Abstract
Long-term exposure to 2-bromoethanesulfonate (BES), an agent known to inhibit methanogenesis, altered the bacterial community structure of an anaerobic enrichment culture that reductively dechlorinated trichloroethene (TCE). BES did not hinder the dechlorination of TCE or other chlorinated ethenes as previously reported, although different intermediates and end products were observed.
Coenzyme M (CoM; HSCH2CH2SO3−) is a cofactor involved in the terminal step of methane biosynthesis, where the methyl group carried by CoM is reduced to methane by methyl-CoM reductase (18). 2-Bromoethanesulfonate (BES; BrCH2CH2SO3−) is a structural analogue of CoM and a potent inhibitor of methanogenesis (9). Because this terminal step is involved in all methane biosynthesis and CoM is present only in methanogens (6), BES has been used and regarded as a methanogen-specific inhibitor (14) in microbiological studies (1–3, 14, 17). It is often assumed implicitly that other organisms in anaerobic mixed cultures are not affected by BES, and data are interpreted accordingly to infer the roles of methanogens and other microorganisms in the consortium.
However, it was found in recent years that BES can inhibit nonmethanogens that reductively dechlorinate polychlorinated biphenyls (PCBs) (19) and chlorinated ethenes (12). Ye et al. (19) reported that BES may compete with PCBs for electrons, since the sulfonate moiety of BES can serve as an alternative electron acceptor for sulfate-reducing bacteria, which were presumably responsible for PCB dechlorination. A study by Löffler et al. (12) showed that, in several nonmethanogenic cultures, complete dechlorination of tetrachloroethene was inhibited by BES, resulting in accumulation of intermediates, such as dichloroethenes (DCEs) and vinyl chloride (VC). These authors therefore concluded that inhibition of dechlorination by BES should not be taken as evidence for the involvement of methanogens in the dechlorination reaction. The mechanism through which BES inhibits microbial reduction of chlorinated ethenes, however, remains unclear (12).
In this paper we present additional evidence, based on denaturing gradient gel electrophoresis (DGGE) analysis, that BES affected bacteria in a trichloroethene (TCE)-dechlorinating enrichment culture. In contrast to the previous observations (12), however, BES treatment did not hinder complete TCE dechlorination to ethene.
Effect of BES on bacteria (DGGE).
A TCE-dechlorinating culture was isolated from a landfill site at Dover Air Force Base (Dover, Del.). This culture was maintained in 120-ml serum bottles (Supelco, Bellefonte, Pa.) containing lactate, acetate, HEPES buffer, vitamins, and TCE, as described in detail elsewhere (11). The stock culture was fed once a month by replacing half of the liquid culture with fresh nutrient medium. The bottles were sealed with Teflon-lined silicone septa and aluminum crimp caps, wrapped in foil, and incubated at room temperature (22 ± 2°C) in an anaerobic glove bag (I2R, Cheltenham, Pa.) under nitrogen. A nonmethanogenic subculture derived from the stock culture was established by including 3 mM BES (Aldrich, Milwaukee, Wis.) in the culture medium for 18 consecutive feeding cycles.
To obtain DNA for PCR and DGGE analysis, cells were collected from the stock culture and the BES culture at the end of the feeding cycle by centrifuging (12,000 × g) 40 ml of each culture for 10 min. Most supernatant was removed, and the cells were vortexed with the remaining liquid (approximately 3 ml). The suspension was then transferred to a 1.5-ml microtube and centrifuged for 2 min (10,000 × g), and the supernatant was discarded. This step was repeated to remove all liquid. One hundred microliters of Tween 20 (0.1%; Bio-Rad, Hercules, Calif.) was added to the microtube and vortexed to promote cell lysis, and the mixture was heated to 100°C for 10 min in an iCycler (Bio-Rad). The cells were resuspended by being vortexed for 10 s and centrifuged for 1 min. Eighty microliters of InstaGene Matrix (Bio-Rad) was used to remove the cell lysis products, and the mixture was vortexed. The sample was incubated at 56°C for 20 min, vortexed, incubated again at 100°C for 8 min, and vortexed again for 10 s. The sample was then centrifuged for 2 min, and the supernatant containing DNA was stored at −20°C before PCR.
