Summary
When corneal epithelial stem cells residing in the corneal limbus become dysfunctional, called a limbal stem cell deficiency (LSCD), corneal transparency is decreased, causing severe vision loss. Transplantation of corneal epithelial cell sheets (CEPS) derived from stem cells, including induced pluripotent stem cells, is a promising treatment for LSCD. However, the potential effect of human leukocyte antigen (HLA) concordance on CEPS transplantation has not been addressed. Here, we show that there is no difference in the immune response to CEPS between HLA-matched and -unmatched peripheral blood mononuclear cells in mixed lymphocyte reactions. CEPS transplantation in cynomolgus monkeys revealed that the immune response to major histocompatibility-unmatched CEPS was not strong and could be controlled by local steroid administration. Furthermore, programmed death ligand 1 was identified as an immunosuppressive molecule in CEPS under inflammatory conditions in vitro. Our results indicate that corneal epithelium has low immunogenicity and allogeneic CEPS transplantation requires mild immunosuppression.
Keywords: corneal epithelium, transplantation, MHC, non-human primate, regenerative medicine, induced pluripotent stem cell, PD-L1, immune rejection, cornea, stem cells
Highlights
-
•
There is no difference in the immune response to CEPS owing to HLA conformity in MLR
-
•
The immune response to MHC-unmatched CEPS is not strong after transplantation
-
•
Local steroid administration could control the immune response to MHC-unmatched CEPS
-
•
PD-L1 was identified as an immunosuppressive molecule in CEPS
In this article, Nishida et al. show that the immune response to MHC-unmatched CEPS was not strong and could be controlled by local steroid administration. They identified PD-L1 as an immunosuppressive molecule in CEPS under inflammatory conditions in vitro. Their results indicate that corneal epithelium has low immunogenicity and allogeneic CEPS transplantation requires mild immunosuppression.
Introduction
The cornea is a transparent tissue covered by the corneal epithelium. Corneal epithelial stem cells reside in the basal epithelial layer of the limbus, between the cornea and the conjunctiva, and supply corneal epithelial cells (CEC) to the central part of the cornea to maintain homeostasis. Limbal stem cell deficiency (LSCD) occurs when the limbus is damaged and the corneal epithelial stem cells are lost or become dysfunctional through ocular surface diseases, such as Stevens-Johnson syndrome or ocular pemphigoid, or by injury, including thermal or chemical burns. LSCD leads to conjunctival invasion, decreases ocular surface transparency, and causes severe vision loss.
LSCD treatment is a prominent issue. Corneal limbal transplantation (LT) (Thoft, 1984) using a donor cornea containing the corneal epithelium, stroma, keratocytes, and antigen-presenting cells (APCs), has been conventionally performed to treat LSCD. However, a shortage of donor corneas (Gain et al., 2016) limits the opportunity for LT, and rejection owing to the mixed cell types, including donor APCs, negatively affects clinical outcomes (Ilari and Daya, 2002). To address these issues, regenerative medicine using autologous cell sources, including cultured corneal epithelial transplantation (Pellegrini et al., 2018) using somatic stem cells and cultivated oral mucosal epithelial transplantation (Nishida et al., 2004a), has recently been implemented. However, these treatments have disadvantages, including the need for the fellow eye to be healthy and the risk of neovascularization in the transplanted eye’s cornea (Nakamura et al., 2004).
A solution is corneal epithelium regeneration using induced pluripotent stem cells (iPSCs). We recently developed the self-formed ectodermal autonomous multi-zone method (Hayashi et al., 2016, 2017) to efficiently induce progenitor cells of various tissues in the eye, including the corneal epithelium, in vitro. Clinical application of corneal regenerative medicine using iPSC-derived CEC sheets (iCEPS) to treat LSCD is in progress. Ideally, autologous iCEPS would be used to avoid rejection. However, the cost of such personalized medicine is prohibitive and exceptionally time consuming. Induction of iCEPS from allogeneic iPSCs can decrease both the cost and manufacturing time. While allogeneic transplantation can be both human leukocyte antigen (HLA)-matched and -unmatched, access to HLA-matched donors is limited. To increase the incidence of HLA-matched transplantation using tissues derived from iPSCs, an HLA homozygous iPSC stock (iPSC bank) has been established and is currently in operation (Gourraud et al., 2012).
The positive effect of HLA adaptation on transplantation was confirmed by MHC compatibility transplantation studies using MHC-controlled cynomolgus monkeys (Ishigaki et al., 2018) in retinal pigment epithelial (RPE) cells (Sugita et al., 2016), cardiomyocytes (Kawamura et al., 2016), and neurons (Morizane et al., 2017). Regarding somatic stem cell-derived CEPS (sCEPS) transplantation, the lower the degree of HLA compatibility, the greater the proportion of graft failure (Zakaria et al., 2014). Conversely, Shimazaki et al. reported no such association (Shimazaki et al., 2002). However, since disease conditions differed in these studies, the effect of HLA conformity in CEPS transplantation remains controversial.
Many immunomodulatory molecules expressed on the cell surface of the anterior segment of the eye were reported (Kunishige and Hori, 2013; Lee et al., 2002). Among them, programmed death ligand 1 (PD-L1) has recently received attention for suppressing T cell activation and downregulating pro-inflammatory cytokine production (Dermani et al., 2019).
Here, we examined in vitro and in vivo effects of MHC conformity in CEPS transplantation for clinical application, focused on PD-L1 among immunosuppressive molecules, and examined its expression in CEPS.
Results
Immune responses of HLA-matched and -unmatched peripheral blood mononuclear cells to iCEPS
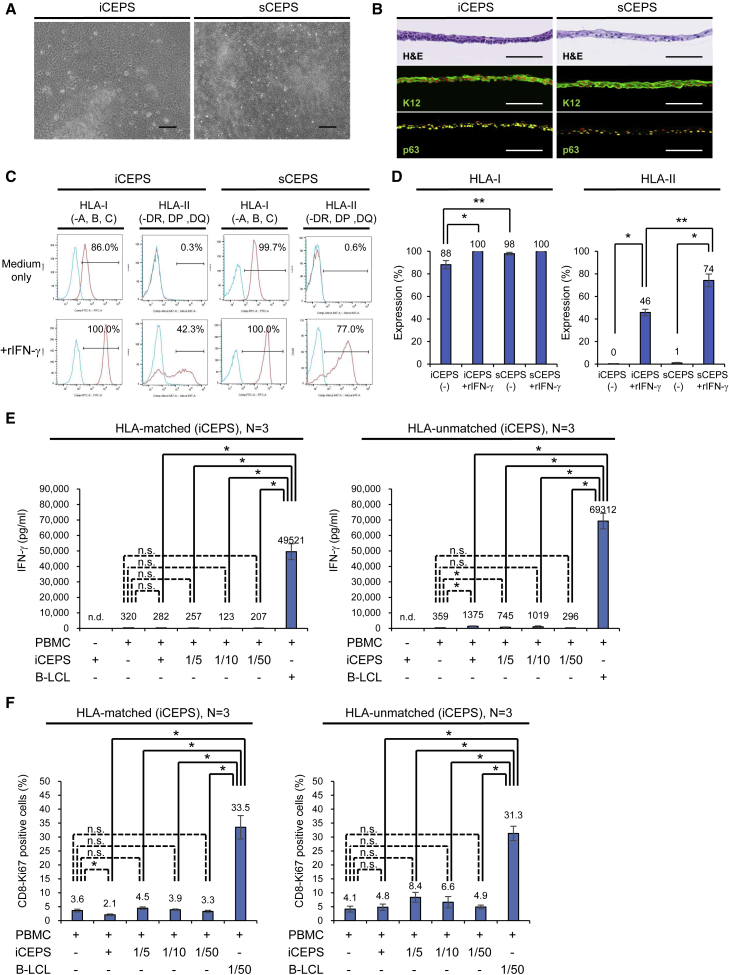
Human iCEPSs (Hayashi et al., 2016, 2017) and sCEPSs (Hayashi et al., 2007) were prepared as previously described (Figures 1A and 1B). To examine the potential immunogenicity of CEPS for transplantation in humans, HLA expression was analyzed using flow cytometry. iCEPS and sCEPS predominantly expressed HLA-I on the cell surface (Figure 1C). CEPS was incubated with human recombinant interferon-γ (rIFN-γ), a pro-inflammatory cytokine, to mimic surgical inflammation. After this treatment, HLA-I and HLA-II expression increased in iCEPS and sCEPS (Figures 1C, 1D, and S1). HLA-I expression without rIFN-γ treatment and HLA-II expression with rIFN-γ treatment were weaker in iCEPS than in sCEPS.
Figure 1.
MLR of PBMCs and iPSC-derived iCEPS
(A) Phase-contrast microscopy of iCEPS and sCEPS. Scale bars, 200 μm.
(B) H&E staining and K12 and p63 (green) immunostaining of iCEPS and sCEPS. Scale bars, 100 μm. Nuclei, red (immunostaining).
