Abstract
Objectives:
Increasing use of transoral robotic surgery (TORS) is likely to impact outcomes for HPV+ oropharyngeal squamous cell carcinomas (OPSCCs). We aimed to describe oncologic outcomes for a large HPV+ OPSCC cohort after TORS and develop a risk prediction model for recurrence under this treatment paradigm.
Materials and Methods:
634 HPV+ OPSCC patients receiving TORS-based therapy at a single institution were reviewed retrospectively to describe survival across the entire cohort and for patients suffering recurrence. Risks for distant metastatic recurrence (DMR) and locoregional recurrence (LRR) were modeled using multivariate logistic regression analyses of case-control sub-cohorts.
Results:
5-year overall and recurrence-free survival were 91.2% and 86.1%, respectively. 5-year overall survival was 52.5% following DMR and 83.3% after isolated LRR (P=.01). In case-control analyses, positive surgical margins were associated with DMR (adjusted OR 5.8, CI 2.1–16.0, P=.001), but not isolated LRR, and increased DMR risk 4.2 fold in patients with early clinical stage disease. By contrast, LRR was associated with not receiving recommended adjuvant therapy (OR 13.4, CI 6.3–28.5, P<.001).
Conclusions:
This study sets a benchmark for oncologic outcomes from HPV+ OPSCC after TORS-based therapy. Under this treatment paradigm, margins are relevant for assessing lethal recurrence risk during clinical trial design and post-treatment surveillance.
Keywords: Head and Neck cancer, HPV, TORS, oropharyngeal cancer, distant metastasis, head and neck squamous cell carcinoma
Graphical Abstract
Introduction
HPV+ OPSCC continues to rise in incidence after its recognition over a decade ago as a distinct subtype of head and neck squamous cell carcinoma[1]. As a result, the oropharynx recently surpassed the cervix as the leading anatomic site for HPV-related cancer in the USA[2]. By 2030, HPV+ OPSCC incidence in the United States is projected to surpass that of all HPV-negative head and neck squamous cell carcinomas[3]. Compared to HPV-negative cases, HPV+ OPSCCs arise in patients with limited or no smoking history and demonstrate more favorable outcomes[1].
High cure rates for HPV+ OPSCCs have inspired efforts to reduce the intensity of treatment, which can leave lifelong treatment-related disabilities in survivors[4]. Multiple phase II studies have shown favorable outcomes after limiting radiation dose and/or cisplatin use during nonsurgical therapy[5–7] or after primary surgery[8–10]. However, de-escalation in clinical practice has been challenging, with some attempts negatively impacting survival. For instance, two phase III trials substituting cetuximab for cisplatin during primary chemoradiation resulted in decreased survival without reducing treatment morbidity[11, 12]. Accurately identifying cases at lowest risk of lethal recurrence would facilitate safe therapy de-escalation for patients who may be over-treated by current standards of care. In addition, prospective identification of recurrence-prone patients would aid in evaluation of novel therapies for high-risk cases and guide post-treatment surveillance.
The trend away from surgery for oropharyngeal cancer in the 1990’s was reversed by advances in minimally invasive tools that can reduce operative morbidity. FDA approval of the Intuitive DaVinci robot for transoral removal of oropharyngeal tumors in 2009 drove widespread adoption of this modality[13]. Recent analysis of the National Cancer Database showed transoral robotic surgery (TORS) to be associated with better survival for oropharyngeal cancer relative to other surgical methods[14]. Availability of TORS for HPV+ OPSCCs has facilitated trials evaluating reduced postoperative radiation and/or elimination of cytotoxic chemotherapy for cases thought to have low recurrence risk[8–10]. However, such trials continue to risk-stratify using 8th edition AJCC pathologic staging, which was derived from older surgical cohorts where non-TORS procedures predominated[15] and may not reflect the populations presently undergoing TORS. To date, improving postoperative risk stratification to guide adjuvant therapy under the TORS treatment paradigm has been impaired by lack of adequate case numbers and followup to capture enough recurrent cases for multivariate analyses.
This study analyzes a large cohort of HPV+ OPSCCs from the first center to advance TORS into routine clinical practice, thus offering uniquely long-term follow-up of patients who received relatively homogeneous therapy. Using this resource, we aimed to describe oncologic outcomes after TORS for the entire cohort and compare survival among patients with distinct subtypes of recurrence. Sufficient recurrence events allowed use of a case-control approach to create a novel risk prediction model for distant metastatic recurrence, which led to most of the poor outcomes in the overall cohort.
Materials and Methods
Patient cohorts
This retrospective study includes 634 treatment-naïve HPV+ OPSCC cases consecutively managed during 2007–2017 with primary TORS plus neck dissection at the University of Pennsylvania. These patients were designated as the TORS Cohort. Within the TORS cohort, two case-control sub-cohorts were created by identifying patients with distant metastatic recurrence (DMR) at any point in their postoperative course (Cohort-1) or with isolated locoregional recurrence (LRR), meaning local and/or regional nodal recurrence in absence of DMR (Cohort-2). We then matched the cases from these two cohorts with recurrence-free patients who had follow-up up-to or beyond the latest recorded recurrence for each cohort. The protocol was approved by the University of Pennsylvania IRB. HPV status was confirmed by p16 immunohistochemistry using College of American Pathology criteria[16].
Preoperative evaluation
All patients underwent endoscopy under anesthesia and head and neck imaging by CT and/or MRI in order to assess TORS candidacy using defined criteria[17]. For locally advanced tumors where a negative margin reesecetion was deemed to feasible, a TORS assisted approach was offered utilizing free tissue transfer to a select number of patients. Patients with primary tumors not identified during preoperative evaluation (18.9%) underwent diagnostic TORS removal of the ipsilateral palatine and lingual tonsil and subsequent pathology-guided re-resections using a described protocol[18]. 98.7% of patients underwent preoperative imaging for distant metastasis by PET/CT (66.1%), chest x-ray (37.6%), or chest CT (4.6%).
