Abstract
Background
PDGFR-inhibition by the tyrosine kinase inhibitor (TKI) nintedanib attenuates the progress of idiopathic pulmonary fibrosis (IPF). However, the effects of PDGF-BB on the airway tone are almost unknown. We studied this issue and the mechanisms beyond, using isolated perfused lungs (IPL) of guinea pigs (GPs) and precision-cut lung slices (PCLS) of GPs and humans.
Methods
IPL: PDGF-BB was perfused after or without pre-treatment with the TKI imatinib (perfused/nebulised) and its effects on the tidal volume (TV), the dynamic compliance (Cdyn) and the resistance were studied. PCLS (GP): The bronchoconstrictive effects of PDGF-BB and the mechanisms beyond were evaluated. PCLS (human): The bronchoconstrictive effects of PDGF-BB and the bronchorelaxant effects of imatinib were studied. All changes of the airway tone were measured by videomicroscopy and indicated as changes of the initial airway area.
Results
PCLS (GP/human): PDGF-BB lead to a contraction of airways. IPL: PDGF-BB decreased TV and Cdyn, whereas the resistance did not increase significantly. In both models, inhibition of PDGFR-(β) (imatinib/SU6668) prevented the bronchoconstrictive effect of PDGF-BB. The mechanisms beyond PDGF-BB-induced bronchoconstriction include activation of MAP2K and TP-receptors, actin polymerisation and Ca2+-sensitisation, whereas the increase of Ca2+ itself and the activation of EP1–4-receptors were not of relevance. In addition, imatinib relaxed pre-constricted human airways.
Conclusions
PDGFR regulates the airway tone. In PCLS from GPs, this regulatory mechanism depends on the β-subunit. Hence, PDGFR-inhibition may not only represent a target to improve chronic airway disease such as IPF, but may also provide acute bronchodilation in asthma. Since asthma therapy uses topical application. This is even more relevant, as nebulisation of imatinib also appears to be effective.
Background
Platelet-derived growth factor (PDGF)-BB and its receptor PDGFR are strongly involved in the pathogenesis of chronic airway disease [1], as both highly promote proliferation in airways [2]. This instance provides for the evidence that PDGFR-inhibition by tyrosine kinase inhibitors (TKIs) appear to be beneficial in chronic airway disease, e.g. idiopathic pulmonary fibrosis (IPF) [3–5] or asthma [6, 7]. Beyond the involvement of PDGF-BB and PDGFR in proliferation of airways and pulmonary vessels [1, 2, 8], PDGF-BB and PDGFR appear to regulate the tone of airways [9, 10].
The receptor tyrosine kinase PDGFR comprises of the two subunits αα, αβ or ββ which are activated by different ligands, e.g., in vivo PDGFR-α is activated by PDGF-AA and PDGF-CC, whereas PDGFR-β is activated by PDGF-BB [2]. In contrast, further possibilities are conceivable in vitro, e.g. the activation of PDGFR-αβ by PDGF-BB [2]. During organogenesis, the various PDGFR-subunits fulfil different functions, e.g., PDGFR-α is involved in the formation of the lungs, the skin, the gonads and the central nervous system, whereas PDGFR-β is responsible for the formation of blood vessel [2]. In respect of proliferative processes, PDGFR-β promotes the remodelling of the pulmonary vascular bed [11], as well as the remodelling in chronic fibrotic lung disease [12, 13] and asthma [14, 15].
This study was designed to evaluate the contractile effects of PDGF-BB on airway parameters in isolated perfused lungs (IPL) of guinea pigs (GPs) [16–19]. Further, we studied the effects of PDGF-BB in precision-cut lung slices (PCLS) of GPs and humans [17–22]. PCLS resembles an ex vivo model which allows to study the tone of pulmonary arteries, pulmonary veins and airways concurrently within their tissue organization excluding the exposure to in vivo factors such as shear stress, vascular filling pressure or thromboembolism [20, 21, 23]. As a major advantage, PCLS allow to compare how pulmonary vessel and airways react to several stimulants within different species [16, 18, 22, 24–26].
With regard to acute and chronic airway diseases, there are multiple open questions concerning the role of PDGF-BB and PDGFR. We addressed the following points: (1) Does PDGF-BB contract GP airways and is this contraction related to PDGFR-β? (2) How are the effects of PDGF-BB on airway parameters? (3) Does PDGF-BB affect airway parameters, if lungs are pre-treated with the TKI imatinib (perfused/inhaled)? (4) Does PDGF-BB contract human airways? (5) Do TKIs exert bronchodilative properties in human airways? (6) What are the mechanisms beyond PDGF-BB-induced contraction?
Methods
Lung tissue from GPs and humans
Female Dunkin Hartley GPs (350 ± 50 g) were obtained from Charles River (Sulzfeld, Germany). All animal care and experimental procedures were approved by the Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen (ID: 84-02.04.2013A146, 8.87-51.05.20.10.245, 50066A4) and strictly performed due the rules of the Directive 2010/63/EU of the European Parliament.
Human PCLS were prepared from patients undergoing thoracic surgery (lobectomy) due to cancer. After pathological inspection, cancer free tissue from a peripheral part of the lung was selected. In functional lung measurements, none of the patients showed relevant signs of chronic airway disease. The study was approved by the ethics committee (EK 61/09) of the Medical Faculty Aachen, Rhenish-Westphalian Technical University (RWTH) Aachen. All patients gave written informed consent.
Isolated perfused lungs of the GP
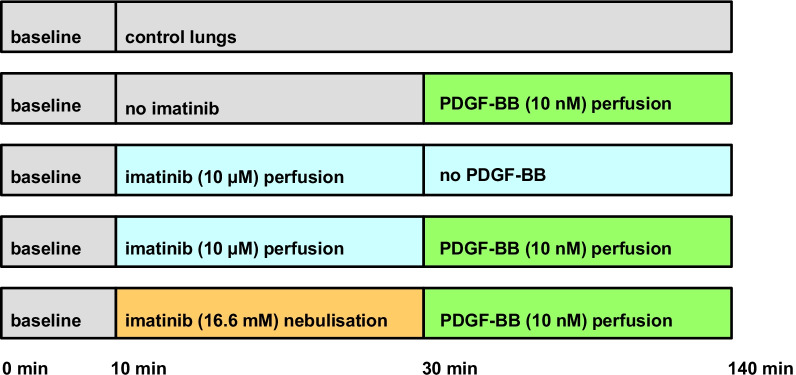
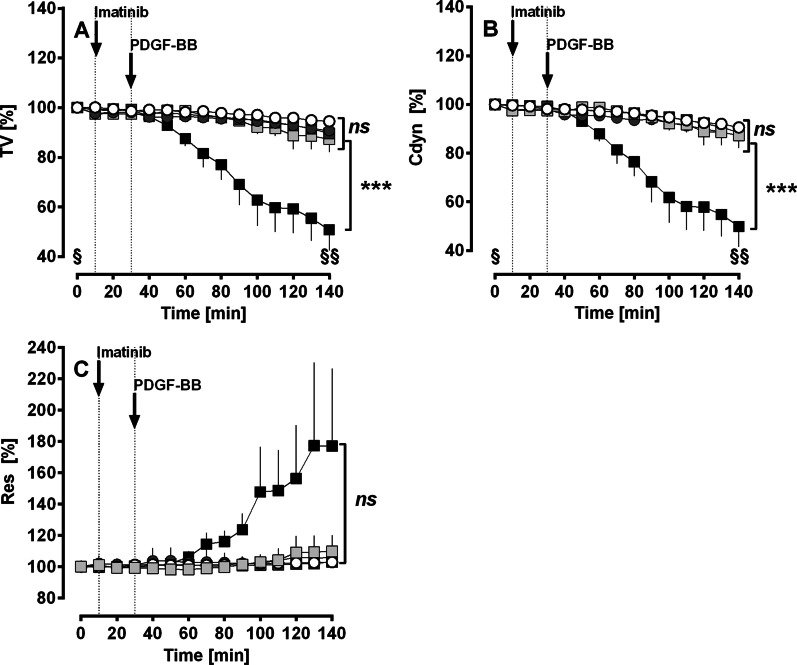
GP lungs were prepared as described previously [17–19, 27]. Briefly, intraperitoneal anaesthesia was performed (pentobarbital: 95 mg/kg) and verified by missing reflexes. The GP was exsanguinated, the trachea cannulated and the lung ventilated with positive pressure with a frequency of 70 breaths/min. Next, the apex of the left ventricle was cut and cannulas were placed in the pulmonary artery (perfusion inflow) and in the left atrium (perfusion outflow). Afterwards, the lung was perfused at constant flow (20 mL/min) with Krebs–Henseleit buffer, containing 2% bovine serum albumin, 0.1% glucose, 0.3% HEPES and 50 nM salbutamol in order to prevent bronchoconstriction [28]. The temperature of the perfusate was maintained at 37 °C with a water bath and the pH was adjusted between limits (7.35 and 7.45) by carbon dioxide. Heart and lungs were withdrawn en-bloc and transferred into a negative-pressure chamber; next, ventilation was switched from positive pressure to negative pressure. To avoid atelectasis of the lung, every 5 min a deep breath was applied. All following parameters were continuously monitored: tidal volume (TV), dynamic compliance (Cdyn), resistance (Res), pulmonal arterial pressure (PPA), left atrial pressure (PLA) and flow. Once respiratory and haemodynamic parameters remained stable over 10 min (baseline), imatinib was either perfused (10 µM) or nebulised (16.6 mM). Control lungs remained untreated. After 30 min, PDGF-BB (10 nM) was added to the recirculating perfusion buffer (total volume 200 mL) and perfused in untreated and in imatinib-pre-treated lungs. The different groups and the timeline of the experiments are illustrated in Fig. 1.
