Abstract
The purpose of this investigation was to identify modifiable risk factors for the development of first-onset chronic neck pain among an inception cohort of healthy individuals working in a high risk occupation. Candidate risk factors identified from previous studies were categorized into psychosocial, physical, and neurophysiological domains, which were assessed concurrently in a baseline evaluation of 171 office workers within the first 3 months of hire. Participants completed monthly online surveys over the subsequent year to identify the presence of chronic interfering neck pain, defined as a Neck Disability Index score ≥5 points for 3 or more months. Data were analyzed using backwards logistic regression to identify significant predictors within each domain, which were then entered into a multivariate regression model adjusted for age, sex, and body mass index. Development of chronic interfering neck pain was predicted by depressed mood (OR=3.36(1.10–10.31), p=0.03), cervical extensor endurance (OR=0.92(0.87–0.97), p=0.001), and diffuse noxious inhibitory control (OR=0.90(0.83–0.98), p=0.02) at baseline. These findings provide the first evidence that individuals with pre-existing impairments in mood and descending pain modulation may be at greater risk for developing chronic neck pain when exposed to peripheral nociceptive stimuli such as that produced during muscle fatigue.
Introduction
Neck pain ranks as the fourth greatest contributor to global disability [31] and affects 30–50% of the general population [30], with an even greater prevalence among individuals in high risk occupations. For example, up to 63% of office workers report at least one episode of neck pain, with 14% indicating significant activity limitations [16, 27]. Importantly, the majority of those who experience an acute episode of neck pain report either persistent (37%), recurrent (23%), or worsening (10%) symptoms up to 12 months later [15]. Individuals who develop chronic neck pain respond poorly to treatment and consume a disproportionate share of healthcare resources [9]. Furthermore, reduced productivity attributed to neck pain and other musculoskeletal disorders in the workplace has been estimated to cost $61.2 billion annually [60]. Recent systematic reviews of longitudinal and case-control studies have reported numerous and often inconsistent risk factors for neck pain [18, 48]. As a result of this heterogeneity, no widely accepted conceptual framework currently exists to guide research on primary prevention of this prevalent and costly disorder.
To better understand how multiple factors may contribute to increased risk of chronic neck pain, we reviewed the literature to identify candidate risk factors in the psychosocial, physical, and neurophysiological domains. These domains encompass previously reported impairments in one emerging (neurophysiological) and two established (psychosocial and physical) areas of investigation that can be targeted for primary prevention in future studies. In the psychosocial domain, job-related and general measures of psychological health have been shown to predict long term symptoms and disability associated with existing neck pain; however, the strength of the relation between psychological health and new onset musculoskeletal pain is much smaller [40]. Although cross-sectional studies commonly report that depression, anxiety, and catastrophization are higher in those with existing pain [22, 29, 53], this observation cannot rule out the possibility that persistent pain perpetuates poor mood [44].
In the physical domain, job-related exposures including poor ergonomic positioning and high physical strain have been suggested to increase the risk of neck pain [1, 2, 20]; however, a systematic review found only limited or conflicting evidence from prospective studies to support the majority of these risk factors among office workers [48]. Limited data from cross-sectional investigations suggest that more general physical characteristics such as routine physical activity and impairments in cervicoscapular strength, mobility, and endurance may also be associated with neck pain [2, 52], yet the majority of these physical characteristics have not been investigated prospectively.
In the neurophysiological domain, recent evidence has shown alterations in pain processing that result in localized and widespread hypersensitivity to mechanical stimuli in individuals with neck pain compared to pain free controls [1, 53]. Diffuse noxious inhibitory control (DNIC) is also thought to be impaired in individuals with chronic pain compared to healthy individuals [39]; however, it is not known whether alterations in endogenous pain inhibition are present prior to, or as a consequence of persistent pain.
Most prospective investigations to date have included participants with a previous or ongoing history of neck pain, precluding identification of causal factors for the initial onset of pain [48]. In addition to prior pain episodes, the most consistently reported risk factors for neck pain include increasing age and female sex [43, 48]. However, these factors cannot be modified and are unlikely to inform prevention and management strategies to reduce the socioeconomic and personal costs of neck related disability. Finally, no study has prospectively examined whether pre-existing neurophysiological factors account for additional risk when considered with more established physical and psychosocial risk factors within the same cohort. Therefore, the purpose of this investigation was to identify modifiable psychosocial, physical, and neurophysiological risk factors for the initial onset of chronic, non-specific neck pain in a healthy cohort of office workers.
Methods
Participants
Participants 18–65 years of age were recruited by convenience sampling from the Denver metropolitan area through print and radio advertisements, new employee orientations, and employee bulletins and flyers posted by local businesses. All participants were within 3 months of their date of hire in a new job that required them to work ≥30 hours per week in an office setting with the use of a computer for at least 75% of the workday. They reported no neck pain or neck-related disorders during the previous year, and were screened for the absence of cervical pathology during a physical examination (see exclusion criteria). To minimize the potential for selection bias due to poor recall of pain symptoms, the Neck Disability Index (NDI) was used to screen for activity limitations caused by pain during the previous year, which are more likely to be remembered than non-interfering neck pain. Individuals with no signs or symptoms of cervical pathology who reported a score <5 points on the NDI were eligible for participation.
Exclusion criteria included: 1) objective signs of structural pathology upon physical examination, including but not limited to shoulder bursitis, impingement, tendonitis, fracture, cervical nerve or disc impairment, radiculopathy, or loss of upper extremity sensory or motor function, 2) history of fibromyalgia or musculoskeletal pain present in more than 4 body regions concurrently, 3) self-reported systemic illness including cancer, rheumatic, cardiovascular, or neurological disease, 4) prior surgery involving the cervical spine or shoulders, 5) acute (<12 weeks prior to study) injury of the neck or shoulders, 6) untreated psychiatric condition, 7) uncontrolled hypertension (resting systolic BP >150 mmHg, or diastolic BP>90 mmHg), 8) pregnancy, and 9) an inability to type or comprehend written and oral instructions in English. All procedures were approved by the local institutional review board and informed consent was obtained from every subject.
Baseline assessment
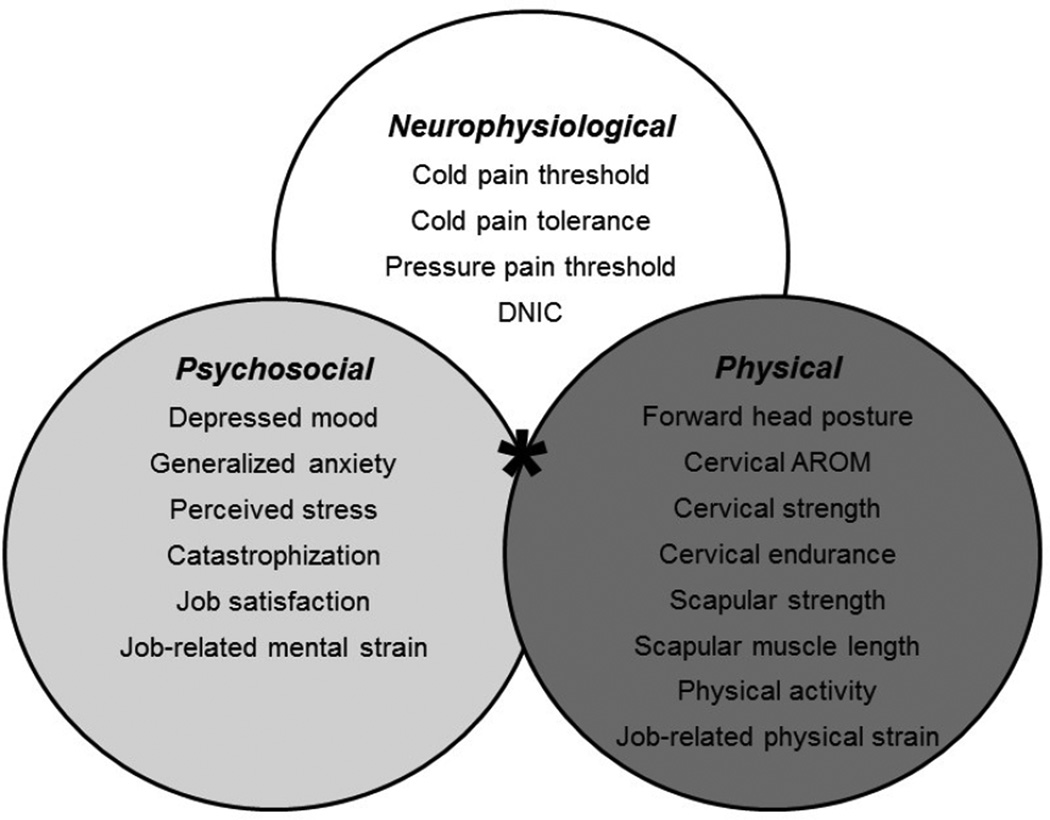
A 2–3 hour baseline assessment was performed for each participant within the first 3 months of hire into an occupation placing them at high risk for the development of neck pain. Data were collected on established personal risk factors for neck pain, including age, sex, body mass index (BMI), and smoking history [43, 48, 49]. Descriptive characteristics of the study sample were also surveyed, including marital status, education level, race/ethnicity, and general medical history. As detailed below, candidate predictors were categorized into psychosocial, physical, and neurophysiological domains based on a theoretical construct derived from existing longitudinal and case-control studies. Figure 1 illustrates the candidate measures that were collected during the baseline assessment for risk analyses within each of the primary measurement domains. In addition to these measures, a subset of participants (Figure 2) agreed to participate in ambulatory worksite monitoring for a sub-study of autonomic and motor responses to experimental and occupational stressors that will be reported elsewhere.
