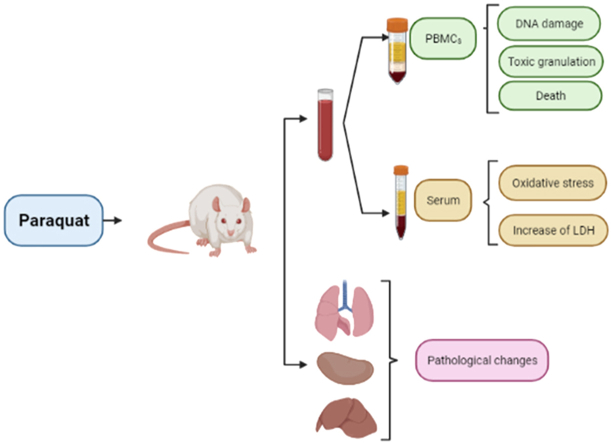
Abstract
Paraquat (PQ) is a herbicide belonging to the group of bipyridylium salts. The objective of this study was to evaluate oxidative stress, DNA damage, and cytotoxicity induced by paraquat in peripheral lymphocyte cells in vivo as well as pathological changes in various tissues. For this purpose, 28 male Wistar rats in 6 different groups were poisoned by paraquat gavage and blood samples were taken from the hearts of rats after during the poisoning period. Oxidative stress, DNA damage, cell membrane integrity, serum lactate dehydrogenase, and cytotoxicity, were investigated by Ferric Reducing Antioxidant Potential (FRAP) test, alkaline comet assay, measuring serum lactate dehydrogenase (LDH), Hoechst staining and flow cytometry with propidium iodide (PI) respectively. The lung, kidney, and liver tissues were also examined pathologically. Paraquat caused dose-dependent DNA damage in peripheral lymphocyte cells and significant oxidative cell membrane damage. The most damage was caused by a single dose of 200 mg/kg b.w of paraquat by gavage. The gradual exposure to a dose of 300 mg/kg b.w of paraquat showed less damage, which could be due to the activation of the antioxidant defense mechanism.
Keywords: Paraquat, Oxidative stress, DNA damage, Cytotoxicity, In vivo
Graphical abstract
Highlights
-
•
Paraquat induced oxidative stress.
-
•
Paraquat increases serum lactate dehydrogenase.
-
•
Oxidative stress Inducted by exposure to paraquat Inducted DNA damage.
Paraquat; Oxidative stress; DNA damage; Cytotoxicity; In vivo.
1. Introduction
Paraquat (PQ, 1, dimethyl-4,4-bipyridylium dichloride-1) in the group of bipyridine herbicides is a rapid contact herbicide that works by diverting electrons from photosystem I (PSI) into the chloroplast. PQ is the second best-selling herbicide available as a 20% solution [1, 2]. Paraquat is used in different regions of Iran under different climatic conditions and different agricultural activities [3]. In North America, paraquat is used to conserve soybeans, cereals, and cotton, in China for rice, Malaysia for crops such as oil palm, the United Kingdom for potatoes and green vegetables, and Brazil for coffee [4]. Despite its advantages for agriculture, the toxicity of PQ in lands and waters raises serious concerns. Paraquat cannot decompose rapidly in the environment, and prolonged exposure to it can cause harmful bio-magnification in humans and mammals. Extensive applications of paraquat lead to widespread residues on soil surfaces and aquatic environments that finally enter the food chain [5]. In many developing countries where the rules governing the sale and use of PQ are not strict, it is difficult to prevent PQ poisoning [6]. In pesticide poisoning, paraquat poisoning is after organophosphorus poisoning. However, paraquat poisoning causes the highest number of deaths and its mortality rate is up to 60–80%. There is currently no specific antidote for the treatment of paraquat poisoning [7]. Due to the toxicity of PQ to humans and its potential effects on the environment, paraquat remains one of the most controversial and studied herbicides in the last 50 years [8]. Paraquat increases the formation of free radicals and oxidative stress [9]. PQ exerts its toxic effects primarily through its redox cycle through the production of superoxide anions in living organisms. Therefore, this leads to an imbalance in cell redox status and causes oxidative damage and cell death [10]. It was found that PQ reduces superoxide dismutase (SOD) activity and increase malondialdehyde (MDA) level [11] Endoplasmic reticulum stress (ERS) is also a cause of many reported diseases caused by this toxin, although mammalian cells have a defense mechanism (unfolded protein response) against ERS-induced apoptosis [12].
Oxidative stress, defined as the overproduction of reactive oxygen species [13], changes the structure and function of nucleic acids, lipids, and proteins [14]. ROS as the cell signaling major regulator is also involved in regulating cell growth, development, inflammation, and apoptosis [15]. Paraquat can cause severe damage to vascular endothelial cells and can interfere with blood clotting when taken orally [16]. PQ is rapidly but incompletely absorbed and then excreted in the urine within 12–24 h. This substance has a severe effect on the lungs and in high doses may damage other important organs such as the heart, kidneys, liver, adrenal glands, central nervous system, muscles, and spleen and cause numerous failures [6]. The lung is the main target organ in paraquat poisoning, and respiratory failure due to lung injury is the most common cause of death due to paraquat poisoning [17]. The polyamine re-uptake system, which is mainly expressed in the membrane of type II pneumocytes, actively adsorbs paraquat ions against the plasma gradient. As a result, the concentration of paraquat in the lung is 10 times higher than in the plasma chamber [1]. Long-term exposure to PQ is a risk factor for age-related neurodegenerative diseases such as Parkinson's disease [18]. Using PQ is currently banned in 32 countries, including the European Union, which is primarily based on human health concerns. However, it is still ordered and used in more than 90 countries [8].
In 2021, paraquat was included in List III of the Rotterdam Convention, and the purchase and sale of this material must be done with prior informed consent [19]. Little information is available on the carcinogenicity of Paraquat. However, in some studies, there is an association between exposure to paraquat and the incidence of some cancers [20, 21, 22, 23, 24, 25]. The existence of an association between occupational exposure to paraquat and the development of non-Hodgkin's lymphoma has been observed in some studies [26, 27]. This association may be due to various injuries caused by exposure to paraquat on lymphocyte cells. Therefore, in this study, we focused more on lymphocyte cells to investigate the destructive effects of paraquat in vivo conditions. Several studies have examined the effect of paraquat on lymphocyte cell culture but the results may be different under in vivo conditions and the presence of different defense mechanisms [28, 29]. The objective of this study was to evaluate oxidative stress, DNA damage, and cytotoxicity induced by paraquat exposure in peripheral lymphocyte cells in rats as well as pathological changes in various tissues.
2. Materials and methods
2.1. Rats
Male Wistar rats weighing 200–300 g were purchased from the laboratory animal care department of Birjand University of Medical Sciences. They were kept in a temperature-controlled place during 12 h of darkness/light and humidity of 40–45% and had free access to water and food. This research was conducted with the supervision of the ethics committee of Kerman University of medical sciences with Ethics Code: IR.KMU.REC.1399.471. and Project code: 99000419.
2.2. Poisoning of rats
28 rats were randomly divided into 5 groups of n = 5 and a group of n = 3 and poisoned for dose-response and comparison of gradual and acute exposure with different doses of paraquat. Several different doses were selected according to similar studies and available information about LD50 of Paraquat and were finalized by a pre-test. Group A was the negative control group by gavage of 2 mL of distilled water one day before blood sampling in rats. Group B was gavaged 30 mg/kg b.w of paraquat (Nanjing Red Sun Biochemistry Co., China) that was dissolved in distilled water with a final volume of 2 mL, once daily for three days, and blood sampling one day after the last gavage. Group C was gavaged 60 mg/kg b.w of paraquat once daily for three days and blood sampling one day after the last gavage. Group D was gavaged 200 mg/kg b.w of paraquat once and blood sampling after one day. Group E was gavaged 50 mg/kg b.w of paraquat for three weeks, twice a week (total gavage = 6 times). Group F was gavaged 200 mg/kg b.w of ethyl methanesulfonate by gavage (Merck, India) 24 and 3 h before blood sampling. All groups consisted of 5 rats except group F which consisted of 3 rats. Group F based on the OECD guideline (OECD guideline for testing of chemicals in vivo mammalian alkaline comet assay) was considered a positive control in the comet assay.
