Abstract
Background
Fatigue is the most frequent and burdensome symptom of patients with diffuse glioma. It is closely linked to decreased health-related quality of life and symptoms such as depression and sleep disturbances. Currently, there is no evidence-based treatment that targets severe fatigue in patients with brain tumours. Cognitive behavioural therapy is aimed at fatigue-maintaining beliefs and behaviour. This therapy has been proven effective in reducing severe fatigue in cancer survivors and patients with multiple sclerosis. A blended therapy program combines sessions with a therapist with therapist-guided web-based therapy modules. The aim of this randomized controlled trial is to determine the efficacy of blended cognitive behavioural therapy in treating severe fatigue in patients with diffuse glioma.
Methods
We will include a maximum of 100 patients with diffuse glioma with clinically and radiologically stable disease and severe fatigue (i.e. Checklist Individual Strength, subscale fatigue severity ≥ 35). Patients will be randomized to blended cognitive behavioural therapy or a waiting list condition. The 12-week intervention GRIP on fatigue consists of five patient-therapist sessions and five to eight individualized web-based therapy modules supported by email contact. The primary outcome measure is fatigue severity. Secondary outcome measures include sleep quality, health-related quality of life, depression, anxiety, functional impairment and subjective and objective cognitive functioning. Primary and secondary outcome measures will be assessed at baseline and after 14 and 24 weeks. Magnetoencephalography and MRI will be used to evaluate potential biomarkers for intervention success. This trial has a Bayesian design: we will conduct multiple interim analyses to test for efficacy or futility of the trial. This is the first trial within the GRIP trial platform: a platform developing four to five different interventions for the most common symptoms in patients with diffuse glioma.
Discussion
The results of the GRIP on fatigue trial will provide information about the efficacy of this intervention on fatigue in patients with diffuse glioma. Multiple other outcomes and possible predictors of treatment success will also be explored.
Trial registration
Netherlands Trial Register NL8711. Registered on 14 June 2020.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13063-022-06485-5.
Keywords: Psychosocial intervention, Glioma, Fatigue, Blended cognitive behavioural therapy, Web-based, Digital health, Online intervention, Cancer, Health-related quality of life
Background
Patients with diffuse glioma are a distinct group within the cancer population, characterized by continuous tumour growth leading to inevitable death. Malignant gliomas are the most common primary brain tumour with an incidence of 6 per 100,000 in Europe [1]. During the disease trajectory, patients experience a multitude of symptoms such as fatigue, cognitive impairment and neurological deficits, resulting in a high symptom burden and impaired health-related quality of life (HRQOL) [2–4].
Fatigue is one of the most frequently reported symptoms in patients with a brain tumour. Cancer-related fatigue (CRF) is “a distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer that is not proportional to recent activity and interferes with usual functioning” [5]. The prevalence of fatigue in diffuse glioma patients varies between studies; it is reported to affect up to 96% of patients in different stages of the disease [6–10]. In a study of long-term survivors with low-grade glioma, 40% of the survivors reported to be severely fatigued [11]. Additionally in a qualitative study, patients with diffuse glioma described their tiredness as the most severe of the multiple symptoms that they experience [12].
The aetiology of fatigue in patients with a brain tumour is complex and poorly understood. Demographic, biomedical, neuropsychological, psychosocial and behavioural factors may contribute to the origin and persistence of fatigue [13]. Fatigue is associated with cognitive complaints and various other symptoms in different symptom clusters, such as depression, anxiety and sleep–wake disturbances, and has a negative influence on role functioning [10]. These different clusters of symptoms have a large impact on everyday life of patients and is linked to a decreased HRQOL [7, 10, 13, 14].
No evidence-based intervention is currently available for the treatment of fatigue in patients with a brain tumour, even though it is thought that effective treatment could improve HRQOL [3, 13]. A 2016 Cochrane review on treating fatigue in primary brain tumour patients could only identify one randomized controlled trial (RCT) that restricted inclusion to severely fatigued patients and had fatigue as the primary outcome measure [8]. This trial was conducted by our group and did not show an effect of the psychostimulant modafinil on fatigue [15]. Inclusion and follow-up rates were lower than expected, because patients were reluctant to take more medication or they experienced side effects. However, several trials that did not limit inclusion to severely fatigued brain tumour patients, but also included brain tumour patients without severe fatigue, have determined positive effects of cognitive rehabilitation and pharmacological treatment on fatigue [16–20]. Despite the lack of evidence-based interventions, the majority of patients seek treatment for their symptoms through complementary medicine [21], indicating a unmet need to alleviate symptoms.
The treatment of CRF has been studied more extensively in non-central nervous system cancer survivors and in palliative cancer patients. Systematic reviews have indicated that Cognitive behavioural therapy (CBT) can reduce fatigue in these patient groups [20, 22]. Fatigue-specific CBT is based on the assumption that cancer treatment and cancer itself can trigger fatigue, but that factors such as sleep disturbances and unhelpful thoughts can contribute to the persistence of fatigue [23, 24]. Treatments targeting such factors may therefore be promising interventions to alleviate fatigue in brain tumour patients. A recent RCT conducted by our group in severely fatigued cancer patients, who were treated with palliative intent, showed a significant reduction of fatigue after a 12-week face-to-face CBT intervention [25]. Cancer survivors who receive CBT for cancer-related fatigue not only report a reduction in fatigue, but also report less cognitive disability and concentration problems [26]. Also, the blended version of the CBT intervention showed a significant reduction of fatigue in breast cancer survivors [27]. CBT as a treatment for fatigue also seems promising in patients with neurological disease. It has shown to be effective in reducing severe fatigue in patients with multiple sclerosis [28] and is studied in treating post-stroke fatigue [29, 30]. Whether fatigued patients with diffuse glioma could benefit from CBT to resolve or reduce fatigue remains to be determined.
The primary objective of this RCT is to determine whether a 12-week therapist-guided blended CBT (BCBT) intervention will reduce severe fatigue post-intervention in patients with diffuse glioma compared to a waiting list condition (WLC). Secondarily, we will determine whether the intervention results in an improvement of sleep quality, HRQOL, depression, anxiety, functional impairment and subjective and objective cognitive functioning. In addition, we will evaluate invested time by patients and therapists and patients’ satisfaction with the treatment and the online platform. Furthermore, we will investigate possible biomarkers for treatment response with exploratory measurements (e.g. advanced neuroimaging and neurophysiology [31–35]).
