Abstract
There is an intriguing association between winter births and subsequent increased risk of schizophrenia. However, little is known about the environmental risk factors that contribute this month-of-birth effect. The aims of this study were to carry out a systematic review and meta-analysis of studies investigating the month-of-birth effect in schizophrenia and to explore possible factors such as latitude, daylight and infections that could explain this epidemiological observation. Medline, Embase and the Cochrane Library were searched for articles published up to December 23, 2021. Study selection, data extraction and analysis were undertaken according to Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines. Generic inverse-variance with random effects models were used to determine the risk ratios (RR) and 95% confidence intervals (CI) for each month-of-birth. Associations between variables latitude and daylight were investigated using linear regression and Kendall's rank correlation coefficients were calculated assess the relationship between monthly infections rates schizophrenia births. Ten studies were included in the meta-analysis encompassing 262,188 schizophrenia patients. We identified significantly higher number of schizophrenia births in December [1.04 (95%CI 1.00–1.08)], January [1.06 (95%CI 1.03–1.1)] and February [1.03 (95%CI 1.00–1.05)]. We did not find any association between latitude and the magnitude of the month-of-birth effect. On the other hand, we found a significant negative correlation between monthly severe enterovirus cases and schizophrenia births (tau −0.57, p = 0.0099) using data from Taiwan. This highlights a role for enterovirus infections in mediating the month-of-birth effect in schizophrenia and these results carry implications for disease prevention strategies.
Keywords: Month, Season, Schizophrenia, Infection, Latitude
Highlights
-
•
This largest study to date identified an excess of schizophrenia births in December, January and February.
-
•
There was no association between latitude and the magnitude of this month-of-birth effect in schizophrenia.
-
•
There was a negative correlation between monthly severe enterovirus cases and schizophrenia births.
-
•
These findings carry implications for disease prevention strategies in schizophrenia.
1. Introduction
The association between winter births and subsequent increased risk of schizophrenia is an intriguing and well-studied area of research (Mortensen et al., 1999; O'Hare et al., 1980). This points towards a seasonally fluctuating risk factor in early life. However, the factors that mediate this effect have remained elusive and little progress has been made in this area of research. In 2003, a meta-analysis of studies from Northern European and North American countries suggested that latitude is linked to the excess winter births (Davies et al., 2003a; Parker et al., 2000; Selten et al., 2000; Suvisaari et al., 2001; Torrey et al., 1977). Accordingly, it was hypothesised that sunlight and more specifically vitamin D levels could play an important role in the disease, but subsequent genetic epidemiology studies have not supported this theory (Taylor et al., 2016). Since then, several new studies investigating the month-of-birth effect in schizophrenia have emerged (Carrion-Baralt et al., 2004; Disanto et al., 2012; Cheng et al., 2013; Karlsson et al., 2019; Munoz-Delgado et al., 2003; Mino and Oshima, 2006). Some of these studies were carried out in low latitude countries where sunlight does not vary substantially throughout the year but the link between winter birth and risk of schizophrenia has persisted in these countries (Carrion-Baralt et al., 2004; Munoz-Delgado et al., 2003). This in turn has brought doubt to the relevance of latitude to the month-of-birth effect in schizophrenia.
The developing brain is particularly susceptible to environmental factors that can influence genetically determined neurodevelopmental processes. Several observational studies have identified an association between maternal infection during pregnancy and subsequent increased risk of schizophrenia in their offspring (Buka et al., 2001, 2008; Babulas et al., 2006; Saatci et al., 2021). Timing of infection is important, and although not comprehensive, infections in the second trimester seem to mediate this increased risk (Saatci et al., 2021). This is further supported by evidence from mouse models of maternal immune activation, in which pregnant mice are challenged with immune insults and their adult offspring show disease relevant behavioural, pathological, and neurochemical changes (Gumusoglu and Stevens, 2019). Interestingly, many infections show fluctuations throughout the year including neurotropic enteroviruses (Pons-Salort et al., 2018), but little has been done to investigate their role in mediating the month-of-birth effect in schizophrenia.
Understanding the factors that contribute to the month-of-birth effect in schizophrenia is an important area of research because it could, not only shed light on the underlying disease mechanisms but also help to identify modifiable risk factors in early life. Accordingly, in this study we 1. carried out an updated systematic review and meta-analysis of population-based studies investigating the link between month of birth and schizophrenia in line with the MOOSE guidelines (Stroup et al., 2000), and 2. explored whether latitude, daylight and infective agents could explain the month-of birth-effect in schizophrenia.
2. Methods
2.1. Search strategy
Medline, EMBASE and the Cochrane Library were searched from inception to December 23, 2021, using the search terms, “month of birth”, “season of birth”, “schizophrenia” and “non-affective psychosis” (full search strategies available in Supplementary Table 1). Reference lists were hand-searched. There were no language restrictions.
