Abstract
Rationale
The legalization of medicinal use of Cannabis sativa in most US states and the removal of hemp from the Drug Enforcement Agency (DEA) controlled substances act has resulted in a proliferation of products containing Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) for oral consumption (e.g., edibles, oils and tinctures) that are being used for recreational and medicinal purposes.
Objective
This study examined the effects of cannabinoids THC and CBD when administered orally on measures of pain sensitivity, body temperature, locomotor activity, and catalepsy (i.e., cannabinoid tetrad) in male and female Sprague Dawley rats.
Methods
Rats (N=24, 6 per sex/drug group) were administered THC (1-20 mg/kg), CBD (3-30 mg/kg), or sesame oil via oral gavage. Thermal and mechanical pain sensitivity (tail flick assay, von Frey test), rectal measurements for body temperature, locomotor activity, and the bar-test of catalepsy were completed. A separate group of rats (N=8/4 per sex) were administered morphine (5-20 mg/kg; intraperitoneal, IP) and evaluated for pain sensitivity as a positive control.
Results
We observed classic tetrad effects of antinociception, hypothermia, hyper- and hypolocomotion, and catalepsy after oral administration of THC that were long lasting (>7 hours). CBD modestly increased mechanical pain sensitivity and produced sex-dependent effects on body temperature and locomotor activity.
Conclusions
Oral THC and CBD produced long lasting effects, that differed in magnitude and time course when compared with other routes of administration. Examination of cannabinoid effects administered via different routes of administration, species, and in both males and females is critical to enhance translation.
Keywords: THC, Cannabidiol, Oral administration, Cannabinoids, Tetrad, Antinociception
Introduction
Changes in federal regulation of hemp, and legalization of medicinal use of Cannabis sativa and its constituents in the majority of US states has resulted in a proliferation of cannabis products. Oral formulations of the cannabinoids Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) are common for people using cannabis medicinally (e.g. Dronabinol/Marinol™, Epidiolex) and oral cannabis-derived products are widely marketed (e.g., edibles, oils and tinctures)(Spindle et al., 2019).
Preclinical research into cannabinoid effects has been ongoing for decades, with a majority of research to date employing injection methods (e.g., intraperitoneal, IP; subcutaneous, SC; intravenous, IV) for drug administration. Some of the foundational preclinical research into cannabinoids came from the development of the classic tetrad test, where behavioral effects of THC and synthetic cannabinoid receptor 1 (CB1R) agonists were linked with their pharmacological activity on CB1R. The test includes measures of pain sensitivity, body temperature, locomotor activity, and catalepsy. IP, IV, and SC administration of THC and other CB1R agonists produce phenotypic responses of antinociception, hypothermia, hypolocomotion, and catalepsy, and these effects are blocked with CB1 antagonists (Compton et al., 1993; Martin et al., 1991; Metna-Laurent et al., 2017). Studies to date have demonstrated that classic tetrad effects are observed in both rats and mice, though sex differences in the effects of THC can differ with respect to species (Wiley et al., 2021) and route of administration (Moore et al., 2021).
Several rat studies have used oral administration to investigate outcomes in the cannabinoid tetrad, such as antinociception or hypolocomotion, but assays were conducted at discrete timepoints (Hlozek et al., 2017; Rohleder et al., 2020; Sofia et al., 1975), and findings are somewhat limited. To our knowledge, no studies have yet examined the time course of all tetrad effects following oral administration of THC in male and female rats. Historically, most studies using the cannabinoid tetrad have been conducted with mice (Metna-Laurent et al., 2017), but similarly, few studies to date have utilized oral routes of administration in mice. Further, species differences have been observed with respect to tetrad outcomes, sex differences in cannabinoid effects, as well as in cannabinoid metabolism (Wiley et al., 2021; Wiley and Burston, 2014) that require extension of evaluations in mice into rat models. As oral routes of administration are increasingly being used for cannabinoid exposure in animal models, data on the classic behavioral tetrad provide the basis for selection of pretreatment times, and optimal dosing to assess behavioral outcomes in rat models.
Use of an oral route of administration is important from a translational approach. While IP injection and oral administration both undergo first pass hepatic metabolism, there are pharmacokinetic differences. When taken orally, THC is slowly and erratically absorbed (Grotenhermen, 2003; Newmeyer et al., 2017; Wall et al., 1983). A pharmacokinetic study of oral, subcutaneous injection, and vapor administration in rats found that oral administration resulted in long-lasting levels of THC in serum and brain, and the highest brain levels of THC compared to other routes of administration (Hlozek et al., 2017). The pharmacokinetic parameters of oral consumption of THC are further affected by the formulation (solution vs. capsule) and food (high fat food vs. fasted state). Further, cannabinoid receptors are distributed throughout the gastrointestinal tract and some cannabinoid effects may be mediated peripherally. Therefore, this study sought to characterize the tetrad effects of THC (0-20 mg/kg) administered orally and in a high fat vehicle in male and females rats over multiple hours post-administration. We also assessed the effects of oral CBD (0-30 mg/kg) on three of the four tetrad behaviors (antinociception, hyperthermia, locomotor activity). Cataleptic behavior was not assessed in animals treated with CBD, as prior studies demonstrated that CBD does not induce catalepsy, but instead has anticataleptic effects (Gomes et al., 2013).
Materials and Methods
Subjects
Adult male and female Sprague Dawley rats (Charles River, Wilmington, MA) were single housed in wire-topped, plastic cages (27 × 48 × 20 cm) with standard enrichment. The vivarium was on a 12hr reverse light/dark cycle (lights off at 9:00 a.m.) and was humidity and temperature controlled. Rats were maintained at 90% of their free feeding weight throughout the experiments; food was given at the same time each day or after tests with drug or vehicle administration were completed on test days. Diet was a corn-based chow (Teklad Diet 2018; Harlan, Indianapolis, IN) and rats had free access to water except during test procedures. All procedures used in this study were approved by the Johns Hopkins Institutional Animal Care and Use Committee. The facilities adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were AAALAC-approved.
Drugs
(−)-trans-delta9-tetrahydrocannabinol (THC; 200 mg/ml in USP ethyl alcohol 95%) and cannabidiol (synthetic) were provided by the National Institute on Drug Abuse (NIDA) Drug Supply Program. THC and CBD were mixed with 100% sesame oil using sonication and vortex for an oral suspension. Sesame oil was used as it dissolves lipid-soluble cannabinoids for increased bioavailability, and is utilized in pharmaceutical oral formulations of THC and CBD (e.g., Marinol and Epidiolex) (Zgair et al., 2016). THC (1, 3, 5.6, 10, and 20 mg/ml), CBD (3, 10, and 30 mg/ml), and the sesame oil vehicle were administered via oral gavage at a volume of 1 ml/kg. Morphine sulfate (Sigma Aldrich, St. Louis, MO, USA) was dissolved in 0.9% sterile saline for final doses of 5, 10, and 20 mg/ml, and was administered at 1 ml/kg via IP injection; morphine doses were calculated based on the salt.
Study design
The effects of administration of THC or CBD via oral gavage (p.o.) were tested in male and female rats (N=24, 6 per sex/drug group). Separate groups of rats were used for THC and CBD tests. Treatments and behavioral tests were conducted once each week, with a minimum of 7 days between each THC/CBD dose. Vehicle treatments and tests were interspersed throughout the treatment period to assess carry-over drug effects and control for possible baseline shifts over time. THC was administered in a blinded, within subject Latin square design (1-20 mg/kg). THC doses were based on prior studies in our lab using i.p. injection. CBD (3-30 mg/kg) was administered in descending order, based on a dose finding strategy (see discussion). The total testing period was 9 and 5 weeks for THC and CBD, respectively. Behavioral testing occurred at baseline (pre-treatment) and at a series of time points for up to 7 hours following treatment with THC, CBD, or vehicle.
In a separate group of rats (N=8, 4 per sex), the effects of morphine (5-20 mg/kg) administered via IP injection were evaluated as a positive control for comparison of pain sensitivity outcomes. Morphine was administered in a blinded, within subject Latin square design (0-20 mg/kg) with testing occurring once per week, with a minimum 7 days apart. The total morphine testing period was 4 weeks.
