Abstract
Cardiovascular disease (CVD) represents a significant and increasing burden on healthcare systems. Artificial intelligence (AI) is a rapidly evolving transdisciplinary field employing machine learning (ML) techniques, which aim to simulate human intuition to offer cost-effective and scalable solutions to better manage CVD. ML algorithms are increasingly being developed and applied in various facets of cardiovascular medicine, including and not limited to heart failure, electrophysiology, valvular heart disease and coronary artery disease. Within heart failure, AI algorithms can augment diagnostic capabilities and clinical decision-making through automated cardiac measurements. Occult cardiac disease is increasingly being identified using ML from diagnostic data. Improved diagnostic and prognostic capabilities using ML algorithms are enhancing clinical care of patients with valvular heart disease and coronary artery disease. The growth of AI techniques is not without inherent challenges, most important of which is the need for greater external validation through multicenter, prospective clinical trials.
Keywords: artificial intelligence, cardiovascular medicine, machine learning, neural networks
Introduction
Cardiovascular disease (CVD) is the leading cause of death in the world and poses an ever-growing challenge.1 The global prevalence of CVD is rapidly rising, increasing from 271 million in 1990 to 523 million in 2019, accompanied by rising mortality, from 12.1 million to 18.6 million during the same period.1 Moreover, the cost of treating CVD is projected to increase from 863 billion United States Dollars (USD) in 2010 to more than 1.044 trillion USD by 2030.2 Cost-effective and scalable solutions are needed to help mitigate CVD burden, and artificial intelligence (AI) can play a critical role.
AI is a rapidly evolving transdisciplinary field which integrates computer science, statistics, psychology, neuroscience, material science, mechanical engineering and computer hardware design to develop algorithms that aim to simulate human intuition, decision-making and object recognition.3 The overarching aims of AI in cardiovascular medicine are threefold: to optimize patient care, improve efficiency and improve clinical outcomes.3 In cardiology, there has been a growth in the potential sources of new patient data, as well as advances in investigations and therapies, which position the field well to uniquely benefit from AI.4
In this review, we introduce the core AI techniques, their historical development and contemporary applications in cardiology. The future prospects and challenges of AI are also discussed.
AI Techniques
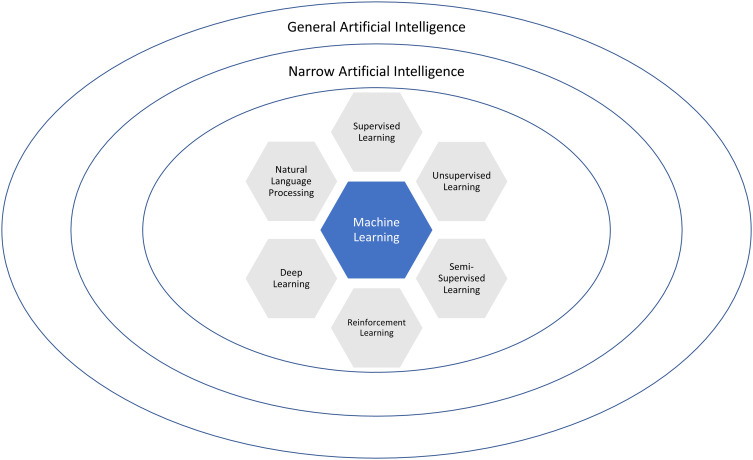
Machine learning (ML) refers to a set of techniques which enable AI, and these techniques vary in their objectives, applications and architectures (Table 1). ML is an iterative process which involves approximating complex mathematical relationships between inputs and outputs by learning from a large data set to predict values from new, unseen data.4 Supervised, unsupervised, semi-supervised and deep learning are terms describing the ways in which ML algorithms learn from data (Figure 1).
Table 1.
Machine Learning (ML) Techniques Which Enable Artificial Intelligence (AI)
| Type of Learning | Machine Learning Algorithm | Outcomes | Strengths | Weaknesses |
|---|---|---|---|---|
|
Supervised Labeled data with defined outputs |
Classification | Categorical | Training data set is reusable if features do not change | Large, accurately labelled training data sets are required. Can be costly and time-consuming At risk of overfitting and does not generalize well if the training data set is heterogenous |
| Regression | Continuous | |||
|
Unsupervised Unlabeled data with unknown outputs |
Clustering | Similarity of Inputs | No previous knowledge of the data set is required and hence the scope of human error is reduced. Faster to perform. | The spectral classes do not necessarily represent features on the ground and can take time to interpret |
| Dimensionality Reduction | Extract Relevant Features | |||
| Association | Co-occurrence Likelihood | |||
| Anomaly Reduction | Outliers | |||
| Semi-Supervised | Generative | Combination of supervised and unsupervised outcomes | Stable algorithm which reduces the time needed to annotate date | Iteration results are not stable and can hence have a low accuracy |
| Reinforcement | Reward based | Sequential decision making | Can self-correct inherent errors introduced during programming | Requires a lot of data and computational power |
Figure 1.
Relationships between artificial intelligence (AI) and machine learning (ML) techniques.