Figure 1.
Candidate risk factors were categorized into psychosocial, physical, and neurophysiological measurement domains, which were assessed concurrently in the same cohort using a combined risk model (*) to investigate their combined contribution (*) to chronic interfering neck pain.
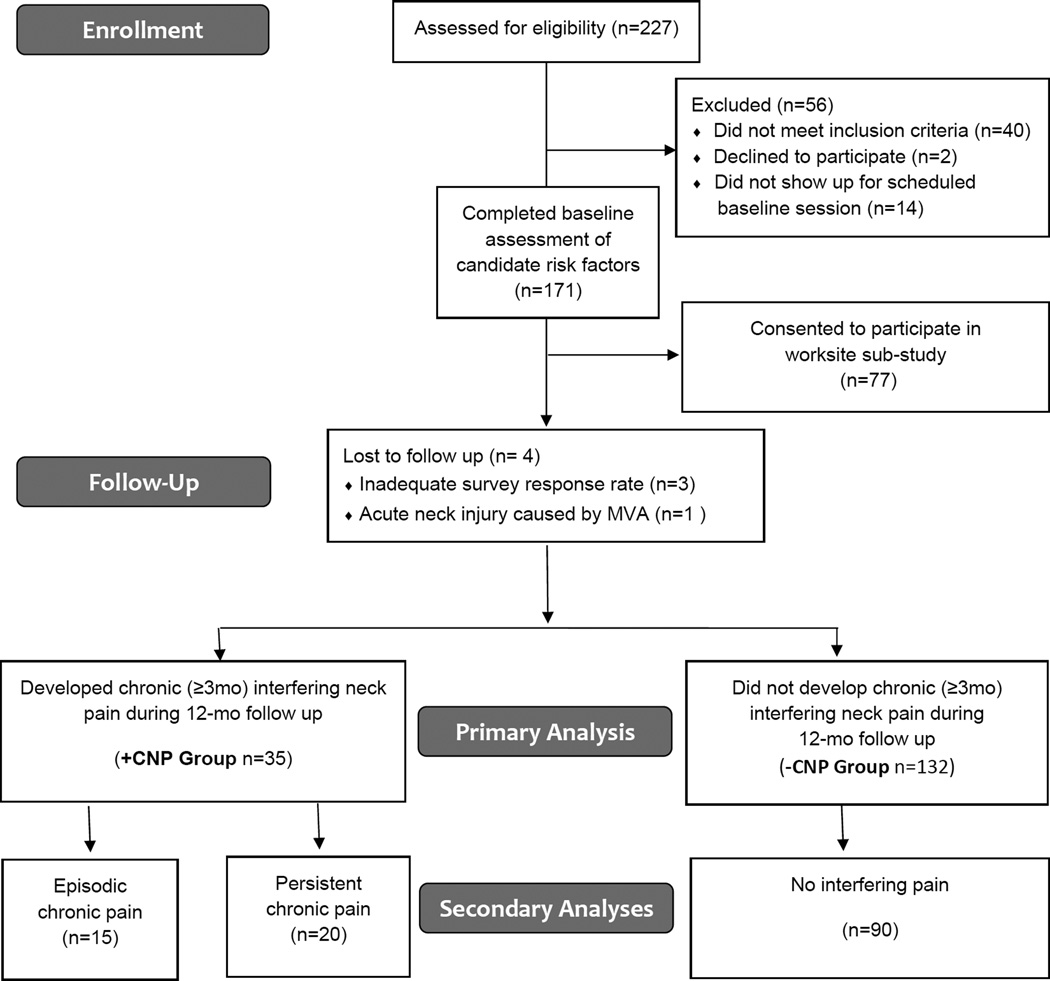
Figure 2.
Flow diagram of participant enrollment, follow up, and analysis.
Psychosocial domain measures
Depression, stress and anxiety, catastrophization, poor job satisfaction, and job-related mental demands/strain are among the most commonly cited psychosocial impairments thought to contribute to spine pain [1, 8, 16, 18, 22, 29, 37, 40, 53], yet the causal role of these factors for neck pain in office workers is still debated [44, 48].
Depressed mood was measured using the Beck Depression Inventory-II (BDI-II), a widely utilized self-report survey that has been shown to have high reliability in multiple populations [66]. The BDI-II includes items that assess symptoms of depression including sadness, agitation, irritability, worthlessness, loss of pleasure, and changes in sleep and appetite. The BDI-II is scored on a 4-point Likert scale, with scores ranging from 0–63 points. Score ranging from 0–13 points are considered to indicate minimal depression, 14–19 points mild depression, 20–28 points moderate depression, and greater than 28 points severe depression [6].
Generalized anxiety was assessed using the trait anxiety scale of the Spielberger State-Trait Anxiety Index (STAI-T), which measures enduring traits of nervousness and fear-based worry that remain relatively stable despite fluctuating contextual factors [56,57]. The STAI-T has been shown to have high reliability in a variety of populations [58]. It is scored using a 4-point Likert scale ranging from 20–80 points, with higher scores indicating greater trait anxiety.
Perceived stress was measured using the Perceived Stress Scale (PSS), which measures the degree to which life situations are perceived to be stressful in the context of individual coping strategies, personal factors, and objective stressful events. The PSS has been shown to have high reliability in similarly aged cohorts [14]. It is scored using a 5-point Likert scale ranging from 0–56 points, with higher scores indicating greater levels of perceived stress.
Catastrophization tendencies were measured using the Pain Catastrophization Scale (PCS), which is designed to assess negative pain-related thoughts such as rumination, magnification, and helplessness associated with painful experiences, in addition to fear of pain. The PCS is scored using a 5-point Likert scale ranging from 0–52 points, with higher scores indicating greater catastrophization tendencies. Scores >30 points are considered to indicate clinically relevant catastrophization. The PCS has been shown to have good reliability in working populations [51, 62]. To assess pain catastrophization in pain-free individuals, the PCS was administered after performing the cold pressor test, and participants were instructed to reference all answers to their experience with this standardized noxious stimulus.
Job satisfaction was measured using the Minnesota Satisfaction Questionnaire (MSQ), which reflects both intrinsic and extrinsic components of job satisfaction, volitional absence, and involvement [67]. The MSQ is scored using a 5-point Likert scale ranging from 0–100 points, with higher scores indicating greater job satisfaction.
Job-related mental strain was assessed using the psychological subscale of the Job Content Questionnaire (JCQ-PSY), which measures mental strain, time demands, and decision latitude in the workplace [35]. The JCQ-PSY subscale is scored using a 4-point Likert scale ranging from 3–33 points, with higher scores indicating more job related mental strain.