Cannabinoid Tetrad Test Battery
Antinociception (thermal pain sensitivity): Tail Flick Assay
Thermal pain sensitivity was assessed using the tail flick (TF) assay. In this test, the distal end of the rat’s tail (~50 mm from the tip) is exposed to radiant heat from a precise photobeam (Harvard Apparatus, Cambridge, MA, USA) and latency (s) to respond to the heat stimulus by flexion of the tail is recorded. The maximum duration was limited to 10s. Prior to testing, the radiant heat setting was calibrated to achieve a group average baseline latency of 5s. Baseline TF latencies were obtained immediately prior to drug administration and averaged across test weeks. Antinociception was calculated as percent of maximum possible effect (% MPE= [(test TF latency– baseline TF latency)/(maximum TF latency – baseline TF latency)] x 100). Rats were tested at 3 time points post oral administration: 60 min, 120 min, and 300 min for THC, CBD, and vehicle. A separate group of animals were tested in the tail flick assay 30 min after i.p. administration of vehicle or morphine (5-20 mg/kg) as a positive control.
Antinociception (mechanical pain sensitivity): von Frey Test
The von Frey test was used to assess drug effects on mechanical pain sensitivity. Rats were placed in clear plastic cubicles on an elevated screen platform. Each hind paw is probed with von Frey filaments (9 filaments, 0.6-15.0 g, beginning with 2.0g) on the plantar surface for 3s. The presence or absence of a response (nocifensive hind paw flexion reflex) is recorded. If no response occurs, a stronger stimulus is presented otherwise the next weaker stimulus is applied. This up-down process is repeated 4 times after the first change in response, and the 50% threshold for paw withdrawal is determined by the individual response pattern and the force of the last von Frey filament tested (Chaplan et al., 1994; Dixon, 1991). Antinociception is demonstrated by an increase in the 50% mechanical withdrawal threshold. Baseline thresholds were obtained immediately prior to drug administration and averaged across test weeks. Antinociception was calculated as percent of maximum possible effect (% MPE= [(test threshold– baseline threshold)/(maximum threshold – baseline threshold)] x 100). Rats were tested at 3 time points post-drug administration, following the tail flick assay: 75 min, 135 min, and 315 min for THC, CBD, and vehicle. A separate group of animals were tested in the von Frey assay 45 min after i.p. administration of vehicle or morphine (5-20 mg/kg) as a positive control.
Locomotor Activity
Rats were placed in standard activity chambers (San Diego Instruments Inc.) where automated activity data was collected using a 4 x16 photobeam array. Tests were conducted in the dark with a white noise machine. Locomotor chambers interfaced with a computer running Photobeam Activity System (PAS) software that automatically recorded all beam interruptions, central peripheral activity, ambulation movements, fine movements, and time stamped (x,y) positions. Locomotor tests were 10-min in duration and occurred at 30, 150, and 270 min post oral administration of THC, CBD, or vehicle.
Catalepsy: Bar Test
Catalepsy, or immobilization, was tested using a bar test (Sanberg et al., 1988). Each individual chamber contained a bar apparatus 12 cm high and 5 mm in diameter (Med Associates, St. Albans, VT). Rats were placed with both front paws in contact with the bar. Experimenters blinded to the treatment condition timed the duration of contact with the bar, stopping the trial once both paws were removed. Total time (seconds) spent in contact with the bar, up to a maximum 180s was recorded. Trials were repeated after 2-min for a total of 3 trials per test. An average time immobile across the 3 trials was used as a measure of catalepsy. Catalepsy was measured at baseline, 90-min, and 330-min post oral administration of THC or vehicle.
Body temperature
Body temperature was determined with a digital rectal thermometer with a lubricated flexible probe across 3-5 time points on the test day. Temperature was measured at 60, 120, 210, 300, and 420 min after THC or vehicle administration, or at 60, 120, 300 min after CBD or vehicle administration.
Statistical Analysis
Thermal and mechanical pain sensitivities were calculated as percent of maximum possible effect (% MPE= [(test threshold- baseline threshold)/(maximum threshold – baseline threshold)] x 100). Maximum thresholds for the tail flick and von Frey assays were 10 and 15, respectively. An average of baseline tests were used in these calculations. Locomotor activity was calculated as a percent change from each animal’s average of weekly vehicle tests. Time immobile (s) in the catalepsy test was logarithmically transformed (ln) prior to analysis (Ferre et al., 1990). Outcome measures analyzed included: %MPE, body temperature change (°C), locomotor activity distance traveled (% of vehicle), and time immobile (ln(s)) in the catalepsy test. Three-way repeated measures ANOVAs were conducted with sex as a between subjects variable and dose and time as within subject variables; for interpretation of interactions with time, Bonferroni post-hoc tests were used. Follow up two-way ANOVAs (sex as a between subjects variable and dose as a within subject variable) were conducted within each time point. Dunnett’s post hoc tests were used to analyze differences in outcomes between THC or CBD dose/condition and vehicle. In the event of a main effect or interaction with sex, post-hoc comparisons between males and females were determined with Sidak’s test. Post-hoc results with sexes collapsed are reported in the text, figures show the average data as well as points for males and females separately. ED50 values were calculated with nonlinear regression using data from the time point where maximum effects were observed. All statistics were performed with Statistica 11 and Graphpad Prism 9.
Results
Effects of oral THC
Thermal Pain Sensitivity
Oral THC administration produced antinociception in the tail flick assay (Fig. 1A) as confirmed by a significant main effect of THC dose (F(5, 45)= 6.67, p<0.001). There was also a main effect of time (F(2, 18) =8.13, p<0.01), as %MPE increased across the testing period, and an interaction of time × sex (F(2, 18) =4.15, p<0.05). The average %MPE of male rats increased over the time course (p’s<0.05) while female %MPE was equivalent across the 3 tests. When analyzed within each time point, THC (5.6-20 mg/kg) produced thermal antinociceptive effects at 120-min (F(3.47, 40.91)= 4.27, p<0.01) and 300 min (F(2.46, 24.07)= 6.11, p<0.01).
Figure 1.
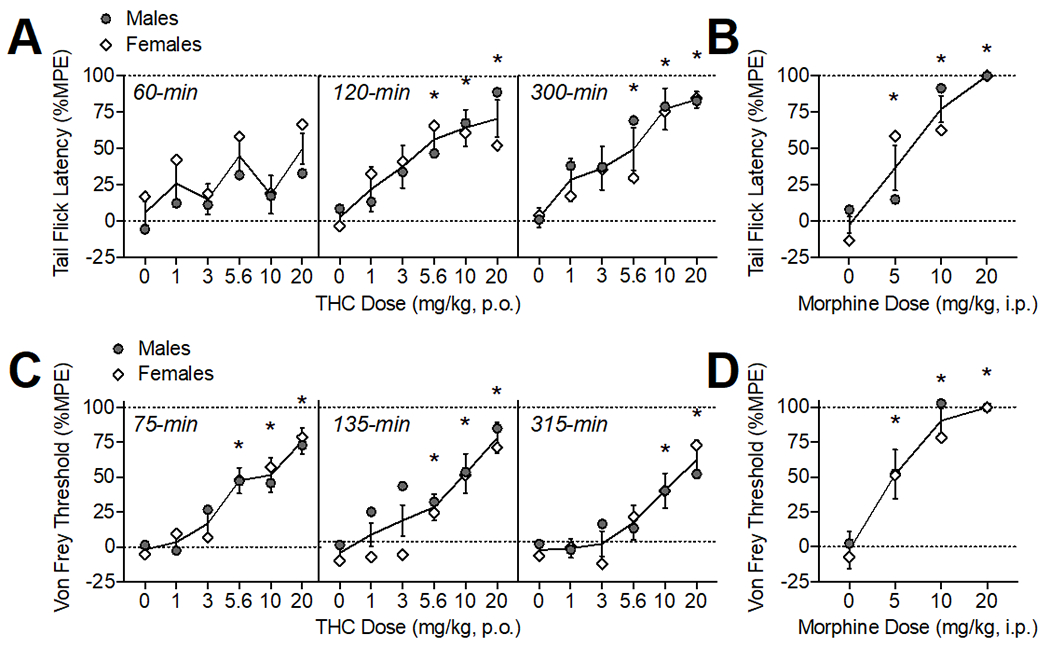
Oral THC effects on thermal (A) and mechanical (C) pain sensitivity. Morphine (i.p.) comparison (B, D). The time of data collection after oral administration is noted in italics. Asterisks (*) represent a significant difference from vehicle. Data are Mean ±SEM; N=6/sex for THC, N=4/sex for morphine.
