Abstract
Background:
The INSIGHT START trial showed that over an average of 3 years of follow-up, immediate antiretroviral therapy (ART) in asymptomatic adults with >500 CD4 cells/μL consistently reduced the relative risk of the primary endpoint of serious AIDS, serious non-AIDS conditions, or death compared to deferring ART until CD4 <350 cells/μL across all patients subgroups. We sought to identify subgroups that showed the greatest absolute benefit from immediate treatment.
Methods:
We estimated event rates and absolute risk reduction (ARR) with immediate compared to deferred ART with 95% confidence intervals (CIs), overall and within patient subgroups defined at baseline. We computed the number need to treat (NNT) immediately for one year to prevent one event. Heterogeneity of the ARR across subgroups was assessed using bootstrap tests.
Findings:
From April 15, 2009 to December 23 2013, 4,684 patients, 28.6% women, with a median age of 36 years were enrolled in 35 countries across five continents. The ARR for the primary endpoint was 0.80 per 100 person-years with immediate treatment (95% CI: 0.48 to 1.13), and the NNT to prevent one event was 126 (95% CI: 89–208). Across subgroups, significant heterogeneity of the ARR with immediate ART was found for subgroups by age (p=0.002), CD4:CD8 ratio (p<0.001), and plasma HIV RNA levels (p=0.03). Patients aged ≥ 50 years, those with a CD4:CD8 ratio < 0.5, and those with a plasma HIV RNA level ≥ 50,000 copies/mL had the highest ARR (2.40, 1.73 and 1.48 per 100 PY, respectively) and lowest NNT (42, 58 and 68, respectively).
Interpretation:
In asymptomatic ART-naïve adults with CD4 levels >500 cells/μL, older participants, those with a low CD4:CD8 ratio and those with high plasma viral load benefited most from immediate treatment over an average of 3 years of follow-up. These patients should be prioritized for immediate ART initiation.
Funding:
by the US National Institute of Allergy and Infectious Diseases and others. NIH grants: UM1-AI068641 and UM1-AI120197.
INTRODUCTION
The Strategic Timing of AntiRetroviral Treatment (START) trial was designed to determine the risks and benefits of initiating antiretroviral therapy (ART) in asymptomatic HIV-positive individuals with CD4 cell counts greater than 500 cells/μL and to answer the long-debated question of “When to start ART” in early asymptomatic HIV-infection.1
The results of this international trial, which showed that immediate treatment reduced the risk of serious AIDS, serious non-AIDS conditions, and death by 57% compared with deferring therapy until the CD4 cell count has declined to 350 cells/μL or AIDS developed, have led to rapid changes in international guidelines now recommending immediate ART for HIV-infection regardless of the CD4 cell count1–4. This clinical benefit of immediate ART was also confirmed in another randomized trial conducted in Cote d’Ivoire. In addition to reducing morbidity and mortality, ART substantially reduces infectivity, an important public health benefit.5–7
In the START trial, hazard ratios for the primary outcome were similar across subgroups formed by demographic and clinical baseline characteristics, with the same relative benefits of early treatment initiation.1 Event rates, however, differed across subgroups. Given similar reductions in relative risk, patients at higher absolute risk would benefit more from immediate ART, as they would have larger absolute risk reductions, which is particularly relevant for clinical practice.
Because of the challenges of providing immediate access to ART to all patients with HIV-infection, identifying patients who might benefit the most from immediate ART would provide critical information to help policy makers and health care providers to prioritize immediate ART access in those with early asymptomatic HIV-infection. In this study, we identified participant characteristics at baseline that were associated with higher absolute risk reduction, lower numbers needed to treat to prevent one clinical event, and, therefore, higher net benefit of immediate ART.
METHODS
Participants and Study Design
The START trial was designed and conducted by the International Network for Strategic Initiatives in Global HIV Trials (INSIGHT) to assess the risks and benefits of immediate versus deferred initiation of ART in early asymptomatic HIV-infection. This international, open-label trial randomized ART-naïve HIV-infected asymptomatic adults who had CD4 cell counts greater than 500 cells/μL and no prior AIDS to immediate ART initiation versus deferring ART until the CD4 count declined to 350 cells/μL, or AIDS or other conditions that required ART developed. ART regimens were selected by the participants and their providers. Details of the design and data collection plans have been published elsewhere.1 Participants were enrolled from 2009 through 2013 in 35 countries. Our study includes data accrued through May 26, 2015, when the results of the START study were unblinded and participants in the deferred ART group were offered ART following a recommendation by the independent DSMB, because immediate ART initiation was found to decrease the risk of the primary endpoint by 57%.
The START trial was approved by the institutional review boards or ethics committees at all clinical sites, and all participants provided written informed consent. ClinicalTrials.gov: NCT00867048.
