Abstract
In this study, we demonstrated that analyzed strains of Vibrio mimicus and Vibrio cholerae could be separated in two groups by using multilocus enzyme electrophoresis (MEE) data from 14 loci. We also showed that the combination of four enzymatic loci enables us to differentiate these two species. Our results showed that the ribosomal intergenic spacer regions PCR-mediated identification system failed, in some cases, to differentiate between V. mimicus and V. cholerae. On the other hand, MEE proved to be a powerful molecular tool for the discrimination of these two species even when atypical strains were analyzed.
Vibrio mimicus is a species closely related to Vibrio cholerae. Phenotypically, most of the features of this organism are identical or similar to those found in V. cholerae, and sucrose fermentation is the main trait differentiating them biochemically (10, 11). They share somatic antigens and virulence-related genes and are associated with sporadic and epidemic cholera diarrhea (4, 10, 15). Both species are natural inhabitants of aquatic environments, such as seawater, freshwater, and brackish water. They may constitute the microbiota of zooplankton, crustaceans, and filter-feeding molluscs but are recognized mainly as human pathogens (6, 9).
In 1991 a large cholera outbreak started in Latin America, and the etiological agent was V. cholerae O1 biotype El Tor. Interestingly, during this epidemic, cases of severe diarrhea associated with the presence of V. mimicus were reported in Costa Rica (5). At the same time, in French Guyana and in the northern part of the Brazilian Amazon region, a sucrose-negative variant of V. cholerae was identified in most of the cholera cases reported (8). Therefore, the emergence of V. mimicus as a pathogen and its coexistence with non-sucrose-fermenting V. cholerae isolates highlight the necessity for precise discrimination between these two species.
After the characterization of V. mimicus as a new pathogenic species, only a few attempts to identify it on a molecular basis have been reported. One of these previous studies applied multilocus enzyme electrophoresis (MEE) to characterize V. cholerae strains, and the results suggested the possibility of using this approach to differentiate V. cholerae from V. mimicus (13). Chun et al. (7) recently developed a PCR-mediated identification system based on the analysis of nucleotide sequences of 16S-23S ribosomal intergenic spacer regions (ISR) that would be useful in distinguishing between these two species. However, it is important to observe that in both studies only a limited number of V. mimicus strains were considered, since V. cholerae was the main interest.
Reported here are the results of an analysis by MEE of V. mimicus isolates from distinct sources and geographic regions. Using these data, we determined the genetic variation within this species and the relationship between V. mimicus and V. cholerae. In addition, we evaluated the efficiency of the ISR-PCR approach in the separation of these two closely related species.
The strains used in the study are described in Table 1. We used routine bacteriological procedures for Vibrio identification as described previously (6, 18). The environmental Brazilian isolates of V. mimicus were also characterized biochemically using the API 20E system (BioMérieux Vitek, Inc., Hazelwood, Mo.) (6). The biochemical characterization of the isolates showed different possible API 20E profile numbers (Table 1).
TABLE 1.