Methods
This intervention, the GRIP on fatigue trial, is part of the Guarding Quality of Survivorship (GRIP) trial platform. The aim of this platform is to develop four to five different interventions for the most common symptoms in patients with diffuse glioma, such as fatigue, cognitive deficits, anxiety and reduced physical fitness [3]. This trial is the first to be launched within this platform. The other trials are under development.
Recruitment, screening and randomization
Patients visiting the CCA Brain Tumour Center Amsterdam (Amsterdam University Medical Centers)—a tertiary referral centre for patients with brain tumours—will be screened by their treating health care professional (e.g. neurologist, neurosurgeon, psychologist). Patients diagnosed with histologically confirmed diffuse glioma (WHO grade 2, 3 or 4) with clinically and radiologically stable disease and an expected survival of at least three months are eligible for inclusion. A set of laboratory tests is performed: haemoglobin, CRP, sedimentation rate, leukocyte count and differentiation, thrombocyte count, TSH, LDH, ASAT, ALAT, gamma-GT, sodium, potassium, urea, creatinine and glucose. These laboratory results will be screened to exclude medically treatable causes of fatigue.
Patients complete screening questionnaires to check for eligibility: Checklist Individual Strength (CIS) [36] and the Beck Depression Inventory Primary Care (BDI-PC) [37]. See Table 1 for all inclusion and exclusion criteria. The presence of severe fatigue is a criterion for inclusion and is reflected by a score ≥ 35 on the CIS subscale fatigue severity (CIS-fatigue) [36]. The Mini-International Neuropsychiatric Interview-depressive disorder [38] will be conducted over the phone if the patient has a BDI-PC score ≥4. If the criteria of a depression are met, the patient will not be included and will be referred for diagnostics and treatment. Demographic characteristics and disease-related determinants will be collected from the medical file. Comorbidities are assessed by the researcher using the Cumulative Illness Rating Scale [40].
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| (1) Histologically confirmed diffuse glioma WHO grade 2, 3 or 4 | (1) Treatable somatic cause that could explain the presence of severe fatigue (other than the underlying disease and its treatment) |
| (2) Age ≥ 18 years | (2) Primary sleep disorders previously diagnosed by a physician |
| (3) CIS subscale fatigue severity ≥ 35 (36) | (3) Current treatment by a psychiatrist or psychologist for a psychiatric disorder |
| (4) Expected survival of at least three months, as determined by treating clinician | (4) Suspected depression by screening with BDI-PC≥4 [37] and The Mini-International Neuropsychiatric Interview depressive disorder [38] |
| (5) No oncological treatment for at least two months prior to inclusion | (5) Pregnancy or given birth in the past three months |
| (6) No signs of radiological or clinical tumour progression at the time of inclusion | (6) Pharmacological treatment for fatigue, started in the past three months |
| (7) Able to speak, read and write Dutch | (7) Karnofsky Performance Status score <70 [39] |
| (8) Access and ability to use the internet | (8) Corticosteroid use |
| (9) Written informed consent |
Abbreviations: CIS Checklist Individual Strength, BDI-PC Beck Depression Inventory – Primary Care
After signing for informed consent and completing the screening procedure, the patient will be randomized to the BCBT intervention or to the WLC group with the use of the web-based computer program, Castor EDC [41]. This program automatically randomizes the patient to one of the two conditions. To keep the number of patients randomized to the two arms balanced throughout the trial, the program uses block randomization with a block size of two and four in random order. A block of two consists of one randomization to the BCGT group and one randomization to the WLC group in random order. A block of four consists of two BCGT randomisations and two WLC group randomisations in random order [42]. After the screening process, the patient is informed on the outcome of the randomization.
Assessments and procedures
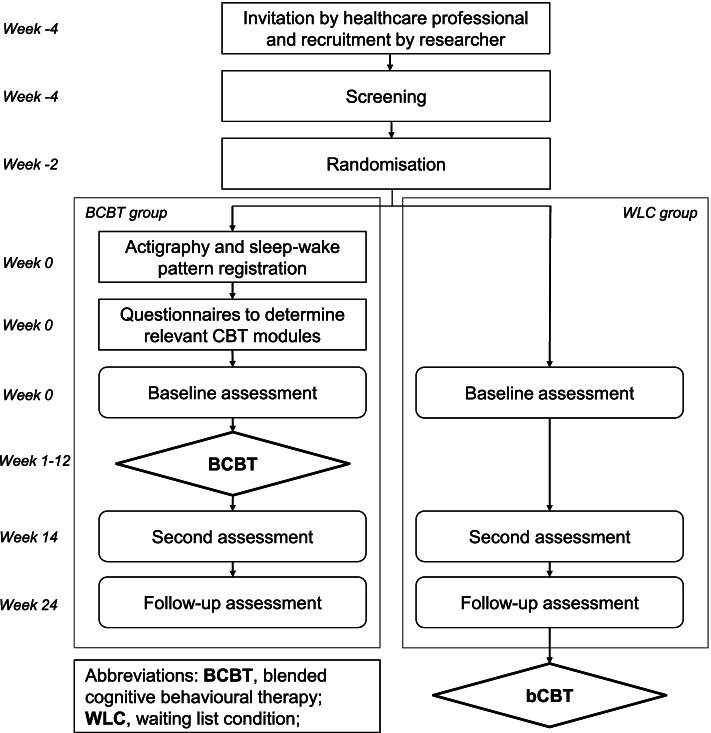
There are three trial assessments (see Fig. 1 for the flowchart): a baseline assessment after randomization and before the start of the intervention, a second assessment 14 weeks after baseline, and a follow-up assessment 24 weeks after baseline. The baseline and second assessments consist of web-based questionnaires, which are completed at home, and several measurements in the hospital (e.g. MRI, magnetoencephalography, neurocognitive testing and neurological examination). The questionnaires and measurements are explained in further detail at the endpoints section. The follow-up assessment only consists of web-based questionnaires.
Fig. 1.
Flowchart of the trial
All patients will be treated in concordance with national and regional glioma clinical practice guidelines of the National Dutch Working Group on Neuro-Oncology [1]. Patients are asked not to follow any other interventions or use pharmaceuticals aimed at treating fatigue during trial participation. Patients assigned to the WLC group are on a waiting list and will have the opportunity to do the BCBT program after the follow-up period (see Fig. 1). Patients can withdraw from the study at any time for any reason. The researcher can withdraw patients from the study in case of tumour progression or incorrect enrolment. Patients that drop out or are withdrawn are asked to still conduct the second and follow-up assessments.
