H.A., Muramoto G.G., Daher P., Meadows S.K., Russell J.L., Doan P., Chi J.T., Salter A.B., Lento W.E., Reya T., et al. Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nat. Med. 2010;16:475–482. doi: 10.1038/nm.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himburg H.A., Termini C.M., Schlussel L., Kan J., Li M., Zhao L., Fang T., Sasine J.P., Chang V.Y., Chute J.P. Distinct bone marrow sources of pleiotrophin control hematopoietic stem cell maintenance and regeneration. Cell Stem Cell. 2018;23:370–381.e5. doi: 10.1016/j.stem.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata Y., Furuhashi K., Ishii H., Li H.W., Pinho S., Ding L., Robson S.C., Frenette P.S., Fujisaki J. CD150(high) bone marrow Tregs maintain hematopoietic stem cell quiescence and immune privilege via adenosine. Cell Stem Cell. 2018;22:445–453.e5. doi: 10.1016/j.stem.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T.T., Dellorusso P.V., Verovskaya E.V., Bakker S.T., Flach J., Smith L.K., Ventura P.B., Lansinger O.M., Hérault A., Zhang S.Y., et al. Aged hematopoietic stem cells are refractory to bloodborne systemic rejuvenation interventions. J. Exp. Med. 2021;218:e20210223. doi: 10.1084/jem.20210223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y.H., Del Toro R., Rivera-Torres J., Rak J., Korn C., García-García A., Macías D., González-Gómez C., Del Monte A., Wittner M., et al. Remodeling of bone marrow hematopoietic stem cell niches promotes myeloid cell expansion during premature or physiological aging. Cell Stem Cell. 2019;25:407–418.e6. doi: 10.1016/j.stem.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper A.T., Butler J.M., Nolan D.J., Kranz A., Iida K., Kobayashi M., Kopp H.G., Shido K., Petit I., Yanger K., et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4:263–274. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover R., Rubin R., Wise G., Warren R. Adhesion of normal and sickle erythrocytes to endothelial monolayer cultures. Blood. 1979;54:872–876. doi: 10.1182/blood.v54.4.872.bloodjournal544872. [DOI] [PubMed] [Google Scholar]
- Ingram V.M. A specific chemical difference between the globins of normal human and sickle-cell anæmia hæmoglobin. Nature. 1956;178:792–794. doi: 10.1038/178792a0. [DOI] [PubMed] [Google Scholar]
- Ingram V.M. Gene mutations in human hæmoglobin: the chemical difference between normal and sickle cell hæmoglobin. Nature. 1957;180:326–328. doi: 10.1038/180326a0. [DOI] [PubMed] [Google Scholar]
- Itkin T., Gur-Cohen S., Spencer J.A., Schajnovitz A., Ramasamy S.K., Kusumbe A.P., Ledergor G., Jung Y., Milo I., Poulos M.G., et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature. 2016;532:323–328. doi: 10.1038/nature17624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Suda T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat. Rev. Mol. Cell Biol. 2014;15:243–256. doi: 10.1038/nrm3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Turcotte R., Cui J., Zimmerman S.E., Pinho S., Mizoguchi T., Arai F., Runnels J.M., Alt C., Teruya-Feldstein J., et al. Self-renewal of a purified Tie2+ hematopoietic stem cell population relies on mitochondrial clearance. Science. 2016;354:1156–1160. doi: 10.1126/science.aaf5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki H., Arai F., Kubota Y., Dahl M., Suda T. Endothelial protein C receptor-expressing hematopoietic stem cells reside in the perisinusoidal niche in fetal liver. Blood. 2010;116:544–553. doi: 10.1182/blood-2009-08-240903. [DOI] [PubMed] [Google Scholar]
- Jaiswal S., Fontanillas P., Flannick J., Manning A., Grauman P.V., Mar B.G., Lindsley R.C., Mermel C.H., Burtt N., Chavez A., et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson F.L., Look A.T., Gockerman J., Ruggiero M.R., Dalla-Pozza L., Billings F.T., 3rd Bone-marrow transplantation in a patient with sickle-cell anemia. N. Engl. J. Med. 1984;311:780–783. doi: 10.1056/NEJM198409203111207. [DOI] [PubMed] [Google Scholar]
- Joneckis C.C., Ackley R.L., Orringer E.P., Wayner E.A., Parise L.V. Integrin alpha 4 beta 1 and glycoprotein IV (CD36) are expressed on circulating reticulocytes in sickle cell anemia. Blood. 1993;82:3548–3555. doi: 10.1182/blood.v82.12.3548.bloodjournal82123548. [DOI] [PubMed] [Google Scholar]
- Jones R.J., DeBaun M.R. Leukemia after gene therapy for sickle cell disease: insertional mutagenesis, busulfan, both, or neither. Blood. 2021;138:942–947. doi: 10.1182/blood.2021011488. [DOI] [PubMed] [Google Scholar]
- Kanter J., Walters M.C., Krishnamurti L., Mapara M.Y., Kwiatkowski J.L., Rifkin-Zenenberg S., Aygun B., Kasow K.A., Pierciey F.J., Jr., Bonner M., et al. Biologic and clinical efficacy of LentiGlobin for sickle cell disease. N. Engl. J. Med. 2022;386:617–628. doi: 10.1056/NEJMoa2117175. [DOI] [PubMed] [Google Scholar]
