Abstract
Background
Many thrombotic complications are linked to coronavirus disease 2019 (COVID-19). Antithrombotic treatments are important for prophylaxis against these thrombotic events.
Objectives
This study was designed to compare enoxaparin and rivaroxaban as prophylactic anticoagulants in moderate cases of COVID-19 in terms of efficacy, safety, and clinical outcomes.
Methods
The study involved 124 patients with moderate COVID-19 (pneumonia without hypoxia) divided into two groups. The first group (G1) comprised 66 patients who received enoxaparin subcutaneously at a dose of 0.5 mg/kg every 12 h until discharge from the hospital. The second group (G2) comprised 58 patients who received oral rivaroxaban at a dose of 10 mg once daily until discharge from the hospital. The outcomes evaluated in this study were as follows: intermediate care unit (IMCU) duration, the number of patients transferred from the IMCU to the intensive care unit (ICU), ICU duration, the total length of hospital stay, in-hospital mortality, and thrombotic and bleeding complications.
Results
No significant differences in IMCU duration (p = 0.39), ICU duration (p = 0.96), and total length of hospital stay (p = 0.73) were observed between the two groups. The percentage of patients requiring ICU admission after hospitalization was 21.2% in G1 and 22.4% in G2 (p = 0.87). The mortality rate was 12.1% in G1 and 10.3% in G2 (p = 0.76). The proportion of patients who had thrombotic complications was 9.1% in G1 and 12.1% in G2 (p = 0.59). The incidence of mild bleeding was 3% in G1 and 1.7% in G2 (p = 0.64).
Conclusion
Either enoxaparin or rivaroxaban may be used as thromboprophylaxis agents in managing patients with moderate COVID-19. Either medication has no clear advantage over the other.
Keywords: Anticoagulants, Thromboprophylaxis, COVID-19, Enoxaparin, Rivaroxaban
1. Introduction
The increased prevalence of coagulation disorders due to coronavirus disease 2019 (COVID-19) has become a worldwide concern; numerous studies have revealed high mortality rates caused by these disorders.1 Studies have demonstrated an increased incidence of thromboembolic events in patients with COVID-19.2 Many hypotheses have been proposed to determine the principal pathophysiology for the progress of a prothrombotic state in COVID-19, including increased inflammatory response, leading to the activation of the coagulation cascade and endothelial injury.3 , 4
Many studies on the importance of using anticoagulants in COVID-19 have been conducted; however, the optimal anticoagulant agent for various cases of COVID-19 has yet to be found. Moreover, the dose, time of treatment, and how long it takes to get the treatment are not definitively identified.1 Cytokine storms and hypercoagulability are responsible for the progression to the severe form of COVID-19 and venous thromboembolism.5 Anticoagulant therapy is associated with a reduction in mortality rates in patients with COVID-19. Prothrombin time (PT) and D-dimer are predictors of prognosis, and adjusting these parameters may be an essential therapeutic aim.6 The most common types of anticoagulants are vitamin K antagonists, direct oral anticoagulants (direct thrombin inhibitors and direct factor Xa inhibitors), and low-molecular-weight heparin (LMWH). Each of them has a specific mechanism for inhibiting the formation of blood clots.7
Rivaroxaban is more convenient for patients and clinicians because of its administration route (oral), its quick start and offset of effect, and lack of requirement for regular monitoring.8 However, its short half-life negative affects efficacy, and its medication acquisition costs are high.9 LMWH is another anticoagulant drug that is administered subcutaneously to inhibit thrombosis and pulmonary embolism. Its mechanism of action is the inhibition of activated factor X (factor Xa) through binding to antithrombin.8 LMWH has a favorable pharmacokinetic and pharmacodynamic profile and a reliable anticoagulant effect. However, the use of this anticoagulant drug can occasionally be accompanied by a condition known as heparin-induced thrombocytopenia, which is characterized by a decrease in platelet count.10 Despite the aforementioned downside, LMWH is the preferred therapy in high-risk patients due to its little influence on PT and whole blood clotting time and its anti-inflammatory and antiviral properties.11 Enoxaparin and rivaroxaban were compared for use as prophylactic anticoagulants in moderate cases of COVID-19 in terms of efficacy, safety, and clinical outcomes in this study.
2. Methods
Study design: This study is a randomized clinical trial adopting an open-label, parallel-group study design. The study was conducted at the Chest Diseases Department, Minia University Hospital where all medical assessments were performed. Follow-up was performed by both physicians and clinical pharmacists. The patients were divided into two parallel groups in a 1:1 ratio.
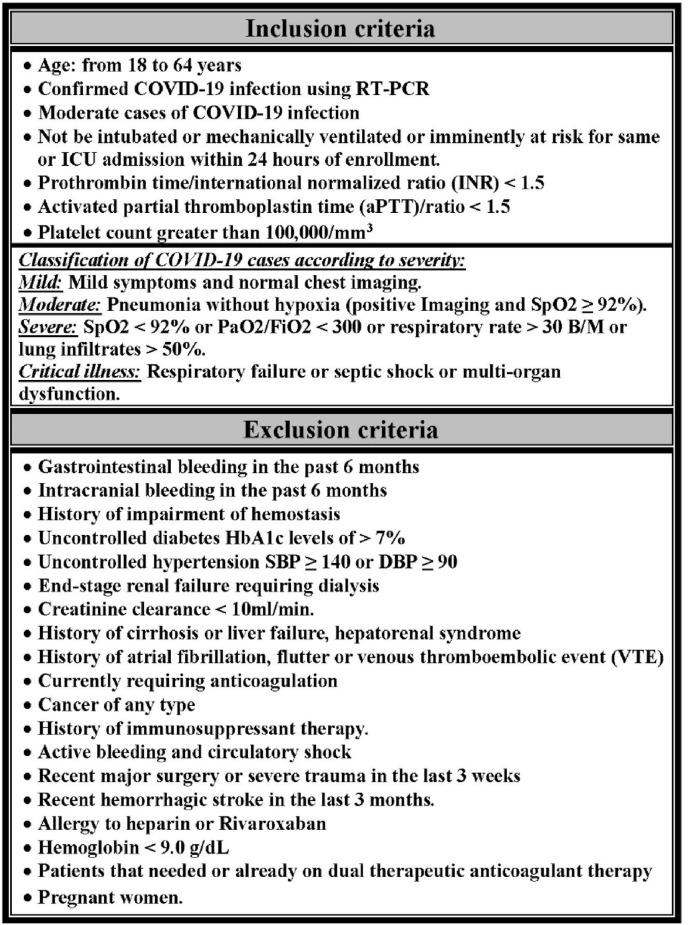
Patients: Adults aged 18–64 years diagnosed with moderate COVID-19 according to the management protocol for COVID-19 in Egypt12 and admitted to the intermediate care unit (IMCU) were deemed eligible for enrollment in this study. The assessment was performed according to the inclusion and exclusion criteria (Fig. 1 ).