2.2. Study selection
We included any comparative cohort study investigating the association between month or season of birth and the outcome of subsequent schizophrenia or non-affective psychotic disorders in the Northern Hemisphere. We excluded unpublished studies, any study without a valid population-control group and outcomes that included affective psychotic disorders or other neurodevelopmental disorders. Two reviewers (LH and DS) independently assessed the eligibility of each title and abstract identified from the search strategy. Full-text articles were retrieved for further assessment of inclusion. Authors were contacted if full text articles were unavailable.
2.3. Data extraction
Data was extracted by two reviewers (LH and DS) using a standardised form (Supplementary Table 2), detailing year of publication, study design, exposures/outcomes of interest, and study-specific summary statistics (i.e., odds ratios (ORs) and relative risks (RRs) with 95% confidence intervals). If summary statistics were unavailable, these were estimated using total number of schizophrenia births and general population births available in each study. Any missing data was addressed by contacting authors. For publications that had overlapping datasets, the ones chosen for inclusion were those with the highest quality studies and the largest datasets.
2.4. Quality assessment
Both reviewers (LH and DS) used the validated Newcastle-Ottawa Scale for observational studies to assess risk of bias in each research study extracted (Wells et al., Tugwell). A score of 7 was deemed a high-quality study with low risk of bias.
2.5. Outcome assessment and exploratory variables
The outcome of interest was the month of birth for schizophrenia patients and healthy controls. The following exploratory variables were defined a priori: age of study (defined as the time from the median of the study period), latitude, daylight, and infections previously implicated in schizophrenia pathogenesis (including influenza, meningococcal disease, enterovirus and toxoplasmosis) as detailed in our protocol published on PROSPERO, ID: CRD42022299737. These variables were selected because of existing evidence of their role in contributing to the underlying seasonal variation of schizophrenia births (Davies et al., 2003a; Parker et al., 2000; Selten et al., 2000; Suvisaari et al., 2001; Torrey et al., 1977; Saatci et al., 2021; Khandaker et al., 2012).,
2.6. Statistical analysis
For our outcome of interest (month-of-schizophrenia births), we used random-effects meta-analyses using the generic inverse variance method were carried out to calculate summary estimates. Heterogeneity, a measure of the percentage of the total observed variance and between-study variation, was calculated using I2. An I2 greater than 50% was considered as high heterogeneity. A priori selected factors (latitude, daylight and infection) that contributed to the heterogeneity were explored using random-effects meta-regression model (mixed-effects model), using the Restricted Maximum Likelihood (REML) estimator. Furthermore, Egger's test were used to evaluate any publication bias. All analyses were carried out using the ‘dmetar’ package in R.
We assessed seasonal variation of schizophrenic births compared to the general population using the Cosinor regression model, using month of birth from each study to represent the time. This generalized linear model fits a linear regression under the Poisson distribution using cosine and sine terms that describe the sinusoid and provides information on the mean, amplitude (distance from mean to the peak) and the phase (the peak month) (Barnett, 2010). We used month of birth from each study to represent time. Analyses were carried out using the ‘season’ package in R.
To further explore the association between schizophrenia seasonality and our selected exploratory variables (latitude, daylight and infection), we carried out two separate statistical analyses. For latitude and daylight, data were publicly available for all study regions through Google Maps and the World Meteorological Organization (Organization), respectively. We employed linear regression to investigate the relationship between latitude/daylight and schizophrenia seasonality. For infective agents, complete monthly infection rates were publicly available for only one country, Taiwan, through their Centres for Disease Control publications (https://www.cdc.gov.tw/En). To explore the strength and direction of association between infection rates and schizophrenia seasonality we used Kendall's rank correlation, as the data is ordinal and non-parametric. We included average rates of infection over 4 years for enterovirus (Suvisaari et al., 1999), influenza (Brown et al., 2004), toxoplasmosis (Brown et al., 2005) and meningococcal disease (Abrahao et al., 2005).
3. Results
A total of 1093 publications were screened to assess their eligibility for inclusion. 35 articles were eligible for full-text review and 10 publications met the inclusion criteria (Fig. 1). The included studies and their population characteristics are presented in Table 1. Quality assessment found that 6 out of 10 studies were of a high quality with low risk of bias. Furthermore, Egger's test did not identify any publication bias (Table 2). The excluded studies and the reasons their exclusion are detailed in Supplementary Table 3.
Fig. 1.
Study flow diagram.
Table 1.