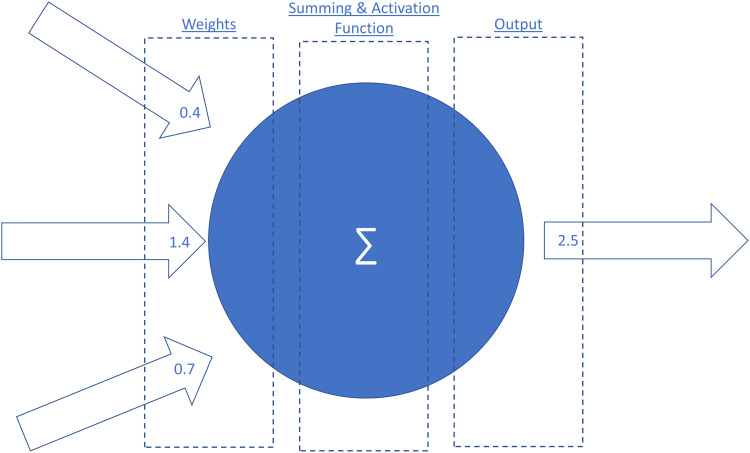
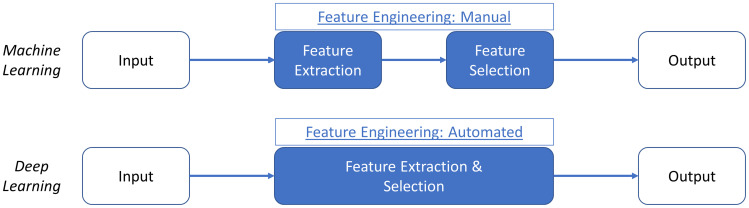
Supervised learning refers to algorithms learning rules between a set of inputs and outputs using a labelled training data set (referred to as mapping) in order to predict outcomes for new inputs. The labelled training data set typically consists of predictor variables, features and labeled outcomes.5 Features refer to pieces of information that make up data and the quality, accuracy and richness of features determines the overall effectiveness of supervised ML algorithms.6 Supervised ML algorithms are especially useful when the research question involves classification or regression-based analysis. A well-labelled training data set can be reused if the pre-specified features do not need to be changed. However, the drawbacks of supervised learning include the often timely and costly process of accurately labelling a large data set. Deep learning is another subfield of ML and refers to algorithms which are modelled on the neural structures of the brain, consisting of inter-connected perceptrons (Figure 2). Compared to traditional ML algorithms, deep learning requires minimal data arbitration or feature engineering (Figure 3).6 An example of supervised learning is a study which used 91,232 single-lead ECGs from 55,549 patients to train an ML algorithm to classify the ECGs into 12 distinct rhythm classes.7 The ECGs contained features suggestive of different arrhythmias and were manually labelled by a consensus committee of board-certified cardiologists before the supervised ML algorithm was trained.7 When compared with the consensus committee of cardiologists, the algorithm had an AUC of 0.97.7
Figure 2.
A perceptron and a simple artificial neural network (ANN).
Figure 3.
Comparing the stepwise approach of machine learning (ML) and deep learning (DL) approaches with respect to feature extraction and selection.
Unsupervised learning is a different form of learning which uses unlabeled data to discover underlying relationships and is often referred to as knowledge discovery.3 Examples of unsupervised learning include principal component analysis, cluster analysis and deep learning.5 In principal component analysis, large, multidimensional datasets are broken into simpler data sets with a smaller number of dimensions through the identification of correlated variables.8 Cluster analysis involves dividing objects into groups that share similar characteristics with the aim of finding subgroups within a larger data set.8 Unsupervised learning is extremely useful in clinical practice where manually labelling large datasets is unfeasible. Moreover, unsupervised learning has reduced scope for incorporating human error. An example is an ML algorithm using principal component and cluster analysis to successfully differentiate heart failure with preserved ejection fraction (HFpEF) from healthy subjects by analyzing LV long axis myocardial velocity patterns on stress echocardiography.9 The study trained an unsupervised ML algorithm from 156 patients using 22 descriptors with 300 features collected from tissue Doppler-derived longitudinal myocardial velocity traces at rest and during exercise.9 The algorithm found naturally occurring patterns and relationships within the details to identify a continuum of disease from normal cardiac function to patients with HFpEF.9 The spectrum varied in their age, body mass index, left ventricular mass index and left ventricular filling pressure and symptom burden as measured by the 6-minute walk test.9 Semi-supervised learning combines supervised and unsupervised learning techniques. It provides the ability to extrapolate and label a large data set from a smaller, manually labelled data set. This is performed by manually labelling a small portion of data and then employing unsupervised learning algorithms to extrapolate from this data.3
Historical Perspectives of AI in Cardiology
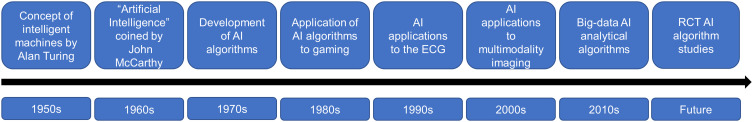
The origins of AI can be traced to Alan Turing who explored the mathematical possibility of AI and described how to build and test intelligent machines in the early 1950s.10 However, computers lacked the functional capabilities to implement this theoretical knowledge at the time. As computing power improved and information could be stored, processed and extracted, ML algorithm development flourished in the 1970s and 1980s.10 Early work focused on AI applications in computer gaming, but its role in clinical decision-making only began taking shape in the 1990s.
In cardiology, the first applications of AI were the development of self-learning neural networks applied to electrocardiography (ECG).11,12 One of the earliest works in 1990 trained a self-learning neural network using 60 ECGs to localize the atrioventricular accessory pathway in patients with Wolff-Parkinson-White Syndrome by using the polarity of the delta waves as an input.12 In a testing cohort of 25 ECGs, the algorithm correctly localized the atrioventricular pathway in 23 patients.12 As AI techniques developed and computing power continued to improve, applications of AI in cardiology expanded to other fields, including cardiac imaging, electrophysiology, heart failure and interventional procedures. In 1999, a study applied artificial neural networks to echocardiography to segment images into either blood or tissue regions.13 The study trained the ML algorithm using 279 images and compared the algorithm to manual segmentation by two independent investigators and found good agreement (R = 0.93).13 More recently, the development of big-data analytical techniques has further opened the field of cardiology. Big-data analytical techniques have focused on integrating and synthesizing various multimodality imaging data points, including various omics data, imaging, ECG and unstructured free text.4
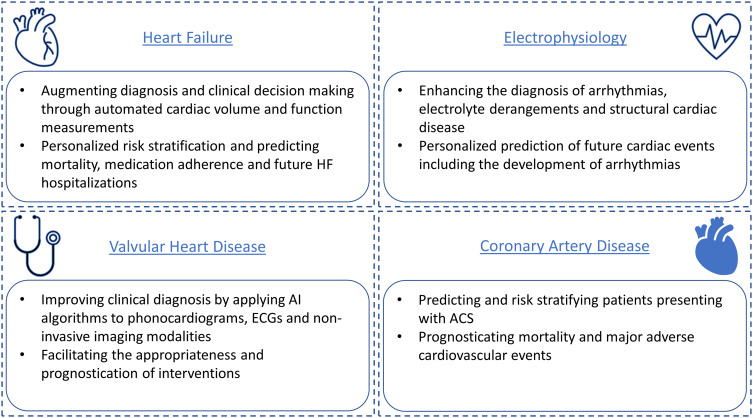
Over the intervening years, AI advancements in cardiology have led to wide-ranging applications, which encompass precise disease stratification, integration of multi-modality imaging, continuous remote monitoring and diagnostics, therapy selection and AI-aided diagnosis (Figure 4). In this review, we distill the broad applications of AI in cardiology into four clinical areas relevant for the practicing cardiologist: heart failure, electrophysiology, valvular heart disease and coronary artery disease (Figure 5).
