Abstract
Nitrogen-fixing bacteria isolated from banana (Musa spp.) and pineapple (Ananas comosus (L.) Merril) were characterized by amplified 16S ribosomal DNA restriction analysis and 16S rRNA sequence analysis. Herbaspirillum seropedicae, Herbaspirillum rubrisubalbicans, Burkholderia brasilensis, and Burkholderia tropicalis were identified. Eight other types were placed in close proximity to these genera and other alpha and beta Proteobacteria.
Associative nitrogen-fixing bacteria such as Azospirillum brasilense, Herbaspirillum seropedicae, and Acetobacter diazotrophicus may benefit their host plants as N biofertilizers and plant growth promoters. The latter two organisms were the first nitrogen-fixing bacteria suggested to be endophytes (1, 4). Several new classified and as-yet-unclassified diazotrophic bacteria have been isolated from economically important mono- and dicotyledonous plants (3, 5), including banana and pineapple (17).
Thirty-eight nitrogen-fixing bacteria isolated from stems, leaves, roots, and fruits of pineapple and banana cultivars from Bahia (BA) and Rio de Janeiro (RJ) States, Brazil, including 14 isolates previously described (17), were analyzed following DNA sequencing and PCR-restriction fragment length polymorphism analysis of the 16S rRNA gene (amplified 16S ribosomal DNA restriction analysis [ARDRA]) to define their phylogenetic positions. Reference strains Z67, Z78, and M2 for H. seropedicae, M4 for Herbaspirillum rubrisubalbicans, M130 for Burkholderia brasilensis, and Ppe8 for Burkholderia tropicalis (1) were from our collection (Table 1). All strains were grown overnight in NFbHPN (8) medium at 30°C at 120 rpm, diluted (1:10), boiled for 5 min, and cooled on ice, and the DNA was amplified (7) in an OmniGene thermocycler from Hybaid Ltd., Teddington, United Kingdom. The primers used were Y1 (5′-TGGCTCAGAACGAACGCTGGCGGC-3′) (19) (positions 20 to 43 of the Escherichia coli 16S rRNA gene) and Y3 (5′-TACCTTGTTACGACTTCACCCCAGTC-3′) (J. P. W. Young, personal communication) (positions 1482 to 1507 of the E. coli 16S rRNA gene) (2), complementary to the ends of the 16S rDNA. The DNA templates extracted from all of the strains produced a single band of approximately 1,500 bp.
TABLE 1.
Characterization of nitrogen-fixing bacteria isolated from Musa spp. and Ananas comosus (L) Merril cultivated in Brazil, as revealed by ARDRA of Y1-Y3-amplified fragments and Y1-Y2 sequence of the 16S rDNA
| Species and straina | Host | Cultivar | Tissue | Geographic origin | Restriction patternb | Type
|
|
|---|---|---|---|---|---|---|---|
| ARDRAc | Y1-Y2 sequenced | ||||||
| H. seropedicae | |||||||
| Z67 | aaaa | 1 | I | ||||
| Z78 | aaaa | 1 | NEf | ||||
| M2 | aaaa | 1 | NE | ||||
| BA153 | Banana | Marmelo | Fruit | Itaguaí, RJ | aaaa | 1 | 1 |
| X8 | NDe | ND | ND | ND | aaaa | 1 | II |
| H. rubrisubalbicans | |||||||
| M4 | baaa | 2 | III | ||||
| AB7 | Pineapple | Alenquer | Leaf | Cruz das Almas, BA | baaa | 2 | I |
| BA10 | Banana | Butuhan | Stem | Cruz das Almas, BA | baaa | 2 | I |