Population and study characteristics of included studies within the meta-analysis.
| Study Author, Year | Study Design | Country | Study Time Period | Number of included schizophrenia patients | Outcome of Interest | Division of year for analysis | Newcastle Ottawa Score | Findings |
|---|---|---|---|---|---|---|---|---|
| Mino et al., 2006 | Population-based | Japan | Schizophrenia Births: 1910–1996 General Population Births: 1940–1996 |
88778 | Schizophrenia | Monthly | 6 | Excess births seen in Winter/Spring for both females and males. |
| Cheng et al., 2013 | Population-based | Taiwan | All births: 1950–1989 | 5047 | Schizophrenia | Monthly | 7 | Excess births seen in Winter/Spring compared to Autumn/Summer. Seen in females. |
| Parker et al., 2000 | Population-based | Singapore | Schizophrenia Births: 1930–1984 General Population Births: 1960–1984 |
9141 | Schizophrenia | Monthly | 6 | No seasonal excess births for schizophrenia patients identified. Trough months include March–April. |
| Selten et al., 2000 | Population-based | Netherlands | All births: 1926–1970 | 29891 | Schizophrenia | Monthly | 8 | Excess late spring/early summer births and deficit in late summer/early autumn in schizophrenia patients. |
| Suvisaari et al., 2001 | Population-based | Finland | All births: 1950–1969 | 15389 | Schizophrenia | Monthly | 8 | Excess winter/spring births in schizophrenia patients. |
| Disanto et al., 2012 | Population-based | UK | Schizophrenia diagnosis: 2003–2011 General Population Births: 1950–1990 |
26676 | Schizophrenia | Monthly | 6 | Excess schizophrenia births in winter (January) and deficit in summer (July). |
| Karlsson et al., 2019 | Population-based | Sweden | All births: 1940–1997 | 30684 | Schizophrenia | Monthly | 8 | Excess winter (December) schizophrenia births. |
| Munoz-Delgado et al., 2003 | Single-centre | Mexico | Schizophrenia diagnosis: 1913–1989 General Population Births: 1991–2001 |
2288 | Schizophrenia | Monthly | 5 | Non-significant increment in Autumn/Winter months. |
| Torrey et al., 1977 | Population-based | USA | All births: 1920–1955 | 53584 | Schizophrenia | Monthly | 7 | Higher schizophrenia births seen from December to May (peak March/April). |
| Carrion-Baralt et al., 2004 | Case-Control | Puerto Rico | All births: 1932–1967 | 710 | Schizophrenia | Monthly | 7 | Higher schizophrenia births seen in February. |
Table 2.
Meta-analysis of risk of schizophrenia by month of birth.
| Months | Number of studies | RR (95%CI) | p-value | Heterogeneity (I2) | Egger's Test (p-value) |
|---|---|---|---|---|---|
| January | 10 | 1.06 (1.03–1.1) | <0.001 | 77% | 0.36 |
| February | 10 | 1.03 (1.00–1.05) | 0.02 | 55% | 0.67 |
| March | 10 | 1.03 (0.98–1.07) | 0.21 | 89% | 0.35 |
| April | 10 | 1.01 (0.99–1.03) | 0.32 | 41% | 0.59 |
| May | 10 | 1.01 (0.99–1.03) | 0.25 | 23% | 0.23 |
| June | 10 | 0.98 (0.95–1.02) | 0.32 | 78% | 0.90 |
| July | 10 | 0.97 (0.94–0.99) | 0.01 | 59% | 0.72 |
| August | 10 | 0.96 (0.93–0.98) | 0.04 | 70% | 0.54 |
| September | 10 | 0.95 (0.92–0.98) | <0.001 | 71% | 0.86 |
| October | 10 | 0.97 (0.95–0.98) | <0.001 | 12% | 0.33 |
| November | 10 | 0.99 (0.96–1.01) | 0.36 | 58% | 0.26 |
| December | 10 | 1.04 (1.00–1.08) | 0.04 | 83% | 0.22 |
3.1. Month of birth and schizophrenia
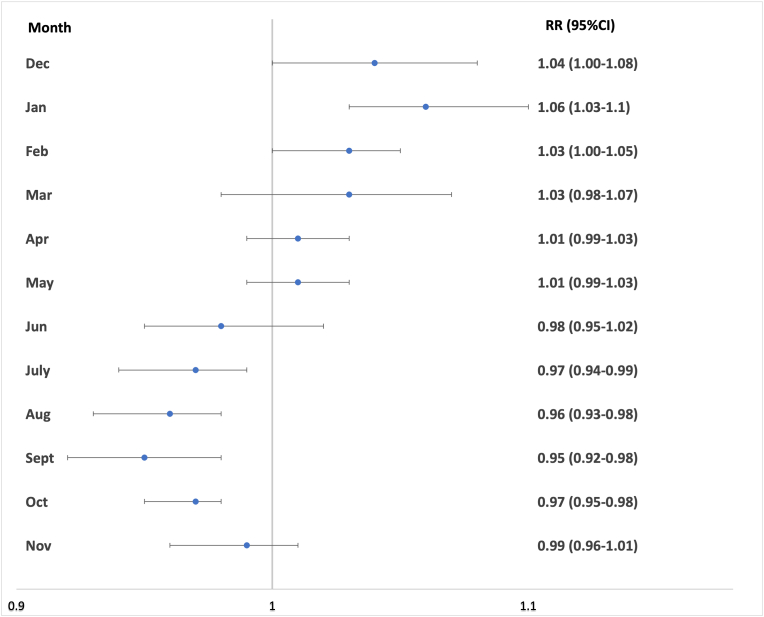
There were 262,188 schizophrenic births in total. We identified significantly higher schizophrenia births in December [1.04 (95%CI 1.00–1.08)], January [1.06 (95%CI 1.03–1.1)] and February [1.03 (95%CI 1.00–1.05)]. There were significantly lower schizophrenia births in July [0.97 (95%CI 0.94–0.99)], August [0.96 (95%CI 0.93–0.98)], September [0.95 (95%CI 0.92–0.98)]and October [0.97 (95%CI 0.95–0.98)] (Table 2, Fig. 2). Heterogeneity was low for October, moderate for April and May, and high for all other months (Table 2). Meta-regression analyses investigating age of study, latitude and daylight individually did not account for the heterogeneity observed in those months with significantly higher schizophrenia births (Table 3). Furthermore, seasonality was detected for schizophrenic births compared to the general population using the Cosinor test with the peak corresponding to February and the trough corresponding to August (p < 0.001, amplitude = 158 births, phase = 2.2, low point = 8.2).