Operative management
TORS tumor resections were performed as previously described[19, 20]. Cases not deemed resectable by TORS alone (10.3%) underwent TORS plus resection of the inferior margin through a lateral pharyngotomy and free tissue reconstruction[21]. All surgical margins were evaluated from the main resection specimen. Re-resections were performed as deemed necessary intraoperatively based on gross exam of the initial specimen and were incorporated into the main specimen prior to pathologic processing. A positive margin was defined as tumor present at the final inked margin. Of note, 3.6% of patients underwent an additional operative procedure to clear a positive or <2mm margin when the margin of concern was exclusively mucosal or after a palatine or lingual tonsillectomy identified an occult primary and created a concerning margin in the process. Neck dissection was performed simultaneously in 19.2% of cases, including the 10.3% undergoing free tissue reconstruction, and as a staged procedure in the remainder.
Adjuvant therapy
For 85.7% of the TORS Cohort, pathology-guided adjuvant therapy conformed with NCCN guidelines[22], with 40.9% of patients receiving radiation and 32.4% receiving radiation plus chemotherapy (80.1% cisplatin, 14.0% cetuximab, 5.9% other systemic agent).12.8% of patients did not receive physician-recommended radiation, and 2.9% of patients did not receive recommended chemotherapy.
Outcomes classification
Recurrences at the primary site and/or cervical lymph nodes were classified as LRR. All other recurrences were classified as DMR. Overall survival (OS) was defined as time from surgery to death or to last known contact prior to death if the date was not precisely documented. Recurrence-free survival (RFS) was defined as time from surgery to first sign of recurrence based on clinical exam and/or imaging. Biopsy confirmation was obtained for 85% of DMRs and 89% of LRRs. Progression-free survival (PFS) was evaluated from the time of recurrence using RECIST 1.1[23].
Statistical Methods
Kaplan-Meier survival curves were compared using the log-rank test. Cumulative incidence curves were estimated for DMR and LRR in the TORS Cohort to account for competing risks and differences between curves assessed using Gray’s test. Characteristics of recurrent cases were compared to those of controls using Pearson’s chi-squared or Fisher’s Exact tests. Complete case analysis was used. Multivariate logistic regression analyses used a backward elimination procedure starting with all variables (age, sex, race, smoking history, charlson comorbidity, overall clinical stage, overall pathologic stage, lymphovascular invasion, perineural invasion, pathologic IV/V nodes, positive surgical margin, and extranodal extension) and thresholds for removal and inclusion in the model of P ≥ .1 and P ≤ .05, respectively. Categorical variables were regrouped to eliminate collinearity or when clinically appropriate (e.g., grouping clinical stage I/II as “early stage” in multivariate analysis). Goodness-of-fit for the model was evaluated using the Hosmer-Lemeshow test. Analyses were performed using STATA/IC v15.0. The bootstrap method was used to assess the variable selection performance of stepwise regression modeling in Cohort-1 (n=302). Using sampling with replacement, 20,000 bootstrap samples of size 302 were selected, and logistic regression using backward elimination was fit to each sample using SAS/STAT v9.4. The number of times each variable appeared in the 20,000 final models was tabulated.
Results
Characteristics of TORS Cohort
Characteristics of the TORS Cohort are in Table 1. Median age was 60 (IQR 53–65), and 86.1% were male. Active or prior smokers with a >10 pack-year history comprised 33.1%, whereas 46.2% were never smokers. Most cases (81.9%) had a Charlson Comorbidity Index (CCI) of 0. 7.6% of the cohort had locally advanced (clinical T3/T4) disease at presentation. Tumors arose from the tonsil (50%), tongue base (39%), or boundary between those sites (8.5%). The primary tumor was never identified for 2.2%. By 8th edition AJCC staging, 89.8% of cases had clinical stage I disease at presentation, whereas clinical stages II and III comprised 5.0% and 5.2%, respectively. Surgery led to pathologic staging of 77.9% as stage I, with pathologic stages II and III accounting for 21.0% and 1.1%, respectively.
