Abstract
Although blunted sensitivity to reward is thought to play a key role in promoting risk for depression, most research on this topic has utilized monetary reward paradigms and focused on currently depressed adults. To address this issue, we analyzed neural reward and β-endorphin data from the Psychobiology of Stress and Adolescent Depression (PSY SAD) Study, which recruited a well-characterized sample of adolescent girls at high vs. low risk for major depressive disorder (MDD) (N = 52, Mage = 14.90, SD = 1.35) based on their mothers’ lifetime history of MDD. As hypothesized, greater striatal activity while receiving positive (vs. neutral) social evaluation was associated with lower depression symptom severity as independently assessed by the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS). This association was present for girls at high but not low risk for MDD, suggesting that this neural response may represent a pre-clinical marker of risk for depression. Consistent with these results, higher post-social evaluation levels of a peripheral marker of reward sensitivity, β-endorphin, were related to lower clinician-rated depression symptom severity. Together, these results indicate that neural and peripheral markers of responsivity to social reward are both related to depression severity, which may have implications for understanding the pathophysiology of depression.
Keywords: Depression, Reward sensitivity, Social evaluation, Striatum, β-endorphin, Adolescence
Highlights
-
•
The neurobiological reward processes underlying adolescent depression risk are unclear.
-
•
To address this issue, we recruited adolescent girls at high vs. low risk for MDD.
-
•
We assessed their striatal activity, β-endorphin levels, & depression symptom severity.
-
•
Striatal activity & β-endorphin levels during social evaluation predicted depression severity.
-
•
These effects differed for high-vs. low-risk youth, suggesting pre-clinical risk biomarkers.
1. Introduction
Depression is one of the most common and costly of all disorders [[1], [2], [3]]. Depression also continues to grow in prevalence [[2], [3], [4]], with rates having increased notably over the past two decades in a variety of demographic groups, with the largest increases being evident for adolescents [5]. It is well known that women become twice as likely to develop depression as men following the pubertal transition [[6], [7], [8]]. Despite this well-known increase in risk, however, our understanding of processes that underlie risk for depression in adolescent girls is poor.
One factor that may be particularly important for understanding risk for depression in adolescent girls is social evaluation [[9], [10], [11]]. Social evaluation can serve as a reward (e.g., a compliment from a peer) or a stressor (e.g., being criticized) [12]. Social stressors (e.g., negative social evaluation) robustly predict increased depression symptom severity [13,14] as well as overall biological and clinical functioning [15,16]. Animal model work has implicated social stressors in the development of depression [17,18]. Fear of social evaluation, in the context of both positive and negative social interactions, may affect risk for depression [[19], [20]], and prior research has examined how negative social evaluation relates to depression [[21], [22]].
In contrast to the work on negative social evaluation, much less is known about the role that positive social evaluation (e.g., praise from a peer) plays in depression (c.f. [23]). A fear of social evaluation might be expected to blunt reward-related neural activity and experiences of reward in what would otherwise be rewarding positive social interactions. Additionally, individual differences in reward preferences should be considered. Although positive evaluation may be rewarding for some, individuals who fear evaluation may be less likely to experience positive feedback as a reward [24,25].
Research on reward sensitivity in depression may provide clues for understanding how neural and peripheral responses to positive social evaluation are related to depression symptoms. Notably, depressed individuals show less activity in the striatum—specifically, the nucleus accumbens (NAcc), caudate nucleus, and putamen, which are known to be critical for experiences of reward—during monetary reward tasks [[26], [27], [28], [29]]. Reward sensitivity has been implicated in several disorders [[30], [31], [32], [33]], and reward hyposensitivity, which is a key factor in anhedonia [34], is thought to be a core characteristic of depression [28,35]. Although historically, anhedonia has been viewed as a key symptom of depression [36], recent research suggests that anhedonia is best thought of as risk factor for the development of depression, as anhedonia has been found to be present prior to the onset of a major depressive episode (MDE) [[37], [38]].
Several studies have shown that children who have not yet experienced a MDE, but who have at least one parent with a history of depression, exhibited blunted reward responsivity to monetary reward [29,[39], [40], [41]]. For example, a maternal history of depression was shown to be associated with a blunted reward response in adolescent girls who were not yet depressed [42]. However, most of this research has been conducted using monetary rewards paradigms that do not include social cues or interactions. Given that there are gender differences in how monetary and social reward are represented in the brain (i.e., female participants exhibit equal activation to monetary and social rewards whereas male participants had greater activation for monetary reward) [43], and that positive social evaluation has been found to be associated with lower depression symptom severity in women [44], investigating how positive social evaluation is processed at the neural level is critical for better understanding how and why depression develops among adolescent girls.
In terms of peripheral biology, experiencing reward has been found to upregulate circulating levels of the endogenous opioid β-endorphin [45,46]. If depression is associated with atypical reward processing during positive social evaluation, then depression should relate to a decrease in β-endorphin levels from pre- to post-social evaluation. Prior research has found that depression is associated with differences in β-endorphin levels [[47], [48], [49], [50]]; however, results have been mixed, as prior studies have found both elevated and decreased β-endorphin levels in depression (for a review, see Ref. [51]). To date, no studies have examined how depression symptom severity relates to β-endorphin levels following positive social evaluation in adolescent girls.
1.1. Present study
In sum, despite research showing that social stressors are related to greater depression symptom severity (e.g. Ref. [10]), and evidence that blunted sensitivity to monetary reward is associated with depression [28,41,[52], [53], [54]], how neural and peripheral markers of social reward responsivity are related to depression in adolescence is unknown. To address this gap, we examined the extent to which risk for depression and current depression symptom severity were associated with differences in striatal activity to positive social evaluation and β-endorphin output following positive social reward in the context of the Psychobiology of Stress and Adolescent Depression (PSY SAD) Study [55]. We hypothesized that both risk for depression (i.e., having a positive maternal history of depression) and current depressive symptoms would be related to blunted striatal activity during positive (vs. neutral) social evaluation, as well as to blunted β-endorphin output following an acute, laboratory-based experience of social evaluation. Moreover, we expected to find that only the girls who have a mother with a history of MDD (i.e., the high-risk girls) would exhibit an association between current depressive symptoms and blunted striatal activity during positive (vs. neutral) social evaluation. This is because we view both maternal history of MDD and blunted neural sensitivity to social reward (i.e., anhedonia) as contributing risk factors for depression. As anhedonia has been found to serve as a pre-clinical risk marker for depression, it is possible that these two factors may confer a greater risk for depression when they are present concurrently.
2. Method
2.1. Participants
Participants in the PSY SAD Study (N = 52, Mage = 14.90, SD = 1.35) were recruited from online advertisements, flyers posted in community locations, social media posts, word of mouth, and announcements made at middle and high schools in the greater Los Angeles area [55]. Inclusion and exclusion criteria included age between 12 and 16 years old, English-speaking, right-handed, not claustrophobic, free of bodily metal (except dental fillings) and other contraindications for MRI, living with their biological mother, and have no current or past history of any Diagnostic and Statistical Manual-IV (DSM-IV) Axis I disorder. We focused on this age range because it is a critical developmental period when risk for major depressive disorder (MDD) increases significantly but before most adolescent girls experience their first MDE [56]. In addition, girls must not have had any recent alcohol or substance use or dependence, not have been pregnant as verified with a pregnancy test, and not have had any history of head trauma or a learning disability. Finally, girls had to be free of past or current inflammatory illness (e.g., multiple sclerosis, lupus), major sleep disturbance (e.g., insomnia), tobacco use, prescription drug use, excessive caffeine use (i.e., >8 cups/day), or a body mass index of ≥30, all of which are confounds for peripheral markers [57]. Seven girls were excluded from fMRI analyses due to excessive motion (see below), leaving a final sample size for fMRI analysis of 45.