The forward and reverse primer sets used for bacteria and methanogens were P63f (5′ CAG GCC TAA CAC ATG CAA GTC 3′) and P518r (5′ ATT ACC GCG GCT GCT GG 3′) and M23f (5′ TGG TTG ATC CTG CCA GAG G 3′) and M440r (5′ CGG CTG GCA CCG GTC TTG C 3′), respectively. P63f had been shown to be applicable to a wide range of bacteria (13). P518r was based on a universally conserved region of bacteria (16). M23f and M440r were designed based on the conserved regions of the methanogenic 16S ribosomal RNA sequence (10). A GC clamp of 40 bases was added to both forward primers. These primer sets were expected to give PCR products of approximately 495 bp (P63f and P518r) and 493 bp (M23f and M440r).
The PCR mixture consisted of 3.5 μl of 10× Taq buffer, 0.28 μl of 100 mM deoxynucleoside triphosphate mix (0.25 mM/base), 0.18 μl of Taq polymerase (5 U/μl), and 7 μl of Q solution (PCR Core Kit; Qiagen, Valencia, Calif.). Primers P63f and P518r (Operon, Alameda, Calif.) were added in the amounts equivalent to 50 pmol per 100 μl of reaction mixture. One microliter of extracted DNA was used as template. Sterile deionized (DI) water was added to make up the mixture volume to 35 μl. PCR was performed using a Bio-Rad iCycler. The temperature program for the bacterial DNA was (i) 95°C for 5 min; (ii) 30 cycles of 92°C for 1 min, 55°C for 1 min, and 72°C for 1 min; and (iii) 72°C for 10 min. The program for methanogen DNA was (i) 94°C for 2 min; (ii) 30 cycles of 94°C for 1 min, 50°C for 1 min, and 72°C for 1.5 min; and (iii) 72°C for 2 min (10).
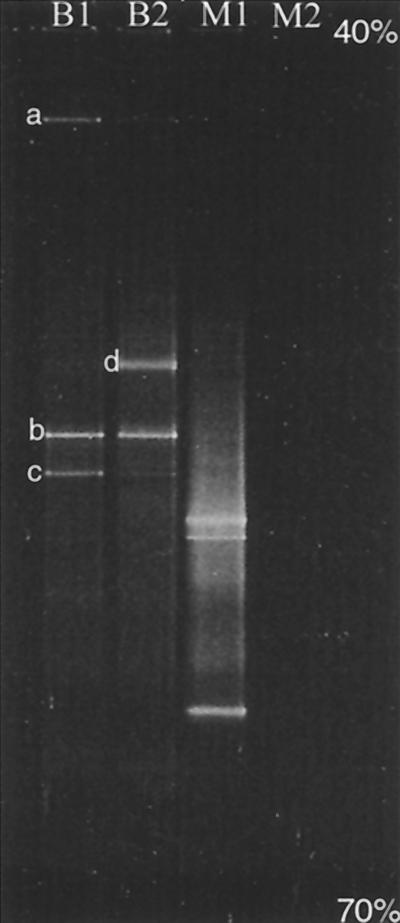
DGGE was performed using a Bio-Rad DCode System. Thirty microliters of PCR products and 5 μl of 6× gel loading dye (6% bromophenol blue, 6% xylene cyanol, 100% glycerol, and DI water) were loaded onto an 8% (wt/vol) polyacrylamide gel in 1× Tris-acetate-EDTA running buffer. The linear 40 to 70% denaturing gradient was created by mixing 7 M urea and 40% formamide (J. T. Baker, Phillipsburg, N.J.). The electrophoresis was performed at 60°C at 75 V for 12 h. After electrophoresis, the gels were soaked for 30 min in a SYBR green I solution (Molecular Probes, Eugene, Oreg.). The stained gel was viewed using a UV transilluminator (VWR, Baltimore, Md.) and photographed with a Polaroid camera (Cambridge, Mass.). The major bacteria bands (Fig. 1, bands a, b, c, and d) were excised with a sterile blade from the polyacrylamide gel, and the DNA was extracted using a Qiagen gel extraction kit. (Several minor bands were also obtained; however, they were too faint to excise precisely and/or to yield reliable sequences.) The DNA was sequenced using an ABI Prism 377 DNA Sequencer (Applied Biosystems, Foster City, Calif.) at the University of Delaware Center for Agricultural Biotechnology. The same PCR primers were used for sequencing. The major bacteria were identified by comparing the sequences of their 16S ribosomal DNA (rDNA) fragments to the sequences of known organisms using BLAST 2.0 (Basic Local Alignment Search Tool) of the National Center for Biotechnology Information (NCBI; http://www.ncbi.nlm.nih.gov). The highest percent match for each of the bands ranged from 88 to 95%.