Fig. 1.
Overview of the timeline. This overview illustrates the different groups and the timeline of all experiments using the IPL
Perfusion and nebulisation of imatinib
Using a buffer volume of 200 mL, perfusion of 10 µM imatinib corresponds to a total dose of 1.18 mg imatinib or to 3.5 mg/kg body weight imatinib. To nebulise imatinib, 29.38 mg imatinib mesylate were solved in 3 ml aqua to obtain a solution of 16.6 mM. Afterwards, the solution of imatinib (16.6 mM) was nebulised over 130 min. Supposing a lung flow of 0.21 L/min (70 breaths by 3 mL) and a pressure of 1.5 bar, the total amount of inhaled imatinib corresponds to less than 4% of the nebulised amount of imatinib [29], namely 1.18 mg, corresponding to 3.5 mg/kg body weight imatinib, respectively.
Precision-cut lung slices (PCLS) from GPs and humans
In GPs, intraperitoneal anaesthesia was performed with 95 mg/kg pentobarbital (Narcoren; Garbsen, Germany) and verified by missing reflexes. The GP was exsanguinated, the trachea cannulated and the diaphragm opened. Thereafter, PCLS were prepared as described before [17–21, 24]. Whole lungs from GP or human lung lobes were filled via the trachea or a main bronchus, respectively with 1.5% low-melting agarose and cooled on ice to harden them. Afterwards, tissue cores (diameter 11 mm) were prepared and cut into 300 µm thick slices with a Krumdieck tissue slicer (Alabama Research & Development, Munford, AL, USA). PCLS were incubated at 37 °C and in order to wash out the agarose, repeated medium changes were performed.
Identification of the airway, histology
Airways from GPs were identified by their anatomical features; (1) beating cilia indicate the airways including their functional integrity and (2) the airways accompanying the pulmonary arteries [18, 21].
Pharmacological interventions, measurements and videomicroscopy
To evaluate the contractile effect of PDGF-BB in airways from GPs or humans, PCLS were exposed for 60 min to 100 nM PDGF-BB (Fig. 3A, B). If a signalling pathway was evaluated (Figs. 4, 5, 6 and 7), PCLS were additionally pre-treated for 60 min with one of the following inhibitors at concentrations about 10–100 fold above the IC50 value of the target: PDGFR-α: 100 nM ponatinib (IC50: 1.1 nM) [30–32]; PDGFR-β: 5 µM SU6668 (IC50: 0.008–0.1 µM) [33–35]; PDGFR-α/β: 100 µM imatinib (IC50: 0.6–1.8 µM) [36], L-Type Ca2+-channels: 100 nM amlodipine (IC50: 1.9 nM) [37]; Rho-Kinase: 10 µM fasudile (IC50: 1.4 µM) [38]; protein kinase C (PKC): 5 µM calphostin C (IC50: 50 nM) [39]; MAP2K: 50 µM PD98059 (IC50: 2–7 µM) [40]; MAP2K: 5 µM U0126 (IC50: 58–72 nM) [41]; actin polymerisation: 10 µM cytochalasin D (IC50: 100 nM) [42]; TP: 10 µM SQ29548 (IC50 10 nM) [43]; IP: 1 µM RO-1138452 (IC50: 5–10 nM) [44]; EP1: 1 µM SC51322 (IC50: 13.8 nM) [45]; EP2: 1 µM PF04418948 (IC50: 2.7 nM) [46, 47]; EP3: 1 µM L798106 (IC50: 10 nM) [43, 48] and EP4: 1 µM L161982 (IC50: 3.2 nM) [43]. To study the relaxing effects of imatinib in human airways (Fig. 3C), human PCLS were incubated with 100 nM Endothelin-1 (ET-1) to induce a stable contraction after 1 h. Subsequently, a concentration–response curve with imatinib was performed. Controls received no further treatment.
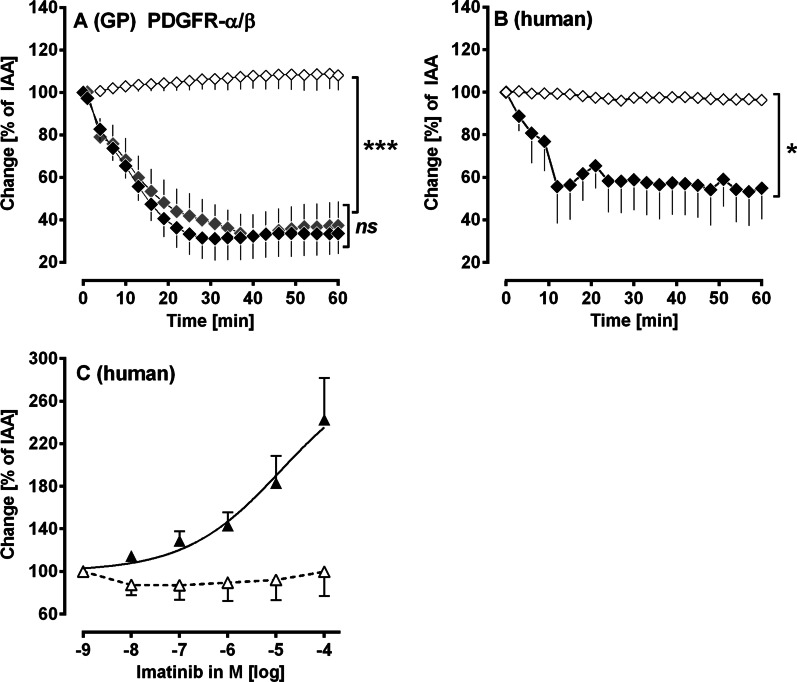
Fig. 3.
(Human and GPs’ PCLS): PDGF-BB regulates the airway tone via activation of PDGFR-(β). A The contractile effect of PDGF-BB in GPs’ airways: (◆) no pre-treatment/100 nM PDGF-BB (n = 7); (
 ) pre-treatment with 100 nM ponatinib/100 nM PDGF-BB (n = 7); (◇) pre-treatment with 5 µM SU6668/100 nM PDGF-BB (n = 7). B PDGF-BB contracts human airways: (◆) 100 nM PDGF-BB (n = 3); (◇) pre-treatment with 100 µM imatinib/100 nM PDGF-BB (n = 3). C Imatinib relaxes human airways: (△) pre-treatment with 100 nM ET-1 (n = 3); (▲) pre-treatment with 100 nM ET-1/imatinib (n = 5). A, B Statistics was performed by a LMM. C Statistics was performed by calculating EC50 values by the standard 4-parameter logistic non-linear regression model (GraphPad). p < 0.05 are considered as significant: *p < 0.05, **p < 0.01 and ***p < 0.001
) pre-treatment with 100 nM ponatinib/100 nM PDGF-BB (n = 7); (◇) pre-treatment with 5 µM SU6668/100 nM PDGF-BB (n = 7). B PDGF-BB contracts human airways: (◆) 100 nM PDGF-BB (n = 3); (◇) pre-treatment with 100 µM imatinib/100 nM PDGF-BB (n = 3). C Imatinib relaxes human airways: (△) pre-treatment with 100 nM ET-1 (n = 3); (▲) pre-treatment with 100 nM ET-1/imatinib (n = 5). A, B Statistics was performed by a LMM. C Statistics was performed by calculating EC50 values by the standard 4-parameter logistic non-linear regression model (GraphPad). p < 0.05 are considered as significant: *p < 0.05, **p < 0.01 and ***p < 0.001
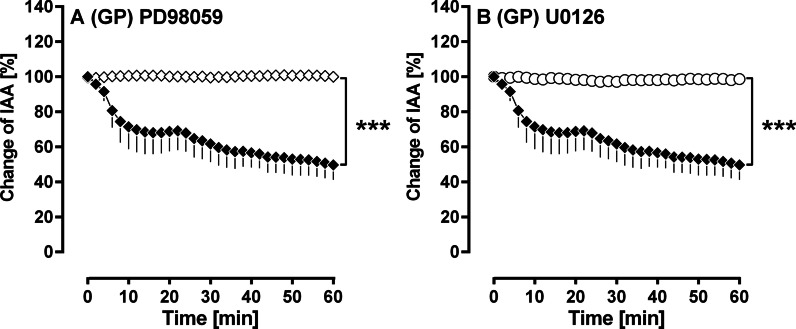
Fig. 4.
(GPs’ PCLS): Activation of MAP2K-signalling in PDGF-BB-induced bronchoconstriction. A Inhibition of MAP2K by PD98059 (◆) no pre-treatment/100 nM PDGF-BB (n = 4); (◇) pre-treatment with 50 µM PD98059/100 nM PDGF-BB (n = 4). B Inhibition of MAP2K by U0126 (◆) no pre-treatment/100 nM PDGF-BB (n = 4); (○) pre-treatment with 5 µM U0126/100 nM PDGF-BB (n = 4). A/B Statistics was performed by a LMM. p < 0.05 are considered as significant: *p < 0.05, **p < 0.01 and ***p < 0.001
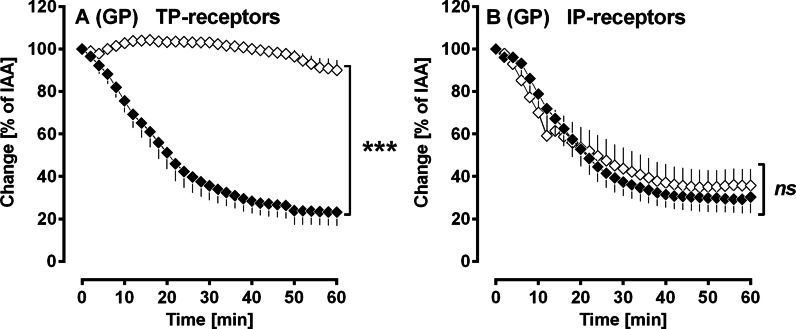
Fig. 5.