Fig. 2.
Meta-analysis of schizophrenia risk by month of birth. Relative risk (RR) and 95% confidence intervals are shown for each month.
Table 3.
Meta-regression analysis for latitude, daylight and age of study.
| Latitude Coefficient (95%CI) | p-value | Variance accounted | Daylight Coefficient (95%CI) | p-value | Variance accounted | Age of Study Coefficient (95%CI) | p-value | Variance accounted (%) | |
|---|---|---|---|---|---|---|---|---|---|
| January | 0.0004 (−0.0014 to 0.0023) | 0.65 | 0% | −0.0002 (−0.0007 to 0.0004) | 0.52 | 0% | −0.0004 [-0.0023 to 0.0016] | 0.19 | 0 |
| February | −0.0007 (−0.0018 to 0.0005) | 0.28 | 41% | 0.00 (−0.0004 to 0.0005) | 0.38 | 0% | 0.0006 [-0.0009 to 0.0020] | 0.84 | 0 |
| March | 0.00 (−0.0023 to 0.0024) | 0.97 | 0% | 0.0005 (−0.0003 to 0.0013) | 0.49 | 4% | 0.0006 [-0.0018 to 0.0030] | 0.87 | 0 |
| April | 0.0011 (−0.0002 to 0.0025) | 0.10 | 0% | 0.0003 (−0.0003 to 0.0009) | 0.46 | 7% | 0.0015 [0.0004 to 0.0026] | 0.006 | 92 |
| May | −0.0003 (−0.0015 to 0.0009) | 0.60 | 0% | −0.0001 (−0.0005 to 0.0003) | 0.58 | 0% | 0.0010 [0.00 to 0.0021] | 0.05 | 96 |
| June | 0.0005 (−0.0015 to 0.0025) | 0.60 | 0% | 0.0003 (−0.0002 to 0.0009) | 0.18 | 3% | 0.0003 [-0.0018 to 0.0024] | 0.75 | 0 |
| July | 0.0004 (−0.0012 to 0.0019) | 0.65 | 0% | 0.0001 (−0.0004 to 0.0005) | 0.68 | 0% | 0.0005 [-0.0011 to 0.0021] | 0.54 | 0 |
| August | 0.00 (−0.0018 to 0.0018) | 0.90 | 0% | 0.0004 (−0.0003 to 0.0011) | 0.09 | 2% | 0.0018 [0.00 to 0.0035] | 0.05 | 18 |
| September | 0.0002 (−0.0015 to 0.0020) | 0.80 | 0% | −0.0005 (−0.0011 to 0.0001) | 0.13 | 16% | −0.0012 [-0.0029 to 0.0005] | 0.15 | 21 |
| October | −0.0003 (−0.0013 to 0.0007) | 0.53 | 0% | 0.00 (−0.0004 to 0.0003) | 0.78 | 0% | −0.0006 [-0.0016 to 0.0004] | 0.26 | 0 |
| November | 0.0013 (−0.0007 to 0.0033) | 0.70 | 0% | −0.0002 (−0.0007 to 0.0003) | 0.36 | 14% | −0.002 [-0.0031 to −0.001] | <0.001 | 100 |
| December | −0.0013 (−0.0045 to 0.0019) | 0.43 | 0% | 0.0006 (−0.0005 to 0.0016) | 0.28 | 0% | −0.0030 [-0.006 to 0.0001] | 0.06 | 15 |
3.2. Candidates that can explain the month-of-birth effect
3.2.1. Latitude
Latitude was reported for all study regions. We did not identify any association between the relative risk of schizophrenia and latitude (Table 4). Further, grouping studies into latitude bands (<20°, 20–39°, 40–60°, and >60°) did not reveal any differences in the within-year fluctuations of schizophrenia births (Supplementary Fig. 1).