Intervention: GRIP on fatigue
GRIP on fatigue is a multimodal Dutch intervention with therapist sessions and online modules with therapist feedback. BCBT for CRF in patients with a brain tumour is directed at the beliefs and behaviour of the patient that contribute to the persistence of fatigue.
The intervention lasts 12 weeks. There are five patient–therapist sessions, of which those at the start and finish of the intervention are face to face. The other sessions can be either face-to-face or via secure video consultation, depending on the patients’ preferences. This is combined with five to eight web-based therapy modules delivered via the web portal Minddistrict (www.minddistrict.nl, CE marking 2017/590-01) hosted by Intermax (SO27001 and NEN-7510 certified). The patient is supported by messaging and feedback from the therapist within the online portal. This portal is available via a web browser on the computer or via an application for tablet and mobile phone.
The information and assignments are based on several evidence-based CBT interventions in different populations targeting fatigue by our research group [27, 43, 44]. These interventions have been adapted by experts on brain tumours and CRF to target patients with a brain tumour. The intervention is based on the assumption that having a brain tumour and/or the operation and treatment may initially trigger fatigue, but that other factors such as sleep disturbance, anxiety, physical inactivity, and dysfunctional thoughts about fatigue are responsible for the persistence of fatigue [13, 23, 43]. The module on fear of recurrence is based on the Conquer Fear program, which has shown to be effective in reducing fear of recurrence in comparison to relaxation training [45]. It is expected that patients spend approximately one hour per day on completing the online intervention.
As there are many individual differences in fatigue maintaining cognitive-behavioural factors, patients who are treated with BCBT undergo assessments to determine the relevant fatigue-maintaining factors and select the right treatment modules addressing these factors. Those instruments form an integral part of the intervention and include a set of questionnaires, actigraphy for fourteen consecutive days and simultaneously keeping a sleep–wake diary on seven consecutive days before the intervention starts. With actigraphy, two different activity patterns will be distinguished: relatively active or low active. The instruments are used for tailoring the treatment, for example the module Activity regulation will be personalized with regards to the activity pattern of the patient. Table 2 gives an overview of all modules of the intervention and their content. Some of the modules are optional and only indicated with specific scores on the relevant questionnaires.
Table 2.
Content of the online modules and assessments
| Online modules | Assessments |
|---|---|
|
1. Introduction and goals The patient formulates positive and tangible goals, which consist of activities they want to do when no longer severely fatigued. |
|
|
2. Sleep and rest The patient makes a sleep schedule and keeps an online diary with sleep and wake times. A regular sleep–wake cycle and sleep hygiene are discussed. Instructions are given on how to improve these. |
- Sickness Impact Profile (subscale sleep and rest) [46] - Registration of bedtime, wake-up time and sleep during the day for seven consecutive days |
|
3. Fatigue-related cognitions Loss of control over fatigue symptoms and thoughts, fatigue catastrophizing and dysfunctional thoughts are assessed. The patient does exercises to address and change their dysfunctional thoughts and keeps an online diary about these thoughts. Patients learn to focus less on fatigue. |
- Fatigue Catastrophizing Scale [47] - Illness Management Questionnaire factor III [48] - Self Efficacy Scale Fatigue [49] |
|
4. Activity regulation The patient with a ‘relatively active’ activity pattern learns to distribute activities more evenly. Then both ‘relatively active’ and ‘low active’ patients systematically increase their physical activity with a graded activity program with walking or cycling. They track their daily progress in an online diary. They learn how to solve problems with activity regulation. The module aims to change activity-impeding beliefs and increase the physical activity level of patients. |
- With actigraphy (actometer around the ankle for 14 consecutive days) the level of activity is objectified [50]. The activity pattern will be rated as ‘Low active’ or ‘Relatively active’. |
|
Submodule 4A: Regulation of social activities (optional) The relationship between cancer, fatigue and a reduction of social activities as well as cognitions about social activities are assessed. The patient increases his/her social activity level. |
- Sickness Impact Profile (indication for this optional submodule: score subscale social activities ≥ 100) [46] |
|
Submodule 4B: Regulation of mental activities (optional) The patient learns about cognitive deficits and how to deal with them. The patient increases their mental activity level. |
- Checklist Individual Strength (indication for this optional submodule: score subscale concentration ≥ 18) [36] |
|
Submodule 4C: Going back to work (optional) The patient makes a plan to return to work or increase working hours. |
(indication for this optional submodule: if the patient has set a goal to return to work or increase working hours) |
|
5. Fear of disease progression (optional) Thoughts and situations that trigger fear regarding the future or tumour growth are assessed. The patient learns to be more accepting towards anxious feelings and to handle these feelings with exercises based on detached mindfulness, meta-cognitive therapy and exposure. |
- Fear of Progression Questionnaire (indication for the module: score ≥ 34) [51] - Beck Anxiety Inventory (indication for the module: score ≥ 36) [52] |
|
6. Social support (optional) Reactions of the partner and significant others to fatigue are assessed. Perceived discrepancy between actual and desired social support, experiences with negative social interactions and unrealistic expectations of others are assessed. The goal of this module is to support emotional independence of others and to become more assertive, as far as fatigue is concerned. |
- Social Support List, subscale discrepancy (indication for the module: score ≥ 50) and subscale negative interactions (indication for the module: score ≥ 14) [53] |
|
7. Living with a brain tumour (optional) This module focuses on uncertainty about the future and how one can deal with the fact that one has an incurable disease. Several elements from meaning-centred psychotherapy, well-being therapy and writing therapy are used to help the patients to deal with the disease trajectory. |
- Illness Cognition Questionnaire, subscale acceptance (indication for the module: score ≤ 12) and subscale helplessness (indication for the module: score ≥ 14) [54] - Impact Event Scale, subscale avoidance (indication for the module: score ≥ 10) and subscale intrusion (indication for the module: score ≥ 10) [55] |
|
8. Realizing goals The patient looks back at the goals set in the first module and makes a plan to realize these goals. The intervention is evaluated. |
The intervention will be provided by two to four trained and experienced cognitive-behavioural therapists, working at the Expert Center for Chronic Fatigue of the Amsterdam UMC, a tertiary treatment centre for fatigue. Every patient is assigned to one therapist, based on the availability of the therapist. The therapists are trained in delivering interventions on fatigue and this intervention specifically. They will be supervised every two weeks by a therapist with experience in BCBT in patients with a brain tumour.