(GPs’ PCLS): Activation of TP- and IP-receptors in PDGF-BB-induced bronchoconstriction. A Inhibition of TP-receptors by SQ29548 (◆) no pre-treatment/100 nM PDGF-BB (n = 5); (◇) pre-treatment with 10 µM SQ29548/100 nM PDGF-BB (n = 5). B Inhibition of IP-receptors by RO-1138 (◆) no pre-treatment/100 nM PDGF-BB (n = 8); (◇) pre-treatment with 1 µM RO-1138/100 nM PDGF-BB (n = 8). A/B Statistics was performed by a LMM. p < 0.05 are considered as significant: *p < 0.05, **p < 0.01 and ***p < 0.001
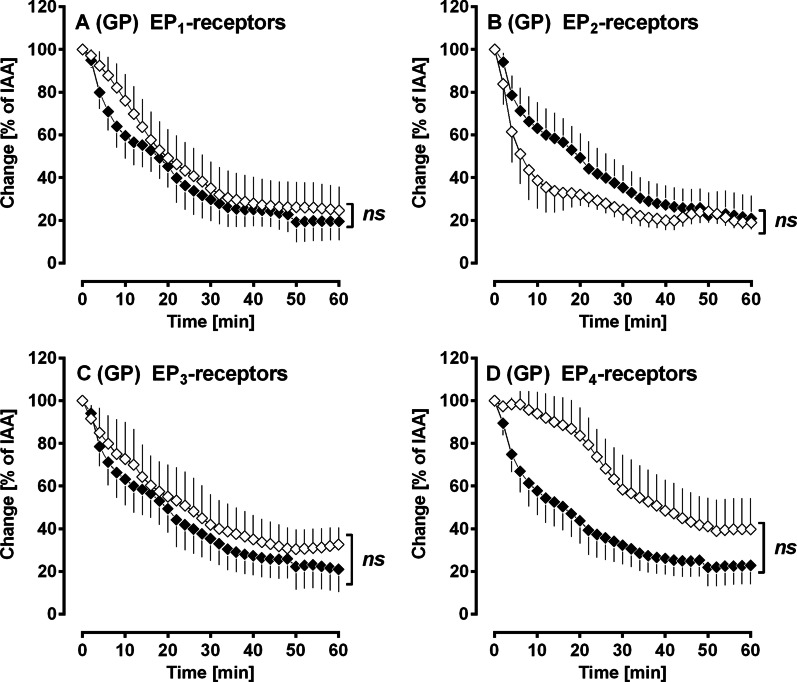
Fig. 6.
(GPs’ PCLS): No role of EP1–4-receptors in PDGF-BB-induced bronchoconstriction. A Inhibition of EP1-receptors by SC51322: (◆) no pre-treatment/100 nM PDGF-BB (n = 6); (◇) pre-treatment with 1 µM SC513222/100 nM PDGF-BB (n = 6). B Inhibition of EP2-receptors by PF0441 (◆) no pre-treatment/100 nM PDGF-BB (n = 5); (◇) pre-treatment with 1 µM PF0441/100 nM PDGF-BB (n = 5). C Inhibition of EP3-receptors by L798106: (◆) no pre-treatment/100 nM PDGF-BB (n = 5); (◇) pre-treatment with 1 µM L798106/100 nM PDGF-BB (n = 5). D Inhibition of EP4-receptors by L161982 (◆) no pre-treatment/100 nM PDGF-BB (n = 6); (◇) pre-treatment with 1 µM L161982/100 nM PDGF-BB (n = 6). A–D Statistics was performed by a LMM. p < 0.05 are considered as significant: *p < 0.05, **p < 0.01 and ***p < 0.001
Fig. 7.
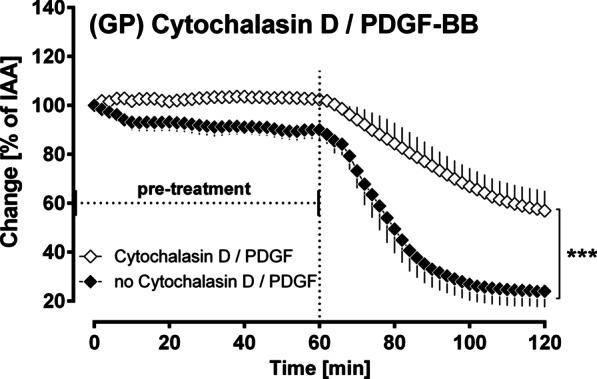
(GPs’ PCLS): The role of actin polymerisation within PDGF-BB-induced bronchoconstriction. Inhibition of actin polymerisation by cytochalasin D: (◆) no pre-treatment/100 nM PDGF-BB (n = 7); (◇) pre-treatment with 10 µM cytochalasin D/100 nM PDGF-BB (n = 7). Statistics was performed by a LMM. p < 0.05 are considered as significant: *p < 0.05, **p < 0.01 and ***p < 0.001
In PCLS, all changes of the initial airway area (IAA) were quantified in % and indicated as “Change [% of IAA]”. Thus, an IAA < 100% indicates contraction and an IAA > 100% indicates relaxation. To compare the contractile effect of PDGF-BB in pre-treated airways, the intraluminal area was defined after pre-treatment again as 100% (exceptional Fig. 4). In the graphs, all pre-treatments were indicated. The intraluminal area of airways was monitored with a digital video camera (Leica Viscam 1280, Leica DFC 280). The images were analysed with Optimas 6.5 (Media Cybernetics, Bothell, WA).
Chemicals
PDGF-BB was provided by Peprotech (Hamburg, Germany). Imatinib mesylate, amlodipine, fasudile, calphostin C, SC51322, PF04418948, L798106 and L161982 were purchased from Tocris Bioscience (Ellisville, Missouri, USA). Ponatinib was acquired from SelleckChem (Munich, Germany). SQ29548, RO-1138452, SU6668, PD98059 and U0126 were acquired from Cayman Europe (via Biomol, Hamburg, Germany). ET-1 was purchased from Biotrends (Wangen, Switzerland). Cytochalasin D or standard laboratory chemicals were provided by Sigma (Steinheim, Germany).
Statistical analysis
Statistics were conducted using SAS software 9.3 (SAS Institute, Cary, North Carolina, USA) and GraphPad Prism 5.01 (GraphPad, La Jolla, USA). All data, except Fig. 3C were analysed using a linear mixed model analysis (LMM) with the covariance structure AR(1). The data in Fig. 3C were analysed by EC50 values (GraphPad Prism). All p-values were adjusted for multiple comparisons by the false discovery rate and are presented as mean ± SEM; n indicates the numbers of animals or human lungs. p < 0.05 is considered as significant.
Results
We studied the effect of PDGF-BB on the airway tone using the IPL and PCLS from humans and GPs.
IPL: effect of PDGF-BB on airway parameters
Perfusion of PDGF-BB decreased the TV (Fig. 2A) and Cdyn (Fig. 2B) up to 50% compared to baseline values and to untreated control lungs (p < 0.001 for all). This effect was completely prevented, if lungs were pre-treated with perfused or nebulised imatinib (Fig. 2A, B). Accordingly, perfusion with PDGF-BB appears to increase Res, but statistical evaluation did not reveal significance (Fig. 2C). Anyhow, pre-treatment with imatinib prevented any changes (Fig. 2C). Imatinib itself had no influence on these airway parameters (Fig. 2A–C).
Fig. 2.
(GPs’ IPL): Effect of PDGF-BB on airway parameters. A Effect of PDGF-BB on TV: (○) control (n = 7); (■) PDGF-BB (n = 7); (
 ) imatinib (n = 7); (
) imatinib (n = 7); (
 ) perfused imatinib/PDGF-BB (n = 7); (
) perfused imatinib/PDGF-BB (n = 7); (
 ) nebulised imatinib/PDGF-BB (n = 6); ■ PDGF-BB: time point 0 (§) vs. 140 (§§) min: p < 0.001. B Effect of PDGF-BB on Cdyn: (○) control (n = 7); (■) PDGF-BB (n = 7); (
) nebulised imatinib/PDGF-BB (n = 6); ■ PDGF-BB: time point 0 (§) vs. 140 (§§) min: p < 0.001. B Effect of PDGF-BB on Cdyn: (○) control (n = 7); (■) PDGF-BB (n = 7); (
 ) imatinib (n = 7); (
) imatinib (n = 7); (
 ) perfused imatinib/PDGF-BB (n = 7); (
) perfused imatinib/PDGF-BB (n = 7); (
 ) nebulised imatinib/PDGF-BB (n = 6); ■ PDGF-BB: time point 0 (§) vs. 140 (§§) min: p < 0.001. C Effect of PDGF-BB on Res: (○) control (n = 7); (■) PDGF-BB (n = 7); (
) nebulised imatinib/PDGF-BB (n = 6); ■ PDGF-BB: time point 0 (§) vs. 140 (§§) min: p < 0.001. C Effect of PDGF-BB on Res: (○) control (n = 7); (■) PDGF-BB (n = 7); (
 ) imatinib (n = 7); (
) imatinib (n = 7); (
 ) perfused imatinib/PDGF-BB (n = 7); (
) perfused imatinib/PDGF-BB (n = 7); (
 ) nebulised imatinib/PDGF-BB (n = 6). A–C Statistics was performed by a LMM. p < 0.05 are considered as significant: *p < 0.05, **p < 0.01 and ***p < 0.001
) nebulised imatinib/PDGF-BB (n = 6). A–C Statistics was performed by a LMM. p < 0.05 are considered as significant: *p < 0.05, **p < 0.01 and ***p < 0.001
PCLS (GP): PDGF-BB contracts airways via activation of PDGFR-β
Next, using PCLS, we tried to find out, if PDGF-BB contracts the airways and if this contraction depends on PDGFR-β, as it was already shown for PVs [17].