Table 4.
Linear regression analysis for latitude and daylight in Northern Hemisphere studies.
| Month-of-Birth Effect | Month | Latitude Coefficient | p-value | Daylight Coefficient | p-value |
|---|---|---|---|---|---|
| Peak | December | −0.0019 | 0.41 | 0.001 | 0.22 |
| January | 0.0011 | 0.25 | −0.0003 | 0.24 | |
| February | −0.00037 | 0.84 | −0.0006 | 0.17 | |
| Trough | July | 0.00010 | 0.23 | 0.00009 | 0.72 |
| August | −0.00023 | 0.85 | 0.00007 | 0.12 | |
| September | −0.00047 | 0.57 | −0.0003 | 0.31 | |
| October | −0.00047 | 0.45 | −0.0002 | 0.31 |
3.2.2. Daylight
Daylight was reported for 8 out of 10 study regions. We did not identify any association between the relative risk of schizophrenia and daylight levels reported for the country of study origin (Table 4).
3.2.3. Infections
We found a significant negative correlation between monthly severe enterovirus cases (peak summer, August) and schizophrenia births (peak winter, January–February) (tau −0.57, p = 0.0099) using data from Taiwan. This corresponds to enterovirus infection during the second trimester of pregnancy. We did not observe any correlation between other monthly infections of severe influenza, toxoplasmosis and meningococcal disease and schizophrenia births (Table 5).
Table 5.
Association between month of birth and monthly infection rates in Taiwan.
| Month of birth schizophrenia | Monthly Influenza cases | Kendal Tau | p-value | Monthly enterovirus cases | Kendal Tau | p-value | Monthly toxoplasma cases | Kendal Tau | p-value | Monthly meningoccocal cases | Kendal Tau | p-value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| January | 486 | 189 | 0.17 | 0.45 | 1.3 | −0.57 | 0.0099 | 0.5 | −0.12 | 0.61 | 1 | 0.29 | 0.23 |
| February | 513 | 277 | 1 | 2 | 0.75 | ||||||||
| March | 425 | 126 | 0.3 | 1.5 | 1.5 | ||||||||
| April | 383 | 50 | 1.7 | 0.75 | 0.25 | ||||||||
| May | 372 | 49 | 3 | 1 | 0.25 | ||||||||
| June | 355 | 94 | 3.2 | 1.5 | 0 | ||||||||
| July | 355 | 124 | 3.2 | 1.5 | 1 | ||||||||
| August | 355 | 82 | 3.7 | 2.25 | 0.75 | ||||||||
| September | 414 | 69 | 2.8 | 0.75 | 0.75 | ||||||||
| October | 444 | 43 | 2.7 | 1.25 | 0.75 | ||||||||
| November | 438 | 51 | 2.2 | 1.5 | 0.75 | ||||||||
| December | 455 | 81 | 3 | 1.5 | 0.75 |
4. Discussion
In this systematic review and meta-analysis, we report an excess of schizophrenia births in December, January and February. We included 262,188 individuals with schizophrenia from 10 studies encompassing a range of regions and latitudes making this the largest study to date. Overall, our findings broadly support the previous work by Davies and colleagues (Davies et al., 2003b) who found an excess of winter-spring births compared to summer-autumn births. However, we were able to expand our analyses to provide more precise risk estimates by month of birth. This is particularly relevant because the four discrete seasons in temperate regions are not well defined in the extremes of latitude, but the month of birth effect has been reported in low latitude counties such as Puerto Rico (Carrion-Baralt et al., 2004) and Mexico (Munoz-Delgado et al., 2003).
The underlying mechanisms mediating the month-of-birth effect in schizophrenia births is not well understood. Previous studies have postulated that latitude and vitamin D levels could play a role in schizophrenia (Eyles et al., 2018; McGrath et al., 2010). In line with this, Davies and colleagues showed that higher latitude countries had more schizophrenia births in the winter months as well as longer periods of risk within the year (Davies et al., 2003a). However, we did not find any association between latitude and the magnitude of the month-of-birth effect or the length of risk period. Furthermore, we found no association between daylight and the magnitude of the month-of-birth effect. Our analyses were better equipped to address this question because more studies from low latitude counties were available for inclusion in this meta-analysis. Collectively, our results suggests that latitude and daylight are unlikely to contribute to the month-of-birth effect in schizophrenia. Thus, further work exploring other candidate environmental risk factors is warranted. It is however important to recognise that our results do not rule out the importance of these factors in the pathogenesis of schizophrenia altogether.