Mechanical Pain Sensitivity
Oral THC administration produced antinociception in the von Frey test (Fig. 1B) as confirmed by a significant main effect of THC dose (F(5, 50) =3.39, p<0.05). There was also a main effect of time (F(2, 20) =11.43, p<0.001), an interaction of time × sex (F(2, 20) =4.81, p<0.05), and an interaction of THC dose × sex (F(10, 100) =4.51, p<0.001). Von Frey thresholds (%MPE) overall were declining by 300-min compared to the first test (p<0.05), particularly in females, indicating effects returning to baseline. When analyzed within each time point, THC (5.6-20 mg/kg) produced significant mechanical antinociceptive effects at 75-min, (F(3.67, 36.69)= 12.51, p<0.001) and 135-min post-administration (F(3.19, 31.90)= 10.54, p<0.001). At 315-min, the highest doses of THC (10-20 mg/kg) continued to show mechanical antinociceptive effects (F(3.45, 34.49)= 8.153, p<0.001).
Body Temperature
Oral THC administration reduced body temperature dose-dependently (Fig. 2) as confirmed by a main effect of THC dose (F(5, 50)= 10.84, p<0.001), time (F(4, 40)= 13.40, p<0.001), and an interaction of THC dose × time (F(20, 200)= 2.44, p<0.001). When analyzed within each time point, oral THC decreased body temperature at 120-min (20 mg/kg), 210-min (10-20 mg/kg), 300 (1, 5.6-20 mg/kg), and 420-min (3, 10-20 mg/kg) post-administration (F’s between 4.18-11.43, p’s<0.05). There were main effects of sex at 120, 210, and 300 (F’s (1,10) = between 9.93-14.63); under vehicle conditions, males showed slightly increasing temperatures (+0.14-0.19°C) throughout the course of the testing period compared to females (−0.04-0.06°C ; p’s<0.05). At 420-min post oral THC, there was an interaction of THC dose and sex (F(5, 50)= 2.87, p<0.05), though post-hoc tests did not isolate sex differences to any specific THC dose.
Figure 2.
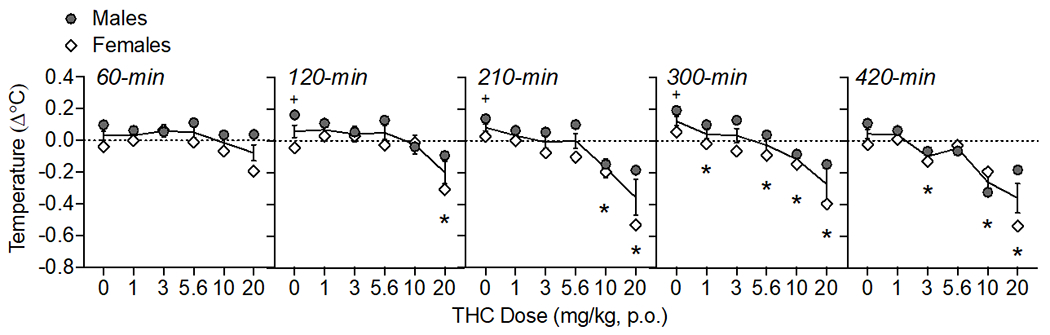
Oral THC effects on body temperature change from baseline (Δ°C). The time of data collection after oral administration is noted in italics. Asterisks (*) represent a significant difference from vehicle. Plus sign (+) indicates a sex difference. Data are Mean ±SEM; N=6/sex.
Locomotor Activity
THC modulated locomotor activity over the testing period (Fig. 3) as confirmed by a main effect of THC dose (F(5, 50)= 3.38, p<0.05) and time (F(2,20)= 11.81, p<0.001). There were also interactions of THC dose × time (F(10, 100)= 4.60, p<0.001) and time × sex (F(2, 20)= 4.54, p<0.05). When collapsed across dose to assess the time × sex interaction, females’ activity was lower at the last time point (270-min) compared to the first time point (30-min; p<0.05). At 30-min post oral administration, THC increased locomotor activity at 5.6 and 20 mg/kg (F(3.29, 32.93)= 6.61, p<0.01). There was an effect of sex at 30-min (F (1, 10)= 5.77, p<0.05), with females showing higher activity than males after 10 mg/kg THC (p<0.05). At 150-min post oral THC administration, there was a main effect of THC dose on locomotor activity (F(3.01, 30.13)= 2.86, p=0.05), though post-hoc tests did not indicate any specific doses were different than vehicle. By 270-min, THC reduced locomotor activity at 3 and 5.6 mg/kg (F(3.47, 34.74)= 3.57, p<0.05).
Figure 3.
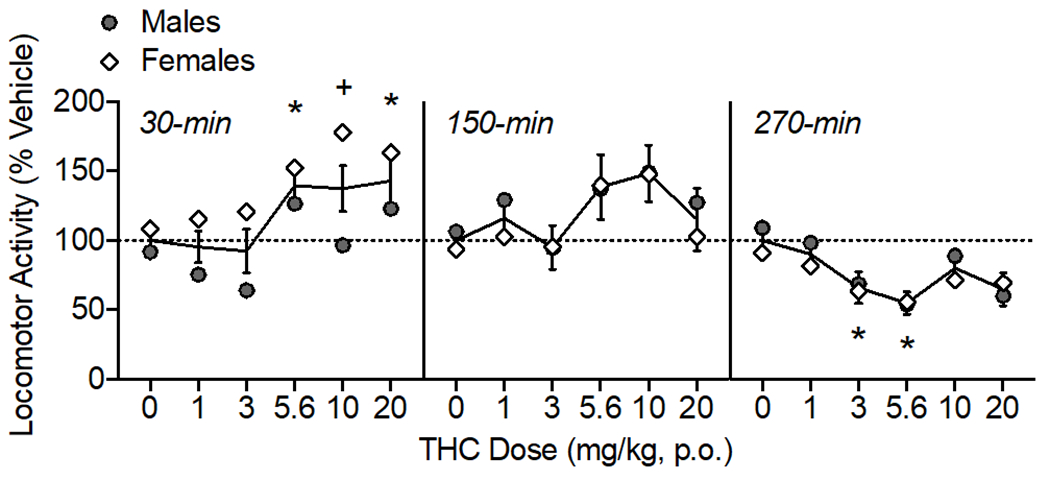
Oral THC effects on locomotor activity, shown as percent of vehicle distance traveled. The time of data collection after oral administration is noted in italics. Asterisks (*) represent a significant difference from vehicle. Plus sign (+) indicates a sex difference. Data are Mean ±SEM; N=6/sex.
Catalepsy
High doses of THC caused increases in catalepsy over the testing period (Fig. 4) as confirmed by a main effect of THC dose (F(5, 50)= 13.54, p<0.001) and time (F(1, 10)= 5.55, p<0.05). At 90-min, 20 mg/kg THC increased catalepsy (F(3.26, 32.57)= 11.20, p<0.001), and at 330-min, 10-20 mg/kg THC increased catalepsy (F(3.04, 30.40)= 6.68, p<0.001).
Figure 4.