Physical domain measures
Poor posture and impaired cervicoscapular muscle performance have been reported in patients with current neck pain compared to pain-free controls [52]; however, it is not clear which, if any, of these impairments precede the development of pain. Furthermore, both general physical activity levels and physical demands in the workplace have been associated with an increased risk of neck pain in some, but not all longitudinal investigations [18, 48].
Forward head posture was quantified as the angle of the cervical spine relative to the horizontal (cervical angle), with smaller cervical angles corresponding to increased forward head posture. Cervical angle was defined as the angle measured from the horizontal plane to a reflective marker located on the tragus of the ear, with the origin at a marker located over the spinous process of the seventh cervical vertebrae. Cervical angle was measured from digital photographs taken while participants were seated at a standardized computer workstation with their eyes fixed on a computer monitor and their hand on a mouse to replicate their natural neck posture in the workplace.
Cervicoscapular impairments were measured during a clinical examination as described in detail by Shahidi et al. [52]. Briefly, the examination included inclinometer measurements of cervical flexion, extension, lateral bending, and rotation active range of motion (AROM); handheld dynamometer measurements of cervical isometric flexion, extension, and lateral bending strength; stopwatch measurements of cervical flexor and extensor muscle endurance; handheld dynamometer measurements of rhomboid and trapezius isometric muscle strength; and flexible ruler measurements of pectoralis minor muscle length. Muscle strength, length, and range of motion were assessed bilaterally and either averaged across sides to report mean muscle strength and length, or summed to report total range of motion. These measures are commonly used in the clinical assessment of neck pain, and reliability has been shown to be fair to excellent in individuals with and without neck pain [52].
Physical activity was assessed using the Baecke Physical Activity (BPA) Index, which includes subscales for work- (BPA-W), leisure- (BPA-L), and sports-related (BPA-S) physical activities [3]. Each BPA subscale is scored on a 5-point Likert scale, with scores ranging from 1–5 points for each category, and a total summated range of 3–15 points. Higher scores within each category indicate higher levels of physical activity.
Job-related physical strain was assessed using the Physical Demand subscale of the Job Content Questionnaire (JCQ-PD) [35]. The physical demand subscale is scored on a 4-point Likert scale and ranges from 3–12 points, with higher scores indicating greater exposure to physical demands in the workplace.
Neurophysiological domain measures
Emerging evidence suggests that alterations in pain processing may increase sensitivity to noxious stimuli among individuals with chronic pain compared to pain-free controls [1, 53, 39]. Several different procedures for quantitative sensory testing of pain sensitivity have been proposed, and it is not currently known which sensory modality is most relevant to musculoskeletal pain, or whether various sensory modalities are differentially affected by factors that alter pain processing. Therefore, pain sensitivity was assessed for both thermal and mechanical stimuli in the present study. Whereas changes in pain sensitivity can result from impairments at multiple levels throughout the neuromuscular system, endogenous pain inhibition reflects modulation of nociceptive signals by the central nervous system. Diffuse noxious inhibitory control (DNIC) is a type of endogenous pain inhibition that can be quantified using a variety of conditioned pain modulation (CPM) protocols [68]. DNIC is thought to involve descending spino-bulbo-spinal circuits that cause a decrease in sensitivity to a painful phasic (aka test) stimulus in the presence of another painful tonic (aka conditioning) stimulus [55, 68].
Cold pain threshold (CPTh) and cold pain tolerance (CPTo) were quantified as the amount of time in seconds required for a cold sensation to change into slight pain or discomfort (CPTh) and intolerable pain (CPTo), respectively, while the participant’s non-dominant hand was submerged to wrist level in a 4 degree Celsius water bath during the cold pressor test. Thermal thresholds demonstrate fair to excellent reliability, with correlation coefficients ranging between 0.4 and 0.9 [46].
Pressure pain threshold (PPT) was measured using a handheld digital pressure algometer (FPIX 50, Wagner Instruments, Greenwich, CT) with a 1-cm diameter tip applied at a rate of 1.0 kgF/sec to the upper trapezius muscle at a standardized location 2-cm lateral to the midpoint between C7 and the acromion process with the participant seated upright in a chair supported by back and arm rests. PPT was defined as the lowest pressure (kgF/cm2) at which the sensation of pressure turned to slight pain or discomfort. PPT measurements were performed 3 times on the dominant and non-dominant upper trapezius, with at least 1 minute rest between trials. Both the participant and examiner were blind to force readings during the assessment, which were digitally recorded and analyzed offline by a research assistant who was blinded to group. The average of all trials on both sides was used in subsequent risk analyses. This specific procedure was selected because mechanical hyperalgesia of the neck musculature has previously been reported among individuals with chronic neck pain, and upper trapezius PPT values are highly reliable with a known minimum detectable change of 0.48 kgF/cm2 [65].
Diffuse noxious inhibitory control (DNIC) was assessed as a measure of endogenous pain inhibition by measuring the change in PPT (phasic test stimulus) for the dominant trapezius muscle during submersion of the non-dominant hand in a 4 degree Celsius ice water bath (tonic conditioning stimulus) compared to a water bath held at room temperature to control for the non-thermal sensory effects of water immersion [39]. The two temperature conditions were presented in random order, with at least 30 minutes rest between conditions to control for residual analgesia and any unknown effects of repeated sensory testing. The change in PPT between temperature conditions was expressed relative to the control (room temperature) condition according to the following formula: %DNIC = [PPT (cold) - PPT (control)]/ PPT control*100, where higher values indicate more efficient pain inhibition. Measurement reliability for DNIC has been reported in two studies to date [12, 39], and test-retest reliability has been reported to be excellent when the cold pressor test is used as the conditioning stimulus [39].
Follow up assessments
After the initial baseline assessment, all participants were followed prospectively for 12 months through administration of a monthly online survey that required 5–10 minutes to complete (REDCap Software, v 5.5.9, Vanderbilt University 2014). A link to a secure, personally identified survey was electronically mailed to participants on the same day of the month for 12 consecutive months; if not completed within 7 days, one additional electronic reminder was sent. The survey contained questions from the NDI, with additional questions related to health care utilization and filing of an insurance claim due to neck pain during the previous month. These questions were used to identify participants who developed interfering neck pain, operationally defined by the Task Force on Neck Pain as the self-reported presence of neck pain causing a person to restrict his or her participation in usual activities, seek health care, and/or file an insurance claim [25]. Activity and participation limitations were identified by a score ≥5 points on the NDI [61]. Seeking health care included individuals who reported spending health care dollars in traditional (e.g. physician, physical therapist) or complementary (e.g. massage therapist, chiropractor) health care settings. Filing a claim included individuals who reported filing a health insurance, auto insurance, workers’ compensation, or personal injury claim related to their neck pain.
The presence of self-reported limitations in daily activities and utilization of health care resources was used to define interfering neck pain in the present study so that, regardless of pain severity, individuals defined as having neck pain perceived their symptoms to be of sufficient intensity to modify their behavior and/or invest economic resources to improve their condition. This definition was intended to distinguish clinically meaningful episodes of neck pain from more commonly occurring symptoms that do not interfere with daily activities and typically resolve without intervention. Individuals who met the definition of interfering neck pain for 3 or more months during the 12-month follow up period were classified as having developed chronic interfering neck pain [63]. It is well known that chronic neck pain often presents as an episodic disorder, with disabling episodes of pain that vary in duration and are separated by a transitory resolution of symptoms [15, 25]. We considered the presence of interfering pain to be a clinically meaningful endpoint regardless of its time course; therefore, our definition of chronic interfering neck pain included individuals who developed either persistent (≥3 consecutive months) or episodic (≥3 non-consecutive months) interfering pain at any point during the 12-month follow up. Consequently, all participants identified as having chronic interfering neck pain in the present study reported symptoms that interfered with their daily activities for at least a quarter of the previous year, irrespective of when these limitations occurred.
Participants were considered to be lost to follow up if they completed fewer than 8 of the 12 monthly surveys, or if they failed to respond to the survey for 3 consecutive months. Individuals were excluded from the study if they moved to a new job that no longer met the inclusion criteria for high risk office work, or developed pain from a known mechanism of injury that did not meet the inclusion criteria for non-specific neck pain (e.g., motor vehicle accident).