Figure 4.
The historical development of artificial intelligence (AI) in cardiology.
Abbreviations: ECG, electrocardiogram; RCT, randomized controlled trial.
Figure 5.
Selected applications of artificial intelligence (AI) in cardiovascular medicine.
Abbreviation: ACS, acute coronary syndrome.
Contemporary Applications of AI in Cardiology
Heart Failure
Heart failure (HF) is a complex and progressive clinical syndrome characterized by the heart’s inability to pump sufficient blood to meet the body’s demand.14 Heart failure can be categorized into three subgroups based on left ventricular ejection fraction (LVEF): HF with reduced EF (HFrEF; EF < 40%), HF with mid-range EF (HFmrEF; EF 40–49%), and preserved EF (EF ≥ 50%).14 The etiologies are often multifactorial but lead to a combination of elevated intracardiac pressure and impaired cardiac output.14 There is a growing appreciation for the role of AI in helping guide clinicians manage patients with HF. A broad range of AI applications revolve around augmenting diagnostic capabilities and clinical decision-making through automated cardiac function measurements and AI-based predictive modeling.
Heart failure is a clinical diagnosis, but echocardiography plays a pivotal role in evaluating underlying cardiac structure and function.14 However, echocardiography is highly dependent on the experience of the sonographer for image acquisition, and precise interpretation by an expert reader. As a result, ML-based platforms are being developed, tested and validated, aiming to augment echocardiography.15 One recent example is the “HeartModel A.I.” which uses AI segmentation algorithms and 3D echocardiography to automatically measure cardiac volumes and function.16 Medvedofsky et al performed a multicenter study validating this technology and including 180 patients from six different sites.16 The algorithm correlated strongly with manual tracings with R-values of 0.97 when measuring left ventricular (LV) end-diastolic volume, 0.97 when measuring LV end-systolic volume, 0.88 when measuring LV ejection fraction (EF) and 0.96 when measuring left atrial volume.16 Another similar example is a CNN-based algorithmic tool developed by Ouyang et al using 10,030 echocardiograms from a single center.17 The testing cohort included 2895 echocardiograms and the study found the algorithm accurately segmented the LV with a Dice similarity coefficient of 0.92, predicted LV ejection fraction (EF) with a mean absolute error of 4.1%, and was able to classify patients with HF with reduced EF with an area under the curve (AUC) of 0.97.17 Identifying LV dysfunction using echocardiography has also been augmented by incorporating 12-lead electrocardiograms (ECGs). Sun et al developed a CNN to identify LV dysfunction and used 12,732 ECG-TTE pairs for training, 2530 pairs for validation and 2530 pairs for testing.18 The authors found the CNN-based model detected LV dysfunction with an accuracy of 73.9%, sensitivity of 69.2%, specificity of 70.5% and AUC of 0.713.18 The positive and negative predictive values of the model were 70.1% and 69.9%, respectively.18
AI algorithms have also been developed and validated for risk stratification and predicting mortality, medication adherence and recurrent hospitalizations in patients with HF. An ANN studied by Ortiz et al in 95 patients found the AI tool was able to predict 1-year all-cause mortality in patients with HFrEF with an accuracy of 90%, specificity of 93% and a sensitivity of 71.4%.19 Son et al used an SVM-based model to predict medication adherence.20 Electronic health record data was collected from 76 patients with HFrEF, and an SVM model was developed to identify variables which best predicted medication adherence.20 The variables included in the model were gender, daily frequency of medications, New York Heart Association (NYHA) class, medication knowledge, and presence of a spouse; the highest detection accuracy for the model was 77.6%.20 Another example is Kang et al who used a combination of decision trees and metaheuristic approaches from 552 patients to predict HF hospital admission.21, 382 patients were included in the training dataset and 184 patients in the validation dataset.21 The study found that the algorithm was able to predict HF admission with a C-statistic of 0.59, a true-positive rate of 0.65 and a false-positive rate of 0.49 with a final accuracy of 77%.21 Further studies have also applied ML algorithms to telemetry data from non-invasive wearable technology to predict HF re-hospitalization. The LINK-HF study used a disposable multisensor patch placed on the chest of 100 study subjects over 3 months.22 The study found that multivariate physiological data points could accurately predict HF hospitalizations with a sensitivity of 76% to 88% and a specificity of 85%.22 AI-based algorithms have also been used to predict the future development of HF. Akbilgic et al developed a CNN-based ECG-AI model using 14,618 patients from a nationwide perspective study to predict the likelihood of HF development within 10 years using standard 12-lead ECG.23 With an AUC of 0.818, the model output, when combined with clinical variables including age, gender, race, BMI, smoking status, coronary heart disease, diabetes mellitus, systolic blood pressure, and heart rate, outperformed traditional HF risk calculators such as ARIC with an AUC of 0.802 and the Framingham Heart Study with an AUC of 0.780.23 Another example is Choi et al who developed a recurrent neural network using 3884 patients with HF and 18,903 patients without HFrEF to predict a future diagnosis of HFrEF using traditional electronic health record system data points.24 The study used a 12-month observation window and found that the algorithm had an AUC of 0.747 for predicting HFrEF.24
Cardiomyopathy
Hypertrophic cardiomyopathy (HCM) is a genetic disorder that occurs when the walls of the heart muscle thicken, resulting in the heart’s reduced ability to pump out and take in blood.25 Though the condition is rare, affecting around 1 in 200 to 500 people, it is a leading cause of death among younger individuals and is associated with serious morbidity in all age groups.26 Given that more than 90% of patients with HCM have ECG abnormalities, ECGs can provide excellent screening for this condition, and AI can be used to detect HCM in imaging.27
Recent studies have shown the efficacy of AI in detecting HCM in both adults and children. Ko et al trained a CNN model on ECGs from 46,901 adult patients, including a majority of controls and a minority of confirmed HCM cases.26 When tested on 12-lead ECGs from 13,400 patients, again with a majority of controls, the model was able to discriminate HCM cases from non-HCM control cases with an AUC of 0.96.26 Siontis et al also used a CNN trained on ECGs from 300 pediatric patients with HCM and 18,439 non-HCM pediatric controls. The model detected HCM from 12-lead ECGs with an AUC of 0.98 and a corresponding sensitivity and specificity of 92% and 95%, respectively.27
Electrophysiology
Cardiac arrhythmias refer to abnormal atrial or ventricular rhythms and are estimated to affect 1.5–5% of the general population, with atrial fibrillation (AF) being the most prevalent arrhythmia in an ageing population.28 ECGs remain a key diagnostic tool for the timely diagnosis and management of arrhythmias.28 AI is increasingly being applied to the ECGs to garner new insights with respect to diagnosis, risk stratification and prediction.