2.2. Materials and procedure
Interested mothers and daughters completed a phone screening and, if eligible, completed an in-person intake session. First, written informed consent and assent was obtained from mothers and daughters, respectively. Then, mothers and daughters were screened separately by trained diagnostic interviewers (all trained by GMS) to ensure they met all of the inclusion criteria, did not meet any of the exclusion criteria, and ascertain the daughter's maternal risk group, which was classified as either low-risk (i.e., mothers with no MDEs; N = 30) or high-risk (i.e., mothers with at least one MDE; N = 22) [55].
Depression symptom severity was evaluated in the daughters using the Kiddie Schedule for Affective Disorders and Schizophrenia for School Aged Children-Present and Lifetime Version (K-SADS-PL) [58]. Severity scores represent the sum of all symptoms (0 = no information; 1 = not present; 2 = sub-threshold; 3 = threshold); given that 23 symptoms were assessed, the maximum depression symptom severity score was 69. In addition to assessing depression symptomology, the K-SADS provided scores for mania, psychotic disorders, generalized anxiety disorder, panic disorder, and eating disorders. Daughters with a lifetime history of any of these disorders were excluded. In turn, mothers were assessed using the Structured Clinical Interview for DSM-IV (SCID-IV) [59] and Beck Depression Inventory-II (BDI-II) to determine their lifetime history of depression [60]. Afterward, daughters completed a 10-min video-recorded interview about their upbringing, opinions, feelings, and autobiographical memories in absence of their mothers (for complete procedural details, see Ref. [55]).
A second, 3.5-h fMRI session was scheduled within approximately 1 month of the initial session (median = 26.5 days). During this second session, blood was drawn at multiple times to assess β-endorphin levels 55 min before a social evaluation stressor (T1; baseline), and 35 min (T2; post-evaluation) and 65 min (T3; not analyzed) after the social evaluation stressor. 3 mL of blood was drawn into an EDTA Vacutainer Tube, immediately placed on ice, and then transferred by the end of each study session to the UCLA Center for Pathology Research Services, which centrifuged the samples for 15 min at 3000 RPMs. Extracted plasma was divided into 1 mL aliquots and frozen at −80 °C until assays were performed by the Olvera-Alvarez Lab. Plasma concentrations of β-endorphin were measured using the Human Neuropeptide Panel (catalog #HNPMAG-35K) from Luminex Corporation (Austin, USA). The lower limit of detection was 85 pg/mL. All controls were within the expected range. The inter-assay CV was 7.40%.
In addition, the daughters completed self-report questionnaires assessing current mood and social perceptions, including the Profile of Mood States (POMS) [61]. Before entering the fMRI scanner, the daughters were introduced to “another participant” (actually a confederate) and told that the person was a participant completing a related study. The confederate was always a female, college-aged research assistant who dressed and acted like a slightly older adolescent. The confederate was thus believably a participant who created an experience of social-evaluative threat for the daughter during the fMRI task.
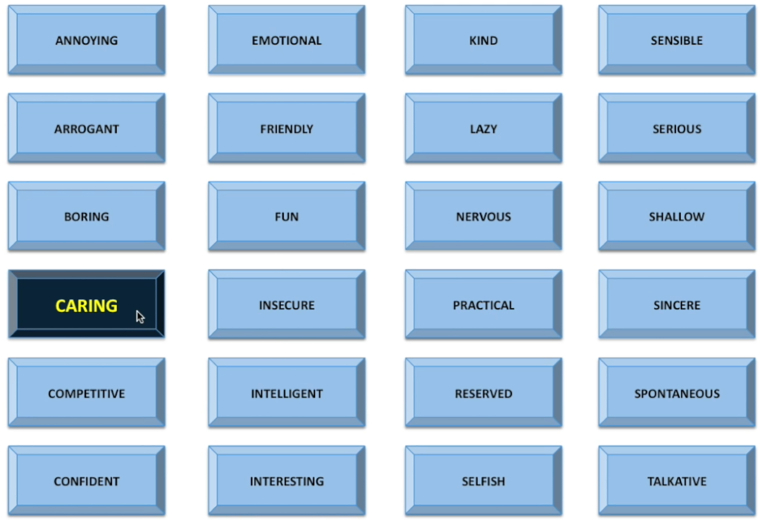
To complete this task, both the daughter and confederate were taken to the MRI scanner control room. They were then told that they would be completing a task based on “social impressions” (i.e., the social evaluation task [62,63]. They were reminded of their recorded interview from the first session and then told that the “other participant” (i.e., the confederate) would be viewing and judging their video by clicking on 1 of 24 potential adjectives (one-third positive, one-third neutral, one-third negative) to describe their impression of the participant (Fig. 1). To enhance the believability of this task, the confederate would always ask “How often should I provide a rating?” whilst receiving instructions. The participant was informed that they would be viewing the adjective ratings in real time, when, in reality, all participants watched the same pre-recorded video in which the adjectives were “selected” in pseudorandom order, with a jittered inter-adjective interval (approximately 10 s) and no more than two similarly valenced words clicked consecutively. This task lasted for 10 min. Prior research has shown that this task engages the amygdala [[63], [64]] and increases self-reported feelings of social evaluation and rejection [9], which are common features of depression in youth [65].
Fig. 1.
A screenshot of the social evaluation task that participants saw while in the fMRI scanner. Adolescent girls were led to believe that their previously recorded interview was being watched and evaluated by another participant (actually a confederate). Roughly every 10 s, an adjective was pressed, and participants were asked to rate how they felt from 1 (very bad) to 4 (very good). Depicted is an example of positive social evaluation word being selected.
After the MRI scan, participants returned to the testing room, where they again completed post-evaluation self-report questionnaires assessing current mood and social perceptions. Finally, participants were fully debriefed and thanked. All procedures were pre-approved by the UCLA Institutional Review Board.
2.3. fMRI image acquisition
Imaging data were acquired using a Prisma 3.0 T whole-body scanner (Siemens Medical Systems, Iselin, New Jersey) at the Staglin One Mind Center for Cognitive Neuroscience at UCLA. High resolution T1-weighted structural images were acquired using a magnetized prepared rapid acquisition gradient echo (MPRAGE) sequence containing 1.1 mm isotropic voxels, TR/TE/flip angle = 2300 ms/2.95 ms/9°, FOV = 270 mm2, 176 slices. Blood oxygenation level-dependent (BOLD) functional images were acquired containing 3 mm isotropic voxels, TR/TE/flip angle = 2000 ms/34 ms/76°, FOV = 208 mm2, 48 slices.
2.4. fMRI preprocessing & analyses
Functional and structural MRI data used in univariate (i.e., BOLD activation) analyses were preprocessed using SPM12. Preprocessing included realignment (and unwarp) of functional files, functional centering to (0,0,0) coordinates (translation), functional slice-timing correction, motion correction, functional segmentation and normalization, structural translation, and structural segmentation and normalization. Participants with >3 mm and/or 3° of movement between slices were excluded prior to fMRI analyses. Contrast images for activity were estimated using SPM12.