2.3. Blood sampling
The rats were anesthetized by intraperitoneal injection of ketamine, xylazine, and acepromazine. After complete anesthesia, the rats' abdomen was opened and blood was drawn from the heart immediately with a heparinized syringe. 2 mL of this blood was transferred to a tube containing clot activating gel to prepare the serum. The serum obtained after centrifugation for 10 min at 1207 × g was kept at 193 K until further tests were performed. 3 mL of blood taken from the heart was diluted by 3 mL of phosphate-buffered saline (PBS). Then, using ficoll peripheral blood mononuclear cell (PBMC) was separated by centrifuge at 1207 × g for 15 min. After washing three times with PBS buffer, it was mixed with 1 mL of this buffer and used for comet assay, Hoechst staining, and flow cytometry.
2.4. Total antioxidant capacity
Free radical damage is associated with the development of many degenerative diseases, including cancer, cardiovascular disease, cataracts, and aging. The balance between production and inhibition of reactive oxygen species (ROS) leads to homeostasis. However, if the balance is somehow shifted towards the formation of free radicals, it leads to accumulated cell damage in time [30].
Total Antioxidant Capacity is an analysis that is often used to evaluate the antioxidant status of biological samples and can evaluate the antioxidant response to free radicals produced due to a particular disease [31]. One of the most common methods for evaluating total antioxidant capacity is the Ferric Reducing Antioxidant Power (FRAP) method, which is relatively simple, fast, sensitive, and inexpensive [32]. In this study, Total Antioxidant Capacity Zantox kit based on the FRAP method was used to measure total antioxidant capacity.
2.5. Comet assay
The comet assay in this study was performed as follows. First, the microscope slide was coated with a thin layer of ordinary 1% agarose (Agar with a normal melting point of 1% w/v in water). Approximately 10–100 μL of agarose with a low melting point of 1% (w/v in PBS) was mixed with the cell suspension, poured onto the previously prepared slide, and spread on the slide surface to coagulate. The prepared slides were transferred to the refrigerator and lysed buffer (5.2 M of sodium chloride, 10 mM of Tris-HCl, 10 M of Na2EDTA, 1 M of 10% dimethyl sulfoxide, 1% Triton X-100, pH = 5.7) was added and kept in the refrigerator for 1 h. After 1 h, a denatured solution (300 mM of NaOH, 1 mM of Na2EDTA, pH = 13) was added to the above slides and stored in the refrigerator for 20 min. This opens DNA strands and reveals DNA damage. The slides were finally slowly removed from the denatured solution, placed side by side in an electrophoresis tank containing denatured buffer, and then exposed to 20 V for 20 min. After electrophoresis, the slides were washed with neutralizing buffer (0.4 M of Tris-HCl, pH = 5.7) for 5 min to neutralize the base media. This stage was repeated three times and then the slides were exposed to methanol for 5 min. Finally, the slides were stained with ethidium bromide. UV fluorescent microscope of wide green (WG) 515–560 nm filter and 590 nm barrier filter were used to observe the damage. The comet score software was used to analyze the images, which determines DNA damage in the cell by measuring the length of DNA migration and percentage. Finally, the comet score software calculates the Olive moment (Olive moment= (tail mean-head mean) × % of DNA in the tail) [33]. The greater amount of Olive moment and % of DNA in the tail, the greater the DNA damage to the cell.
2.6. Lactate dehydrogenase test
Lactate dehydrogenase (LDH) is an oxidoreductase that participates in the glycolysis of carbohydrates. This enzyme catalyzes reactions of converting pyruvic to lactic acid (under anaerobic conditions) and vice versa and lactic to pyruvic acid under aerobic conditions [34]. Due to tissue damage, cells release LDH into the bloodstream. Depending on the type of tissue damage, the enzyme can stay in the bloodstream for up to 7 days. The serum LDH increased as a result of organ destruction due to significant cell death leading to cytoplasmic loss [35]. This study measured serum LDH levels by clinical-chemical analyzer Prestige 24i.
2.7. Hoechst 33258 staining
Hoechst is a cell-permeable dye that usually binds to the adenine-thymine regions of DNA, is excited in ultraviolet light, and emits blue fluorescence at 460–490 nm [36]. 30 μL of the obtained PBMC was transferred to a microtubule and centrifuged at 850 × g for 10 min. The supernatant was then drained and added to the remaining 50 μL of Hoechst dye (neutral formalin (10%) dye-containing Hoechst 33258 (6.25 ng/mL) and kept in the dark at 277 K for 24 h. After 24 h, a drop of the suspension was placed on the slide and covered with a slide. 200 cells were Examined in magnification of 40X the percentage of apoptotic cells was calculated. The nuclei of healthy cells are generally spherical and DNA is evenly distributed [37]. The morphological properties of apoptosis include reduced cell volume, chromatin density, and nuclear fragmentation [38].
2.8. Flow cytometry
Propidium iodide (PI) is a small fluorescent molecule that binds to DNA but cannot pass through cells that have intact plasma membranes. PI uptake compared to non-uptake can be used to detect dead cells, in which plasma membranes are damaged regardless of the mechanism of death, while they cannot penetrate living cells with healthy membranes. PI is excited at wavelengths between 400 and 600 nm and emits light between 600 and 700 nm, and is therefore compatible with lasers and photodetectors commonly found in flow cytometers [39]. This method was used to evaluate the cytotoxicity of peripheral lymphocytes after exposure to paraquat.
2.9. Pathological examinations
Liver, kidney, and lung tissues were isolated and transferred to 10% formalin at room temperature. After tissue stabilization and dehydration with alcohol, xylene was used for clarification. Then, paraffin blocks with sections with a diameter of 6 μm were prepared and examined by an optical microscope. The results were semi-quantitative as follows:
2.9.1. Inflammation of the lungs
Several fields on each slide were examined for inflammation and scored on a scale of 1–4 as follows:
1 = Minimal change, including one or more small foci in the alveoli or around the conducting airways, 2 = Mild change, including small to medium foci, 3 = Medium change, containing frequent and medium foci, and 4 = Change marked with extensive features and the intersection of foci that affect most of the tissue [40].
2.9.2. Hyaline membrane
For the Hyaline membrane in the lung, the whole lung was divided into 10 parts and examined for the presence of hyaline membranes. 0 = none, 1 = identified in Fields 1 or 2, 2 = in 3 or 4 Fields, 3 = in 5 or 6 Fields, 4 = in 7 or 8 Fields, and 5 = in 9 or 10 Fields [41].
2.9.3. Renal tubular lesions
Renal tubular tissue damage was evaluated based on the percentage of tubules that showed dilatation, atrophy, and/or necrosis of epithelial cells, and the assigned scores were as follows:
0 = normal state, 1 = less than 10%, 2 = 10–20%, 3 = 26–50%, 4 = 51–75%, and 5 = more than 75%
10 renal parts with a magnification of 20X were randomly selected for each kidney for evaluation and the mean score for each kidney was recorded [42].
2.9.4. Glomerular atrophy
The number of glomerular atrophy (GA) of atrophic glomeruli in each high-potency field (HPF) was counted, and the mean number of at least 20 parts was randomly selected for each kidney and examined with a 40X representation [43].
2.9.5. Inflammation of the liver parenchyma and portal ducts
0 = none, 1 = focal and mild lesions, 2 = multifocal lesions, moderate, 3 = multifocal lesions, severe, and 4 = multifocal degeneration or necrosis [44].