| BA11 | Banana | Butuhan | Leaf | Cruz das Almas, BA | baaa | 2 | I |
| BA12 | Banana | Yangambi | Root | Cruz das Almas, BA | baaa | 2 | I |
| BA14 | Banana | Yangambi | Stem | Cruz das Almas, BA | baaa | 2 | I |
| BA15 | Banana | Prata Anã | Root | Cruz das Almas, BA | baaa | 2 | I |
| BA16 | Banana | Prata Anã | Stem | Cruz das Almas, BA | baaa | 2 | I |
| BA17 | Banana | Butuhan | Stem | Cruz das Almas, BA | baaa | 2 | NE |
| BA134 | Banana | Maçã | Stem | Itaguaí, RJ | baaa | 2 | I |
| BA149 | Banana | Maçã | Leaf | Itaguaí, RJ | baaa | 2 | NE |
| BA161 | Banana | Maçã | Root | Itaguaí, RJ | baaa | 2 | I |
| B. brasilensis | |||||||
| M130 | cbbb | 3 | IV | ||||
| BA124 | Banana | Prata Manteiga | Stem | Macaé, RJ | cbbb | 3 | NE |
| B. tropicalis | |||||||
| Ppe8 | dccb | 4 | V | ||||
| AB98 | Pineapple | Pérola | Fruit | Macaé, RJ | dccb | 4 | V |
| AB147 | Pineapple | Smooth cayenne | Stem | Quissamã, RJ | dccb | 4 | V |
| Unknown | |||||||
| O1 | Banana | Yangambi | ND | Cruz das Almas, BA | geed | 5 | VI |
| BA22 | Banana | Prata Anã | Leaf | Cruz das Almas, BA | geed | 5 | VI |
| BA23 | Banana | Yangambi | Stem | Cruz das Almas, BA | geed | 5 | VI |
| BA25 | Banana | Prata Anã | Stem | Cruz das Almas, BA | geed | 5 | NE |
| BA27 | Banana | Yangambi | Leaf | Cruz das Almas, BA | geed | 5 | VI |
| BA88 | Banana | Maçã | Leaf | Itaguaí, RJ | geed | 5 | NE |
| BA104 | Banana | Prata Anã | Stem | Itaguaí, RJ | geed | 5 | NE |
| BA106 | Banana | Maçã | Leaf | Itaguaí, RJ | geed | 5 | NE |
| BA128 | Banana | Prata | Fruit | Itaguaí, RJ | geed | 5 | NE |
| BA136 | Banana | Prata | Leaf | Itaguaí, RJ | geed | 5 | VI |
| AB117 | Pineapple | Smooth Cayenne | Root | Quissamã, RJ | gfcc | 6 | VII |
| AB120 | Pineapple | Pérola | Stem | Macaé, RJ | gfcc | 6 | VII |
| BA123 | Banana | Prata Manteiga | Root | Macaé, RJ | dgce | 7 | VIII |
| BA126 | Banana | D'água | Root | Itaguaí, RJ | dgce | 7 | VIII |
| AB48 | Pineapple | Perolera | Root | Cruz das Almas, BA | ghcc | 8 | IX |
| AB71 | Pineapple | Perolera | Stem | Cruz das Almas, BA | ghcc | 8 | IX |
| AB119 | Pineapple | Pérola | Leaf | Macaé, RJ | dibb | 9 | NE |
| Ala | ND | ND | ND | ND | fddc | 10 | X |
| BA131 | Banana | D'água | Leaf | Itaguaí, RJ | bjf– | 11 | XI |
| A2a | ND | ND | ND | ND | eddc | 12 | NE |
| A3b | ND | ND | ND | ND | eddc | 12 | NE |
| A8b | ND | ND | ND | ND | eddc | 12 | NE |
The reference strains analyzed were Z67 and M2 from H. seropedicae, M4 from H. rubrisubalbicans, Ppe8 from B. tropicalis, and M130 from B. brasilensis.
Restriction pattern obtained for the endonuclease AluI, HaeIII, HinfI, and RsaI. The four-letter code refers to the restriction patterns produced by the four restriction endonucleases. Each letter defines a common pattern for a given endonuclease.
The ARDRA genotype represents the combination of the four endonucleases.
The Y1-Y2 sequenced region covers approximately 300 bp in the 5′ end of the 16S rDNA molecule. Numbers represent genotypes with 100% identical sequences.
ND, no data.