Fig. 1.
Inclusion and exclusion criteria.
All patients were subjected to complete history taking, medical examination, laboratory, and radiological investigations.
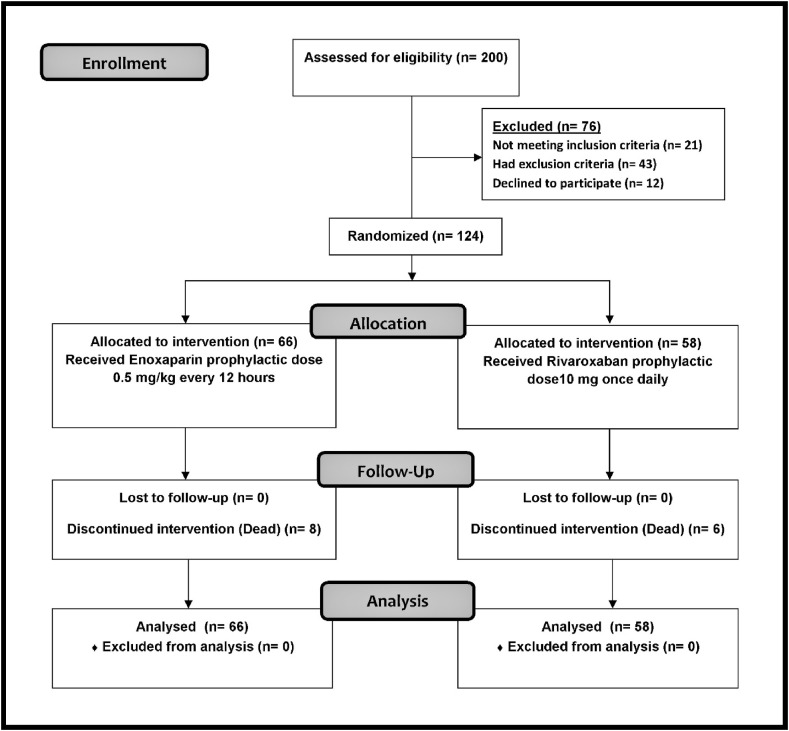
Randomization and procedure: Simple randomization was used. The sample size was calculated using a sample size calculator program (version: 2.0.4; Android application) with standard error of 0.05 and confidence level of 95%. The diagnosis of COVID-19 was confirmed by positive real-time reverse transcription-polymerase chain reaction (rRT-PCR) and chest radiology. The patients (n = 124) were randomly divided into two groups: G1 (n = 66) and G2 (n = 58). G1 received 0.5 mg/kg enoxaparin every 12 h, and G2 received 10 mg rivaroxaban once daily. All groups received the standard treatment for patients with COVID-19 according to the management protocol in Egypt.12 The study protocol was approved by the Ethics Committee of Minia University. Written informed consent was obtained from the patients or their legal caregivers after describing this study's goals and advantages.
Outcomes: Timeline: This study was conducted from the patients’ admission to the hospital until discharge or death. IMCU duration, the number of patients transferred from the IMCU to the intensive care unit (ICU), ICU duration, the total length of hospital stay, in-hospital mortality rate, and the rate of patients experiencing bleeding or thrombotic complications were the outcomes evaluated in this study. Bleeding complications were classified into the following: severe or life-threatening (intracerebral hemorrhage or resulting in substantial hemodynamic compromise requiring treatment), moderate (needed blood transfusion and not causing in hemodynamic compromise), and mild (the above conditions do not apply).13
Statistical analysis: Continuous variables with a normal distribution were expressed as means ± standard deviations. The unpaired t-test was used to assess the difference between two continuous variables with a normal distribution. The chi-square test was used to compare two groups with qualitative data. Pearson's correlation coefficients were used to measure the statistical relationship or association between two continuous variables. Differences with p-values of less than or equal to 0.05 were considered statistically significant. The effect size is low if the r value is approximately 0.1, medium if the r value is approximately 0.3, and large if the r value is more than 0.5.14 All statistical analyses were performed and graphs were made using Statistical Package for the Social Sciences, version 26.
3. Results
Between August 2021 and October 2021, 200 hospitalized patients with COVID-19 were screened for enrollment. Twelve patients declined to participate in the study and 64 patients did not meet the inclusion and exclusion criteria (Fig. 2 ).
Fig. 2.
Flow Diagram of the studied sample.
Table 1 shows the baseline descriptive characteristics of the patients. No significant differences in demographic data, clinical symptoms, and medical examination and laboratory test values were observed between the two groups.
Table 1.