2.10. Statistical analysis
SPSS and Excel software was used for statistical analysis of the obtained data and drawing of graphs. If the data were normal, an ANOVA test and then, if the variances were homogeneous, the Tukey post hoc test was used. A T-test was used to statistically analyze the variables in both groups. For ranking data, the Kruskal-Wallis test was used first. Then, using the Mann-Whitney test, each group alone was compared with the control group in terms of significant differences.
3. Results
3.1. Results of evaluation of total antioxidant capacity
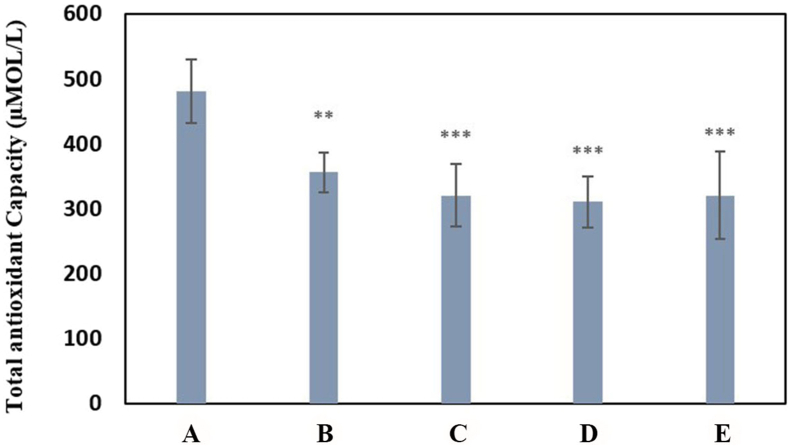
Evaluation of total antioxidant capacity showed oxidative stress induction due to PQ exposure. The reduction of TAC in exposure to paraquat was significant in all groups (Group B (P < 0.01) and groups C, D, E (P < 0.001)) compared to the negative control group. The average total antioxidant capacity in different groups is compared in Chart 1.
Chart 1.
Mean comparison of total antioxidant capacity by FRAP method for experimental groups compared with the negative control group. Group A: negative control group with 2 mL of distilled water gavage, Group B: 30 mg/kg b.w of PQ by gavage, once daily for three days, Group C: 60 mg/kg b.w of PQ by gavage, once daily for three days, Group D: 200 mg/kg b.w of PQ once by gavage, Group E: 50 mg/kg b.w of PQ for three weeks, twice a week by gavage (total 6 times) (∗∗(P<0.01), ∗∗∗(P<0.001)).
3.2. Results of comet assay
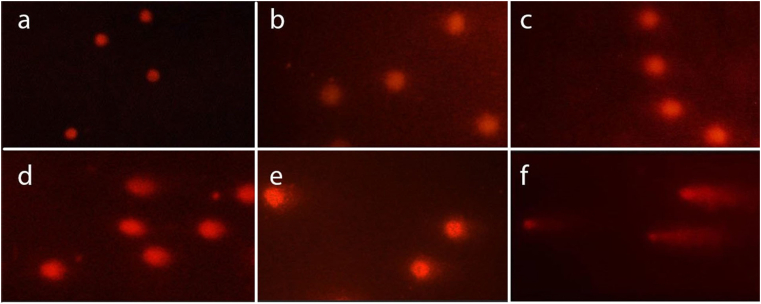
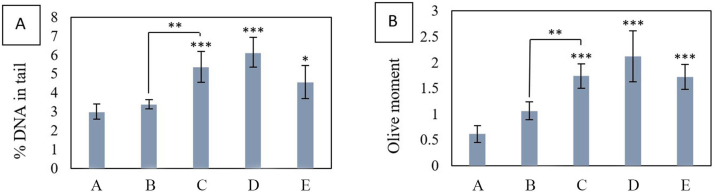
Comet images of the lymphocyte cells stained with Ethidium bromide and observed under the fluorescent microscope are shown in Figure 1(a, b, c, d, e, f). Migration of fragmented DNA away from the nucleus was observed in the positive control group (Figure 1f). In contrast, in the negative control group, DNA was accumulating in the head and the tail was not visible (Figure 1a). Comet images for different groups were analyzed by Comet Score software. % DNA in tail and olive moment for 100 cells per rat were calculated by comet score software. The mean of this value was calculated for each animal in each group. Mean values of % DNA in tail and Olive tail moment in different groups showed that experimental groups increased significantly compared to the controls, except for exposure to 30 mg/kg b.w of PQ once daily for three days (Group B) (Chart 2). The % DNA in tail and olive moment values are presented in Table 1. Each of these values is the average of 5 independent experiments.
Figure 1.
Comet images of the lymphocyte cells stained with Ethidium bromide and observed under a fluorescent microscope. The photos a-f belong to the A-F group, respectively. Group A: negative control group with 2 mL of distilled water gavage, Group B: 30 mg/kg b.w of PQ by gavage, once daily for three days, Group C: 60 mg/kg b.w of PQ by gavage, once daily for three days, Group D: 200 mg/kg b.w of PQ once by gavage, Group E: 50 mg/kg b.w of PQ for three weeks, twice a week by gavage (total 6 times), F: 200 mg/kg b.w of ethyl methanesulfonate by gavage 24 and 3 h before blood sampling (positive control).
Chart 2.
(a) Comparison of the mean % DNA in tail for experimental groups compared to the negative control group. (b) comparison of the mean olive moment for experimental groups compared to the negative control group. A: negative control group with 2 mL of distilled water gavage, Group B: mg/kg b.w of PQ by gavage, once daily for three days, Group C: 60 mg/kg b.w of PQ by gavage, once daily for three days, Group D: 200 mg/kg b.w of PQ once by gavage, Group E: 50 mg/kg b.w of PQ for three weeks, twice a week by gavage (total 6 times) (∗(P<0.05), ∗∗(P<0.01),∗∗∗ (P<0.001)).
Table 1.
% DNA in tail and olive moment for different groups. The table expresses the mean of the values obtained from 5 independent experiments.
| group | % DNA in tail | p-value | olive moment | p-value | ||
|---|---|---|---|---|---|---|
| A | 2.958615 | 0.617857 | ||||
| B | 3.379965 | p-value | 0.910 | 1.062152 | p-value | 0.222 |
| C | 5.35085 | 0.006 | 0.001 | 1.739478 | 0.024 | 0.000 |
| D | 6.113208 | 0.000 | 2.121375 | 0.000 | ||
| E | 4.553258 | 0.030 | 1.722134 | 0.000 | ||
3.3. Results of lactate dehydrogenase
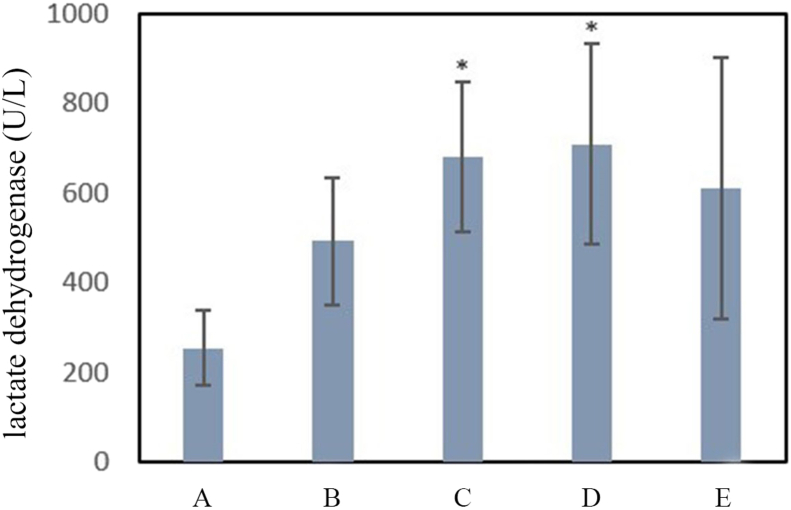
The levels of LDH in serum samples were measured as an indicator of lipid peroxidation and loss of cell membrane integrity. As shown in Chart 3, serum samples’ LDH levels were increased in PQ-treated rats compared to those in the control group. This increase was significant (P < 0.05) at doses of 60 mg/kg b.w of PQ three times and 200 mg/kg b.w of PQ once (Groups C and D).