NE, not examined.
Y1-Y3 PCR products (10 μl) were digested with AluI, HaeIII, HinfI, or RsaI (5 U) as specified by Life Technologies, and the fragments were separated on a 2.5% agarose gel and stained with ethidium bromide (0.5 μg/ml). Three to seven fragments and 5 to 10 unique restriction patterns were produced by each endonuclease.
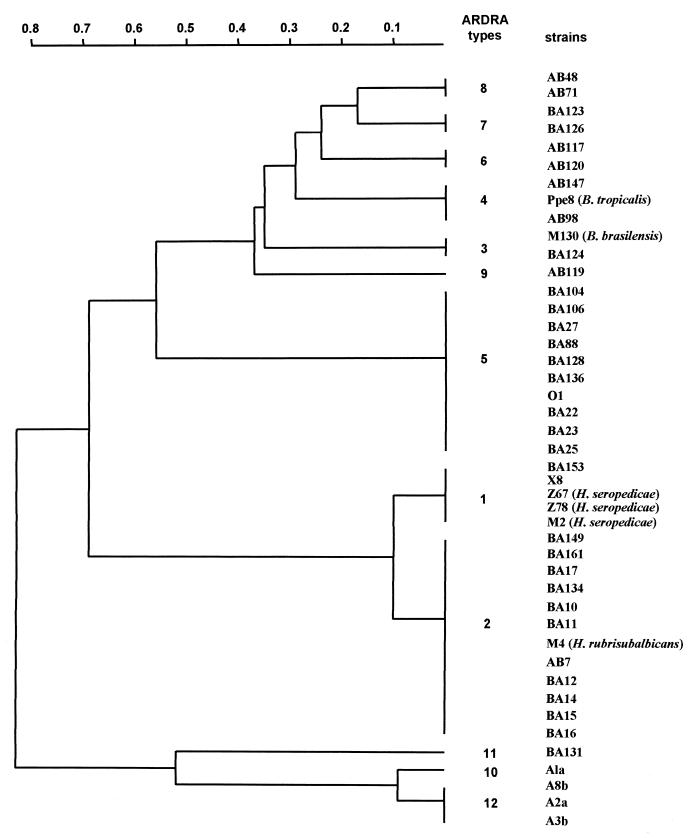
A combination of the restriction digests produced 12 unique banding patterns or ARDRA types (Table 1). Isolates BA153 and X8 shared the same pattern as H. seropedicae type strains Z67, Z78, and M2. Isolates AB7, BA10, BA11, BA12, BA14, BA15, BA16, BA17, BA134, BA149, and BA161 had the same pattern as H. rubrisubalbicans strain M4. Isolate BA124 showed the same pattern as B. brasilensis strain M130. Finally, AB98 and AB147 had the same pattern as B. tropicalis strain Ppe8. The remaining 22 isolates produced eight new ARDRA types, types 5 to 12.
Restriction analysis with endonucleases AluI and HaeIII was sufficient to allocate the strains into the 12 types. Moreover, HaeIII alone was capable of resolving the most types (10 types), followed by AluI (7 types), and HinfI and RsaI (5 types) (Table 1).
ARDRA types 1 (H. seropedicae) and 2 (H. rubrisubalbicans) shared all but one DNA fragment in the AluI restriction pattern. Types 10 and 12 were differentiated by only two AluI restriction fragments, and types 6 and 8 were differentiated only by the HaeIII restriction pattern. A dendrogram, constructed from restriction patterns by using the TreeCon program (15), illustrated these tight relationships and showed three major clusters (Fig. 1). The first was formed by types 1 and 2 and included H. seropedicae and the H. rubrisubalbicans reference strains, the second was formed by types 3 to 9 and included Burkholderia reference strains M130 (type 3) and Ppe8 (type 4), and the third was formed by types 10 to 12 and was distant from the other two. Type 5 was separated from the other types in the cluster formed by types 3 to 9 and separated the Burkholderia and Herbaspirillum clusters (Fig. 1).
FIG. 1.