Several recent studies have trained neural networks on single, 3 and 12-lead ECGs to accurately differentiate and diagnose arrhythmias. Hannun et al used 92,000 single-lead ECG tracings from 53,877 patients wearing adhesive patches to train a deep neural network (DNN) to classify an ECG into 12 rhythm classes.7 The algorithm was validated against a group of board-certified cardiologists and found to have an AUC of 0.837.7 Similarly, Raghunath et al trained a DNN to predict 1-year all-cause mortality from an ECG.29 The study trained the algorithm using 1,169,662 resting ECGs from 253,397 patients and tested the model on a sample of 168,914 patients.29 The algorithm was found to have an AUC of 0.88 when predicting all-cause mortality at 1-year from a single ECG.29
Electrolyte derangements can alter cardiac membranes and lead to arrhythmias. These derangements are often manifested with subtle ECG changes. Recent studies have developed and validated algorithms to help diagnose electrolyte derangements from the ECG. A recent study trained a DNN using 1,576,581 ECGs from 449, 380 patients to detect serum potassium levels of 5.5 mEq/l or less.30 The algorithm was validated using 61,965 patents with stage 3 or greater chronic kidney disease (CKD) and was found to have an AUC of 0.853 to 0.883.30 Another study trained a separate algorithm using 83,449 ECGs from 48,356 patients to diagnose various electrolyte derangements.31 The study was comprised of internal and external validation groups with the AUCs for predicting hyperkalemia, hypokalemia, hypernatremia, hyponatremia, hypercalcemia, and hypocalcemia being 0.873, 0.857, 0.839, 0.856, 0.831, and 0.813, respectively.31
ML algorithms have also been applied to the ECG to predict the future onset of arrhythmias. Attia et al developed a CNN-based algorithm using 649,931 ECG traces from 180,922 patients to predict the future development of AF.32 The study found a single AI-enabled ECG was able to identify AF with an AUC of 0.87, sensitivity of 79.0%, specificity of 79.5%, and an overall accuracy of 79.4%.32 The hypothesis proposed for this AI-powered insight is that cardiac substrate modulations such as atrial dilation often precede the onset of AF. Hence, these substrate changes result in subtle ECG changes, which can be detected by the DL algorithms to predict the future onset of AF.32 The notion of AI-ECG being able to detect occult and manifest structural changes has been studied in patients with LV dysfunction, hypertrophy, amyloid heart disease and peripartum cardiomyopathy.33–36 For example, Grogan et al trained a neural network using 12-lead ECG data from 2541 patients to predict the presence of cardiac amyloidosis, and found the CNN had an AUC of 0.91 (CI, 0.90 to 0.93), with a positive predictive value of 0.86.34
Valvular Heart Disease
Valvular heart disease (VHD) encompasses many important cardiovascular conditions related to the heart’s valvular apparatus, and accounts for 10–20% of all cardiac procedures in the US.37 AI supported algorithms have been developed and validated to enhance the diagnosis, prognostication and treatment of patients with valvular heart disease.
VHD gives rise to characteristic murmurs, and recent studies have applied AI algorithms to phonocardiograms obtained from digital stethoscopes to augment clinical decision-making. A recent study developed an SVM classifier using 49 patients with VHD, and 325 healthy controls to help classify abnormal heart sounds.38 The algorithm was proven to successfully classify aortic regurgitation, mitral regurgitation and pulmonary stenosis with an accuracy of 98.8%, 98.4% and 98.7%, respectively.38 Another example is a study which used an automated batch processing protocol to classify heart sounds.39 The authors trained the algorithm using 3180 heart sounds from 603 outpatient visits, and found the algorithm had a sensitivity, specificity and accuracy of 93% (95% confidence intervals (CI): 90–95%), 81% (CI: 75–85%) and 88% (CI: 85–91%), respectively.39
AI algorithms are capable of identifying subtle changes and patterns in pixel density and have increasingly been applied to echocardiography. A recent study developed and tested an ML algorithm in 52 patients with aortic stenosis (AS) undergoing transcatheter aortic valve replacement (TAVR), which aimed to measure the aortic annulus dimensions.40 When compared with measurements obtained from cardiac computed tomography (CT), the study found that there was less than 10% variability in the ML derived measurements.40 Another study compared an ML-based algorithm with manual tracings for measuring the mitral annulus area.41 The study found that the ML algorithm had a good correlation with manual measurements, but the time for analysis was significantly shorter (260 ± 65 vs 381 ± 68 seconds, P < 0.001).41 Measuring valvular dimensions has extended beyond echocardiography, and recent studies have shown its applicability to cardiac CT. A recent study compared a semi-automated ML algorithm with manual measurements for measuring the aortic annulus in 74 patients. The study found that the ML algorithm was highly correlated with manual annulus measurements in both systole (r = 0.94) and diastole (r = 0.93).42
AI-based algorithms have also been developed to help guide clinicians on the prognosis of patients with VHD who undergo intervention. A recent study used a combination of artificial neural network, logistic regression, and random forest machine learning algorithms to predict in-hospital mortality after TAVR using patient data from 10,883 patients from 2012 to 2015.43 All models showed a good AUC for predicting in-hospital mortality (AUC = 0.92 (95% CI: 0.89–0.95); 0.85 (95% CI: 0.82–0.88); 0.90 (95% CI: 0.88–0.92) and 0.90 (95% CI: 0.87–0.93)), respectively.43
Coronary Artery Disease
Coronary artery disease (CAD) is the most common type of heart disease and was the cause of death in 360,900 people in 2019.44 ML algorithms play a far-reaching role in the risk stratification, diagnosis, prognostication and management of patients with CAD.