Chart 3.
Comparison of the mean level of lactate dehydrogenase for the experimental groups compared to the negative control group. A: negative control group with 2 mL of distilled water gavage, Group B: mg/kg b.w of PQ by gavage, once daily for three days, Group C: 60 mg/kg b.w of PQ by gavage, once daily for three days, Group D: 200 mg/kg b.w of PQ once by gavage, Group E: 50 mg/kg b.w PQ for three weeks, twice a week by gavage (total 6 times) (∗(P<0.05)).
3.4. Results of hoechst staining
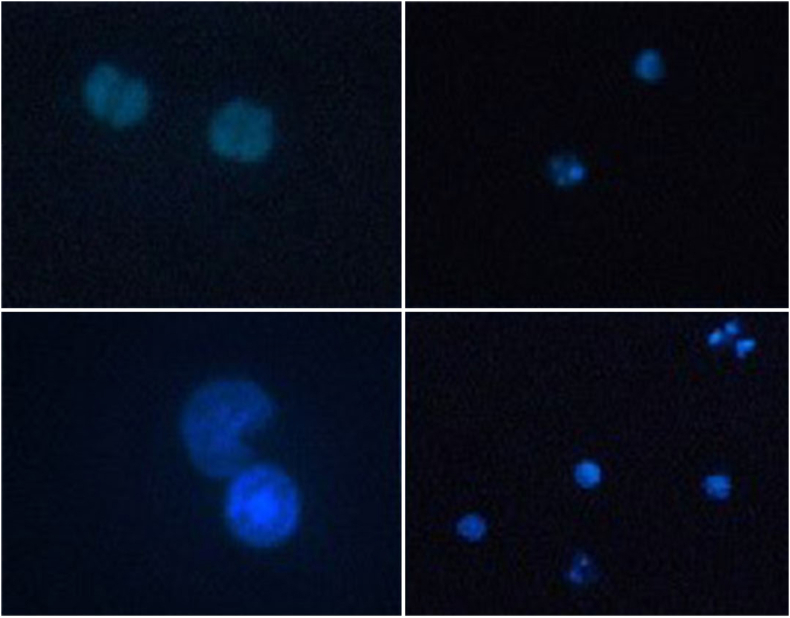
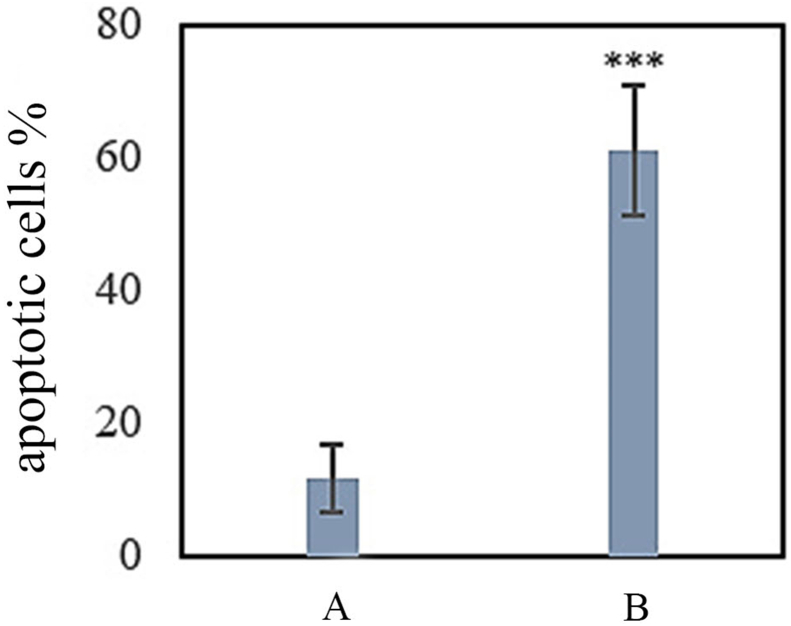
The number of apoptotic cells was significantly greater in Group E (exposed to 50 mg/kg b.w of PQ for three weeks) than in the control group. Figure 2 shows images of Hoechst stained cells. The percentage of apoptotic cells in different groups is compared in Chart 4.
Figure 2.
Imaging obtained from UV fluorescent microscope by Hoechst staining with magnification 40 and 100X, showing apoptosis in peripheral lymphocytes exposed to PQ (Group E: exposure to 50 mg/kg b.w of PQ for three Weeks, twice a week (total 6 times)).
Chart 4.
Percentage of apoptotic cells in Group A and Group E. A: negative control group with 2 mL of distilled water gavage; E: 50 mg/kg b.w of PQ for three weeks, twice a week by gavage (total 6 times) (∗∗∗ (P<0.001)).
3.5. Results of flow cytometry
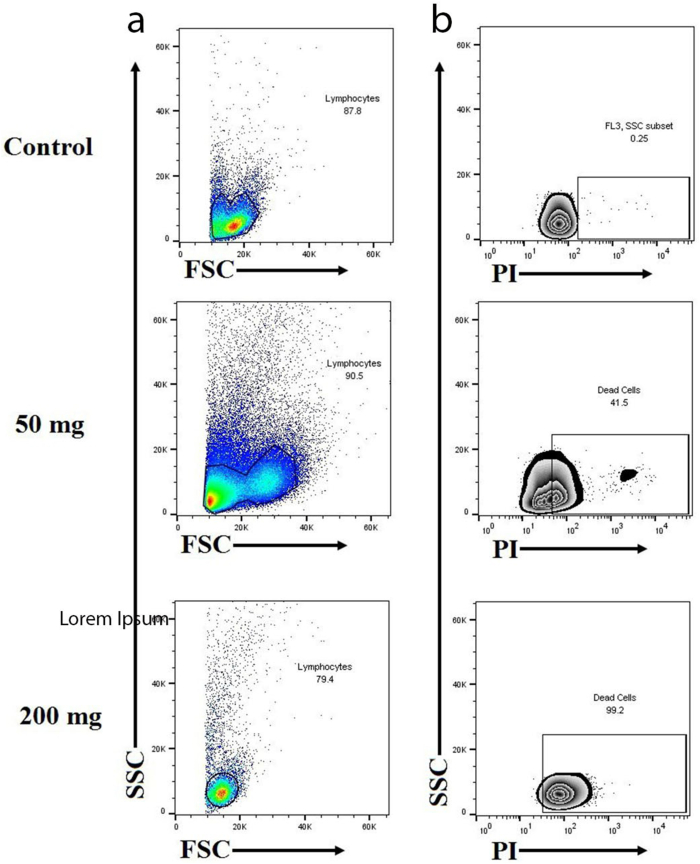
This test was performed for Groups A, D, and E. Figure 3 shows the scatter analysis of lymphocytes by flow cytometry in section a and the death rate of lymphocytes in section b. The results of flow cytometry showed an increased toxic granulation when exposed to 50 mg/kg b.w of PQ paraquat for three Weeks, twice a week (Groups E). Flow cytometry after staining with PI showed deat in different groups. Exposure to paraquat led to increased death of lymphocytes by 41.5% in group D (200 mg/kg b.w of PQ once by gavage) and 99.2% in group E (Figure 3).
Figure 3.
(a) The scatter analysis of lymphocytes by flow cytometry is shown in section a. The horizontal axis (side scatter [SSC]) showed increased toxic granularity when exposed to 50 mg/kg b.w of paraquat for three Weeks, twice a week (Groups E). (b) The plot in section b shows the death rate of lymphocytes. Exposure to PQ led o increased eath of lymphocytes by 41.5% in group D (200 mg/kg b.w of PQ once by gavage) and 99.2% in group E.