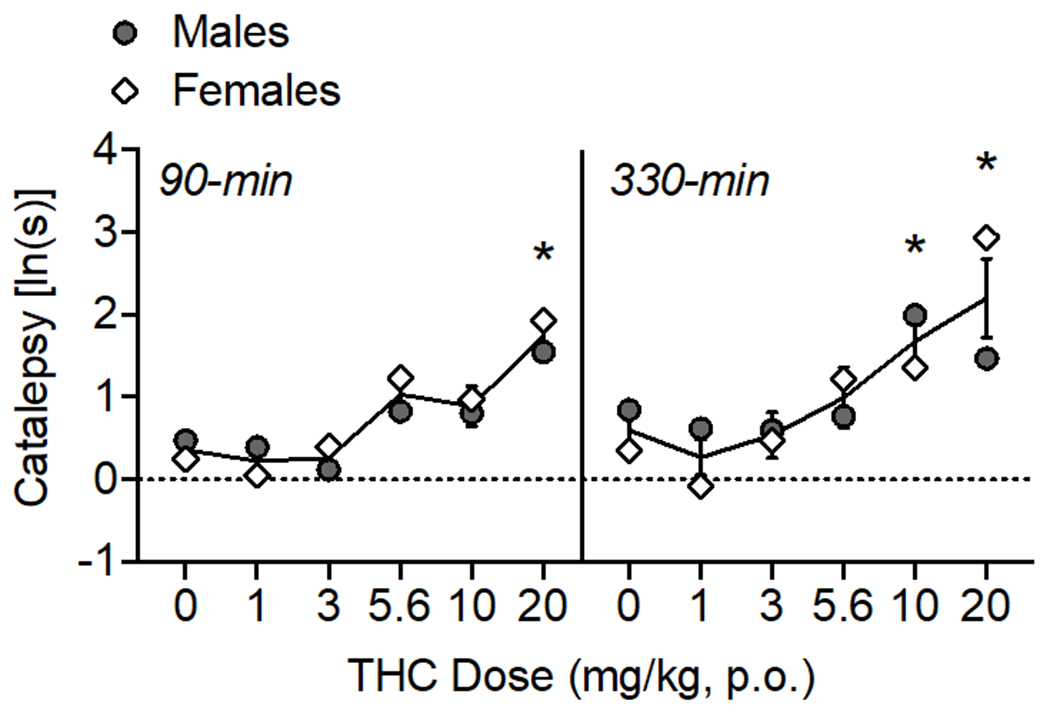
Oral THC effects on catalepsy. The time of data collection after oral administration is noted in italics. Asterisks (*) represent a significant difference from vehicle (males or females). Data are Mean ±SEM; N=6/sex.
ED50 values
ED50 values were calculated at the time point where effects were maximal (see Table 1). The ED50 values for THC’s thermal antinociceptive effects were 1.66 and 4.34 mg/kg for males and females, respectively. In comparison, the ED50 values observed for morphine in the tail flick test were 9.00 and 4.76 mg/kg for males and females. For mechanical antinociceptive effects, ED50 values were 4.32 and 6.40 mg/kg for males and females, which was less potent than morphine (ED50 = 2.42 and 2.96 mg/kg for males and females). Notably, oral THC was less efficacious at producing thermal and mechanical antinociception, with maximum responses in the tail flick and von Frey test around 80% compared with 100% efficacy observed with morphine.
Table 1.
ED50 values of THC and Morphine. ED50 was calculated for the time point in which maximal effects were observed.
| Drug | Outcome | Time | Sex | ED50: mg/kg (95%CI) |
|---|---|---|---|---|
| Morphine | Tail Flick (%MPE) | 30-min | M | 9.00 (5.5-9.5) |
| F | 4.76 (3.8-5.3) | |||
| Von Frey (%MPE) | 45-min | M | 2.42 (0.0-7.5) | |
| F | 2.96 (1.0-6.2) | |||
| THC | Tail Flick (%MPE) | 300-min | M | 1.66 (0.5-4.3) |
| F | 4.34 (1.6-10.7)* | |||
| Von Frey (%MPE) | 135-min | M | 4.32 (1.9-9.0) | |
| F | 6.40 (3.3-12.6) | |||
| Hypothermia (Δ°C) | 420-min | M | 4.54 (2.1-9.7) | |
| F | 11.14 (6.5-19.8) | |||
| Hyperlocomotion (% Vehicle) | 30-min | M | 4.57 (1.2-15.4) | |
| F | 4.23 (1.4-11.9) | |||
| Hypolocomotion (% Vehicle) | 270-min | M | 2.19 (0.5-7.1) | |
| F | 1.81 (0.0-13.4) | |||
| Catalepsy (ln(s)) | 330-min | M | 9.47 (2.3-56.2) | |
| F | 7.53 (4.2-13.8) |
An asterisk (*) is used to denote non-overlapping 95% confidence intervals between males and females.
The IC50 for hypothermic effects of THC were calculated to be 4.54 and 11.14 mg/kg THC for males and females, respectively. IC50 values for hyperlocomotive effects (observed at 30-min) were 4.57 and 4.23 mg/kg for males and females, while the ED50 values for hypolocomotive effects (270-min) were 2.19 and 1.81 mg/kg for males and females. ED50 for cataleptic effects was 9.47 and 7.53 mg/kg for males and females, respectively.
Effects of oral CBD
Thermal and Mechanical Pain Sensitivity
Oral CBD administration had modest effects on pain sensitivity as measured in the tail flick assay and von Frey test (Fig. 5A–B). There was a main effect of CBD dose on tail flick latencies (%MPE) (F(3, 30)= 3.73, p<0.05); however, post-hoc tests did not isolate any doses that had significant differences from the vehicle condition at any time point tested. There was a CBD dose × time × sex interaction on von Frey thresholds (%MPE; F(6, 60)= 2.87, p<0.05). CBD (30 mg/kg) increased pain sensitivity at 315-min (F(1.88, 18.75)= 4.29, p<0.05). At 315-min, there was also an interaction of CBD dose × sex (F(3, 30)= 3.13, p<0.05), and while there were no significant sex differences determined by post-hoc tests, this interaction was likely driven by a greater effect of 30 mg/kg CBD in increasing mechanical nociception in females at this time point.
Figure 5.
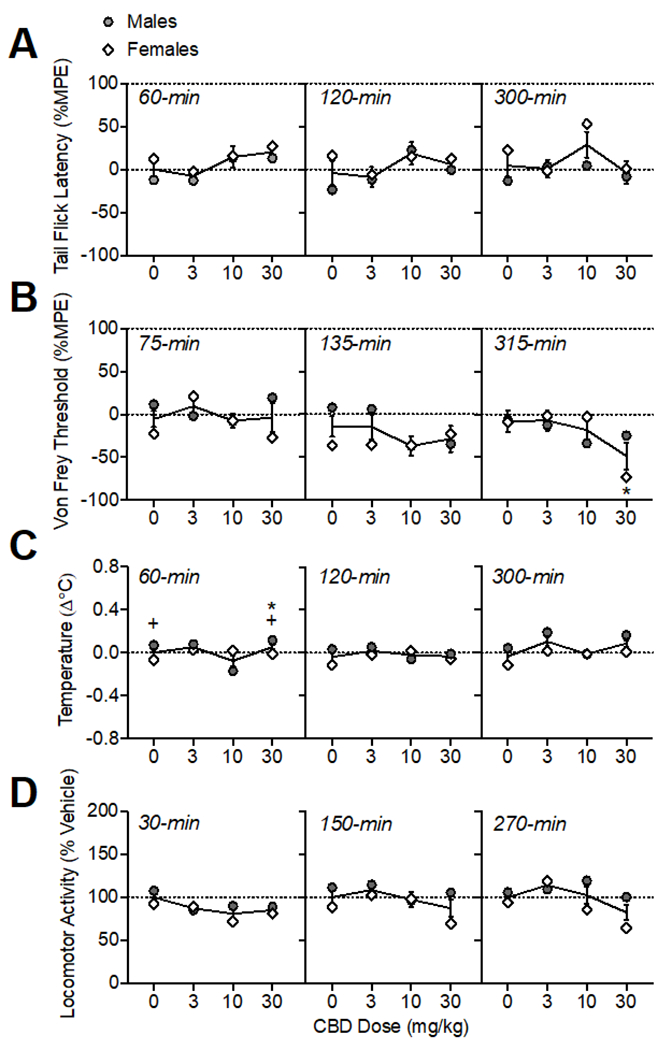
Oral CBD effects on thermal (A) and mechanical (B) pain sensitivity, body temperature (C), and locomotor activity (D). The time of data collection after oral administration is noted in italics. Asterisks (*) represent a significant difference from vehicle. Plus sign (+) indicates a sex difference. Data are Mean ±SEM; N=6/sex.