Recent research has focused on applying AI algorithms to patients in the emergency department (ED) presenting with acute coronary syndrome (ACS). A recent study developed and validated a CNN algorithm to predict which patients presenting to the ED with ACS would require invasive angiography.45 The study retrospectively evaluated 2204 patients and developed a CNN using 40 variables including the patient’s ECG, cardiac biomarkers and clinical history.45 The ML network was found to have a sensitivity of 88.1% (95% CI: 84.8% to 91.4%) and a specificity of 86.2% (95% CI: 84.6% to 87.7%) in predicting which patients presenting to the ED with chest pain had cardiac ischemia.45 Another study included 269 patients presenting with chest pain to predict which patients were likely to have a non-ST elevation myocardial infarct (NSTEMI) using a CNN derived from 42 demographic and clinical features.46 The sensitivity, specificity, positive predictive value and negative predictive value of the CNN model to predict which patients had a NSTEMI were 90.91%, 93.33%, 76.92%, and 97.67%, respectively.46
AI algorithms have also been studied to predict short- and long-term mortality in patients with ACS. A recent study developed an SVM to predict in-hospital mortality in patients admitted with ACS.47 A total of 4871 patients were included in the analysis and 668 in-hospital deaths occurred; the receiver operating characteristic (ROC) scores for discrimination of the SVM ranged from 0.71 to 0.99.47 In a larger, registry-based, study of 9066 consecutive ACS patients, models were developed to predict 6-month mortality using two ML methods: logistic regression and extreme gradient boosting.48 The XBboost and logistic regression had better performance compared to the GRACE score: AUCs of 0.890 (95% CI: 0.864–0.916) and 0.867 (95% CI: 0.837–0.897), respectively, versus 0.822 (95% CI: 0.785–0.859).48
AI-based algorithms are also being applied to noninvasive cardiac imaging to help risk stratify patients. A recent study developed a random forest ML model to automatically quantify coronary artery calcification (CAC) from non-contrast cardiac CT scans and tested the algorithm on 530 images.49 Compared to manual measurements, the ML algorithm demonstrated a high level of correlation with a correlation coefficient of 0.95. When stratified by coronary anatomy, the correlation coefficients were 0.98, 0.69 and 0.95, for the left anterior descending artery (LAD), left circumflex artery (LCx) and right coronary artery (RCA), respectively.49 Importantly, the algorithm also shortened processing times when compared with manual interpretation (45 vs 128 seconds).49 Another example is a similar study by Van Velzen et al which employed two consecutive CNNs to quantify CAC from 7240 CT scans with the algorithm having a correlation coefficient of 0.9 when compared to manual scoring.50
Studies have also applied similar ML-based algorithms to quantify epicardial adipose tissue. A recent multicenter study trained a CNN-based ML algorithm on 850 CT scans to quantify epicardial adipose tissue, and tested the algorithm on 141 CT scans.51 Compared to three expert radiologists, the ML algorithm was found to be quicker (1.57 seconds ± 0.49 seconds vs 15 mins for manual expert review) and showed a strong correlation with expert opinion (r = 0.974; P < 0.001).51 AI algorithms have also been developed to assess plaque morphology to identify high-risk features. In a recent study, a CNN was compared with an expert reader in detecting luminal area, stenosis and contrast density in 716 diseased coronary segments in 156 patients. The study found an excellent correlation for all values (R = 0.98, 0.96, 0.97, respectively).52
Recent advances in AI have also enabled algorithms to be developed to predict obstructive CAD. In a recent study, a boosted ensemble ML algorithm (LogitBoost) was used to analyze myocardial perfusion single-photon emission CT (SPECT) images of 713 consecutive cases with suspected CAD and 468 with low likelihood of CAD (<5%).53 The diagnostic accuracy of the model for prediction of obstructive CAD ≥70% was compared to standard prone/supine quantification and to visual analysis by two experienced readers utilizing all imaging, quantitative, and clinical data.53 The diagnostic accuracy of ML (87.3% ± 2.1%) was similar to Expert 1 (86.0% ± 2.1%), but superior to combined supine/prone total perfusion defect (82.8% ± 2.2%) and Expert 2 (82.1% ± 2.2%) (P < 0.01).53
There is a growing appreciation of AI algorithms for prognosticating outcomes for patients with ACS. In a study of 2619 patients who underwent exercise or a pharmacological stress test with SPECT myocardial perfusion imaging, 239 patients developed major adverse cardiovascular events (MACE) during a mean follow-up of 3.2 years.54 The study found that the ML-based algorithm had a higher predictive accuracy compared to conventional physician-diagnosis (AUC 0.81 vs 0.62, P < 0.01).54 Another study trained an ML algorithm using 25 clinical variables and 44 cardiac CT angiography parameters on 10,030 patients to predict 5-year all-cause mortality.55 The study found that the ML score (AUC: 0.79) outperformed the Framingham risk score (AUC: 0.61; P < 0.001).55
Future Prospects
Cardiac disease remains an important cause of global morbidity and mortality. Timely and effective diagnosis, management, and follow-up are essential in improving patient outcomes and ensuring effective use of health resources. Fortunately, the data-driven nature of managing many complex cardiac diseases, and the abundant use of cardiac imaging techniques including ECG, echocardiography, CT and nuclear imaging make cardiovascular medicine a prime field for AI. As with all interventions, clinical trials are the cornerstones for demonstrating clinical utility. As such, the bulk of studies thus far have been developed in silico, using single-center homogenous study groups. There will invariably be a shift from initial validation studies to external validation using multicenter clinical trials. The first AI-ECG prospective clinical trial was recently published to study the real-world application of AI-ECG algorithms.56 The study randomized 120 primary care teams to a control arm, which utilized an AI ECG algorithm to predict LV dysfunction in a control arm without access to the AI algorithm, and found that the study arm had increased diagnostic accuracy.56 Apart from AI applications in ECG, published literature in the cardiology is largely restricted to current research context, rather than routine clinical use. Increased funding for AI research studies coinciding with a rapid growth in clinical databases, medical smartphone applications and wearable devices provides a perfect environment for widening the study of AI platforms in cardiology.