Strains of Vibrio used in this study
| Strain | Species | Sourcea | Origin, locale | Other designation(s) | Zymovar |
|---|---|---|---|---|---|
| 121 | V. cholerae O1 E1 Tor | C. A. Salles | Human, India | 4507 | 014 A |
| 519 | V. cholerae O1 E1 Tor S(−)b | C. A. Salles | Human, Brazil | API20E 5347124 | 014B |
| 200 | V. cholerae O1 Classical | C. A. Salles | Human, India | GP48 | 013 |
| 017 | V. cholerae non-O1 | C. A. Salles | Water, Brazil | 458 | 015 |
| 090 | V. cholerae non-O1 | C. A. Salles | Human, Ghana | 968/79 | 006 |
| 093 | V. cholerae non-O1 | C. A. Salles | Sewage, Brazil | A7 | 117 |
| 298 | V. cholerae non-O1 | P. Desmarchelier | Human, Australia | N50 | 045 |
| 328 | V. cholerae non-O1 S(−) | P. Desmarchelier | Human, Australia | N128 | 058 |
| 490 | V. cholerae non-O1 | C. A. Salles | Human, France | 930181 | 108 |
| 518 | V. cholerae non-O1 | This study | Fish, Brazilc | API20E 5047124 | 111 |
| 537 | V. cholerae non-O1 S(−) | This study | Crabb-uca, Brazilc | API20E 5147124 | 118 |
| 580 | V. cholerae non-O1 | C. A. Salles | Human, India | PG 128 | 145 |
| 478 | V. cholerae non-O1 | C. A. Salles | Water, Bolivia | 920135 | 153 |
| 621 | V. cholerae non-O1 | C. A. Salles | Water, Peru | N-8 | 158 |
| 160 | V. mimicus | ATCC | Human, United States | ATCC 33653 | 020 |
| 161 | V. mimicus | ATCC | Water, United States | ATCC 33654 | 021 |
| 162 | V. mimicus | ATCC | Human, United States | ATCC 33655 | 019 |
| 207 | V. mimicus | S. Shinoda | Water, Japan | E-26 | 125 |
| 275 | V. mimicus | P. Desmarchelier | Human, Australia | VS33 | 034 |
| 284 | V. mimicus | P. Desmarchelier | Human, Australia | N142Sm | 040 |
| 285 | V. mimicus | S. Shinoda | Water, Japan | E-28 | 126 |
| 327 | V. mimicus | P. Desmarchelier | Water, Australia | VS31-B | 127 |
| 337 | V. mimicus | S. Shinoda | Water, Japan | H-26 | 128 |
| 339 | V. mimicus | S. Shinoda | Water, Japan | H-31 | 129 |
| 343 | V. mimicus | S. Shinoda | Water, Japan | H-43 | 130 |
| 428 | V. mimicus | S. Shinoda | Water, Japan | O-21 | 131 |
| 441 | V. mimicus | S. Shinoda | Water, Japan | J-24 | 132 |
| 449 | V. mimicus | S. Shinoda | Water, Japan | O-12 | 133 |
| 461 | V. mimicus | S. Shinoda | Water, Japan | K-45 | 134 |
| 468 | V. mimicus | S. Shinoda | Water, Japan | E-33 | 135 |
| 532 | V. mimicus | This study | Fish, Brazilc | API20E 5146104 | 148 |
| 535 | V. mimicus | This study | Water, Brazilc | API20E 7246105 | 149 |
| 542 | V. mimicus | This study | Oyster, Brazilc | API20E 5146104 | 150 |
| 543 | V. mimicus | This study | Oyster, Brazilc | API20E 5146104 | 151 |
| 573 | V. mimicus | E. M. Bik | Human, India | Vm 4053 | 136 |
| 601 | V. mimicus | This study | Water, Brazild | API20E 4146104 | 137 |
| 602 | V. mimicus | This study | Water, Brazild | API20E 5346104 | 138 |
| 603 | V. mimicus | This study | Water, Brazild | API20E 5146104 | 139 |
| 605 | V. mimicus | This study | Water, Brazild | API20E 4144104 | 139 |
| 606 | V. mimicus | This study | Water, Brazild | API20E 4146104 | 140 |
C. A. Salles, Laboratório de Sistemática Bioquímica, Oswaldo Cruz Institute, Rio de Janeiro, Brazil; ATCC, American Type Collection Culture, Manassas, Va.; P. Desmarchelier, Commonwealth Institute of Health, The University of Sydney, Sydney, New South Wales, Australia; S. Shinoda, Faculty of Pharmaceutical Sciences, Okayama, Japan; E. M. Bik, National Institute of Public Health and Environment, Bilthoven, The Netherlands.
S(−), non-sucrose fermenting.
Isolated from Rio de Janeiro state.
Isolated from Pará/Amazonia region.