Baseline characteristics.
| Demographic | Total N = 124 |
G1 (enoxaparin) N = 66 |
G2 (rivaroxaban) N = 58 |
P |
|---|---|---|---|---|
| Age (years), mean ± SD | 42 ± 11.8 | 42.4 ± 12.8 | 41.5 ± 10.6 | 0.68 |
| Gender (male/female) | 72/52 | 40/26 | 32/26 | 0.54 |
| Residence (urban/rural) | 71/53 | 38/28 | 33/25 | 0.94 |
| Smoking, n (%) | 25 (20.2%) | 12 (18.2%) | 13 (22.4%) | 0.56 |
| Mean time from symptom onset to hospitalization (day) | 5.5 ± 1.9 | 5.55 ± 1.9 | 5.38 ± 1.9 | 0.62 |
| Clinical symptoms | ||||
| Fever, n (%) | 85 (68.5%) | 47 (71.2%) | 38 (65.5%) | 0.5 |
| Cough, n (%) | 88 (71%) | 49 (74.2%) | 39 (67.2%) | 0.39 |
| Fatigue, n (%) | 59 (47.6%) | 28 (42.4%) | 31 (53.4%) | 0.22 |
| Shortness of breath, n (%) | 49 (39.5%) | 26 (39.4%) | 23 (39.7%) | 0.98 |
| Myalgia or arthralgia, n (%) | 19 (15.3%) | 12 (18.2%) | 7 (12.1%) | 0.35 |
| Headache, n (%) | 17 (13.7%) | 6 (9.1%) | 11 (19%) | 0.11 |
| Diarrhea, n (%) | 7 (5.6%) | 4 (6.1%) | 3 (5.2%) | 0.83 |
| Medical examination | ||||
| Heart rate (beats per minute), mean ± SD | 115.6 ± 18.2 | 116.9 ± 19 | 114.2 ± 17.3 | 0.42 |
| Systolic B.P. (mmHg), mean ± SD | 109.1 ± 10.4 | 109.6 ± 10.4 | 108.5 ± 10.5 | 0.56 |
| Diastolic BP (mmHg), mean ± SD | 72.3 ± 5 | 72.7 ± 4.7 | 71.8 ± 5.27 | 0.35 |
| Respiratory rate (breaths/min) | 22.4 ± 3.8 | 22.4 ± 3.8 | 22.41 ± 3.8 | 0.94 |
| Oxygen saturation, mean ± SD | 96 ± 1.9 | 96.1 ± 1.9 | 95.9 ± 1.9 | 0.57 |
| PaO2/FiO2 ratio, mean ± SD | 351.6 ± 22.4 | 354.6 ± 24 | 348.24 ± 20 | 0.12 |
| Laboratory tests | ||||
| HbA1c (%), mean ± SD | 6.7 ± 1.2 | 6.6 ± 1.3 | 6.674 ± 1.2 | 0.84 |
| RBC (106/μL), mean ± SD | 4.8 ± 0.6 | 4.8 ± 0.54 | 4.8 ± 0.62 | 0.1 |
| Hemoglobin (g/dL), mean ± SD | 14.8 ± 1.7 | 14.8 ± 1.7 | 14.7 ± 1.7 | 0.67 |
| WBC (103/μL), mean ± SD | 7.2 ± 1.8 | 7.3 ± 1.8 | 7.1 ± 1.9 | 0.55 |
| Platelets (103/μL), mean ± SD | 281.8 ± 72.8 | 288.2 ± 72.5 | 274.5 ± 73.2 | 0.3 |
| ALT(U/L), mean ± SD | 24.3 ± 10.3 | 23.4 ± 9.8 | 25.4 ± 10.8 | 0.29 |
| AST(U/L), mean ± SD | 31.8 ± 14.2 | 30.9 ± 14.6 | 32.8 ± 13.8 | 0.48 |
| Serum Albumin (g/dL), mean ± SD | 3.2 ± 1.4 | 3.1 ± 1.5 | 3.2 ± 1.3 | 0.8 |
| Urea (mg/dL), mean ± SD | 14.1 ± 4.2 | 14.5 ± 4.05 | 13.6 ± 4.3 | 0.24 |
| Creatinine (mg/dl), mean ± SD | 1 ± 0.2 | 1.1 ± 0.2 | 1 ± 0.2 | 0.55 |
| ESR (mm/h), mean ± SD | 34.4 ± 13.6 | 36.2 ± 13.6 | 32.2 ± 13.4 | 0.1 |
| CRP (mg/L), mean ± SD | 5.4 ± 2.7 | 5 ± 2.9 | 5.9 ± 2.5 | 0.06 |
| D-dimer (ng/mL), mean ± SD | 259.8 ± 128 | 245.6 ± 139 | 275.8 ± 114 | 0.19 |
| LDH (mg/dL), mean ± SD | 226.6 ± 52.4 | 224.3 ± 55.3 | 229.1 ± 49.2 | 0.61 |
| Ferritin (ng/mL), mean ± SD | 450 ± 289.7 | 485.5 ± 297.4 | 409.7 ± 277.7 | 0.15 |
| PT/INR | 1.2 ± 0.18 | 1.2 ± 0.2 | 1.2 ± 0.2 | 0.43 |
| aPPT ratio | 1.2 ± 0.17 | 1.2 ± 0.2 | 1.2 ± 0.2 | 0.73 |
SD, standard deviation; BP, blood pressure; PaO2/FiO2 ratio, arterial pO2 divided by fraction (percent) of inspired oxygen; HbA1c, glycated hemoglobin; RBC, red blood cell; WBC, white blood cell; ALT, alanine transaminase; AST, aspartate aminotransferase; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; LDH, lactate dehydrogenase; PT/INR, prothrombin time (international normalized ratio); aPPT ratio, activated partial thromboplastin time ratio.
This study included 124 patients with moderate COVID-19. Among them, 72 patients (58.1%) were male and 52 patients (41.9%) were female. Moreover, 53 (42.7%) patients were living in urban areas and 71 (57.3%) patients were living rural in areas. Ninety-nine (79.8%) patients were non-smokers and 25 (20.2%) were smokers.