In PCLS, PDGF-BB contracted the airways up to 30% of IAA (p < 0.001) and this effect was prevented, if PCLS were pre-treated with the PDGFR-β-inhibitor SU6668 (Fig. 3A; p < 0.001). In contrast, inhibition of PDGFR-α by ponatinib did not alter PDGF-BB induced bronchoconstriction (Fig. 3A).
Human PCLS: activation or inhibition of PDGFR-α/β alters the airway tone
PDGF-BB contracted human airways up to 53% of IAA (p < 0.05) and this bronchoconstriction was completely prevented if human airways were pre-treated with the PDGFR-α/β-inhibitor imatinib (Fig. 3B; p < 0.05). Next, we studied if imatinib also relaxes human airways. Therefore, airways were pre-constricted with 100 nM ET-1 prior to the application of increasing concentrations of imatinib (Fig. 3C). Imatinib relaxed human airways strongly up to 260% of IAA (Fig. 3C).
PCLS (GP): mechanisms for PDGF-BB-induced bronchoconstriction
Activation of MAP2K in PDGF-BB-induced bronchoconstriction
Inhibition of MAP2K by 50 µM PD98059 (Fig. 4A) or 5 µM U0126 (Fig. 4B) completely prevented the contractile effect of PDGF-BB (p < 0.001).
Activation of TP- and IP-receptors in PDGF-BB-induced bronchoconstriction
Inhibition of TP-receptors with 10 µM SQ29548 (Fig. 5A) prevented PDGF-BB-induced contraction, whereas inhibition of IP-receptors with 1 µM RO-1138 (Fig. 5B) had no effect on PDGF-BB-induced bronchoconstriction.
No role of EP1–4-receptors in PDGF-BB-induced bronchoconstriction
Neither inhibition of EP1-receptors with 1 µM SC51322 (Fig. 6A), nor inhibition of EP2-receptors with 1 µM PF0441 (Fig. 6B), nor inhibition of EP3-receptors with 1 µM L796106 (Fig. 6C) influenced PDGF-BB-induced contraction. In contrast, inhibition of EP4-receptors with 1 µM L161982 (Fig. 6D) appears to lower the contractile effect of PDGF-BB; however, this effect was without statistical significance.
The role of actin polymerisation within PDGF-BB-induced bronchoconstriction
The control airways slightly contracted over the pre-treatment period of 60 min, whereas airways pre-treated with cytochalasin D neither contracted nor relaxed and the airway tone remained stable. After treatment with PDGF-BB, control airways strongly contracted to 23% of IAA (Fig. 4). In contrast, the airways which passed through inhibition of actin polymerisation by cytochalasin D, contracted only to 56% of IAA (p < 0.001; Fig. 7).
The role of Ca2+ and Ca2+-sensitisation within PDGF-BB-induced bronchoconstriction
Pre-treatment with the Ca2+-channel blocker amlodipine (100 nM) did not significantly influence PDGF-BB-induced contraction (Fig. 8A). In contrast, inhibition of Rho-Kinase by fasudile (10 µM) significantly reduced the contractile effect of PDGF-BB (Fig. 8B), as does inhibition of PKC by calphostin C (5 µM) (Fig. 8C).
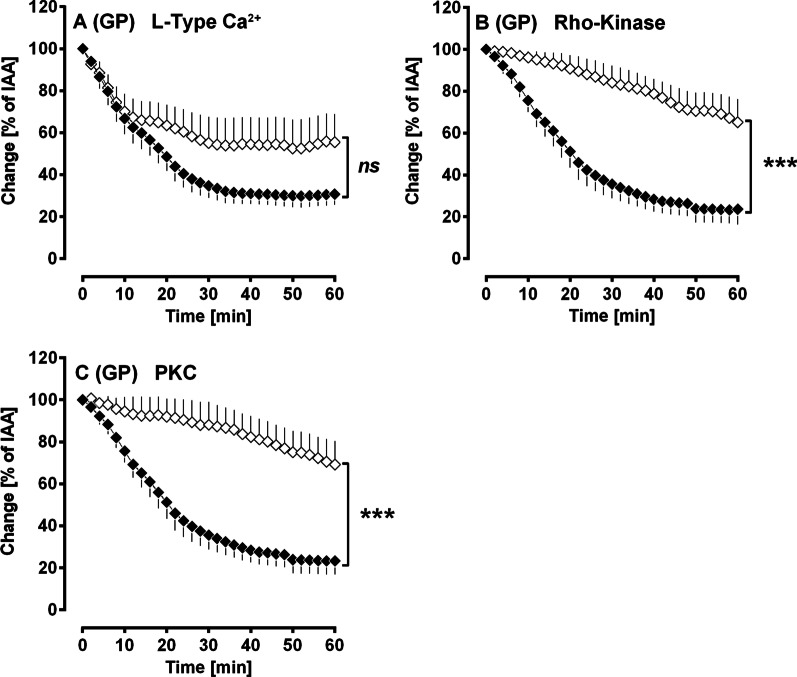
Fig. 8.
(GPs’ PCLS): The role of Ca2+ and Ca2+-sensitisation within PDGF-BB-induced bronchoconstriction. A Inhibition of voltage-gated Ca2+-channels by amlodipine: (◆) no pre-treatment/100 nM PDGF-BB (n = 7); (◇) pre-treatment with 100 nM amlodipine/100 nM PDGF-BB (n = 7). B Inhibition of Rho-Kinase by fasudile: (◆) no pre-treatment/100 nM PDGF-BB (n = 5); (◇) pre-treatment with 10 µM fasudile/100 nM PDGF-BB (n = 5). C Inhibition of PKC by calphostin C: (◆) no pre-treatment/100 nM PDGF-BB (n = 5); (◇) pre-treatment with 5 µM calphostin C/100 nM PDGF-BB (n = 5). A–C Statistics was performed by a LMM. p < 0.05 are considered as significant: *p < 0.05, **p < 0.01 and ***p < 0.001
Discussion
PDGF and PDGFR play a critical role within the remodelling in chronic airway diseases [1, 49]. Additionally, TKIs are increasingly focused as possible therapeutic agents [3–7, 50]. In contrast to these considerations, the acute effects of PDGF-BB and TKIs on the airway tone, e.g. constriction or relaxation have not yet been studied intensively. Here, we show that PDGF-BB contracts airways of GPs and humans via activation of PDGFR. Vice versa, PDGF-BB-induced airway contraction was prevented by TKIs. Further, the TKI imatinib even relaxed ET-1 pre-constricted human airways.
Effect of PDGF-BB on airway parameters
In the IPL (GP), PDGF-BB significantly reduced the tidal volume (Fig. 2A) and the dynamic compliance (Fig. 2B) of the lung. In addition, resistance tends to increase, however without statistical significance (Fig. 2C). The appearance of this bronchoconstrictive effect of PDGF-BB is supported by our results from GPs’ PCLS, where PDGF-BB also exerted distinct bronchoconstriction (Fig. 3A). In both experimental models, PDGF-BB-induced bronchoconstriction was completely prevented by TKIs. For example, in IPLs (Fig. 2A–C) pre-treatment with the PDGFR-α/β inhibitor imatinib completely avoided PDGF-BB-induced bronchoconstriction. Further in GPs’ PCLS, inhibition of PDGFR-β by SU6668 prevented PDGF-BB-induced bronchoconstriction (Fig. 3A), whereas inhibition of PDGFR-α by ponatinib had no effect (Fig. 3A), suggesting a dominant role of PDGFR-β within the contractile effect of PDGF-BB. The relevance of our findings is reinforced by the fact that PDGF-BB strongly contracted human airways (Fig. 3B) in PCLS, which was prevented by the PDGFR-α/β inhibitor imatinib (Fig. 3B). Further, imatinib also relaxed human airways after pre-constriction with ET-1, suggesting a relevant role of PDGFR within the regulation of the airway tone (Fig. 3C). This observation might be useful for approaches in asthma therapy.
Research in this field is scarce. Solely, Schaafsma et al. [51] demonstrated the bronchoconstrictive effect of PDGF-BB in GP tracheal strips, whereas Zhou et al. [10] proved the role of PDGFR within bronchoconstriction in rats lung slices.