Challenges
Despite the promise of AI in cardiology, there are a number of inherent challenges to this technology which need to be addressed before AI models become integrated into clinical care pathways. AI algorithms require large volumes of accurately labelled training data sets to extract the most value and overcome the variety, complexity and potential noise involved in applying algorithms to external patient cohorts.6 Such datasets are hard to curate and data-sharing agreements often restrict multicenter collaborations. Additionally, storing and sharing sensitive datasets needed for multi-center trials is challenging with data privacy, compliance and potential data breaches representing real risks.4 Naturally, current frameworks and guidelines are limited due to the novelty of this technology, and updated guidelines are needed to enable larger multicenter studies and collaborations. The lack of standardization and heterogeneity of AI techniques employed in studies raises the additional challenge of systematically comparing and critiquing AI research studies.
The aim of ML algorithms is to optimize their generalizability such that the model defines the underlying relationship but not to the extent of introducing inadvertent bias from poorly curated data sets.3 Hence, a variance-bias trade-off exists where variance refers to how well the model performs with new, subtly different data, and bias refers to how well the model fits to the training data.3 Overfitting refers to a model that has a high bias and a low variance. In other words, a model is overfitted when it struggles to adapt to new, subtly different data. This represents a significant challenge for AI research in cardiology, as the majority of existing studies have only been trained and tested in research settings. The introduction of biases in curating data sets for AI is another challenge. Specifically, DNNs are vulnerable to adversarial attacks, in which algorithms are presented with inputs that looks “normal” to humans but cause misclassification by models due to minute differences both during and after training.57 Fortunately, researchers have developed a regularization method that reduces the impact of adversarial noise upon the output of a DNN model.57 Beyond such solutions, regular feedback from clinicians and potential operators is essential in the development of a balanced and robust model, which is not just applicable to a selected subgroup of potential patients. However, machine learning algorithms are designed and modified by human experts whose proficiency may significantly differ among centers, affecting the robustness of the model. At some level, all AI algorithmic applications in cardiology are susceptible to the “black-box” phenomenon. This refers to the notion that despite the input and output variables being known, knowledge about how the AI/ML algorithm works is often obscure to the clinician.32 Therefore, there is a clear tradeoff between a model’s accuracy and its interpretation.58 While a model may be very accurate, its operations may be difficult to interpret by a clinician.58 On the other hand, a model that has easily interpretable features within its algorithm may be less accurate, fostering a debate about AI/ML algorithm adoption in clinical practice.58 Finally, current DNNs are not robust to out-of-distribution inputs, which are samples that are not similar to those in the training set. Since AI models are limited in what they can evaluate, they may not replace human experts in the near future.
Conclusions
This review highlights the key advances in AI algorithms within cardiovascular medicine with respect to heart failure, electrophysiology, valvular heart disease and coronary artery disease. The growth in the number, quality and funding of clinical studies means AI algorithms will increasingly become more complex, powerful and able to draw on the rapidly growing pool of clinical data derived from clinical reports, mobile monitors and clinical diagnostic data. Although stringent efforts must be taken to ensure biases are minimized in these platforms through the use of multicenter, heterogeneous patient populations, the wider adoption of AI technology in cardiovascular medicine seems eventually inevitable in the digital world of 21st century healthcare.
Funding Statement
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors have no conflicts of interest to declare. The work described in this case report has not been published previously, is not under consideration for publication elsewhere, and has been approved by all authors responsible for the work carried out.
References
- 1.Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mela A, Rdzanek E, Poniatowski LA, et al. Economic costs of cardiovascular diseases in Poland Estimates for 2015–2017 Years. Front Pharmacol. 2020;11:1231. doi: 10.3389/fphar.2020.01231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haq IU, Haq I, Xu B. Artificial intelligence in personalized cardiovascular medicine and cardiovascular imaging. Cardiovasc Diagn Ther. 2021;11:911–923. doi: 10.21037/cdt.2020.03.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu B, Kocyigit D, Grimm R, Griffin BP, Cheng F. Applications of artificial intelligence in multimodality cardiovascular imaging: a state-of-the-art review. Prog Cardiovasc Dis. 2020;63:367–376. doi: 10.1016/j.pcad.2020.03.003 [DOI] [PubMed] [Google Scholar]
- 5.Nabi W, Bansal A, Xu B. Applications of artificial intelligence and machine learning approaches in echocardiography. Echocardiography. 2021;38:982–992. doi: 10.1111/echo.15048 [DOI] [PubMed] [Google Scholar]
- 6.Johnson KW, Torres Soto J, Glicksberg BS, et al. Artificial intelligence in cardiology. J Am Coll Cardiol. 2018;71:2668–2679. doi: 10.1016/j.jacc.2018.03.521 [DOI] [PubMed] [Google Scholar]
- 7.Hannun AY, Rajpurkar P, Haghpanahi M, et al. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat Med. 2019;25:65–69. doi: 10.1038/s41591-018-0268-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nichols JA, Herbert Chan HW, Baker MAB. Machine learning: applications of artificial intelligence to imaging and diagnosis. Biophys Rev. 2019;11:111–118. doi: 10.1007/s12551-018-0449-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez-Martinez S, Duchateau N, Erdei T, et al. Machine learning analysis of left ventricular function to characterize heart failure with preserved ejection fraction. Circ Cardiovasc Imaging. 2018;11:e007138. doi: 10.1161/CIRCIMAGING.117.007138 [DOI] [PubMed] [Google Scholar]