3.6. Pathological results
Lung, kidney, and liver tissues were examined and due to the multiplicity of pathological complications, only a semi-quantitative analysis of some cases was adequate.
3.6.1. Lung tissue
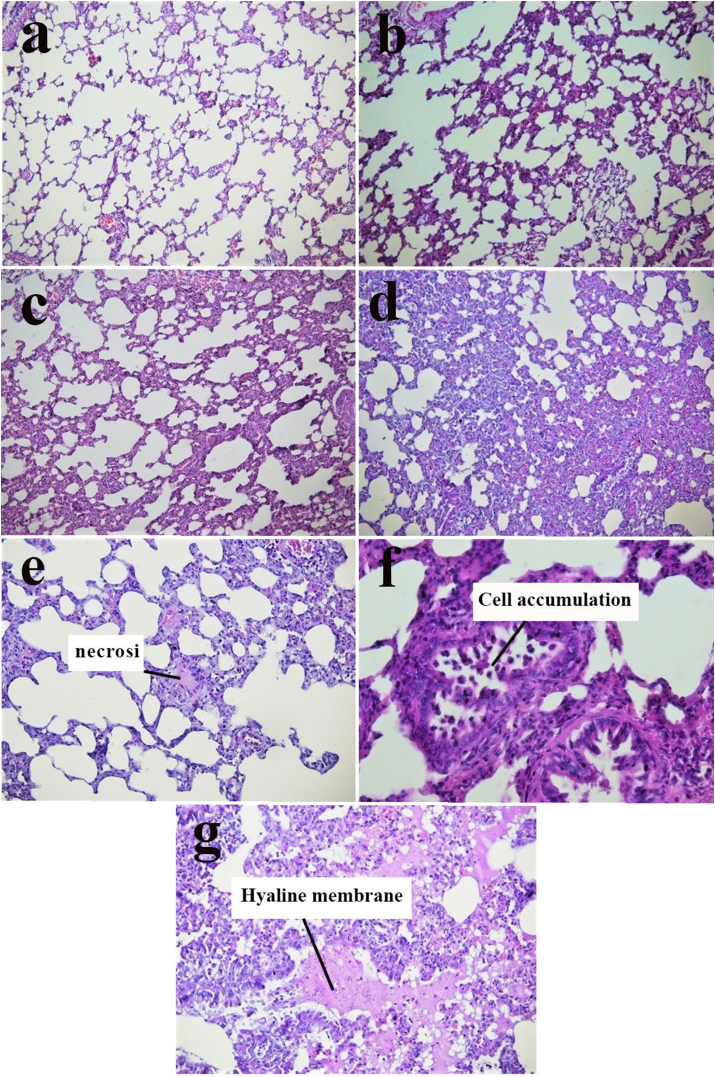
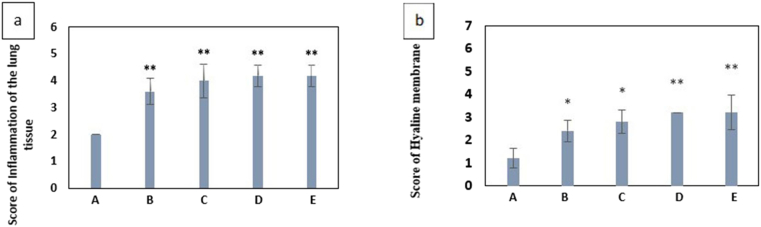
Figure 4a presents the histopathological examination of the lung tissues of normal control mice, showing normal alveolar septa. The lung tissues of mice injected with PQ showed an increase in macrophages, the thickness of alveolar septa (Figure 4a-d), inflammation, necrosis (Figure 4e), Cell accumulation in the alveoli (Figure 4f), and hyaline membrane formation (Figure 4g). The mean scores related to alveolar inflammation and hyaline membrane formation in different groups are given in Chart 5, respectively. UV fluorescent microscope images of lung sections are shown in Figure 4.
Figure 4.
In iImages a-d, an increase was of observed in alveolar septa (optical microscope image of lung sections stained with H&E with magnification 10X). The recorded scores are as follows: a = 1 (negative control), b = 2 (Exposure 30 mg/kg b.w of PQ), c = 3 (Exposure 60 mg/kg b.w of PQ), and d = 4 (Exposure 200 mg/kg b.w of PQ), e. lung tissue necrosis in the Exposure 200 mg/kg b.w of PQ (magnification 10X), f. the accumulation of cells in the alveoli in the Exposure 200 mg/kg b.w of PQ (magnification 40X), g. the formation of a hyaline membrane in the Exposure 200 mg/kg b.w of PQ (magnification 20X).
Chart 5.
(a) Mean value of alveolar inflammation scores due to exposure to paraquat in different groups compared to the control group. (b) Mean Score value of Hyaline membrane scores due to exposure to paraquat in different groups compared to the control group A: negative control group with 2 mL of distilled water gavage, Group B: mg/kg b.w of PQ by gavage, once daily for three days, Group C: 60 mg/kg b.w of PQ by gavage, once daily for three days, Group D: 200 mg/kg b.w of PQ once by gavage, Group E: 50 mg/kg b.w of PQ for three weeks, twice a week by gavage (total 6 times) (∗(P < 0.05), ∗∗ (P < 0.01)).
3.6.2. Kidney tissue
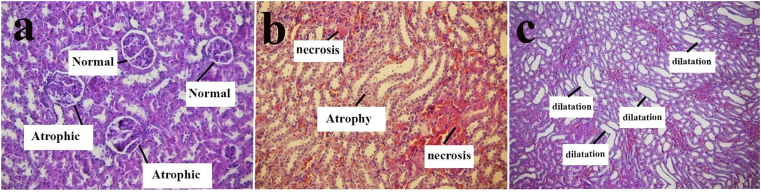
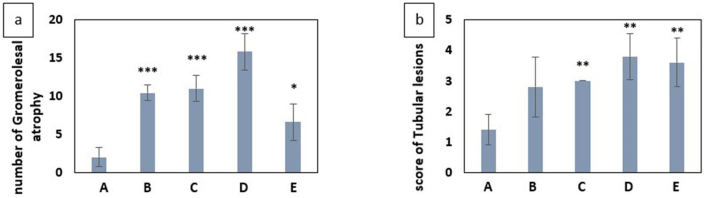
Examination of renal tissue showed glomerular atrophy, dilatation, atrophy, and necrosis of tabulation. Figure 5 shows glomerular atrophy (Figure 5a), atrophy, necrotic (Figure 5b) and dilatation (Figure 5c) in renal tabulation. Chart 6 shows the mean comparison of the number of glomerular atrophy in different groups and score of tabulation damage in different groups.
Figure 5.
Images of kidney tissue under the optical microscope with magnification 20X After exposure to 200 mg/kg b.w of PQ, (a) glomerular atrophy in the Exposure of paraquat, (b) renal tubular necrosis and atrophy in the presence of paraquat, (c) dilatation of Renal tubules in the presence of paraquat.
Chart 6.
(a) Comparison of the mean number of glomerular atrophy in different groups exposed to paraquat and control group. (b) Mean Score of Tubular lesions due to exposure to paraquat in different groups compared to the control group A: negative control group with 2 mL of distilled water gavage, Group B: mg/kg b.w of PQ by gavage, once daily for three days, Group C: 60 mg/kg b.w of PQ by gavage, once daily for three days, Group D: 200 mg/kg b.w of PQ once by gavage, Group E: 50 mg/kg b.w of PQ for three weeks, twice a week by gavage (total 6 times) (∗(P<0.05),∗∗(P<0.01),∗∗∗(P<0.001)).