(C and D) HLA-I and HLA-II expression of iCEPS and sCEPS with or without rIFN-γ (for 48 h) analyzed using flow cytometry. Blue histograms represent isotype control staining (mouse IgG, C). Representative data (C) and means ± SEM (D) of ten experiments are presented. ∗p < 0.05 compared to two groups using the Wilcoxon signed-rank test. ∗∗p < 0.05 compared with two groups using the Mann-Whitney U test. See also Figure S1.
(E and F) PBMCs were co-cultured with allogeneic iCEPS at a ratio of 1 × 105 (1:1) to 2 × 103 (1:1/50) cells/well. As a positive control for allogeneic immune rejection, isolated and B-LCL were prepared from an HLA-unmatched healthy adult donor, and co-cultured with PBMCs at a ratio of 1 × 105 (1:1, E) and 2 × 103 (1/50:1, F) cells/well. After 7 days, supernatants were harvested and assessed for IFN-γ secretion using ELISA (E), and the PBMCs were harvested for flow cytometric analysis (F). Three healthy adult donors were used for each HLA-matched and -unmatched experiments. Means ± SEM of 12 ELISA determinations (four ELISA determinations in each experiment, E), and means ± SEM data from six flow cytometric analyses (data from two flow cytometric analyses in each experiment, F) are presented. ∗p < 0.05 compared two groups using the Mann-Whitney U test. n.s., not significant. n.d., not detected. See also Figure S2.
We used a mixed lymphocyte reaction (MLR) to investigate the differences in immune response owing to CEPS HLA compatibility. Human iCEPS were co-cultured with peripheral blood mononuclear cells (PBMCs) (three HLA-matched and three HLA-unmatched) for 7 days, after which IFN-γ, interleukin-17 (IL-17, another pro-inflammatory cytokine), and IL-10 (an anti-inflammatory cytokine) secretions were assessed using ELISA, and CD8-Ki67 double-positive cells were analyzed using flow cytometry. HLA-unmatched iCEPS induced more IFN-γ secretion in PBMCs than HLA-matched iCEPS in a specific mixing ratio, but the IFN-γ levels were much lower than in transformed B cells (B-LCL) derived from an HLA-unmatched healthy adult donor (Figure 1E). HLA-matched and -unmatched iCEPS slightly induce IL-17 or IL-10 in a specific mixing ratio, but no significant increase was observed (Figures S2A and S2B). HLA-matched and -unmatched iCEPS did not increase the ratios of CD8 and Ki67 double-positive cells (Figure 1F). The observed ratios were lower than in B-LCL. These results suggest that allogeneic immune reaction against iCEPS in vitro was negligible, irrespective of HLA compatibility.
CEPS transplantation to cynomolgus monkeys
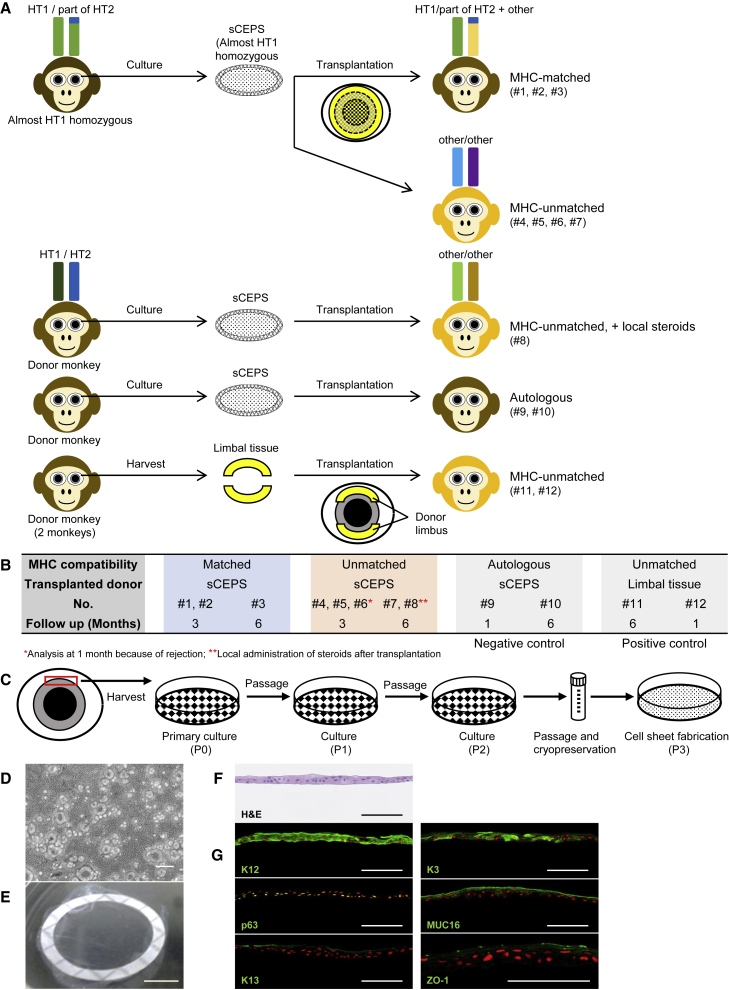
We used cynomolgus monkeys to examine the immune response to CEPS and the influence of MHC conformity on cell engraftment in vivo. Since a corneal epithelial stem cell induction method from cynomolgus monkey iPSCs has not yet been established, we used sCEPS (Figures 2A and 2B). The cynomolgus monkey LSCD model was created by surgical dissection of the limbal tissue (lamellar keratectomy) (Figure S3A). The absence of corneal epithelium regeneration and conjunctival invasion into the cornea in the LSCD model were confirmed by assessing K12 (a specific differentiation marker of CEC) and K13 (a conjunctival epithelial marker) expression using PCR and immunostaining (Figures S3B–S3D). The sCEPS was prepared from the limbus of the cynomolgus monkey (Figure 2C) (details in the experimental procedures). They were stratified (about three cell layers) similarly to humans, and 3.12 ± 0.46 × 105 cells/cm2 (mean ± SEM) per sheet (Figures 2D–2F). K12 and p63 (a stratified epithelial stem cell marker) were observed in all layers (Figure 2G). Other differentiation markers of CEC, namely K3, MUC16 (membrane-type mucin), and ZO-1 (a cell-cell adhesion protein), were expressed in sCEPS. K13 was observed locally in the outermost layer of sCEPS. The sCEPS was transplanted onto the corneas of three MHC-matched (#1–3) and five MHC-unmatched (#4–8) animals (Figures 2A and 2B; Tables S1 and S2). To characterize different immune responses, steroids were systemically administered only during the perioperative period, and immunosuppressive agents were not used. In one MHC-unmatched monkey (#8), steroids were injected subconjunctivally weekly after sCEPS transplantation assuming clinical practice; topical corticosteroids are usually administered continuously after corneal transplantation to suppress rejection. The observation period was 3 months (#1, 2: MHC-matched monkeys and #4–6: MHC-unmatched monkeys) or 6 months (#3: MHC-matched monkey, #7: MHC-unmatched monkey, and #8: MHC-unmatched monkey with local steroids). When rejection occurred, the monkey was euthanized, and histological analyses were performed. As negative controls of rejection, autologous sCEPS transplantation was performed (#9 and #10, details in Figures S4A–S4F). To date, there have been no detailed reports on corneal epithelial transplantation rejection in cynomolgus monkeys. Therefore, we performed allogeneic LT (#11 and #12) beforehand to confirm rejection and determine the corneal epithelial transplantation rejection signs in cynomolgus monkeys (Table S3).
Figure 2.
sCEPS transplantation and corneal LT in cynomolgus monkeys
(A) Experimental design of sCEPS transplantation and LT in cynomolgus monkeys. In LT (#11 and #12), the donor limbal tissue from healthy monkeys was divided into two transplanted to the recipient cornea’s upper and lower sides. MHC-unmatched transplantation using healthy monkeys was performed in #8. See also Figures S3–S5.
(B) Transplantation schedule. See also Tables S1–S3.
(C) Schematic representation of sCEPS preparation. P, passage.
(D) Phase-contrast image of sCEPS. Scale bar, 200 μm.
(E) Image of harvested sCEPS with PVDF membrane. Scale bar, 5 mm.
(F) H&E staining of sCEPS. Scale bars, 100 μm.
(G) K12, p63, K13, K3, MUC16, and ZO-1 immunostaining of sCEPS (green). Nuclei, red. Scale bars, 100 μm.