Study endpoints
The primary endpoint in START was a composite outcome with two major components: Serious AIDS-defining illnesses or death from AIDS, and serious non-AIDS illnesses (including cardiovascular, end-stage renal and liver diseases, non-AIDS defining cancers) or non-AIDS-related death. The specific conditions included in the serious AIDS and serious non-AIDS endpoints were described previously.1 All reported events were reviewed by an endpoint review committee using pre-established criteria, blinded to the treatment group assignment.1 Events that were adjudicated as confirmed or probable were included. Deaths were classified according to the CoDe system. 1
Subgroups
In order to assess which participants benefitted most from immediate initiation of ART, we considered subgroups formed according to participants’ baseline characteristics. Eight of the subgroup analyses were specified a-priori in the protocol: by age, sex and mode of infection, race, geographic region (high income: North America, Europe and Israel, Australia; low/moderate income: South America, Mexico, Asia, Africa), baseline CD4 cell count, baseline plasma HIV RNA level, smoking status (currently smoking or not), and Framingham 10-year coronary heart disease (CHD) risk (calculated with the use of the risk assessment tool from the Framingham Heart study). In addition, we investigated the following subgroups post-hoc: by baseline CD8 cell count, CD4:CD8 ratio; hepatitis B (defined as a positive Hepatitis B surface antigen within one year prior to enrollment) and C co-infection (defined as a positive hepatitis C antibody test); anemia (hemoglobin level <12 g/dL in women and <13 g/dL in men); nadir CD4 cell count; hypertension; hyperlipidemia; and baseline levels of IL-6 and D-dimers, since those subgroups have been associated with morbidity and mortality in patients with HIV-infection, as well as subgroups by pre-specified ART regimen.8–14 In order to form subgroups based on continuous-valued characteristics, approximate terciles were chosen as cut-points, unless different cut-points were clinically meaningful (e.g., age ≥ 50 years). Interleukin (IL)-6 and D-dimer levels were measured in centrally stored plasma samples collected at baseline. IL-6 was measured using high sensitivity ELISA (R&D Systems) and D-dimer using VIDAS system (Biomerieux).
Statistical Analysis
We censored follow-up on May 26, 2015, the day before the results of the START trial were unblinded. Four additional primary events (all in the deferred group) that occurred prior to May 26, 2015 were reported since the primary result manuscript was published. Thus, event counts in this paper differ slightly from those previously reported.1 We considered three endpoints: the START primary endpoint, and its two components of serious AIDS (non-fatal and fatal) and serious non-AIDS conditions (non-fatal and death due to causes other than AIDS). We estimated event rates per 100 person-years in the immediate and deferred ART groups, overall and within baseline subgroups, by dividing event counts by the person-years accrued, times 100. Absolute risks reductions (ARR), the event rate difference between the deferred minus the immediate arms, were estimated for each subgroup, and 95% confidence intervals (CI) were computed using Wilson’s score method.15 Heterogeneity of the ARR across subgroups was tested using Q-statistics and bootstrap estimates of the p-values.16 We used bootstrap because Poisson models for ARRs failed to converge for several subgroups. The numbers needed to treat (NNT) for one year in the immediate arm to prevent one event relative to the deferred arm were calculated as NNT=100/ARR. The upper and lower limits of 95% CIs for the NNT were calculated as 100 times the inverse of the lower and upper limits of the CI for ARR, respectively. 17
For the primary endpoint, we calculated Kaplan-Meier estimates for the cumulative percentage of participants with events, for subgroups by age, CD4:CD8 ratio and HIV RNA, to illustrate how the treatment effect differed across the subgroups.
All comparisons between the immediate and deferred ART groups were by intention-to-treat. Because the statistical analyses may be unstable for very low event numbers, only subgroup categories with at least 10 primary events were considered. We did not adjust for multiple comparisons; therefore, due to inflation of type I error, borderline significant effects need to be interpreted with caution. Also, the method we used to test for heterogeneity of the ARRs may be sensitive to the placement of the subgroup cut-points.
Statistical analyses were performed using SAS software (Version 9.3, SAS Institute, Cary, NC, USA) and R. All P-values cited are 2-sided and P ≤ 0.05 was the threshold for statistical significance.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
A total of 4684 HIV-positive participants were enrolled in 35 countries from 15 April 2009 to 23 December 2013. Baseline characteristics by treatment group that were previously reported and some that were not reported are shown in Table 1.1 The median age was 36 years, 26.8% of participants were women, the median CD4 cell count was 651 cells/μL, the median CD4:CD8 ratio was 0.64 and the median plasma HIV RNA level was 12,761 copies/mL. Baseline characteristics by CD4:CD8 ratio are shown in the Appendix (pages 1–2, Table S1). In the immediate ART group, 97% of participants had started ART by month 4, and ART was used for 96.8% of follow-up time accrued.1 In the deferred ART group, by design, participants started ART gradually as the CD4 cell counts declined; median time to ART initiation was 3.0 years, and ART was used for 29.1% of the follow-up time accrued. Almost all participants who reported using ART had suppressed viral load, which suggests high adherence.1
Table 1.