FIG. 1.
DGGE profiles of the 16S rDNA fragments of the stock culture amplified with universal bacterial PCR primers (lane B1) and with methanogen-specific primers (lane M1) and of the BES culture amplified with universal bacterial primers (lane B2). No PCR products were obtained from the BES culture using methanogen-specific primers (lane M2).
Figure 1 shows the DGGE profiles of the PCR-amplified 16S rDNA fragments from the stock culture and the BES-amended culture. Using the bacterium-specific primers, the stock culture was found to contain three dominant bacteria (bands a, b, and c in lane B1). Sequencing results showed that bands a (93%) and b (88%) corresponded to uncultured bacteria in the Cytophaga-Flavobacter-Bacteroides phylum (5; E. Stackebrandt, submitted to Deutsche Sammlung von Mikroorganismen und Zellkulturen, accession no. AJ287664). No specific information was available for band a. Band b was closely related to the genus Porphyromonas (5), which belongs to the family Bacteroidaceae, a group of gram-negative, obligate anaerobes. Band c matched (95%) a bacterium found at a site contaminated with hydrocarbons and chlorinated solvents, which was most likely a gram-positive Syntrophomonas (7). For the culture receiving BES, bands a and c essentially disappeared, whereas band b was not affected. Interestingly, a new band (band d in lane B2), which was very faint in lane B1, became dominant as a result of BES treatment. This new band matched (92%) an uncultured bacterium found in an anaerobic digester (8). Three prominent bands were obtained from the stock culture with the methanogen-specific primers (lane M1). These methanogen bands, which matched Methanosaeta concilii (82 to 98%), were eliminated completely by BES, as expected. No PCR products were obtained for DGGE analysis from the BES-treated culture using the methanogen-specific primers (lane M2).
The data in Fig. 1 show that long-term exposure to BES not only eliminated methanogenic archaea but also altered the bacterial community structure. It is possible that BES affected some of these bacteria directly by acting as a competing substrate (19) or through other mechanisms (12). Alternatively, bacteria which consume and/or produce substrates for methanogenesis, such as H2 and acetate, would be affected indirectly by BES through the elimination of methanogens. That is, one cannot expect to specifically remove one group of organisms without eventually affecting the metabolically associated members in the community. Therefore, exposure time should be taken into account when using BES as a specific inhibitor for methanogens. For short-term experiments, caution should also be exercised in interpreting the data, as BES has been shown to directly inhibit certain bacteria. Further studies are necessary to fully understand the limitations of using BES as an inhibitor in enzymatic and microbial ecology studies.
Effect of BES treatment on TCE dechlorination.
The effect of BES treatment on the culture's ability to reductively dechlorinate TCE was investigated by using batch reactors similar to those described elsewhere (11). The experiment was conducted using 63-ml amber reactors (Alltech, Deerfield, Ill.) set up in duplicate at room temperature under anaerobic and light-excluded conditions. Immediately after feeding, 31.5 ml of the stock or BES culture was placed into each reactor, leaving 31.5 ml of N2 headspace. Each reactor was capped with a Mininert valve (Precision, Baton Rouge, La.), sealed with low-permeability vinyl tape (3M, St. Paul, Minn.), and incubated in an inverted position on a shaker at 150 rpm. At different elapsed times, 100 μl of headspace samples were withdrawn with a gas-tight syringe and analyzed using a Hewlett Packard 6890 gas chromatograph (Wilmington, Del.) equipped with a flame-ionization detector and a 30-m GS-GasPro capillary column (J&W, Folson, Calif.). The temperature program used was 40°C for 2 min, 25°C/min to 115°C, 10°C/min to 200°C, and 200°C for 1 min. The analytes had the following retention times: methane, 0.422 min; ethane, 0.625 min; ethene, 0.791 min; acetylene, 1.58 min; VC, 5.87 min; 1,1-DCE, 7.74 min; trans-DCE, 8.60 min; cis-DCE, 9.83 min; and TCE, 11.0 min. Quantification of peak areas was based on external calibration standards.