MHC-unmatched LT-evoked rejection in cynomolgus monkeys
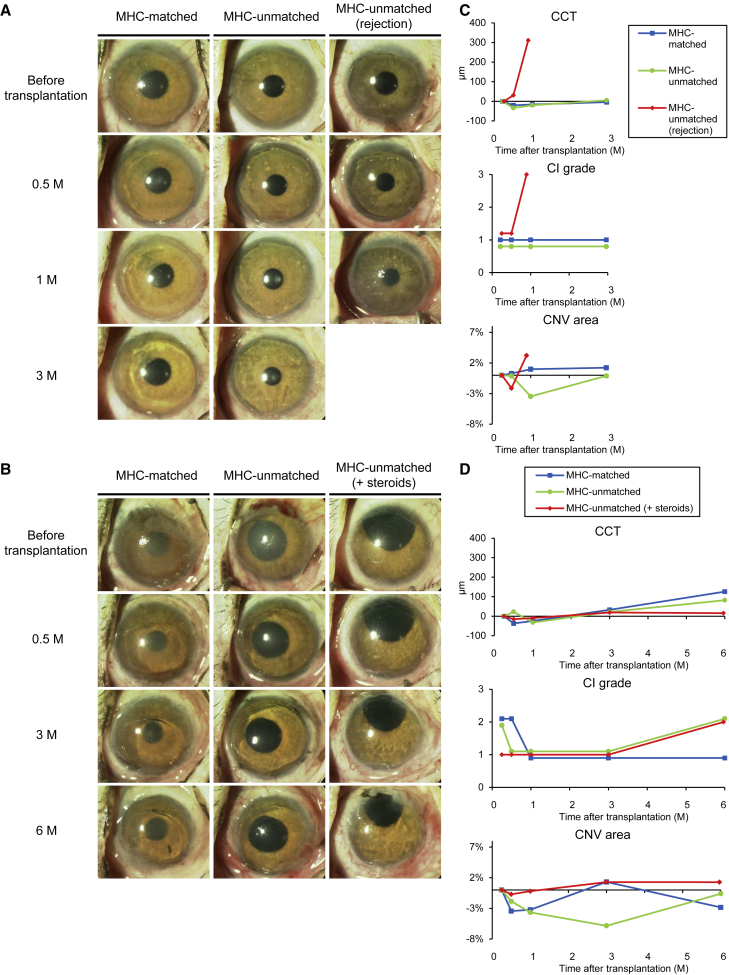
In MHC-unmatched LT, similar to humans (Daya et al., 2000), sharply increased central corneal thickness (CCT), risen conjunctival injection (CI) grade, and expanded corneal neovascularization (CNV) area were simultaneously observed at 28 days in #11 or at 20 days in #12 (details in Figures S5A–S5D). Therefore, we determined these findings as indicators of rejection.
Immune reaction after sCEPS transplantation in MHC-matched or -unmatched recipients
We summarize the clinical findings after sCEPS transplantation in all monkeys (#1–#8) in Tables 1 and 2 and show the representative anterior segment photograph and clinical data in Figure 3. The corneal surface covered by transplanted sCEPS became clear and smooth with no corneal epithelial defects, and ocular surface inflammation was slight in all monkeys examined by ophthalmoscopy 2 weeks after transplantation (Figures 3A and 3B). Until 3 months, none of the MHC-matched monkeys (#1–#3) showed rejection, while two of three monkeys presented an increased CI grade or an expanded CNV area (Table 1). In MHC-unmatched monkeys (#4–#8), one of four monkeys (#6) presented a sharply increased CCT, an increased CI grade, and expanded CNV area simultaneously, similar to monkeys with rejection after allogeneic LT, indicating rejection (Figure 3C). The eye showing rejection was enucleated for histological analyses. In other MHC-unmatched monkeys, rejection did not occur, while two of three monkeys presented an expanded CNV area. MHC-unmatched monkey with continuous local steroids did not show rejection or immune response during 3 months if follow-up. At 3 months, histological analyses were performed on two of three MHC-matched and two of three MHC-unmatched monkeys. One MHC-matched monkey (#3), one MHC-unmatched monkey (#7), and one MHC-unmatched monkey with local steroids (#8) were followed until 6 months. In the MHC-matched monkey, slowly increased CCT was observed, but no rejection occurred. In the MHC-unmatched monkey, immune responses such as slowly increased CCT, increased CI grade, and expanded CNV area were observed, but no rejection occurred. The MHC-unmatched monkey treated with continuous topical steroids did not show rejection and had less immune response than the MHC-unmatched monkey (Figure 3D and Table 2). To summarize, MHC-matched monkeys and MHC-unmatched monkey with local steroids did not show rejection, but presented the same degree of immune responses as autologous sCEPS transplantation. In MHC-unmatched monkeys, one of the four monkeys showed rejection. Furthermore, the immune response in MHC-unmatched monkeys without rejection was similar to that of MHC-matched monkeys at 3 months, but was stronger at 6 months.
Table 1.
Summary of clinical findings until 3 months after sCEPS transplantation to cynomolgus monkeys
| No. | MHC compatibility | Evaluation time point (months) | Sharply increased CCT | Risen CI grade | Expanded CNV area | Rejection |
|---|---|---|---|---|---|---|
| 1 | Matched | 3 | No | No | No | No |
| 2 | Matched | 3 | No | Yes | No | No |
| 3 | Matched | 3 | No | No | Yes | No |
| 4 | Unmatched | 3 | No | No | Yes | No |
| 5 | Unmatched | 3 | No | No | Yes | No |
| 6 | Unmatched | 1a | Yes | Yes | Yes | Yes |
| 7 | Unmatched | 3 | No | No | No | No |
| 8 | Unmatched(+steroids) | 3 | No | No | No | No |
| 9 | Autologous | 1b | No | No | No | No |
| 10 | Autologous | 3 | No | No | Yes | No |
Histological analyses were performed at 1 month owing to rejection.
Histological analyses were performed at 1 month as a negative control of rejection.
Table 2.
The summary of clinical findings 6 months after sCEPS transplantation to cynomolgus monkeys
| No. | MHC compatibility | Evaluation time point (months) | Sharply increased CCT | Risen CI grade | Expanded CNV area | Rejection |
|---|---|---|---|---|---|---|
| 3 | Matched | 6 | Noa | No | Nob | No |
| 7 | Unmatched | 6 | Noa | Yes | Yes | No |
| 8 | Unmatched(+steroids) | 6 | No | Yes | No | No |
| 10 | Autologous | 6 | No | No | Nob | No |
Slowly (not sharply) increased CCT was observed.
Expanded CNV area was observed until 3 months, but not after 3 months.
Figure 3.
Clinical findings after sCEPS transplantation to MHC-matched or MHC-unmatched cynomolgus monkeys
(A and B) Representative anterior segment photograph after sCEPS transplantation to MHC-matched or MHC-unmatched monkeys scheduled for 3 months (A) or 6 months (B) observation period. MHC-matched, #1; MHC-unmatched, #4; MHC-unmatched (rejection), #6 (A). MHC-matched, #3; MHC-unmatched, #7; MHC-unmatched (+steroids), #8 (B).
(C and D) Graphs of CCT, CI grade, and the CNV area in the central cornea after sCEPS transplantation in the same monkeys as Figures 3A, 3C, or Figures 3B, 3D. The value of difference from the CCT (μm) at 1 week (as the baseline) was displayed. The severity of CI was assessed from 0 through 3: CI grade 0 = none, grade 1 = mild, grade 2 = moderate, and grade 3 = severe. The value of difference from the CNV area (%) at 1 week (as the baseline) was displayed.
(C) In MHC-matched monkey (blue), CCT, CI grade, and CNV area did not change. In MHC-unmatched monkey (green), CCT and CI grade did not change, but the CNV area expanded to the baseline from 1 to 3 months. In MHC-unmatched (rejection) monkey (red), CCT increased sharply, CI grade rose, and CNV area expanded simultaneously at 1 month.
(D) In MHC-matched monkey (blue), CCT slowly increased from 3 to 6 months, CI grade did not change, and CNV area expanded from 1 to 3 months and then reduced to 6 months. In MHC-unmatched monkey (green), CCT slowly increased from 3 to 6 months, CI grade rose at 6 months, and CNV area expanded to the baseline from 3 to 6 months. In MHC-unmatched (+steroids) monkey (red), CCT and CNV area did not change, and CI grade rose at 6 months. See also Tables S1 and S2.