Dendrogram inferred from AluI, HaeIII, HinfI, and RsaI restriction pattern data from Y1-Y3 16S rDNA PCR-amplified fragments obtained for the types shown in Table 1. Distances were calculated for all pairwise patterns with Nei-Li coefficient (9), and the dendrogram was established by the unweighted pair-group method with arithmetic average (12).
The Y1-Y3 PCR products were purified using Nucleon QC (Amersham Pharmacia Biotech) and sequenced using dye terminator chemistry and an ABI PRISM 310 sequencer (Applied Biosystems). Primers Y1 and Y2 (5′-CCCACTGCTGCCTCCCGTAGGAGT-3′) (19) were used to sequence both strands of the variable region (approximately 300 bp) located at the 5′ end of the 16S rRNA gene. The length of the Y1-Y2 region varied from 286 to 290 bp for types I to IX (see below), as reported for beta Proteobacteria (11), and was 260 and 259 bp for types X and XI, respectively, as reported for alpha Proteobacteria (16, 19).
The 30 bacterial isolates examined were allocated into 11 different groups (types I to XI [Table 1]), with each group consisting of isolates with the identical sequence. These sequences defined types which agreed well with the ARDRA-defined types, showing no apparent polymorphism within the types. However, two disagreements were observed with Herbaspirillum types: ARDRA type 1 contained the sequence-defined types I and II, and ARDRA type 2 contained the sequence-defined types I and III. While reference strains of H. seropedicae and H. rubrisubalbicans had distinct ARDRA (types 1 and 2) and sequence (types I and III) types, 11 isolates had the same H. rubrisubalbicans ARDRA type while showing 100% sequence identity to H. seropedicae in the Y1-Y2 region (Table 1). These isolates failed to hybridize with an H. seropedicae 23S rDNA species-specific probe (17), and the present molecular data support that these may constitute a new Herbaspirillum cluster.
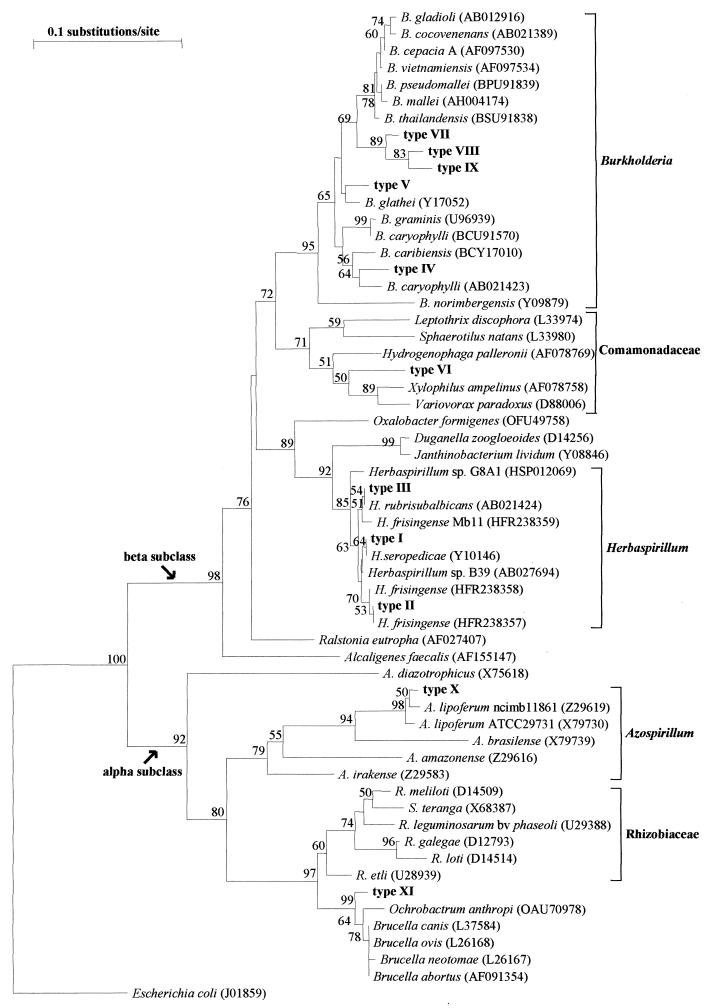
A phylogenetic tree was constructed using the type I to XI sequences plus 47 sequences of 16S rDNAs of alpha and beta Proteobacteria available in the GenBank database (Fig. 2). The sequences were aligned with the ClustalX program (13), and the phylogenetic tree was reconstructed with the TreeCon program (15). A close relationship of types I, II, and III (Table 1) to the Herbaspirillum cluster was evident (Fig. 2). High bootstrap values supported types IV, V, VII, VIII, and IX being clustered within the Burkholderia genus. Type VI clustered within the Comamonadaceae, a family that originated from the Pseudomonas rRNA group III (14), as Burkholderia originated from Pseudomonas rRNA group II (18), and which also contains phytopathogenic species. Finally, types X and XI clustered within the alpha Proteobacteria, the former close to Azospirillum lipoferum and the latter close to Ochrobactrum anthropi.