Primary and secondary endpoints
The primary outcome measure is the CIS-fatigue score at the second assessment, 14 weeks after baseline. The eight CIS-fatigue items are rated on a seven-point Likert scale with a total score ranging from 8 to 56. A score ≥ 35 indicates the presence of severe fatigue [36]. See Additional file 1 for an overview of all primary, secondary and exploratory assessments.
The set of secondary outcomes consists of several symptoms, quality of life and level of functioning measured by questionnaires at baseline, after 14 weeks and in follow-up, including depression, HRQOL and anxiety.
Neurocognitive functioning is tested using a standardized clinical test battery that is normally used for clinical care to evaluate preoperative and postoperative cognitive status. It consists of widely used standardized psychometric instruments with published normative data, such as the Stroop Color Word Test [56] and the Rey Complex Figure Test [57].
Furthermore, data about the usage of the online platform are collected, such as the number of logins, start and finish date of a module, completion date of the diaries and the amount of time spent on the platform. Therapists will register the time they invest per patient. Patients’ expectations of intervention outcome [58] and their satisfaction with the intervention and the platform [59] will be assessed before and after the intervention, respectively.
Exploratory measurements
At baseline and after 14 weeks several exploratory measurements will be conducted, see Additional file 1 for an overview. These measurements are used to identify possible biomarkers for intervention success.
Brain connectivity and network topology will be assessed with magnetoencephalography, resting-state functional MRI, and diffusion MRI. Magnetoencephalography provides a non-invasive tool to study the brain’s neuronal networks and functional connectivity [60, 61]. Properties of baseline network topology of the brain may predict outcome of CBT [34, 35].
Furthermore, patients will undergo neurological examination, using the Neurologic Assessment in Neuro-Oncology Scale [62]. This is a standardized method of neurological physical examination in neuro-oncology patients and an objective clinician-reported outcome of neurologic function.
Trial and data management
Personal data will be handled confidentially and data collection will comply with the EU General Data Protection Regulation and the Dutch Act on Implementation of the General Data Protection Regulation. Participants will be assigned a study identification number, which will be used on all research documents. This number is stored in a master file, only accessible to the principal investigator, co-investigators and study monitor. The web-based application Castor EDC will be used for coded data entry and storage [41]. All the questionnaires are conducted digitally with the use of this platform and automatically stored. Data concerning the usage of the online platform Minddistrict are stored within the online platform itself. Neuropsychological assessments and informed consent forms will be stored in locked file cabinets at the Department of Medical Psychology of the Amsterdam UMC. All magnetoencephalography and MRI files will be stored on a network server at the hospital. Data will be stored until 15 years after the completion of the project. This study will be subject to independent on-site monitoring in accordance with the Dutch Federation of University Medical Centers quality assurance advice regarding research involving human subjects.
Sample size calculation and Bayesian design
A multicentre trial by our group on a 12-week BCBT intervention for fatigue among severely fatigued patients with advanced cancer showed that 14 weeks after baseline, patients in the intervention group reported significantly lower CIS-fatigue scores compared to patients receiving care as usual (−7.22, 97.5% CI −12.73 to −1.72; p=0.003, Cohen’s d=0.72) [25]. Based on these outcomes we anticipated a standardized effect size (Cohen’s d) of 0.7 on the CIS-fatigue.
The required number of patients was calculated as 40 per arm. As we expect a maximum of 20% to drop out before the second assessment at 14 weeks after baseline, we aim to recruit a maximum of 100 patients. The trial uses a Bayesian two-arm multistage trial design, where repeated evaluations for efficacy and futility take place after the second assessment CIS-fatigue scores have been observed for 20, 25, 30 and 35 patients in each arm. Frequentist operating characteristics of the trial were evaluated by means of simulation. In the Bayesian model we assumed CIS-fatigue scores to be normally distributed and weakly informative conjugate normal-gamma priors will be used. Parameters for the prior distribution were chosen such that the impact of the prior on the posterior means for the mean and standard deviation for the CIS fatigue scores were negligible. The empirical one-sided significance level was 2.4% and below the desired one-sided significance level of 2.5%. Empirical power was 79% when the standardized effect size was 0.7 (Cohen’s d). The expected sample size is 27.4 per arm (54.8 in total) in case of equal means and 31.5 (63 in total) in case standardized effect size is 0.7. A standard two-arm trial without interim analyses would require 43 patients per arm, accounting for 20% drop-out.
Termination for efficacy or futility
Both at the interim analyses and final analysis, efficacy will be concluded when the posterior probability that the mean CIS-fatigue scores for BCBT are lower than the mean CIS-fatigue scores for usual care exceeds 99%. If this happens at an interim analysis, the trial is stopped and efficacy is concluded. The trial will be stopped for futility as soon as an interim analysis shows that the predictive probability of concluding efficacy at the end of the trial drops below 10%.
Statistical analysis
The final analysis is conducted on an intention-to-treat basis. Efficacy of the intervention will be concluded based on the posterior probability as outlined in the previous paragraph. Cohen’s d will be calculated as an effect size for CIS-fatigue scores where multiple imputation will be used to deal with missing second measurements. Sensitivity analyses will be performed to assess the robustness of the standardized effect size under different imputation strategies.
In addition, analysis of covariance (ANCOVA) will be performed for the secondary outcomes of the second assessment, with the baseline score on the dependent measure as covariate and group allocation as the fixed factor. A p-level of 0.05 will be used. Longer-term follow-up effects will also be tested using ANCOVA, with the baseline score on the dependent measure as covariate. Different tumour types will be added to the model as covariates. Cohen’s d will be calculated as effect sizes for the secondary outcomes.
Discussion
Fatigue is the most prevalent symptom of brain tumour patients and is linked to several symptoms, such as depression, sleep disturbances, anxiety, and decreased HRQOL. Patients with diffuse glioma have poor survival and high symptom burden, so the quality of survivorship should be an important factor of treatment. A significant part of care for quality of survivorship is symptom management [63]. Cognitive behavioural interventions seem promising in reducing fatigue severity based on their effectiveness in cancer survivors and palliative cancer patients.