Histological analyses after sCEPS transplantation at 3 months
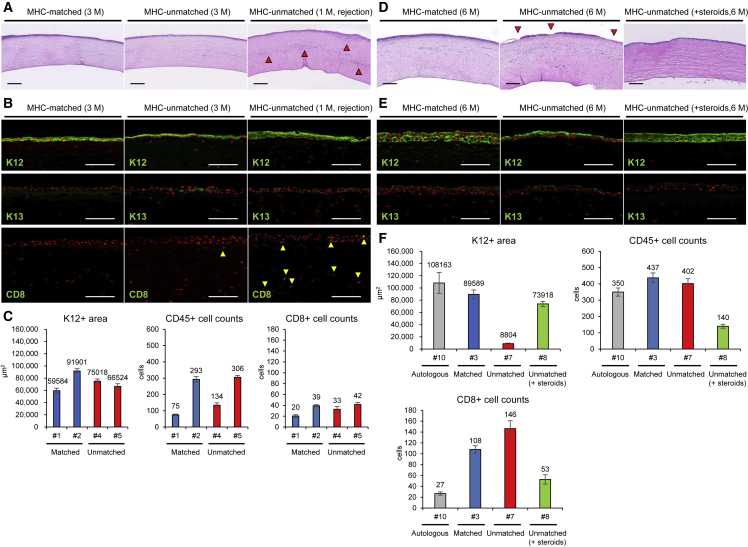
Hematoxylin and eosin (H&E) staining and immunostaining were performed to examine the difference in donor engraftment and inflammatory cell infiltration owing to MHC conformity after sCEPS transplantation. H&E staining revealed a uniformly stratified epithelium on the central corneal surface in all monkeys scheduled for a 3-month observation period (Figure 4A). The stratified epithelium was thinner in MHC-unmatched monkey with clinical rejection (#6) than in monkey after autologous sCEPS transplantation (#9, Figure S4C). Although infiltrated inflammatory cells were found in the corneal stroma, no difference was observed between MHC-matched (#1 and #2) and -unmatched monkeys (#4 and #5), except for #6, in which numerous infiltrated inflammatory cells were noted in the corneal stroma (Figure 4A). Immunostaining revealed that the stratified epithelium in the central cornea was positive for K12 and locally positive for K13 in the outermost layer, similar to sCEPS, in all monkeys (Figure 4B). In #6, there was no K12-positive stratified epithelium defects, but the K12-positive area was smaller than that in #9 (Figure S6). In addition, unlike other monkeys (#1, #2, #4, #5, and #9), many CD8 positive cells (cytotoxic T cells) were observed in the stratified epithelium and stroma of the central cornea. Many infiltrated CD8-positive cells, and no defect of K12-positive stratified epithelium suggest the condition immediately after rejection and before the donor sCEPS dropping out. Among MHC-matched (#1 and #2) and -unmatched (#4 and #5) monkeys at 3 months, there was no difference in the K12-positive area and CD45 (a marker for pan-leukocytes) and CD8-positive cell counts (Figure 4C). Histological analyses and clinical findings indicate that the same degree of immune response occurred in MHC-matched and -unmatched monkeys without rejection at 3 months.
Figure 4.
Histological analyses after sCEPS transplantation to MHC-matched or MHC-unmatched cynomolgus monkeys
Histological analyses on monkeys, scheduled for a 3 months (A–C) or 6 months (D–F) observation period.
(A) H&E staining of the central cornea after sCEPS transplantation in the same monkeys as Figure 3A. The arrowheads show strong cell infiltration in the corneal stroma and sub-epithelium. Scale bars, 200 μm.
(B) Immunostaining of the central cornea for K12, K13, and CD8 (green) after sCEPS transplantation in the same monkeys as Figure 3A. Arrowheads indicate CD8-positive cell infiltration in the central cornea. Nuclei, red. Scale bars, 100 μm.
(C) K12-positive area and CD45- and CD8-positive cell counts in the central cornea (4 mm diameter central zone) at 3 months after transplantation. Means ± SEM of the data from 10 slides are presented. The intervals between each sample were more than 70 μm.
(D) H&E staining of the central cornea after sCEPS transplantation. The arrowheads indicate monolayered epithelium covering part of the central corneal surface. Scale bars, 200 μm.
(E) Immunostaining of the central cornea for K12 and K13 (green) after sCEPS transplantation. Nuclei, red. Scale bars, 100 μm.
(F) K12-positive area and CD45- and CD8-positive cell counts in the central cornea (4 mm diameter central zone) at 6 months after transplantation. Means ± SEM of the data from 10 slides are presented. The intervals between each sample were more than 70 μm. See also Figures S4 and S6; Tables S1 and S2.
Histological analyses after sCEPS transplantation at 6 months
H&E staining in all monkeys at 6 months (#3, #7, and #8) revealed a uniformly stratified epithelium on the central corneal surface in an MHC-matched monkey (#3) and MHC-unmatched monkey with local steroids (#8) (Figure 4D). In MHC-unmatched monkey (#7), the central corneal surface was mainly covered with stratified epithelium, but partly with monolayer epithelium (as shown by the arrowheads in Figure 4D). Inflammatory cell infiltration in the corneal stroma was observed in all monkeys. Immunostaining revealed that the epithelium of the central cornea, including the monolayer epithelium in #7, was K12 positive and locally positive for K13 in the outermost layer, similar to sCEPS, indicating that the donor remained (Figure 4E). The K12-positive area in #7 was smaller than that in #3 (Figure 4F). Combined with the results at 3 months, these histological findings indicate that the immune response in MHC-unmatched monkeys was slightly stronger than that in MHC-matched monkeys. CD45- and CD8-positive cell counts in #8 were less than that in #7, and the K12-positive area in #8 was larger than in #7 and almost equal to #3. These histological findings indicate that the immune response in MHC-unmatched monkeys with local steroids was weaker than that in MHC-unmatched monkeys and was about the same strength as that in MHC-matched monkeys. These data suggest that the immune response to MHC-unmatched sCEPS can be controlled by the continuous administration of local steroids to the same extent as the immune response to MHC-matched sCEPS.
PD-L1 expression in human iCEPS and sCEPS
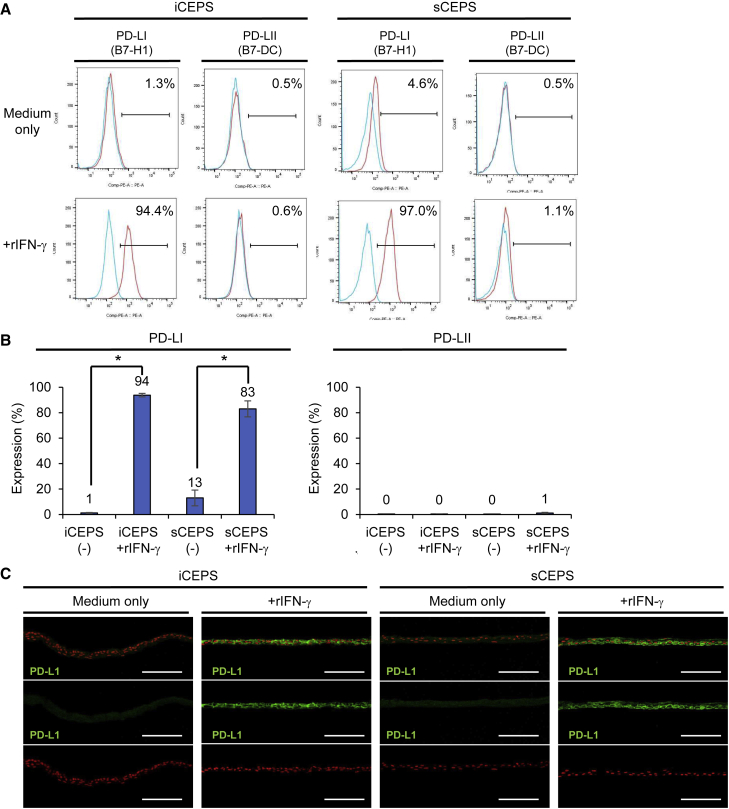
IFN-γ expression was negligible in MLR, even when PBMCs were co-cultured with HLA-unmatched iCEPS. Moreover, we have shown that rejection is unlikely to occur in sCEPS transplantation, even when MHC is unmatched. Therefore, we hypothesized that CEPS express immunosuppressive molecules. To address this hypothesis, we performed RNA sequencing of human iCEPS and sCEPS with or without rIFN-γ treatment. Examination of immunomodulatory molecules expressed on the cell surface of the anterior segment of the eye (Kunishige and Hori, 2013; Lee et al., 2002) (Figure S7) revealed that, in the presence of rIFN-γ, PD-L1 expression increased in both iCEPS and sCEPS. PD-L1 is an immunosuppressive molecule and is a ligand of PD-1. Flow cytometric analyses also revealed that PD-L1 expression was similarly increased in both iCEPS and sCEPS after rIFN-γ treatment (Figures 5A and 5B). PD-L2 expression, another PD-1 ligand, was not observed. Immunostaining revealed PD-L1 expression in all iCEPS and sCEPS layers after rIFN-γ treatment (Figure 5C). These data suggest that PD-L1 is one of the molecules involved in suppressing the immune response to HLA-unmatched CEPS under inflammatory conditions.
Figure 5.
PD-L1 expression in human iCEPS and sCEPS
(A and B) PD-L1 and PD-L2 expression of iCEPS and sCEPS with or without rIFN-γ (for 48 h) analyzed using flow cytometry. Blue histograms represent isotype control staining (mouse IgG, A). Representative data (A) and means ± SEM (B) of 10 (PD-L1 in iCEPS and sCEPS), 9 (PD-L2 in iCEPS), or 8 (PD-L2 in sCEPS) experiments are presented. ∗p < 0.05 compared with two groups using the Wilcoxon signed-rank test.