MEE was performed as described by Salles and Momen (13). Fourteen enzyme loci were assayed for allelic variation: aconitate hydratase (EC 2.4.2.1.3), alanine dehydrogenase (EC 1.4.1.1), isocitrate dehydrogenase (IDH; EC 1.1.1.40), malic enzyme (EC 1.1.1.39), carboxylesterase (NSE; EC 3.1.1.1), 6-phosphogluconate dehydrogenase (EC 1.1.1.44), malate dehydrogenase (EC 1.1.1.37), phosphoglucomutase (EC 2.7.5.1), glucose phosphate isomerase (GPI; EC 5.3.1.9), glucose-6-phosphate dehydrogenase (EC, 1.1.1.49), proline dipeptidase (EC 3.4.13.9), leucylleucyl peptidase (EC 3.4.11), leucylalanine peptidase (EC 3.4.11.1), and leucine aminopeptidase (LAP; EC 3.4.1.1). The distinctive electromorphs (mobility variants) of each enzyme were numbered in order of increasing rate of anodal migration and were equated with alleles at corresponding structural gene loci, and strains having identical allelic profiles for all 14 loci were designated as a zymovar. The numerical analysis was performed using the NTSYS-pc software package (F. James Rohlf, version 1.7, Exeter Software, Setauket, N.Y.). The Jaccard coefficient (16) was used to determine the relationships between the zymovars. The similarity matrix was transformed into a dendrogram by the unweighted pair group method with arithmetic averages (UPGMA). Cophenetic correlation coefficients were determined (16) to assess the agreement between similarity values implied by the phenogram and those of the original similarity matrix. Genetic diversity was estimated as described by Selander et al. (14).
The PCR conditions and primers were described by Chun et al. (7). All strains listed in Table 1 were screened by PCR with two primers, prVC-F and VCM-R, under high-stringency conditions. Identical bands of 295- to 310-bp ISR amplicon were detected by 1.5% agarose gel electrophoresis and visualized by UV transillumination after being stained with ethidium bromide.
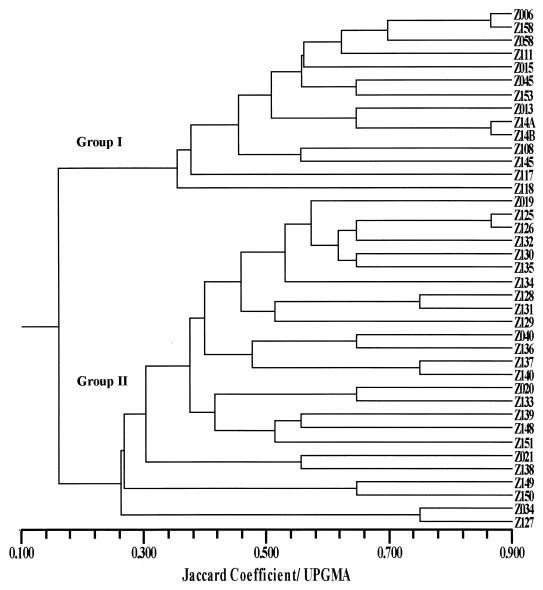
Genetic information derived from MEE can be used to differentiate closely related organisms. Several studies (1, 19) have shown that MEE data support the taxonomic groups that have been proposed on the basis of DNA relatedness, a method considered the standard reference technique in bacterial species classification (17). Salles and Momen (13) have shown that the MEE method can have an application in the differentiation of V. mimicus from V. cholerae; however, in their work only five strains of V. mimicus were analyzed. In this study, we examined 26 strains of V. mimicus by this method. All 14 enzymatic loci assayed were polymorphic among the Vibrio strains tested. The allelic profiles of the Vibrio strains and the distribution of the strains into zymovars are given in Table 2. The relationships of the zymovars are shown in a dendrogram (Jaccard/UPGMA) and are supported by a high cophenetic correlation (r = 0.88) (Fig. 1). There was no sharing of zymovars among the species studied. The zymovars were distributed into two major groups (I and II) at the 0.158 SJ level, corresponding to V. cholerae and V. mimicus, respectively. The V. cholerae group consists of 13 representative zymovars, some of which were reported earlier (13).
TABLE 2.