FIG. 2.
Phylogenetic tree inferred for genotype sequences I to X and representative organisms of the alpha and beta subclasses of Proteobacteria available in the GenBank database (accession numbers are in parentheses). The Y1-Y2 region was used to reconstruct the tree by the neighbor-joining method (10) from distances calculated by the method of Jukes and Cantor (6). A bootstrap analysis with 100 repetitions was performed, and only values above 50 are shown. The sequence of E. coli (a member of the gamma subclass of Proteobacteria) was used to root the tree.
The 14 isolates described by Weber et al. (17) used in this work were originally assigned to six groups related to Herbaspirillum and Burkholderia. Isolates AB48, AB98, AB119, AB120, AB147, BA123, BA124, and BA126, originally present within the same morphological and physiological group as strain M130 of B. brasilensis (17), clustered into six ARDRA groups (types 3, 4, 6, 7, 8, and 9), with only isolate BA124 being related to strain M130 (Table 1). Isolates AB98 and AB147 were similar to B. tropicalis Ppe8, while isolates AB48, AB119, AB120, BA123, and BA126 clustered within the Burkholderia genus. Isolates BA22 and BA23 failed to hybridize to oligonucleotide probes specific for Azospirillum spp., Herbaspirillum spp., Burkholderia spp., and Acetobacter diazotrophicus (17). The present results showed that these two isolates and eight new isolates, sharing the same ARDRA type, were related to Comamonadaceae.
In this paper we redefined 14 isolates described by Weber et al. (17) and the 24 new isolates into 12 genotypes. The discovery of eight new nitrogen-fixing bacterial genotypes, in addition to H. seropedicae, H. rubrisubalbicans, B. brasilensis, and B. tropicalis, in a few bacterial isolates from banana and pineapple revealed the great diversity of nitrogen-fixing bacteria associated with these fruit crops.
Nucleotide sequence accession numbers.
The sequences of the Y1-Y2 region of the 16S rDNA have been deposited in the GenBank database under accession numbers AF164042 through AF164065 and AF213248.