Epidemiological studies have identified maternal infections during pregnancy as an important environmental risk factor in schizophrenia (Buka et al., 2001, 2008; Babulas et al., 2006; Saatci et al., 2021). In particular, infections during the second trimester seem to mediate this increased risk in schizophrenia (Saatci et al., 2021). This is supported by observations that maternal immune activation in mice at a time point analogous to the second trimester leads to downregulation of schizophrenia associated genes in the foetal mouse brain (Handunnetthi et al., 2021) and behavioural deficits in adult offspring (Meyer et al., 2008). This window of vulnerability corresponds to disease relevant neurodevelopmental processes such neurogenesis, neuronal cell migration and synaptogenesis (Gumusoglu and Stevens, 2019). Therefore, we explored the relationship between several infections implicated in schizophrenia and the month -of-birth effect using data from Taiwan. Intriguingly, we identified a strong negative correlation between monthly enterovirus cases and schizophrenia births, with the peaks of enterovirus infection in August and schizophrenia births in January–February. Furthermore, the seasonality of non-polio enterovirus infections appears to be similar across different regions with a peak in the summer months (van der Sanden et al., 2009; Bubba et al., 2020; Kadambari et al., 2014) and have consistently shown this seasonal variation over the last five decades (van der Sanden et al., 2009). Central nervous system involvement in enterovirus infections during childhood is well established, with polio causing long-term neuropsychiatric complications (Nielsen et al., 2007) and non-polio enteroviruses resulting in meningitis and subsequent neurodevelopmental complications (Chang et al., 2007; de Ceano-Vivas et al., 2021). Our findings for the first time connect this neurotropic enterovirus infection to the window of vulnerability in the second trimester, bringing together two independent lines of research in schizophrenia pathogenesis.
There were several limitations to this study. Firstly, despite efforts to account for between-study variance, there remained a high level of heterogeneity in our meta-analysis. We were unable to fully explain this heterogeneity in meta-regression analyses through age of study, latitude and daylight, indicating unidentified study-level factors are likely to contribute to the heterogeneity. Further, the limited number of available studies meant that some of our meta-regression analyses may have been subject to overfitting. Secondly, as this is meta-analysis based on observational studies, there remains the possibility of bias introduced from studies of varying quality. Thirdly, our analyses examining the factors that contribute to the month-of-birth effect were exploratory and we were limited by the completeness and quality of available data on these risk factors. Specifically, we only had infection data from one country and our finding will need further exploration and replication. We acknowledge that several environmental risk factors, including many other neurotropic infections that were not explored in this study, could play a role in mediating the month-of-birth-effect and that further studies are needed.
5. Conclusion
Overall, we carried out an updated meta-analysis of studies investigating the month-of-birth effect and schizophrenia to highlight increased schizophrenia births in December, January and February. We subsequently explored factors that could explain this month-of-birth effect and provided novel insight into the role of enteroviruses in mediating this long held observation in schizophrenia. Further research is warranted to expand these exploratory findings and to understand how enterovirus infections could exert deleterious effects on neurodevelopmental processes. It would also be interesting investigate if infections can interact with other factors such as latitude to mediate the month-of birth effect. Importantly, treatment and prevention of maternal infection during pregnancy are tractable health goals and thus these findings carry clear implications for future prevention strategies in schizophrenia.
Author contributions
DS: Methodology, Data curation, Formal analysis, Writing – original draft; Writing – review & editing; TJ: Methodology, Writing – original draft; Writing – review & editing; MS: Methodology, Writing – original draft; Writing – review & editing; AvN: Methodology, Writing – original draft; Writing – review & editing; LH: Conceptualization, Funding acquisition, Methodology, Writing – original draft; Writing – review & editing.
Funding
This work is supported by National Institute for Health Research U.K. and John Fell Fund from the University of Oxford.