Table 1.
Features of the complete TORS Cohort (n=634)
| Variable | Categories | |
|---|---|---|
| Median months follow-up (range) | (continuous) | 51.6 (0.2–155.8) |
| Median age (range) | (continuous) | 60 (32–89) |
| Sex, N (%) | Male | 546 (86.1) |
| Female | 88 (13.9) | |
| Race | White | 586 (92.4) |
| Non-white | 48 (7.6) | |
| Charlson comorbidity | 0 | 519 (81.9) |
| ≥1 | 115 (18.1) | |
| Smoking history | Never | 288 (46.2) |
| ≤10 Pack-years | 129 (20.7) | |
| >10 Pack-years | 206 (33.1) | |
| Overall clinical stage | Stage I | 569 (89.7) |
| Stage II | 32 (5.0) | |
| Stage III | 33 (5.2) | |
| Clinical T-stage | cTx | 120 (18.9) |
| cT1 | 166 (26.2) | |
| cT2 | 300 (47.3) | |
| cT3 | 17 (2.7) | |
| cT4 | 31 (4.9) | |
| Clinical N-stage | cN0 | 93 (14.7) |
| cN1 | 520 (82.0) | |
| cN2 | 19 (3.0) | |
| cN3 | 2 (0.3) | |
| Overall pathologic stage | Stage I | 494 (77.9) |
| Stage II | 133 (21.0) | |
| Stage III | 7 (1.1) | |
| Pathologic T-stage | pT0 | 14 (2.2) |
| pT1 | 265 (41.9) | |
| pT2 | 303 (47.9) | |
| pT3 | 41 (6.5) | |
| pT4 | 9 (1.4) | |
| Pathologic N-stage | pN0 | 74 (11.7) |
| pN1 | 464 (73.3) | |
| pN2 | 95 (15.0) | |
| Primary tumor site | Tonsil | 318 (50.2) |
| Tongue base | 245 (38.6) | |
| Overlap | 54 (8.5) | |
| Unknown primary | 14 (2.2) | |
| Two synchronous sites | 3 (0.5) | |
| Pathologic level IV and/or V nodes | No | 570 (90.8) |
| Yes | 58 (9.2) | |
| Margin status | Negative | 584 (94.5) |
| Positive | 34 (5.5) | |
| Lymphovascular invasion | No | 411 (68.4) |
| Yes | 190 (31.6) | |
| Perineural invasion | No | 503 (83.7) |
| Yes | 98 (16.3) | |
| Pathologic Extranodal extension | No | 453 (71.6) |
| Yes | 180 (28.4) |
Survival outcomes and cumulative incidence of relapse in the TORS Cohort
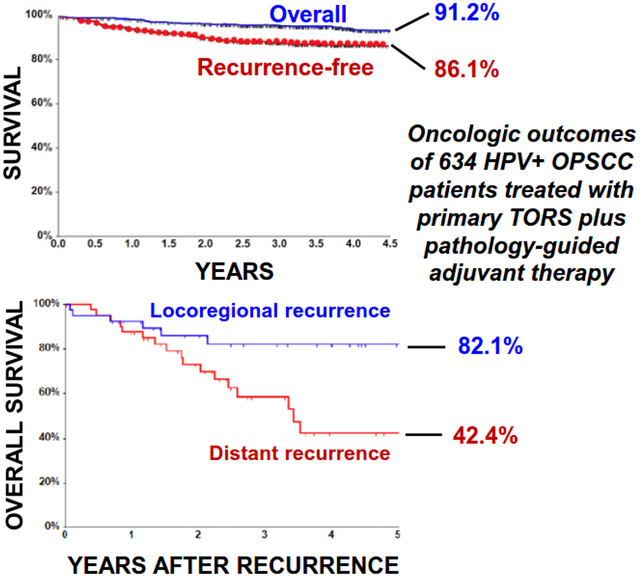
Median follow-up for the TORS cohort was 4.3 years (range 0.02–13, IQR 2.9–5.7). Five-year OS and RFS were 91.2% (95% CI, 88.1%−93.5%) and 86.1% (95% CI, 82.9%−88.7%), respectively (Figure 1A). The 80 patients developing tumor recurrence at any site comprised 12.6% of the TORS cohort, with the latest documented recurrence occurring at 4.5 years. Using competing risk analysis, the 4.5-year cumulative incidence (CI) was estimated to be 7.5% for the LRR events and 6.4% for the DMR events (Figure 1B). Because a segment of the TORS cohort received only a CXR (37.6%) or no imaging (1.3%) for distant staging before surgery, DMR rates may have been inflated by distant metastases that potentially were detectable preoperatively. To address this concern, the DMR CI curves for patients with preoperative CXR or unknown distant staging were compared to those for patients staged by chest CT or PET/CT (Supplementary Figure 1). Lack of significant difference between the CI curves (Gray’s Test p-value 0.123) supported retention of cases lacking preoperative chest CT or PET/CT in further analyses. Taken together, these findings confirm highly favorable locoregional and distant recurrence profiles for HPV+ OPSCCs after TORS in a large cohort with extended follow-up.
Figure 1. Categories of recurrence in the TORS Cohort and comparison of their survival outcomes.
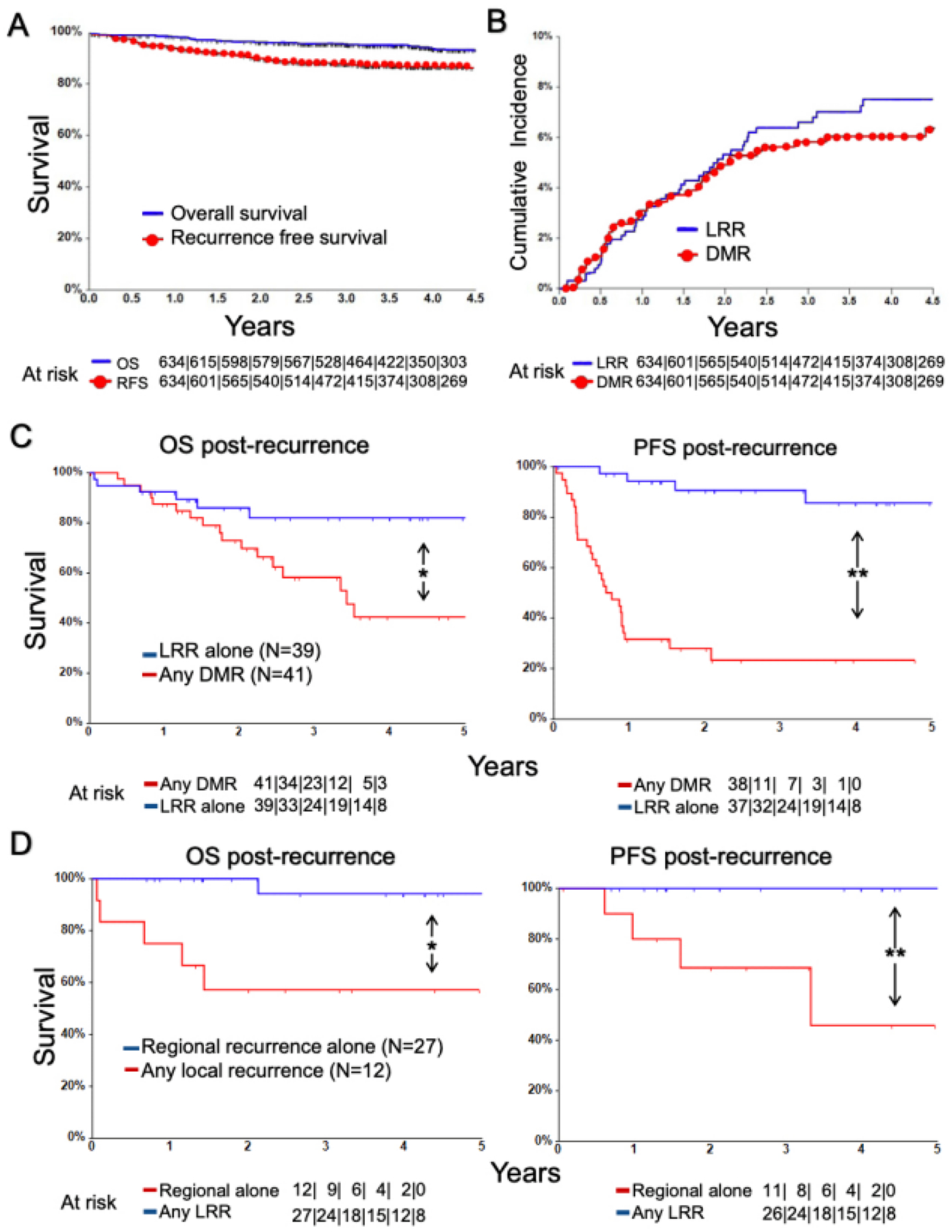
(A) Kaplan Meier curves for OS and RFS for the TORS cohort. (B) Cumulative incidence function estimates for DMR and LRR in the TORS cohort. (C) Kaplan Meier curves for post-relapse OS and PFS for cases with any DMR vs. isolated LRR *P = .01, **P < .001 by Log Rank test. (D) Kaplan Meier curves for post-relapse OS and PFS for cases any local recurrence vs. isolated regional recurrence. *P = .002, **P < .001 by Log Rank test.