REFERENCES
- 1.Baldani J I, Caruso L, Baldani V L D, Goi S R, Döbereiner J. Recent advances in BNF with non-legume plants. Soil Biol Biochem. 1997;29:911–922. [Google Scholar]
- 2.Brosius J, Dull T J, Sleeter D D, Noller H F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981;148:107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- 3.Ferreira A C, Cozzolino K, Carvalho A R V, Döbereiner J. International Symposium on Sustainable Agriculture for the Tropics—the Role of Biological Nitrogen Fixation 1995. Rio de Janeirio, Brazil: Angra dos Reis; 1995. Isolation and characterization of diazotrophic bacteria in oil palm trees; p. 210. [Google Scholar]
- 4.James E K, Olivares F L. Infection and colonization of sugar cane and other graminaceous plants by endophytic diazotrophs. Crit Rev Plant Sci. 1997;17:77–119. [Google Scholar]
- 5.Jimenez-Salgado T, Fuentes-Ramirez L E, Tapia-Hernandez A, Mascarua-Esparza M A, Martinez-Romero E, Caballero-Mellado J. Coffea arabica L., a new host plant for Acetobacter diazotrophicus, and isolation of other nitrogen-fixing acetobacteria. Appl Environ Microbiol. 1997;63:3676–3683. doi: 10.1128/aem.63.9.3676-3683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jukes T H, Cantor C R. Evolution in protein molecules. In: Munro H H, editor. Mammalian protein metabolism. New York, N.Y: Academic Press; 1969. pp. 21–132. [Google Scholar]
- 7.Laguerre G, Allard M, Revoy F, Amarger N. Rapid identification of rhizobia by restriction fragment length polymorphism analysis of PC-amplified 16S rRNA genes. Appl Environ Microbiol. 1994;60:56–63. doi: 10.1128/aem.60.1.56-63.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Machado H B, Funayama S, Rigo L U, Pedrosa F O. Excretion of ammonium by Azospirillum brasilense mutants resistant to ethylenediamine. Can J Microbiol. 1991;37:549–553. [Google Scholar]
- 9.Nei M, Li W-H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA. 1979;76:5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 11.Seal S E, Jackson L A, Young J P W, Daniels M J. Differentiation of Pseudomonas solanacearum, Pseudomonas syzygii, rRNA sequencing: construction of oligonucleotide primers for sensitive detection by polymerase chain reaction. J Gen Microbiol. 1993;139:1587–1594. doi: 10.1099/00221287-139-7-1587. [DOI] [PubMed] [Google Scholar]
- 12.Sneath P H A, Sokal R R. Numerical taxonomy. W. H. San Francisco, Calif: Freeman; 1973. [Google Scholar]
- 13.Thompson J D, Gibson T J, Plewniak F, Jeanmougin F, Higgins D G. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandamme P, Pot B, Gillis M, De Vos P, Kersters K, Swings J. Polyphasic taxonomy, a consensus approach to bacterial systematics. Microbiol Rev. 1996;60:407–438. doi: 10.1128/mr.60.2.407-438.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van de Peer Y, De Wachter R. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput Appl Biosci. 1994;10:569–570. doi: 10.1093/bioinformatics/10.5.569. [DOI] [PubMed] [Google Scholar]
- 16.Vinuesa P, Rademaker J L W, De Bruijn F J, Werner D. Genotypic characterization of Bradyrhizobium strains nodulating endemic woody legumes of the Canary Islands by PCR-restriction fragment length polymorphism analysis of genes encoding 16S rRNA (16S rDNA) and 16S–23S rDNA intergenic spacers, repetitive extragenic palindromic PCR genomic fingerprinting, and partial 16S rDNA sequencing. Appl Environ Microbiol. 1998;64:2096–2104. doi: 10.1128/aem.64.6.2096-2104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber O B, Baldani V L D, Teixeira K R S, Kirchhof G, Baldani J I, Döbereiner J. Isolation and characterization of diazotrophic bacteria in banana and pineapple plants. Plant Soil. 1999;210:103–113. [Google Scholar]
- 18.Yabuuchi E, Kosako Y, Oyaizu H, Yano I, Hotta H, Hashimoto Y, Ezaki T, Arakawa M. Proposal of Burkholderia gen. nov. and transfer of seven species of the genus Pseudomonas homology group II to the new genus, with the type species Burkholderia cepacia (Palleroni and Holmes 1981) comb. nov. Microbiol Immunol. 1992;36:1251–1275. doi: 10.1111/j.1348-0421.1992.tb02129.x. [DOI] [PubMed] [Google Scholar]
- 19.Young J P W, Downer H L, Eardly B D. Phylogeny of the phototrophic Rhizobium strain BTAi1 by polymerase chain reaction-based sequencing of a 16S rRNA gene segment. J Bacteriol. 1991;173:2271–2277. doi: 10.1128/jb.173.7.2271-2277.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]