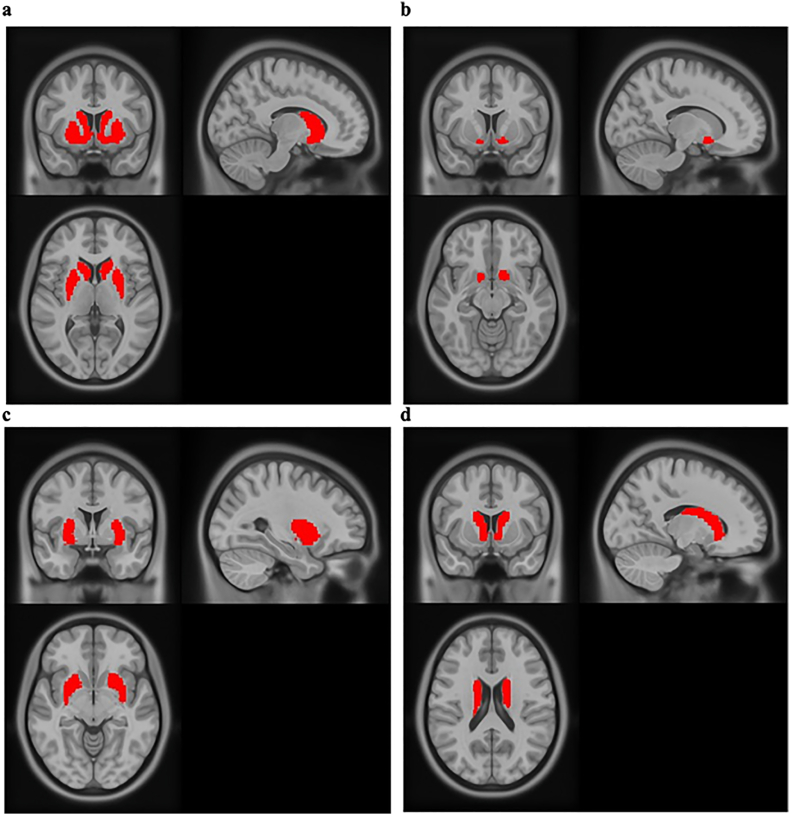
Masks were created for the striatum from its components (i.e., the caudate nucleus, the putamen, and nucleus accumbens [NAcc]) using the WFU PickAtlas software extension for SPM12 (see Fig. 2).1 Signal intensities within ROIs were extracted for use in analyses.
Fig. 2.
The ROI masks used for fMRI analyses. All four masks were created using the WFU PickAtlas and represent the (A) striatum, (B) nucleus accumbens, (C) putamen, and (D) caudate nucleus.
2.5. Data analysis
Difference scores between the positive minus neutral contrasts were calculated in SPM12, before extraction for analysis in R, version 4.1.1. General linear models were fit to examine relations among clinical depression symptom severity, striatal activity, and β-endorphin when striatal activity or β-endorphin was the outcome, and Poisson regressions were used in models with clinical depression symptom severity (a count variable; the minimum observed score was subtracted from all scores to meet Poisson distribution assumptions) as the outcome. Striatal activity (positive – neutral; standardized as the difference score) and β-endorphin (post-evaluation) were standardized prior to analyses using the scale() function in R, ensuring that incidence rate ratios with clinical depression symptom severity represented the raw increase in clinical depression symptom severity for every one-SD increase in striatal activity or β-endorphin. Outliers were determined using studentized residuals (with any |studentized residuals| > 3 being excluded), and the results below are presented both with and without including outliers.
3. Results
Descriptive differences between the high-risk and low-risk groups are reported in Table 1.
Table 1.
Sample characteristics.
| Variable | High-Risk Girls n = 22 Mean (SD) |
Low-Risk Girls n = 30 Mean (SD) |
Group Difference p |
|---|---|---|---|
| Age | 14.68 (1.39) | 15.07 (1.31) | .31 |
| Body mass index | 24.87 (6.70) | 22.24 (4.51) | .10 |
| Depression symptom severity (K-SADS) | 26.63 (6.79) | 25.08 (3.67) | .34 |
| Mothers' depressive symptoms (BDI-II) | 9.91 (10.41) | 4.95 (4.94) | .03 |
| Race | |||
| Black or African American | 9% | 7% | |
| Asian or Asian American | 0% | 7% | |
| White/Caucasian | 23% | 40% | |
| Hispanic/Latino/a | 32% | 27% | |
| Other | 5% | 7% | |
| Mixed/Multiple | 32% | 13% | |
Note: K-SADS, Kiddie Schedule for Affective Disorders and Schizophrenia for School Aged Children-Present and Lifetime Version; BDI-II, Beck Depression Inventory-II. Demographics are for the entire sample, including those who were excluded from fMRI analyses for excessive movement.
3.1. Affect ratings during evaluation
To determine if the girls believed the fMRI-based social evaluation task, they provided an affect rating each time they received social feedback (see Fig. S1). As expected, girls reported greater positive affect when they received positive social evaluation than when they received neutral social evaluation, t(49) = 8.63, p < .001.
3.2. Neural markers of reward
We initially tested whether striatal activity differed while receiving positive vs. neutral social evaluation across the entire sample. Contrary to hypotheses, there was no significant difference in striatal activity between the social evaluation conditions, t(44) = 0.96, p = .339. Notably, however, this lack of an overall difference does not preclude the possibility of individual differences in striatal activity during social evaluation being related to biological or clinical processes.
3.2.1. Neural markers of reward in relation to depression
Our primary analyses examined the extent to which depression risk group, depression symptom severity, and change in self-reported depressed feelings (as assessed by the POMS) across the social evaluation task were related to neural and peripheral markers of reward during social evaluation. We first tested whether striatal activity during positive (vs. neutral) social evaluation differed for low- vs. high-risk girls. Contrary to hypotheses, low-risk girls did not differ from high-risk girls in their striatal responses to positive vs. neutral social evaluation, t(43) = -0.43, p = .668. Similarly, this striatal activity was unrelated to change in self-reported depressed mood (as assessed by the POMS) in response to the social evaluation task (see Table S2).
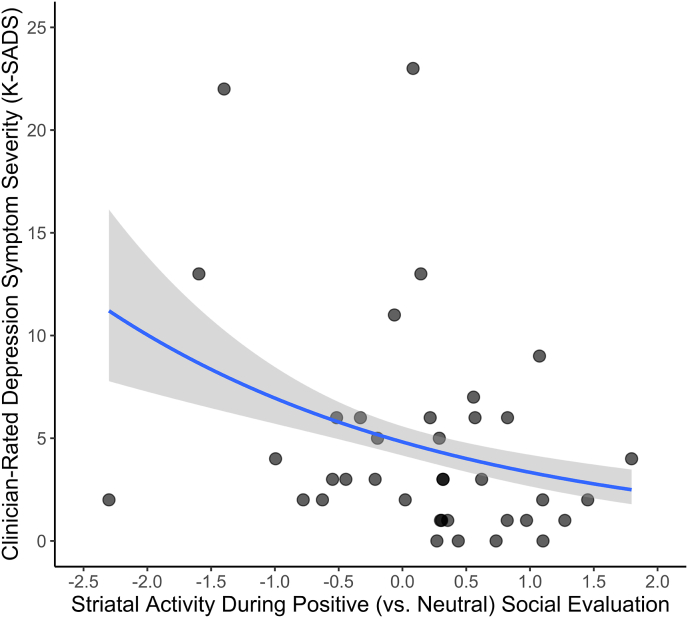
We next examined whether striatal activity (and its subregions) during positive (vs. neutral) social evaluation related to depression symptom severity (see Table 2). As hypothesized, greater striatal activity during positive (vs. neutral) social evaluation was associated with lower depression symptom severity, B = −0.366, IRR = 0.692, p < .001 (Fig. 3). This association remained significant while controlling for age, race, ethnicity, BMI, and whether the daughters were sick within the prior week. As described in the Supplemental Materials, we conducted sensitivity analyses to ensure that outliers were not driving the aforementioned association. Doing this identified four potential outliers according to studentized residuals. Importantly, however, greater striatal activity during positive (vs. neutral) social evaluation continued to be related to less depression symptom severity even when these outliers were removed, B = −0.385, p < .001. Moreover, imputing 0s using mean replacement produced similar results. Additionally, we found that striatal activity during negative (vs. neutral) social evaluation was not related to depression symptom severity, p = .513 (see Table S1).