Statistical analyses
Demographic characteristics of the study sample were described by means (SD) or percentages. Due to the heterogeneity of candidate risk factors for neck pain identified by previous studies and the relative lack of information on predictors of first onset chronic pain, we first used a backwards selection approach in three separate multivariate logistic regression models to identify significant predictors within each of the primary domains in our theoretical construct (psychosocial, physical, and neurophysiological). Cut-off levels for the backwards selection were conservatively set at α≤0.05. All variables with significant odds ratios identified in the backwards logistic regression for each domain were then simultaneously entered into a single multivariate logistic regression model to test our a priori hypothesis that a combination of psychosocial, physical, and neurophysiological factors contribute to the risk of chronic interfering neck pain. The final model was adjusted for previously established covariates of age, sex, and BMI [43, 48, 49]. Goodness of fit statistics were performed using the Hosmer-Lemeshow test, and multi-colinearity was assessed using variance inflation factors [17]. BDI-II scores were log-transformed prior to model entry to correct for a skewed distribution of raw scores. Odds ratios for cervical extensor endurance were reported using 10 second intervals, and odds ratios for DNIC were reported using 5% intervals.
The definition of chronic interfering neck pain selected for our primary analysis was intended to identify participants who developed neck pain severe enough to cause a change in behavior, regardless of whether their pain was persistent or episodic in nature. This was done so that future studies can target high-risk individuals to minimize the economic and personal impact of neck-related disability. However, it is important to note that the group classified as having no neck pain included participants who experienced more benign and self-limiting symptoms that did not meet our stringent criteria for chronic interfering neck pain. Thus, we conducted a post-hoc sensitivity analysis using an identical statistical approach to examine risk factors for chronic interfering neck pain relative to a sub-group of participants who reported no interfering neck pain during the follow up period (NDI<5 for 12 consecutive months). As described above, the group classified as having chronic interfering neck pain in the primary analysis included individuals with both persistent and episodic interfering neck pain. Thus, a second sensitivity analysis excluded the subgroup of participants with episodic neck pain to determine if risk factors differed for those who developed persistent pain with no intermittent resolution of symptoms. Additionally, we examined whether risk factors differed for the subgroup of participants who continued to report interfering pain at the 12-month time point to facilitate comparison with studies using a single endpoint to define the presence or absence of chronic pain at one year. All statistical analyses were performed using SAS statistical software (SAS v 9.3, Cary, North Carolina).
Results
Incidence of chronic neck pain
A flow diagram of participant enrollment, follow up, and analysis is provided in Figure 2. A total of 171 participants (135 females, 36 males) were enrolled in the study. Four participants (2.3%) were lost to follow up, including 3 individuals who failed to respond to the neck pain surveys for at least 8 of the 12 months, and 1 individual who was excluded from analysis due to an acute neck injury associated with a motor vehicle accident. Of the remaining 167 participants, 35 (21%) developed chronic interfering neck pain. Out of a total of 2,052 follow up surveys administered (171 participants x 12 monthly surveys), 1,970 surveys were returned for an overall capture rate of 96.0%. Demographic characteristics for the groups who did (+CNP) and did not (−CNP) develop chronic interfering neck pain are provided in Table 1.
Table 1.
Demographic characteristics of study participants who did (+CNP) and did not (−CNP) develop chronic interfering neck pain
| −CNP Group (N=132) |
+CNP Group (N=35) |
|
|---|---|---|
| Age (years) | 30.2 (8.3) | 29.8 (6.8) |
| Sex (F:M) | 102:30 | 31:4 |
| Body mass index (kg/m2) | 24.0 (4.2) | 23.8 (3.9) |
| Computer time (hrs/week) | 32.7 (6.4) | 33.0 (8.1) |
| Education (% group) | ||
| High school | 6 | 11 |
| Associate’s degree | 5 | 6 |
| Bachelor’s degree | 61 | 66 |
| Graduate degree | 28 | 17 |
| Smoking history (% group) | ||
| Current | 3 | 6 |
| Past | 16 | 12 |
| None | 81 | 82 |
| Marital status (% group) | ||
| Single | 59 | 63 |
| Married | 38 | 37 |
| Divorced | 3 | 0 |
| Race/Ethnicity (% group) | ||
| Caucasian | 85 | 69 |
| Asian | 5 | 12 |
| African American | 2 | 6 |
| Hispanic | 4 | 11 |
| Mixed Race/other | 4 | 2 |
CNP = Chronic interfering neck pain
Mixed race/other = individuals who identified more than one racial/ethnic category or did not identify a specific category
Multivariate prediction model for chronic interfering neck pain
Descriptive statistics (mean (SD)) for all variables assessed in the backwards logistic regression models for each measurement domain are provided in Tables 2–4. In the psychosocial domain (Table 2), depressed mood on the BDI-II scale was a significant risk factor for developing chronic interfering neck pain (OR (95% CI) = 4.15(1.45–11.86), p<0.01). In the physical domain (Table 3), greater cervical extensor endurance (OR (95% CI) =0.93(0.88–0.97), p<0.01) and leisure physical activity (BPA-L) (OR (95%CI)=0.44(0.21–0.89), p=0.02) were protective against development of chronic interfering neck pain. In the neurophysiological domain (Table 4), more efficient DNIC was protective against developing neck pain (OR (95%CI) = 0.91(0.84–0.98), p=0.02). When all significant predictors (Figure 3) were entered into a combined multivariate model adjusted for age, sex, and BMI, only depressed mood (OR(95%CI)=3.36(1.10–10.31), p=0.03), cervical extensor endurance (OR(95% CI)=0.92(0.87–0.97), p=0.001), and DNIC (OR(95% CI)=0.90(0.83–0.98), p=0.02) remained as significant risk factors for chronic interfering neck pain (Table 5). Goodness of fit statistics indicated that model fitting was appropriate for all regression models (p>0.45).
Table 2.
Group mean (SD) for psychosocial domain variables
| −CNP (N=132) |
+CNP (N=35) |
|
|---|---|---|
|
Depressed mood (BDI-II score) |
2.9 (3.9) | 4.6 (5.4) |
|
Generalized anxiety (STAI-Trait score) |
31.4 (7.9) | 34.6 (9.4) |
|
Perceived Stress (PSS score) |
10.8 (5.7) | 13.1 (5.7) |
|
Catastrophization (PCS score) |
12.3 (8.7) | 13.8 (10.0) |
|
Job Satisfaction (MSQ score) |
76.9 (11.7) | 75.9 (12.1) |
|
Job mental strain (JCQ-PSY score) |
16.4 (4.6) | 15.6 (4.5) |
CNP = Chronic interfering neck pain
BDI = Beck Depression Inventory
STAI-Trait = Speilberger State Trait Inventory – Trait index
PSS = Perceived Stress Scale
PCS = Pain Catastrophization Scale
MSQ = Minnesota Satisfaction Questionnaire
JSQ-PHY = Job Content Questionnaire – Psychological subscale
Table 4.
Group mean (SD) for neurophysiological domain variables
| −CNP (N=132) |
+CNP (N=35) |
|
|---|---|---|
| Cold Pain Threshold (sec) | 17.4 (13.9) | 16.6 (16.7) |
| Cold Pain Tolerance (sec) | 119.4 (95.7) | 110.2 (94.0) |
| Mean Pressure Pain Threshold (kgF) | 3.7 (1.6) | 3.9 (1.8) |
| DNIC (%) | 28.5 (27.1) | 16.7 (20.5) |
CNP = Chronic interfering neck pain
DNIC = Diffuse noxious inhibitory control
Table 3.