Mechanisms for PDGF-BB-induced bronchoconstriction
The role of MAP2K-signalling in PDGF-BB-induced bronchoconstriction
Our results indicate that MAP2K-signalling is of pivotal role within the bronchoconstrictive effect of PDGF-BB, as inhibition of MAP2K-signalling by PD98059 and U0126 (Fig. 4A, B) completely prevented PDGF-BB-induced bronchoconstriction. Accordingly, Schaafsma et al. [51] proved the contractile effect of PDGF-BB in the trachea of GPs, just as the involvement of MAP2K. In addition, PDGF-BB-induced contraction of pulmonary veins also depends on MAP2K-signalling [19]. These findings are in line with the role of TP-receptors within the contractile effect of PDGF-BB in GPs’ airways (Fig. 5A) and pulmonary veins [19]. Further, they are explained by the fact that PDGF-BB induces the activation of MAP2K, which itself stimulates phospholipase A2 (PLA2) and subsequent prostaglandin synthesis [52–55]. Consequently, MAP2K-signalling is highly involved in PDGF-BB-induced prostaglandin synthesis.
The role of prostanoids in PDGF-BB-induced bronchoconstriction
TP-receptors are highly involved within PDGF-BB-induced bronchoconstriction, as inhibition of TP-receptors by SQ29548 completely prevented the contractile effect of PDGF-BB (Fig. 5A). TP-receptors are primarily linked to Gαq/11 and Gα12/13. Induction of Gαq/11 leads to the formation of IP3 and—by the release of Ca2+ from the sarcoplasmic reticulum—to increased intracellular Ca2+ levels [56, 57]; whereas activation of Gα12/13 mediates the induction of RhoA/ROCK which itself inhibits the myosin light chain phosphatase. Finally, both signalling pathways provoke sustained contraction [58].
In contrast to the role of TP-receptors, PDGF-BB-induced activation of IP-receptors (Fig. 5B) appears to be without relevance, as inhibition of IP-receptors by RO-1138 did not alter the contractile effect of PDGF-BB. Additionally, activation of EP1–4-receptors by PDGF-BB (Fig. 6A–D) seems to be at most of minor impact. Our results are partly in contrast to those from Schaafsma et al. [51] who found that PDGF-BB-induced bronchoconstriction (GPs) depends on the activation of EP1-receptors [51]. Further, Zhou et al. [10] showed that PDGFR-downstream signalling involves the activation of EP3-receptors. Our present results are further in contrast to a former study in pulmonary veins of GPs. There, PDGF-BB-induced contraction of SMCs was shown to depend on the activation of TP-receptors, as well as on the activation of EP1/3/4-receptors [19].
Comparing between the findings of our present work on GP airways and a former work on GP pulmonary veins [19], it is evident that TP-receptors play a pivotal role within PDGF-BB-induced contraction. This is also supported by the fact that thromboxane B2 (TXB2), the inactive metabolite of thromboxane A2 (TXA2), is strongly increased in the perfusate of IPLs after treatment with PDGF-BB. This effect is prevented if IPLs were pre-treated with imatinib [19].
The role of actin polymerisation within PDGF-BB-induced bronchoconstriction
Actin polymerisation is an important process within the regulation of the tone of smooth muscle cells (SMC) [59]. In this context, activation of PDGFR-β contributes—via the stimulation of SRC and abelson tyrosine kinase (Abl) (Fig. 9)—to actin polymerisation [60, 61]. Conversely, inhibition of PDGFR-β, e.g. by SU6668 or imatinib prevents this process. In addition, imatinib acts as a direct inhibitor of Abl [62]. Consecutively, imatinib prevents Abl downstream signalling such as actin polymerisation [62] and activation of RhoA/ROCK [63, 64]. Our data indicate that actin polymerisation is involved within PDGF-BB-induced bronchoconstriction, as its inhibition by cytochalasin D reduced the contractile effect of PDGF-BB (Fig. 7). Our results are supported by the facts that (1) actin polymerisation is of impact for the contractile effect of PDGF-BB in pulmonary veins (GP) [19] and (2) that actin polymerisation is of relevance for airway hyperresponsiveness [62, 65]. Further, Nayak et al. [66] recently demonstrated the additive effects of beta-agonists and Abl-inhibitors on the airway tone in murine PCLS and in mice with the flexiVent system [66].
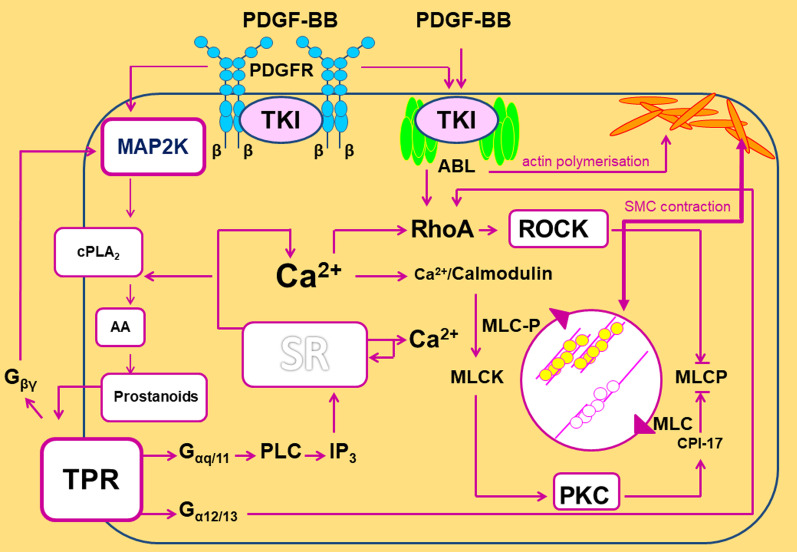
Fig. 9.
Mechanisms for PDGF-BB-induced bronchoconstriction. The contractile effect of PDGF-BB depends on the activation TP-receptors which are mainly coupled to Gα12/13 [56, 57] activating Rho/ROCK and inhibiting thereby MLCP [58]. Further, TP-receptors (TPR) are coupled to Gαq/11, leading to the formation of IP3 and to the release of calcium from the sarcoplasmic reticulum (SR) [56, 57]. Increased cytosolic calcium levels are leading to the activation of PKC which itself inhibits the myosin light chain phosphatase (MLCP), hence the actomyosin system remains activated and contraction of SMCs is intensified [58]. In addition, increased cytosolic calcium levels provoke the activation of Rho/ROCK [58, 67]. Last, TP-receptors (TPR) are linked to Gβγ activating MAPK-signalling. MAPK-signalling supports the activation of TP-receptors, as it strengthens the activation of the cytosolic PLA2 [52, 53], which leads to the formation of arachidonic acid (AA), serving as a substrate for the production of TXA2 [77]. Moreover, both PDGF-BB and PDGFR stimulate the abelson tyrosine kinase (Abl) [60, 61, 78] which acts downstream on Rho/ROCK [63, 64]. In contrast, the TKI imatinib, but not SU6668, inhibits Abl [62]. The stimulation of Abl by PDGF-BB and PDGFR is important for SMCs’ contraction, as Abl supports the polymerisation of subcortical actin filaments [59] strengthening the membrane for the transmission of the force generated by the actomyosin system. Hence, the stabilization of the cytoskeleton and the crossbridge cycling reinforce each other, leading to enhanced contraction [59]
Ca2+- release and Ca2+-sensitisation within PDGF-BB-induced bronchoconstriction
Studying the mechanisms of PDGF-BB-induced bronchoconstriction in GP PCLS, we found that inhibition of L-Type Ca2+ channels by amlodipine seems to play an at best minor role within the contractile effect of PDGF-BB (Fig. 8A). In contrast, inhibition of Rho-Kinase by fasudile (Fig. 8B) as well as inhibition of PKC by calphostin C (Fig. 8C) significantly reduced the contractile effect of PDGF-BB, suggesting a relevant role of Ca2+-sensitisation within the bronchoconstrictive effect of PDGF-BB. Our results are conflicting as Ca2+-sensitisation depends in part also on the increase of intracellular Ca2+ (Fig. 9) [58, 67]; e.g. Rho kinase mediated contraction of SMCs can be activated by Ca2+-independent and Ca2+-dependent mechanisms (Fig. 9), however activation of PKC is absolutely coupled on the release of Ca2+. Consecutively, Ca2+ should be involved within PDGF-BB-induced bronchoconstriction anyhow.
Our results are in contrast to those of Zhou et al. [10], who showed in rat lung slices that PDGF-BB-associated airway constriction depends on increased Ca2+-levels. The diverging results might be explainable by the different species, GPs versus rats. Yet, this aspect should not be the only reason for a possible discrepant regulation of the tone of SMCs. In fact, in contrast to our present results, L-Type Ca2+-channels play a dominant role within PDGF-BB-induced constriction in pulmonary veins of GPs, whereas Ca2+-sensitisation does not [19].
The bronchorelaxant effect of Imatinib
Beyond the contractile effects of PDGF-BB, PDGFR-inhibition by imatinib exerts bronchorelaxation in ET-1-pre-constricted human airways. In contrast, if airways were not pre-constricted, imatinib did not mediate relaxation, but it prevented PDGF-BB-induced contraction. Our results are supported by those of Chopra et al. [50] who proved a relaxant effect of the TKIs ST638, genistein and tyrphostin A47 in isolated bronchioles of rats. Recently, Nayak et al. [66] showed in murine PCLS and in a murine in vivo model (flexiVent) that TKIs and β-agonist act synergistic within the context of bronchorelaxation. Beyond, the relaxant effects of several TKIs, e.g. nilotinib, imatinib and nintedanib (unpublished data) have been proven in different tissue of various species [17, 22, 68–70].