One of the challenges in brain tumour research is the low rate of patient inclusion. Multiple provider reported barriers could explain these low rates, including concerns about the costs and time for patients, frequent hospital visits, suboptimal discussion of possibilities of trial participation and barriers in completing follow-up [64, 65]. This trial tackles some of these barriers by using a blended care intervention with telemedicine limiting visits and travel time. The recruiting clinicians are part of the research team, ensuring a good workflow for patients and clinicians. A researcher will coordinate recruitment, limiting the time spent on recruitment by clinicians. Also, by randomizing patients to a WLC group in the trial, we overcome a potential barrier for patients in enrolling in a trial compared to trials with a care as usual control group. If recruitment is slower than expected after a year, we will include more hospitals for recruitment.
Furthermore, this trial incorporates a Bayesian trial design with a flexible number of patients to be included, ensuring that only the number of patients actually needed to show relevant results will be included. Our trial will include patients with different types of diffuse glioma. This can result in different survival rates, rates of tumour recurrence and dropouts. Subgroup analyses per tumour type may help to explore potentially varying treatment effects.
Potential limitations of the current study design are the inability to blind participants and researchers for the given intervention and the lack of a true placebo condition in the WLC group. These are issues that in general arise in all psychotherapy trials [66]. In this study, patients randomized to the WLC group can be treated for fatigue after the follow-up measurements. It has been hypothesized that a WLC could both result in a placebo and a nocebo response. Provided reasons include that a WLC with future treatment could provide patients with hope for future symptom reduction thus inducing a placebo response, but also that a WLC might result in a nocebo response because the patient has already expressed interest in the therapy and therefore is less motivated to change their behaviour awaiting the promised treatment [66–68]. However, not including a waiting list condition in research in this population is questionable, since patients suffer from severe fatigue with currently no other effective treatment available. Considering this, a WLC seems to be appropriate for this group of vulnerable patients.
The GRIP on fatigue trial will provide information on the efficacy of BCBT compared to a WLC in reducing severe fatigue in patients with diffuse glioma. Secondary outcome measures will include different questionnaires concerning HRQOL, subjective cognitive impairments and symptoms such as depression and anxiety. We additionally aim to investigate exploratory measurements that may relate to treatment response and ultimately predict treatment outcome. If proven effective, we aim to offer the intervention as part of usual care for this group of patients.
Trial status
The protocol reported here is version 8 dated 23 March 2020. Recruitment of patients has started on 11 June 2020 and will continue until at least 1 March 2024. At submission of this paper, the first twelve patients were enrolled in the study.
Supplementary Information
Additional file 1: Table S1. Schedule of enrolment, interventions, and assessments.
Acknowledgements
Not applicable.
Abbreviations
- BCBT
Blended cognitive behavioural therapy
- BDI-PC
Beck Depression Inventory Primary Care
- CBT
Cognitive behavioural therapy
- CIS
Checklist Individual Strength
- CIS-fatigue
Checklist Individual Strength, subscale fatigue severity
- CRF
Cancer-related fatigue
- GRIP
Guarding quality of survivorship
- HRQOL
Health-related quality of life
- RCT
Randomized controlled trial
- WLC
Waiting list condition
Authors’ contributions
JR is the coordinating researcher and responsible for recruitment, data collection and drafting of this manuscript. LD and MK supervise the trial. HK, JR and MK designed the intervention. JR designed the therapy modules with help from HK’s experience with therapeutic guided blended cognitive behavioural interventions and MK’s experience with psychosocial problems in neuro-oncology. HK provided the training and supervision of therapists. PV designed the Bayesian method of this trial and the statistical analysis. PWH, MK, LD, TW and MK founded and designed the GRIP trial platform. JR, LD, HK, PWH, MK, TW, PV, LS and MK have contributed to the trial conception and design. The authors read and approved the final manuscript.
Funding
This trial and the GRIP trial platform are funded by the Stichting Anita Veldman Foundation. The foundation is not involved in the design of the study; collection, analysis, and interpretation of data; and in writing the manuscript.
Availability of data and materials
A core anonymised dataset of the primary and secondary outcome measurements will be published online after completion and publication of this trial. The data generated during this trial will also be available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The medical research ethics committee of the Amsterdam University Medical Centers reviewed and approved the protocol (reference no. 2019.714). Any future substantial changes to the protocol will be reviewed and approved by the same medical research ethics committee. The aims, timelines, and outcome measures of the trial will be explained to all eligible patients. They will be informed that they can drop out at any time without consequences. To indicate consent, the participant will sign the informed consent form. Any changes to the study protocol of interest to the participants will be communicated accordingly.
Consent for publication
Patients will be informed about the process of data collection and that collected data will be used for publication and sign for informed consent.
Competing interests
This trial is funded by the Stichting Anita Veldman Foundation. The authors declare that they have no other competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jantine Geertruida Röttgering, Email: j.rottgering@amsterdamumc.nl.
Linda Douw, Email: l.douw@amsterdamumc.nl.
Philip C. de Witt Hamer, Email: p.dewitthamer@amsterdamumc.nl
Mathilde C. M. Kouwenhoven, Email: m.kouwenhoven@amsterdamumc.nl
Tom Würdinger, Email: t.wurdinger@amsterdamumc.nl.
Peter M. van de Ven, Email: p.vandeven@amsterdamumc.nl
Louise Sharpe, Email: louise.sharpe@sydney.edu.au.
Hans Knoop, Email: hans.knoop@amsterdamumc.nl.
Martin Klein, Email: m.klein@amsterdamumc.nl.
References
- 1.National Working group on Neuro-Oncology (LWNO). Richtlijn gliomen. 2015. [cited 2019 December 22] https://richtlijnendatabase.nl/richtlijn/gliomen/gliomen_-_startpagina.html.