3.6.3. Liver
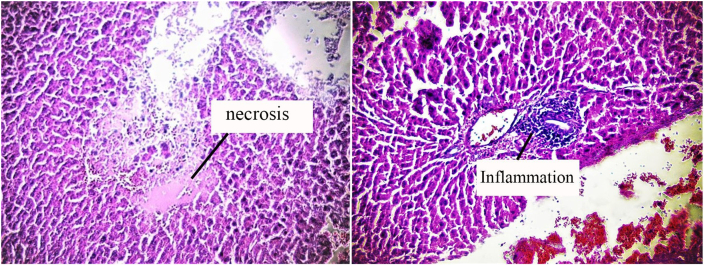
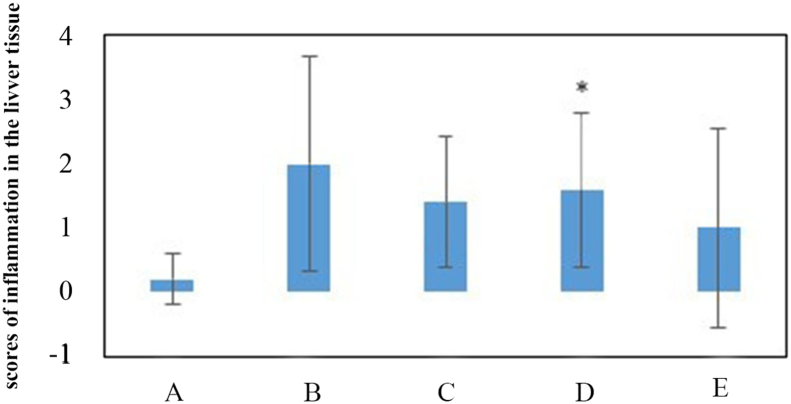
Examination of liver tissue showed necrosis and inflammation in several cases and the mean score assigned to inflammation in all groups increased compared to the control group. Figure 6 shows images of necrosis (Figure 6a) and inflammation (Figure 6b) in liver tissue. Chart 7 shows a comparison of the scores for inflammation in different groups.
Figure 6.
Images of liver tissue under an optical microscope After exposure to 50 mg/kg b.w of PQ for three weeks, twice a week by gavage (total 6 times), (a) necrosis of liver tissue in the presence of paraquat (magnification 20X), (b) inflammation in the liver tissue in the presence of paraquat (magnification 20X).
Chart 7.
Mean score of inflammation in liver tissue in different groups exposed to paraquat compared to the control group. A: negative control group with 2 mL of distilled water gavage, Group B: mg/kg b.w of PQ by gavage, once daily for three days, Group C: 60 mg/kg b.w of PQ by gavage, once daily for three days, Group D: 200 mg/kg b.w of PQ once by gavage, Group E: 50 mg/kg b.w of PQ for three weeks, twice a week by gavage (total 6 times) (∗(P < 0.05)).
4. Discussion
Oxidative stress is one of the most common denominators of toxicity [45]. Evaluation of total antioxidant capacity in the presence of paraquat showed induction of oxidative stress in the face of all doses tested (Chart 1). There was no significant difference in total antioxidant capacity in the face of different doses of paraquat, but the greatest decrease in antioxidant capacity was observed in the face of 200 mg/kg b.w of PQ and in the exposure of 50 mg/kg b.w of PQ 6 times in three weeks (Group E). Despite the increase in total paraquat received, total antioxidant capacity did not decrease more than exposure to 200 mg/kg b.w of PQ, which could be due to the large distance between exposure times and activation of the mechanism of adaptation to oxidative stress [46]. Petrovská, et al. made similar observations in their study, the results of which confirmed the finding that paraquat induces oxidative stress, but also stimulates antioxidant protection within a certain concentration range [47].
A major consequence of oxidative stress is DNA damage [48]. DNA is known to be frequently damaged in cells exposed to reactive oxygen species [49]. DNA damage is any change in the structure of DNA that changes its encoding properties or interferes with the cell cycle [48]. The comet method is a sensitive and rapid method for detecting DNA strand breakage in single cells [50]. The present study in vivo showed that paraquat can cause DNA damage in rat lymphocyte cells in the form of strand breakage. The increase in the olive moment and % DNA in tail was significant at doses of 60 mg/kg b.w of PQ three times (C), 200 mg/kg b.w of PQ once (D) and 50 mg/kg b.w of PQ 6 times in three weeks (E) compared to the negative control group but insignificant at 30 mg/kg b.w of PQ three times (Chart 2). The increase in the olive moment and %DNA in tail in Group C compared to Group B is significant and shows a dose-dependent trend of DNA damage.
Ross et al. also observed a dose-dependent trend of paraquat DNA damage in vitro in mammalian cells [13]. A comparison of Groups D (200 mg/kg b.w of PQ once) and E (50 mg/kg b.w of PQ, 6 times in three weeks) showed that exposure to paraquat reduced its effectiveness in the long run, and Group E received a total of 300 mg/kg b.w not only did not increase olive moment and % DNA in tail compared to 200 mg/kg b.w of PQ but also reduced to some extent. Petrovská et al. also observed that exposure to lower concentrations of paraquat for 1 h caused dose-dependent DNA damage in Hep G2 cells and human peripheral lymphocytes, but that DNA damage was reduced at higher concentrations. No reduction in DNA damage was found in HeLa cells after exposure for 1–24 h [47].
It should be noted that the extracellular activity of lactate dehydrogenase increases under oxidative stress because cell integrity can be disrupted during the lipid peroxidation process [34]. One of the main mechanisms of paraquat poisoning is lipid peroxidation of the cell membrane and degradation of cell membrane structure [51]. In the present study, the integrity of the cell membrane in presence of PQ was investigated by the LDH method as a result of cell lysis. The serum LDH at doses of 60 mg/kg b.w of PQ three times (C) and 200 mg/kg b.w of PQ once (D) showed a significant increase compared to the control group (A). Also at doses of 30 mg/kg b.w of PQ once daily for three days (C) and 50 mg/kg b.w of PQ 6 times in three weeks (E), LDH showed an increasing trend (Chart 3) compared to the control group.
The highest oxidative stress, DNA damage, and loss of cell membrane integrity occurred in Group E (200 mg/kg b.w of PQ once), indicating that paraquat had the highest toxicity to lymphocytes in acute exposure. PQ generates free oxygen radicals in combination with nicotinamide adenine dinucleotide phosphate in a single-electron cycle of reduction/redox reaction, followed by cell death due to lipid peroxidation of the cell membrane [52]. Evidence from the present study showed that paraquat-induced oxidative stress caused cell membrane degradation and DNA strand breakage, but whether DNA damage and cell membrane degradation are relevant needs further studies. In a study, Alberto et al. provided direct evidence that oxidative DNA damage disrupted the integrity of human cell membranes by disrupting the expression of the cytoskeleton gene [53]. This hypothesis may also be true for paraquat that, in addition to lipid peroxidation, changes in gene expression contribute to the process of cell membrane degradation.
Sue Kyung et al. observed a significant relevance between the risk of non-Hodgkin's lymphoma (NHL) and paraquat use. Also, they considered the occurrence of chromosomal aberrations and gene mutations observed in human lymphocytes exposed to paraquat in vitro as one of the possible mechanisms [26]. DNA damage observed in our study could also confirm this.
The results of Hoechst staining showed that exposure to paraquat could significantly kill lymphocyte cells by inducing apoptosis in vivo in rats (Chart 4). Hoechst staining at the early stages of apoptosis as nuclei is compacted and as it progresses, it can be seen as fragmented nuclei.