Table 2.
Associations between striatal (and striatal subregion) activity during positive vs. neutral social evaluation and clinician-rated depression symptom severity, as assessed by the K-SADS.
| Region |
Estimate |
SE |
95% CI |
p |
|
|---|---|---|---|---|---|
| LL | CL | ||||
| K-SADSa | |||||
| Regions | |||||
| Striatum | −0.366 | 0.078 | −0.519 | −0.209 | <.001*** |
| NAccb | −0.257 | 0.062 | −0.377 | −0.133 | <.001*** |
| Caudate | −0.291 | 0.060 | −0.406 | −0.172 | <.001*** |
| Putamen | −0.276 | 0.079 | −0.428 | −0.119 | <.001*** |
| K-SADS x MDD Risk interaction | |||||
| Regions | |||||
| Striatum | −0.602 | 0.172 | −0.943 | −0.270 | <.001*** |
| NAcc | 0.054 | 0.144 | 0.225 | 0.336 | .707 |
| Caudate | −0.330 | 0.138 | −0.600 | −0.061 | .016* |
| Putamen | −0.528 | 0.171 | −0.867 | −0.196 | .002** |
Note: K-SADS, Kiddie Schedule for Affective Disorders and Schizophrenia for School Aged Children-Present and Lifetime Version.
* p-value <.05.
** p-value <.01.
*** p-value <.001.
Depression symptom severity; higher scores indicate more severe symptoms.
Nucleus accumbens.
Fig. 3.
Associations between striatal activity and clinical depression symptom severity. Greater striatal responses to positive (vs. neutral) social evaluation were significantly associated with less depression symptom severity as independently assessed by the clinician-rated K-SADS, p < .001.
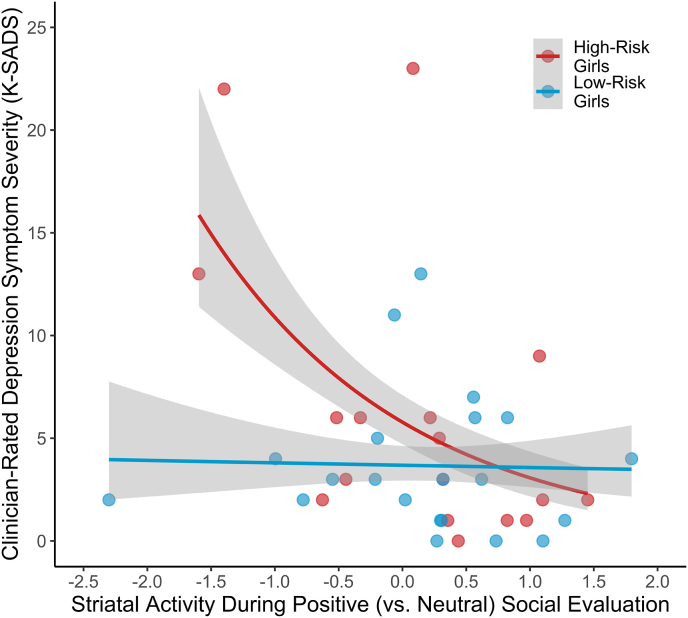
Next, we probed for a neural sensitivity to social evaluation by MDD risk group interaction in predicting depression symptom severity. As hypothesized, analyses revealed a significant Striatum × MDD Risk Group interaction, revealing that low striatal activity during positive (vs. neutral) social evaluation was related to depression symptom severity only for high-risk girls, B = −0.601, p < .001 (Fig. 4). This analysis was robust to removal of four outliers identified by studentized residuals, B = −0.594, p < .001. These results thus suggest that blunted neural sensitivity to positive evaluation interacts with risk for depression in predicting depression symptom severity.
Fig. 4.
Striatal responses to positive (vs. neutral) social evaluation in adolescent girls at high vs. low risk for depression, as determined by having (vs. not having) a SCID-IV evaluated maternal lifetime history of MDD. Less striatal activity during positive (vs. neutral) social evaluation was associated with less depression symptom severity as independently assessed by the clinician-rated K-SADS for girls at high but not low risk for MDD, p < .001.
3.3. Peripheral reward marker analyses
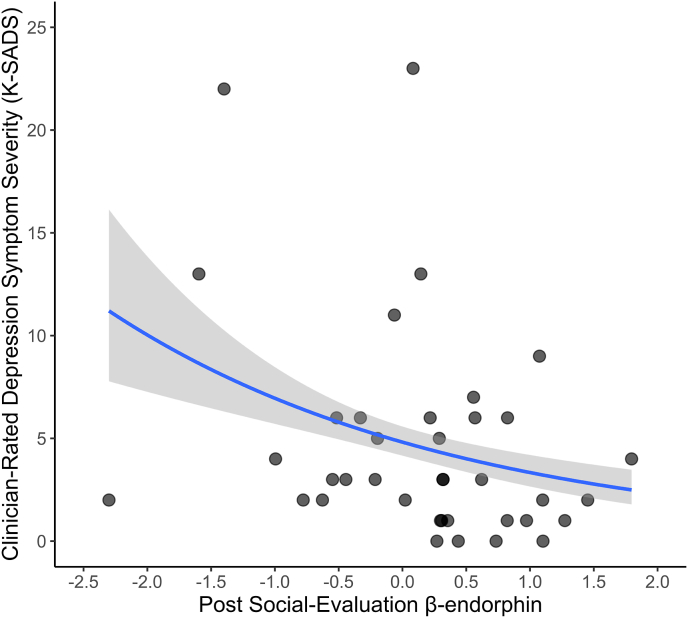
We then examined the extent to which risk for depression, change in self-reported depressed across the social evaluation task, and depression symptom severity were associated with post-evaluation levels of β-endorphin, which is a peripheral marker of reward sensitivity. Consistent with the results described above, neither depression risk status, t(46) = -1.07, p = .289, nor change in self-reported depressed feeling across the social evaluation task, β = 0.064, p = .668, were associated with post-evaluation levels of β-endorphin. As hypothesized, however, higher post-evaluation β-endorphin levels were related to less depression symptom severity, B = −0.198, IRR = 0.821 p = .012 (Fig. 5).
Fig. 5.