Group mean (SD) for physical domain variables
| −CNP (N=132) |
+CNP (N=35) |
|
|---|---|---|
| Forward head posture | ||
| Cervical angle (degrees) | 45.0 (5.8) | 45.8 (4.6) |
| Cervical AROM (degrees) | ||
| Flexion | 60.2 (9.3) | 61.7 (11.3) |
| Extension | 68.6 (13.2) | 70.3 (10.7) |
| Side bend (total) | 90.2 (15.1) | 93.0 (13.1) |
| Rotation (total) | 169.7 (16.4) | 170.0 (17.4) |
| Cervical isometric strength (kgF) | ||
| Flexion | 10.9 (4.5) | 10.3 (3.9) |
| Extension | 17.7 (5.3) | 16.5 (5.7) |
| Side bend (mean) | 14.8 (5.2) | 14.3 (4.2) |
| Cervical endurance (sec) | ||
| Flexion | 39.3 (22.5) | 36.9 (17.3) |
| Extension | 257.9 (66.8) | 196.3 (85.8) |
| Scapular isometric strength (kgF) | ||
| Rhomboid (mean) | 14.9 (5.8) | 13.0 (5.2) |
| Middle Trapezius (mean) | 12.6 (11.6) | 10.4 (4.5) |
| Lower Trapezius (mean) | 9.6 (4.0) | 8.9 (4.1) |
| Muscle length (cm) | ||
| Pectoralis Minor (mean) | 6.5 (1.2) | 6.5 (1.4) |
| Routine physical activity (points) | ||
| BPA-Work | 1.6 (0.3) | 1.7 (0.3) |
| BPA-Sport | 2.8 (0.8) | 2.7 (0.8) |
| BPA-Leisure | 3.1 (0.6) | 2.8 (0.7) |
| Job-related physical strain (points) | ||
| JCQ-PD | 4.6 (1.8) | 4.9 (1.8) |
CNP = Chronic interfering neck pain
AROM = Active range of motion
BPA = Baecke Physical Activity Questionnaire
JCQ-PD = Job Content Questionnaire – Physical Demand subscale
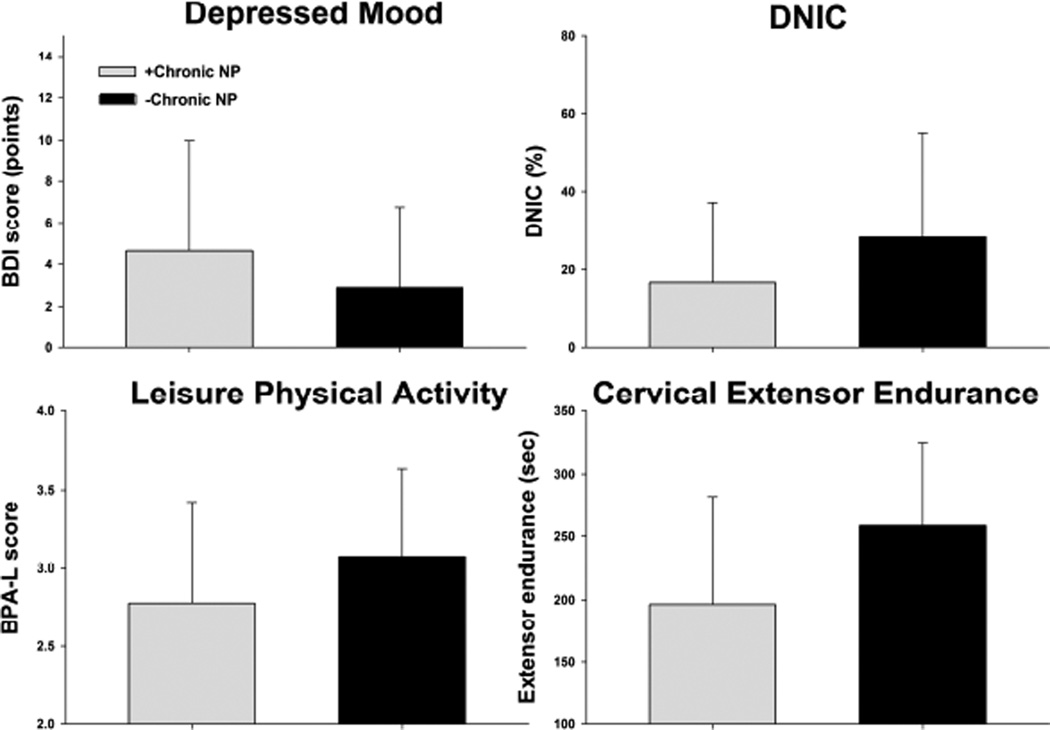
Figure 3.
Group differences in significant risk factors identified from unadjusted risk models for individuals who did (+Chronic NP) and did not (−Chronic NP) develop chronic interfering neck pain during the 12 month follow up. Values are group mean (SD). BDI = Beck Depression Inventory, DNIC = Diffuse noxious inhibitory control, BPA-L = Leisure subscale of the Baecke Physical Activity Questionnaire
Table 5.
Odds ratios (OR) from adjusted multivariate prediction model for the development of chronic interfering neck pain
| OR (95% CI) | p-value | |
|---|---|---|
| Depressed mood (BDI-II score) | 3.36 (1.10–10.31) | 0.03 |
| Cervical extensor endurance (sec) | 0.92 (0.87–0.97) | 0.001 |
| DNIC (%) | 0.90 (0.83–0.98) | 0.02 |
BDI = Beck Depression Inventory, DNIC = Diffuse noxious inhibitory control
Secondary sub-group analyses
To determine the sensitivity of our findings to group categorization definitions, we repeated the same statistical procedures using a reference subgroup of 90 participants who reported no interfering neck pain throughout the entire follow up period (NDI <5 for 12 consecutive months). Using this more conservative definition for the comparison group, depressed mood (OR(95%CI)=8.73(1.27–59.99), p=0.03) and cervical extensor endurance (OR(95% CI)=0.83(0.74–0.94), p=0.002) remained significant predictors of chronic interfering neck pain in the adjusted multivariate model; however, DNIC (OR(95% CI)=0.99(0.97–1.00), p=0.12) was no longer significant. Removing individuals with episodic pain to examine risk factors only for those who developed chronic persistent neck pain (3 or more consecutive months; n=20) indicated a significant risk of high perceived stress (PSS OR(95% CI)=1.01(1.01–1.21), p=0.03) and a trend for DNIC (OR(95% CI)=0.89(0.79–1.01), p=0.08) in the adjusted multivariate model. Finally, cervical extensor endurance (OR(95% CI)=0.90 (0.86–0.96), p<0.001) emerged as the only significant risk factor for chronic interfering neck pain at the 12-month time point (n=17) in the adjusted multivariate model.
Discussion
Incidence of neck pain in office workers
Twenty-one percent of healthy office workers developed chronic interfering neck pain that limited daily activities for 3 or more months during their first year of employment. This is lower than previously reported incidence rates of 30–50% in the general population [16, 30], which likely reflects the varied criteria used to define neck pain across studies. We used monthly self-reports of neck-related disability to minimize recall bias and exclude more benign episodes of neck pain that did not result in activity limitations or utilization of health care resources. Prior studies of neck pain associated with activity limitations or disability have reported a 14–23% incidence among frequent computer users and office workers [16, 27, 34, 47], consistent with findings from the present investigation.
Risk factors for new onset chronic interfering neck pain
The strongest predictor for chronic interfering neck pain was depressed mood. This finding is consistent with numerous cross-sectional investigations correlating depressed mood with the severity and chronicity of existing pain, and further strengthens evidence from the few prospective studies indicating that depression can increase the risk of developing new onset neck pain up to fourfold [11, 40]. It is important to note that self-reported depression scores were low in the present study, despite their association with a three-fold risk of chronic neck pain. Thus, even low levels of depressed mood may be important to identify in high risk populations, as subclinical symptoms appear to have a large impact on the development and chronicity of pain.
Catastrophization was not found to be a risk factor for chronic neck pain, which is in contrast to literature supporting a fear-avoidance model in pain chronicity [8, 64]. This finding may be attributed to completion of the PCQ in response to an experimental thermal pain stimulus by pain-free individuals in the present study, which may not generalize to patients with musculoskeletal pain conditions. Additionally, pain catastrophization may be important in the chronicity, but not the initial onset of pain. Perceived stress and trait anxiety also did not predict chronic interfering pain, in contrast to findings from a prior prospective investigation [24]. Previous investigators have questioned the ability to distinguish between the constructs of stress, anxiety, and depression, and have argued that these psychological factors are highly correlated and may all contribute to chronic pain [40, 41].
Interestingly, job-related psychosocial factors were not found to predict chronic neck pain, contrary to much of the previous literature identifying risk factors such as low job satisfaction and high mental job strain and distress [18, 59]. This discrepancy is likely attributed to the short duration of exposure to participants’ new work environment (< 3 months) when job-related surveys were administered in the present study. There is some evidence that general psychological characteristics including depressed mood and perceived stress may facilitate increasingly negative feelings toward work environments over time, ultimately contributing to low work satisfaction and distress [8]. Future research investigating the time course of interactions between general psychological health and job related psychosocial behaviors is required to elucidate these relationships.