Post-recurrence survival according to recurrence pattern
The 80 patients with recurrence had a post-relapse 5-year OS of 62.3% (95% CI, 48.0%−73.7%) and PFS of 54.4% (95% CI, 41.4%−65.7%). Cases with DMR (n=41) were initially managed using surgery (28.2%), surgery plus adjuvant therapy (2.6%), radiotherapy (18.0%), systemic therapy (46.2%), or supportive care alone (5.1%). Cases with isolated LRR (n=39) were managed with surgery (31.6%), surgery plus adjuvant therapy (21.1%), chemoradiation (34.2%), systemic therapy (10.5%), or supportive care alone (2.6%). The majority of patients with DMR (83%) did not have an LRR event, indicating the two recurrence patterns to be largely independent phenomena. Thus, the post-relapse OS and PFS curves for patients who had LRR alone (n=39) were compared to those for patients who had DMR in presence or absence of LRR (n=41). Patients with DMR had significantly worse post-relapse PFS and OS outcomes than patients with isolated LRRs (post-relapse 5-year OS 42.4% [95% CI, 22.7%−60.8%] vs. 82.1% [95% CI, 63.8%−91.7%], P=0.01; post-relapse 5-year PFS 24.7% [95% CI, 11.4%−40.7%] vs 85.7% [95% CI, 65.1%−94.6%], P<0.001) (Figure 1C). The 39 isolated LRRs were further analyzed as two subgroups: the isolated regional recurrences and the local recurrences with or without regional recurrence. Isolated regional recurrences (n=27) occurred in 4.3% of the TORS Cohort and were readily salvaged, with these patients achieving 5-year post-relapse PFS and OS of 100% and 94.1% (95% CI, 65.0%−99.2%), respectively. Local recurrences in presence (n=6) or absence (n=6) of regional recurrence had worse 5-year post-relapse PFS (45.7%; 95% CI, 8.2%−78.3%, P<0.001) and OS (57.1%; 95% CI, 25.4%−79.6%, P=0.002) (Figure 1D) but comprised only 1.9% of the TORS Cohort. The low local failure rate and favorable outcomes of regional recurrences after salvage therapy provide evidence that DMR is the prevailing mechanism of disease-specific mortality after TORS.
Risk prediction model for DMR
Identifying sufficient recurrences after TOR allowed for pursuit of multivariable models for postoperative recurrence risk prediction based on sub-cohorts containing patients with 41 DMR cases (Cohort-1) and 39 isolated LRR (Cohort-2). This number of recurrent cases within our cohorts allowed us to create regression models using up to four variables without overfitting. These two sub-cohorts incorporated the non-recurrent controls from the TORS Cohort that had follow-up beyond the latest recurrence event (Figure 2). Most characteristics for patients excluded from each sub-cohort were similar to those for the patients that were included (see Supplementary Table 1). Stage, margin status, level IV/V lymph node involvement, and lymphovascular invasion differed significantly between cases and controls in univariate analysis of Cohort-1, which contained 12.9% DMRs (Table 2). Multivariate analysis of Cohort-1 using backward elimination logistic regression (Table 3) revealed independent association of DMR with advanced clinical stage (adjusted OR 8.4; 95% CI, 2.4–29.3, P=.001), positive margins (adjusted OR 5.8; 95% CI, 2.1–16.0, P=.001), and pathologically positive level IV/V lymph nodes (adjusted OR 3.4; 95% CI, 1.4–8.6, P=.009). These features were thus used to create a logistic regression model (Table 4) that emphasizes risk stratification for the early clinical stage patients, who comprised 95% of the overall TORS Cohort and 96% of Cohort-1. Whereas there were no positive margins or positive level IV/V nodes among clinical advanced stage cases, risk of DMR increased in the clinical early stage group 4.2-fold from 9.6% if a positive margin was absent to 40.0% if a positive margin was present. Presence of pathologically positive level 4/5 nodes had a more modest effect, increasing DMR risk 2.8-fold from 9.7% to 27.3%. In the absence of a suitable external validation cohort, a bootstrap analysis of Cohort-1 was performed. This analysis identified margin status to be the most frequent factor selected when modeling DMR risk in Cohort-1 (see Supplementary Table 2). Together, these findings indicate positive margins to be a predictor of DMR after TORS and the variable with greatest utility for early clinical stage cases, which represent most TORS-treated patients and most HPV+ OPSCCs in general [24].
Figure 2. CONSORT diagram describing case-control cohorts.