Declaration of competing interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2022.100486.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abrahao A.L., Focaccia R., Gattaz W.F. Childhood meningitis increases the risk for adult schizophrenia. World J. Biol. Psychiatr. 2005;6(Suppl. 2):44–48. doi: 10.1080/15622970510030063. [DOI] [PubMed] [Google Scholar]
- Babulas V., Factor-Litvak P., Goetz R., Schaefer C.A., Brown A.S. Prenatal exposure to maternal genital and reproductive infections and adult schizophrenia. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't. American Journal of Psychiatry. May. 2006;163(5):927–929. doi: 10.1176/ajp.2006.163.5.927. [DOI] [PubMed] [Google Scholar]
- Barnett A. Analysing Seasonal Health Data. 2010:75–92. [Google Scholar]
- Brown A.S., Begg M.D., Gravenstein S., et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61(8):774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- Brown A.S., Schaefer C.A., Quesenberry C.P., Jr., Liu L., Babulas V.P., Susser E.S. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am J Psychiatry. 2005;162(4):767–773. doi: 10.1176/appi.ajp.162.4.767. [DOI] [PubMed] [Google Scholar]
- Bubba L., Broberg E.K., Jasir A., Simmonds P., Harvala H., Enterovirus study c Circulation of non-polio enteroviruses in 24 EU and EEA countries between 2015 and 2017: a retrospective surveillance study. Lancet Infect Dis. 2020;20(3):350–361. doi: 10.1016/S1473-3099(19)30566-3. [DOI] [PubMed] [Google Scholar]
- Buka S.L., Tsuang M.T., Torrey E.F., Klebanoff M.A., Bernstein D., Yolken R.H. Maternal infections and subsequent psychosis among offspring. Arch Gen Psychiatry. 2001;58(11):1032–1037. doi: 10.1001/archpsyc.58.11.1032. [DOI] [PubMed] [Google Scholar]
- Buka S.L., Cannon T.D., Torrey E.F., Yolken R.H. Collaborative study group on the perinatal origins of severe psychiatric D. Maternal exposure to herpes simplex virus and risk of psychosis among adult offspring. Multicenter study research support. N.I.H., Extramural Research Support, Non-U.S. Gov't. Biological Psychiatry. 2008;63(8):809–815. doi: 10.1016/j.biopsych.2007.09.022. [DOI] [PubMed] [Google Scholar]
- Carrion-Baralt J.R., Fuentes-Rivera Z., Schmeidler J., Silverman J.M. A case-control study of the seasonality effects on schizophrenic births on a tropical island. Schizophr Res. 2004;71(1):145–153. doi: 10.1016/j.schres.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Chang L.Y., Huang L.M., Gau S.S., et al. Neurodevelopment and cognition in children after enterovirus 71 infection. N Engl J Med. 2007;356(12):1226–1234. doi: 10.1056/NEJMoa065954. [DOI] [PubMed] [Google Scholar]
- Cheng C., Loh el W., Lin C.H., Chan C.H., Lan T.H. Birth seasonality in schizophrenia: effects of gender and income status. Psychiatr. Clin. Neurosci. Sep 2013;67(6):426–433. doi: 10.1111/pcn.12076. [DOI] [PubMed] [Google Scholar]
- Davies G., Welham J., Chant D., Torrey E.F., McGrath J. A systematic review and meta-analysis of Northern Hemisphere season of birth studies in schizophrenia. Schizophr. Bull. 2003;29(3):587–593. doi: 10.1093/oxfordjournals.schbul.a007030. [DOI] [PubMed] [Google Scholar]
- Davies G., Welham J., Chant D., Torrey E.F., McGrath J. A systematic review and meta-analysis of Northern Hemisphere season of birth studies in schizophrenia. Review. Schizophr. Bull. 2003;29(3):587–593. doi: 10.1093/oxfordjournals.schbul.a007030. [DOI] [PubMed] [Google Scholar]
- de Ceano-Vivas M., Garcia M.L., Velazquez A., et al. Neurodevelopmental outcomes of infants younger than 90 Days old following enterovirus and parechovirus infections of the Central nervous system. Front Pediatr. 2021;9 doi: 10.3389/fped.2021.719119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disanto G., Morahan J.M., Lacey M.V., et al. Seasonal distribution of psychiatric births in England. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0034866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyles D.W., Trzaskowski M., Vinkhuyzen A.A.E., et al. The association between neonatal vitamin D status and risk of schizophrenia. Sci. Rep. Dec 6 2018;8(1) doi: 10.1038/s41598-018-35418-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumusoglu S.B., Stevens H.E. Maternal inflammation and neurodevelopmental programming: a review of preclinical outcomes and implications for translational psychiatry. Biol. Psychiatr. Jan 15 2019;85(2):107–121. doi: 10.1016/j.biopsych.2018.08.008. [DOI] [PubMed] [Google Scholar]
- Handunnetthi L., Saatci D., Hamley J.C., Knight J.C. Maternal immune activation downregulates schizophrenia genes in the foetal mouse brain. Brain Commun. 2021;3(4):fcab275. doi: 10.1093/braincomms/fcab275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.cdc.gov.tw/En
- Kadambari S., Bukasa A., Okike I.O., et al. Enterovirus infections in England and Wales, 2000-2011: the impact of increased molecular diagnostics. Clin. Microbiol. Infect. Dec 2014;20(12):1289–1296. doi: 10.1111/1469-0691.12753. [DOI] [PubMed] [Google Scholar]