Physical risk factors for chronic interfering neck pain included poor cervical extensor endurance and low leisure physical activity. It seems logical that computer work would require high endurance of the cervical extensors to stabilize the neck and head throughout the workday. Only two studies have specifically investigated cervical extensor endurance as a risk factor for musculoskeletal pain, with conflicting results [26, 32]. However, longitudinal studies of related muscle groups support the notion that poor cervicoscapular muscle endurance can increase the risk of neck pain [4, 28]. Collectively, these findings suggest that future research should examine the efficacy of cervicoscapular endurance training to prevent the development of chronic neck pain in susceptible individuals. Interestingly, our findings demonstrate that other commonly observed cervicoscapular impairments such as range of motion and strength [52] do not precede the onset of pain, and are more likely to reflect pain-related adaptations than a direct cause of neck pain in office workers.
Low leisure physical activity also predicted the development of chronic neck pain in the unadjusted physical risk model, but was not retained in the final model after adjusting for factors that are strongly associated with physical activity such as age, sex, and BMI. Evidence supporting physical activity as a risk factor is conflicting to date, with some studies reporting that high physical activity reduces the risk of neck pain [32, 36] and other studies demonstrating no effect [10]. A recent systematic review concluded that there is insufficient evidence to support the protective benefits of physical activity in reducing the risk of neck pain [48]; however, the present results suggest otherwise and may indicate physical activity interventions for the primary prevention of chronic neck pain. Increasing leisure time physical activity may be particularly beneficial for individuals who spend the majority of their workday in a sedentary occupation.
Descending pain modulation has not been previously investigated in individuals with neck pain, yet several studies have reported impaired DNIC among patients with fibromyalgia, chronic widespread pain, and temporomandibular disorder (TMD) [33, 42, 50]. A large prospective investigation recently concluded that heightened mechanical pain sensitivity predicts chronicity of persistent TMD symptoms, but does not predict first onset of pain [54]; however, this study did not investigate endogenous pain inhibition as a candidate risk factor. Increased pain sensitivity may be indicative of low nociceptive thresholds resulting from an imbalance between facilitatory and inhibitory pain modulation pathways that contribute to peripheral and central sensitization [5]. Results of the present study provide the first evidence that pre-existing impairments in endogenous pain inhibition in otherwise healthy individuals precede changes in mechanical pain sensitivity and increase the risk of developing a chronic musculoskeletal pain condition. Because we excluded individuals with prior musculoskeletal injuries or chronic pain, these factors cannot explain DNIC impairments, suggesting that endogenous pain inhibition may reflect an individual or genetic predisposition that is present prior to any pain condition. Interestingly, serotonin receptor and transporter genes (HTR2A and SLC6A4) have been directly linked to higher depression and anxiety [19], and several neuromodulatory pathways for descending pain inhibition are also thought to involve serotonergic signaling [13]. Additional research is needed to delineate mechanisms underlying the concomitant presence of depressed mood and impaired DNIC among individuals at risk for chronic pain.
Risk factors for persistent and episodic neck pain subgroups
Depressed mood and poor cervical extensor endurance, but not DNIC, remained as significant predictors of chronic interfering neck pain when examined relative to a smaller subgroup of individuals who reported no episodes of interfering neck pain during their first year of employment. When only individuals with persistent neck pain were included in this analysis, perceived stress (highly correlated with depressed mood in our sample) emerged as the only significant predictor, along with a trend for less efficient DNIC. Finally, cervical extensor endurance predicted the presence of ongoing neck pain at the 12-month follow up. Although the limited number of cases available for post hoc subgroup analyses likely resulted in insufficient power to detect all relevant predictors, these findings demonstrate similar risk factor profiles across multiple outcome definitions for chronic neck pain and suggest that findings from the primary analysis are generally robust.
Study Limitations
This is one of the few prospective, inception cohort studies to identify modifiable risk factors for new onset chronic musculoskeletal pain. Methodological strengths included the concurrent assessment of physical, psychological, and neurophysiological risk factors in a large cohort of individuals with a high response rate on monthly surveys indicating the presence or absence of neck-related disability. One of the biggest limitations of this study was the inclusion of a relatively homogenous sample of highly educated, predominantly Caucasian female office workers who developed relatively mild (yet still interfering) neck pain. Future studies are necessary to determine whether the risk factors identified in this study generalize to other demographic populations and professions, and to the progression of more severe symptoms typically seen in clinical populations seeking treatment for resolution and secondary prevention of neck pain.
Although our sample size can be considered large for a non-epidemiologic study that included an extensive physical examination of all participants at baseline, we did not conduct an a priori power analysis for candidate predictors included in the present study, and we may have been underpowered to detect predictors with a smaller effect size. This is a particular concern for secondary subgroup analyses, for which all findings should be considered preliminary. Due to sample size concerns, we were not able to test for potential interactions among risk factors, which should be examined in future studies that target a fewer number of predictors. To our knowledge, this is the first study to report that impaired DNIC is a pre-existing risk factor for the future development of chronic musculoskeletal pain. However, we included only a limited number of quantitative sensory tests in our baseline examination, and the role of other pain processing measures (e.g. temporal summation) in risk assessment for chronic pain remain to be determined.
Implications for future research on preventing the transition to chronic neck pain
Preventing the transition from acute pain - a necessary and normal response to protect the body from injury - to a pathologic state of chronic pain is a major goal of pain research. Our results indicate that poor cervical extensor endurance increases the risk of developing chronic, non-specific neck pain among office workers. This finding suggests that sensitization of peripheral nociceptors by algesic metabolites [21, 23] produced during postural muscle fatigue may play an important role in the transition to chronic pain in susceptible individuals. Depressed mood and DNIC were also found to significantly increase the risk of chronic neck pain. It seems plausible that these predisposing factors could reduce the ability to suppress pain through descending inhibition and cognitive processing of nociceptive input, such as that arising from sensitized muscle chemoreceptors. Low physical activity, which was also identified in the unadjusted risk model for chronic neck pain, directly contributes to impairments in both muscle endurance and depressed mood [7] and may also influence DNIC, although this association has not been sufficiently investigated. Future studies are needed to determine whether physical activity interventions to improve muscle endurance are effective in remediating the primary physical, psychological, and neurophysiological risk factors for chronic neck pain among high risk groups.
PERSPECTIVE.
Depressed mood, poor muscle endurance, and impaired endogenous pain inhibition are predisposing factors for the development of new onset chronic neck pain of nonspecific origin in office workers. These findings may assist with primary prevention by allowing clinicians to screen for individuals at risk of developing chronic neck pain.
HIGHLIGHTS.