Beyond the Abl-inhibiting properties of imatinib [62], it should be considered that imatinib relaxed human airways pre-constricted with ET-1. Within this context it is relevant that ET-1 downstream signalling [71–74] involves the release of TXA2 and subsequently the activation of TP-receptors, as a common pathway of ET-1-induced contraction. Furthermore, it is possible that imatinib interacts directly with TP-receptors. Since long, TP-receptors are focused as possible targets in the therapy of chronic asthma [75, 76]. Imatinib might contribute to generate new therapeutic approaches in asthma. Within this context inhaled imatinib may enlarge the existing repertoire of topical application.
In conclusion, (1) PDGF-BB contracts airways. (2) Imatinib (perfused/nebulised) prevents the contractile effects of PDGF-BB. (3) Imatinib relaxes ET-1 pre-constricted human airways. Finally, PDGFR might act as a central platform in chronic airway disease, e.g. IPF or asthma. Within this context, MAP2K-signalling, activation of TP-receptors, actin polymerisation and Ca2+-sensitisation appear to play a central role. Thus, TKI-inhibition might be a beneficial and prospective strategy in asthma and airway hyperresponsiveness.
Acknowledgements
This work was supported by the START programme of the RWTH-Aachen. We further gratefully acknowledge Hanna Czajkowska for excellent technical assistance.
Abbreviations
- Abl
Abelson tyrosine kinase
- Cdyn
Dynamic compliance
- EP-receptor
Prostaglandin E1–4 receptor
- ET-1
Endothelin-1
- GPs
Guinea pigs
- IAA
Initial airway area
- IPF
Idiopathic pulmonary fibrosis
- IPL
Isolated perfused lung
- IP-receptor
Prostacyclin receptor
- LMM
Linear mixed model analyses
- PCLS
Precision-cut lung slices
- PDGF
Platelet derived growth factor
- PDGFR
PDGF-receptor
- PKC
Protein kinase C
- PLA
Left atrial pressure
- PPA
Pulmonal arterial pressure
- Res
Resistance
- SMC
Smooth muscle cell
- TKIs
Tyrosine kinase inhibitors
- TP-receptor
Thromboxane prostanoid receptor
- TV
Tidal volume
- TXB2
Thromboxane B2, inactive metabolite of thromboxane A2
- TXA2
Thromboxane A2
Author contributions
ADR designed the study, performed the experiments, analysed the data, interpreted the data and wrote the manuscript. SS, EV performed the experiments, analysed the data and interpreted the data. CA, NAB performed the experiments. JWS, SK provided the human lung tissue and critically reviewed the manuscript. SvS, TB helped with the human lung tissue and critically reviewed the manuscript. SU analysed the data, interpreted the data and critically reviewed the manuscript. CM designed the study, analysed the data, interpreted the data and critically reviewed the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was funded by the START programme (Grant 109/14 (691440)) of the RWTH-Aachen. The funders had no influence of the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Availability of data and materials
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Female Dunkin Hartley GPs (350 ± 50 g) were delivered from Charles River (Sulzfeld, Germany). All animal experiments were approved by the Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen (ID: 84-02.04.2013A146, 8.87-51.05.20.10.245 and 50066A4) and strictly performed according to the rules of the Directive 2010/63/EU of the European Parliament.
Intraperitoneal anaesthesia was performed (pentobarbital: 95 mg/kg, Narcoren: Garbsen, Germany) and verified by missing reflexes, afterwards the animal was exsanguinated.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Annette D. Rieg, Email: arieg@ukaachen.de
Said Suleiman, Email: suleiman.aachen@gmail.com.
Carolin Anker, Email: carolin.anker@rwth-aachen.de.
Nina A. Bünting, Email: nbuenting@ukaachen.de
Eva Verjans, Email: everjans@ukaachen.de.
Jan Spillner, Email: jspillner@ukaachen.de.
Sebastian Kalverkamp, Email: skalverkamp@ukaachen.de.
Saskia von Stillfried, Email: svonstillfri@ukaachen.de.
Till Braunschweig, Email: tbraunschweig@ukaachen.de.
Stefan Uhlig, Email: suhlig@ukaachen.de.
Christian Martin, Email: chmartin@ukaachen.de.
References
- 1.Ingram JL, Bonner JC. EGF and PDGF receptor tyrosine kinases as therapeutic targets for chronic lung diseases. Curr Mol Med. 2006;6:409–421. doi: 10.2174/156652406777435426. [DOI] [PubMed] [Google Scholar]
- 2.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grimminger F, Gunther A, Vancheri C. The role of tyrosine kinases in the pathogenesis of idiopathic pulmonary fibrosis. Eur Respir J. 2015;45:1426–1433. doi: 10.1183/09031936.00149614. [DOI] [PubMed] [Google Scholar]
- 4.Wollin L, Maillet I, Quesniaux V, Holweg A, Ryffel B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J Pharmacol Exp Ther. 2014;349:209–220. doi: 10.1124/jpet.113.208223. [DOI] [PubMed] [Google Scholar]
- 5.Wollin L, Wex E, Pautsch A, Schnapp G, Hostettler KE, Stowasser S, Kolb M. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur Respir J. 2015;45:1434–1445. doi: 10.1183/09031936.00174914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Humbert M, De BF, Garcia G, Prud'homme A, Leroyer C, Magnan A, Tunon-de-Lara JM, Pison C, Aubier M, Charpin D, Vachier I, Purohit A, Gineste P, Bader T, Moussy A, Hermine O, Chanez P. Masitinib, a c-kit/PDGF receptor tyrosine kinase inhibitor, improves disease control in severe corticosteroid-dependent asthmatics. Allergy. 2009;64:1194–1201. doi: 10.1111/j.1398-9995.2009.02122.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee HY, Hur J, Kim IK, Kang JY, Yoon HK, Lee SY, Kwon SS, Kim YK, Rhee CK. Effect of nintedanib on airway inflammation and remodeling in a murine chronic asthma model. Exp Lung Res. 2017;43:187–196. doi: 10.1080/01902148.2017.1339141. [DOI] [PubMed] [Google Scholar]
- 8.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N, Seeger W, Grimminger F. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115:2811–2821. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suganuma N, Ito S, Aso H, Kondo M, Sato M, Sokabe M, Hasegawa Y. STIM1 regulates platelet-derived growth factor-induced migration and Ca2+ influx in human airway smooth muscle cells. PLoS ONE. 2012;7:e45056. doi: 10.1371/journal.pone.0045056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou J, Alvarez-Elizondo MB, Botvinick E, George SC. Adenosine A1 and prostaglandin EP3 receptors mediate global airway contraction following local epithelial injury. Am J Respir Cell Mol Biol. 2012 doi: 10.1165/rcmb.2012-0174OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perros F, Montani D, Dorfmuller P, Durand-Gasselin I, Tcherakian C, Le PJ, Mazmanian M, Fadel E, Mussot S, Mercier O, Herve P, Emilie D, Eddahibi S, Simonneau G, Souza R, Humbert M. Platelet-derived growth factor expression and function in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;178:81–88. doi: 10.1164/rccm.200707-1037OC. [DOI] [PubMed] [Google Scholar]
- 12.Aono Y, Kishi M, Yokota Y, Azuma M, Kinoshita K, Takezaki A, Sato S, Kawano H, Kishi J, Goto H, Uehara H, Izumi K, Nishioka Y. Role of platelet-derived growth factor/platelet-derived growth factor receptor axis in the trafficking of circulating fibrocytes in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2014;51:793–801. doi: 10.1165/rcmb.2013-0455OC. [DOI] [PubMed] [Google Scholar]
- 13.Kishi M, Aono Y, Sato S, Koyama K, Azuma M, Abe S, Kawano H, Kishi J, Toyoda Y, Okazaki H, Ogawa H, Uehara H, Nishioka Y. Blockade of platelet-derived growth factor receptor-beta, not receptor-alpha ameliorates bleomycin-induced pulmonary fibrosis in mice. PLoS ONE. 2018;13:e0209786. doi: 10.1371/journal.pone.0209786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhee CK, Lee SH, Yoon HK, Kim SC, Lee SY, Kwon SS, Kim YK, Kim KH, Kim TJ, Kim JW. Effect of nilotinib on bleomycin-induced acute lung injury and pulmonary fibrosis in mice. Respiration. 2011;82:273–287. doi: 10.1159/000327719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhee CK, Kang JY, Park CK, Lee SY, Kwon SS, Kim YK, Yoon HK. Effect of nilotinib on airway remodeling in a murine model of chronic asthma. Exp Lung Res. 2014;40:199–210. doi: 10.3109/01902148.2013.831959. [DOI] [PubMed] [Google Scholar]
- 16.Held HD, Uhlig S. Basal lung mechanics and airway and pulmonary vascular responsiveness in different inbred mouse strains. J Appl Physiol (1985) 2000;88:2192–2198. doi: 10.1152/jappl.2000.88.6.2192. [DOI] [PubMed] [Google Scholar]