Baseline characteristics of the START participants.
| Immediate ART Group (N=2,325) | Deferred ART Group (N=2,359) | All participants (N=4,684) | |
|---|---|---|---|
|
| |||
| Age (years), median (IQR) | 36 [29, 44] | 36 [29, 44] | 36 [29, 44] |
| Women, N (%) | 624 (26.8) | 633 (26.8) | 1257 (26.8) |
| Race, N (%) | |||
| Asian | 198 (8.5) | 190 (8.1) | 388 (8.3) |
| Black | 701 (30.2) | 707 (30.0) | 1408 (30.1) |
| Latino/Hispanic | 319 (13.7) | 318 (13.5) | 637 (13.6) |
| White | 1016 (43.7) | 1071 (45.4) | 2087 (44.6) |
| Other | 91 (3.9) | 73 (3.1) | 164 (3.5) |
| Geographic region, N (%) | |||
| High income | 1067 (45.9) | 1088 (46.1) | 2155 (46.0) |
| Low/moderate income | 1258 (54.1) | 1271 (53.9) | 2529 (54.0) |
| Mode of HIV infection, N (%) | |||
| MSM | 1300 (55.9) | 1287 (54.6) | 2587 (55.2) |
| Heterosexual | 871 (37.5) | 917 (38.9) | 1788 (38.2) |
| Injection drug use | 37 (1.6) | 27 (1.1) | 64 (1.4) |
| Blood products, other, or unknown | 117 (5.0) | 128 (5.4) | 245 (5.2) |
| Years since HIV diagnosis, median [IQR] | 1.0 [0.4, 3.0] | 1.1 [0.4, 3.1] | 1.0 [0.4, 3.1] |
| CD4 count† (cells/μL), median [IQR] | 652 [585, 765] | 651 [582, 764] | 651 [584, 765] |
| Nadir CD4 count (cells/μL), median [IQR] | 552 [485, 660] | 553 [490, 650] | 553 [488, 654] |
| CD8 count (cells/μL), median [IQR] | 1039 [774,1383] | 1049 [780, 1422] | 1041 [777,1402] |
| CD4:CD8 ratio (cells/μL), median [IQR] | 0.65 [0.47, 0.86] | 0.64 [0.47, 0.88] | 0.64 [0.47, 0.87] |
| HIV RNA (copies/mL), median [IQR] | 13000 [3128, 43837] | 12550 [2976, 42567] | 12761 [3025, 43482] |
| IL-6 (pg/mL), median [IQR] | 1.4 [1.0, 2.2] | 1.4 [1.0, 2.1] | 1.4 [1.0, 2.1] |
| D-dimer (μg/mL), median [IQR} | 0.3 [0.2, 0.5] | 0.3 [0.2, 0.5] | 0.3 [0.2, 0.5] |
| Framingham 10-year CHD risk‡ (%), median (IQR) | 1.9 [0.5, 5.0] | 1.9 [0.5, 5.3] | 1.9 [0.5, 5.1] |
| Current smoker, N (%) | 732 (31.5) | 767 (32.5) | 1499 (32.0) |
| Hepatitis B, N (%) | 64 (2.8) | 65 (2.8) | 129 (2.8) |
| Hepatitis C, N (%) | 89 (3.9) | 82 (3.6) | 171 (3.7) |
| Anemia, N (%) | 321 (13.8) | 348 (14.8) | 669 (14.3) |
| Pre-specified ART regimen, N (%) | |||
| NNRTI + 2NRTIs | 1845 (79.4) | 1841 (78.0) | 3686 (78.7) |
| PI + 2NRTIs | 386 (16.6) | 429 (18.2) | 815 (17.4) |
| INSTI + 2NRTIs | 94 (4.0) | 89 (3.8) | 183 (3.9) |
The median CD4 cell count is based on the average of two CD4 counts obtained at screening for each participant.
The 10-year risk of coronary heart disease (CHD) was calculated with the use of the risk-assessment tool from the Framingham Heart Study.
Abbreviations: HIV=human immunodeficiency virus, INSTI=integrase inhibitor, IQR=interquartile range, NNRTI=non-nucleoside transcriptase inhibitor, NRTI=nucleoside transcriptase inhibitor, PI=protease inhibitor.
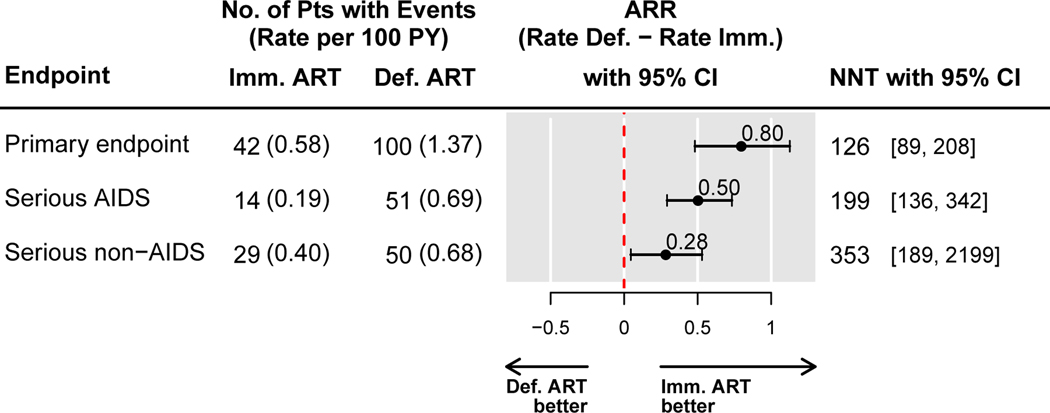
In the immediate ART group, 42 primary events occurred over a mean follow-up of 3.2 years, compared with 100 in the deferred group, at rates of 0.58 and 1.37 per 100 PY, respectively. The difference between these rates, 0.80 per 100 person-years (95% CI: 0.48 to 1.13) estimates the ARR with immediate ART compared with deferred ART, and favors immediate ART. Consequently, 126 patients (95% CI: 89–208) would need to initiate treatment immediately versus deferring treatment in order to prevent one primary event per year (NTT=126) (Figure 1). For serious AIDS events and serious non-AIDS events, the corresponding estimates are shown in Figure 1.