(C) Immunostaining for PD-L1 (green) on iCEPS and sCEPS with or without 48 h rIFN-γ treatment. Nuclei, red. Scale bars, 100 μm. See also Figure S7.
Discussion
Transplantation of CEPS derived from stem cells, including HLA homozygous iPSCs, is a promising treatment for LSCD. In this study, we obtained two novel findings related to the effects of MHC conformity on CEPS transplantation. First, the difference in immune response after CEPS transplantation owing to MHC conformity was not large, and the immune response to MHC-unmatched CEPS could be controlled by the continuous administration of local steroids. Second, PD-L1 is expressed in CEPS and could be involved in suppressing the immune response to HLA-unmatched CEPS.
In this study, the rejection rate in MHC-unmatched CEPS transplantation was very low (one in four monkeys), compared with MHC-unmatched LT (two of two monkeys) as a positive control. Moreover, the immune response in MHC-unmatched CEPS transplantation could be controlled by local steroid administration. In organ transplantation, including the cornea, donor APCs in the graft directly present the donor MHC to recipient T cells, allowing the recipient to recognize donor alloantigen (direct pathway) (Denton et al., 1999; Hos et al., 2019). Therefore, the number of APCs in the graft is a critical risk factor for rejection (Miller et al., 2016). In many organ transplantations, such as kidneys (Opelz et al., 1992) and heart (Opelz and Wujciak, 1994), where the graft contains many APCs, the lower the HLA compatibility, the more graft failure. Similarly, in corneal transplantation, the lower HLA compatibility, the higher the rejection rate in LT (Kwitko et al., 1995), where the graft consists of corneal epithelium and limbal stroma with many mature APCs (Mayer et al., 2007). In contrast, in CEPS transplantation, the graft is a cell sheet of cultured corneal epithelium and contains almost no mature APCs from the donor (Shortt et al., 2007), which is probably a reason why MHC-unmatched CEPS transplantation had a lower rejection rate than LT and a controllable immune response with local steroid administration.
Our results indicate that local steroid administration could control the immune response in MHC-unmatched CEPS transplantation to the same extent as MHC-matched CEPS transplantation. In addition to the direct pathway, in alloantigen recognition in organ transplantation, including cornea, recipient APCs recognize donor antigens and transport them to the lymph nodes (indirect pathway) (Hos et al., 2019). Therefore, the recipient APC infiltration from blood vessels in the graft bed is an important risk factor for rejection. Steroids are administered after organ transplantation to suppress rejection, and the mechanisms include repression of hemangiogenesis and lymphangiogenesis (Hos et al., 2011) by obstructing pro-inflammatory cytokine expression and decreasing immune cell infiltration (Brostjan et al., 1997) by inhibiting adhesion molecule expression on vascular endothelial cells. In our study, the CNV area in #8 (MHC-unmatched monkey with local steroids) did not change, expanding gradually in #7 (MHC-unmatched monkey). Moreover, the number of inflammatory cells infiltrating into the recipient cornea in #8 was less than in #7. These effects of suppressing the recipient APC infiltration into the graft by steroids was observed in CEPS transplantation by local administration. This is probably one of the reasons why the immune response in MHC-unmatched CEPS transplantation could be controlled by only local steroid administration.
In MLR, we found that the immune response of PBMCs to MHC-unmatched CEPS was negligible. In addition, rejection is unlikely to occur in CEPS transplantation, even when MHC is unmatched. These results indicate that CEPS express immunosuppressive molecules and suppresses the immune response. We focused on PD-L1 among immunosuppressive molecules. PD-L1 is expressed on human corneal endothelial (HCE) cells (Sugita et al., 2009a) and RPE cells (Sugita et al., 2009b) and has recently received attention for suppressing T cell activation and downregulating pro-inflammatory cytokine production (Dermani et al., 2019). PD-L1 is a PD-1 ligand and belongs to the B7 family. PD-L1 binds to PD-1 expressed on the surface of activated T cells and suppresses cellular responses, inducing immune tolerance. Moreover, as Francisco and associates have reported, PD-L1 is involved in suppressing immune rejection by introducing regulatory T (Treg) cells from naive T cells (Francisco et al., 2009). Whether or not PD-L1 is expressed on normal corneal epithelium depends on the report (Hori et al., 2006; Shen et al., 2007). However, PD-L1 expression is enhanced when human CEC are cultured with rIFN-γ (Yang et al., 2009). In the current study, we found that iCEPS and sCEPS express PD-L1 in the same manner after rIFN-γ treatment. Therefore, PD-L1 could be involved in suppressing the immune response to HLA-unmatched CEPS, as it does in HCE and RPE cells. Since iCEPS did not induce significant IL-10 secretion in MLR, PD-L1 pathways, other than Treg cell induction, could be involved in suppressing the immune response. Further studies on molecules including PD-L1 and secreted cytokines are expected to elucidate the immunosuppressive mechanisms of CEPS.
MHC-unmatched CEPS transplantation had a lower rejection rate than MHC-unmatched LT and a controllable immune response with local steroid administration. Like other transplanted organs, allogeneic LT, as a conventional treatment, requires immunosuppressants such as cyclosporin (Ilari and Daya, 2002) and tacrolimus (Sloper et al., 2001) in addition to systemic steroids to suppress rejection, but the side effects of these agents are clinically challenging. For example, local and systemic immunocompromised conditions increase the risk of eye and systemic infections. In addition, systemic side effects, such as renal damage and myelosuppression, require a decrease in or discontinuation of immunosuppressants, increasing the risk of rejection. Our results indicate that systemic immunosuppression could be decreased in allogeneic CEPS transplantation than conventional corneal transplantation in clinical practice. In that case, the risks of the above complications owing to the side effects decrease, and clinical outcomes are expected to improve. Furthermore, the indication may be extended to patients who could not receive immunosuppressants owing to systemic diseases. Further studies are needed to determine the extent of immunosuppression after allogeneic CEPS transplantation in clinical practice.
Our results suggest that allogeneic CEPS transplantation is a useful treatment to decrease post-operative immunosuppression compared with allogeneic LT, conventional treatment. When allogeneic CEPS transplantation is considered, sCEPS, prepared by culturing donor corneal epithelium, and iCEPS, prepared by culturing corneal epithelial progenitor/stem cells differentiated from allogeneic iPSCs, are candidates for the graft. Differentiated derivatives from embryonic stem cells and iPSCs generally show a lower HLA-I expression and are less antigenic than somatic cells (Araki et al., 2013; Drukker et al., 2006; Liu et al., 2013). Consistently, HLA-I expression was weaker in iCEPS than in sCEPS in the current study. Moreover, iCEPS with rIFN-γ treatment showed weaker HLA-II expression than sCEPS. RNA sequencing results showed that iCEPS after rIFN-γ treatment had higher expression levels of HLA-E, -F, and -G, CD55, CD46, and CD276 and lower expression levels of TNFSF10 than sCEPS. These results indicate that immunomodulatory molecule expression is higher in iCEPS than in sCEPS. In addition, while sCEPS may contain mature APCs from donors, iCEPS is grown from a pure population of corneal epithelial progenitor/stem cells obtained by fluorescence-activated cell sorting and is unlikely to contain APCs. Therefore, recipients are less likely to recognize alloantigens in iCEPS transplantation than in sCEPS transplantation. Altogether, the risk of rejection in allogeneic iCEPS transplantation is expected to be less than in allogeneic sCEPS transplantation. Regarding the graft supply, allogeneic sCEPS transplantation requires quality control for each donor owing to the limited number of grafts that can be prepared from one donor, making it difficult to provide a stable graft supply. In contrast, for allogeneic iCEPS transplantation, a stable graft supply is feasible once master cells with guaranteed quality are established. Therefore, allogeneic iCEPS transplantation using an iPSC bank may be a more appropriate treatment than allogeneic sCEPS transplantation, because it can stably supply quality-guaranteed grafts to many patients.
Our experimental system has some limitations. First, owing to the small number of MHC-matched monkeys, we were unable to perform statistical analyses in each intervention group. However, by performing detailed analyses on each monkey (clinical and histological assessments), we obtained novel results regarding the immune response after CEPS transplantation. Second, we did not include animals with LSCD that received no donor cells as a negative control group. Since the objective of this study was to examine the difference in immune response owing to MHC conformity, autologous transplantation alone was considered sufficient as a negative control. Third, we could not directly distinguish between donor and recipient monkeys after transplantation. However, all K12-positive cells on the ocular surface after CEPS transplantation are considered to be derived from the donor cells, because we confirmed that there were no K12-positive cells on the cornea before CEPS transplantation in all monkeys. Fourth, since there is no anti-PD-L1 antibody that cross-reacts with cynomolgus monkeys, its expression in the cornea after transplantation could not be investigated. Further studies on the expression of PD-L1 in the cornea and the effect of PD-L1 neutralizing antibody on transplantation are needed.