Association between post-social evaluation β-endorphin levels and depression symptom severity as independently assessed by the clinician-rated K-SADS. Higher levels of β-endorphin following the social evaluation task were significantly associated with less K-SADS rated depression symptom severity, p = .012.
3.4. Relation between neural and peripheral reward markers
Finally, we assessed the extent to which striatal activity while receiving positive (vs. neutral) social evaluation was associated with post-evaluation levels of β-endorphin. Contrary to hypotheses, we found that greater striatal activity while receiving positive (vs. neutral) social evaluation was not significantly related to greater post-evaluation β-endorphin, r(39) = 0.281, p = .075. There were no significant correlations between greater activity in the putamen and nucleus accumbens while receiving positive evaluation, ps < .24. However, a positive correlation was observed between activity in the caudate nucleus during positive evaluation and post-evaluation β-endorphin, r(39) = 0.341, p = .029. Importantly, changes in β-endorphin from pre- to post-social evaluation were not significantly related to any striatal activity (including subregions), ps < .103.
As described in the Supplemental Materials, sensitivity analyses separating baseline β-endorphin (pre-evaluation) from social evaluation-related reactivity in β-endorphin (i.e., 35 min post-evaluation minus pre-evaluation) in the same model revealed that baseline β-endorphin was a significant predictor of depression symptom severity, B = −0.197, IRR = 0.821, p = .029, whereas β-endorphin reactivity to social evaluation was not, B = 0.031, IRR = 1.032, p = .696. Moreover, in this model, baseline β-endorphin was a better inverse predictor of depression symptom severity than β-endorphin reactivity, p = .027 (one-tailed), suggesting that pre-social evaluation β-endorphin levels may be more relevant for modulating depression severity than β-endorphin level changes in response to an acute social stressor.
3.5. Alternative analytic approaches
Different analytic approaches such as using a negative binomial model, zero-inflated poisson model, and log-transformed variables in normal models did not alter any of the striatal activity results; in contrast, β-endorphin was unrelated to depression symptom severity when using these alternative analytic approaches (ps > .069). Full details of the alternative analytic approaches can be found in the Supplemental Materials.
4. Discussion
Despite substantial evidence showing that social stressors are strongly related to increases in depression symptoms [10] and the known relation between monetary reward processing and depression [28], little is known about how neural and peripheral responses to social reward are associated with depression risk status and severity in adolescence. To address that gap, we examined neural and peripheral markers of reward during positive and neutral social evaluation in relation to youths' depression risk status, clinician-rated depression symptom severity, and self-reported depression in the PSY SAD Study. We found that neural activity indicative of reward processing in response to positive (vs. neutral) social evaluation was related to depression symptom severity but not MDD risk group or self-reported feelings of depression. Moreover, we found that blunted neural sensitivity to positive social evaluation was related to depression symptom severity in girls at high risk of developing depression, indicating that blunted neural sensitivity to positive social evaluation may forecast the development of depression in high-risk youth. We also found that a peripheral marker of reward sensitivity, β-endorphin, was significantly related to depression symptom severity and marginally related to participants' neural responses to positive (vs. neutral) social evaluation. In short, therefore, both neural and peripheral markers of reward processing during positive social evaluation were related to youths' clinician-rated depression symptom severity.
Several studies have examined how positive social interactions differentially affect depressed individuals at a behavioral level [[66], [67]]. This research has generally found that depression is associated with blunted feelings of reward in real or imagined positive social evaluation, as well as with a fear of positive social evaluation (e.g., would request no praise in a public setting) [24,25]. Our results support and extend these behavioral findings, as we found that blunted neural activity indicative of reward in response to positive (vs. neutral) social evaluation was related to depression symptom severity as independently determined by the K-SADS. Whether blunted reward related neural activity is a cause or consequence of fear of positive social evaluation may be a fruitful topic for future research and could be addressed by conducting longitudinal research on this topic.
In addition to improving our understanding of the neural representation of positive social evaluation, these results help to clarify neural differences associated with risk for depression in a well-characterized sample of adolescent girls who are at high or low risk for MDD, but who have not yet experienced a MDE. Importantly, we found that striatal responses to positive (vs. neutral) social evaluation were related to clinician-rated depression symptom severity only for girls at high risk of developing MDD. Our finding showing that blunted striatal activity during positive social evaluation is related to depression symptom severity thus identifies a neural marker that is associated with heightened pre-clinical risk for MDD. Moreover, in conjunction with depression risk, this blunted neural response to positive social evaluation may be an important prodromal predictor of a developing a MDD—a topic worthy of future investigation.
To date, we know of only one prior study that has examined social evaluative reward-related neural processing in the context of depression. Specifically, Schaefer et al. [68] showed participants affective and neutral images and found reduced activity in the striatum in response to images of positive social interaction. However, this study did not investigate pre-clinical risk mechanisms as all participants were already depressed. Moreover, Schaefer et al. (2006) studied adults, not adolescents. The present findings thus replicate and extend Schaefer et al. (2006) by showing that blunted reward-related neural activity is present and related to depression not only in adults who have experienced at least one MDE but also in adolescent girls who are at high-risk for MDD but who have never been depressed.
None of the girls in the PSY SAD Study had yet experienced a lifetime MDE, but did exhibit some symptoms of depression [55]. As the K-SADS-PL is considered the gold-standard for childhood assessment of psychiatric symptoms [58], finding significant associations between a highly specified brain region and clinician-evaluated symptoms of depression may be useful in extending our understanding of how the brain processes reward, leading to depression. Looking forward, longitudinal research is required to examine if the associations between blunted reward responsivity to social reward and MDD risk predict the subsequent development of MDD.
These findings are consistent with theories of depression which posit that reward processing plays a central role in anhedonia (e.g., Ref. [69]). Additionally, given that none of the girls in this sample had yet experienced a MDE, we believe our findings support the claim that anhedonia may be a risk factor for depression, not simply a symptom [37]. This formulation is supported in so far as we found that despite only the girls at high-risk for depression exhibited a blunted striatal response to positive (vs. neutral) evaluation, they did not differ from low-risk girls with respect to their overall depression symptom severity, as assessed by the K-SADS.
Contrary to hypotheses, positive social evaluation was not related to greater striatal activity as compared to neutral social evaluation. This may be due to the sample characteristics. In particular, because depression is associated with blunted reward processing [28] and the sample included many girls who exhibited one or more symptoms of depression (albeit without having experienced a MDE), the prevalence of depression symptoms in the sample may have reduced our ability to observe a difference in striatal activity during the processing of positive, relative to neutral, social evaluation. Nonetheless, we found that girls reported greater positive affect in response to positive feedback, relative to either neutral or negative feedback.
Despite its known role in neural reward responsivity [46], there is very limited research on how the neural reward system interacts with β-endorphins in the context of depression [70]. Our findings from the PSY SAD Study suggest that while post-evaluation β-endorphin is related to less depression symptom severity, this relation appears to stem from individual differences in baseline β-endorphin, as we found that baseline β-endorphin levels, but not β-endorphin social evaluation-related reactivity levels, were related to depression symptom severity. However, it should be noted that β-endorphin was not associated with depression symptoms when alternative analytic methods were used (see Supplemental Materials). Therefore, the extent to which β-endorphin is a robust predictor of depression symptom severity requires further study. Additional research is also needed to understand what role, if any, β-endorphin plays in influencing subsequent risk for developing MDD.