Body Temperature
Oral CBD administration altered temperature in a sex-dependent manner (Fig. 5C; main effects of CBD dose F(3, 30)= 6.31, p<0.001; sex: F(1, 10)= 9.43, p<0.05; and a CBD dose × sex interaction: (F(3, 30)= 8.50, p<0.001)). At 60-min, 30 mg/kg CBD increased body temperature (F(1.33, 13.33)= 6.73, p<0.05). There was also an interaction of CBD dose × sex (F(3, 30)= 10.48, p<0.001), with males showing higher temperature changes than females under vehicle conditions and after 30 mg/kg CBD, though these differences were small (mean difference: 0.12-0.14°C). At 300-min post oral CBD administration, there was a main effect of CBD dose (F(1.72, 17.15)= 3.79, p<0.01), though post-hoc tests did not indicate differences from vehicle for any specific dose.
Locomotor Activity
Oral CBD administration modulated locomotor activity (Fig. 5D; main effect of CBD dose: (F(3,30)= 9.59, p<0.001) and interactions of CBD dose × sex (F(3, 30)= 5.00, p<0.001), CBD dose × time (F(6, 60)= 2.75, p<0.05), and CBD dose × time × sex (F(6, 60)= 3.18, p<0.01). Analysis within each time point determined that CBD (10-30 mg/kg) reduced locomotor activity at 30-min (F(2.60, 25.99)= 6.89, p<0.01. At 270-min post oral CBD administration, there was a main effect of CBD dose (F(2.28, 22.83)= 5.72, p<0.01); though post-hoc tests did not indicate differences from vehicle for any specific dose. There was also a CBD dose × sex interaction (F(3, 30)= 3.75, p<0.05); likely due to lower activity in females than males at 10-30 mg/kg, though sex differences were not significant according to posthoc tests.
Discussion
Oral THC produced typical behavioral effects in the classic tetrad: antinociception, hypothermia, hypolocomotion, and catalepsy. These outcomes were mostly equivalent in males and females and time course of effects were quite prolonged, lasting up to 7 hours post administration. While the present study did not include any direct comparisons with different routes of administration, we have previously published on effects of IP injected and vaporized THC effects on thermal antinociception and body temperature in male and female Sprague-Dawley rats across a five-hour time course (Moore et al., 2021).
In this study, dose-dependent thermal antinociceptive effects in the tail flick test were observed at 120 and 300 minutes following oral THC administration, with maximal effects seen at 300-min. There were sex differences in the time course of thermal antinociception: in males, antinociceptive effects of THC increased across the testing period, while antinociception in females was equal across time points. The potency of the thermal antinociceptive effects at 300-min, when effects were maximal, was higher in males compared to females. Dose-dependent mechanical antinociceptive effects were also observed in the von Frey test when tested at 75, 135, and 315 minutes following oral THC administration, with maximal effects seen at 135-min. There were sex differences in the time course of mechanical antinociception: in females, antinociceptive effects were lower after 315-min compared to the 75-min time point, indicating a return to baseline levels, while the %MPE in males was equal across time points. Taken together, antinociceptive effects were observed in both tests of pain sensitivity, though overall, mechanical antinociceptive effects were observed earlier and reached their maximum at an earlier time point compared with thermal antinociceptive effects, which peaked later. However, this time course of antinociceptive effects was sex-dependent: males primarily showed a slower onset of thermal antinociceptive effects and prolonged mechanical antinociception compared with females, who had a faster onset (thermal antinociception) and offset (mechanical antinociception).
Notably, when compared with morphine (20 mg/kg, IP), oral THC (20/mg/kg) was less efficacious at producing thermal and mechanical antinociception, with maximum responses in the tail flick and von Frey test around 80% compared with 100% efficacy observed with morphine. The potency of oral THC and IP morphine to produce thermal antinociception were roughly equivalent in females (ED50s ~ 4 mg/kg), while the ED50 in males was lower for oral THC compared with morphine (1.66 vs 9.00 mg/kg, respectively). The potency of morphine was around two-fold higher than THC for producing mechanical antinociceptive effects (ED50 ~2 and 3 mg/kg for morphine and ~4 and 6 mg/kg for THC, in males and females respectively). It should be noted that we selected the standard route of injection (IP) for morphine as our positive control to produce maximal antinociceptive effects in our assays. Thus, interpretation of efficacy of IP morphine vs oral THC are limited by differences in route of administration, however, our prior study examining i.p. administered THC (20 mg/kg) also produced a ~85% MPE, comparable to what was observed in this study with orally administered THC.
In the current study, there were modest sex differences observed in the antinociceptive effects of oral THC, particularly in the time course of effects. In previous studies using IP injected THC, we and others have shown sex differences in thermal and mechanical antinociception in rats, where females show greater sensitivity to IP THC (i.e. effects at lower doses, greater magnitude of effects, longer lasting effects) compared to males (Craft et al., 2019; Moore et al., 2021; Tseng and Craft, 2001). Sex differences observed in rats after IP THC were consistent across multiple types of antinociception assays, including thermal (warm water tail withdrawal test, focused light beam tail flick test) and mechanical (von Frey, paw pressure tests) (Craft et al., 2019; Moore et al., 2021; Tseng and Craft, 2001). In studies using mice, however , females have demonstrated reduced sensitivity to the antinociceptive effects of IP THC compared to male mice (Henderson-Redmond et al., 2021). Using a vapor exposure route of administration, THC produced equivalent thermal antinociceptive effects in male and female rats (Moore et al., 2021). Our current results indicating a lower potency of oral THC to produce thermal antinociceptive effects in females compared with males suggests sex differences may depend on route of THC administration, however this would need to be more directly tested. To date, there are only a few studies looking at antinociceptive effects of oral THC in both males and females. In one such study using oral THC, sex differences in antinociceptive effects were observed in a neuropathic pain model, where chronic oral THC reduced hypersensitivity in male rats, but had no effect in female rats (Linher-Melville et al., 2020). A study of volitional consumption of oral THC in gelatin (~2 mg/kg), equivalent antinociception was observed in male and female rats when tested once immediately after 1-hr access to THC (Kruse et al., 2019). These two studies of oral THC consumption, taken with results from the current study demonstrate that the magnitude and/or expression of sex effects may depend on the time course of drug effects when using oral administration. This could be due in part to sex differences in pharmacokinetics that may differ depending on the route of administration. Studies using IP injection have observed higher levels of the active metabolite 11-OH-THC in female rats compared with males (Wiley et al., 2021). Higher 11-OH-THC has also been observed in women compared with men after oral consumption of cannabis extract, indicating a similar metabolic sex differences between these two routes (Lunn et al., 2019; Nadulski et al., 2005). Our data suggest lower potency and a differential time course in females’ response to THC, although another possibility is that our study design resulted in increased tolerance to oral THC in females compared with males. We gave active doses of THC seven days apart, as previous studies using this schedule with IP or vaporized THC have not shown development of tolerance in rats (Javadi-Paydar et al., 2018; Taffe et al., 2015). However preclinical evidence suggests that when given chronically, females develop tolerance to THC faster than males (Cooper and Craft, 2018; Nguyen et al., 2018; Wakley et al., 2014) and CB1 receptors are downregulated more rapidly (Farquhar et al., 2019). Further studies are needed to determine if there are sex differences in the development of tolerance to oral THC, and under what dosing parameters.
We observed modest, orderly decreases in body temperature over time, with the temperature nadir of −0.4°C observed 7 hours after oral THC administration. By comparison, in our recent study (Moore et al., 2021), we observed temperature nadirs of −1.0°C, 300-min after administration of 20 mg/kg IP THC and −0.7°C, 60-min after ~100 mg THC vapor exposure. A separate study measuring body temperatures after voluntary oral consumption of THC in gelatin (~2 mg/kg) showed a similar magnitude of decrease when measured immediately following 1-hr access in adolescent male and female rats (Kruse et al., 2019). There were no clear sex differences in the hypothermic effects of THC in the present study. In other studies that examined sex differences of THC hypothermic effects, female rats have shown greater (Nguyen et al., 2018) and longer lasting (Javadi-Paydar et al., 2018) reductions in body temperature following THC vapor. In a study using mice, the hypothermic effects of IP THC were similar in males and females (Wiley et al., 2021). Sex differences in hypothermic effects of THC may therefore be dependent on species and/or route of administration.