- 10.Kaul V, Enslin S, Gross SA. History of artificial intelligence in medicine. Gastrointest Endosc. 2020;92:807–812. doi: 10.1016/j.gie.2020.06.040 [DOI] [PubMed] [Google Scholar]
- 11.Dassen WR, Mulleneers R, Smeets J, et al. Self-learning neural networks in electrocardiography. J Electrocardiol. 1990;23:200–202. doi: 10.1016/0022-0736(90)90102-8 [DOI] [PubMed] [Google Scholar]
- 12.Dassen WR, Mulleneers RG, Den Dulk K, et al. An artificial neural network to localize atrioventricular accessory pathways in patients suffering from the Wolff-Parkinson-White syndrome. Pacing Clin Electrophysiol. 1990;13:1792–1796. doi: 10.1111/j.1540-8159.1990.tb06892.x [DOI] [PubMed] [Google Scholar]
- 13.Binder T, Sussner M, Moertl D, et al. Artificial neural networks and spatial temporal contour linking for automated endocardial contour detection on echocardiograms: a novel approach to determine left ventricular contractile function. Ultrasound Med Biol. 1999;25:1069–1076. doi: 10.1016/S0301-5629(99)00059-9 [DOI] [PubMed] [Google Scholar]
- 14.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 15.Romiti S, Vinciguerra M, Saade W, Anso Cortajarena I, Greco E. Artificial Intelligence (AI) and cardiovascular diseases: an unexpected alliance. Cardiol Res Pract. 2020;2020:4972346. doi: 10.1155/2020/4972346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medvedofsky D, Mor-Avi V, Amzulescu M, et al. Three-dimensional echocardiographic quantification of the left-heart chambers using an automated adaptive analytics algorithm: multicentre validation study. Eur Heart J Cardiovasc Imaging. 2018;19:47–58. doi: 10.1093/ehjci/jew328 [DOI] [PubMed] [Google Scholar]
- 17.Ouyang D, He B, Ghorbani A, et al. Video-based AI for beat-to-beat assessment of cardiac function. Nature. 2020;580:252–256. doi: 10.1038/s41586-020-2145-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun JY, Qiu Y, Guo HC, et al. A method to screen left ventricular dysfunction through ECG based on convolutional neural network. J Cardiovasc Electrophysiol. 2021;32:1095–1102. doi: 10.1111/jce.14936 [DOI] [PubMed] [Google Scholar]
- 19.Ortiz J, Ghefter CG, Silva CE, Sabbatini RM. One-year mortality prognosis in heart failure: a neural network approach based on echocardiographic data. J Am Coll Cardiol. 1995;26:1586–1593. doi: 10.1016/0735-1097(95)00385-1 [DOI] [PubMed] [Google Scholar]
- 20.Son YJ, Kim HG, Kim EH, Choi S, Lee SK. Application of support vector machine for prediction of medication adherence in heart failure patients. Healthc Inform Res. 2010;16:253–259. doi: 10.4258/hir.2010.16.4.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang Y, McHugh MD, Chittams J, Bowles KH. Utilizing home healthcare electronic health records for telehomecare patients with heart failure: a decision tree approach to detect associations with Rehospitalizations. Comput Inform Nurs. 2016;34:175–182. doi: 10.1097/CIN.0000000000000223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stehlik J, Schmalfuss C, Bozkurt B, et al. Continuous wearable monitoring analytics predict heart failure hospitalization: the LINK-HF multicenter study. Circ Heart Fail. 2020;13:e006513. doi: 10.1161/CIRCHEARTFAILURE.119.006513 [DOI] [PubMed] [Google Scholar]
- 23.Akbilgic O, Butler L, Karabayir I, et al. ECG-AI: electrocardiographic artificial intelligence model for prediction of heart failure. Eur Heart J Digit Health. 2021;2:626–634. doi: 10.1093/ehjdh/ztab080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi E, Schuetz A, Stewart WF, Sun J. Using recurrent neural network models for early detection of heart failure onset. J Am Med Inform Assoc. 2017;24:361–370. doi: 10.1093/jamia/ocw112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marian AJ, Braunwald E. Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res. 2017;121:749–770. doi: 10.1161/CIRCRESAHA.117.311059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ko WY, Siontis KC, Attia ZI, et al. Detection of hypertrophic cardiomyopathy using a convolutional neural network-enabled electrocardiogram. J Am Coll Cardiol. 2020;75:722–733. doi: 10.1016/j.jacc.2019.12.030 [DOI] [PubMed] [Google Scholar]
- 27.Siontis KC, Liu K, Bos JM, et al. Detection of hypertrophic cardiomyopathy by an artificial intelligence electrocardiogram in children and adolescents. Int J Cardiol. 2021;340:42–47. doi: 10.1016/j.ijcard.2021.08.026 [DOI] [PubMed] [Google Scholar]
- 28.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the heart rhythm society in collaboration with the society of thoracic surgeons. Circulation. 2019;140:e125–e151. doi: 10.1161/CIR.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 29.Raghunath S, Ulloa Cerna AE, Jing L, et al. Prediction of mortality from 12-lead electrocardiogram voltage data using a deep neural network. Nat Med. 2020;26:886–891. doi: 10.1038/s41591-020-0870-z [DOI] [PubMed] [Google Scholar]
- 30.Galloway CD, Valys AV, Shreibati JB, et al. Development and validation of a deep-learning model to screen for hyperkalemia from the electrocardiogram. JAMA Cardiol. 2019;4:428–436. doi: 10.1001/jamacardio.2019.0640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwon JM, Jung MS, Kim KH, et al. Artificial intelligence for detecting electrolyte imbalance using electrocardiography. Ann Noninvasive Electrocardiol. 2021;26:e12839. doi: 10.1111/anec.12839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Attia ZI, Noseworthy PA, Lopez-Jimenez F, et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet. 2019;394:861–867. doi: 10.1016/S0140-6736(19)31721-0 [DOI] [PubMed] [Google Scholar]
- 33.Adedinsewo D, Carter RE, Attia Z, et al. Artificial intelligence-enabled ECG algorithm to identify patients with left ventricular systolic dysfunction presenting to the emergency department with dyspnea. Circ Arrhythm Electrophysiol. 2020;13:e008437. doi: 10.1161/CIRCEP.120.008437 [DOI] [PubMed] [Google Scholar]