Figure 1.
Absolute risk reduction (ARR) in the immediate versus the deferred ART groups, and numbers needed to treat (NNT) to prevent one event in the START study, for the primary endpoint and its components, serious AIDS and serious non-AIDS conditions including death.
Abbreviations: ARR = absolute risk reduction, CI = confidence interval, NNT = number needed to treat to prevent one event with immediate ART.
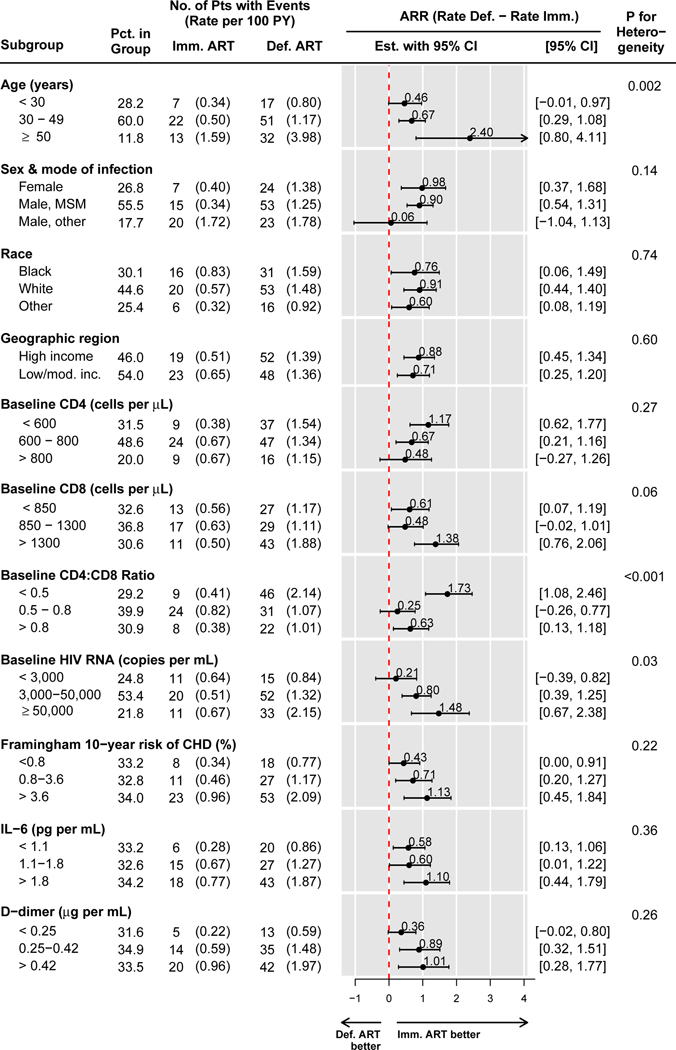
Figure 2 shows event rates for the primary endpoint by treatment group and subgroups, as well as the corresponding ARR. For all subgroups, the estimated ARR was consistently above 0, favoring the immediate arm. The ARRs varied significantly by age (p=0.002), by baseline CD4:CD8 ratio (p<0.001), and by baseline HIV RNA level (p=0.03). Across these subgroups, the ARR was largest, and the NNT smallest, in the 11.8% of participants aged 50 years or older, at 2.40 per 100 person-years (95% CI: 0.80–4.11), NNT=42 (95% CI: 24 to 125); among the 29.2% of participants with a baseline CD4:CD8 ratio below 0.5, with an ARR of 1.73 per 100 person-years (95 CI: 1.08–2.46), NNT=58 (95% CI: 40 to 93), and among the 21.8% of participants with plasma HIV RNA levels at or above 50,000 copies per mL, with an ARR of 1.48 per 100 person-years (95% CI: 0.67–2.38) and NNT=68 (95% CI: 42 to 149). In each case, there was a clear risk gradient in the deferred ART group, with higher event rates among those who were older, had lower CD4:CD8 ratios, and higher HIV RNA levels. Only 63 (1.4%) participants were in the subgroups with highest ARR levels for all three variables (age ≥ 50 years, CD4:CD8 ratio < 0.5, and HIV RNA ≥ 50,000 copies per mL); among participants who were in the highest ARR category for 2 or more out of the three variables, the ARR was 2.57 (95% CI: 1.27 to 4.04) and NNT=39 (95% CI: 25 to 79) (Appendix, page 3, Figure S1).
Figure 2.
Rates of the START primary endpoint (serious AIDS or serious non-AIDS conditions including all-cause death) per 100 person-years, and absolute risk reduction (ARR) in the immediate versus the deferred ART groups within subgroups formed by baseline characteristics.