Allelic profiles at 14 enzyme loci for the zymovars of Vibrio
| Zymovar | No. of strains | Species | Allele at indicated enzyme locusa
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACO | ADH | IDH | ME | NSE | PGD | MDH | PGM | GPI | G6P | PD | P1 | P2 | LAP | |||
| 006 | 1 | V. cholerae | 1 | 1 | 1 | 2 | 5 | 3 | 3 | 2 | 3 | 4 | 4 | 2 | 1 | 2 |
| 013 | 1 | V. cholerae | 1 | 1 | 1 | 2 | 4 | 2 | 3 | 2 | 3 | 5 | 4 | 2 | 1 | 2 |
| 014 A | 1 | V. cholerae | 1 | 1 | 1 | 2 | 4 | 3 | 3 | 2 | 2 | 5 | 4 | 2 | 1 | 1 |
| 014 B | 1 | V. cholerae | 1 | 1 | 1 | 2 | 4 | 3 | 3 | 2 | 2 | 5 | 4 | 2 | 1 | 3 |
| 015 | 1 | V. cholerae | 1 | 1 | 1 | 2 | 5 | 3 | 3 | 2 | 3 | 3 | 1 | 1 | 1 | 2 |
| 045 | 1 | V. cholerae | 2 | 1 | 1 | 2 | 5 | 3 | 3 | 2 | 3 | 5 | 3 | 2 | 1 | 2 |
| 058 | 1 | V. cholerae | 1 | 1 | 1 | 2 | 5 | 3 | 3 | 2 | 3 | 4 | 5 | 3 | 1 | 2 |
| 108 | 1 | V. cholerae | 1 | 2 | 1 | 2 | 5 | 4 | 3 | 2 | 3 | 5 | 5 | 2 | 1 | 2 |
| 111 | 1 | V. cholerae | 1 | 1 | 1 | 2 | 5 | 3 | 3 | 1 | 2 | 4 | 4 | 2 | 1 | 1 |
| 117 | 1 | V. cholerae | 0.5 | 1 | 1 | 2 | 5 | 4 | 3 | 1 | 2 | 4 | 5 | 1 | 1 | 2 |
| 118 | 1 | V. cholerae | 1 | 1 | 2 | 1 | 5 | 3 | 3 | 2 | 3 | 5 | 3 | 2 | 2 | 1 |
| 145 | 1 | V. cholerae | 2 | 1 | 1 | 2 | 5 | 4 | 3 | 2 | 3 | 4 | 5 | 2 | 2 | 2 |
| 153 | 1 | V. cholerae | 1 | 1 | 1 | 2 | 5 | 3 | 3 | 2 | 3 | 5 | 2 | 2 | 1 | 1 |
| 158 | 1 | V. cholerae | 1 | 1 | 1 | 2 | 5 | 3 | 3 | 1 | 3 | 4 | 4 | 2 | 1 | 2 |
| 019 | 1 | V. mimicus | 1 | 1 | 2 | 2 | 1 | 6 | 4 | 3 | 4 | 5 | 2 | 1 | 3 | 5 |
| 020 | 1 | V. mimicus | 1 | 1 | 2 | 2 | 3 | 6 | 3 | 3 | 6 | 7 | 3 | 3 | 1 | 3 |
| 021 | 1 | V. mimicus | 2 | 1 | 2 | 1 | 1 | 4 | 2 | 3 | 5 | 5 | 1 | 2 | 2 | 4 |
| 034 | 1 | V. mimicus | 2 | 3 | 2 | 2 | 1 | 3 | 3 | 1 | 5 | 5 | 4 | 3 | 3 | 4 |
| 040 | 1 | V. mimicus | 1 | 1 | 2 | 2 | 1 | 6 | 3 | 2 | 5 | 5 | 2 | 2 | 4 | 5 |
| 125 | 1 | V. mimicus | 1 | 1 | 2 | 2 | 1 | 6 | 2 | 3 | 5 | 5 | 2 | 1 | 5 | 5 |
| 126 | 1 | V. mimicus | 1 | 1 | 2 | 2 | 1 | 6 | 2 | 3 | 5 | 6 | 2 | 1 | 5 | 5 |
| 127 | 1 | V. mimicus | 2 | 3 | 2 | 2 | 1 | 3 | 3 | 1 | 5 | 5 | 2 | 3 | 3 | 5 |
| 128 | 1 | V. mimicus | 1 | 1 | 3 | 2 | 1 | 6 | 3 | 3 | 5 | 5 | 4 | 1 | 2 | 5 |
| 129 | 1 | V. mimicus | 1 | 1 | 2 | 2 | 1 | 6 | 2 | 3 | 5 | 5 | 3 | 3 | 2 | 4 |
| 130 | 1 | V. mimicus | 1 | 1 | 2 | 2 | 0.5 | 6 | 2 | 3 | 5 | 5 | 2 | 2 | 3 | 5 |
| 131 | 1 | V. mimicus | 1 | 1 | 3 | 2 | 1 | 6 | 3 | 3 | 5 | 5 | 3 | 4 | 2 | 5 |