- Katayama Y., Battista M., Kao W.M., Hidalgo A., Peired A.J., Thomas S.A., Frenette P.S. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- Kato G.J., Hebbel R.P., Steinberg M.H., Gladwin M.T. Vasculopathy in sickle cell disease: biology, pathophysiology, genetics, translational medicine, and new research directions. Am. J. Hematol. 2009;84:618–625. doi: 10.1002/ajh.21475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato G.J., Piel F.B., Reid C.D., Gaston M.H., Ohene-Frempong K., Krishnamurti L., Smith W.R., Panepinto J.A., Weatherall D.J., Costa F.F., Vichinsky E.P. Sickle cell disease. Nat. Rev. Dis. Prim. 2018;4:18010. doi: 10.1038/nrdp.2018.10. [DOI] [PubMed] [Google Scholar]
- Kato G.J., Steinberg M.H., Gladwin M.T. Intravascular hemolysis and the pathophysiology of sickle cell disease. J. Clin. Invest. 2017;127:750–760. doi: 10.1172/JCI89741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul D.K., Finnegan E., Barabino G.A. Sickle red cell-endothelium interactions. Microcirculation. 2009;16:97–111. doi: 10.1080/10739680802279394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenswil K.J.G., Pisterzi P., Sánchez-Duffhues G., van Dijk C., Lolli A., Knuth C., Vanchin B., Jaramillo A.C., Hoogenboezem R.M., Sanders M.A., et al. Endothelium-derived stromal cells contribute to hematopoietic bone marrow niche formation. Cell Stem Cell. 2021;28:653–670.e11. doi: 10.1016/j.stem.2021.01.006. [DOI] [PubMed] [Google Scholar]
- Khan J.A., Mendelson A., Kunisaki Y., Birbrair A., Kou Y., Arnal-Estapé A., Pinho S., Ciero P., Nakahara F., Ma'ayan A., et al. Fetal liver hematopoietic stem cell niches associate with portal vessels. Science. 2016;351:176–180. doi: 10.1126/science.aad0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel M.J., Radice G.L., Morrison S.J. Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell. 2007;1:204–217. doi: 10.1016/j.stem.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Kiel M.J., Yilmaz Ö.H., Iwashita T., Yilmaz O.H., Terhorst C., Morrison S.J. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Butler J.M., O'Donnell R., Kobayashi M., Ding B.S., Bonner B., Chiu V.K., Nolan D.J., Shido K., Benjamin L., Rafii S. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat. Cell Biol. 2010;12:1046–1056. doi: 10.1038/ncb2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli D.R., Li Y., Khasabov S.G., Gupta P., Kehl L.J., Ericson M.E., Nguyen J., Gupta V., Hebbel R.P., Simone D.A., Gupta K. Pain-related behaviors and neurochemical alterations in mice expressing sickle hemoglobin: modulation by cannabinoids. Blood. 2010;116:456–465. doi: 10.1182/blood-2010-01-260372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkaliaris K.D., Kunz L., Cabezas-Wallscheid N., Christodoulou C., Renders S., Camargo F., Trumpp A., Scadden D.T., Schroeder T. Adult blood stem cell localization reflects the abundance of reported bone marrow niche cell types and their combinations. Blood. 2020;136:2296–2307. doi: 10.1182/blood.2020006574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisaki Y., Bruns I., Scheiermann C., Ahmed J., Pinho S., Zhang D., Mizoguchi T., Wei Q., Lucas D., Ito K., et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637–643. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumbe A.P., Ramasamy S.K., Adams R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507:323–328. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumbe A.P., Ramasamy S.K., Itkin T., Mäe M.A., Langen U.H., Betsholtz C., Lapidot T., Adams R.H. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature. 2016;532:380–384. doi: 10.1038/nature17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson C., Oronsky B., Carter C.A., Oronsky A., Knox S.J., Sher D., Reid T.R. TGF-beta: a master immune regulator. Expert Opin. Ther. Targets. 2020;24:427–438. doi: 10.1080/14728222.2020.1744568. [DOI] [PubMed] [Google Scholar]
- Lewis K., Yoshimoto M., Takebe T. Fetal liver hematopoiesis: from development to delivery. Stem Cell Res. Ther. 2021;12:139. doi: 10.1186/s13287-021-02189-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Georgakopoulou A., Mishra A., Gil S., Hawkins R.D., Yannaki E., Lieber A. In vivo HSPC gene therapy with base editors allows for efficient reactivation of fetal gamma-globin in beta-YAC mice. Blood Adv. 2021;5:1122–1135. doi: 10.1182/bloodadvances.2020003702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S.H., Morris A., Li K., Fitch A.C., Fast L., Goldberg L., Quesenberry M., Sprinz P., Methé B. Intestinal microbiome analysis revealed dysbiosis in sickle cell disease. Am. J. Hematol. 2018;93:E91–E93. doi: 10.1002/ajh.25019. [DOI] [PubMed] [Google Scholar]