Flow cytometry with PI shows only apoptosis at the late stages and necrosis. PI is a dye impenetrable to the cell membrane, so at the early and middle stages of apoptosis, when the cell membrane is integrated, it is not possible to enter the cell and bind it to DNA. Flow cytometry with PI in this study showed that paraquat caused death and toxic granulation in some living cells (Figure 3). For Hoechst staining, Group E, received the highest dose in total, was compared with the negative control group (A), but after analyzing the data, the highest oxidative stress and DNA damage in a single exposure to 200 mg/kg b.w of PQ was found. It seems that lung damage is due to the extensive production of reactive oxygen species such as superoxide anion, hydroxyl, and peroxide radicals by PQ in the lung parenchyma, which rapidly overcame any antioxidant defense [54]. Lung tissue pathology examination in this study shows a strong effect of paraquat on the lungs in all experimental groups. Inflammation and hyaline membrane in lung tissue were analyzed semi-quantitatively, which increased significantly in all groups compared to the control group (Chart 5).
Paraquat is not actively metabolized in the body and more than 90% is excreted unchanged through the kidneys [17]. In this study, atrophy of glomeruli and renal tubular lesions were observed, which is consistent with the observations of Eatemad A et al. who observed glomerular atrophy, degradation, and necrosis of renal tubules [55]. Dehong et al. observed the activation of lysosomal proteases associated with acute kidney damage in the presence of paraquat. Oxidative stress and inflammation are closely related. Oxidative stress and ROS cause inflammation through immune detection by molecules released from dying cells. Long-term expression of inflammatory mediators leads to increased production and activation of lysosomal proteases that split collagen, elastin, and proteins of proteoglycan nuclei by serine and metalloproteinases, leading to irreversible tissue damage [56].
The liver is another major target of paraquat poisoning [57]. Examination of liver tissue showed several cases of necrosis and inflammation and in all groups, the mean score assigned to liver inflammation increased compared to the control group (A). This increase showed a significant difference between the group exposed to 200 mg/kg b.w of PQ for one time (group D) and the control group (A). Eatemad et al. also investigated the pathological changes of paraquat in liver and kidney tissue and observed necrosis in some liver cells [55].
5. Conclusion
Paraquat induced oxidative stress and activated antioxidant protection. This oxidative stress induced breaking DNA strands in peripheral lymphocytes in vivo. Evaluation of serum lactate dehydrogenase level showed loss of cell membrane integrity in the presence of paraquat. Besides, LDH levels showed oxidative stress which caused DNA damage and cell death. Regarding the widespread side effects of this toxin, especially oxidative stress and DNA damage that can be effective on carcinogenesis, it is suggested to make and/or introduce more appropriate and less dangerous alternatives instead of this pesticide to the process of banning the use of this substance with the assistance of the people current consumers.
Declarations
Author contribution statement
Soheila Alizadeh: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper
Gholamreza Anani Sarab: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Hoda Amiri: Analyzed and interpreted the data.
Majid Hashemi: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
Dr Majid Hashemi was supported by Kerman University of Medical Sciences [99000419].
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
This research is the result of a master's thesis in Environmental toxicology with project code: 99000419 and Ethics Code: IR.KMU.REC.1399.471. This research was supported by the Vice-Chancellor for Research and Technology of Kerman University of Medical Sciences, Kerman, Iran.
Contributor Information
Gholamreza Anani-sarab, Email: ghansa@yahoo.com.
Majid Hashemi, Email: mhashemi120@gmail.com.
References
- 1.Khazraei S., Marashi S.M., Sanaei-Zadeh H. Ventilator settings and outcome of respiratory failure in paraquat-induced pulmonary injury. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-52939-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hawkes T.R. Mechanisms of resistance to paraquat in plants. Pest Manag. Sci. 2014;70(9):1316–1323. doi: 10.1002/ps.3699. [DOI] [PubMed] [Google Scholar]
- 3.Kermani M., et al. Using H2O2-based photochemical oxidation (UV/H2O2) in eliminating paraquat from aqueous solutions. J. North Khorasan Univ. Med. Sci. 2018;10(1):36–45. [Google Scholar]
- 4.Roberts T.R., Dyson J.S., Lane M.C. Deactivation of the biological activity of paraquat in the soil environment: a review of long-term environmental fate. J. Agric. Food Chem. 2002;50(13):3623–3631. doi: 10.1021/jf011323x. [DOI] [PubMed] [Google Scholar]
- 5.Huang Y., et al. Paraquat degradation from contaminated environments: current achievements and perspectives. Front. Microbiol. 2019;10:1754. doi: 10.3389/fmicb.2019.01754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oghabian Z., et al. Clinical features, treatment, prognosis, and mortality in paraquat poisonings: a hospital-based study in Iran. J. Res. Pharm. Pract. 2019;8(3):129–136. doi: 10.4103/jrpp.JRPP_18_71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J., et al. Identify the early predictor of mortality in patients with acute paraquat poisoning. BioMed Res. Int. 2020;2020 doi: 10.1155/2020/8894180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sartori F., Vidrio E. Environmental fate and ecotoxicology of paraquat: a California perspective. Toxicol. Environ. Chem. 2018;100(5-7):479–517. [Google Scholar]
- 9.Ranjbar A., et al. Role of cerium oxide nanoparticles in a paraquat-induced model of oxidative stress: emergence of neuroprotective results in the brain. J. Mol. Neurosci. 2018;66(3):420–427. doi: 10.1007/s12031-018-1191-2. [DOI] [PubMed] [Google Scholar]
- 10.Liu B., et al. Protective mechanism of 1-methylhydantoin against lung injury induced by paraquat poisoning. PLoS One. 2019;14(9):e0222521. doi: 10.1371/journal.pone.0222521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao L., et al. Toxicology of paraquat and pharmacology of the protective effect of 5-hydroxy-1-methylhydantoin on lung injury caused by paraquat based on metabolomics. Sci. Rep. 2020;10(1):1790. doi: 10.1038/s41598-020-58599-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omura T., et al. Sodium tauroursodeoxycholate prevents paraquat-induced cell death by suppressing endoplasmic reticulum stress responses in human lung epithelial A549 cells. Biochem. Biophys. Res. Commun. 2013;432(4):689–694. doi: 10.1016/j.bbrc.2013.01.131. [DOI] [PubMed] [Google Scholar]
- 13.Ross W.E., Block E.R., Chang R.-Y. Paraquat-induced DNA damage in mammalian cells. Biochem. Biophys. Res. Commun. 1979;91(4):1302–1308. doi: 10.1016/0006-291x(79)91208-7. [DOI] [PubMed] [Google Scholar]
- 14.Pisoschi A.M., Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur. J. Med. Chem. 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Z., et al. Klotho alleviates lung injury caused by paraquat via suppressing ROS/P38 MAPK-regulated inflammatory responses and apoptosis. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/1854206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu X., et al. Increased plasma prothrombin time is associated with poor prognosis in patients with paraquat poisoning. J. Clin. Lab. Anal. 2018;32(9):e22597. doi: 10.1002/jcla.22597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sittipunt C. Paraquat poisoning. Respir. Care. 2005;50(3):383. [PubMed] [Google Scholar]
- 18.Chang Z.-S., et al. Forkhead box O3 protects the heart against paraquat-induced aging-associated phenotypes by upregulating the expression of antioxidant enzymes. Aging Cell. 2019;18(5):e12990. doi: 10.1111/acel.12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.http://www.pic.int/TheConvention/Chemicals/Recommendedforlisting/Paraquatdichloride