This study has several strengths, including the use of a gold-standard instrument for diagnosing MDD in youth (i.e., the K-SADS-PL [58]), analyzing the whole striatum as well as its subregions, conducting sensitivity analyses to evaluate the robustness of results, combining neural and peripheral markers of reward cognition, and sampling well-characterized youth at high risk for MDD who have never had a MDE. However, several limitations should also be noted. First, neither the neural nor peripheral markers of reward examined here related to adolescents' self-reported feelings of depressed mood; moreover, the reason for the discrepancy between this finding and the fact that both of these markers were associated with youths’ clinician-rated depression symptom severity remains unclear. Second, the present results are cross-sectional, and we did not investigate how these markers predicted subsequent risk for developing MDD. Third, the sample was well-characterized but limited in size, which can reduce the robustness of findings and increase the likelihood of observing spurious associations. This is especially relevant for the interaction observed. Although we cannot rule out the possibility that the moderation of the association between striatal activity and depression by MDD risk group was spurious, as described in the introduction, we believe that this finding is consistent with existing theory. Finally, although the PSY SAD sample is highly diverse (see Ref. [55]), it is still relatively Western, Educated, Industrialized, Rich, and Democratic (WEIRD) [71]. Therefore, additional research is needed to understand the generalizability of these results as well as potential cross-cultural differences in how neural and peripheral reward processes vary and are related to depression across different populations.
4.1. Conclusion
In conclusion, although social reward processing is hypothesized to play a role in determining risk for MDD, no studies to date have investigated this issue in adolescents using non-monetary reward paradigms. In addressing this issue, we found that positive social evaluation-induced reward activity in the striatum, as well as peripheral marker of reward cognition (i.e., β-endorphin), were both related to exhibiting fewer symptoms of depression as independently assessed by the K-SADS. Moreover, blunted striatal activity to positive (vs. neutral) social evaluation was related to depression symptom severity but only for girls at high risk of developing MDD, suggesting that altered social reward processing may potentially play a role in the etiology of this common and costly disorder. Looking forward, additional research is needed to investigate the predictive utility of these neural and peripheral risk markers, and to investigate the generalizability of these findings in larger samples and other age groups, populations, and cultures.
Funding
This study was supported by a Society in Science—Branco Weiss Fellowship, NARSAD Young Investigator Grant 23958 from the Brain & Behavior Research Foundation, and National Institutes of Health (NIH) grant K08 MH103443 to GMS. SS was supported by National Science Foundation GRFP Grant DGE-2034835. MV is supported by the Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, Department of Veterans Affairs. The UCLA Clinical and Translational Research Center is supported by NIH grant UL1 TR001881. These organizations had no role in designing or planning this study; in collecting, analyzing, or interpreting the data; in writing the article; or in deciding to submit this article for publication.
Declarations of competing interest
None.
Acknowledgements
We thank the mothers and daughters who participated in this study. We also thank the many students, trainees, and fellows who helped with various aspects of this study, including: Jacob Allely, Sammy Benavidez, Kaitlyn Breiner, Ashley Chipoletti, Kelly Costa, Desiree Delavary, Micah Dombroe, Kishan Ghadiya, Kirsten Gimse, Connie Ha, Marzia Hazara, Kean Hsu, Ashley Huynh, Mark Libowitz, Roman Liccini, Kristy Lin, Abigail Looi, Oria Mimi Lu, Kaivalya Molugu, Riya Mukhopadhyay, Rachel Ogata, Kelly Sun, Evelyn Valencia, Ruben Valentin, Kevin Walsh, and Hilary Wilson. Finally, we thank Keely Muscatell for providing the Social Evaluation Task, three reviewers who provided excellent comments on an earlier version of this article, and several centers at UCLA for supporting the study including the Cousins Center for Psychoneuroimmunology (especially Michael Irwin), Staglin One Mind Center for Cognitive Neuroscience, UCLA Clinical and Translational Research Center, UCLA Neuroscience Genomics Core, and Center for Pathology Research Services.
Footnotes
Analyses using individual ROIs rather than the overall striatum did not alter any of the primary results.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cpnec.2022.100149.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Friedrich M.J. Depression is the leading cause of disability around the world. JAMA. 2017;317:1517. doi: 10.1001/jama.2017.3826. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg P.E., Fournier A.-A., Sisitsky T., Simes M., Berman R., Koenigsberg S.H., Kessler R.C. The economic burden of adults with major depressive disorder in the United States (2010 and 2018) Pharmacoeconomics. 2021;39:653–665. doi: 10.1007/s40273-021-01019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Q., He H., Yang J., Feng X., Zhao F., Lyu J. Changes in the global burden of depression from 1990 to 2017: findings from the global burden of disease study. J. Psychiatr. Res. 2020;126:134–140. doi: 10.1016/j.jpsychires.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 4.O'Leary K. Global increase in depression and anxiety. Nat. Med. 2021 doi: 10.1038/d41591-021-00064-y. [DOI] [PubMed] [Google Scholar]
- 5.Weinberger A.H., Gbedemah M., Martinez A.M., Nash D., Galea S., Goodwin R.D. Trends in depression prevalence in the USA from 2005 to 2015: widening disparities in vulnerable groups. Psychol. Med. 2018;48:1308–1315. doi: 10.1017/S0033291717002781. [DOI] [PubMed] [Google Scholar]
- 6.Nolen-Hoeksema S., Girgus J.S. The emergence of gender differences in depression during adolescence. Psychol. Bull. 1994;115:424–443. doi: 10.1037/0033-2909.115.3.424. [DOI] [PubMed] [Google Scholar]
- 7.Piccinelli M., Wilkinson G. Gender differences in depression: critical review. Br. J. Psychiatry. 2000;177:486–492. doi: 10.1192/bjp.177.6.486. [DOI] [PubMed] [Google Scholar]
- 8.Weller E.B., Kloos A., Kang J., Weller R.A. Depression in children and adolescents: does gender make a difference? Curr. Psychiatr. Rep. 2006;8:108–114. doi: 10.1007/s11920-006-0007-1. [DOI] [PubMed] [Google Scholar]
- 9.Dedovic K., Slavich G.M., Muscatell K.A., Irwin M.R., Eisenberger N.I. Dorsal anterior cingulate cortex responses to repeated social evaluative feedback in young women with and without a history of depression. Front. Behav. Neurosci. 2016;10 doi: 10.3389/fnbeh.2016.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slavich G.M., Irwin M.R. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol. Bull. 2014;140:774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanhalst J., Luyckx K., Scholte R.H.J., Engels R.C.M.E., Goossens L. Low self-esteem as a risk factor for loneliness in adolescence: perceived - but not actual - social acceptance as an underlying mechanism. J. Abnorm. Child Psychol. 2013;41:1067–1081. doi: 10.1007/S10802-013-9751-Y. [DOI] [PubMed] [Google Scholar]