We observed both hyper- and hypolocomotor effects of oral THC depending on the time of the activity test post THC administration. When compared to vehicle, oral THC administration initially increased locomotor activity at 30 min and then resulted in decreases in locomotor activity after 5 hours. In contrast, studies using male mice IP injected with THC have found only hypolocomotion, observable 30-min after injection and lasting up to 420-min (Martin et al., 1991; Metna-Laurent et al., 2017; Puighermanal et al., 2013; Tai et al., 2015; Wiley et al., 2021). In rats, both hypolocomotive and hyperlocomotive effects of THC have been reported with use of multiple routes of administration. A study using IP THC observed “triphasic” effects, where very low doses and high doses reduced locomotor and mid-range doses stimulated activity immediately after injection (0-60 min) (Sanudo-Pena et al., 2000). Studies utilizing THC vapor exposure have observed either no effect (Javadi-Paydar et al., 2018; Nguyen et al., 2018) or hypolocomotion (Nguyen et al., 2016) when measuring home-cage activity for 3 hours. Several rat studies using oral administration of THC have observed either increases or decreases depending on the time point tested. A study of voluntary oral consumption of low dose THC (~2 mg/kg) in gelatin observed hyperlocomotion immediately following 1-hr access to THC in a 10-min activity test, and increased activity was similar in males and female rats (Kruse et al., 2019). In a separate study, 10 mg/kg oral THC in sesame oil overall reduced locomotor activity in a 5-min test when measured 120 minutes after administration (Hlozek et al., 2017). Finally, two studies of rats given oral or intragastric THC (0.1 - 10 mg/kg) found no effects on locomotor activity when measured 40-100 minutes after administration (Dow-Edwards and Zhao, 2008; Rock et al., 2016). In summary, though less often reported, the hyperlocomotor effects of THC have been observed in rats using various routes of administration, though typically at lower doses of THC, and observed early in the time course. Hypolocomotor effects of THC appear at higher doses and later in the time course.
Results from the present study also indicate sex differences in locomotor effects of THC, specifically, greater hyperlocomotion at 30-min in female rats. A previous study found that subcutaneously injected THC (30 mg/kg) produced hypolocomotion in male, but not female rats, when tested 30-min after injection (Marusich et al., 2014); though other studies using IP injection have observed no sex differences in rats when tested after 30-min (Wiley et al., 2007). In a study of vaporized THC, hypolocomotion was observed in male, but not female rats (Javadi-Paydar et al., 2018). Some sex differences have been reported in locomotor effects in mice, where females showed higher activity than male mice after 3-30 mg/kg THC administered IP (Wiley et al., 2021). To our knowledge, this is the first study reporting both increases and decreases in activity after oral THC administration, and the first to observe sex differences in locomotor effects of oral THC.
In the current study, catalepsy was observed at the highest doses of oral THC tested (10-20 mg/kg) and this peaked at 5.5h after administration. Prior studies have shown that cataleptic effects of THC depend on route of administration (Marshell et al., 2014). While IP THC and synthetic CB1 agonists reliably produce catalepsy, cataleptic effects were not observed in male mice after exposure to vaporized THC or synthetic cannabinoids JWH-018 and JWH-073 (Marshell et al., 2014). The present data concurs with what has been shown using IP injection: catalepsy produced by 10 mg/kg IP THC was shown to last up to 6 hours in male rats (Prescott et al., 1992). A study using oral THC (50 mg/kg) observed catalepsy in female mice when tested at 60-min post administration (Burstein et al., 1987). Sex differences in the cataleptic effects of THC have also been reported: 10 mg/kg IP THC produced greater amounts of catalepsy in female rats compared to male rats, and effects peaked at 60-min post injection (Tseng and Craft, 2001). A study using mice observed no differences in the cataleptic effects of IP THC in males vs. females (Wiley et al., 2021). In the present study, there were no sex differences observed in the cataleptic effects of oral THC.
In the present study, oral CBD had only modest effects on behaviors in the tetrad, which was expected. No antinociceptive effects of CBD were detected in the tail flick test, and in fact, increased pain sensitivity was observed after the highest dose of CBD (30 mg/kg) in the von Frey test 300-min after administration. In the von Frey assay, CBD effects were not dose-dependent or consistent across time points. While CBD has been shown to reduce or prevent hyperalgesia in inflammatory and neuropathic pain models (for review, see Finn et al., 2021; Jesus et al., 2019; King et al., 2017; Mlost et al., 2020), acute antinociceptive effects were not observed following IP CBD administration in pain-naive rats (1.25-50 mg/kg) (Booker et al., 2009; Britch et al., 2017) or mice (30 mg/kg) (Abraham et al., 2020). Similarly, no antinociceptive effects were observed after vaporized CBD in rats (Javadi-Paydar et al., 2019) or CBD smoke exposure in mice (Varvel et al., 2006). To our knowledge, oral CBD effects on acute pain sensitivity has not been assessed in rats; replication of these effects is necessary for determining whether oral CBD reliably increases mechanical nociception in female rats.
We observed oral CBD effects on temperature in a sex-dependent manner; however, these changes in temperature were small (<0.1°C) and would not be considered meaningful. CBD injected IP has been shown to have modest hypothermic (Long et al., 2010) or no effects (Varvel et al., 2006) on body temperature. Vaporized CBD has been shown to reduce body temperature in rats (Javadi-Paydar et al., 2019; Javadi-Paydar et al., 2018). A lack of hypothermic effects in the present study suggests that reductions in body temperature often observed after IP or vaporized CBD is less likely to be observed under conditions of oral CBD administration.
We observed effects of CBD on locomotor activity: specifically, hypolocomotion 30-min following oral administration of 10-30 mg/kg CBD. Locomotor activity after 270-min was modulated by CBD in a sex-dependent manner, where female’s activity was overall lower compared to males, though sex effects were not isolated to any particular CBD dose. Similar to THC, effects of CBD on locomotor activity seem to depend on species, dose and perhaps route of administration, with studies in mice reporting no effects at low doses (1-5 mg/kg), increases and decreases in locomotor activity after high CBD doses (10-30 mg/kg) via injection (IP, intravenous) (Long et al., 2010; Varvel et al., 2006). In rats administered CBD via IP injection, hyperlocomotive effects were observed after 10 and 30 mg/kg, but only after 240-360 min post administration, and these effects were equivalent in males and females (Britch et al., 2017). Vaporized CBD has been shown to produce hypolocomotion immediately following 1-hr exposure, which returned to control levels after 30-min (Javadi-Paydar et al., 2019). A study in rats did not observe any effects of 10 mg/kg oral CBD when tested once, 120-min after administration (Hlozek et al., 2017). Taken together with results from the present study, route of administration may have differential effects on locomotor response to CBD and the time course of those effects. It is important to note that our treatment strategy for CBD included an element of ‘dose-finding’, in contrast to our THC doses which were chosen based on our prior studies (Moore et al., 2021). We initially tested our hypothesized ‘maximal’ effective dose (30 mg/kg) for tetrad activity. Based on observed effects on mechanical pain sensitivity and activity, we continued to test lower doses in a descending order to assess any dose-related effects, as bell-shaped dose response curves are often reported for CBD effects(Gallily et al., 2015; Guimaraes et al., 1990). Overall, minimal dose-orderly effects were observed on tetrad outcomes after CBD administration.
Conclusions
The findings from the current study on the time course of dose-effects of orally administered cannabinoids in the tetrad provide foundational data for future studies for selection of THC and CBD dose and timing of behavioral measures when using the oral route of administration. Results from this study, in conjunction with our prior study assessing time course of behavioral effects of IP and vaporized THC (Moore et al., 2021), demonstrate the importance of consideration of time course when evaluating behavioral effects, particularly when using a nontraditional route of drug administration.