- Lo Celso C., Fleming H.E., Wu J.W., Zhao C.X., Miake-Lye S., Fujisaki J., Côté D., Rowe D.W., Lin C.P., Scadden D.T. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–96. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas D., Bruns I., Battista M., Mendez-Ferrer S., Magnon C., Kunisaki Y., Frenette P.S. Norepinephrine reuptake inhibition promotes mobilization in mice: potential impact to rescue low stem cell yields. Blood. 2012;119:3962–3965. doi: 10.1182/blood-2011-07-367102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas D., Scheiermann C., Chow A., Kunisaki Y., Bruns I., Barrick C., Tessarollo L., Frenette P.S. Chemotherapy-induced bone marrow nerve injury impairs hematopoietic regeneration. Nat. Med. 2013;19:695–703. doi: 10.1038/nm.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H., Mu W.C., Karki R., Chiang H.H., Mohrin M., Shin J.J., Ohkubo R., Ito K., Kanneganti T.D., Chen D. Mitochondrial stress-initiated aberrant activation of the NLRP3 inflammasome regulates the functional deterioration of hematopoietic stem cell aging. Cell Rep. 2019;26:945–954.e4. doi: 10.1016/j.celrep.2018.12.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach D.B., Rogers S.D., Sabino M.C., Luger N.M., Schwei M.J., Pomonis J.D., Keyser C.P., Clohisy D.R., Adams D.J., O'Leary P., Mantyh P.W. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience. 2002;113:155–166. doi: 10.1016/s0306-4522(02)00165-3. [DOI] [PubMed] [Google Scholar]
- Mahesri M., Schneeweiss S., Globe D., Mutebi A., Bohn R., Achebe M., Levin R., Desai R.J. Clinical outcomes following bone marrow transplantation in patients with sickle cell disease: a cohort study of US Medicaid enrollees. Eur. J. Haematol. 2021;106:273–280. doi: 10.1111/ejh.13546. [DOI] [PubMed] [Google Scholar]
- Maryanovich M., Zahalka A.H., Pierce H., Pinho S., Nakahara F., Asada N., Wei Q., Wang X., Ciero P., Xu J., et al. Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat. Med. 2018;24:782–791. doi: 10.1038/s41591-018-0030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui N.M., Borsig L., Rosen S.D., Yaghmai M., Varki A., Embury S.H. P-selectin mediates the adhesion of sickle erythrocytes to the endothelium. Blood. 2001;98:1955–1962. doi: 10.1182/blood.v98.6.1955. [DOI] [PubMed] [Google Scholar]
- Mayer E.A., Knight R., Mazmanian S.K., Cryan J.F., Tillisch K. Gut microbes and the brain: paradigm shift in neuroscience. J. Neurosci. 2014;34:15490–15496. doi: 10.1523/JNEUROSCI.3299-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGann P.T., Ware R.E. Hydroxyurea therapy for sickle cell anemia. Expet Opin. Drug Saf. 2015;14:1749–1758. doi: 10.1517/14740338.2015.1088827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvinsky A., Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- Méndez-Ferrer S., Lucas D., Battista M., Frenette P.S. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- Méndez-Ferrer S., Michurina T.V., Ferraro F., Mazloom A.R., Macarthur B.D., Lira S.A., Scadden D.T., Ma'ayan A., Enikolopov G.N., Frenette P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonça R., Silveira A.A.A., Conran N. Red cell DAMPs and inflammation. Inflamm. Res. 2016;65:665–678. doi: 10.1007/s00011-016-0955-9. [DOI] [PubMed] [Google Scholar]
- Mentzer W.C., Heller S., Pearle P.R., Hackney E., Vichinsky E. Availability of related donors for bone marrow transplantation in sickle cell anemia. Am. J. Pediatr. Hematol. Oncol. 1994;16:27–29. [PubMed] [Google Scholar]
- Metcalf B., Chuang C., Dufu K., Patel M.P., Silva-Garcia A., Johnson C., Lu Q., Partridge J.R., Patskovska L., Patskovsky Y., et al. Discovery of GBT440, an orally bioavailable R-state stabilizer of sickle cell hemoglobin. ACS Med. Chem. Lett. 2017;8:321–326. doi: 10.1021/acsmedchemlett.6b00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C., Burmeister T., Gröger D., Tsaur G., Fechina L., Renneville A., Sutton R., Venn N.C., Emerenciano M., Pombo-de-Oliveira M.S., et al. The MLL recombinome of acute leukemias in 2017. Leukemia. 2018;32:273–284. doi: 10.1038/leu.2017.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney R.D., Desbonnet L., Clarke G., Dinan T.G., Cryan J.F. The microbiome: stress, health and disease. Mamm. Genome. 2014;25:49–74. doi: 10.1007/s00335-013-9488-5. [DOI] [PubMed] [Google Scholar]
- Moore K.A., Ema H., Lemischka I.R. In vitro maintenance of highly purified, transplantable hematopoietic stem cells. Blood. 1997;89:4337–4347. doi: 10.1182/blood.v89.12.4337. [DOI] [PubMed] [Google Scholar]
- Morrison S.J., Hemmati H.D., Wandycz A.M., Weissman I.L. The purification and characterization of fetal liver hematopoietic stem cells. Proc. Natl. Acad. Sci. U S A. 1995;92:10302–10306. doi: 10.1073/pnas.92.22.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A.M., Medvinsky A., Strouboulis J., Grosveld F., Dzierzakt E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1994;1:291–301. doi: 10.1016/1074-7613(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Nagasawa T., Kikutani H., Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc. Natl. Acad. Sci. U S A. 1994;91:2305–2309. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura-Ishizu A., Ito K., Suda T. Hematopoietic stem cell metabolism during development and aging. Dev. Cell. 2020;54:239–255. doi: 10.1016/j.devcel.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura-Ishizu A., Takubo K., Fujioka M., Suda T. Megakaryocytes are essential for HSC quiescence through the production of thrombopoietin. Biochem. Biophys. Res. Commun. 2014;454:353–357. doi: 10.1016/j.bbrc.2014.10.095. [DOI] [PubMed] [Google Scholar]