Regarding symptoms, fever was present in 85 (68.5%) patients. Moreover, among the 124 patients, 88, 59, 49, 19, 17, and 7 had cough, fatigue, shortness of breath, myalgia or arthralgia, headache, and diarrhea, respectively.
The clinical parameters evaluated in this study were respiratory rate (22.4 ± 3.8 breaths/min), oxygen saturation (96 ± 1.9), and PaO2/FiO2 ratio (351.6 ± 22.4).
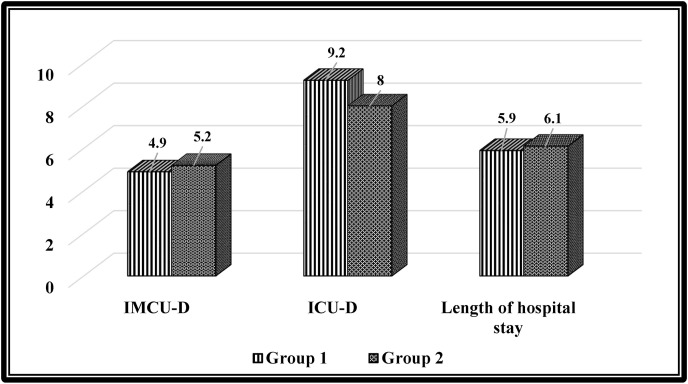
Table 2 shows no significant differences in IMCU duration (p = 0.39), ICU duration (p = 0.96), and total length of hospital stay (p = 0.73) between the two groups (see Fig. 3 ).
Table 2.
Comparison between the outcomes and adverse events in the two groups.
| Outcomes | Total N = 124 |
G1 (enoxaparin) N = 66 |
G2 (rivaroxaban) N = 58 |
P |
|---|---|---|---|---|
| IMCU duration (day), mean ± SD | 5 ± 1.4 | 4.9 ± 1.4 | 5.2 ± 1.4 | 0.39 |
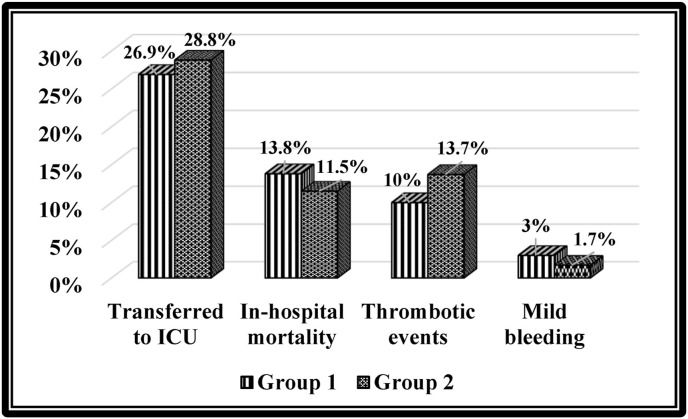
| Transferred to ICU, n (%) | 27 (21.8%) | 14 (21.2%) | 13 (22.4%) | 0.87 |
| ICU duration (day), mean ± SD | 8.6 ± 1.9 | 9.2 ± 1.6 | 8 ± 2.2 | 0.32 |
| Length of hospital stay (day), mean ± SD | 6 ± 2.9 | 5.9 ± 3.1 | 6.1 ± 2.8 | 0.73 |
| In-hospital mortality, n (%) | 14 (11.3%) | 8 (12.1%) | 6 (10.3%) | 0.76 |
| Thrombotic events, n (%) | 13 (10.5%) | 6 (9.1%) | 7 (12.1%) | 0.59 |
| Adverse bleeding events, n (%) | ||||
| Mild bleeding, n (%) | 3 (2.4%) | 2 (3%) | 1 (1.7%) | 0.64 |
| Moderate bleeding, n | 0 | 0 | 0 | .a |
| Severe bleeding, n | 0 | 0 | 0 | .a |
IMCU, intermediate care unit; transferred to, patients transferred from the IMCU to the intensive care unit.
a. No statistics are computed because moderate and severe bleeding is a constant.
N.B. IMCU duration, ICU duration, and length of hospital stay were calculated for patients who recovered only after excluding those who died.
Fig. 3.
Comparison of the outcomes between the two groups. (Intermediate care unit duration, intensive care unit duration, and length of hospital stay).
The number of patients who needed to be transferred from the IMCU to the ICU was 27 (21.8%). Fourteen patients died during hospitalization (11.3%). Thirteen patients (10.5%) experienced thrombotic events. Mild bleeding was present in three (2.4%) patients (see Fig. 4 ).
Fig. 4.
Comparison of the outcomes and adverse events between the two groups.
The number of patients who needed to be transferred from the IMCU to the ICU after hospitalization was 14 in G1 and 13 in G2 without significant difference (p = 0.87). The number of patients who died was 8 in G1 and 6 in G2 without significant difference (p = 0.76). Six patients experienced thrombotic complications in G1, whereas seven patients had thrombotic complications in G2; the difference between the two groups was insignificant (p = 0.59). The number of patients who had mild bleeding was 2 in G1 and 1 in G2 without significant difference (p = 0.64).