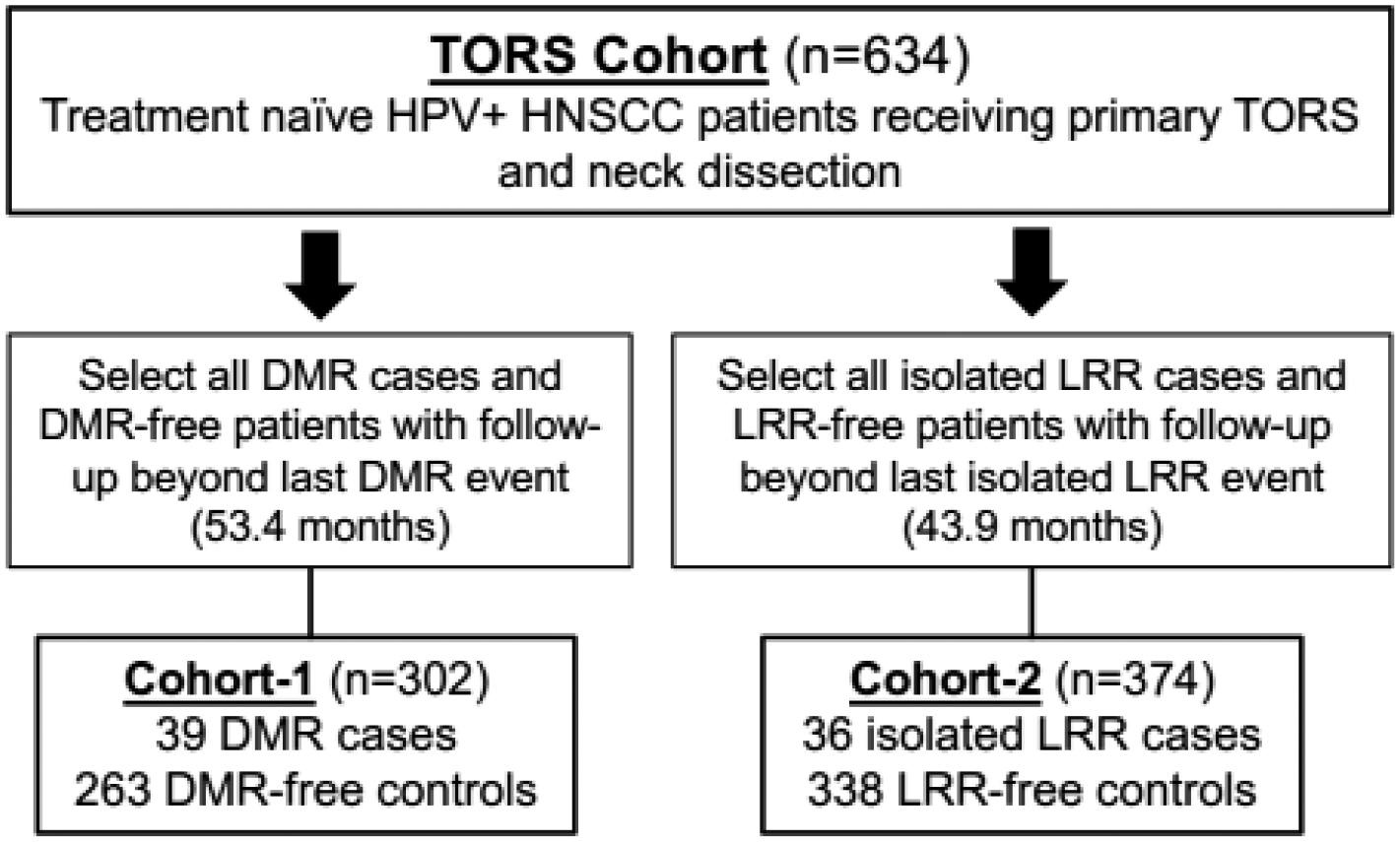
Methodology used to define Cohort-1 and Cohort-2 from the TORS Cohort
Table 2.
Features of Cohort-1 and Cohort-2 with univariate analysis
| Cohort-1 (n = 302) | Cohort-2 (n = 374) | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Category | DMR (−) (N=263) | DMR (+) (N=39) | P valuea | LRR (−) (N=338) | LRR (+) (N=36) | P valuea |
| Median age (range) | (continuous) | 58 (34–84) | 62 (32–84) | .09 | 58 (32–84) | 62 (42–78) | .016 |
| Sex, N (%) | Male | 229 (87.1) | 34 (87.2) | .99 | 298 (88.2) | 31 (86.1) | .72 |
| Female | 34 (12.9) | 5 (12.8) | 40 (11.8) | 5 (13.9) | |||
| Race | White | 241 (91.6) | 38 (97.4) | .33 | 310 (91.7) | 33 (91.7) | > .99 |
| Non-white | 22 (8.4) | 1 (2.6) | 28 (8.3) | 3 (8.3) | |||
| Charlson comorbidity | 0 | 218 (82.9) | 30 (76.9) | .36 | 282 (83.4) | 25 (69.4) | .037 |
| ≥1 | 45 (17.1) | 9 (23.1) | 56 (16.6) | 11 (30.6) | |||
| Smoking history | Never | 124 (47.1) | 19 (48.7) | .98 | 161 (47.6) | 16 (44.4) | .38 |
| ≤10 PY | 54 (20.5) | 8 (20.5) | 71 (21.0) | 5 (13.9) | |||
| >10 PY | 85 (32.3) | 12 (30.8) | 106 (31.4) | 15 (41.7) | |||
| Overall clinical stage | Stage I | 239 (90.9) | 31 (79.5) | .02 | 304 (89.9) | 32 (88.9) | .82 |
| Stage II | 17 (6.5) | 3 (7.7) | 20 (5.9) | 2 (5.6) | |||
| Stage III | 7 (2.7) | 5 (12.8) | 14 (4.1) | 2 (5.6) | |||
| Overall pathologic stage | Stage I | 204 (77.6) | 21 (53.8) | .005 | 258 (76.3) | 30 (83.3) | .62 |
| Stage II | 57 (21.7) | 17 (43.6) | 78 (23.1) | 6 (16.7) | |||
| Stage III | 2 (0.8) | 1 (2.6) | 2 (0.6) | 0 (0.0) | |||
| Pathologic level IV or V nodes | No | 239 (90.9) | 30 (76.9) | .009 | 303 (89.6) | 35 (97.2) | .23 |
| Yes | 24 (9.1) | 9 (23.1) | 35 (10.4) | 1 (2.8) | |||
| Margin status | Negative | 251 (95.4) | 31 (79.5) | < .001 | 316 (93.5) | 34 (94.4) | > .99 |
| Positive | 12 (4.6) | 8 (20.5) | 22 (6.5) | 2 (5.6) | |||
| Lymphovascular invasion | No | 192 (73.0) | 20 (51.3) | .006 | 240 (71.0) | 27 (75.0) | .61 |
| Yes | 71 (27.0) | 19 (48.7) | 98 (29.0) | 9 (25.0) | |||
| Perineural invasion | No | 219 (83.3) | 32 (82.1) | .85 | 286 (84.6) | 29 (80.6) | .53 |
| Yes | 44 (16.7) | 7 (17.9) | 52 (15.4) | 7 (19.4) | |||
| Extranodal extension | No | 188 (71.5) | 23 (59.0) | .11 | 241 (71.3) | 26 (72.2) | .91 |
| Yes | 75 (28.5) | 16 (41.0) | 97 (28.7) | 10 (27.8) | |||
Abbreviations: PY, Pack-years; DMR, Distant metastatic recurrence; LRR, Locoregional recurrence
Pearson’s chi-square statistic or Fisher’s exact test for categorical variables, Wilcoxon rank-sum test for continuous variables
Table 3.