- Nakamura-Ishizu A., Takubo K., Kobayashi H., Suzuki-Inoue K., Suda T. CLEC-2 in megakaryocytes is critical for maintenance of hematopoietic stem cells in the bone marrow. J. Exp. Med. 2015;212:2133–2146. doi: 10.1084/jem.20150057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel J.V. The inheritance of sickle cell anemia. Science. 1949;110:64–66. doi: 10.1126/science.110.2846.64. [DOI] [PubMed] [Google Scholar]
- Nevitt S.J., Jones A.P., Howard J. Hydroxyurea (hydroxycarbamide) for sickle cell disease. Cochrane Database Syst. Rev. 2017;4:CD002202. doi: 10.1002/14651858.CD002202.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby G.A., Yen J.S., Woodard K.J., Mayuranathan T., Lazzarotto C.R., Li Y., Sheppard-Tillman H., Porter S.N., Yao Y., Mayberry K., et al. Base editing of haematopoietic stem cells rescues sickle cell disease in mice. Nature. 2021;595:295–302. doi: 10.1038/s41586-021-03609-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niihara Y., Matsui N.M., Shen Y.M., Akiyama D.A., Johnson C.S., Sunga M.A., Magpayo J., Embury S.H., Kalra V.K., Ho Cho S., Tanaka K.R. L-glutamine therapy reduces endothelial adhesion of sickle red blood cells to human umbilical vein endothelial cells. BMC Blood Disord. 2005;5:4. doi: 10.1186/1471-2326-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niihara Y., Miller S.T., Kanter J., Lanzkron S., Smith W.R., Hsu L.L., Gordeuk V.R., Viswanathan K., Sarnaik S., Osunkwo I., et al. A phase 3 trial of l-glutamine in sickle cell disease. N. Engl. J. Med. 2018;379:226–235. doi: 10.1056/nejmoa1715971. [DOI] [PubMed] [Google Scholar]
- Niihara Y., Zerez C.R., Akiyama D.S., Tanaka K.R. Oral L-glutamine therapy for sickle cell anemia: I. Subjective clinical improvement and favorable change in red cell NAD redox potential. Am. J. Hematol. 1998;58:117–121. doi: 10.1002/(sici)1096-8652(199806)58:2<117::aid-ajh5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Nilsson S.K., Johnston H.M., Coverdale J.A. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood. 2001;97:2293–2299. doi: 10.1182/blood.v97.8.2293. [DOI] [PubMed] [Google Scholar]
- Nilsson S.K., Johnston H.M., Whitty G.A., Williams B., Webb R.J., Denhardt D.T., Bertoncello I., Bendall L.J., Simmons P.J., Haylock D.N. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005;106:1232–1239. doi: 10.1182/blood-2004-11-4422. [DOI] [PubMed] [Google Scholar]
- Noguchi C.T., Rodgers G.P., Serjeant G., Schechter A.N. Levels of fetal hemoglobin necessary for treatment of sickle cell disease. N. Engl. J. Med. 1988;318:96–99. doi: 10.1056/NEJM198801143180207. [DOI] [PubMed] [Google Scholar]
- Novelli E.M., Gladwin M.T. Crises in sickle cell disease. Chest. 2016;149:1082–1093. doi: 10.1016/j.chest.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksenberg D., Dufu K., Patel M.P., Chuang C., Li Z., Xu Q., Silva-Garcia A., Zhou C., Hutchaleelaha A., Patskovska L., et al. GBT440 increases haemoglobin oxygen affinity, reduces sickling and prolongs RBC half-life in a murine model of sickle cell disease. Br. J. Haematol. 2016;175:141–153. doi: 10.1111/bjh.14214. [DOI] [PubMed] [Google Scholar]
- Olson T.S., Caselli A., Otsuru S., Hofmann T.J., Williams R., Paolucci P., Dominici M., Horwitz E.M. Megakaryocytes promote murine osteoblastic HSC niche expansion and stem cell engraftment after radioablative conditioning. Blood. 2013;121:5238–5249. doi: 10.1182/blood-2012-10-463414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omatsu Y., Seike M., Sugiyama T., Kume T., Nagasawa T. Foxc1 is a critical regulator of haematopoietic stem/progenitor cell niche formation. Nature. 2014;508:536–540. doi: 10.1038/nature13071. [DOI] [PubMed] [Google Scholar]
- Omatsu Y., Sugiyama T., Kohara H., Kondoh G., Fujii N., Kohno K., Nagasawa T. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33:387–399. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Park S.Y., Matte A., Jung Y., Ryu J., Anand W.B., Han E.Y., Liu M., Carbone C., Melisi D., Nagasawa T., et al. Pathologic angiogenesis in the bone marrow of humanized sickle cell mice is reversed by blood transfusion. Blood. 2020;135:2071–2084. doi: 10.1182/blood.2019002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszty C., Brion C.M., Manci E., Witkowska H.E., Stevens M.E., Mohandas N., Rubin E.M. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science. 1997;278:876–878. doi: 10.1126/science.278.5339.876. [DOI] [PubMed] [Google Scholar]