The p-value is for a bootstrap test for heterogeneity of the ARR across subgroups.
There was no evidence for differences in ARR (and NNT) across subgroups by the following baseline characteristics: sex and likely mode of HIV infection, race, geographic region, CD4 and CD8 cell counts, IL-6, and D-dimers levels (Figure 2); as well as across all other investigated subgroups, including by nadir CD4 cell count, smoking status, hepatitis B or C, presence of anemia, hyperlipidemia, hypertension, or pre-specified ART regimen (Appendix, page 4, Figure S2).
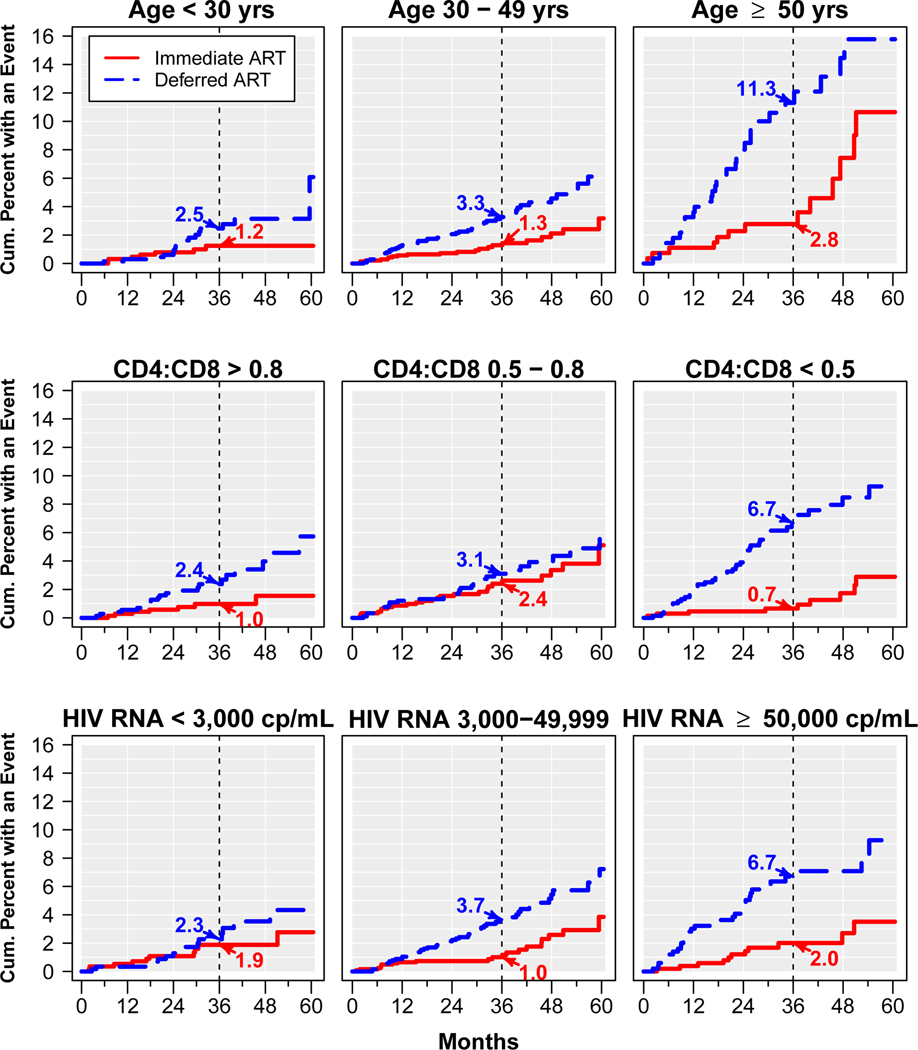
Figure 3 shows Kaplan-Meier estimates for the cumulative proportion of participants with a primary event in the immediate and deferred ART groups within subgroups by age, CD4:CD8 ratio, and by HIV RNA level; estimates at 36 months are cited. At month 36, the difference in the estimated cumulative event rates between the immediate and deferred ART groups was largest (and the absolute benefit of immediate ART was highest) among those aged 50 years or older (2.8% vs 11.3%), those with a CD4:CD8 ratio < 0.5 (0.7% vs 6.7%), and those with plasma HIV RNA levels of 50,000 cp/mL or higher (2.0% vs 6.7%).
Figure 3.
Kaplan-Meier estimates for the cumulative percent of participants who experienced a primary event (serious AIDS or serious non-AIDS conditions including all-cause death) in the immediate and deferred ART groups, within subgroups formed at baseline by age, CD4:CD8 ratio, and plasma HIV RNA.
When considering the major components of the primary endpoint separately, event counts are lower, resulting in lower power to detect heterogeneity of the ARR across subgroups. Similar to the primary endpoint, estimated rates of serious AIDS were consistently lower with immediate compared to deferred ART across all subgroups, but the ARR varied significantly only across subgroups by the CD4:CD8 ratio (p=0.003) and CD8 cell counts (p=0.02) (Appendix, page 5, Figure S3). For serious non-AIDS events, higher age was associated with larger ARR with immediate ART (p<0.001), with an ARR of 1.60 per 100 PY (95% CI: 0.23–3.07) in those age 50 years or older (Appendix, page 6, Figure S4).