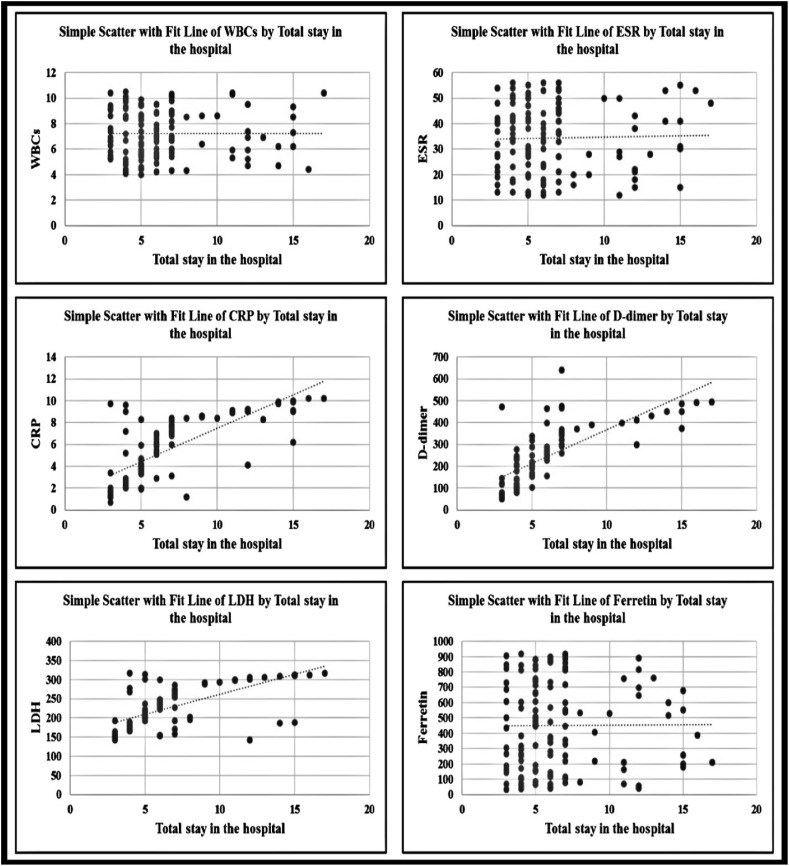
Table 3 shows a significant correlation between length of hospital stay and C-reactive protein (CRP) (0.75), D-dimer (0.73), and lactate dehydrogenase (LDH) (0.66) levels with a large effect size; however, no significant correlation was found between length of hospital stay and white blood cell (WBC) count (0.006), ESR (0.105), and ferritin levels (0.04) with a small effect size.
Table 3.
Correlation between length of hospital stay and some laboratory data.
| WBCs | ESR | CRP | D-dimer | LDH | Ferritin | ||
|---|---|---|---|---|---|---|---|
| Length of hospital stay | r | 0.006 | 0.105 | 0.75** | 0.73 ** | 0.66** | 0.04 |
| P | 0.954 | 0.276 | <0.001 | <0.001 | <0.001 | 0.681 | |
r, Pearson's correlation coefficient.
WBC, white blood cells; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; LDH, lactate dehydrogenase.
Figure (5) shows that there was a significant correlation between length of hospital stay and CRP, D-dimer, and LDH levels with a large effect size; however, no but a non-significant correlation was observed between length of hospital stay and WBCs count, ESR, and ferritin levels with a low small effect size.
Fig. 5.
Correlation between total length of hospital stay and some laboratory data.
4. Discussion
This study found that COVID-19 infection affected males more than females; this is consistent with the results of a previous study that revealed higher incidence and mortality rates in males than in females.15 The rate of infection in urban areas in this study was more than that in rural areas (57.3% vs. 42.7%). These results agree with the findings reported in another study that revealed that urban counties, on average, had substantially higher prevalence of COVID-19.16 The proportion of smokers was 20.2% of the total sample in this study. Several studies have reported that smoking is one of the causes of COVID-19 infection.16 The mean duration from symptom onset to hospitalization was 5.5 ± 1.9 days, which differs from those reported in many studies as it was 2.62 days in Singapore, 4.41 days in Hong Kong, and 5.14 days in the UK.17 , 18
A hallmark of COVID-19 is its wide range of severity, ranging from asymptomatic infection to life-threatening illness.19 In this study, all patients with COVID-19 were hospitalized and had moderate COVID-19 (pneumonia without hypoxia), which required admission to the IMCU. Many variable symptoms of COVID-19 without any significant difference between the two groups were observed. The most common symptoms were fever, cough, fatigue, shortness of breath, myalgia or arthralgia, headache, and diarrhea. Patients with COVID-19 experience varying symptoms, and this was evident in several studies.20 , 21 Fever, dry cough, dyspnea, myalgia, sputum, weakness, headache, and chest discomfort were all usual clinical manifestations of COVID-19 infection.22, 23, 24 COVID-19 cases rarely had diarrhea or nausea. Most patients first present with one or more symptoms. A considerable number of infected individuals experienced variable symptoms, such as widespread weakness and headache. Some patients with COVID-19 had obvious upper respiratory tract complaints (e.g., rhinorrhea, sneezing, or sore throat), whereas others had not, suggesting that the receptors are found in the lower airway according to prior findings.22, 23, 24, 25, 26, 27
In this study, the mean hemoglobin level was 14.8 g/DL; other studies revealed that anemia and altered iron homeostasis were common in hospitalized patients with COVID-19, and initial anemia was associated with increased mortality.28 There was neither leukocytosis nor leukocytopenia in most cases in this study, and another study supported this result and showed that in the early stages of COVID-19 infection, when symptoms are non-specific, peripheral WBCs and lymphocytes are normal or slightly lowered.29 In one study, the lymphocyte count was lower in patients with acute respiratory distress syndrome (ARDS), those with severe illness who needed ICU admission, and those who died.30
At the beginning of the COVID-19 pandemic, it has been clear that coagulopathy and thromboembolism are common among hospitalized and severely sick patients.31 Coagulopathy with elevated plasma concentrations of D-dimer is associated with increased COVID-19 mortality even in the absence of a clinical diagnosis of thromboembolism.32 Postmortem findings from patients with COVID-19 have demonstrated a high frequency of pulmonary microvascular platelet-fibrin thrombi, suggesting that coagulopathy contributes to respiratory failure and death in COVID-19 even in the absence of a clinical diagnosis of thromboembolism.33 , 34
In this study, a significant correlation between CRP, D-dimer, LDH, and length of hospital stay was found; however, only a weak insignificant correlation between WBC count, ESR, and ferritin levels and length of hospital stay was found (Fig. 5 and Table 3). Several other studies have reported similar results; for example, a study has reported that abnormally high D-dimer levels are associated with poor prognosis.35
A study showed that D-dimer is correlated with disease degree and is a dependent prognostic indicator of in-hospital mortality in cases of COVID-19.36 High D-dimer levels are associated with community-acquired pneumonia and its clinical outcomes.37 , 38 A study found a link between D-dimer values and illness severity, as measured by the area of affected lungs on chest computed tomography, oxygenation index, and in-hospital fatality rates.36 A study reported that D-dimer levels of more than 1 μg/mL are associated with a higher risk of mortality.33 LDH levels were elevated in approximately 40% of the patients. High LDH levels have been linked to an increased risk of ARDS, ICU admission, and death.29