- Pattabhi S., Lotti S.N., Berger M.P., Singh S., Lux C.T., Jacoby K., Lee C., Negre O., Scharenberg A.M., Rawlings D.J. In vivo outcome of homology-directed repair at the HBB gene in HSC using alternative donor template delivery methods. Mol. Ther. Nucleic Acids. 2019;17:277–288. doi: 10.1016/j.omtn.2019.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauling L., Itano H.A., Singer S.J., Wells I.C. Sickle cell anemia a molecular disease. Science. 1949;110:543–548. doi: 10.1126/science.110.2865.543. [DOI] [PubMed] [Google Scholar]
- Pecker L.H., Darbari D.S. Psychosocial and affective comorbidities in sickle cell disease. Neurosci. Lett. 2019;705:1–6. doi: 10.1016/j.neulet.2019.04.011. [DOI] [PubMed] [Google Scholar]
- Piel F.B., Patil A.P., Howes R.E., Nyangiri O.A., Gething P.W., Dewi M., Temperley W.H., Williams T.N., Weatherall D.J., Hay S.I. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet. 2013;381:142–151. doi: 10.1016/S0140-6736(12)61229-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel F.B., Steinberg M.H., Rees D.C. Sickle cell disease. N. Engl. J. Med. 2017;376:1561–1573. doi: 10.1056/NEJMra1510865. [DOI] [PubMed] [Google Scholar]
- Pierce H., Zhang D., Magnon C., Lucas D., Christin J.R., Huggins M., Schwartz G.J., Frenette P.S. Cholinergic signals from the CNS regulate G-CSF-mediated HSC mobilization from bone marrow via a glucocorticoid signaling relay. Cell Stem Cell. 2017;20:648–658.e4. doi: 10.1016/j.stem.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras E.M., Mirantes-Barbeito C., Fong S., Loeffler D., Kovtonyuk L.V., Zhang S., Lakshminarasimhan R., Chin C.P., Techner J.M., Will B., et al. Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nat. Cell Biol. 2016;18:607–618. doi: 10.1038/ncb3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho S., Frenette P.S. Haematopoietic stem cell activity and interactions with the niche. Nat. Rev. Mol. Cell Biol. 2019;20:303–320. doi: 10.1038/s41580-019-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho S., Lacombe J., Hanoun M., Mizoguchi T., Bruns I., Kunisaki Y., Frenette P.S. PDGFRα and CD51 mark human Nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J. Exp. Med. 2013;210:1351–1367. doi: 10.1084/jem.20122252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho S., Marchand T., Yang E., Wei Q., Nerlov C., Frenette P.S. Lineage-biased hematopoietic stem cells are regulated by distinct niches. Dev. Cell. 2018;44:634–641.e4. doi: 10.1016/j.devcel.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt O.S. Sickle cell anemia as an inflammatory disease. J. Clin. Invest. 2000;106:337–338. doi: 10.1172/JCI10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt O.S., Brambilla D.J., Rosse W.F., Milner P.F., Castro O., Steinberg M.H., Klug P.P. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N. Engl. J. Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- Platt O.S., Thorington B.D., Brambilla D.J., Milner P.F., Rosse W.F., Vichinsky E., Kinney T.R. Pain in sickle cell disease. Rates and risk factors. N. Engl. J. Med. 1991;325:11–16. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
- Polanowska-Grabowska R., Wallace K., Field J.J., Chen L., Marshall M.A., Figler R., Gear A.R., Linden J. P-selectin-mediated platelet-neutrophil aggregate formation activates neutrophils in mouse and human sickle cell disease. Arterioscler. Thromb. Vasc. Biol. 2010;30:2392–2399. doi: 10.1161/ATVBAHA.110.211615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postler T.S., Ghosh S. Understanding the holobiont: how microbial metabolites affect human health and shape the immune system. Cell Metabol. 2017;26:110–130. doi: 10.1016/j.cmet.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos M.G., Guo P., Kofler N.M., Pinho S., Gutkin M.C., Tikhonova A., Aifantis I., Frenette P.S., Kitajewski J., Rafii S., Butler J.M. Endothelial Jagged-1 is necessary for homeostatic and regenerative hematopoiesis. Cell Rep. 2013;4:1022–1034. doi: 10.1016/j.celrep.2013.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos M.G., Ramalingam P., Gutkin M.C., Llanos P., Gilleran K., Rabbany S.Y., Butler J.M. Endothelial transplantation rejuvenates aged hematopoietic stem cell function. J. Clin. Invest. 2017;127:4163–4178. doi: 10.1172/JCI93940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafii S., Butler J.M., Ding B.S. Angiocrine functions of organ-specific endothelial cells. Nature. 2016;529:316–325. doi: 10.1038/nature17040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafii S., Shapiro F., Pettengell R., Ferris B., Nachman R.L., Moore M.A., Asch A.S. Human bone marrow microvascular endothelial cells support long-term proliferation and differentiation of myeloid and megakaryocytic progenitors. Blood. 1995;86:3353–3363. doi: 10.1182/blood.v86.9.3353.bloodjournal8693353. [DOI] [PubMed] [Google Scholar]