Discussion
In the START trial, immediate ART decreased the risk of the primary composite endpoint of serious AIDS, serious non-AIDS illnesses and all-cause death by 57%, and this relative risk reduction was consistent and similar across subgroups defined at baseline, underscoring the main finding of the study, that all asymptomatic HIV-infected patients with a CD4 cell count above 500 cells/μL will draw about the same relative benefit from the immediate initiation of ART.1 WHO and US DHHS guidelines now recommend starting ART immediately, irrespective of CD4 cell counts, and expanding these recommendation to asymptomatic HIV-positive people with CD4 cell counts above 500 cells/mL was based in large part on the results of the INSIGHT START trial.3–6
While the relative risk reduction was similar across all subgroups of participants, we found that the reduction of absolute risk is greater among older participants, those with low baseline CD4:CD8 ratios and those with high plasma HIV RNA, corresponding to lower numbers needed to treat immediately to prevent serious AIDS and non-AIDS events. We assessed the absolute risk reduction across subgroups in order to help identify patients who might benefit the most from immediate ART initiation, and inform doctors and policy makers, who may have to prioritize ART access in low-resource environments. This analysis alludes to the cost-effectiveness of the immediate treatment initiation strategy with respect to preventing serious clinical events, but does not take into account the beneficial effect of immediate treatment on preventing HIV transmission, which could substantially increase the overall cost-effectiveness of immediate ART. 6
Advanced age, usually defined as 50 years or more in HIV-infected patients, has been independently associated with more rapid disease progression and mortality in cohort studies in both untreated patients and in those starting ART.18–20 The reasons for this more rapid disease progression are likely to be multifactorial, including the role of comorbidities associated with aging and the direct role of HIV. Edwards et al., in their recent modeling study based on retrospective and observational data also showed that the effect of delaying ART on mortality was age dependent, with the greatest absolute benefit of early ART projected in the oldest age group, those above 45 years21. In our study population, where all had CD4 cell counts above 500 cells/μL at study entry, those aged 50 years or older (11.8% of the total population) were at greater absolute risk for events, and had greater ARR (2.40 vs 0.46 per 100 PY among those aged under 30 years) with immediate ART, and lower NNT to prevent one primary endpoint (NNT= 42). Interestingly, the higher absolute risk reduction among older participants was dominated by the reduction in serious non-AIDS-related events (Supplemental Figure S2). It appears that patients entering care at older age are more vulnerable to the consequences of delaying ART initiation than younger patients, and our data now confirm, in the setting of a randomized trial, that initiating ART in patients aged ≥ 50 years is urgent. In fact, in the US in 2009, 92% of HIV-infected patients above 55 years of age who were under care were prescribed ART, and this should be the case also elsewhere.22 Finally, these data also underline the need for improving HIV testing rates in older populations, since up to 18% of new HIV diagnosis are reported in people aged 50 years or older, but only 37% of persons aged 45–64 had ever had an HIV test compared with 57% in those aged 25 to 44 years in the US in 2010.23
We also found that participants with low CD4:CD8 ratio at baseline were at increased risk of events in the deferred ART group, and benefited more from immediate treatment. Indeed, among those with a CD4:CD8 ratio < 0.5 (29.2% of the total population), the ARR for the primary endpoint was 1.73 per 100 person-years (and the NNT was only 58) as compared to 0.63 per 100 person-years, respectively, among participants with a CD4:CD8 ratio above 0.8. Interestingly, the higher absolute risk reduction among participants with a CD4:CD8 ratio < 0.5 was dominated by the reduction in serious-AIDS-related events (Supplemental Figure S1). Our results are consistent with previous findings in untreated HIV-infected patients showing that low CD4:CD8 ratios are associated with increased risk of AIDS and death, but differ from studies conducted among well-suppressed HIV-infected patients under antiretroviral therapy in whom a low CD4:CD8 ratio was also associated with a higher risk of non-AIDS events.10–13. In summary, ART-naïve patients with a low CD4:CD8 ratio tend to progress more rapidly to the composite of serious AIDS, non-AIDS events or death and may be prioritized for immediate treatment. Indeed, earlier initiation of ART has been reported to increase the probability of restoring normal CD4 counts and normal CD4:CD8 ratios, and to lower levels of immune activation and inflammation11, 24–26. The mechanisms driving a low CD4:CD8 ratio in untreated HIV-infection are not fully understood but are likely to be related to innate and adaptive immune dysfunction and/or activation in the peripheral blood and in the gut induced by HIV and also CMV replication, leading to the expansion of CD8 T-cells.27 Indeed, as shown in Table S1, patients with low CD4:CD8 ratios tended to have not only lower CD4 T-cell counts, but also higher CD8 cell counts and higher plasma HIV RNA levels. In patients with untreated HIV-infection, most CD8 T-cells are HIV-specific T-cells. It is therefore likely that the expansion of CD8 T-cells is a marker of immune dysfunction predictive of AIDS or non-AIDS events as previously reported. 