- 17.Maihofer NA, Suleiman S, Dreymuller D, Manley PW, Rossaint R, Uhlig S, Martin C, Rieg AD. Imatinib relaxes the pulmonary venous bed of guinea pigs. Respir Res. 2017;18:32. doi: 10.1186/s12931-017-0514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rieg AD, Suleiman S, Perez-Bouza A, Braunschweig T, Spillner JW, Schroder T, Verjans E, Schalte G, Rossaint R, Uhlig S, Martin C. Milrinone relaxes pulmonary veins in guinea pigs and humans. PLoS ONE. 2014;9:e87685. doi: 10.1371/journal.pone.0087685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rieg AD, Suleiman S, Anker C, Verjans E, Rossaint R, Uhlig S, Martin C. PDGF-BB regulates the pulmonary vascular tone: impact of prostaglandins, calcium. Respir Res. 2018;19:120. doi: 10.1186/s12931-018-0829-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rieg AD, Rossaint R, Verjans E, Maihöfer NA, Uhlig S, Martin C. Levosimendan relaxes pulmonary arteries and veins in precision-cut lung slices—the role of KATP-channels, cAMP and cGMP. PLoS ONE. 2013;8(6):e66195. doi: 10.1371/journalpone0066195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rieg AD, Rossaint R, Uhlig S, Martin C. Cardiovascular agents affect the tone of pulmonary arteries and veins in precision-cut lung slices. PLoS ONE. 2011;6:e29698. doi: 10.1371/journal.pone.0029698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rieg AD, Bunting NA, Cranen C, Suleiman S, Spillner JW, Schnoring H, Schroder T, von Stillfried S, Braunschweig T, Manley PW, Schalte G, Rossaint R, Uhlig S, Martin C. Tyrosine kinase inhibitors relax pulmonary arteries in human and murine precision-cut lung slices. Respir Res. 2019;20:111. doi: 10.1186/s12931-019-1074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanderson MJ. Exploring lung physiology in health and disease with lung slices. Pulm Pharmacol Ther. 2011;24:452–465. doi: 10.1016/j.pupt.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ressmeyer A, Larsson A, Vollmer E, Dahlen S, Uhlig S, Martin C. Characterisation of guinea pig precision-cut lung slices: comparison with human tissues. Eur Respir J. 2006;28:603–611. doi: 10.1183/09031936.06.00004206. [DOI] [PubMed] [Google Scholar]
- 25.Schleputz M, Rieg AD, Seehase S, Spillner J, Perez-Bouza A, Braunschweig T, Schroeder T, Bernau M, Lambermont V, Schlumbohm C, Sewald K, Autschbach R, Braun A, Kramer BW, Uhlig S, Martin C. Neurally mediated airway constriction in human and other species: a comparative study using precision-cut lung slices (PCLS) PLoS ONE. 2012;7:e47344. doi: 10.1371/journal.pone.0047344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suleiman S, Klassen S, Katz I, Balakirski G, Krabbe J, von Stillfried S, Kintsler S, Braunschweig T, Babendreyer A, Spillner J, Kalverkamp S, Schroder T, Moeller M, Coburn M, Uhlig S, Martin C, Rieg AD. Argon reduces the pulmonary vascular tone in rats and humans by GABA-receptor activation. Sci Rep. 2019;9:1902. doi: 10.1038/s41598-018-38267-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uhlig S, Wollin L. An improved setup for the isolated perfused rat lung. J Pharmacol Toxicol Methods. 1994;31:85–94. doi: 10.1016/1056-8719(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 28.Atzori L, Bannenberg G, Corriga AM, Moldeus P, Ryrfeldt A. Sulfur dioxide-induced bronchoconstriction in the isolated perfused and ventilated guinea-pig lung. Respiration. 1992;59:16–21. doi: 10.1159/000196018. [DOI] [PubMed] [Google Scholar]
- 29.Hugo Sachs Elektronik Harvard Apparatus GmbH, inventors. Operating Instructions for PARI Jet-Nebulizer No. 73-1963. United States patent application. 2008.
- 30.Gozgit JM, Wong MJ, Wardwell S, Tyner JW, Loriaux MM, Mohemmad QK, Narasimhan NI, Shakespeare WC, Wang F, Druker BJ, Clackson T, Rivera VM. Potent activity of ponatinib (AP24534) in models of FLT3-driven acute myeloid leukemia and other hematologic malignancies. Mol Cancer Ther. 2011;10:1028–1035. doi: 10.1158/1535-7163.MCT-10-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang WS, Metcalf CA, Sundaramoorthi R, Wang Y, Zou D, Thomas RM, Zhu X, Cai L, Wen D, Liu S, Romero J, Qi J, Chen I, Banda G, Lentini SP, Das S, Xu Q, Keats J, Wang F, Wardwell S, Ning Y, Snodgrass JT, Broudy MI, Russian K, Zhou T, Commodore L, Narasimhan NI, Mohemmad QK, Iuliucci J, Rivera VM, Dalgarno DC, Sawyer TK, Clackson T, Shakespeare WC. Discovery of 3-[2-(imidazo[1,2-b]pyridazin-3-yl)ethynyl]-4-methyl-N-{4-[(4-methylpiperazin-1-y l)methyl]-3-(trifluoromethyl)phenyl}benzamide (AP24534), a potent, orally active pan-inhibitor of breakpoint cluster region-abelson (BCR-ABL) kinase including the T315I gatekeeper mutant. J Med Chem. 2010;53:4701–4719. doi: 10.1021/jm100395q. [DOI] [PubMed] [Google Scholar]
- 32.O'Hare T, Shakespeare WC, Zhu X, Eide CA, Rivera VM, Wang F, Adrian LT, Zhou T, Huang WS, Xu Q, Metcalf CA, III, Tyner JW, Loriaux MM, Corbin AS, Wardwell S, Ning Y, Keats JA, Wang Y, Sundaramoorthi R, Thomas M, Zhou D, Snodgrass J, Commodore L, Sawyer TK, Dalgarno DC, Deininger MW, Druker BJ, Clackson T. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16:401–412. doi: 10.1016/j.ccr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Godl K, Gruss OJ, Eickhoff J, Wissing J, Blencke S, Weber M, Degen H, Brehmer D, Orfi L, Horvath Z, Keri G, Muller S, Cotten M, Ullrich A, Daub H. Proteomic characterization of the angiogenesis inhibitor SU6668 reveals multiple impacts on cellular kinase signaling. Cancer Res. 2005;65:6919–6926. doi: 10.1158/0008-5472.CAN-05-0574. [DOI] [PubMed] [Google Scholar]
- 34.Laird AD, Vajkoczy P, Shawver LK, Thurnher A, Liang C, Mohammadi M, Schlessinger J, Ullrich A, Hubbard SR, Blake RA, Fong TA, Strawn LM, Sun L, Tang C, Hawtin R, Tang F, Shenoy N, Hirth KP, McMahon G, Cherrington, SU6668 is a potent antiangiogenic and antitumor agent that induces regression of established tumors. Cancer Res. 2000;60:4152–4160. [PubMed] [Google Scholar]
- 35.Laird AD, Christensen JG, Li G, Carver J, Smith K, Xin X, Moss KG, Louie SG, Mendel DB, Cherrington JM. SU6668 inhibits Flk-1/KDR and PDGFRbeta in vivo, resulting in rapid apoptosis of tumor vasculature and tumor regression in mice. FASEB J. 2002;16:681–690. doi: 10.1096/fj.01-0700com. [DOI] [PubMed] [Google Scholar]
- 36.Medarametla V, Festin S, Sugarragchaa C, Eng A, Naqwi A, Wiedmann T, Zisman LS. PK10453, a nonselective platelet-derived growth factor receptor inhibitor, prevents the progression of pulmonary arterial hypertension. Pulm Circ. 2014;4:82–102. doi: 10.1086/674881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burges RA, Gardiner DG, Gwilt M, Higgins AJ, Blackburn KJ, Campbell SF, Cross PE, Stubbs JK. Calcium channel blocking properties of amlodipine in vascular smooth muscle and cardiac muscle in vitro: evidence for voltage modulation of vascular dihydropyridine receptors. J Cardiovasc Pharmacol. 1987;9:110–119. [PubMed] [Google Scholar]
- 38.Pfitzer G, Sonntag-Bensch D, Brkic-Koric D. Thiophosphorylation-induced Ca(2+) sensitization of guinea-pig ileum contractility is not mediated by Rho-associated kinase. J Physiol. 2001;533:651–664. doi: 10.1111/j.1469-7793.2001.t01-2-00651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pollack IF, Kawecki S. The effect of calphostin C, a potent photodependent protein kinase C inhibitor, on the proliferation of glioma cells in vitro. J Neurooncol. 1997;31:255–266. doi: 10.1023/A:1005729626354. [DOI] [PubMed] [Google Scholar]
- 40.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 41.Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 42.Saito SY, Hori M, Ozaki H, Karaki H. Cytochalasin D inhibits smooth muscle contraction by directly inhibiting contractile apparatus. J Smooth Muscle Res. 1996;32:51–60. doi: 10.1540/jsmr.32.51. [DOI] [PubMed] [Google Scholar]
- 43.Jones RL, Giembycz MA, Woodward DF. Prostanoid receptor antagonists: development strategies and therapeutic applications. Br J Pharmacol. 2009;158:104–145. doi: 10.1111/j.1476-5381.2009.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones RL, Wise H, Clark R, Whiting RL, Bley KR. Investigation of the prostacyclin (IP) receptor antagonist RO1138452 on isolated blood vessel and platelet preparations. Br J Pharmacol. 2006;149:110–120. doi: 10.1038/sj.bjp.0706841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Durocher Y, Perret S, Thibaudeau E, Gaumond MH, Kamen A, Stocco R, Abramovitz M. A reporter gene assay for high-throughput screening of G-protein-coupled receptors stably or transiently expressed in HEK293 EBNA cells grown in suspension culture. Anal Biochem. 2000;284:316–326. doi: 10.1006/abio.2000.4698. [DOI] [PubMed] [Google Scholar]