- Rai P., Ataga K.I. Drug therapies for the management of sickle cell disease. F1000Res. 2020;9 doi: 10.12688/f1000research.22433.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D.C., Williams T.N., Gladwin M.T. Sickle-cell disease. Lancet. 2010;376:2018–2031. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- Reiter C.D., Wang X., Tanus-Santos J.E., Hogg N., Cannon R.O., 3rd, Schechter A.N., Gladwin M.T. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat. Med. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- Renders S., Svendsen A.F., Panten J., Rama N., Maryanovich M., Sommerkamp P., Ladel L., Redavid A.R., Gibert B., Lazare S., et al. Niche derived netrin-1 regulates hematopoietic stem cell dormancy via its receptor neogenin-1. Nat. Commun. 2021;12:608. doi: 10.1038/s41467-020-20801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repka T., Hebbel R.P. Hydroxyl radical formation by sickle erythrocyte membranes: role of pathologic iron deposits and cytoplasmic reducing agents. Blood. 1991;78:2753–2758. doi: 10.1182/blood.v78.10.2753.2753. [DOI] [PubMed] [Google Scholar]
- Ribeil J.A., Hacein-Bey-Abina S., Payen E., Magnani A., Semeraro M., Magrin E., Caccavelli L., Neven B., Bourget P., El Nemer W., et al. Gene therapy in a patient with sickle cell disease. N. Engl. J. Med. 2017;376:848–855. doi: 10.1056/NEJMoa1609677. [DOI] [PubMed] [Google Scholar]
- Roh J.S., Sohn D.H. Damage-associated molecular patterns in inflammatory diseases. Immune. Netw. 2018;18:e27. doi: 10.4110/in.2018.18.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosanwo T.O., Bauer D.E. Editing outside the body: ex vivo gene-modification for beta-hemoglobinopathy cellular therapy. Mol. Ther. 2021;29:3163–3178. doi: 10.1016/j.ymthe.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan T.M., Ciavatta D.J., Townes T.M. Knockout-transgenic mouse model of sickle cell disease. Science. 1997;278:873–876. doi: 10.1126/science.278.5339.873. [DOI] [PubMed] [Google Scholar]
- Saçma M., Pospiech J., Bogeska R., de Back W., Mallm J.P., Sakk V., Soller K., Marka G., Vollmer A., Karns R., et al. Haematopoietic stem cells in perisinusoidal niches are protected from ageing. Nat. Cell Biol. 2019;21:1309–1320. doi: 10.1038/s41556-019-0418-y. [DOI] [PubMed] [Google Scholar]
- Salinas Cisneros G., Thein S.L. Research in sickle cell disease: from bedside to bench to bedside. Hemasphere. 2021;5:e584. doi: 10.1097/HS9.0000000000000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjuan-Pla A., Macaulay I.C., Jensen C.T., Woll P.S., Luis T.C., Mead A., Moore S., Carella C., Matsuoka S., Jones T.B., et al. Platelet-biased stem cells reside at the apex of the haematopoietic stem-cell hierarchy. Nature. 2013;502:232–236. doi: 10.1038/nature12495. [DOI] [PubMed] [Google Scholar]
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cell. 1978;4:7–25. [PubMed] [Google Scholar]
- Seike M., Omatsu Y., Watanabe H., Kondoh G., Nagasawa T. Stem cell niche-specific Ebf3 maintains the bone marrow cavity. Genes Dev. 2018;32:359–372. doi: 10.1101/gad.311068.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serjeant G.R. The natural history of sickle cell disease. Cold Spring Harb. Perspect. Med. 2013;3:a011783. doi: 10.1101/cshperspect.a011783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setty B.N.Y., Kulkarni S., Stuart M.J. Role of erythrocyte phosphatidylserine in sickle red cell-endothelial adhesion. Blood. 2002;99:1564–1571. doi: 10.1182/blood.v99.5.1564. [DOI] [PubMed] [Google Scholar]
- Shen B., Tasdogan A., Ubellacker J.M., Zhang J., Nosyreva E.D., Du L., Murphy M.M., Hu S., Yi Y., Kara N., et al. A mechanosensitive peri-arteriolar niche for osteogenesis and lymphopoiesis. Nature. 2021;591:438–444. doi: 10.1038/s41586-021-03298-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shet A.S., Lizarralde-Iragorri M.A., Naik R.P. The molecular basis for the prothrombotic state in sickle cell disease. Haematologica. 2020;105:2368–2379. doi: 10.3324/haematol.2019.239350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein L., Goncalves K.A., Kharchenko P.V., Turcotte R., Kfoury Y., Mercier F., Baryawno N., Severe N., Bachand J., Spencer J.A., et al. Proximity-based differential single-cell analysis of the niche to identify stem/progenitor cell regulators. Cell Stem Cell. 2016;19:530–543. doi: 10.1016/j.stem.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh L., Brennan T.A., Russell E., Kim J.H., Chen Q., Brad Johnson F., Pignolo R.J. Aging alters bone-fat reciprocity by shifting in vivo mesenchymal precursor cell fate towards an adipogenic lineage. Bone. 2016;85:29–36. doi: 10.1016/j.bone.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipkins D.A., Wei X., Wu J.W., Runnels J.M., Côté D., Means T.K., Luster A.D., Scadden D.T., Lin C.P. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435:969–973. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer J.A., Ferraro F., Roussakis E., Klein A., Wu J., Runnels J.M., Zaher W., Mortensen L.J., Alt C., Turcotte R., et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508:269–273. doi: 10.1038/nature13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatoyannopoulos G. Control of globin gene expression during development and erythroid differentiation. Exp. Hematol. 2005;33:259–271. doi: 10.1016/j.exphem.2004.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg M.H. Predicting clinical severity in sickle cell anaemia. Br. J. Haematol. 2005;129:465–481. doi: 10.1111/j.1365-2141.2005.05411.x. [DOI] [PubMed] [Google Scholar]