28 In our study, participants with higher baseline plasma HIV RNA levels also benefitted more from immediate ART than those with lower levels (Figure 2), and patients with plasma HIV RNA levels of 50,000 copies/mL or more (21.8% of the total population) had the greatest ARR and lowest NNT to prevent one primary endpoint. The greater absolute risk reduction can be explained by higher event rates for participants with higher baseline HIV RNA levels in the deferred ART group, both of serious AIDS and serious non-AIDS events, while immediate ART resulted in similarly low event rates across all baseline HIV RNA categories. High plasma HIV RNA levels have been independently associated with higher probabilities of progression to AIDS and death.8,29 Also, a high plasma HIV RNA level is associated with an increased risk of HIV transmission, and since ART can significantly reduce viremia and HIV transmission to sexual partners, this is another good reason to urgently treat these patients. 6,7,30 In our study, there was no evidence for differences in the absolute risk reduction with immediate ART across subgroups by sex, mode of HIV infection, race, geographic region, Framingham risk of coronary heart disease, IL-6 or D-dimers levels, CD4 or CD8 cell counts. We do not have a clear explanation to why the CD4:CD8 ratio appeared to have a stronger prognostic capacity than IL-6 and D-dimer in this study as compared to previous studies, except that previous studies were conducted among HIV-infected patients already on antiretroviral therapy contrary to the current study conducted among antiretroviral naïve patients.14
Strengths of our study include the randomized design, the large sample size, and the uniform reporting and central adjudication of events. The main limitations are that duration of follow-up is only 3.2 years and, consequently, event counts in most subgroups are small, particularly when considering the component endpoints of AIDS and serious non-AIDS illnesses separately. Low event numbers in individual subgroups result in substantial uncertainty in the estimates of the ARR, reflected in large confidence intervals, and limited power to detect heterogeneity of treatment effect across subgroups. We also considered a large number of subgroups, some of which were not pre-specified, without adjustment for type I error, which may have led to chance findings. We have chosen to focus on subgroups for which tests for interaction in ARR were significant, but we acknowledge that our heterogeneity tests are also sensitive to the cut-points used to form subgroups.
In summary, this subgroup analysis of START is consistent with the main study findings that all patients irrespective of their baseline characteristics will draw the same relative benefit from immediate initiation of ART, and supports the recommendation of starting ART in all patients with HIV-infection as soon as possible. Moreover, we have identified subgroups of participants who had the highest reduction in absolute risk from immediate ART: those ≥ 50 years of age, those with a CD4:CD8 ratio < 0.5, and those with a plasma HIV RNA level ≥ 50,000 copies/mL. In such patients, the immediate initiation of ART at diagnosis is most urgent.
Supplementary Material
Acknowledgements:
We would like to thank the START participants without whom this work would not be possible. The INSIGHT START Study Group represents the investigators of the START trial, listed in the main results publication (ref).
The primary funder of the INSIGHT START trial was the National Institute of Allergy and Infectious Diseases (NIH grants: UM1-AI068641 and UM1-AI120197).
Additional funding was derived from NIH Clinical Center, National Cancer Institute, National Heart, Lung and Blood Institute, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Mental Health, National Institute of Neurological Disorders and Stroke, National Institute of Arthritis and Musculoskeletal and Skin Diseases, Agence Nationale de Recherches sur le SIDA et les Hépatites Virales (France), National Health and Medical Research Council (Australia), National Research Foundation (Denmark), Bundesministerium für Bildung und Forschung (Germany), European AIDS Treatment Network, Medical Research Council (United Kingdom), National Institute for Health Research, National Health Service (United Kingdom).
Antiretroviral drugs were donated to the central drug repository by: AbbVie, Inc., Bristol-Myers Squibb, Gilead Sciences, Inc., GlaxoSmithKline/ViiV Healthcare, Janssen Scientific Affairs, LLC, and Merck & Co., Inc.
The members of the START study group are cited in the Acknowledgments section.
Footnotes
Declaration of Interests::
JM Molina has participated to advisory board with Gilead Sciences, Merck, Janssen, ViiV and Teva and has received grants from Gilead Sciences and Merck. .
References
- 1.Lundgren D, Babiker AG, Gordin F, Emery S, Sharma S, Avihingsanon AC, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med 2015; 373(9):795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European AIDS Clinical Society. Guidelines Version 8.1 October 2016. [Online] Available at: http://www.eacsociety.org/files/guidelines_8.1-english.pdf [Accessed: 29/10/2016].