- 20.Wesseling C., et al. Cancer in banana plantation workers in Costa Rica. Int. J. Epidemiol. 1996;25(6):1125–1131. doi: 10.1093/ije/25.6.1125. [DOI] [PubMed] [Google Scholar]
- 21.Monge P., et al. Parental occupational exposure to pesticides and the risk of childhood leukemia in Costa Rica. Scand. J. Work. Environ. Health. 2007;33(4):293–303. doi: 10.5271/sjweh.1146. [DOI] [PubMed] [Google Scholar]
- 22.Lee W.J., et al. Agricultural pesticide use and risk of glioma in Nebraska, United States. Occup. Environ. Med. 2005;62(11):786–792. doi: 10.1136/oem.2005.020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bae I., et al. BRCA1 induces antioxidant gene expression and resistance to oxidative stress. Cancer Res. 2004;64(21):7893–7909. doi: 10.1158/0008-5472.CAN-04-1119. [DOI] [PubMed] [Google Scholar]
- 24.Jee S.H., et al. Photodamage and skin cancer among paraquat workers. Int. J. Dermatol. 1995;34(7):466–469. doi: 10.1111/j.1365-4362.1995.tb00611.x. [DOI] [PubMed] [Google Scholar]
- 25.Wesseling C., et al. Geographical differences of cancer incidence in Costa Rica in relation to environmental and occupational pesticide exposure. Int. J. Epidemiol. 1999;28(3):365–374. doi: 10.1093/ije/28.3.365. [DOI] [PubMed] [Google Scholar]
- 26.Park S.K., et al. Cancer incidence among paraquat exposed applicators in the agricultural health study: prospective cohort study. Int. J. Occup. Environ. Health. 2009;15(3):274–281. doi: 10.1179/oeh.2009.15.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferri G.M., et al. Risk of lymphoma subtypes by occupational exposure in Southern Italy. J. Occup. Med. Toxicol. 2017;12(1):31. doi: 10.1186/s12995-017-0177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Abidin Salam A.Z., et al. The mutagenicity of Gramoxone (paraquat) on different eukaryotic systems. Mutat. Res. Genet. Toxicol. 1993;319(2):89–101. doi: 10.1016/0165-1218(93)90067-n. [DOI] [PubMed] [Google Scholar]
- 29.Ribas G., et al. Genotoxic evaluation of the herbicide paraquat in cultured human lymphocytes. Teratog. Carcinog. Mutagen. 1997;17(6):339–347. [PubMed] [Google Scholar]
- 30.Poljsak B., Šuput D., Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid. Med. Cell. Longev. 2013;2013 doi: 10.1155/2013/956792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubio C.P., et al. Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum: an update. BMC Vet. Res. 2016;12(1):166. doi: 10.1186/s12917-016-0792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benzie I.F., Choi S.W. Antioxidants in food: content, measurement, significance, action, cautions, caveats, and research needs. Adv. Food Nutr. Res. 2014;71:1–53. doi: 10.1016/B978-0-12-800270-4.00001-8. [DOI] [PubMed] [Google Scholar]
- 33.Mozaffarieh M., et al. Comet assay analysis of single-stranded DNA breaks in circulating leukocytes of glaucoma patients. Mol. Vis. 2008;14:1584–1588. [PMC free article] [PubMed] [Google Scholar]
- 34.Jovanovic P., et al. Lactate dehydrogenase and oxidative stress activity in primary open-angle glaucoma aqueous humour. Bosn. J. Basic Med. Sci. 2010;10(1):83–88. doi: 10.17305/bjbms.2010.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farhana A., Lappin S.L. StatPearls. StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC; Treasure Island (FL: 2021. Biochemistry, lactate dehydrogenase. [Google Scholar]
- 36.Atale N., et al. Cell-death assessment by fluorescent and nonfluorescent cytosolic and nuclear staining techniques. J. Microsc. 2014;255(1):7–19. doi: 10.1111/jmi.12133. [DOI] [PubMed] [Google Scholar]
- 37.Crowley L.C., Marfell B.J., Waterhouse N.J. Analyzing cell death by nuclear staining with Hoechst 33342. Cold Spring Harb. Protoc. 2016;2016(9) doi: 10.1101/pdb.prot087205. [DOI] [PubMed] [Google Scholar]
- 38.Abedini M.R., et al. Possible role of FLICE-like inhibitory protein (FLIP) in chemoresistant ovarian cancer cells in vitro. Oncogene. 2004;23(42):6997–7004. doi: 10.1038/sj.onc.1207925. [DOI] [PubMed] [Google Scholar]
- 39.Crowley L.C., et al. Measuring cell death by propidium iodide uptake and flow cytometry. Cold Spring Harb. Protoc. 2016;2016(7) doi: 10.1101/pdb.prot087163. [DOI] [PubMed] [Google Scholar]
- 40.Nelson C., et al. Therapeutic efficacy of esomeprazole in cotton smoke-induced lung injury model. Front. Pharmacol. 2017;8(16) doi: 10.3389/fphar.2017.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pourfathi M., et al. Lung metabolism and inflammation during mechanical ventilation; an imaging approach. Sci. Rep. 2018;8(1):3525. doi: 10.1038/s41598-018-21901-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen J., et al. Lysyl oxidases expression and histopathological changes of the diabetic rat nephron. Mol. Med. Rep. 2018;17(2):2431–2441. doi: 10.3892/mmr.2017.8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun P., et al. Clusterin deficiency predisposes C57BL/6j mice to cationic bovine serum albumin-induced glomerular inflammation. J. Inflamm. Res. 2020;13:969–983. doi: 10.2147/JIR.S285985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martino A.T., et al. Tolerance induction to cytoplasmic beta-galactosidase by hepatic AAV gene transfer: implications for antigen presentation and immunotoxicity. PLoS One. 2009;4(8):e6376. doi: 10.1371/journal.pone.0006376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gagné F. In: Biochemical Ecotoxicology. Gagné F., editor. Academic Press; Oxford: 2014. Chapter 6 - oxidative stress; pp. 103–115. [Google Scholar]
- 46.Pickering A.M., et al. Oxidative stress adaptation with acute, chronic, and repeated stress. Free Radic. Biol. Med. 2013;55:109–118. doi: 10.1016/j.freeradbiomed.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petrovská H., Dušinská M. Oxidative DNA damage in human cells induced by paraquat. Altern Lab Anim. 1999;27(3):387–395. doi: 10.1177/026119299902700314. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez-Hunt C., Wadhwa M., Sanders L. DNA damage by oxidative stress: measurement strategies for two genomes. Curr. Opin. Toxicol. 2018;7 [Google Scholar]
- 49.Demirbag R., Yilmaz R., Kocyigit A. Relationship between DNA damage, total antioxidant capacity and coronary artery disease. Mutat. Res. 2005;570(2):197–203. doi: 10.1016/j.mrfmmm.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Liao W., McNutt M.A., Zhu W.G. The comet assay: a sensitive method for detecting DNA damage in individual cells. Methods. 2009;48(1):46–53. doi: 10.1016/j.ymeth.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 51.Kavousi-Gharbi S., et al. Discernment scheme for paraquat poisoning: a five-year experience in Shiraz, Iran. World J. Exp. Med. 2017;7(1):31–39. doi: 10.5493/wjem.v7.i1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marashi S.M., et al. One-lung circumvention, an interventional strategy for pulmonary salvage in acute paraquat poisoning: an evidence-based review. Tzu Chi Med. J. 2015;27(3):99–101. [Google Scholar]
- 53.Rubfiaro A.S., et al. Scanning ion conductance microscopy study reveals the disruption of the integrity of the human cell membrane structure by oxidative DNA damage. ACS Appl. Bio Mater. 2021;4(2):1632–1639. doi: 10.1021/acsabm.0c01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buendía J.A., et al. Comparison of four pharmacological strategies aimed to prevent the lung inflammation and paraquat-induced alveolar damage. BMC Res. Notes. 2019;12(1):584. doi: 10.1186/s13104-019-4598-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Awadalla E.A. Efficacy of vitamin C against liver and kidney damage induced by paraquat toxicity. Exp. Toxicol. Pathol. 2012;64(5):431–434. doi: 10.1016/j.etp.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 56.Tan D., et al. Betanin attenuates oxidative stress and inflammatory reaction in kidney of paraquat-treated rat. Food Chem. Toxicol. 2015;78:141–146. doi: 10.1016/j.fct.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 57.Liu Z., et al. Hydrogen Sulfide Protects against Paraquat-Induced Acute Liver Injury in Rats by Regulating Oxidative Stress, Mitochondrial Function, and Inflammation. Oxid. Med. Cell. Longev. 2020 doi: 10.1155/2020/6325378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.