Ferritin levels in patients with COVID-patients have generated ambiguous outcomes in several studies. Whether it is a bystander or a true characteristic of the disease is unclear.39 Two studies showed that ferritin had a little influence in determining ICU admission and the requirement for ventilation and failed to predict death.40 , 41 However, another study and a meta-analysis found that ferritin levels are associated with fatal illness and death.41 , 42
A meta-analysis revealed that high serum CRP, procalcitonin (PCT), D-dimer, and ferritin levels were associated with poor outcomes, including death, severe COVID-19, ARDS, and ICU admission, in COVID-19 cases. The outcome was unrelated to sex, age, cardiovascular morbidity, diabetes, and chronic obstructive pulmonary disease.42
A retrospective study revealed that high serum CRP levels were related to 30-day mortality rate,43 whereas some studies revealed opposite results.44, 45, 46 Moreover, new evidence has revealed that serum CRP levels might be used in predicting the severity of COVID-19 cases.47
A meta-analysis concluded that higher levels of inflammatory markers, such as WBC, CRP, PCT, ESR, interleukin (IL)-6, and IL-10, are related to the severity of COVID-19 and therefore could be used as significant prognostic factors for the disease.24
The optimal approach for prophylactic anticoagulation therapy in patients with COVID-19 is under active investigation and remains a challenging clinical conundrum.2 In this study, no significant differences in the safety or efficacy between enoxaparin and rivaroxaban in preventing in-hospital death, thrombosis, or bleeding were found. Moderate or severe bleeding in patients in either group was not observed.
In this study, no significant differences in the effects between enoxaparin and rivaroxaban in terms of IMCU duration, number of patients who required ICU admission, ICU duration, length of hospital stay, and in-hospital mortality were found. Another study revealed that weight-adjusted intermediate-dose enoxaparin was not more effective than standard-dose enoxaparin in preventing death or thrombosis in a population of hospitalized adults with severe COVID-19.48
Another study examined 150 patients randomized to take enoxaparin or oral rivaroxaban while hospitalized and after discharge for 28 days. This trial assessed different rivaroxaban doses (i.e., 10, 15, and 20 mg once daily), and the outcomes were combinations of mortality, mechanical ventilation, intubation, and admission to the ICU.49
In a randomized clinical trial, therapeutic enoxaparin improved gas exchange, reduced D-dimer levels, and increased the ratio of weaning from mechanical ventilation in cases of respiratory failure in COVID-19.48
Two other studies have revealed the clinical advantage of anticoagulants in COVID-19 cases. A retrospective study showed a low 28-day mortality rate in severe COVID-19 cases that received anticoagulant therapy for 7 days or more, particularly those with increased sepsis-induced coagulopathy score (≥4) or increased D-dimer levels (≥3.0 mg/L).50
In this study, adverse bleeding events were present in only 2.4% of the patients in the form of mild bleeding. Although COVID-19 is accompanied by coagulopathy, this condition has a low risk of bleeding.51 In COVID-19, PT and activated partial thromboplastin time prolongation is less prevalent than in bacterial-sepsis driven coagulopathy, and thrombocytopenia is minimal.51 According to one study, among individuals who received systemic anticoagulation, 3% had bleeding incidents, whereas only 1.9% of those who did not receive systemic anticoagulation had bleeding incidents (p = 0.2).52 In another study, the overall rate of major bleeding events in patients with COVID-19 with the most diverse anticoagulation regimes was 4.8%.32
A comparative study between the extended duration of rivaroxaban and the standard duration of enoxaparin in decreasing venous thromboembolism (VTE) involving 8101 patients53 revealed that the standard duration of rivaroxaban administration was not inferior to the standard duration of enoxaparin in terms of VTE inhibition. However, the extended duration of rivaroxaban use was superior to enoxaparin and was accompanied by a greater rate of clinically relevant bleeding complications.54
In another trial, the safety and efficacy of rivaroxaban were compared with those of enoxaparin in 3173 medically ill hospitalized patients due to acute infectious disease. That study provided evidence of the superiority of rivaroxaban over enoxaparin in terms of efficacy (4.4% vs. 6.6%; RR = 0.50, 95% confidence interval = 0.45–0.92). However, enoxaparin showed a more favorable profile in terms of safety.55
A sub-study of MARINER, evaluating the efficacy of rivaroxaban, as a composite of symptomatic VTE, MI, non-hemorrhagic stroke, and cardiovascular death, revealed a 28% reduction in fatal and non-major thromboembolic events (1.28% in rivaroxaban vs. 1.77% in placebo).56
This study has some limitations. First, it was a single-center study, so the population size should be increased with more demographic locations. Second, this study was limited to moderate cases of COVID-19. Third, there was a lack of previous studies on the same research topic. Finally, this study was not designed to examine long-term outcomes. Therefore, additional studies are needed to confirm the study findings and assess the long-term effects of various types of anticoagulant therapies on patients with COVID-19.