In conclusion, we found that the difference in immune response after CEPS transplantation owing to MHC conformity was not large, and the immune response to MHC-unmatched CEPS could be controlled by continuous administration of local steroids. Our results indicate that allogeneic CEPS transplantation can reduce post-operative immunosuppression compared with allogeneic LT, conventional treatment. Moreover, further studies on the immunological properties of CEPS, including PD-L1 expression, are expected to elucidate the immune reaction mechanism in CEPS transplantation.
Experimental procedures
Non-human primates
Sixteen female cynomolgus monkeys (Macaca fascicularis; 33–150 months old, 2.3–4.0 kg) purchased from Ina Research (Shiga, Japan) or Shiga University of Medical Science (Shiga, Japan) were used. Animals were individually housed in species-specific rooms with environmental controls set to maintain 25 ± 1.5°C, 45 ± 15% relative humidity, and a 12-h light-dark cycle (light: 8:00 am to 8:00 pm). The daily diet consisted of about 80–100 g feeds (PS-A; Oriental. Yeast Co., Ltd., Tokyo, Japan), fresh fruit, and water ad libitum. All animal experimentation was performed per the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and was approved by the animal ethics committees of Osaka University (reference no. 27-091-007).
Preparation of non-human primate sCEPS
Portions of the corneal limbus were collected, shredded with micro scissors, processed with Accutase (ThermoFisher Scientific, Waltham, MA) for 70 min, seeded on LN511E8 (Nippi, Tokyo, Japan) coated (0.5 μg/cm2 for 1 h) plates, and cultured in corneal epithelium maintenance medium (CEM; DMEM/F12 [2:1], ThermoFisher Scientific) containing 2% B27 supplement (ThermoFisher Scientific), 1% penicillin-streptomycin solution, 10–20 ng/mL KGF, and 10 μM Y-27632 (primary culture). Once the epithelial cells became subconfluent, they were passaged and cryopreserved using STEM-CELLBANKER (Nippon Zenyaku Kogyo Co., Ltd, Fukushima, Japan) at passage two. For transplantation, epithelial cells were thawed on a 12-well temperature-responsive culture plate (UpCell, CellSeed, Tokyo, Japan) coated with LN511E8 (0.5 μg/cm2 for 1 h) and cultured in CEM until confluent. To promote maturation, the epithelial cells were cultivated in the corneal epithelium maturation medium (Hayashi et al., 2016, 2017) (KCM medium containing 10–20 ng/mL KGF and 10 μM Y-27632) for an additional 4 or 5 days after CEM culture. After confirming the stratification of the sheet using a phase-contrast microscope as an index, transplantation was performed. We identified passage three as optimal for use based on preliminary experiments on CEC culture from other monkeys, in which cell proliferation slowed down, the morphology became irregular after four passages, and cells could not be sheeted after five passages in some strains. The corneal limbus of the cynomolgus monkey with almost homozygous MHC haplotypes (HT-1 and part of HT-2) was independently collected five times to generate cell lines for sCEPS.
Transplantation of sCEPS
sCEPS transplantation was performed more than 5 weeks after LSCD model preparation. To prepare the graft bed, the tissue covering the corneal surface was removed and scraped using a scleral knife for 3 min and a quick medical absorber for 1 min sCEPS cultivated on temperature-responsive dishes were released from their substrate by reducing the temperature to 20°C (PVDF membrane; Merck KGaA, Hessen, Germany) was used as the carrier (Nishida et al., 2004b), and the sCEPS were transplanted.
The sCEPS was prepared from the limbus of the cynomolgus monkey with almost homozygous MHC haplotypes (HT-1 and part of HT-2) and was transplanted onto the corneas of three MHC-matched (#1–#3) and four MHC-unmatched (#4–#7) animals. MHC-unmatched sCEPS transplantation using a healthy monkey was performed in #8 (MHC-unmatched monkey with local steroids).
Systemic steroid was administered for 4 days (#1, #2, #4, #5, #6, and #8) from the preoperative day to the second post-operative day, or for 7 days (#3 and #7: the preoperative day, and the baseline, first, second, third, seventh, and 10th post-operative days). Only #8 received additional steroid treatment. In #8, subconjunctival steroid injections were performed once a week after sCEPS transplantation. After transplantation, ocular surface observation using a slit lamp or fluorescein staining, and CCT measurement using anterior segment optical coherence tomography (SS-1000, CASIA, Tomey Corporation, Aichi, Japan), were performed once a week for 2 weeks, then twice a week for 6 weeks (until 8 weeks), and once a week thereafter. The severity of CI was assessed from 0 to 3 based on the grading system for Stevens-Johnson syndrome (Sotozono et al., 2007): CI grade 0 = none, grade 1 = mild, grade 2 = moderate, and grade 3 = severe. The proportion of the CNV area in the central cornea (8-mm diameter) was measured using ImageJ software at 1–4, 6, 8, 10, 12, 16, 20, and 24 weeks.
Immunostaining and histological analyses
Abraded cynomolgus monkey corneal epithelium samples were fixed in 4% paraformaldehyde (PFA), mounted in O.C.T. Compound (Sakura Finetek Japan. Co., Ltd., Tokyo, Japan), and 5-μm frozen sections were prepared using a CM3050 S cryomicrotome (Leica Biosystems, Tokyo, Japan). Frozen sections were washed with Tris-buffered saline (TBS, TaKaRa Bio. Inc., Shiga, Japan) for 5 min and incubated with TBS containing 5% donkey serum and 0.3% Triton X-100 for 1 h to block non-specific reactions. Sections were then incubated at 4°C overnight with the antibodies: cytokeratin 12 (N-16, 1 μg/mL, Santa Cruz Biotechnology, Dallas TX), cytokeratin 13 (AE8, 5 μg/mL, Abcam, Cambridgeshire, UK), and p63 (4A4, 1 μg/mL, Santa Cruz Biotechnology). Sections were washed twice with TBS for 10 min and incubated with Alexa Fluor 488-, 568-, and 647-conjugated secondary antibodies as appropriate (20 μg/mL, ThermoFisher Scientific) for 1 h at room temperature (20–28°C). Counterstaining was performed with Hoechst 33342 (Merck KgaA) before fluorescence microscopy (ELYRA S.1, Zeiss, Baden-Württemberg, Germany).
Cynomolgus monkeys were euthanized by intravenous administration of sodium pentobarbitone, after which their eyes were immediately enucleated for histological analyses. The cornea, containing the limbus, was divided into two. One piece of the cornea containing limbus was mounted in O.C.T. compound for immunostaining, and the other was fixed with 10% formaldehyde neutral buffer solution (Nacalai Tesque, Kyoto, Japan) and embedded in paraffin for H&E staining. The paraffin-embedded cornea and CEPS (sCEPS of cynomolgus monkey, and iCEPS and sCEPS of human origin) were cut into 7- and 5-μm-thick sections, respectively. Sections were stained with H&E after deparaffinization and hydration and observed with a NanoZoomer-XR C12000 (Hamamatsu Photonics, Shizuoka, Japan). Sections were also immunoassayed using cytokeratin 12 (N-16, Santa Cruz Biotechnology), cytokeratin 13 (AE8, Abcam), CD45 (D9M8I, 0.25 μg/mL, Cell Signaling Technology, Danvers, MA), purified anti-human CD8a (2.5 μg/mL, BioLegend, San Diego, CA), Keratin K3/K76 (AE5, 5 μg/mL, PROGEN Biotechnik GmbH, Baden-Württemberg, Germany), anti-MUC16 antibody (OC125, 0.25 μg/mL, Abcam), mouse anti-ZO-1 (2.5 μg/mL, ThermoFisher Scientific), and CD274 (PD-L1, B7-H1) monoclonal antibody (MIH1, 10 μg/mL, ThermoFisher Scientific). For samples from #8, frozen sections were fixed in 4% PFA and stained with H&E.
The K12-positive area and CD45- and CD8-positive cell counts in the central cornea (4-mm diameter central zone) were compared from ten slides of each sample with intervals of more than 70 μm.
Preparation of human iCEPS or sCEPS
All human studies were performed according to the Declaration of Helsinki and were approved by the ethics review committee of Osaka University (reference no. 16279-6). The HLA homozygous human iPSC strain (YZWJ14) was provided by the Center for iPS Cell Research and Application, Kyoto University. The methods for maintenance, differentiation, and CEPS preparation from human iPSCs were previously described (Hayashi et al., 2016, 2017). Human somatic stem cells obtained from corneoscleral rims (CorneaGen, Seattle, WA) were cultivated on LN511E8 (Nippi) coated (0.5 μg/cm2, 1 h) temperature-responsive culture plates as reported previously (Hayashi et al., 2007). Maturation promotion and removal of the sCEPS were performed similarly to the non-human primate sCEPS above. iCEPS or sCEPS were co-cultured with human recombinant IFN-γ (100 ng/mL) (Bio-Techne, Minneapolis, MN) for 48 h before flow cytometric analysis or RNA sequencing. iCEPS or sCEPS were treated with 0.25% trypsin-EDTA (ThermoFisher Scientific) for 15–20 min at 37°C to obtain single cells for MLR or flow cytometric analysis. Total RNA was obtained from iCEPS or sCEPS using the RNeasy total RNA kit and the QIAzol reagent (Qiagen, Hilden, Germany) for RNA sequencing.