- 2.Taphoorn MJ, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159–168. doi: 10.1016/S1474-4422(04)00680-5. [DOI] [PubMed] [Google Scholar]
- 3.Boele FW, Klein M, Reijneveld JC, Verdonck-de Leeuw IM, Heimans JJ. Symptom management and quality of life in glioma patients. CNS Oncol. 2014;3(1):37–47. doi: 10.2217/cns.13.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taphoorn MJ, Sizoo EM, Bottomley A. Review on quality of life issues in patients with primary brain tumors. Oncologist. 2010;15(6):618–626. doi: 10.1634/theoncologist.2009-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fabi A, Bhargava R, Fatigoni S, Guglielmo M, Horneber M, Roila F, et al. Cancer-related fatigue: ESMO Clinical Practice Guidelines for diagnosis and treatment. Ann Oncol Off J Eur Soc Med Oncol. 2020;31(6):713–723. doi: 10.1016/j.annonc.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong TS, Cron SG, Bolanos EV, Gilbert MR, Kang DH. Risk factors for fatigue severity in primary brain tumor patients. Cancer. 2010;116(11):2707–2715. doi: 10.1002/cncr.25018. [DOI] [PubMed] [Google Scholar]
- 7.Fox SW, Lyon D, Farace E. Symptom clusters in patients with high-grade glioma. J Nurs Scholarsh Off Publ Sigma Theta Tau Int Honor Soc Nurs. 2007;39(1):61–67. doi: 10.1111/j.1547-5069.2007.00144.x. [DOI] [PubMed] [Google Scholar]
- 8.Day J, Yust-Katz S, Cachia D, Wefel J, Katz LH, Tremont I, et al. Interventions for the management of fatigue in adults with a primary brain tumour. Cochrane Database Syst Rev. 2016;4:CD011376. doi: 10.1002/14651858.CD011376.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Coevorden-van Loon EMP, Coomans MB, Heijenbrok-Kal MH, Ribbers GM, van den Bent MJ. Fatigue in patients with low grade glioma: systematic evaluation of assessment and prevalence. J Neuro-Oncol. 2017;133(2):237–246. doi: 10.1007/s11060-017-2454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coomans MB, Dirven L, Aaronson NK, Baumert BG, Van Den Bent M, Bottomley A, et al. Symptom clusters in newly diagnosed glioma patients: which symptom clusters are independently associated with functioning and global health status? Neuro Oncol. 2019;21(11):1447–1457. doi: 10.1093/neuonc/noz118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Struik K, Klein M, Heimans JJ, Gielissen MF, Bleijenberg G, Taphoorn MJ, et al. Fatigue in low-grade glioma. J Neurooncol. 2009;92(1):73–78. doi: 10.1007/s11060-008-9738-7. [DOI] [PubMed] [Google Scholar]
- 12.Molassiotis A, Wilson B, Brunton L, Chaudhary H, Gattamaneni R, McBain C. Symptom experience in patients with primary brain tumours: a longitudinal exploratory study. Eur J Oncol Nurs. 2010;14(5):410–416. doi: 10.1016/j.ejon.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Asher A, Fu JB, Bailey C, Hughes JK. Fatigue among patients with brain tumors. CNS Oncol. 2016;5(2):91–100. doi: 10.2217/cns-2015-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelletier G, Verhoef MJ, Khatri N, Hagen N. Quality of life in brain tumor patients: the relative contributions of depression, fatigue, emotional distress, and existential issues. J Neurooncol. 2002;57(1):41–49. doi: 10.1023/A:1015728825642. [DOI] [PubMed] [Google Scholar]
- 15.Boele FW, Douw L, de Groot M, van Thuijl HF, Cleijne W, Heimans JJ, et al. The effect of modafinil on fatigue, cognitive functioning, and mood in primary brain tumor patients: a multicenter randomized controlled trial. Neuro Oncol. 2013;15(10):1420–1428. doi: 10.1093/neuonc/not102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gehring K, Sitskoorn MM, Gundy CM, Sikkes SA, Klein M, Postma TJ, et al. Cognitive rehabilitation in patients with gliomas: a randomized, controlled trial. J Clin Oncol. 2009;27(22):3712–3722. doi: 10.1200/JCO.2008.20.5765. [DOI] [PubMed] [Google Scholar]
- 17.Kaleita TA, Wellisch DK, Graham CA, Steh B, Nghiemphu P, Ford JM, et al. Pilot study of modafinil for treatment of neurobehavioral dysfunction and fatigue in adult patients with brain tumors. J Clin Oncol. 2006;24(18_suppl):1503. doi: 10.1200/jco.2006.24.18_suppl.1503. [DOI] [Google Scholar]
- 18.Gehring K, Patwardhan SY, Collins R, Groves MD, Etzel CJ, Meyers CA, et al. A randomized trial on the efficacy of methylphenidate and modafinil for improving cognitive functioning and symptoms in patients with a primary brain tumor. J Neurooncol. 2012;107(1):165–174. doi: 10.1007/s11060-011-0723-1. [DOI] [PubMed] [Google Scholar]
- 19.Shaw EG, Rosdhal R, D'Agostino RB, Jr, Lovato J, Naughton MJ, Robbins ME, et al. Phase II study of donepezil in irradiated brain tumor patients: effect on cognitive function, mood, and quality of life. J Clin Oncol. 2006;24(9):1415–1420. doi: 10.1200/JCO.2005.03.3001. [DOI] [PubMed] [Google Scholar]
- 20.Kangas M. Psychotherapy Interventions for Managing Anxiety and Depressive Symptoms in Adult Brain Tumor Patients: A Scoping Review. Front. Oncol. 2015;5:116. doi: 10.3389/fonc.2015.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Randazzo DM, McSherry F, Herndon JE, Affronti ML, Lipp ES, Flahiff C, et al. Complementary and integrative health interventions and their association with health-related quality of life in the primary brain tumor population. Complement Ther Clin Pract. 2019;36:43–48. doi: 10.1016/j.ctcp.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Jacobsen PB, Donovan KA, Vadaparampil ST, Small BJ. Systematic review and meta-analysis of psychological and activity-based interventions for cancer-related fatigue. Health Psychol. 2007;26(6):660–667. doi: 10.1037/0278-6133.26.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Servaes P, Verhagen S, Bleijenberg G. Determinants of chronic fatigue in disease-free breast cancer patients: a cross-sectional study. Ann Oncol. 2002;13(4):589–598. doi: 10.1093/annonc/mdf082. [DOI] [PubMed] [Google Scholar]