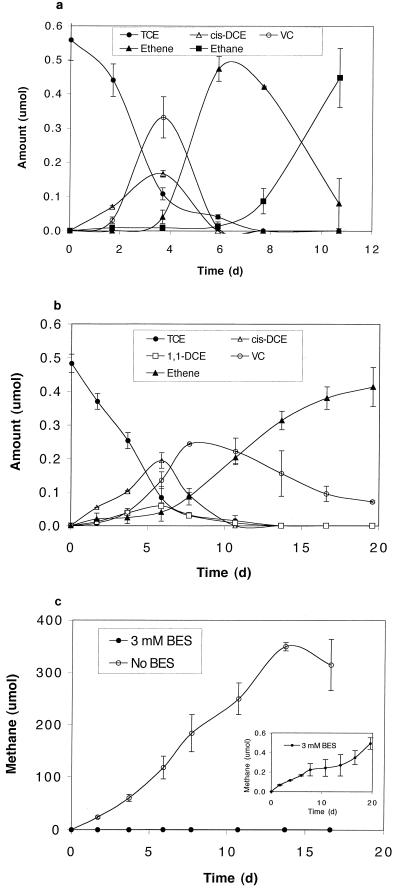
The stock culture dechlorinated TCE quantitatively to ethane via cis-DCE, VC, and ethene as intermediates (Fig. 2a). No 1,1-DCE and only trace amounts of trans-DCE were formed. Upon complete conversion of TCE to ethane, additional ethene was spiked, which was reduced to ethane quantitatively, indicating that ethene was the precursor of ethane (data not shown). Large quantities of methane were produced throughout the experiment (Fig. 2c). In comparison, the BES-treated culture dechlorinated TCE to ethene (but not ethane) via a mixture of cis- and 1,1-DCE (approximately 3:1 ratio) and VC as intermediates (Fig. 2b). Dechlorination of cis-DCE and VC by the BES culture was not hindered in the presence of BES (3 mM), in contrast to the nonmethanogenic, dechlorinating cultures studied previously (12). The appearance of 1,1-DCE suggests that BES either selected for organisms that dechlorinated TCE to 1,1-DCE or inhibited organisms that dechlorinated 1,1-DCE in the stock culture. Methanogens were probably not involved in the observed dechlorination, as BES reduced methanogenic activities by three orders of magnitude and only minute amounts of methane were formed (Fig. 2c, insert).
FIG. 2.
Reductive dechlorination of TCE by the stock culture (a) and the BES culture (b) and methane production by the two cultures (c).
The inhibitory effect of BES on ethene reduction to ethane has been reported elsewhere (4), but the organism(s) involved and the role of methanogens remain unknown. Interestingly, we observed, under various conditions, that ethane was formed only during active methanogenesis; however, the reverse was not true. That is, high methanogenic activities did not guarantee ethane formation. For example, when H2 (instead of lactate) was used as the electron donor to stimulate methanogenic and dechlorination activities, ethene was the final product (data not shown).
In summary, our results show that extended exposure to BES altered the bacterial community structure of a TCE-dechlorinating consortium but did not affect the culture's ability to completely dechlorinate TCE, even in the presence of 3 mM BES. Different reduction intermediates and products of TCE were observed, which suggests that dehalogenators (and other organisms) were influenced by BES. Therefore, data from studies involving the use of BES as a methanogen inhibitor should be interpreted with care.
Acknowledgments
We thank Michael D. Lee for sharing the stock culture, Bruce Kingham for sequencing the DNA, and Mark Radosevich for reviewing the manuscript.
This study was supported in part by the National Science Foundation (award no. 9984669).