In summary, in this open-label randomized trial, a prophylactic dose of enoxaparin was not more effective than a prophylactic dose of rivaroxaban in preventing death or thrombosis in hospitalized patients with moderate COVID-19 and vice versa. These prospective data must be interpreted in the context of other trials investigating strategies for thromboprophylaxis in patients with different severities of COVID-19.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
Asmaa: Conceptualization, Data curation, Methodology, Project administration, Supervision, Roles/Writing - original draft, Writing - review & editing.
Hosam: Conceptualization, Data curation, Formal Analysis, Methodology, Roles/Writing - original draft, Writing - review & editing.
Alyaa: Conceptualization, Methodology, Data curation.
Fatma: Conceptualization, Methodology, Resources.
Ali: Conceptualization, Writing - review & editing.
Ahmed: Conceptualization, Methodology, Project administration, Resources, Supervision, Writing - review & editing.
Declaration of competing interest
All authors have no conflicts of interest to disclose.
Acknowledgments
The authors thank all participants of this study.
Data availability
The datasets generated and/or analyzed in this study are available from the corresponding author on reasonable request.
References
- 1.Chandra A., Chakraborty U., Ghosh S., Dasgupta S. Anticoagulation in COVID-19: current concepts and controversies. Postgrad Med. 2021 doi: 10.1136/postgradmedj-2021-139923. [DOI] [PubMed] [Google Scholar]
- 2.Miesbach W., Makris M. COVID-19: coagulopathy, risk of thrombosis, and the rationale for anticoagulation. Clin Appl Thromb Hemost. 2020;26 doi: 10.1177/1076029620938149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed S., Zimba O., Gasparyan A.Y. Clinical rheumatology; 2020. Thrombosis in Coronavirus Disease 2019 (COVID-19) through the Prism of Virchow's Triad; pp. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourgonje A.R., Abdulle A.E., Timens W., et al. Angiotensin‐converting enzyme 2 (ACE2), SARS‐CoV‐2 and the pathophysiology of coronavirus disease 2019 (COVID‐19) J Pathol. 2020;251(3):228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klok F., Kruip M., Van der Meer N., et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thachil J. The versatile heparin in COVID‐19. J Thromb Haemostasis. 2020;18(5):1020–1022. doi: 10.1111/jth.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amaraneni A., Chippa V., Rettew A.C. 2020. Anticoagulation Safety. StatPearls [Internet] [PubMed] [Google Scholar]
- 8.Young A.M., Marshall A., Thirlwall J., et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D) J Clin Oncol. 2018 doi: 10.1200/JCO.2018.78.8034. [DOI] [PubMed] [Google Scholar]
- 9.Bauer K.A. Pros and cons of new oral anticoagulants. Hematology. 2013;2013(1):464–470. doi: 10.1182/asheducation-2013.1.464. [DOI] [PubMed] [Google Scholar]
- 10.Arepally G.M. Heparin-induced thrombocytopenia. Blood. J Am Soc Hematol. 2017;129(21):2864–2872. doi: 10.1182/blood-2016-11-709873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neki, N., COVID-19 and Low Molecular Weight Heparin (LMWH).
- 12.Management Protocol for COVID-19 Patients. Egypt Ministry of Health and population; Egypt: 2021. [Google Scholar]
- 13.Mehran R., Rao S.V., Bhatt D.L., et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- 14.Cohen J. Statistical power analysis for the behavioral sciences; 1988. The Effect Size; pp. 77–83. [Google Scholar]
- 15.Ramírez-Soto M.C., Arroyo-Hernández H., Ortega-Cáceres G. Sex differences in the incidence, mortality, and fatality of COVID-19 in Peru. PLoS One. 2021;16(6):e0253193. doi: 10.1371/journal.pone.0253193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paul R., Arif A.A., Adeyemi O., Ghosh S., Han D. Progression of COVID‐19 from urban to rural areas in the United States: a spatiotemporal analysis of prevalence rates. J Rural Health. 2020;36(4):591–601. doi: 10.1111/jrh.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pellis L., Scarabel F., Stage H.B., et al. Challenges in control of Covid-19: short doubling time and long delay to effect of interventions. Phil. Transact. Royal Soc. B. 2021;376(1829) doi: 10.1098/rstb.2020.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraemer M.U., Yang C.-H., Gutierrez B., et al. The effect of human mobility and control measures on the COVID-19 epidemic in China. Science. 2020;368(6490):493–497. doi: 10.1126/science.abb4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 20.Larsen J.R., Martin M.R., Martin J.D., Kuhn P., Hicks J.B. Modeling the onset of symptoms of COVID-19. Front Public Health. 2020;8:473. doi: 10.3389/fpubh.2020.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pullen M.F., Skipper C.P., Hullsiek K.H., et al. Open Forum Infectious Diseases. Oxford University Press US; 2020. Symptoms of COVID-19 outpatients in the United States. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peiris J.S.M., Chu C.-M., Cheng V.C.-C., et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jernigan J.A., Low D.E., Helfand R.F. Combining clinical and epidemiologic features for early recognition of SARS. Emerg Infect Dis. 2004;10(2):327. doi: 10.3201/eid1002.030741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou L., Ruan F., Huang M., et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu W.J., Zhao M., Liu K., et al. T-cell immunity of SARS-CoV: implications for vaccine development against MERS-CoV. Antivir Res. 2017;137:82–92. doi: 10.1016/j.antiviral.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao J., Zhao J., Mangalam A.K., et al. Airway memory CD4+ T cells mediate protective immunity against emerging respiratory coronaviruses. Immunity. 2016;44(6):1379–1391. doi: 10.1016/j.immuni.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Wit E., Van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samprathi M., Jayashree M. Biomarkers in COVID-19: an up-to-date review. Front. Pediatr. 2020;8 doi: 10.3389/fped.2020.607647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terpos E., Ntanasis‐Stathopoulos I., Elalamy I., et al. Hematological findings and complications of COVID‐19. Am J Hematol. 