| 132 | 1 | V. mimicus | 1 | 1 | 2 | 2 | 1 | 6 | 2 | 3 | 5 | 7 | 2 | 4 | 3 | 5 |
| 133 | 1 | V. mimicus | 1 | 1 | 2 | 2 | 1 | 6 | 3 | 3 | 6 | 7 | 3 | 3 | 2 | 5 |
| 134 | 1 | V. mimicus | 1 | 0.5 | 2 | 2 | 1 | 6 | 2 | 3 | 5 | 6 | 1 | 1 | 3 | 5 |
| 135 | 1 | V. mimicus | 1 | 1 | 3 | 2 | 1 | 6 | 2 | 3 | 5 | 5 | 2 | 3 | 3 | 5 |
| 136 | 1 | V. mimicus | 1 | 1 | 2 | 2 | 1 | 6 | 4 | 2 | 4 | 5 | 3 | 2 | 4 | 5 |
| 137 | 1 | V. mimicus | 1 | 1 | 3 | 2 | 1 | 6 | 3 | 2 | 5 | 6 | 1 | 1 | 4 | 5 |
| 138 | 1 | V. mimicus | 1 | 1 | 2 | 2 | 1 | 4 | 3 | 2 | 5 | 5 | 1 | 2 | 2 | 4 |
| 139 | 2 | V. mimicus | 1 | 1 | 2 | 2 | 1 | 6 | 3 | 3 | 5 | 6 | 4 | 4 | 3 | 5 |
| 140 | 1 | V. mimicus | 1 | 1 | 2 | 1 | 1 | 6 | 3 | 2 | 5 | 6 | 1 | 1 | 4 | 5 |
| 148 | 1 | V. mimicus | 2 | 1 | 2 | 2 | 1 | 6 | 3 | 3 | 6 | 6 | 4 | 2 | 3 | 4 |
| 149 | 1 | V. mimicus | 2 | 1 | 3 | 3 | 1 | 6 | 2 | 3 | 6 | 7 | 3 | 3 | 4 | 4 |
| 150 | 1 | V. mimicus | 2 | 1 | 2 | 3 | 1 | 6 | 2 | 3 | 6 | 7 | 4 | 3 | 3 | 4 |
| 151 | 1 | V. mimicus | 2 | 1 | 2 | 2 | 1 | 6 | 3 | 3 | 6 | 6 | 2 | 3 | 4 | 5 |
ACO, aconitate hydratase; ADH, alanine dehydrogenase; IDH, isocitrate dehydrogenase; ME, malic enzyme, NSE, carboxylesterase; PGD, 6-phosphogluconate dehydrogenase; MDH, malate dehydrogenase; PGM, phosphoglucomutase; GPI, glucose phosphate isomerase; G6P, glucose-6-phosphate dehydrogenase; PD, proline dipeptidase; P1, leucylleucyl peptidase; P2, leucylalanine peptidase; LAP, leucine aminopeptidase. Characteristic alleles for specific species are in boldface.
FIG. 1.
Dendrogram showing the relationship among zymovars of V. cholerae (group I) and V. mimicus (group II).
We also established that although the 14 enzymatic loci were effective in separating these related species, specific combinations of 4 enzymatic loci are enough to differentiate V. mimicus from V. cholerae. The IDH, NSE, GPI, and LAP loci showed characteristic alleles for the different species studied (Table 2). Some alleles for GPI, NSE, and LAP only found in V. mimicus strains in this study were also found within the 135 zymovars of V. cholerae (unpublished data). The NSE-3 was a rare allele found only in one zymovar of V. cholerae. GPI-4 and LAP-3 were found in three and six zymovars of the V. cholerae respectively. IDH-2 and IDH-3 were found only in three zymovars of V. cholerae. Therefore, the diagnostic value of the combinations of these loci can be very useful in ecological, clinical, and epidemiological studies.