Psychosocial, physical, and neurophysiological factors affect risk for neck pain
Depressed mood is the strongest predictor for development of chronic neck pain
Diffuse noxious inhibitory control is impaired in those developing chronic neck pain
Poor cervical extensor endurance increases risk for developing chronic neck pain
Acknowledgments
The authors would like to acknowledge Jaclyn Balter, Ashley Haight, and Cynthia Armstrong for assistance with participant recruitment and data collection. This research was supported by NIH R01 AR056704 awarded to K.S.M., and a Promotion of Doctoral Studies scholarship from the Foundation for Physical Therapy awarded to B.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest
References
- 1.Andersen J, Kaergaard A, Frost P, Thomsen JF, Bonde JP, Fallentin N, Borg V, Mikkelsen S. Physical, psychosocial, and individual risk factors for neck/shoulder pain with pressure tenderness in the muscles among workers performing monotonous, repetitive work. Spine. 2002;27:660–667. doi: 10.1097/00007632-200203150-00017. [DOI] [PubMed] [Google Scholar]
- 2.Ariens G, van Mechelen W, Bongers P, Bouter L, van der Wal G. Physical risk factors for neck pain. Scan J Work Environ Health. 2000;26:7–19. doi: 10.5271/sjweh.504. [DOI] [PubMed] [Google Scholar]
- 3.Baecke J, Burena J, Frijters J. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 4.Barnekow-Bergkvist M, Hedberg G, Janlert U, Jansson E. Determinants of self-reported neck-shoulder and low back symptoms in a general population. Spine. 1998;23:235–243. doi: 10.1097/00007632-199801150-00017. [DOI] [PubMed] [Google Scholar]
- 5.Basbaum A, Bushnell C. Science of Pain. Boston, MA: Elsevier; 2009. [Google Scholar]
- 6.Beck A, Steer R, Ball R, Ranieri W. Comparison of Beck Depression Inventories-IA and -II in Psychiatric Outpatients. J Personality Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 7.Bishop-Bailey D. Mechanisms governing the health and performance benefits of exercise. Br J Pharmacol. 2013;170:1153–1166. doi: 10.1111/bph.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bongers P, Winter C, Kompier M, Hidebrandt V. Psychosocial factors at work and musculoskeletal disease. Scand J Work Environ Health. 1993;19:297–312. doi: 10.5271/sjweh.1470. [DOI] [PubMed] [Google Scholar]
- 9.Borghouts JA, Koes BW, Vondeling H, Bouter LM. Cost-of-illness of neck pain in The Netherlands in 1996. Pain. 1999;80:629–636. doi: 10.1016/S0304-3959(98)00268-1. [DOI] [PubMed] [Google Scholar]
- 10.Brandt L, Andersen J, Lassen C, Kryger A, Overgaard E, Vilstrup I, Mikkelsen S. Neck and shoulder symptoms and disorders among Danish computer workers. Scand J Work Environ Health. 2004;30:399–409. doi: 10.5271/sjweh.828. [DOI] [PubMed] [Google Scholar]
- 11.Carroll L, Cassidy D, Cote P. Depression as a risk factor for onset of an episode of troublesome neck and low back pain. Pain. 2004;107:134–139. doi: 10.1016/j.pain.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Cathcart S, Winefield A, Roan P, Lushington K. Reliability of temporal summation and diffuse noxious inhibitory control. Pain Res Manag. 2009;14:433–438. doi: 10.1155/2009/523098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chitour D, Dickenson A, Le Bars D. Pharmacological evidence for the involvement of serotonergic mechanisms in diffuse noxious inhibitory controls (DNIC) Brain Res. 1982;236:329–337. doi: 10.1016/0006-8993(82)90718-1. [DOI] [PubMed] [Google Scholar]
- 14.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 15.Cote P, Cassidy JD, Carroll LJ, Kristman V. The annual incidence and course of neck pain in the general population: a population-based cohort study. Pain. 2004;112:267–273. doi: 10.1016/j.pain.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Cote P, van der Velde G, Cassidy JD, Carroll LJ, Hogg-Johnson S, Holm LW, Carragee EJ, Haldeman S, Nordin M, Hurwitz EL, Guzman J, Peloso PM. The burden and determinants of neck pain in workers: results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. J Manipulative & Physiol Ther. 2008;32:S70–S86. doi: 10.1016/j.jmpt.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Craney T, Surles J. Model-Dependent Variance Inflation Factor Cutoff Values. Quality Engineering. 2007;14:391–403. [Google Scholar]
- 18.Da Costa B, Vieira E. Risk factors for work-related musculoskeletal disorders: a systematic review of recent longitudinal studies. Am J Ind Med. 2010:1–39. doi: 10.1002/ajim.20750. [DOI] [PubMed] [Google Scholar]
- 19.Diatchenko L, Fillingimm R, Smith S, Maixneer W. The phenotypic and genetic signatures of common musculoskeletal pain conditions. Nat Rev Rheumatol. 2013;9:340–350. doi: 10.1038/nrrheum.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eltayeb S, Staal J, Hassan A, de Bie R. Work related risk factors for neck, shoulder and arms complaints: a cohort study among dutch computer office workers. J Occup Rehabil. 2009;19:315–322. doi: 10.1007/s10926-009-9196-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerdle B, Kristiansen J, Larsson B, Saltin B, Sogaard K, Sjogaard G. Algogenic substances and metabolic status in work-related Trapezius myalgia: a multivariate explorative study. BMC Musculoskelet Disord. 2014;15:357. doi: 10.1186/1471-2474-15-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gore M, Sadosky A, Stacey BR, Tai KS, Leslie D. The burden of chronic low back pain: clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine. 2012;37:E668–E677. doi: 10.1097/BRS.0b013e318241e5de. [DOI] [PubMed] [Google Scholar]
- 23.Graven-Nielsen T, Mense S. The peripheral apparatus of muscle pain: evidence from animal and human studies. Clin J Pain. 2001;17:2–10. doi: 10.1097/00002508-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Grimby-Ekman A, Andersson E, Hagberg M. Analyzing musculoskeletal neck pain, measured as present pain and periods of pain, with three different regression models: a cohort study. BMC Musculoskelet Disord. 2009;10:73. doi: 10.1186/1471-2474-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guzman J, Hurwitz EL, Carroll LJ, Haldeman S, Cote P, Carragee EJ, Peloso PM, van der Velde G, Holm LW, Hogg-Johnson S, Nordin M, Cassidy JD. A new conceptual model of neck pain: linking onset, course, and care: the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine. 2008;33:S14–S23. doi: 10.1097/BRS.0b013e3181643efb. [DOI] [PubMed] [Google Scholar]
- 26.Hamberg van Reenen H, Ariens G, Blatter B, van der Beek J, Twisk W, Mechellen W, Bongers P. Is an imbalance between physical capacity and exposure to work-related physical factors associated with low-back, neck or shoulder pain? Scand J Work Environ Health. 2006;32:190–197. doi: 10.5271/sjweh.998. [DOI] [PubMed] [Google Scholar]
- 27.Hansson G, Nordander C, Asterland P, Ohlsson K, Stromberg U, Skerfing S, Rempel D. Sensitivity of trapezius electromyography to differences between work tasks-influence of gap definition and normalization methods. J Electromyogr Kinesiol. 2000;10:103–115. doi: 10.1016/s1050-6411(99)00030-9. [DOI] [PubMed] [Google Scholar]
- 28.Hanvold T, Waersted M, Mengshoel A, Bjerness E, Stigum H, Twisk J, Veiersted K. The effect of work-related sustained trapezius muscle activity on the development of neck and shoulder pain among young adults. Scand J Work Environ Health. 2014;39:390–400. doi: 10.5271/sjweh.3357. [DOI] [PubMed] [Google Scholar]
- 29.Hilderink P, Burger H, Deeg D, Beekman A, Oude Voshaar R. The temporal relation between pain and depression: results from the longitudinal aging study Amsterdam. Psychosom Med. 2012;74:945–951. doi: 10.1097/PSY.0b013e3182733fdd. [DOI] [PubMed] [Google Scholar]
- 30.Hogg-Johnson S, van der Velde G, Carroll LJ, Holm LW, Cassidy JD, Guzman J, Cote P, Haldeman S, Ammendolia C, Carragee E, Hurwitz E, Nordin M, Peloso P. The burden and determinants of neck pain in the general population: results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. J Manipulative Physiol Ther. 2008;32:S46–S60. doi: 10.1016/j.jmpt.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 31.Hoy D, March L, Woolf A, Blyth F, Brooks P, Smith E, Vos T, Barendregt J, Blore J, Murray C, Burstein R, Buchbinder R. The global burden of neck pain: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2008;73:1309–1315. doi: 10.1136/annrheumdis-2013-204431. [DOI] [PubMed] [Google Scholar]