REFERENCES
- 1.Alperin M J, Reeburgh W S. Inhibition experiments on anaerobic methane oxidation. Appl Environ Microbiol. 1985;50:940–945. doi: 10.1128/aem.50.4.940-945.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradley P M, Chapelle F H. Acetogenic microbial degradation of vinyl chloride. Environ Sci Technol. 2000;34:2761–2763. [Google Scholar]
- 3.Bradley P M, Chapelle F H, Lovley D R. Humic acids as electron acceptors for anaerobic microbial oxidation of vinyl chloride and dichloroethene. Appl Environ Microbiol. 1998;44:3102–3105. doi: 10.1128/aem.64.8.3102-3105.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Bruin W P, Kotterman M J J, Posthumus M A, Schraa G, Zehnder A J B. Complete biological reductive transformation of tetrachloroethene to ethane. Appl Environ Microbiol. 1992;58:1996–2000. doi: 10.1128/aem.58.6.1996-2000.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dewhirst F E, Chien C C, Paster B J, Ericson R L, Orcutt R P, Schauer D B, Fox J G. Phylogeny of the defined murine microbiota: altered Schaedler flora. Appl Environ Microbiol. 1999;65:3287–3292. doi: 10.1128/aem.65.8.3287-3292.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiMarco A A, Bobik T A, Wolfe R S. Unusual coenzymes of methanogenesis. Annu Rev Biochem. 1990;59:355–394. doi: 10.1146/annurev.bi.59.070190.002035. [DOI] [PubMed] [Google Scholar]
- 7.Dojka M A, Hugenholtz P, Haack S K, Pace N R. Microbial diversity in a hydrocarbon- and chlorinated solvent-contaminated aquifer undergoing intrinsic bioremediation. Appl Environ Microbiol. 1998;64:3869–3877. doi: 10.1128/aem.64.10.3869-3877.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godon J-J, Zumstein E, Dabert P, Habouzit F, Moletta R. Molecular microbial diversity of an anaerobic digester as determined by small subunit rDNA sequence analysis. Appl Environ Microbiol. 1997;63:2802–2813. doi: 10.1128/aem.63.7.2802-2813.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunsalus R P, Romesser J A, Wolfe R S. Preparation of coenzyme M analogues and their activity in the methylcoenzyme M reductase system of Methanobacterium thermoautotrophicum. Biochemistry. 1978;17:2374–2377. doi: 10.1021/bi00605a019. [DOI] [PubMed] [Google Scholar]
- 10.Hiraishi A, Kamgata Y, Nakamura K. Polymerase chain reaction amplification and restriction fragment length polymorphism analysis of 16S rRNA genes from methanogens. J Ferment Bioeng. 1995;79:523–529. [Google Scholar]
- 11.Lampron K J, Chiu P C, Cha D K. Biological reduction of trichloroethene supported by Fe(0) Bioremediation. 1998;2:175–181. [Google Scholar]
- 12.Löffler F E, Ritalahti K M, Tiedje J M. Dechlorination of chloroethenes is inhibited by 2-bromoethanesulfonate in the absence of methanogens. Appl Environ Microbiol. 1997;63:4982–4985. doi: 10.1128/aem.63.12.4982-4985.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchesi J R, Sato T, Weightman A J, Martin T A, Fry J C, Hiom S J, Wade W G. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl Environ Microbiol. 1998;64:795–799. doi: 10.1128/aem.64.2.795-799.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nollet L, Demeyer D, Verstraete W. Effect of 2-bromoethanesulfonic acid and Peptostreptococcus productus ATCC 35244 addition on stimulation of reductive acetogenesis in the ruminal ecosystem by selective inhibition of methanogenesis. Appl Environ Microbiol. 1997;63:194–200. doi: 10.1128/aem.63.1.194-200.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oremland R S, Capone D G. Use of “specific” inhibitors in biogeochemistry and microbial ecology. Adv Microbiol Ecol. 1988;10:285–383. [Google Scholar]
- 16.O/vreås L, Forney L, Daae F L, Torsvik V. Distribution of bacterioplankton in Meromictic Lake Sælenvannet, as determined by denaturing gradient gel electrophoresis of PCR-amplified gene fragments coding for 16S rRNA. Appl Environ Microbiol. 1997;63:3367–3373. doi: 10.1128/aem.63.9.3367-3373.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scholten J C M, Conrad R, Stams A J M. Effect of 2-bromo-ethane sulfonate, molybdate and chloroform on acetate consumption by methanogenic and sulfate-reducing populations in freshwater sediment. FEMS Microbiol Ecol. 2000;32:35–42. doi: 10.1111/j.1574-6941.2000.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 18.Vogels G D, Keltjens J T, van der Drift C. Biochemistry of methane production. In: Zehnder A J B, editor. Biology of anaerobic microorganisms. New York, N.Y: John Wiley & Sons; 1988. pp. 707–770. [Google Scholar]
- 19.Ye D, Quensen III J F, Tiedje J M, Boyd S A. 2-Bromoethanesulfonate, sulfate, molybdate, and ethanesulfonate inhibit anaerobic dechlorination of polychlorobiphenyls by pasteurized microorganisms. Appl Environ Microbiol. 1999;65:327–329. doi: 10.1128/aem.65.1.327-329.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]