- 12.Bhanji J.P., Delgado M.R. The social brain and reward: social information processing in the human striatum. Wiley Interdiscip Rev Cogn Sci. 2014;5(1):61–73. doi: 10.1002/wcs.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slavich G.M., Thornton T., Torres L.D., Monroe S.M., Gotlib I.H. Targeted rejection predicts hastened onset of major depression. J. Soc. Clin. Psychol. 2009;28:223–243. doi: 10.1521/jscp.2009.28.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slavich G.M., O'Donovan A., Epel E.S., Kemeny M.E. Black sheep get the blues: a psychobiological model of social rejection and depression. Neurosci. Biobehav. Rev. 2010;35:39–45. doi: 10.1016/j.neubiorev.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slavich G.M. Social safety theory: a biologically based evolutionary perspective on life stress, health, and behavior. Annu. Rev. Clin. Psychol. 2020;16:265–295. doi: 10.1146/annurev-clinpsy-032816-045159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slavich G.M. Social Safety Theory: understanding social stress, disease risk, resilience, and behavior during the COVID-19 pandemic and beyond. Curr. Opin. Psychol. 2022;45 doi: 10.1016/j.copsyc.2022.101299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rygula R., Abumaria N., Flügge G., Fuchs E., Rüther E., Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav. Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi A., Flanigan M.E., McEwen B.S., Russo S.J. Aggression, social stress, and the immune system in humans and animal models. Front. Behav. Neurosci. 2018;12:56. doi: 10.3389/fnbeh.2018.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menne-Lothmann C., Decoster J., van Winkel R., Collip D., Rutten B., Delespaul P., De Hert M., Derom C., Thiery E., Jacobs N., van Os J., Wichers M. Psychological and biological validation of a novel digit social peer evaluation experiment (digi-spee) Noro. Psikiyatr. Ars. 2017;54(1):3–10`. doi: 10.5152/npa.2017.19318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W., Hsu W., Chiu Y., Liang C. The hierachical model of social interaction anxiety and depression: the critical roles of fears of evaluation. J. Anxiety Disord. 2012;26(1):215–224. doi: 10.1016/J.JANXDIS.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Dedovic K., Duchesne A., Engert V., Lue S.D., Andrews J., Efanov S.I., Beaudry T., Pruessner J.C. Psychological, endocrine, and neural responses to social evaluation in subclinical depression. Soc. Cognit. Affect Neurosci. 2014;9:1632–1644. doi: 10.1093/scan/nst151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winer E., Salem T. Reward devaluation: dot-probe meta-analytic evidence of avoidance of positive information in depressed persons. Psychol. Bull. 2016;142(1):18–78. doi: 10.1037/bul0000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nezlek J., Imbrie M., Shean G. Depression and everyday social interaction. J. Pers. Soc. Psychol. 1994;67(6):1101–1111. doi: 10.1037//0022-3514.67.6.1101. [DOI] [PubMed] [Google Scholar]
- 24.Jordan D.G., Winer E.S., Salem T., Kilgore J. Longitudinal evaluation of anhedonia as a mediator of fear of positive evaluation and other depressive symptoms. Cognit. Emot. 2018;32:1437–1447. doi: 10.1080/02699931.2017.1289895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reichenberger J., Wiggert N., Agroskin D., Wilhelm F.H., Blechert J. No praise, please: depressive symptoms, reactivity to positive social interaction, and fear of positive evaluation. J. Behav. Ther. Exp. Psychiatr. 2017;54:186–194. doi: 10.1016/j.jbtep.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Caseras X., Lawrence N.S., Murphy K., Wise R.G., Phillips M.L. Ventral striatum activity in response to reward: differences between bipolar I and II disorders. Am. J. Psychiatr. 2013;170:533–541. doi: 10.1176/appi.ajp.2012.12020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCabe C., Cowen P.J., Harmer C.J. Neural representation of reward in recovered depressed patients. Psychopharmacology. 2009;205:667–677. doi: 10.1007/s00213-009-1573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pizzagalli D.A., Holmes A.J., Dillon D.G., Goetz E.L., Birk J.L., Bogdan R., Dougherty D.D., Iosifescu D. v, Rauch S.L., Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am. J. Psychiatr. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rappaport B.I., Kandala S., Luby J.L., Barch D.M. Brain reward system dysfunction in adolescence: current, cumulative, and developmental periods of depression. Am. J. Psychiatr. 2020;177:754–763. doi: 10.1176/appi.ajp.2019.19030281. [DOI] [PubMed] [Google Scholar]
- 30.Alloy L.B., Olino T., Freed R.D., Nusslock R. Role of reward sensitivity and processing in major depressive and bipolar spectrum disorders. Behav. Ther. 2016;47:600–621. doi: 10.1016/j.beth.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alloy L.B., Abramson L.B. The role of the behavioral approach system (BAS) in bipolar spectrum disorders. Curr. Dir. Psychol. Sci. 2010;19:189–194. doi: 10.1177/0963721410370292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gruber J., Gilbert K.E., Youngstrom E., Youngstrom J.K., Feeny N.C., Findling R.L. Reward dysregulation and mood symptoms in an adolescent outpatient sample. J. Abnorm. Child Psychol. 2013;41:1053–1065. doi: 10.1007/s10802-013-9746-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volkow N.D., Wang G.-J., Fowler J.S., Tomasi D., Telang F., Baler R. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain's control circuit. Bioessays. 2010;32:748–755. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCabe C., Woffindale C., Harmer C., Cowen P. Neural processing of reward and punishment in young people at increased familiar risk of depression. Biol. Psychiatr. 2012;72:588–594. doi: 10.1016/j.biopsych.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 35.Pizzagalli D.A. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu. Rev. Clin. Psychol. 2014;10:393–423. doi: 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Psychiatric Association American. fifth ed. 2013. Diagnostic and Statistical Manual of Mental Disorders. [DOI] [Google Scholar]
- 37.Enneking V., Krüssel P., Zaremba D., Kohm K., Grotegerd D., Förster K., Meinert S., Bürger C., Dzonyar F., Leehr E.J., Böhnlein J., Repple J., Opel N., Winter N.R., Redlich R., Dannlowski U. Social anhedonia in major depressive disorder: a symptom-specific neuroimaging approach. Neuropsychopharmacology. 2019;44:883–889. doi: 10.1038/s41386-018-0283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rawal A., Collishaw S., Tharpar A., Rice F. ‘The risks of playing it safe’: a prospective longitudinal study of response to reward in the adolescent offspring of depressed parents. Psychol. Med. 2013;43(1):27–38. doi: 10.1017/S00332917112001158. [DOI] [PubMed] [Google Scholar]
- 39.Bress J.N., Foti D., Kotov R., Klein D.N., Hajcak G. Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology. 2013;50:74–81. doi: 10.1111/J.1469-8986.2012.01485.X. [DOI] [PubMed] [Google Scholar]