Multivariable logistic regression models for DMR (Cohort-1) and isolated LRR (Cohort-2)
| Recurrence type | Significant feature | Unadjusted odds ratio | 95% CI | P valuea | Adjusted odds ratio | 95% CI | P valuea |
|---|---|---|---|---|---|---|---|
| DMRs (Cohort-1) | Positive marginb | 5.4 | 2.0–14.2 | .001 | 5.9 | 2.2–16.2 | .001 |
| Clinical advanced stagec | 5.5 | 1.6–18.4 | .006 | 8.1 | 2.4–27.9 | .001 | |
| Positive level IV/V nodesd | 3.0 | 1.3–7.0 | .012 | 3.3 | 1.3–8.1 | .011 | |
| Isolated LRRs (Cohort-2) | Age | 1.04 | 1.0–1.1 | .02 |
Wald Test
Negative margin used as reference
Clinical stage I/II used as reference
No positive level IV/V nodes used as reference
Table 4.
Risk prediction model for DMR based on Cohort 1 (n=302)
| Advanced stage | Positive margin | Positive level IV/V nodes | Total patients (N) | DMR absent (N) | DMR present (N) | Observed Proportion with DMR | Predicted Risk of DMR | Confidence Interval for Predicted Risk |
|---|---|---|---|---|---|---|---|---|
| No | No | No | 241 | 222 | 19 | 0.079 | 0.081 | 0.053 – 0.122 |
| Yes | No | No | 12 | 7 | 5 | 0.417 | 0.417 | 0.185 – 0.692 |
| No | Yes | No | 16 | 10 | 6 | 0.375 | 0.346 | 0.169 – 0.571 |
| No | No | Yes | 29 | 22 | 7 | 0.241 | 0.223 | 0.113 – 0.395 |
| No | Yes | Yes | 4 | 2 | 2 | 0.500 | 0.630 | 0.346 – 0.846 |
| Yes | No | Yes | 0 | - | - | - | - | |
| Yes | Yes | No | 0 | - | - | - | - | |
| Yes | Yes | Yes | 0 | - | - | - | - |
Abbreviations: DMRs, Distant metastatic recurrences
Coefficient in logistic regression model. The intercept is −2.5
Wald Test
Risk prediction for isolated LRR
Multivariate analysis of Cohort-2, which contained 9.6% isolated LRRs, did not identify clinically meaningful independent associations between isolated LRR and features that are known immediately after surgery (Table 3). However, 93 patients (14.7%) in the TORS Cohort subsequently failed to receive physician-recommended postoperative adjuvant therapy in the form of radiation (n=80) and/or chemotherapy (n=19) or had unknown adjuvant therapy (n=3). Suboptimal adjuvant therapy was received by the majority (56.4%) of those with isolated LRR cases but only 12.5% of DMR cases and was strongly associated in univariate analysis with LRR in Cohort-2 but not DMR in Cohort-1 (Table 5). Adding suboptimal adjuvant therapy as a feature for multivariate analysis confirmed independent association with isolated LRR (OR 13.4, CI 6.3–28.5, P<.001), where it increased risk 8.5-fold from 5.0% to 42.0% in Cohort 2 but did not alter the associations with DMR found for Cohort-1. These findings suggest that suboptimal adjuvant therapy contributes substantially to isolated LRRs but has minimal impact on DMR risk.
Table 5.
Association of suboptimal adjuvant therapy with isolated LRR but not DMR
| Cohort | Recurrence status | Standard therapy N (%) | Suboptimal therapy N (%) | Total N (%) | P valuea |
|---|---|---|---|---|---|
| Cohort-1 | No DMR | 226 (86.9) | 36 (87.8) | 262 (87.0) | .88 |
| DMR | 34 (13.1) | 5 (12.2) | 39 (13.0) | ||
| Cohort-2 | No isolated LRR | 306 (95.3) | 32 (60.4) | 338 (90.4) | < .001 |
| Isolated LRR | 15 (4.7) | 21 (39.6) | 36 (9.6) |
Abbreviations: DMR, Distant metastatic recurrence; LRR, Locoregional recurrence
Pearson’s chi-square statistic
Discussion
Primary TORS has an expanding role as an alternative to primary chemoradiation[13] for the subset of HPV+ OPSCCs that can be excised transorally without severe morbidity. Although the precise oncologic and functional dividends from TORS over chemoradiation remain unclear, the TORS paradigm can help deintensify therapy by eliminating radiation use in a minority of cases, obviating need for chemotherapy for the majority, and allowing reductions in radiation dose. Here we set a benchmark for favorable oncologic outcomes after TORS based upon the largest single-institution cohort of TORS-treated HPV+ OPSCCs reported to date. Upon characterizing recurrences in this cohort, local failures were noted to be rare, and regional failures were readily controlled. By contrast, DMR accounted for most of the disease-related mortality. For the first time, availability of a sufficient number of DMR cases allowed creation of a risk prediction model, which showed positive surgical margin to be the dominant factor in increasing DMR risk for the 95% of patients presenting with AJCC 8th edition clinical stage I/II disease. This finding identifies patients with positive margins after TORS as a subgroup that may not be appropriate for de-escalation trials and might warrant more intensive treatment and surveillance during follow-up.