- 24.Abrahams HJG, Gielissen MFM, Verhagen C, Knoop H. The relationship of fatigue in breast cancer survivors with quality of life and factors to address in psychological interventions: A systematic review. Clin Psychol Rev. 2018;63:1–11. doi: 10.1016/j.cpr.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Poort H, Peters MEWJ, van der Graaf WTA, Nieuwkerk PT, van de Wouw AJ, Nijhuis-van der Sanden MWG, et al. Cognitive behavioral therapy or graded exercise therapy compared with usual care for severe fatigue in patients with advanced cancer during treatment: a randomized controlled trial. Ann Oncol. 2020;31(1):115–122. doi: 10.1016/j.annonc.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Goedendorp MM, Knoop H, Gielissen MF, Verhagen CA, Bleijenberg G. The effects of cognitive behavioral therapy for postcancer fatigue on perceived cognitive disabilities and neuropsychological test performance. J Pain Symptom Manag. 2014;47(1):35–44. doi: 10.1016/j.jpainsymman.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 27.Abrahams HJG, Gielissen MFM, Donders RRT, Goedendorp MM, van der Wouw AJ, Verhagen C, et al. The efficacy of Internet-based cognitive behavioral therapy for severely fatigued survivors of breast cancer compared with care as usual: A randomized controlled trial. Cancer. 2017;123(19):3825–3834. doi: 10.1002/cncr.30815. [DOI] [PubMed] [Google Scholar]
- 28.van den Akker LE, Beckerman H, Collette EH, Twisk JW, Bleijenberg G, Dekker J, et al. Cognitive behavioral therapy positively affects fatigue in patients with multiple sclerosis: Results of a randomized controlled trial. Mult Scler (Houndmills, Basingstoke, England). 2017;23(11):1542–1553. doi: 10.1177/1352458517709361. [DOI] [PubMed] [Google Scholar]
- 29.Gillespie DC, Barber M, Brady MC, Carson A, Chalder T, Chun Y, et al. Study protocol for POSITIF, a randomised multicentre feasibility trial of a brief cognitive-behavioural intervention plus information versus information alone for the treatment of post-stroke fatigue. Pilot Feasibility Stud. 2020;6:84. doi: 10.1186/s40814-020-00622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen S, Wong D, McKay A, Rajaratnam SMW, Spitz G, Williams G, et al. Cognitive behavioural therapy for post-stroke fatigue and sleep disturbance: a pilot randomised controlled trial with blind assessment. Neuropsychol Rehabi. 2019;29(5):723–738. doi: 10.1080/09602011.2017.1326945. [DOI] [PubMed] [Google Scholar]
- 31.Li P, Yang X, Greenshaw AJ, Li S, Luo J, Han H, et al. The effects of cognitive behavioral therapy on resting-state functional brain network in drug-naive patients with obsessive-compulsive disorder. Brain Behav. 2018;8(5):e00963. doi: 10.1002/brb3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moody TD, Morfini F, Cheng G, Sheen C, Tadayonnejad R, Reggente N, et al. Mechanisms of cognitive-behavioral therapy for obsessive-compulsive disorder involve robust and extensive increases in brain network connectivity. Transl Psychiatry. 2017;7(9):e1230. doi: 10.1038/tp.2017.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Z, Gu S, Honnorat N, Linn KA, Shinohara RT, Aselcioglu I, et al. Network changes associated with transdiagnostic depressive symptom improvement following cognitive behavioral therapy in MDD and PTSD. Mol Psychiatry. 2018;23(12):2314–2323. doi: 10.1038/s41380-018-0201-7. [DOI] [PubMed] [Google Scholar]
- 34.Ellard KK, Gosai AG, Bernstein EE, Kaur N, Sylvia LG, Camprodon JA, et al. Intrinsic functional neurocircuitry associated with treatment response to transdiagnostic CBT in bipolar disorder with anxiety. J Affect Disord. 2018;238:383–391. doi: 10.1016/j.jad.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reggente N, Moody TD, Morfini F, Sheen C, Rissman J, O'Neill J, et al. Multivariate resting-state functional connectivity predicts response to cognitive behavioral therapy in obsessive-compulsive disorder. Proc Natl Acad Sci U S A. 2018;115(9):2222–2227. doi: 10.1073/pnas.1716686115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Worm-Smeitink M, Gielissen M, Bloot L, van Laarhoven HWM, van Engelen BGM, van Riel P, et al. The assessment of fatigue: Psychometric qualities and norms for the Checklist individual strength. J Psychosom Res. 2017;98:40–46. doi: 10.1016/j.jpsychores.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Beck AT, Guth D, Steer RA, Ball R. Screening for major depression disorders in medical inpatients with the Beck Depression Inventory for Primary Care. Behav Res Ther. 1997;35(8):785–791. doi: 10.1016/S0005-7967(97)00025-9. [DOI] [PubMed] [Google Scholar]
- 38.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33;quiz 4-57. [PubMed] [Google Scholar]
- 39.Martin RC, Gerstenecker A, Nabors LB, Marson DC, Triebel KL. Impairment of medical decisional capacity in relation to Karnofsky Performance Status in adults with malignant brain tumor. Neurooncol Pract. 2015;2(1):13–19. doi: 10.1093/nop/npu030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parmelee PA, Thuras PD, Katz IR, Lawton MP. Validation of the Cumulative Illness Rating Scale in a geriatric residential population. J Am Geriatr Soc. 1995;43(2):130–137. doi: 10.1111/j.1532-5415.1995.tb06377.x. [DOI] [PubMed] [Google Scholar]
- 41.Castor EDC. Castor Electronic Data Capture 2019. 2019. [Google Scholar]
- 42.Suresh K. An overview of randomization techniques: An unbiased assessment of outcome in clinical research. J Hum Reprod Sci. 2011;4(1):8–11. doi: 10.4103/0974-1208.82352. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Poort H, Verhagen CAHHVM, Peters MEWJ, Goedendorp MM, Donders ART, Hopman MTE, et al. Study protocol of the TIRED study: a randomised controlled trial comparing either graded exercise therapy for severe fatigue or cognitive behaviour therapy with usual care in patients with incurable cancer. BMC Cancer. 2017;17(1):81. doi: 10.1186/s12885-017-3076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Houniet-de Gier M, Beckerman H, van Vliet K, Knoop H, de Groot V. Testing non-inferiority of blended versus face-to-face cognitive behavioural therapy for severe fatigue in patients with multiple sclerosis and the effectiveness of blended booster sessions aimed at improving long-term outcome following both therapies: study protocol for two observer-blinded randomized clinical trials. Trials. 2020;21(1):98. doi: 10.1186/s13063-019-3825-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Butow PN, Turner J, Gilchrist J, Sharpe L, Smith AB, Fardell JE, et al. Randomized Trial of ConquerFear: A Novel, Theoretically Based Psychosocial Intervention for Fear of Cancer Recurrence. J Clin Oncol. 2017;35(36):4066–4077. doi: 10.1200/JCO.2017.73.1257. [DOI] [PubMed] [Google Scholar]