- 32.Hush J, Michaleff Z, Maher CH, Refshauge K. Individual, physical and psychological risk factors for neck pain in Australian office workers: a 1-year longitudinal study. Eur Spine J. 2009;18:1532–1540. doi: 10.1007/s00586-009-1011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Julien N, Goffaux P, Arsenault P, Marchand S. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain. 2005;114:295–302. doi: 10.1016/j.pain.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 34.Kanchanomai S, Janwantanakul P, Pensri P, Jiamjarasrangsi W. Risk factors for the onset and persistence of neck pain in undergraduate students: 1-year prospective cohort study. BMC public health. 2011;11:566. doi: 10.1186/1471-2458-11-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karasek R, Brisson C, Kawakami N, Houtman I, Bongers P, Amich B. The Job Content Questionnaire (JCQ): An instrument for internationally comparative assessments of psychosocial job characteristics. J Occup Health Psychol. 1998;3:322–355. doi: 10.1037//1076-8998.3.4.322. [DOI] [PubMed] [Google Scholar]
- 36.Korhonen T, Ketola R, Toivonen R, Luukkonen R, Hakkanen M, Viikari-Juntura E. Work related and individual predictors for incident neck pain among office employees working with video display units. Occup Environ Med. 2003;60:475–482. doi: 10.1136/oem.60.7.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kraatz S, Lang J, Kraus T, Munster E, Ochsmann E. The incremental effect of psychosocial workplace factors on the development of neck and shoulder disorders: a systematic review of longitudinal studies. Int Arch Occup Environ Health. 2013;86:375–395. doi: 10.1007/s00420-013-0848-y. [DOI] [PubMed] [Google Scholar]
- 38.Leeuw M, Goossens M, Linton S, Crombez G, Boersma K, Vlaeyen J. The fear-avoidance model of musculoskeletal pain: a current state of scientific evidence. J Behav Med. 2007;30:77–94. doi: 10.1007/s10865-006-9085-0. [DOI] [PubMed] [Google Scholar]
- 39.Lewis G, Heales L, Rice D, Rome K, McNair P. Reliability of the conditioned pain modulation paradigm to assess endogenous inhibitory pain pathways. Pain Res Manag. 2012;17:98–102. doi: 10.1155/2012/610561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Linton S. A review of psychological risk factors in back and neck pain. Spine. 2000;9:1148–1156. doi: 10.1097/00007632-200005010-00017. [DOI] [PubMed] [Google Scholar]
- 41.Lovibond P, Lovibond S. The structure of negative emotional states: comparison of the depression anxiety stress scales (DASS) with the beck depression and anxiety inventories. Behav Res Ther. 1995;33:335–343. doi: 10.1016/0005-7967(94)00075-u. [DOI] [PubMed] [Google Scholar]
- 42.Maixner W, Fillingim R, Booker D, Sigurdsson A. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain. Pain. 1995;63:341–351. doi: 10.1016/0304-3959(95)00068-2. [DOI] [PubMed] [Google Scholar]
- 43.Manchikanti L, Singh V, Datta S, Cohen SP, Hirsch JA. Comprehensive review of epidemiology, scope, and impact of spinal pain; American Society of Interventional Pain Physicians. Pain Physician. 2009;12:E35–E70. [PubMed] [Google Scholar]
- 44.Mantyselka P, Lupsakko T, Kautiainen H, Vanhala M. Neck-shoulder pain and depressive symptoms: a cohort study with a 7-year follow up. Eur J Pain. 2010;14:189–1893. doi: 10.1016/j.ejpain.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Martin BI, Deyo RA, Mirza SK, Turner JA, Comstock BA, Hollingworth W, Sullivan SD. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299:656–664. doi: 10.1001/jama.299.6.656. [DOI] [PubMed] [Google Scholar]
- 46.Moloney N, Hall T, Doody C. Reliability of thermal quantitative sensory testing: A systematic review. J Rehabil Res Dev. 2012;49:191–208. doi: 10.1682/jrrd.2011.03.0044. [DOI] [PubMed] [Google Scholar]
- 47.Paksaichol A, Janwantanakul P, Lawsirirat C. Development of a neck pain risk score for predicting nonspecific neck pain with disability in office workers: a 1-year prospective cohort study. J Manipulative Physiol Ther. 2014;37:468–475. doi: 10.1016/j.jmpt.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 48.Paksaichol A, Janwantanakul P, Purepong N, Pensri P, van der Beek A. Office workers’ risk factors for the development of non-specific neck pain: a systematic review of prospective cohort studies. Occup Environ Med. 2012;69:610–618. doi: 10.1136/oemed-2011-100459. [DOI] [PubMed] [Google Scholar]
- 49.Palmer K, Syddall H, Cooper C, Coggon D. Smoking and musculoskeletal disorders: findings from a British national survey. Ann Rheum Dis. 2003;62:33–36. doi: 10.1136/ard.62.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pielsticker A, Haag G, Zaudig M, Lautenbacher S. Impairment of pain inhibition in chronic tension-type headache. Pain. 2005;118:215–223. doi: 10.1016/j.pain.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 51.Quartana P, Campbell C, Edwards R. Pain catastrophizing: a critical review. Expert Rev Neurother. 2009;9:745–58. doi: 10.1586/ERN.09.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shahidi B, Johnson C, Curran-Everett D, Maluf K. Reliability and group differences in quantitative cervicothoracic measures among individuals with and without chronic neck pain. BMC Musculoskelet Disord. 2012;13:215. doi: 10.1186/1471-2474-13-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sjors A, Larsson B, Persson A, Gerdle B. An increased response to experimental muscle pain is related to psychological status in women with chronic non-traumatic neck-shoulder pain. BMC Musculoskelet Disord. 2011;12:230. doi: 10.1186/1471-2474-12-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slade G, Sanders A, Ohrbach R, Fillingim R, Dubner R, Gracely R, Bair E, Maixner W, Greenspan J. Pressure pain thresholds fluctuate with, but do not usefully predict, the clinical course of painful temporomandibular disorder. Pain. 2014;155:2134–2143. doi: 10.1016/j.pain.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sluka K. Mechanisms and management of pain for the physical therapist. Seattle: IASP Press; 2009. [Google Scholar]
- 56.Spielberger CD, Gorsuch RL. State-Trait Anxiety Inventory for adults: Manual and sample: Manual, Instrument and Scoring Guide. Palo Alto: Consulting Psychologists Press; 1983. [Google Scholar]
- 57.Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Inventory. Palo Alto: Consulting Psychologists Press; 1970. [Google Scholar]
- 58.Stanley M, Beck J, Zebb B. Psychometric properties of four anxiety measures in older adults. Behav Res Ther. 1996;34:827–838. doi: 10.1016/0005-7967(96)00064-2. [DOI] [PubMed] [Google Scholar]
- 59.Sterud T, Johannessen, Tynes T. Work-related psychosocial and mechanical risk factors for neck/shoulder pain: a 3-year follow-up study of the general working population of Norway. Int Arch Occup Environ Health. 2014;87:471–481. doi: 10.1007/s00420-013-0886-5. [DOI] [PubMed] [Google Scholar]
- 60.Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. JAMA. 2003;290:2443–2454. doi: 10.1001/jama.290.18.2443. [DOI] [PubMed] [Google Scholar]
- 61.Stratford PW, Riddle DL, Binkley JM, Spadoni G, Westaway MD, Padfield B. Using the Neck Disability Index to make decisions concerning individual patients. Physiother Can. 1999;51:107–112. [Google Scholar]
- 62.Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- 63.van Tulder M, Furlan A, Bombardier C, Bouter L. Updated method guidelines for systematic reviews in the Cochrane Collaboration back review group. Spine. 2003;28:1290–1299. doi: 10.1097/01.BRS.0000065484.95996.AF. [DOI] [PubMed] [Google Scholar]
- 64.Vlaeyen J, Linton S. Fear-avoidance model of chronic musculoskeletal pain: 12 years on. Pain. 2012;153:1144–1147. doi: 10.1016/j.pain.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 65.Walton DM, Macdermid JC, Nielson W, Teasell RW, Chiasson M, Brown L. Reliability, standard error, and minimum detectable change of clinical pressure pain threshold testing in people with and without acute neck pain. J Orthop Sports Phys Ther. 2011;41:644–650. doi: 10.2519/jospt.2011.3666. [DOI] [PubMed] [Google Scholar]
- 66.Wang Y, Gorenstein C. Psychometric properties of the Beck Depression Inventory-II: a comprehensive review. Rev Bras Psiquiatr. 2013;35:416–431. doi: 10.1590/1516-4446-2012-1048. [DOI] [PubMed] [Google Scholar]
- 67.Weiss D, Dawis R, England G. Manual for the Minnesota Satisfaction Questionnaire. Minnesota Studies in Vocational Rehabilitation. 1967;22:120. [Google Scholar]
- 68.Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol. 2010;23:611–615. doi: 10.1097/ACO.0b013e32833c348b. [DOI] [PubMed] [Google Scholar]