MLR with human iCEPS
After informed consent was obtained, PBMCs (1 × 105 cells/well in 96-well plates) were isolated from healthy adult donors and co-cultured with single iCEPS cells for 7 days. As a positive control of allogeneic immune rejection, B-LCL were prepared from HLA-unmatched healthy adult donor, as previously reported (Fujiki et al., 2007). The culture medium used was RPMI-1640 medium (Nacalai Tesque) containing 10% fetal bovine serum (ThermoFisher Scientific), 10 IU/mL human recombinant IL-2 (Shionogi & Co., Ltd., Osaka, Japan), 55 μM 2-mercaptoethanol (ThermoFisher Scientific), 1% MEM non-essential amino acids solution (ThermoFisher Scientific), 1% Glutamax Supplement (ThermoFisher Scientific), 1 mM sodium pyruvate (ThermoFisher Scientific), and 1% penicillin-streptomycin (ThermoFisher Scientific). Before the assay, B cells and single iCEPS cells were incubated for 45 min at 37°C with 50 μg/mL mitomycin C to inactivate their proliferative activity. Supernatants were harvested after 7 days and assessed for IFN-γ secretion using ELISA (Bio-Techne). The PBMCs and PBMCs co-cultured with single iCEPS cells or B-LCL were harvested for flow cytometric analysis.
Flow cytometry
iCEPS or sCEPS single-cell suspensions were stained for 20 min on ice with antibodies: fluorescein isothiocyanate anti-HLA-A, -B, and -C antibody (mouse IgG2a, κ, Biolegend); Alexa Fluor 647 anti-HLA-DR, -DP, and -DQ antibody (mouse IgG2a, κ, Biolegend); PE anti-CD274 antibody (PD-L1, B7-H1, Mouse IgG1, κ, ThermoFisher Scientific); and PE anti-CD 273 antibody (PD-L2, MIH1, Mouse IgG1, κ, ThermoFisher Scientific). The harvested PBMCs in MLR were incubated with APC anti-CD8a antibody (mouse IgG1, κ, ThermoFisher Scientific) for 1 h at room temperature. Intracellular staining by PE anti-human Ki-67 antibody (mouse IgG1, κ, BioLegend) was performed after using fixation and permeabilization solution (BD Biosciences, Franklin Lakes, NJ). All samples were analyzed on a FACSAria II or FACSVerse flow cytometer (BD Biosciences). Data were analyzed by FlowJo software (TreeStar, Ashland, OR).
Statistics and reproducibility
At least four independent experiments were performed for all in vitro assays. All data were presented as means ± SEM. Statistical analyses in flow cytometry were Wilcoxon signed-rank test or Mann-Whitney U test as appropriate. Statistical analyses in MLR were Mann-Whitney U tests. Values were considered statistically significant if the p value was less than 0.05.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author contributions
T.So., T.Sh., K.O., R.H., and K.N. designed the research. Y.Y., T.So., S.A., Y.H., T.K., Y.S., and H.T. performed experiments and analyzed data. K.M. and N.H. supervised the project. Y.Y., T.So., and R.H. wrote the paper.
Conflicts of interest
The authors declare no competing interests.
Acknowledgments
The authors acknowledge Daisuke Okuzaki and the NGS core facility of the Genome Information Research Center at the Research Institute for Microbial Diseases, Osaka University, for support in RNA-sequencing. This research was supported by the Japan Agency for Medical Research and Development (AMED) under Grant Number JP19bk0104080h and JP19bm0504005h and by an Osaka Eye Bank Research Grant 2020.
Published: June 23, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2022.05.018.
Supplemental information
References
- Araki R., Uda M., Hoki Y., Sunayama M., Nakamura M., Ando S., Sugiura M., Ideno H., Shimada A., Nifuji A., et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 2013;494:100–104. doi: 10.1038/nature11807. [DOI] [PubMed] [Google Scholar]
- Brostjan C., Anrather J., Csizmadia V., Natarajan G., Winkler H. Glucocorticoids inhibit E-selectin expression by targeting NF-kappaB and not ATF/c-Jun. J. Immunol. 1997;158:3836–3844. [PubMed] [Google Scholar]
- Daya S.M., Bell R.D., Habib N.E., Powell–Richards A., Dua H.S. Clinical and pathologic findings in human keratolimbal allograft rejection. Cornea. 2000;19:443–450. doi: 10.1097/00003226-200007000-00007. [DOI] [PubMed] [Google Scholar]
- Denton M.D., Magee C.C., Sayegh M.H. Immunosuppressive strategies in transplantation. Lancet. 1999;353:1083–1091. doi: 10.1016/s0140-6736(98)07493-5. [DOI] [PubMed] [Google Scholar]
- Dermani F.K., Samadi P., Rahmani G., Kohlan A.K., Najafi R. PD-1/PD-L1 immune checkpoint: potential target for cancer therapy. J. Cell. Physiol. 2019;234:1313–1325. doi: 10.1002/jcp.27172. [DOI] [PubMed] [Google Scholar]
- Drukker M., Katchman H., Katz G., Even-Tov Friedman S., Shezen E., Hornstein E., Mandelboim O., Reisner Y., Benvenisty N. Human embryonic stem cells and their differentiated derivatives are less susceptible to immune rejection than adult cells. Stem Cell. 2006;24:221–229. doi: 10.1634/stemcells.2005-0188. [DOI] [PubMed] [Google Scholar]
- Francisco L.M., Salinas V.H., Brown K.E., Vanguri V.K., Freeman G.J., Kuchroo V.K., Sharpe A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki F., Oka Y., Tsuboi A., Kawakami M., Kawakatsu M., Nakajima H., Elisseeva O.A., Harada Y., Ito K., Li Z., et al. Identification and characterization of a WT1 (Wilms tumor gene) protein-derived HLA-DRB1∗0405-restricted 16-mer helper peptide that promotes the induction and activation of WT1-specific cytotoxic T lymphocytes. J. Immunother. 2007;30:282–293. doi: 10.1097/01.cji.0000211337.91513.94. [DOI] [PubMed] [Google Scholar]
- Gain P., Jullienne R., He Z., Aldossary M., Acquart S., Cognasse F., Thuret G. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 2016;134:167. doi: 10.1001/jamaophthalmol.2015.4776. [DOI] [PubMed] [Google Scholar]
- Gourraud P.-A., Gilson L., Girard M., Peschanski M. The role of human leukocyte antigen matching in the development of multiethnic “haplobank” of induced pluripotent stem cell lines. Stem Cell. 2012;30:180–186. doi: 10.1002/stem.772. [DOI] [PubMed] [Google Scholar]
- Hayashi R., Yamato M., Sugiyama H., Sumide T., Yang J., Okano T., Tano Y., Nishida K. N-cadherin is expressed by putative stem/progenitor cells and melanocytes in the human limbal epithelial stem cell niche. Stem Cell. 2007;25:289–296. doi: 10.1634/stemcells.2006-0167. [DOI] [PubMed] [Google Scholar]
- Hayashi R., Ishikawa Y., Sasamoto Y., Katori R., Nomura N., Ichikawa T., Araki S., Soma T., Kawasaki S., Sekiguchi K., et al. Co-ordinated ocular development from human iPS cells and recovery of corneal function. Nature. 2016;531:376–380. doi: 10.1038/nature17000. [DOI] [PubMed] [Google Scholar]
- Hayashi R., Ishikawa Y., Katori R., Sasamoto Y., Taniwaki Y., Takayanagi H., Tsujikawa M., Sekiguchi K., Quantock A.J., Nishida K. Coordinated generation of multiple ocular-like cell lineages and fabrication of functional corneal epithelial cell sheets from human iPS cells. Nat. Protoc. 2017;12:683–696. doi: 10.1038/nprot.2017.007. [DOI] [PubMed] [Google Scholar]
- Hori J., Wang M., Miyashita M., Tanemoto K., Takahashi H., Takemori T., Okumura K., Yagita H., Azuma M. B7-H1-Induced apoptosis as a mechanism of immune privilege of corneal allografts. J. Immunol. 2006;177:5928–5935. doi: 10.4049/jimmunol.177.9.5928. [DOI] [PubMed] [Google Scholar]
- Hos D., Saban D.R., Bock F., Regenfuss B., Onderka J., Masli S., Cursiefen C. Suppression of inflammatory corneal lymphangiogenesis by application of topical corticosteroids. Arch. Ophthalmol. 2011;129:445–452. doi: 10.1001/archophthalmol.2011.42. [DOI] [PubMed] [Google Scholar]
- Hos D., Matthaei M., Bock F., Maruyama K., Notara M., Clahsen T., Hou Y., Le V.N.H., Salabarria A.-C., Horstmann J., et al. Immune reactions after modern lamellar (DALK, DSAEK, DMEK) versus conventional penetrating corneal transplantation. Prog. Retin. Eye Res. 2019;73:100768. doi: 10.1016/j.preteyeres.2019.07.001. [DOI] [PubMed] [Google Scholar]
