Introduction
The percentage of positive SARS-CoV-2 tests has been used with other metrics to reflect community transmission and guide community prevention strategies [1,2]. The Centers for Disease Control and Prevention (CDC) recommends calculating percent positivity as the number of positive nucleic acid amplification tests (NAAT) divided by the total number of NAAT results reported during a specified period (e.g., 7 days).1
Individuals may have repeat testing for COVID-19 during or following their infection, which is not reflective of new cases in the community. This analysis assesses the impact on percent positivity of deduplicating and censoring SARS-CoV-2 test results at the person level.
Materials & methods
Using SARS CoV-2 NAAT and antigen diagnostic test results reported to the New Mexico Department of Health during February 9–22, 2022, the following methods of calculating percent positivity were compared after excluding false positive antigen tests: (1) only NAAT test results; (2) only antigen test results; (3) NAAT and antigen test results; (4) deduplicating multiple NAAT tests on the same day and retaining the positive result if any; and (5) excluding all tests for persons with a positive result for 90 days following the positive specimen collection date.
Results
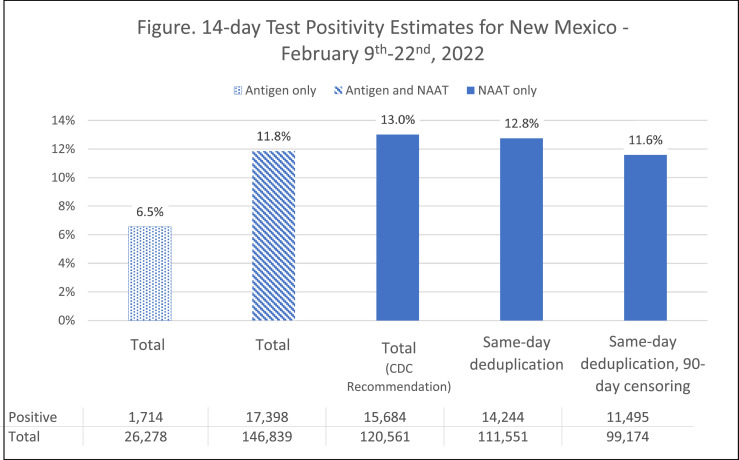
We identified 146,839 unique test results, of which 82% (n = 120,561) were NAAT (Fig. 1). Using the CDC-recommended calculation, 14 day percent positivity was 13.0%. Positivity by antigen test results was 6.5%. Including antigen test results decreased overall 14 day percent positivity to 11.8%. Six (0.02%) false-positive antigen results were identified. The percent positivity decreased from 13.0% to 12.8% after applying same day deduplication and further dropped to 11.6% after applying 90 day censoring.
Fig. 1.
Percent positivity is highest when using the CDC recommended calculation and reduces after applying same-day deduplication of antigen and NAAT tests and applying both same-day deduplication and 90-day censoring.
Discussion
The percent positivity for antigen tests was half that for NAAT tests before same day deduplication. Antigen tests are less sensitive than NAAT tests and are preferred when rapid results are needed, such as screening in higher risk settings [4]. Therefore, the positivity rate is expected to be lower with a less sensitive test and with testing for screening purposes versus testing due to exposure or symptoms.
Excluding test results for 90 days following specimen collection date reduces overall percent positivity. Persons who test positive for COVID-19 can continue to test positive with NAAT for 30 days on average even though they are no longer infectious, and risk of re-infection during this time is low [3]. Thus, a new case of COVID-19 is counted when the onset of a previous infection is greater than 90 days prior.2
Our analysis is limited by changes in testing practices and variant types, which can impact test sensitivity. This study was performed during relatively high-volume testing in the decline of the Omicron variant surge and may not reflect results in other times of the pandemic.
In New Mexico, percent positivity has been a useful metric for identifying deficiencies in testing capacity and understanding the underestimation of official case rates. When percent positivity increases, access to testing may not be sufficient and a higher proportion of cases are missed.3 Antigen test results serve as an important testing resource in hard-to-reach areas, and their performance improves in high-incidence settings; however, inclusion of antigen test results reduces the jurisdictional percent positivity relative to the national standard calculation method. Although censoring of repeat tests following infection provides a more accurate estimate of community transmission, censoring had a small impact on the percent positivity. Consistently applying the national calculation method over time should identify changing trends in adequacy of testing capacity and variation of percent positivity across communities.
Disclaimer
The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy.4
Footnotes
The authors have no conflicts of interest to disclose.
COVID-19 Testing: Understanding the “Percent Positive” | Johns Hopkins (jhu.edu)
See e.g., 45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq.
References
- 1.Oster A.M., Caruso E., DeVies J., Hartnett K.P., Boehmer T.K. Transmission dynamics by age group in COVID-19 hotspot counties - United States. MMWR Morb Mortal Wkly Rep. 2020;69(41):1494–1496. doi: 10.15585/mmwr.mm6941e1. April-September 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christie A., Brooks J.T., Hicks L.A., Sauber-Schatz E.K., Yoder J.S., Honein M.A. Guidance for implementing COVID-19 prevention strategies in the context of varying community transmission levels and vaccination coverage. MMWR Recommend Rep. 2021;70(30) doi: 10.15585/mmwr.mm7030e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biggerstaff B.J., Akinbami L.J., Hales C., Chan P.A., Petersen L.R. Duration of viral nucleic acid shedding and early reinfection with severe respiratory syndrome coronavirus 2 in healthcare workers and first responders. J Infect Dis. 2021;224(11):1873–1877. doi: 10.1093/infdis/jiab504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinnes J., Deeks J.J., Berhane S., et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Coch Database Syst Rev. 2021;(3) doi: 10.1002/14651858.CD013705.pub2. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]