- Karlsson H., Dal H., Gardner R.M., Torrey E.F., Dalman C. Birth month and later diagnosis of schizophrenia. A population-based cohort study in Sweden. J Psychiatr Res. 2019;116:1–6. doi: 10.1016/j.jpsychires.2019.05.025. [DOI] [PubMed] [Google Scholar]
- Khandaker G.M., Zimbron J., Dalman C., Lewis G., Jones P.B. Childhood infection and adult schizophrenia: a meta-analysis of population-based studies. Schizophr Res. 2012;139(1–3):161–168. doi: 10.1016/j.schres.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J.J., Eyles D.W., Pedersen C.B., et al. Neonatal vitamin D status and risk of schizophrenia: a population-based case-control study. Arch Gen Psychiatry. 2010;67(9):889–894. doi: 10.1001/archgenpsychiatry.2010.110. [DOI] [PubMed] [Google Scholar]
- Meyer U., Murray P.J., Urwyler A., Yee B.K., Schedlowski M., Feldon J. Adult behavioral and pharmacological dysfunctions following disruption of the fetal brain balance between pro-inflammatory and IL-10-mediated anti-inflammatory signaling. Mol Psychiatry. 2008;13(2):208–221. doi: 10.1038/sj.mp.4002042. [DOI] [PubMed] [Google Scholar]
- Mino Y., Oshima I. Seasonality of birth in patients with schizophrenia in Japan. Psychiatry and Clinical Neurosciences. 2006;60(2):249–252. doi: 10.1111/j.1440-1819.2006.01493.x. [DOI] [PubMed] [Google Scholar]
- Mortensen P.B., Pedersen C.B., Westergaard T., et al. Effects of family history and place and season of birth on the risk of schizophrenia. N Engl J Med. 1999;340(8):603–608. doi: 10.1056/NEJM199902253400803. [DOI] [PubMed] [Google Scholar]
- Munoz-Delgado J., Perez-Rincon H., Nicolini H., et al. Season of birth in schizophrenia in the Mexico City area: Comparison with general population. Biological rhythm research. 2003;34(5):485–492. [Google Scholar]
- Nielsen N.M., Rostgaard K., Hjalgrim H., Askgaard D., Skinhoj P., Aaby P. Psychiatric hospitalizations in a cohort of Danish polio patients. Am J Epidemiol. 2007;165(3):319–324. doi: 10.1093/aje/kwk003. [DOI] [PubMed] [Google Scholar]
- O'Hare A., Walsh D., Torrey F. Seasonality of schizophrenic births in Ireland. Br J Psychiatry. 1980;137:74–77. doi: 10.1192/bjp.137.1.74. [DOI] [PubMed] [Google Scholar]
- Organization W.M. http://data.un.org/Data.aspx?d=CLINO&f=ElementCode%3A15%3BCountryCode%3AKO#CLINO
- Parker G., Mahendran R., Koh E.S., Machin D. Season of birth in schizophrenia: No latitude at the equator. Br. J. Psychiatry. 2000;176(JAN):68–71. doi: 10.1192/bjp.176.1.68. [DOI] [PubMed] [Google Scholar]
- Pons-Salort M., Oberste M.S., Pallansch M.A., et al. The seasonality of nonpolio enteroviruses in the United States: patterns and drivers. Proc Natl Acad Sci U S A. 2018;115(12):3078–3083. doi: 10.1073/pnas.1721159115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatci D., van Nieuwenhuizen A., Handunnetthi L. Maternal infection in gestation increases the risk of non-affective psychosis in offspring: a meta-analysis. J Psychiatr Res. 2021;139:125–131. doi: 10.1016/j.jpsychires.2021.05.039. [DOI] [PubMed] [Google Scholar]
- Selten J.P., Van Der Graaf Y., Dijkgraaf M., Edlinger M., Kahn R. Seasonality of schizophrenia and stillbirths in The Netherlands. Schizophr. Res. 2000;44(2):105–111. doi: 10.1016/S0920-9964(99)00202-9. [DOI] [PubMed] [Google Scholar]
- Stroup D.F., Berlin J.A., Morton S.C., et al. Meta-analysis of observational studies in EpidemiologyA proposal for reporting. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Suvisaari J., Haukka J., Tanskanen A., Hovi T., Lonnqvist J. Association between prenatal exposure to poliovirus infection and adult schizophrenia. Am J Psychiatry. 1999;156(7):1100–1102. doi: 10.1176/ajp.156.7.1100. [DOI] [PubMed] [Google Scholar]
- Suvisaari J.M., Haukka J.K., Lonnqvist J.K. Season of birth among patients with schizophrenia and their siblings: evidence for the procreational habits hypothesis. Am. J. Psychiatr. May 2001;158(5):754–757. doi: 10.1176/appi.ajp.158.5.754. [DOI] [PubMed] [Google Scholar]
- Taylor A.E., Burgess S., Ware J.J., et al. Investigating causality in the association between 25(OH)D and schizophrenia. Sci. Rep. 2016;6 doi: 10.1038/srep26496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey E.F., Torrey B.B., Peterson M.R. Seasonality of schizophrenic births in the United States. Arch Gen Psychiatry. 1977;34(9):1065–1070. doi: 10.1001/archpsyc.1977.01770210079007. [DOI] [PubMed] [Google Scholar]
- van der Sanden S., Koopmans M., Uslu G., van der Avoort H. Dutch working group for Clinical V. Epidemiology of enterovirus 71 in The Netherlands, 1963 to 2008. J Clin Microbiol. 2009;47(9):2826–2833. doi: 10.1128/JCM.00507-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells G.A.B.S., D O'Connell, Peterson J., Welch V., Losos M., Tugwell P. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.