- 34.Grogan M, Lopez-Jimenez F, Cohen-Shelly M, et al. Artificial intelligence-enhanced electrocardiogram for the early detection of cardiac amyloidosis. Mayo Clin Proc. 2021;96:2768–2778. doi: 10.1016/j.mayocp.2021.04.023 [DOI] [PubMed] [Google Scholar]
- 35.Adedinsewo DA, Johnson PW, Douglass EJ, et al. Detecting cardiomyopathies in pregnancy and the postpartum period with an electrocardiogram-based deep learning model. European Heart Journal - Digital Health. 2021;2(4):586–596. doi: 10.1093/ehjdh/ztab078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hopkins CB, Suleman J, Cook C. An artificial neural network for the electrocardiographic diagnosis of left ventricular hypertrophy. Crit Rev Biomed Eng. 2000;28:435–438. doi: 10.1615/CritRevBiomedEng.v28.i34.140 [DOI] [PubMed] [Google Scholar]
- 37.Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2021;143:e72–e227. doi: 10.1161/CIR.0000000000000923 [DOI] [PubMed] [Google Scholar]
- 38.Sun S, Huang T, Zhang B, et al. A novel intelligent system based on adjustable classifier models for diagnosing heart sounds. Sci Rep. 2022;12:1283. doi: 10.1038/s41598-021-04136-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson WR, Reinisch AJ, Unterberger MJ, Schriefl AJ. Artificial intelligence-assisted auscultation of heart murmurs: validation by virtual clinical trial. Pediatr Cardiol. 2019;40:623–629. doi: 10.1007/s00246-018-2036-z [DOI] [PubMed] [Google Scholar]
- 40.Mediratta A, Addetia K, Medvedofsky D, et al. 3D echocardiographic analysis of aortic annulus for transcatheter aortic valve replacement using novel aortic valve quantification software: comparison with computed tomography. Echocardiography. 2017;34:690–699. doi: 10.1111/echo.13483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kagiyama N, Toki M, Hara M, et al. Efficacy and accuracy of novel automated mitral valve quantification: three-dimensional transesophageal echocardiographic study. Echocardiography. 2016;33:756–763. doi: 10.1111/echo.13135 [DOI] [PubMed] [Google Scholar]
- 42.Guez D, Boroumand G, Ruggiero NJ, Mehrotra P, Halpern EJ. Automated and manual measurements of the aortic annulus with ECG-gated cardiac CT angiography prior to transcatheter aortic valve replacement: comparison with 3D-transesophageal echocardiography. Acad Radiol. 2017;24:587–593. doi: 10.1016/j.acra.2016.12.008 [DOI] [PubMed] [Google Scholar]
- 43.Hernandez-Suarez DF, Kim Y, Villablanca P, et al. Machine learning prediction models for in-hospital mortality after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2019;12:1328–1338. doi: 10.1016/j.jcin.2019.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 45.Baxt WG, Shofer FS, Sites FD, Hollander JE. A neural network aid for the early diagnosis of cardiac ischemia in patients presenting to the emergency department with chest pain. Ann Emerg Med. 2002;40:575–583. doi: 10.1067/mem.2002.129171 [DOI] [PubMed] [Google Scholar]
- 46.Wu CC, Hsu WD, Islam MM, et al. An artificial intelligence approach to early predict non-ST-elevation myocardial infarction patients with chest pain. Comput Methods Programs Biomed. 2019;173:109–117. doi: 10.1016/j.cmpb.2019.01.013 [DOI] [PubMed] [Google Scholar]
- 47.Pinaire J, Chabert E, Aze J, Bringay S, Landais P. Sequential pattern mining to predict medical in-hospital mortality from administrative data: application to acute coronary syndrome. J Healthc Eng. 2021;2021:5531807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hernesniemi JA, Mahdiani S, Tynkkynen JA, et al. Extensive phenotype data and machine learning in prediction of mortality in acute coronary syndrome - the MADDEC study. Ann Med. 2019;51:156–163. doi: 10.1080/07853890.2019.1596302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolterink JM, Leiner T, Takx RA, Viergever MA, Isgum I. Automatic coronary calcium scoring in non-contrast-enhanced ECG-Triggered Cardiac CT with ambiguity detection. IEEE Trans Med Imaging. 2015;34:1867–1878. doi: 10.1109/TMI.2015.2412651 [DOI] [PubMed] [Google Scholar]
- 50.van Velzen SGM, Lessmann N, Velthuis BK, et al. Deep learning for automatic calcium scoring in CT: validation using multiple cardiac CT and Chest CT Protocols. Radiology. 2020;295:66–79. doi: 10.1148/radiol.2020191621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Commandeur F, Goeller M, Razipour A, et al. Fully Automated CT quantification of epicardial adipose tissue by deep learning: a multicenter study. Radiol Artif Intell. 2019;1:e190045. doi: 10.1148/ryai.2019190045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hong Y, Commandeur F, Cadet S, et al. Deep learning-based stenosis quantification from coronary CT Angiography. Proc SPIE Int Soc Opt Eng. 2019;10949:643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arsanjani R, Dey D, Khachatryan T, et al. Prediction of revascularization after myocardial perfusion SPECT by machine learning in a large population. J Nucl Cardiol. 2015;22:877–884. doi: 10.1007/s12350-014-0027-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Betancur J, Otaki Y, Motwani M, et al. Prognostic value of combined clinical and myocardial perfusion imaging data using machine learning. JACC Cardiovasc Imaging. 2018;11:1000–1009. doi: 10.1016/j.jcmg.2017.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Motwani M, Dey D, Berman DS, et al. Machine learning for prediction of all-cause mortality in patients with suspected coronary artery disease: a 5-year multicentre prospective registry analysis. Eur Heart J. 2017;38:500–507. doi: 10.1093/eurheartj/ehw188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yao X, Rushlow DR, Inselman JW, et al. Artificial intelligence-enabled electrocardiograms for identification of patients with low ejection fraction: a pragmatic, randomized clinical trial. Nat Med. 2021;27:815–819. doi: 10.1038/s41591-021-01335-4 [DOI] [PubMed] [Google Scholar]
- 57.Ma L, Liang L. A regularization method to improve adversarial robustness of neural networks for ECG signal classification. Comput Biol Med. 2022;144:105345. doi: 10.1016/j.compbiomed.2022.105345 [DOI] [PubMed] [Google Scholar]
- 58.Quer G, Arnaout R, Henne M, Arnaout R. Machine learning and the future of cardiovascular care: JACC State-of-the-art review. J Am Coll Cardiol. 2021;77:300–313. doi: 10.1016/j.jacc.2020.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]