- Steinberg M.H. Sickle cell anemia, the first molecular disease: overview of molecular etiology, pathophysiology, and therapeutic approaches. Sci. World J. 2008;8:1295–1324. doi: 10.1100/tsw.2008.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg M.H. Genetic etiologies for phenotypic diversity in sickle cell anemia. Sci. World J. 2009;9:46–67. doi: 10.1100/tsw.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg M.H. Fetal hemoglobin in sickle cell anemia. Blood. 2020;136:2392–2400. doi: 10.1182/blood.2020007645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C., Jang T., Mo G., Mohamed N., Poplawska M., Egini O., Dutta D., Lim S.H. Antibiotics to modify sickle cell disease vaso-occlusive crisis? Blood Rev. 2021;50:100867. doi: 10.1016/j.blre.2021.100867. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Kohara H., Noda M., Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Sundd P., Gladwin M.T., Novelli E.M. Pathophysiology of sickle cell disease. Annu. Rev. Pathol. 2019;14:263–292. doi: 10.1146/annurev-pathmechdis-012418-012838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerlick R.A., Eckman J.R., Kumar A., Jeitler M., Wick T.M., KumAr A. Alpha 4 beta 1-integrin expression on sickle reticulocytes: vascular cell adhesion molecule-1-dependent binding to endothelium. Blood. 1993;82:1891–1899. doi: 10.1182/blood.v82.6.1891.1891. [DOI] [PubMed] [Google Scholar]
- Takeda S., Elefteriou F., Levasseur R., Liu X., Zhao L., Parker K.L., Armstrong D., Ducy P., Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- Tang A., Strat A.N., Rahman M., Zhang H., Bao W., Liu Y., Shi D., An X., Manwani D., Shi P., et al. Murine bone marrow mesenchymal stromal cells have reduced hematopoietic maintenance ability in sickle cell disease. Blood. 2021;138:2570–2582. doi: 10.1182/blood.2021012663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari S., Brousse V., Piel F.B., Menzel S., Rees D.C. Environmental determinants of severity in sickle cell disease. Haematologica. 2015;100:1108–1116. doi: 10.3324/haematol.2014.120030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikhonova A.N., Dolgalev I., Hu H., Sivaraj K.K., Hoxha E., Cuesta-Domínguez Á., Pinho S., Akhmetzyanova I., Gao J., Witkowski M., et al. The bone marrow microenvironment at single-cell resolution. Nature. 2019;569:222–228. doi: 10.1038/s41586-019-1104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres L., Conran N. Emerging pharmacotherapeutic approaches for the management of sickle cell disease. Expet Opin. Pharmacother. 2019;20:173–186. doi: 10.1080/14656566.2018.1548610. [DOI] [PubMed] [Google Scholar]
- Torres L.S., Teles L.I.M., Shaul M.E., Fridlender Z.G., Santos I., Leonardo F.C., de Melo Campos P., Benites B.D., Olalla Saad S.T., Costa F.F., Conran N. Accelerated low-density neutrophil transition in sickle cell anaemia may contribute to disease pathophysiology. Br. J. Haematol. 2021;197:232–235. doi: 10.1111/bjh.18009. [DOI] [PubMed] [Google Scholar]
- Turhan A., Weiss L.A., Mohandas N., Coller B.S., Frenette P.S. Primary role for adherent leukocytes in sickle cell vascular occlusion: a new paradigm. Proc. Natl. Acad. Sci. U S A. 2002;99:3047–3051. doi: 10.1073/pnas.052522799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eeden S.F., Kitagawa Y., Klut M.E., Lawrence E., Hogg J.C. Polymorphonuclear leukocytes released from the bone marrow preferentially sequester in lung microvessels. Microcirculation. 1997;4:369–380. doi: 10.3109/10739689709146801. [DOI] [PubMed] [Google Scholar]
- Vekilov P.G. Sickle-cell haemoglobin polymerization: is it the primary pathogenic event of sickle-cell anaemia? Br. J. Haematol. 2007;139:173–184. doi: 10.1111/j.1365-2141.2007.06794.x. [DOI] [PubMed] [Google Scholar]
- Vichinsky E., Hoppe C.C., Ataga K.I., Ware R.E., Nduba V., El-Beshlawy A., Hassab H., Achebe M.M., Alkindi S., Brown R.C., et al. A phase 3 randomized trial of voxelotor in sickle cell disease. N. Engl. J. Med. 2019;381:509–519. doi: 10.1056/NEJMoa1903212. [DOI] [PubMed] [Google Scholar]
- Vichinsky E.P., Neumayr L.D., Earles A.N., Williams R., Lennette E.T., Dean D., Nickerson B., Orringer E., McKie V., Bellevue R., et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. N. Engl. J. Med. 2000;342:1855–1865. doi: 10.1056/NEJM200006223422502. [DOI] [PubMed] [Google Scholar]
- Ware R.E., de Montalembert M., Tshilolo L., Abboud M.R. Sickle cell disease. Lancet. 2017;390:311–323. doi: 10.1016/S0140-6736(17)30193-9. [DOI] [PubMed] [Google Scholar]