- 46.Af Forselles KJ, Root J, Clarke T, Davey D, Aughton K, Dack K, Pullen N. In vitro and in vivo characterization of PF-04418948, a novel, potent and selective prostaglandin EP(2) receptor antagonist. Br J Pharmacol. 2011;164:1847–1856. doi: 10.1111/j.1476-5381.2011.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Birrell MA, Maher SA, Buckley J, Dale N, Bonvini S, Raemdonck K, Pullen N, Giembycz MA, Belvisi MG. Selectivity profiling of the novel EP2 receptor antagonist, PF-04418948, in functional bioassay systems: atypical affinity at the guinea pig EP2 receptor. Br J Pharmacol. 2013;168:129–138. doi: 10.1111/j.1476-5381.2012.02088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Juteau H, Gareau Y, Labelle M, Sturino CF, Sawyer N, Tremblay N, Lamontagne S, Carriere MC, Denis D, Metters KM. Structure–activity relationship of cinnamic acylsulfonamide analogues on the human EP3 prostanoid receptor. Bioorg Med Chem. 2001;9:1977–1984. doi: 10.1016/S0968-0896(01)00110-9. [DOI] [PubMed] [Google Scholar]
- 49.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, Kim DS, Kolb M, Nicholson AG, Noble PW, Selman M, Taniguchi H, Brun M, Le MF, Girard M, Stowasser S, Schlenker-Herceg R, Disse B, Collard HR. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014 doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 50.Chopra LC, Hucks D, Twort CH, Ward JP. Effects of protein tyrosine kinase inhibitors on contractility of isolated bronchioles of the rat. Am J Respir Cell Mol Biol. 1997;16:372–378. doi: 10.1165/ajrcmb.16.4.9115747. [DOI] [PubMed] [Google Scholar]
- 51.Schaafsma D, Gosens R, Bos IS, Meurs H, Zaagsma J, Nelemans SA. Role of contractile prostaglandins and Rho-kinase in growth factor-induced airway smooth muscle contraction. Respir Res. 2005;6:85. doi: 10.1186/1465-9921-6-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bornfeldt KE, Campbell JS, Koyama H, Argast GM, Leslie CC, Raines EW, Krebs EG, Ross R. The mitogen-activated protein kinase pathway can mediate growth inhibition and proliferation in smooth muscle cells. Dependence on the availability of downstream targets. J Clin Invest. 1997;100:875–885. doi: 10.1172/JCI119603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boulven I, Palmier B, Robin P, Vacher M, Harbon S, Leiber D. Platelet-derived growth factor stimulates phospholipase C-gamma 1, extracellular signal-regulated kinase, and arachidonic acid release in rat myometrial cells: contribution to cyclic 3',5'-adenosine monophosphate production and effect on cell proliferation. Biol Reprod. 2001;65:496–506. doi: 10.1095/biolreprod65.2.496. [DOI] [PubMed] [Google Scholar]
- 54.Gijon MA, Leslie CC. Regulation of arachidonic acid release and cytosolic phospholipase A2 activation. J Leukoc Biol. 1999;65:330–336. doi: 10.1002/jlb.65.3.330. [DOI] [PubMed] [Google Scholar]
- 55.Karpova AY, Abe MK, Li J, Liu PT, Rhee JM, Kuo WL, Hershenson MB. MEK1 is required for PDGF-induced ERK activation and DNA synthesis in tracheal myocytes. Am J Physiol. 1997;272:L558–L565. doi: 10.1152/ajplung.1997.272.3.L558. [DOI] [PubMed] [Google Scholar]
- 56.Markovic T, Jakopin Z, Dolenc MS, Mlinaric-Rascan I. Structural features of subtype-selective EP receptor modulators. Drug Discov Today. 2017;22:57–71. doi: 10.1016/j.drudis.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 57.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 58.Puetz S, Lubomirov LT, Pfitzer G. Regulation of smooth muscle contraction by small GTPases. Physiology (Bethesda) 2009;24:342–356. doi: 10.1152/physiol.00023.2009. [DOI] [PubMed] [Google Scholar]
- 59.Gunst SJ, Zhang W. Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am J Physiol Cell Physiol. 2008;295:C576–C587. doi: 10.1152/ajpcell.00253.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beazely MA, Weerapura M, MacDonald JF. Abelson tyrosine kinase links PDGFbeta receptor activation to cytoskeletal regulation of NMDA receptors in CA1 hippocampal neurons. Mol Brain. 2008;1:20. doi: 10.1186/1756-6606-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hantschel O, Superti-Furga G. Regulation of the c-Abl and Bcr-Abl tyrosine kinases. Nat Rev Mol Cell Biol. 2004;5:33–44. doi: 10.1038/nrm1280. [DOI] [PubMed] [Google Scholar]
- 62.Tang DD. Critical role of actin-associated proteins in smooth muscle contraction, cell proliferation, airway hyperresponsiveness and airway remodeling. Respir Res. 2015;16:134. doi: 10.1186/s12931-015-0296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zandy NL, Playford M, Pendergast AM. Abl tyrosine kinases regulate cell-cell adhesion through Rho GTPases. Proc Natl Acad Sci USA. 2007;104:17686–17691. doi: 10.1073/pnas.0703077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zandy NL, Pendergast AM. Abl tyrosine kinases modulate cadherin-dependent adhesion upstream and downstream of Rho family GTPases. Cell Cycle. 2008;7:444–448. doi: 10.4161/cc.7.4.5452. [DOI] [PubMed] [Google Scholar]
- 65.Cleary RA, Wang R, Wang T, Tang DD. Role of Abl in airway hyperresponsiveness and airway remodeling. Respir Res. 2013;14:105. doi: 10.1186/1465-9921-14-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nayak AP, Lim JM, Arbel E, Wang R, Villalba DR, Nguyen TL, Schaible N, Krishnan R, Tang DD, Penn RB. Cooperativity between beta-agonists and c-Abl inhibitors in regulating airway smooth muscle relaxation. FASEB J. 2021;35:e21674. doi: 10.1096/fj.202100154R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu C, Zuo J, Pertens E, Helli PB, Janssen LJ. Regulation of Rho/ROCK signaling in airway smooth muscle by membrane potential and [Ca2+]i. Am J Physiol Lung Cell Mol Physiol. 2005;289:L574–L582. doi: 10.1152/ajplung.00134.2005. [DOI] [PubMed] [Google Scholar]
- 68.Abe K, Toba M, Alzoubi A, Koubsky K, Ito M, Ota H, Gairhe S, Gerthoffer WT, Fagan KA, McMurtry IF, Oka M. Tyrosine kinase inhibitors are potent acute pulmonary vasodilators in rats. Am J Respir Cell Mol Biol. 2011;45:804–808. doi: 10.1165/rcmb.2010-0371OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ozgur-Akdemir A, Demirturk K, Karabakan M, Volkan-Oztekin C, Abdulkadir NA, Cetinkaya M, Gur S, Hellstrom WJ. Imatinib mesylate (Gleevec) as protein-tyrosine kinase inhibitor elicits smooth muscle relaxation in isolated human prostatic tissue. Urology. 2011;78:968–976. doi: 10.1016/j.urology.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 70.Pankey EA, Thammasibon S, Lasker GF, Baber S, Lasky JA, Kadowitz PJ. Imatinib attenuates monocrotaline pulmonary hypertension and has potent vasodilator activity in the pulmonary and systemic vascular beds of the rat. Am J Physiol Heart Circ Physiol. 2013;305:1288–1296. doi: 10.1152/ajpheart.00329.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Filep JG, Battistini B, Sirois P. Pharmacological modulation of endothelin-induced contraction of guinea-pig isolated airways and thromboxane release. Br J Pharmacol. 1991;103:1633–1640. doi: 10.1111/j.1476-5381.1991.tb09840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park SJ, Lee JJ, Vanhoutte PM. Endothelin-1 releases endothelium-derived endoperoxides and thromboxane A2 in porcine coronary arteries with regenerated endothelium. Zhongguo Yao Li Xue Bao. 1999;20:872–878. [PubMed] [Google Scholar]
- 73.Taddei S, Vanhoutte PM. Endothelium-dependent contractions to endothelin in the rat aorta are mediated by thromboxane A2. J Cardiovasc Pharmacol. 1993;22(Suppl 8):S328–S331. doi: 10.1097/00005344-199322008-00086. [DOI] [PubMed] [Google Scholar]
- 74.Wilkes BM, Mento PF, Hollander AM, Maita ME, Sung S, Girardi EP. Endothelin receptors in human placenta: relationship to vascular resistance and thromboxane release. Am J Physiol. 1990;258:E864–E870. doi: 10.1152/ajpendo.1990.258.5.E864. [DOI] [PubMed] [Google Scholar]
- 75.Hernandez JM, Janssen LJ. Revisiting the usefulness of thromboxane-A2 modulation in the treatment of bronchoconstriction in asthma. Can J Physiol Pharmacol. 2015;93:111–117. doi: 10.1139/cjpp-2014-0364. [DOI] [PubMed] [Google Scholar]
- 76.Martin C, Uhlig S, Ullrich V. Cytokine-induced bronchoconstriction in precision-cut lung slices is dependent upon cyclooxygenase-2 and thromboxane receptor activation. Am J Respir Cell Mol Biol. 2001;24:139–145. doi: 10.1165/ajrcmb.24.2.3545. [DOI] [PubMed] [Google Scholar]
- 77.Gryglewski RJ. Prostacyclin among prostanoids. Pharmacol Rep. 2008;60:3–11. [PubMed] [Google Scholar]
- 78.Jia L, Wang R, Tang DD. Abl regulates smooth muscle cell proliferation by modulating actin dynamics and ERK1/2 activation. Am J Physiol Cell Physiol. 2012;302:C1026–C1034. doi: 10.1152/ajpcell.00373.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.