The differentiation of these two species has been based largely on the inability of V. mimicus to ferment sucrose. Phenotypic studies have reported other tests, such as the Voges-Proskauer reaction, lipase production (corn oil), Jordan tartrate test, and polymyxin sensitivity (10, 18), to be of limited value in distinguishing between these two species. Desmarchelier and Reichelt (11) analyzed sucrose-negative strains by DNA relatedness and determined that they belonged to the V. cholerae species. Seven of these strains possessed traits described for V. cholerae, not considering sucrose fermentation, and two strains were phenotypically closely related to V. mimicus. Therefore, sucrose-negative strains of V. cholerae might be misidentified as V. mimicus; interestingly, in 1995 an outbreak of a pathogenic sucrose-negative O1 toxigenic variant of V. cholerae was detected in the Amazon region (8).
Our MEE results also showed that even atypical strains of V. cholerae and V. mimicus could be correctly identified. The two sucrose-negative V. cholerae non-O1 isolates and the sucrose-negative, toxigenic V. cholerae O1 isolate clustered within the species as did the 535 strain, with a nontypical phenotypic profile when typed using the API 20E biochemical system, within V. mimicus species (Fig. 1). These results are in agreement with the previous study of Chowdhury et al. (6) which reported the inefficacy of the API 20E system alone in the identification of V. mimicus strains and suggested some complementary tests for use in conjunction.
According to MEE data, V. mimicus is a heterogeneous genetic group of microorganisms. The mean genetic diversity per locus was 0.431, comparable to the value of 0.436 found by Beltran et al. (2) for V. cholerae strains. These degrees of variation were less than the 0.52 reported for the Escherichia coli reference collection (12). Our analysis of clinical and environmental isolates of V. mimicus revealed that the environmental Japanese strains subgrouped at the 0.45 SJ level (Fig. 1). The great genetic variability of clinical strains of V. mimicus from different geographic regions had been also reported by Bi et al. (3), using arbitrarily primed PCR.
The ISR-PCR identification system was proposed to distinguish V. cholerae from V. mimicus. However, our results show that the V. cholerae diagnostic amplicon is present in 11% of V. mimicus isolates, among them 339, 602, and the biochemically atypical 535. The genetic diversity found within these species led us to believe that it is necessary to have a representative sample of strains to evaluate any identification system.
In conclusion, the MEE method has provided an accurate molecular approach for differentiation between V. cholerae and V. mimicus that is particularly useful for the identification of atypical and environmental samples.
We thank Fernanda dos Santos Freitas of the Biochemical Systematic Laboratory, FIOCRUZ, Brazil, for the technical assistance.
This research was supported in part by CAPES and FAPERJ fellowships and a PAPES/FIOCRUZ grant.
REFERENCES
- 1.Balmelli T, Piffaretti J. Analysis of the genetic polymorphism of Borrelia burgdorferi sensu latu by multilocus enzyme electrophoresis. Int J Syst Bacteriol. 1996;46:167–172. doi: 10.1099/00207713-46-1-167. [DOI] [PubMed] [Google Scholar]
- 2.Beltran P, Delgado G, Navarro A, Trujillo F, Selander R K, Craviotto A. Genetic diversity and population structure of Vibrio cholerae. J Clin Microbiol. 1999;37:581–590. doi: 10.1128/jcm.37.3.581-590.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bi K, Shi L, Maehara Y, Miyoshi S, Tomochika K, Shinoda S. Analysis of Vibrio mimicus clinical strains by arbitrarily primed polymerase chain reaction. Microbiol Immunol. 2000;44:149–153. doi: 10.1111/j.1348-0421.2000.tb01258.x. [DOI] [PubMed] [Google Scholar]