- 46.Jacobs HM, Luttik A, Touw-Otten FW, de Melker RA. The sickness impact profile; results of an evaluation study of the Dutch version. Ned Tijdschr Geneeskd. 1990;134(40):1950–1954. [PubMed] [Google Scholar]
- 47.Jacobsen PB, Andrykowski MA, Thors CL. Relationship of catastrophizing to fatigue among women receiving treatment for breast cancer. J Consult Clin Psychol. 2004;72(2):355–361. doi: 10.1037/0022-006X.72.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ray C, Weir W, Stewart D, Miller P, Hyde G. Ways of coping with chronic fatigue syndrome: development of an illness management questionnaire. Soc Sci Med. 1993;37(3):385–391. doi: 10.1016/0277-9536(93)90268-9. [DOI] [PubMed] [Google Scholar]
- 49.Prins JB, Bleijenberg G, Bazelmans E, Elving LD, de Boo TM, Severens JL, et al. Cognitive behaviour therapy for chronic fatigue syndrome: a multicentre randomised controlled trial. The Lancet. 2001;357(9259):841–847. doi: 10.1016/S0140-6736(00)04198-2. [DOI] [PubMed] [Google Scholar]
- 50.Gresham G, Schrack J, Gresham LM, Shinde AM, Hendifar AE, Tuli R, et al. Wearable activity monitors in oncology trials: Current use of an emerging technology. Contemp Clin Trials. 2018;64:13–21. doi: 10.1016/j.cct.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 51.Goebel S, Mehdorn HM. Fear of disease progression in adult ambulatory patients with brain cancer: prevalence and clinical correlates. Support Care Cancer. 2019;27(9):3521–3529. doi: 10.1007/s00520-019-04665-9. [DOI] [PubMed] [Google Scholar]
- 52.Ke Y, Ng T, Yeo HL, Shwe M, Gan YX, Chan A. Psychometric properties and measurement equivalence of the English and Chinese versions of the Beck Anxiety Inventory in patients with breast cancer. Support Care Cancer. 2017;25(2):633–643. doi: 10.1007/s00520-016-3452-3. [DOI] [PubMed] [Google Scholar]
- 53.van Sonderen E. Sociale Steun Lijst-Interacties (SSL-I) en Sociale Steun Lijst - Discrepanties (SSL-D) Groningen: Noordelijk Centrum voor Gezondheidsvraagstukken; 1993. [Google Scholar]
- 54.Evers AW, Kraaimaat FW, van Lankveld W, Jongen PJ, Jacobs JW, Bijlsma JW. Beyond unfavorable thinking: the illness cognition questionnaire for chronic diseases. J Consult Clin Psychol. 2001;69(6):1026–1036. doi: 10.1037/0022-006X.69.6.1026. [DOI] [PubMed] [Google Scholar]
- 55.van der Ploeg E, Mooren TT, Kleber RJ, van der Velden PG, Brom D. Construct validation of the Dutch version of the impact of event scale. Psychol Assess. 2004;16(1):16–26. doi: 10.1037/1040-3590.16.1.16. [DOI] [PubMed] [Google Scholar]
- 56.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18(6):643. doi: 10.1037/h0054651. [DOI] [Google Scholar]
- 57.Boone KB, Lesser IM, Hill-gutierrez E, Berman NG, D'Elia LF. Rey-osterrieth complex figure performance in healthy, older adults: Relationship to age, education, sex, and IQ. Clin Neuropsychol. 1993;7(1):22–28. doi: 10.1080/13854049308401884. [DOI] [PubMed] [Google Scholar]
- 58.Heins MJ, Knoop H, Bleijenberg G. The role of the therapeutic relationship in cognitive behaviour therapy for chronic fatigue syndrome. Behav Res Ther. 2013;51(7):368–376. doi: 10.1016/j.brat.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 59.Bangor A, Kortum PT, Miller JT. An empirical evaluation of the system usability scale. Int J Hum Comput Interact. 2008;24(6):574–594. doi: 10.1080/10447310802205776. [DOI] [Google Scholar]
- 60.Derks J, Dirkson AR, de Witt Hamer PC, van Geest Q, Hulst HE, Barkhof F, et al. Connectomic profile and clinical phenotype in newly diagnosed glioma patients. Neuroimage Clin. 2017;14:87–96. doi: 10.1016/j.nicl.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Douw L, Miller JJ, Steenwijk MD, Stufflebeam SM, Gerstner ER. Altered structural hub connectivity and its clinical relevance in glioma. bioRxiv. 2019;610618.
- 62.Nayak L, DeAngelis LM, Brandes AA, Peereboom DM, Galanis E, Lin NU, et al. The Neurologic Assessment in Neuro-Oncology (NANO) scale: a tool to assess neurologic function for integration into the Response Assessment in Neuro-Oncology (RANO) criteria. Neuro Oncol. 2017;19(5):625–635. doi: 10.1093/neuonc/nox029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amidei C. Symptom-based interventions to promote quality survivorship. Neuro-Oncol. 2018;20:vii27–vii39. doi: 10.1093/neuonc/noy100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rogers JL, Acquaye A, Vera E, Bates A, Wen PY, Armstrong TS. Provider-reported challenges and barriers to referring patients to neuro-oncology clinical trials: a report from the Society for Neuro-Oncology member survey. Neuro-Oncol Pract. 2019;7(1):38–51. doi: 10.1093/nop/npz038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee EQ, Chukwueke UN, Hervey-Jumper SL, de Groot JF, Leone JP, Armstrong TS, et al. Barriers to Accrual and Enrollment in Brain Tumor Trials. Neuro Oncol. 2019. [DOI] [PMC free article] [PubMed]
- 66.Enck P, Zipfel S. Placebo effects in psychotherapy: a framework. Front Psychiatr. 2019;10(456). [DOI] [PMC free article] [PubMed]
- 67.Gold SM, Enck P, Hasselmann H, Friede T, Hegerl U, Mohr DC, et al. Control conditions for randomised trials of behavioural interventions in psychiatry: a decision framework. Lancet Psychiatr. 2017;4(9):725–732. doi: 10.1016/S2215-0366(17)30153-0. [DOI] [PubMed] [Google Scholar]
- 68.Furukawa TA, Noma H, Caldwell DM, Honyashiki M, Shinohara K, Imai H, et al. Waiting list may be a nocebo condition in psychotherapy trials: a contribution from network meta-analysis. Acta Psychiatr Scand. 2014;130(3):181–192. doi: 10.1111/acps.12275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Schedule of enrolment, interventions, and assessments.
Data Availability Statement
A core anonymised dataset of the primary and secondary outcome measurements will be published online after completion and publication of this trial. The data generated during this trial will also be available from the corresponding author upon reasonable request.