2020;95(7):834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang I., Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J. intensive care. 2020;8:1–10. doi: 10.1186/s40560-020-00453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Middeldorp S., Coppens M., van Haaps T.F., et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemostasis. 2020;18(8):1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Samkari H., Karp Leaf R.S., Dzik W.H., et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dolhnikoff M., Duarte‐Neto A.N., de Almeida Monteiro R.A., et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID‐19. J Thromb Haemostasis. 2020 doi: 10.1111/jth.14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu B., Li X., Chen J., et al. Evaluation of variation in D-dimer levels among COVID-19 and bacterial pneumonia: a retrospective analysis. J Thromb Thrombolysis. 2020;50(3):548–557. doi: 10.1007/s11239-020-02171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao Y., Cao J., Wang Q., et al. D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study. J. intensive care. 2020;8(1):1–11. doi: 10.1186/s40560-020-00466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Querol-Ribelles J.M., Tenias J.M., Grau E., et al. Plasma d-dimer levels correlate with outcomes in patients with community-acquired pneumonia. Chest. 2004;126(4):1087–1092. doi: 10.1378/chest.126.4.1087. [DOI] [PubMed] [Google Scholar]
- 38.Dai R.-X., Kong Q.-H., Mao B., et al. The mortality risk factor of community acquired pneumonia patients with chronic obstructive pulmonary disease: a retrospective cohort study. BMC Pulm Med. 2018;18(1):1–10. doi: 10.1186/s12890-018-0587-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kappert K., Jahić A., Tauber R. Assessment of serum ferritin as a biomarker in COVID-19: bystander or participant? Insights by comparison with other infectious and non-infectious diseases. Biomarkers. 2020;25(8):616–625. doi: 10.1080/1354750X.2020.1797880. [DOI] [PubMed] [Google Scholar]
- 40.Bellmann-Weiler R., Lanser L., Barket R., et al. Prevalence and predictive value of anemia and dysregulated iron homeostasis in patients with COVID-19 infection. J Clin Med. 2020;9(8):2429. doi: 10.3390/jcm9082429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin Z., Long F., Yang Y., Chen X., Xu L., Yang M. Serum ferritin as an independent risk factor for severity in COVID-19 patients. J Infect. 2020;81(4):647–679. doi: 10.1016/j.jinf.2020.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang I., Pranata R., Lim M.A., Oehadian A., Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14 doi: 10.1177/1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koozi H., Lengquist M., Frigyesi A. C-reactive protein as a prognostic factor in intensive care admissions for sepsis: a Swedish multicenter study. J Crit Care. 2020;56:73–79. doi: 10.1016/j.jcrc.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 44.Song J., Park D.W., Moon S., et al. Diagnostic and prognostic value of interleukin-6, pentraxin 3, and procalcitonin levels among sepsis and septic shock patients: a prospective controlled study according to the Sepsis-3 definitions. BMC Infect Dis. 2019;19(1):1–11. doi: 10.1186/s12879-019-4618-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miguel-Bayarri V., Casanoves-Laparra E., Pallás-Beneyto L., et al. Prognostic value of the biomarkers procalcitonin, interleukin-6 and C-reactive protein in severe sepsis. Med Intensiva. 2012;36(8):556–562. doi: 10.1016/j.medin.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 46.Ryoo S.M., Han K.S., Ahn S., et al. The usefulness of C-reactive protein and procalcitonin to predict prognosis in septic shock patients: a multicenter prospective registry-based observational study. Sci Rep. 2019;9(1):1–8. doi: 10.1038/s41598-019-42972-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Creamer A.W., Kent A.E., Albur M. Procalcitonin in respiratory disease: use as a biomarker for diagnosis and guiding antibiotic therapy. Breathe. 2019;15(4):296–304. doi: 10.1183/20734735.0258-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lemos A.C.B., do Espírito Santo D.A., Salvetti M.C., et al. Therapeutic versus prophylactic anticoagulation for severe COVID-19: a randomized phase II clinical trial (HESACOVID) Thromb Res. 2020;196:359–366. doi: 10.1016/j.thromres.2020.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramacciotti E., Agati L.B., Calderaro D., et al. Medically ill hospitalized patients for COVID-19 THrombosis extended ProphyLaxis with rivaroxaban ThErapy: rationale and design of the MICHELLE trial. Am Heart J. 2021;242:115–122. doi: 10.1016/j.ahj.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemostasis. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iba T., Levy J.H., Levi M., Thachil J. Coagulopathy in COVID‐19. J Thromb Haemostasis. 2020;18(9):2103–2109. doi: 10.1111/jth.14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paranjpe I., Fuster V., Lala A., et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76(1):122–124. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen A.T., Spiro T.E., Büller H.R., et al. Extended-duration rivaroxaban thromboprophylaxis in acutely ill medical patients: MAGELLAN study protocol. J Thromb Thrombolysis. 2011;31(4):407–416. doi: 10.1007/s11239-011-0549-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cohen A.T., Spiro T.E., Büller H.R., et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368:513–523. doi: 10.1056/NEJMoa1111096. [DOI] [PubMed] [Google Scholar]
- 55.Cohoon K., De Sanctis Y., Haskell L., McBane R., Spiro T. Rivaroxaban for thromboprophylaxis among patients recently hospitalized for acute infectious diseases: a subgroup analysis of the MAGELLAN study. J Thromb Haemostasis. 2018;16(7):1278–1287. doi: 10.1111/jth.14146. [DOI] [PubMed] [Google Scholar]
- 56.Spyropoulos A.C., Ageno W., Albers G.W., et al. Rivaroxaban for thromboprophylaxis after hospitalization for medical illness. N Engl J Med. 2018;379(12):1118–1127. doi: 10.1056/NEJMoa1805090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed in this study are available from the corresponding author on reasonable request.