- 4.Boyd E F, Moyer K E, Shi L, Waldor M K. Infectious CTXφ and the Vibrio pathogenicity island prophage in Vibrio mimicus: evidence for recent horizontal transfer between V. mimicus and V. cholerae. Infect Immun. 2000;68:1507–1513. doi: 10.1128/iai.68.3.1507-1513.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campos E, Bolanos H, Acuna M T, Díaz G, Matamoros M C, Raventos H, Sánchez L M, Sánchez O, Barquero C. Vibrio mimicus diarrhea following ingestion of raw turtle eggs. Appl Environ Microbiol. 1996;62:1141–1144. doi: 10.1128/aem.62.4.1141-1144.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chowdhury M A R, Yamanaka H, Myoshi S, Aziz K M S, Shinoda S. Ecology of Vibrio mimicus in aquatic environments. Appl Environ Microbiol. 1989;55:2073–2078. doi: 10.1128/aem.55.8.2073-2078.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chun J, Huq A, Colwell R R. Analysis of 16S–23S rRNA intergenic spacer regions of Vibrio cholerae and Vibrio mimicus. Appl Environ Microbiol. 1999;65:2202–2208. doi: 10.1128/aem.65.5.2202-2208.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coelho, A., J. R. C. Andrade, M. A. S. Batista, A. C. P. Vicente, L. R. Ferraz, and C. A. Salles. Genomic fingerprints of Vibrio cholerae using arbitrary primer PCR are useful epidemiological tools, p. 203–211. In G. T. Keusch and M. Kawakami (ed.), Cytokines, gut, and cholera, 11th ed. IOS Press, Amsterdam, The Netherlands.
- 9.Colwell R R, Huq A. Environmental reservoir of Vibrio cholerae. The causative agent of cholera. Ann N Y Acad Sci. 1994;740:44–54. doi: 10.1111/j.1749-6632.1994.tb19852.x. [DOI] [PubMed] [Google Scholar]
- 10.Davis B R, Fanning G R, Madden J M, Steigerwalt A G, Bradford H B, Jr, Smith H L, Jr, Brenner D J. Characterization of biochemically atypical Vibrio cholerae and designation of a new pathogenic species, Vibrio mimicus. J Clin Microbiol. 1981;14:631–639. doi: 10.1128/jcm.14.6.631-639.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desmarchelier P M, Reichelt J L. A phenotypic and genetic study of sucrose nonfermenting strains of Vibrio mimicus and Vibrio cholerae. Curr Microbiol. 1984;10:41–48. [Google Scholar]
- 12.Ochman H, Whittham T S, Caugant D A, Selander R K. Enzyme polymorphism and genetic population structure in Escherichia coli and Shigella. J Gen Microbiol. 1983;129:2715–2726. doi: 10.1099/00221287-129-9-2715. [DOI] [PubMed] [Google Scholar]
- 13.Salles C A, Momen H. Identification of Vibrio cholerae by enzyme electrophoresis. Trans R Soc Trop Med Hyg. 1991;85:544–547. doi: 10.1016/0035-9203(91)90251-s. [DOI] [PubMed] [Google Scholar]
- 14.Selander R K, Caugant D A, Ochaman H, Musser J M, Gilmour M N, Whittan T S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986;51:873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi L, Miyoshi S, Hiura M, Tomochika K, Shimada T, Shinoda S. Detection of genes encoding cholera toxin (CT), Zonula occludens toxin (ZOT), accessory cholera enterotoxin (ACE) and heat-stable enterotoxin (ST) in Vibrio mimicus clinical strains. Microbiol Immunol. 1998;42:823–828. doi: 10.1111/j.1348-0421.1998.tb02357.x. [DOI] [PubMed] [Google Scholar]
- 16.Sneath P H A, Sokal R R, editors. Numerical taxonomy. W. H. San Francisco, Calif: Freeman; 1973. [Google Scholar]
- 17.Stackebrandt E B, Goebel M. Taxonomic note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int J Syst Bacteriol. 1994;44:846–884. [Google Scholar]
- 18.Tison D. Vibrio. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: American Society for Microbiology; 1999. pp. 497–504. [Google Scholar]
- 19.Vieira V V, Teixeira L M, Zahner V, Momen H, Facklam R R, Steigerwalt A G, Brenner D J, Castro A C D. Genetic relationships among the different phenotypes of Streptococcus dysgalactiae strains. Int J Syst Bacteriol. 1998;48:1231–1243. doi: 10.1099/00207713-48-4-1231. [DOI] [PubMed] [Google Scholar]