The association of margin status with DMR in absence of LRR suggests a causal relationship between the tumor biology underlying DMR and difficulty clearing margins using TORS. In this context, standard adjuvant therapy that presently adds chemotherapy for positive margins appears adequate to mitigate LRR risk but not DMR risk. Although routine availability of surgical margin status makes it an appealing biomarker, sensitivity of margin analysis to practice variations among surgeons when inking and orienting specimens[25] is an important caveat. Whereas our standardized approach to specimen orientation produced a positive margin rate (5.5%) that discriminated cases with high DMR risk, orientation practices that produce substantially higher or lower rates may fail to do so. Validation of this association in an external dataset would be desirable but was hampered by unavailability of other TORS-treated HPV+ OPSCC cohorts with comparable size and follow-up. This limitation led to reliance on validation by bootstrapping analysis and prevents direct prognostic application of margin status at present.
Challenges in standardizing conditions of surgical practice that can impact margin status support the need for molecular biomarkers, which may better capture the biology of DMR. Studies have suggested that certain genetic alterations mostly found in HPV-negative HNSCCs predispose HPV+ HNSCCs to treatment failure[26]. Likewise, some multi-gene expression profiles have shown prognostic potential[27, 28]. However, relevant studies suffer from small case numbers, lack of carefully matched controls, and/or wide primary treatment variations. Thus, biomarker development may benefit from our ongoing efforts to compare the genetic and transcriptomic features of tumors in the TORS Cohort that recurred to well-matched controls from non-recurrent patients.
A need for molecular risk stratification to guide adjuvant therapy is further supported by the modest utility of conventional clinical criteria for most of the HPV+ OPSCC patients currently receiving primary TORS, including lack of independent association between 8th edition AJCC pathologic stage and recurrence. Although advanced AJCC 8th edition clinical stage markedly increased risk of DMR, this feature was present in only 5% of the TORS Cohort, which is representative of the HPV+ OPSCCs typically undergoing surgery in the modern era. Absence of association of DMR with >10 pack-year smoking history contrasts with the worse survival of smokers after primary chemoradiation that is apparent in some[29, 30] but not all[31] large historical series. The increased risk of DMR in presence of pathologic level IV/V nodes, which is not part of AJCC staging, is comparable to an association with radiographic low-lying neck disease observed in non-surgically treated cases49 and might warrant consideration for future staging updates.
In summary, this study describes oncologic outcomes from a modern surgical treatment paradigm for HPV+ HNSCC based on long-term follow-up of a large cohort. Excellent locoregional disease control achieved by TORS-based treatment contributes to lethal events being mostly confined to cases suffering DMR. Because margin status was the dominant predictor of DMR in early-stage cases, patients with positive margins may warrant exclusion from de-escalation trials. A current standard of care for positive margin cases is administration of adjuvant radiation plus cisplatin, which has been shown to decrease locoregional recurrences but not distant failures[32], thus highlighting a need for novel adjuvant approaches to address subclinical distant metastases.
Supplementary Material
High curative salvage rates for locoregional recurrences contribute to excellent long-term oncologic outcomes after TORS-based therapy for HPV+ oropharyngeal cancers
Distant recurrences cause most of the disease-specific mortality for these patients.
Positive surgical margins from TORS indicate risk of distant recurrence even if locoregional control is achieved.
Acknowledgements
Funding:
This work was supported by National Institute of Health/National Institute of Dental and Craniofacial Research [R01DE027185, 2018–2022]
Role of funding source:
DB and PG receieved salary support from National Institute of Health/National Institute of Dental and Craniofacial Research [R01DE027185, 2018–2022]
Declaration of interests
G.S. Weinstein receive royalties from Olympus Inc. through the University of Pennsylvania for the FK-WO retractor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sonawane K, Suk R, Chiao EY, Chhatwal J, Qiu P, Wilkin T, et al. Oral Human Papillomavirus Infection: Differences in Prevalence Between Sexes and Concordance With Genital Human Papillomavirus Infection, NHANES 2011 to 2014. Ann Intern Med. 2017;167:714–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Machtay M, Moughan J, Trotti A, Garden AS, Weber RS, Cooper JS, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26:3582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chen AM, Felix C, Wang PC, Hsu S, Basehart V, Garst J, et al. Reduced-dose radiotherapy for human papillomavirus-associated squamous-cell carcinoma of the oropharynx: a single-arm, phase 2 study. Lancet Oncol. 2017;18:803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Marur S, Li S, Cmelak AJ, Gillison ML, Zhao WJ, Ferris RL, et al. E1308: Phase II Trial of Induction Chemotherapy Followed by Reduced-Dose Radiation and Weekly Cetuximab in Patients With HPV-Associated Resectable Squamous Cell Carcinoma of the Oropharynx- ECOG-ACRIN Cancer Research Group. J Clin Oncol. 2017;35:490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].SS Y, JJ C, JN W, ML G, MT T, R J, et al. NRG-HN002: A Randomized Phase II Trial for Patients with p16-Positive, Non-Smoking-Associated, Locoregionally Advanced Oropharyngeal Cancer. Int J Radiat Oncol 2019. p. 684–5. [Google Scholar]