- Ilari L., Daya S.M. Long-term outcomes of keratolimbal allograft for the treatment of severe ocular surface disorders. Ophthalmology. 2002;109:1278–1284. doi: 10.1016/s0161-6420(02)01081-3. [DOI] [PubMed] [Google Scholar]
- Ishigaki H., Shiina T., Ogasawara K. MHC-identical and transgenic cynomolgus macaques for preclinical studies. Inflamm. Regen. 2018;38:30. doi: 10.1186/s41232-018-0088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura T., Miyagawa S., Fukushima S., Maeda A., Kashiyama N., Kawamura A., Miki K., Okita K., Yoshida Y., Shiina T., et al. Cardiomyocytes derived from MHC-homozygous induced pluripotent stem cells exhibit reduced allogeneic immunogenicity in MHC-matched non-human primates. Stem Cell Rep. 2016;6:312–320. doi: 10.1016/j.stemcr.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunishige T., Hori J. Immune privilege as new therapeutic strategies for success of corneal transplantation. Inflamm. Regen. 2013;33:274–282. doi: 10.2492/inflammregen.33.274. [DOI] [Google Scholar]
- Kwitko S., Marinho D., Barcaro S., Bocaccio F., Rymer S., Fernandes S., Neumann J. Allograft conjunctival transplantation for bilateral ocular surface disorders. Ophthalmology. 1995;102:1020–1025. doi: 10.1016/s0161-6420(95)30918-9. [DOI] [PubMed] [Google Scholar]
- Lee H.O., Herndon J.M., Barreiro R., Griffith T.S., Ferguson T.A. TRAIL: a mechanism of tumor surveillance in an immune privileged site. J. Immunol. 2002;169:4739–4744. doi: 10.4049/jimmunol.169.9.4739. [DOI] [PubMed] [Google Scholar]
- Liu P., Chen S., Li X., Qin L., Huang K., Wang L., Huang W., Li S., Jia B., Zhong M., et al. Low immunogenicity of neural progenitor cells differentiated from induced pluripotent stem cells derived from less immunogenic somatic cells. PLoS One. 2013;8:e69617. doi: 10.1371/journal.pone.0069617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer W.J., Irschick U.M., Moser P., Wurm M., Huemer H.P., Romani N., Irschick E.U. Characterization of antigen-presenting cells in fresh and cultured human corneas using novel dendritic cell markers. Invest. Ophthalmol. Vis. Sci. 2007;48:4459–4467. doi: 10.1167/iovs.06-1184. [DOI] [PubMed] [Google Scholar]
- Miller M.L., Chen J., Daniels M.D., McKeague M.G., Wang Y., Yin D., Vu V., Chong A.S., Alegre M.L. Adoptive transfer of tracer-alloreactive CD4+ T cell receptor transgenic T cells alters the endogenous immune response to an allograft. Am. J. Transplant. 2016;16:2842–2853. doi: 10.1111/ajt.13821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizane A., Kikuchi T., Hayashi T., Mizuma H., Takara S., Doi H., Mawatari A., Glasser M.F., Shiina T., Ishigaki H., et al. MHC matching improves engraftment of iPSC-derived neurons in non-human primates. Nat. Commun. 2017;8:385. doi: 10.1038/s41467-017-00926-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Inatomi T., Sotozono C., Amemiya T., Kanamura N., Kinoshita S. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br. J. Ophthalmol. 2004;88:1280–1284. doi: 10.1136/bjo.2003.038497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida K., Yamato M., Hayashida Y., Watanabe K., Yamamoto K., Adachi E., Nagai S., Kikuchi A., Maeda N., Watanabe H., et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 2004;351:1187–1196. doi: 10.1056/nejmoa040455. [DOI] [PubMed] [Google Scholar]
- Nishida K., Yamato M., Hayashida Y., Watanabe K., Maeda N., Watanabe H., Yamamoto K., Nagai S., Kikuchi A., Tano Y., et al. Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation. 2004;77:379–385. doi: 10.1097/01.tp.0000110320.45678.30. [DOI] [PubMed] [Google Scholar]
- Opelz G., Wujciak T. The influence of HLA compatibility on graft survival after heart transplantation. N. Engl. J. Med. 1994;330:816–819. doi: 10.1056/nejm199403243301203. [DOI] [PubMed] [Google Scholar]
- Opelz G., Mytilineos J., Wujciak T., Schwarz V., Back D. Current status of HLA matching in renal transplantation. The Collaborative Transplant Study. Clin. Investig. 1992;70:767–772. doi: 10.1007/BF00180746. [DOI] [PubMed] [Google Scholar]
- Pellegrini G., Ardigò D., Milazzo G., Iotti G., Guatelli P., Pelosi D., De Luca M. Navigating market authorization: the path holoclar took to become the first stem cell product approved in the European union. Stem Cells Transl. Med. 2018;7:146–154. doi: 10.1002/sctm.17-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L., Jin Y., Freeman G.J., Sharpe A.H., Dana M.R. The function of donor versus recipient programmed death-ligand 1 in corneal allograft survival. J. Immunol. 2007;179:3672–3679. doi: 10.4049/jimmunol.179.6.3672. [DOI] [PubMed] [Google Scholar]
- Shimazaki J., Aiba M., Goto E., Kato N., Shimmura S., Tsubota K. Transplantation of human limbal epithelium cultivated on amniotic membrane for the treatment of severe ocular surface disorders. Ophthalmology. 2002;109:1285–1290. doi: 10.1016/s0161-6420(02)01089-8. [DOI] [PubMed] [Google Scholar]
- Shortt A.J., Secker G.A., Notara M.D., Limb G.A., Khaw P.T., Tuft S.J., Daniels J.T. Transplantation of ex vivo cultured limbal epithelial stem cells: a review of techniques and clinical results. Surv. Ophthalmol. 2007;52:483–502. doi: 10.1016/j.survophthal.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Sloper C.L., Powell R.J., Dua H.S. Tacrolimus (FK506) in the management of high-risk corneal and limbal grafts. Ophthalmology. 2001;108:1838–1844. doi: 10.1016/s0161-6420(01)00759-x. [DOI] [PubMed] [Google Scholar]
- Sotozono C., Ang L.P.K., Koizumi N., Higashihara H., Ueta M., Inatomi T., Yokoi N., Kaido M., Dogru M., Shimazaki J., et al. New grading system for the evaluation of chronic ocular manifestations in patients with Stevens–Johnson syndrome. Ophthalmology. 2007;114:1294–1302. doi: 10.1016/j.ophtha.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Sugita S., Usui Y., Horie S., Futagami Y., Yamada Y., Ma J., Kezuka T., Hamada H., Usui T., Mochizuki M., et al. Human corneal endothelial cells expressing programmed death-ligand 1 (PD-L1) suppress PD-1 + T helper 1 cells by a contact-dependent mechanism. Investig. Opthalmol. Vis. Sci. 2009;50:263–272. doi: 10.1167/iovs.08-2536. [DOI] [PubMed] [Google Scholar]
- Sugita S., Usui Y., Horie S., Futagami Y., Aburatani H., Okazaki T., Honjo T., Takeuchi M., Mochizuki M. T-cell suppression by programmed cell death 1 ligand 1 on retinal pigment epithelium during inflammatory conditions. Investig. Opthalmol. Vis. Sci. 2009;50:2862. doi: 10.1167/iovs.08-2846. [DOI] [PubMed] [Google Scholar]
- Sugita S., Iwasaki Y., Makabe K., Kamao H., Mandai M., Shiina T., Ogasawara K., Hirami Y., Kurimoto Y., Takahashi M. Successful transplantation of retinal pigment epithelial cells from MHC homozygote iPSCs in MHC-matched models. Stem Cell Rep. 2016;7:635–648. doi: 10.1016/j.stemcr.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoft R.A. Keratoepithelioplasty. Am. J. Ophthalmol. 1984;97:1–6. doi: 10.1016/0002-9394(84)90438-0. [DOI] [PubMed] [Google Scholar]
- Yang W., Li H., Chen P.W., Alizadeh H., He Y., Hogan R.N., Niederkorn J.Y. PD-L1 expression on human ocular cells and its possible role in regulating immune-mediated ocular inflammation. Opthalmol. Vis. Sci. 2009;50:273–280. doi: 10.1167/iovs.08-2397. [DOI] [PubMed] [Google Scholar]
- Zakaria N., Possemiers T., Dhubhghaill S., Leysen I., Rozema J., Koppen C., Timmermans J.-P., Berneman Z., Tassignon M.-J. Results of a phase I/II clinical trial: standardized, non-xenogenic, cultivated limbal stem cell transplantation. J. Transl. Med. 2014;12:58. doi: 10.1186/1479-5876-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.