Acknowledgements
All experiments were supported by the National Institute on Drug Abuse of the National Institutes of Health grant numbers R21DA046154 (EW) and the Johns Hopkins University Dalio Fund in Decision Making and the Neuroscience of Motivated Behaviors (EW). The authors have no conflicts of interest to disclose.
Abbreviations:
- THC
Δ9-tetrahydrocannabinol
- CBD
Cannabidiol
- CB1R
cannabinoid receptor 1
- IP
intraperitoneal
- SC
subcutaneous
- IV
Intravenous
- TF
tail flick
- MPE
Maximum possible effect
BIBLIOGRAPHY
- 1.Abraham AD, Leung EJY, Wong BA, Rivera ZMG, Kruse LC, Clark JJ, Land BB, 2020. Orally consumed cannabinoids provide long-lasting relief of allodynia in a mouse model of chronic neuropathic pain. Neuropsychopharmacology 45(7), 1105–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Booker L, Naidu PS, Razdan RK, Mahadevan A, Lichtman AH, 2009. Evaluation of prevalent phytocannabinoids in the acetic acid model of visceral nociception. Drug Alcohol Depend. 105(1-2), 42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Britch SC, Wiley JL, Yu Z, Clowers BH, Craft RM, 2017. Cannabidiol-Delta(9)-tetrahydrocannabinol interactions on acute pain and locomotor activity. Drug Alcohol Depend. 175, 187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burstein S, Hunter SA, Latham V, Renzulli L, 1987. A major metabolite of delta 1-tetrahydrocannabinol reduces its cataleptic effect in mice. Experientia 43(4), 402–403. [DOI] [PubMed] [Google Scholar]
- 5.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL, 1994. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 53(1), 55–63. [DOI] [PubMed] [Google Scholar]
- 6.Compton DR, Rice KC, De Costa BR, Razdan RK, Melvin LS, Johnson MR, Martin BR, 1993. Cannabinoid structure-activity relationships: correlation of receptor binding and in vivo activities. The Journal of pharmacology and experimental therapeutics 265(1), 218–226. [PubMed] [Google Scholar]
- 7.Cooper ZD, Craft RM, 2018. Sex-Dependent Effects of Cannabis and Cannabinoids: A Translational Perspective. Neuropsychopharmacology 43(1), 34–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craft RM, Britch SC, Buzitis NW, Clowers BH, 2019. Age-related differences in Delta(9)-tetrahydrocannabinol-induced antinociception in female and male rats. Exp Clin Psychopharmacol 27(4), 338–347. [DOI] [PubMed] [Google Scholar]
- 9.Dixon WJ, 1991. Staircase bioassay: the up-and-down method. Neurosci. Biobehav. Rev. 15(1), 47–50. [DOI] [PubMed] [Google Scholar]
- 10.Dow-Edwards D, Zhao N, 2008. Oral THC produces minimal behavioral alterations in preadolescent rats. Neurotoxicol. Teratol. 30(5), 385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farquhar CE, Breivogel CS, Gamage TF, Gay EA, Thomas BF, Craft RM, Wiley JL, 2019. Sex, THC, and hormones: Effects on density and sensitivity of CB1 cannabinoid receptors in rats. Drug Alcohol Depend. 194, 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferre S, Guix T, Prat G, Jane F, Casas M, 1990. Is experimental catalepsy properly measured? Pharmacol. Biochem. Behav. 35(4), 753–757. [DOI] [PubMed] [Google Scholar]
- 13.Finn DP, Haroutounian S, Hohmann AG, Krane E, Soliman N, Rice ASC, 2021. Cannabinoids, the endocannabinoid system, and pain: a review of preclinical studies. Pain 162(Suppl 1), S5–S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallily R, Yekhtin Z, Hanuš LO, 2015. Overcoming the bell-shaped dose-response of cannabidiol by using cannabis extract enriched in cannabidiol. Pharmacology & Pharmacy 6(02), 75. [Google Scholar]
- 15.Gomes FV, Del Bel EA, Guimaraes FS, 2013. Cannabidiol attenuates catalepsy induced by distinct pharmacological mechanisms via 5-HT1A receptor activation in mice. Prog Neuropsychopharmacol Biol Psychiatry 46, 43–47. [DOI] [PubMed] [Google Scholar]
- 16.Grotenhermen F, 2003. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet 42(4), 327–360. [DOI] [PubMed] [Google Scholar]
- 17.Guimaraes FS, Chiaretti TM, Graeff FG, Zuardi AW, 1990. Antianxiety effect of cannabidiol in the elevated plus-maze. Psychopharmacology (Berl) 100(4), 558–559. [DOI] [PubMed] [Google Scholar]
- 18.Henderson-Redmond AN, Sepulveda DE, Ferguson EL, Kline AM, Piscura MK, Morgan DJ, 2021. Sex-specific mechanisms of tolerance for the cannabinoid agonists CP55,940 and delta-9-tetrahydrocannabinol (Delta(9)-THC). Psychopharmacology (Berl). [DOI] [PMC free article] [PubMed]
- 19.Hlozek T, Uttl L, Kaderabek L, Balikova M, Lhotkova E, Horsley RR, Novakova P, Sichova K, Stefkova K, Tyls F, Kuchar M, Palenicek T, 2017. Pharmacokinetic and behavioural profile of THC, CBD, and THC+CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. Eur Neuropsychopharmacol 27(12), 1223–1237. [DOI] [PubMed] [Google Scholar]
- 20.Javadi-Paydar M, Creehan KM, Kerr TM, Taffe MA, 2019. Vapor inhalation of cannabidiol (CBD) in rats. Pharmacol. Biochem. Behav. 184, 172741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Javadi-Paydar M, Nguyen JD, Kerr TM, Grant Y, Vandewater SA, Cole M, Taffe MA, 2018. Effects of Delta9-THC and cannabidiol vapor inhalation in male and female rats. Psychopharmacology (Berl) 235(9), 2541–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jesus CHA, Redivo DDB, Gasparin AT, Sotomaior BB, de Carvalho MC, Genaro K, Zuardi AW, Hallak JEC, Crippa JA, Zanoveli JM, da Cunha JM, 2019. Cannabidiol attenuates mechanical allodynia in streptozotocin-induced diabetic rats via serotonergic system activation through 5-HT1A receptors. Brain Res. 1715, 156–164. [DOI] [PubMed] [Google Scholar]
- 23.King KM, Myers AM, Soroka-Monzo AJ, Tuma RF, Tallarida RJ, Walker EA, Ward SJ, 2017. Single and combined effects of Delta(9) -tetrahydrocannabinol and cannabidiol in a mouse model of chemotherapy-induced neuropathic pain. Br. J. Pharmacol. 174(17), 2832–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kruse LC, Cao JK, Viray K, Stella N, Clark JJ, 2019. Voluntary oral consumption of Delta(9)-tetrahydrocannabinol by adolescent rats impairs reward-predictive cue behaviors in adulthood. Neuropsychopharmacology 44(8), 1406–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linher-Melville K, Zhu YF, Sidhu J, Parzei N, Shahid A, Seesankar G, Ma D, Wang Z, Zacal N, Sharma M, Parihar V, Zacharias R, Singh G, 2020. Evaluation of the preclinical analgesic efficacy of naturally derived, orally administered oil forms of Delta9-tetrahydrocannabinol (THC), cannabidiol (CBD), and their 1:1 combination. PLoS One 15(6), e0234176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long LE, Chesworth R, Huang XF, McGregor IS, Arnold JC, Karl T, 2010. A behavioural comparison of acute and chronic Delta9-tetrahydrocannabinol and cannabidiol in C57BL/6JArc mice. Int J Neuropsychopharmacol 13(7), 861–876. [DOI] [PubMed] [Google Scholar]