- 3.Panel on Antiretroviral guidelines for adults and adolescents. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. Department of health and Human services. Available at http://aidsinfo.nih.gov/contentFiles/AdultandAdolescentGL.pdf. [Accessed on 29/10/2016]. [Google Scholar]
- 4.World Health Organisation. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Available at: http://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf?ua=1 [Accessed: 29/10/2016]. [PubMed]
- 5.Danel C, Moh R, Gabillard D, Badje A, Le Carrou J, Ouassa T, et al. A Trial of Early Antiretrovirals and Isoniazid Preventive Therapy in Africa. N Engl J Med 2015; 373(9):808–822. [DOI] [PubMed] [Google Scholar]
- 6.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011;365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodger A, Cambiano V, Bruun T, et al. Risk of HIV transmission in serodifferent couples not using condoms when the HIV positive partner is using suppressive antiretroviral therapy. JAMA JAMA. 2016; 316: 171–81 [DOI] [PubMed] [Google Scholar]
- 8.Mocroft A, Kirk O, Barton SE, Dietrich M, Proenca R, Colebunders R, Pradier C, dArminio MA, Ledergerber B, Lundgren JD. Anaemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe. EuroSIDA study group. AIDS. 1999;13: 943–950 [DOI] [PubMed] [Google Scholar]
- 9.Nikolopoulos GK, Paraskevis D, Hatzitheodorou E, et al. Impact of hepatitis B virus infection on the progression of AIDS and mortality in HIV-infected individuals: a cohort study and meta-analysis. Clin Infect Dis 2009, 48: 1763. –1771 [DOI] [PubMed] [Google Scholar]
- 10.Margolick JB, Gange SJ, Detels R, O’Gorman MR, Rinaldo CR Jr, Lai S. Impact of inversion of the CD4/CD8 ratio on the natural history of HIV-1 infection. J Acquir Immune Defic Syndr. 2006. 15;42:620–6. [DOI] [PubMed] [Google Scholar]
- 11.Serrano-Villar S, Sainz T, Lee SA, et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog. 2014;10:e1004078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serrano-Villar S, Pérez-Elías MJ, Dronda F, et al. Increased risk of serious non-AIDS-related events in HIV-infected subjects on antiretroviral therapy associated with a low CD4/CD8 ratio. PLoS One. 2014;9:e85798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mussini C, Lorenzini P, Cozzi-Lepri A, et al. CD4/CD8 ratio normalisation and non-AIDS-related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: an observational cohort study. Lancet HIV. 2015. ;2 :e98–106. [DOI] [PubMed] [Google Scholar]
- 14.Kuller LH, Tracy R, Belloso W , et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008. Oct 21;5(10):e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Statistics in Medicine. 1998;17:873–90 [DOI] [PubMed] [Google Scholar]
- 16.Woodward M. Epidemiology: study design and data analysis. 2nd ed. Boca Raton: Chapman & Hall/CRC; 2005. [Google Scholar]
- 17.Bender R. Calculating confidence intervals for the number needed to treat. Controlled clinical trials. 2001;22:102–10. [DOI] [PubMed] [Google Scholar]
- 18.Egger M, May M, Chêne G, et al. ; ART Cohort Collaboration. Prognosis of HIV-1 infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–29. [DOI] [PubMed] [Google Scholar]
- 19.Muñoz A, Sabin CA, Phillips AN. The incubation period of AIDS. AIDS. 1997;11 Suppl A:S69–76. [PubMed] [Google Scholar]
- 20.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med 2009;360:1815–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards JK, Cole SR, Westreich D, Mugavero MJ, Eron JJ, Moore RD, Mathews WC, Hunt P, Williams C; for the Centers for AIDS Research Network of Integrated Clinical Systems investigators. Age at entry into care, timing of antiretroviral therapy initiation and 10-year mortality among HIV-seropositive adults in the United States. Clin Infect Dis. 2015. ;61(7):1189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson AS, Beer L, Sionean C, et al. HIV Infection – United States 2008 and 2010. Morbidity and Mortality Weekly Report. Suppl 2013: 62, 112–9. [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. CDC fact sheet: HIV testing in the United States, September 2015. Atlanta, GA. accessed May 15 , 2016 at: http://www.cdc.gov/nchhstp/newsroom/docs/factsheets/hiv-testing-us-508.pdf. [Google Scholar]
- 24.Mocroft A, Phillips AN, Gatell J, et al. Normalisation of CD4 counts in patients with HIV-1 infection and maximum virological suppression who are taking combination antiretroviral therapy: an observational cohort study. Lancet. 2007;370(9585):407–413. [DOI] [PubMed] [Google Scholar]
- 25.Kelley CF, Kitchen CM, Hunt PW, et al. Incomplete peripheral CD4+ cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin Infect Dis. 2009; 48(6):787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jain V, Hartogensis W, Bacchetti P, et al. Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. J Infect Dis. 2013;208(8):1202–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith DM, Nakazawa M, Freeman ML, et al. Asymptomatic CMV replication during early human immunodeficiency virus (HIV) infection is associated with lower CD4/CD8 ratio during HIV treatment. Clin Infect Dis. 2016; 63: 1517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. .Kvale D, Aukrust P, Osnes K, Müller F, Frøland SS. CD4+ and CD8+ lymphocytes and HIV RNA in HIV infection: high baseline counts and in particular rapid decrease of CD8+ lymphocytes predict AIDS. AIDS. 1999;13:195–201. [DOI] [PubMed] [Google Scholar]
- 29.Wood E, Hogg RS, Yip B, Quercia R, Harrigan PR, O’Shaughnessy MV, Montaner JS. Higher baseline levels of plasma Human Immunodeficiency Virus Type 1 RNA are associated with increased mortality after initiation of triple –drug antiretroviral therapy. J Infect Dis. 2003. Nov 15;188(10):1421–5. [DOI] [PubMed] [Google Scholar]
- 30.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, Meehan MO, Lutalo T, Gray RH. Vieral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai project study group. N Engl J Med. 2000; 342(13):921–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.