- [8].Hargreaves S, Beasley M, Hurt C, Jones TM, Evans M. Deintensification of Adjuvant Treatment After Transoral Surgery in Patients With Human Papillomavirus-Positive Oropharyngeal Cancer: The Conception of the PATHOS Study and Its Development. Front Oncol. 2019;9:936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ferris RL, Flamand Y, Holsinger FC, Weinstein GS, Quon H, Mehra R, et al. A novel surgeon credentialing and quality assurance process using transoral surgery for oropharyngeal cancer in ECOG-ACRIN Cancer Research Group Trial E3311. Oral Oncol. 2020;110:104797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ma DJ, Price KA, Moore EJ, Patel SH, Hinni ML, Garcia JJ, et al. Phase II Evaluation of Aggressive Dose De-Escalation for Adjuvant Chemoradiotherapy in Human Papillomavirus-Associated Oropharynx Squamous Cell Carcinoma. J Clin Oncol. 2019;37:1909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mehanna H, Robinson M, Hartley A, Kong A, Foran B, Fulton-Lieuw T, et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet. 2019;393:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gillison ML, Trotti AM, Harris J, Eisbruch A, Harari PM, Adelstein DJ, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. 2019;393:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cracchiolo JR, Baxi SS, Morris LG, Ganly I, Patel SG, Cohen MA, et al. Increase in primary surgical treatment of T1 and T2 oropharyngeal squamous cell carcinoma and rates of adverse pathologic features: National Cancer Data Base. Cancer. 2016;122:1523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nguyen AT, Luu M, Mallen-St Clair J, Mita AC, Scher KS, Lu DJ, et al. Comparison of Survival After Transoral Robotic Surgery vs Nonrobotic Surgery in Patients With Early-Stage Oropharyngeal Squamous Cell Carcinoma. JAMA Oncol. 2020;6:1555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Haughey BH, Sinha P, Kallogjeri D, Goldberg RL, Lewis JS, Piccirillo JF, et al. Pathology-based staging for HPV-positive squamous carcinoma of the oropharynx. Oral Oncol. 2016;62:11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lewis JS, Beadle B, Bishop JA, Chernock RD, Colasacco C, Lacchetti C, et al. Human Papillomavirus Testing in Head and Neck Carcinomas: Guideline From the College of American Pathologists. Arch Pathol Lab Med. 2018;142:559–97. [DOI] [PubMed] [Google Scholar]
- [17].Weinstein GS, O’Malley BW, Rinaldo A, Silver CE, Werner JA, Ferlito A. Understanding contraindications for transoral robotic surgery (TORS) for oropharyngeal cancer. Eur Arch Otorhinolaryngol. 2015;272:1551–2. [DOI] [PubMed] [Google Scholar]
- [18].Hatten KM, O’Malley BW, Bur AM, Patel MR, Rassekh CH, Newman JG, et al. Transoral Robotic Surgery-Assisted Endoscopy With Primary Site Detection and Treatment in Occult Mucosal Primaries. JAMA Otolaryngol Head Neck Surg. 2017;143:267–73. [DOI] [PubMed] [Google Scholar]
- [19].Weinstein GS, O’Malley BW Jr., Snyder W, Sherman E, Quon H. Transoral robotic surgery: radical tonsillectomy. Arch Otolaryngol Head Neck Surg. 2007;133:1220–6. [DOI] [PubMed] [Google Scholar]
- [20].O’Malley BW, Weinstein GS, Snyder W, Hockstein NG. Transoral robotic surgery (TORS) for base of tongue neoplasms. Laryngoscope. 2006;116:1465–72. [DOI] [PubMed] [Google Scholar]
- [21].Parhar HS, Brody RM, Shimunov D, Rajasekaran K, Rassekh CH, Basu D, et al. Retropharyngeal Internal Carotid Artery Management in TORS Using Microvascular Reconstruction. Laryngoscope. 2020. [DOI] [PubMed] [Google Scholar]
- [22].Pfister DG, Spencer S, Adelstein D, Adkins D, Anzai Y, Brizel DM, et al. Head and Neck Cancers, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2020;18:873–98. [DOI] [PubMed] [Google Scholar]
- [23].Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- [24].O’Sullivan B, Huang SH, Su J, Garden AS, Sturgis EM, Dahlstrom K, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol. 2016;17:440–51. [DOI] [PubMed] [Google Scholar]
- [25].Li MM, Puram SV, Silverman DA, Old MO, Rocco JW, Kang SY. Margin Analysis in Head and Neck Cancer: State of the Art and Future Directions. Ann Surg Oncol. 2019;26:4070–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Harbison RA, Kubik M, Konnick EQ, Zhang Q, Lee SG, Park H, et al. The mutational landscape of recurrent versus nonrecurrent human papillomavirus-related oropharyngeal cancer. JCI Insight. 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gleber-Netto FO, Rao X, Guo T, Xi Y, Gao M, Shen L, et al. Variations in HPV function are associated with survival in squamous cell carcinoma. JCI Insight. 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liu X, Liu P, Chernock RD, Kuhs KAL, Lewis JS, Luo J, et al. A prognostic gene expression signature for oropharyngeal squamous cell carcinoma. EBioMedicine. 2020;61:102805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Contrera KJ, Smile TD, Mahomva C, Wei W, Adelstein DJ, Broughman JR, et al. Locoregional and distant recurrence for HPV-associated oropharyngeal cancer using AJCC 8 staging. Oral Oncol. 2020;111:105030. [DOI] [PubMed] [Google Scholar]
- [30].Weller MA, Ward MC, Berriochoa C, Reddy CA, Trosman S, Greskovich JF, et al. Predictors of distant metastasis in human papillomavirus-associated oropharyngeal cancer. Head Neck. 2017;39:940–6. [DOI] [PubMed] [Google Scholar]
- [31].O’Sullivan B, Huang SH, Siu LL, Waldron J, Zhao H, Perez-Ordonez B, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31:543–50. [DOI] [PubMed] [Google Scholar]
- [32].Bernier J, Cooper JS, Pajak TF, van Glabbeke M, Bourhis J, Forastiere A, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck. 2005;27:843–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