- 27.Lunn S, Diaz P, O’Hearn S, Cahill SP, Blake A, Narine K, Dyck JRB, 2019. Human Pharmacokinetic Parameters of Orally Administered Delta(9)-Tetrahydrocannabinol Capsules Are Altered by Fed Versus Fasted Conditions and Sex Differences. Cannabis Cannabinoid Res 4(4), 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshell R, Kearney-Ramos T, Brents LK, Hyatt WS, Tai S, Prather PL, Fantegrossi WE, 2014. In vivo effects of synthetic cannabinoids JWH-018 and JWH-073 and phytocannabinoid Delta9-THC in mice: inhalation versus intraperitoneal injection. Pharmacol. Biochem. Behav. 124, 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, Johnson MR, Melvin LS, Mechoulam R, Ward SJ, 1991. Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol. Biochem. Behav. 40(3), 471–478. [DOI] [PubMed] [Google Scholar]
- 30.Marusich JA, Lefever TW, Antonazzo KR, Craft RM, Wiley JL, 2014. Evaluation of sex differences in cannabinoid dependence. Drug Alcohol Depend. 137, 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metna-Laurent M, Mondesir M, Grel A, Vallee M, Piazza PV, 2017. Cannabinoid-Induced Tetrad in Mice. Curr Protoc Neurosci 80, 9 59 51–59 59 10. [DOI] [PubMed] [Google Scholar]
- 32.Mlost J, Bryk M, Starowicz K, 2020. Cannabidiol for Pain Treatment: Focus on Pharmacology and Mechanism of Action. Int J Mol Sci 21(22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore CF, Davis CM, Harvey EL, Taffe MA, Weerts EM, 2021. Appetitive, antinociceptive, and hypothermic effects of vaped and injected Delta-9-tetrahydrocannabinol (THC) in rats: exposure and dose-effect comparisons by strain and sex. Pharmacol. Biochem. Behav. 202, 173116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nadulski T, Pragst F, Weinberg G, Roser P, Schnelle M, Fronk EM, Stadelmann AM, 2005. Randomized, double-blind, placebo-controlled study about the effects of cannabidiol (CBD) on the pharmacokinetics of Delta9-tetrahydrocannabinol (THC) after oral application of THC verses standardized cannabis extract. Ther Drug Monit 27(6), 799–810. [DOI] [PubMed] [Google Scholar]
- 35.Newmeyer MN, Swortwood MJ, Andersson M, Abulseoud OA, Scheidweiler KB, Huestis MA, 2017. Cannabis Edibles: Blood and Oral Fluid Cannabinoid Pharmacokinetics and Evaluation of Oral Fluid Screening Devices for Predicting Delta(9)-Tetrahydrocannabinol in Blood and Oral Fluid following Cannabis Brownie Administration. Clin. Chem. 63(3), 647–662. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen JD, Aarde SM, Vandewater SA, Grant Y, Stouffer DG, Parsons LH, Cole M, Taffe MA, 2016. Inhaled delivery of Delta(9)-tetrahydrocannabinol (THC) to rats by e-cigarette vapor technology. Neuropharmacology 109, 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen JD, Grant Y, Kerr TM, Gutierrez A, Cole M, Taffe MA, 2018. Tolerance to hypothermic and antinoceptive effects of 9-tetrahydrocannabinol (THC) vapor inhalation in rats. Pharmacol. Biochem. Behav. 172, 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prescott WR, Gold LH, Martin BR, 1992. Evidence for separate neuronal mechanisms for the discriminative stimulus and catalepsy induced by delta 9-THC in the rat. Psychopharmacology (Berl) 107(1), 117–124. [DOI] [PubMed] [Google Scholar]
- 39.Puighermanal E, Busquets-Garcia A, Gomis-Gonzalez M, Marsicano G, Maldonado R, Ozaita A, 2013. Dissociation of the pharmacological effects of THC by mTOR blockade. Neuropsychopharmacology 38(7), 1334–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rock EM, Connolly C, Limebeer CL, Parker LA, 2016. Effect of combined oral doses of Delta(9)-tetrahydrocannabinol (THC) and cannabidiolic acid (CBDA) on acute and anticipatory nausea in rat models. Psychopharmacology (Berl) 233(18), 3353–3360. [DOI] [PubMed] [Google Scholar]
- 41.Rohleder C, Pahlisch F, Graf R, Endepols H, Leweke FM, 2020. Different pharmaceutical preparations of Delta(9) -tetrahydrocannabinol differentially affect its behavioral effects in rats. Addict. Biol. 25(3), e12745. [DOI] [PubMed] [Google Scholar]
- 42.Sanberg PR, Bunsey MD, Giordano M, Norman AB, 1988. The catalepsy test: its ups and downs. Behav. Neurosci. 102(5), 748–759. [DOI] [PubMed] [Google Scholar]
- 43.Sanudo-Pena MC, Romero J, Seale GE, Fernandez-Ruiz JJ, Walker JM, 2000. Activational role of cannabinoids on movement. Eur. J. Pharmacol. 391(3), 269–274. [DOI] [PubMed] [Google Scholar]
- 44.Sofia RD, Vassar HB, Knobloch LC, 1975. Comparative analgesic activity of various naturally occurring cannabinoids in mice and rats. Psychopharmacologia 40(4), 285–295. [DOI] [PubMed] [Google Scholar]
- 45.Spindle TR, Bonn-Miller MO, Vandrey R, 2019. Changing landscape of cannabis: novel products, formulations, and methods of administration. Curr Opin Psychol 30, 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taffe MA, Creehan KM, Vandewater SA, 2015. Cannabidiol fails to reverse hypothermia or locomotor suppression induced by Delta(9) -tetrahydrocannabinol in Sprague-Dawley rats. Br. J. Pharmacol. 172(7), 1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tai S, Hyatt WS, Gu C, Franks LN, Vasiljevik T, Brents LK, Prather PL, Fantegrossi WE, 2015. Repeated administration of phytocannabinoid Delta(9)-THC or synthetic cannabinoids JWH-018 and JWH-073 induces tolerance to hypothermia but not locomotor suppression in mice, and reduces CB1 receptor expression and function in a brain region-specific manner. Pharmacol. Res. 102, 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tseng AH, Craft RM, 2001. Sex differences in antinociceptive and motoric effects of cannabinoids. Eur. J. Pharmacol. 430(1), 41–47. [DOI] [PubMed] [Google Scholar]
- 49.Varvel SA, Wiley JL, Yang R, Bridgen DT, Long K, Lichtman AH, Martin BR, 2006. Interactions between THC and cannabidiol in mouse models of cannabinoid activity. Psychopharmacology (Berl) 186(2), 226–234. [DOI] [PubMed] [Google Scholar]
- 50.Wakley AA, Wiley JL, Craft RM, 2014. Sex differences in antinociceptive tolerance to delta-9-tetrahydrocannabinol in the rat. Drug Alcohol Depend. 143, 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wall ME, Sadler BM, Brine D, Taylor H, Perez-Reyes M, 1983. Metabolism, disposition, and kinetics of delta-9-tetrahydrocannabinol in men and women. Clin Pharmacol Ther 34(3), 352–363. [DOI] [PubMed] [Google Scholar]
- 52.Wiley JL, Barrus DG, Farquhar CE, Lefever TW, Gamage TF, 2021. Sex, species and age: Effects of rodent demographics on the pharmacology of (9)-tetrahydrocanabinol. Prog Neuropsychopharmacol Biol Psychiatry 106, 110064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiley JL, Burston JJ, 2014. Sex differences in Delta(9)-tetrahydrocannabinol metabolism and in vivo pharmacology following acute and repeated dosing in adolescent rats. Neurosci. Lett. 576, 51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wiley JL, O’Connell M M, Tokarz ME, Wright MJ Jr., 2007. Pharmacological effects of acute and repeated administration of Delta(9)-tetrahydrocannabinol in adolescent and adult rats. J. Pharmacol. Exp. Ther. 320(3), 1097–1105. [DOI] [PubMed] [Google Scholar]
- 55.Zgair A, Wong JC, Lee JB, Mistry J, Sivak O, Wasan KM, Hennig IM, Barrett DA, Constantinescu CS, Fischer PM, Gershkovich P, 2016. Dietary fats and pharmaceutical lipid excipients increase systemic exposure to orally administered cannabis and cannabis-based medicines. Am J Transl Res 8(8), 3448–3459. [PMC free article] [PubMed] [Google Scholar]