- 40.Nelson B.D., McGowan S.K., Sarapas C., Robison-Andrew E.J., Altman S.E., Campbell M.L., Gorka S.M., Katz A.C., Shankman S.A. Biomarkers of threat and reward sensitivity demonstrate unique associations with risk for psychopathology. J. Abnorm. Psychol. 2013;122:662–671. doi: 10.1037/a0033982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Callaghan G., Stringaris A. Reward processing in adolescent depression across neuroimaging modalities: a review. Z. Kinder JugenPsychiatr. Psychother. 2019 doi: 10.1024/14224917/a000663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Belleau E.L., Kremens R., Ang Y.-S., Pisoni A., Bondy E., Durham K., Auerbach R.P., Pizzagalli D.A. Reward functioning abnormalities in adolescents at high familial risk for depressive disorders. Biol. Psychiatr.: Cogn. Neurosci. Neuroimaging. 2021;6:270–279. doi: 10.1016/j.bpsc.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spreckelmeyer K., Krach S., Kohls G., Rademacher L., Irmak A., Konrad K., Kircher T., Gründer G. Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Soc. Cogn. Affect. Neurosci. 2009;4(2):158–165. doi: 10.1093/SCAN/NSN051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wareham S., Fowler K., Pike A. Determinants of depression severity and duration in Canadian adults: the moderating effects of gender and social support. J. Appl. Soc. Psychol. 2007;37:2951–2979. doi: 10.1111/J.1559-1816.2007.00289.X. [DOI] [Google Scholar]
- 45.Fu Y., Depue R.A. A novel neurobehavioral framework of the effects of positive early postnatal experience on incentive and consummatory reward sensitivity. Neurosci. Biobehav. Rev. 2019;107:615–640. doi: 10.1016/j.neubiorev.2019.09.026. [DOI] [PubMed] [Google Scholar]
- 46.Roth-Deri I., Green-Sadan T., Yadid G. β-Endorphin and drug-induced reward and reinforcement. Prog. Neurobiol. 2008;86:1–21. doi: 10.1016/j.pneurobio.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 47.Al-Hakeim H.K., Al-Fadhel S.Z., Aldujali A.H., Maes M. In major depression, increased kappa and mu opioid receptor levels are associated with immune activation. Acta Neuropsychiatr. 2020;32:99–108. doi: 10.1017/neu.2019.47. [DOI] [PubMed] [Google Scholar]
- 48.Dinas P.C., Koutedakis Y., Flouris A.D. Effects of exercise and physical activity on depression. Ir. J. Med. Sci. 2011;180:319–325. doi: 10.1007/s11845-010-0633-9. [DOI] [PubMed] [Google Scholar]
- 49.Esel E. Plasma levels of beta-endorphin, adrenocorticotropic hormone and cortisol during early and late alcohol withdrawal. Alcohol Alcohol. 2001;36:572–576. doi: 10.1093/alcalc/36.6.572. [DOI] [PubMed] [Google Scholar]
- 50.Navinés R., Martín-Santos R., Gómez-Gil E., Martínez de Osaba M.J., Gastó C. Interaction between serotonin 5-HT1A receptors and β-endorphins modulates antidepressant response. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2008;32:1804–1809. doi: 10.1016/j.pnpbp.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 51.Hegadoren K.M., O’Donnell T., Lanius R., Coupland N.J., Lacaze-Masmonteil N. The role of β-endorphin in the pathophysiology of major depression. Neuropeptides. 2009;43:341–353. doi: 10.1016/j.npep.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 52.Forbes E.E., Dahl R.E. Altered reward function in adolescent depression: what, when, and how? JCPP (J. Child Psychol. Psychiatry) 2012;53:3–15. doi: 10.1111/j.1469-7610-2011.02477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luking K.R., Pagliaccio D., Luby J.L., Barch D.M. Reward processing and risk for depression across development. Trends Cognit. Sci. 2016;20:456–468. doi: 10.1016/j.tics.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pizzagalli D.A., Iosifescu D., Hallett L.A., Ratner K.G., Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J. Psychiatr. Res. 2008;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sichko S., Bui T.Q., Vinograd M., Shields G.S., Saha K., Devkota S., Olvera-Alvarez H.A., Carroll J.E., Cole S.W., Irwin M.R., Slavich G.M. Psychobiology of stress and adolescent depression (PSY SAD) study: protocol overview for an fMRI-based multi-method investigation. Brain Behav Immun - Health. 2021;17 doi: 10.1016/j.bbih.2021.100334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Angold A., Costello E.J., Worthman C.M. Puberty and depression: the roles of age, pubertal status and pubertal timing. Psychol. Med. 1998;28:51–61. doi: 10.1017/S003329179700593X. [DOI] [PubMed] [Google Scholar]
- 57.O'Connor M.F., Bower J.E., Cho H.J., Creswell J.D., Dimitrov S., Hamby M.E., Hoyt M.A., Martin J.L., Robles T.F., Sloan E.K., Thomas K.M.S., Irwin M.R. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav. Immun. 2009;23:887. doi: 10.1016/J.BBI.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P., Williamson D., Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 59.First M.B., Spitzer, Gibbon M., Williams Davies, Borus J., Howes M.J., Kane J., Pope H.G., Rounsaville B. The structured clinical interview for DSM-IV Axis I disorders-patient edition. Biomet. Res. Depart. 1995 [Google Scholar]
- 60.Beck A., Steer R., Brown G. Psychological Corporation; San Antonio, TX: 1996. Manual for the Beck Depression Inventory-II. [DOI] [Google Scholar]
- 61.Curran S.L., Andrykowski M.A., Studts J.L. Short form of the Profile of Mood States (POMS-SF): psychometric information. Psychol. Assess. 1995;7:80–83. doi: 10.1037/1040-3590.7.1.80. [DOI] [Google Scholar]
- 62.Eisenberger N.I., Inagaki T.K., Muscatell K.A., Byrne Haltom K.E., Leary M.R. The neural sociometer: brain mechanisms underlying state self-esteem. J. Cognit. Neurosci. 2011;23:3448–3455. doi: 10.1162/jocn_a_00027. [DOI] [PubMed] [Google Scholar]
- 63.Muscatell K.A., Dedovic K., Slavich G.M., Jarcho M.R., Breen E.C., Bower J.E., Irwin M.R., Eisenberger N.I. Greater amygdala activity and dorsomedial prefrontal–amygdala coupling are associated with enhanced inflammatory responses to stress. Brain Behav. Immun. 2015;43:46–53. doi: 10.1016/j.bbi.2014.06.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shields G.S., Vinograd M., Bui T.Q., Sichko S., Irwin M.R., Slavich G.M. 2022. Heightened neural activity and functional connectivity responses to social rejection in adolescent girls at risk for depression: testing the Social Signal Transduction Theory of Depression. In preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Platt B., Kadosh K., Lau J. The role of peer rejection in adolescent depression. Depress. Anxiety. 2013;30(9):809–821. doi: 10.1002/da.22120. [DOI] [PubMed] [Google Scholar]
- 66.Nezlek J.B., Hampton C.P., Shean G.D. Clinical depression and day-to-day social interaction in a community sample. J. Abnorm. Psychol. 2000;109:11–19. doi: 10.1037/0021-843X.109.1.11. [DOI] [PubMed] [Google Scholar]
- 67.Weeks J.W. Replication and extension of a hierarchical model of social anxiety and depression: fear of positive evaluation as a key unique factor in social anxiety. Cognit. Behav. Ther. 2014;44:103–116. doi: 10.1080/16506073.2014.990050. [DOI] [PubMed] [Google Scholar]
- 68.Schaefer H., Putnam K., Benca R., Davidson R. Event-related functional magnetic resonance imaging measures of neural activity to positive social stimuli in pre- and post-treatment depression. Biol. Psychiatry. 2006;60(9):974–986. doi: 10.1016/j.biopsych.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 69.Eshel N., Roiser J.P. Reward and punishment processing in depression. Biol. Psychiatr. 2010;68:118–124. doi: 10.1016/j.biopsych.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 70.Yim I.S., Glynn L.M., Schetter C.D., Hobel C.J., Chicz-DeMet A., Sandman C.A. Prenatal βendorphin as an early predictor of postpartum depressive symptoms in euthymic women. J. Affect. Disord. 2010;125:128–133. doi: 10.1016/j.jad.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Henrich J., Heine S.J., Norenzayan A. The weirdest people in the world? Behav. Brain Sci. 2010;33:61–83. doi: 10.1017/S0140525X0999152X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.