- Wei A.H., Döhner H., Pocock C., Montesinos P., Afanasyev B., Dombret H., Ravandi F., Sayar H., Jang J.H., Porkka K., et al. Oral azacitidine maintenance therapy for acute myeloid leukemia in first remission. N. Engl. J. Med. 2020;383:2526–2537. doi: 10.1056/NEJMoa2004444. [DOI] [PubMed] [Google Scholar]
- Wineman J., Moore K., Lemischka I., Muller-Sieburg C. Functional heterogeneity of the hematopoietic microenvironment: rare stromal elements maintain long-term repopulating stem cells. Blood. 1996;87:4082–4090. doi: 10.1182/blood.v87.10.4082.bloodjournal87104082. [DOI] [PubMed] [Google Scholar]
- Winkler I.G., Sims N.A., Pettit A.R., Barbier V., Nowlan B., Helwani F., Poulton I.J., van Rooijen N., Alexander K.A., Raggatt L.J., Lévesque J.P. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116:4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- Wun T., Cordoba M., Rangaswami A., Cheung A.W., Paglieroni T. Activated monocytes and platelet-monocyte aggregates in patients with sickle cell disease. Clin. Lab. Haematol. 2002;24:81–88. doi: 10.1046/j.1365-2257.2002.t01-1-00433.x. [DOI] [PubMed] [Google Scholar]
- Xie Y., Yin T., Wiegraebe W., He X.C., Miller D., Stark D., Perko K., Alexander R., Schwartz J., Grindley J.C., et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 2009;457:97–101. doi: 10.1038/nature07639. [DOI] [PubMed] [Google Scholar]
- Xu C., Gao X., Wei Q., Nakahara F., Zimmerman S.E., Mar J., Frenette P.S. Stem cell factor is selectively secreted by arterial endothelial cells in bone marrow. Nat. Commun. 2018;9:2449. doi: 10.1038/s41467-018-04726-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Gulinello M., Frenette P.S. Nociceptors protect sickle cell disease mice from vaso-occlusive episodes and chronic organ damage. J. Exp. Med. 2021;218 doi: 10.1084/jem.20200065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Lee S.K., Zhang D., Frenette P.S. The gut microbiome regulates psychological-stress-induced inflammation. Immunity. 2020;53:417–428.e4. doi: 10.1016/j.immuni.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S., Ema H., Karlsson G., Yamaguchi T., Miyoshi H., Shioda S., Taketo M.M., Karlsson S., Iwama A., Nakauchi H. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147:1146–1158. doi: 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- Yu V.W., Saez B., Cook C., Lotinun S., Pardo-Saganta A., Wang Y.H., Lymperi S., Ferraro F., Raaijmakers M.H., Wu J.Y., et al. Specific bone cells produce DLL4 to generate thymus-seeding progenitors from bone marrow. J. Exp. Med. 2015;212:759–774. doi: 10.1084/jem.20141843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahalka A.H., Frenette P.S. Nerves in cancer. Nat. Rev. Cancer. 2020;20:143–157. doi: 10.1038/s41568-019-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J., Wu Y., Ren C., Bonanno J., Shen A.H., Shea D., Gehrke J.M., Clement K., Luk K., Yao Q., et al. Therapeutic base editing of human hematopoietic stem cells. Nat. Med. 2020;26:535–541. doi: 10.1038/s41591-020-0790-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.C., Kaba M., Ge G., Xie K., Tong W., Hug C., Lodish H.F. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat. Med. 2006;12:240–245. doi: 10.1038/nm1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Chen G., Manwani D., Mortha A., Xu C., Faith J.J., Burk R.D., Kunisaki Y., Jang J.E., Scheiermann C., et al. Neutrophil ageing is regulated by the microbiome. Nature. 2015;525:528–532. doi: 10.1038/nature15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Gao X., Li H., Borger D.K., Wei Q., Yang E., Xu C., Pinho S., Frenette P.S. The microbiota regulates hematopoietic stem cell fate decisions by controlling iron availability in bone marrow. Cell Stem Cell. 2022;29:232–247.e7. doi: 10.1016/j.stem.2021.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Niu C., Ye L., Huang H., He X., Tong W.G., Ross J., Haug J., Johnson T., Feng J.Q., et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- Zhao M., Perry J.M., Marshall H., Venkatraman A., Qian P., He X.C., Ahamed J., Li L. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat. Med. 2014;20:1321–1326. doi: 10.1038/nm.3706. [DOI] [PubMed] [Google Scholar]
- Zhao M., Tao F., Venkatraman A., Li Z., Smith S.E., Unruh J., Chen S., Ward C., Qian P., Perry J.M., et al. N-Cadherin-Expressing bone and marrow stromal progenitor cells maintain reserve hematopoietic stem cells. Cell Rep. 2019;26:652–669.e6. doi: 10.1016/j.celrep.2018.12.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B.O., Ding L., Morrison S.J. Hematopoietic stem and progenitor cells regulate the regeneration of their niche by secreting Angiopoietin-1. Elife. 2015;4:e05521. doi: 10.7554/eLife.05521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B.O., Yue R., Murphy M.M., Peyer J.G., Morrison S.J. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014;15:154–168. doi: 10.1016/j.stem.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]