Abstract
Objectives
India introduced BBV152/Covaxin and AZD1222/Covishield vaccines in January 2021. We estimated the effectiveness of these vaccines against severe COVID-19 among individuals aged ≥45 years.
Methods
We did a multi-centric, hospital-based, case-control study between May and July 2021. Cases were severe COVID-19 patients, and controls were COVID-19 negative individuals from 11 hospitals. Vaccine effectiveness (VE) was estimated for complete (2 doses ≥ 14 days) and partial (1 dose ≥ 21 days) vaccination; interval between two vaccine doses and vaccination against the Delta variant. We used the random effects logistic regression model to calculate the adjusted odds ratios (aOR) with a 95% confidence interval (CI) after adjusting for relevant known confounders.
Results
We enrolled 1143 cases and 2541 control patients. The VE of complete vaccination was 85% (95% CI: 79-89%) with AZD1222/Covishield and 71% (95% CI: 57-81%) with BBV152/Covaxin. The VE was highest for 6-8 weeks between two doses of AZD1222/Covishield (94%, 95% CI: 86-97%) and BBV152/Covaxin (93%, 95% CI: 34-99%). The VE estimates were similar against the Delta strain and sub-lineages.
Conclusion
BBV152/Covaxin and AZD1222/Covishield were effective against severe COVID-19 among the Indian population during the period of dominance of the highly transmissible Delta variant in the second wave of the pandemic. An escalation of two-dose coverage with COVID-19 vaccines is critical to reduce severe COVID-19 and further mitigate the pandemic in the country.
Keywords: COVID-19 vaccines, BBV152/Covaxin, AZD1222/Covishield, Delta variant, Effectiveness, India
Introduction
India's drugs regulator authorized emergency use for BBV152/Covaxin (Bharat Biotech Limited, India) and AZD1222/Covishield (ChAdOx1-Recombinant, Serum Institute of India Limited, India) in January 2021. BBV152/Covaxin was developed using the whole virion SARS-CoV-2 vaccine strain NIV-2020-770 (spike variant Asp614Gly) inactivated with β-propiolactone, containing a 6 μg vaccine dose with a toll-like receptor 7/8 agonist molecule (imidazoquinoline; IMDG) adsorbed to alum (Algel-IMDG) (Ella et al., 2021a). It is administered as two doses of intramuscular injection on day 0 and day 28. AZD1222/Covishield constitutes a recombinant, replication-deficient chimpanzee adenovirus vector encoding the SARS-CoV-2 Spike (S) glycoprotein and is produced in genetically modified human embryonic kidney 293 cells. Two doses as intramuscular injections are administered 4-12 weeks apart (Serum Institute of India, 2021). India rolled out vaccination beginning with the healthcare and frontline workers and cascading from those ≥ 60 years to all aged above 18 years (The Hindu, 2021, Ministry of Health and Family Welfare, 2021a). By October 2021, 88% of the total 1 billion vaccinees had received AZD1222/Covishield (Ministry of Health and Family Welfare, 2021b).
Vaccine effectiveness (VE) might differ in specific areas or demographics against various disease outcomes and newly emerging SARS-CoV-2 variants. From the healthcare perspective, it is imperative to know if the vaccine is effective against severe COVID-19 and eventual hospitalization. In the Indian context, real-world effectiveness of AZD1222/Covishield and BBV152/Covaxin against SARS-CoV-2 infection and/or COVID-19 with varying severity has been reported among healthcare workers and policemen (Ghosh et al., 2021; Jaiswal et al., 2021; Satwik et al., 2021; Victor et al., 2021). However, vaccine evaluation in a larger population context will provide post-authorization confirmation of the effectiveness of conditionally approved products for regulatory bodies. Hence, we conducted a multi-centric study among the general population to estimate the effectiveness of BBV152/Covaxin and AZD1222/Covishield vaccines against severe COVID-19 and the Delta variant.
Methods
Study design
We conducted a hospital-based, case-control study among individuals aged ≥ 45 years recruited from 11 tertiary care hospitals across India from May - July 2021. We defined cases as severe COVID-19 positive (based on Reverse transcriptase- polymerase chain reaction [RT-PCR]/Rapid Antigen Test [RAT]/GeneXpert/TrueNat test at admission or documented within 14 days before hospitalization) patients hospitalized with signs and symptoms of fever, cough, dyspnoea, fast breathing, respiratory rate > 30 breaths/min or SpO2 < 90% on room air anytime during hospitalization. (World Health Organization, 2020) We defined controls as individuals attending the COVID-19 testing facility of the same study hospital irrespective of symptom status with a negative RT-PCR report for SARS-CoV-2. We excluded individuals who reported negative RT-PCR but were highly suggestive of COVID-19 on CT scan or were unwilling to participate.
Sample size
We needed 1300 cases and 2600 controls assuming VE of 75%, 15% controls vaccinated with two doses, 95% confidence interval (CI), 20% relative width of 95% CI, and a 1:2 case-control ratio. We calculated the sample size of cases (N1) as N1 = (z/d)2[1/A(1–A)+1/CP2(1–P2)], where C (control to case ratio) was 2:1; P2 (prevalence of vaccine exposure in the control group) was assumed to be 15%; A = P2(1- VE)/[1-P2(VE)] where VE denotes the anticipated vaccine effectiveness taken as 75%; Z = 1.96 (based on α = 0.05); d is determined by solving the equation where denotes the CI width; relative width of 95% CI considered as 20% (15% absolute width) and number of controls needed = C*N1 (O'Neill, 1988).
Data collection
Trained investigators screened hospitalized COVID-19 patients and individuals attending COVID-19 testing facilities for the eligibility criteria. All RT-PCR negative individuals were telephonically contacted 7 days after enrolment to confirm any change in COVID-19 test status following the initial negative report. Data was collected through face-to-face interviews with participants or their family members and a review of hospital/laboratory/vaccination records using a pre-tested, pre-coded standardized paper-based form. We collected COVID-19 vaccination status based on data from India's COVID-19 vaccination portal (https://www.cowin.gov.in)/vaccination certificate/text message on a mobile phone or based on participant recall if documents were unavailable. Previous SARS-CoV-2 infection was documented based on reported history and RT-PCR/RAT report.
We collected the nasal/throat swabs from cases and controls and transported them to ICMR-National Institute of Virology, Pune. The swabs were processed for viral RNA screening and Next-Generation Sequencing (NGS) of COVID-19 positive samples to determine the lineages of the sequences (Supplementary appendix).
Statistical analyses
We entered the data in Research Electronic Data Capture (REDCap) software (https://www.project-redcap.org/) after the investigators verified the completeness and errors of completely filled paper forms. We compared the demographics, vaccination status, and risk behaviors of cases and controls using the Chi-square test for categorical, and median test for non-normal continuous variables.
For the primary VE analysis, we compared the proportion of fully vaccinated (two-dose recipients, with an interval between second dose and COVID-19 testing/hospitalization of ≥14 days) and unvaccinated individuals (non-receipt of any COVID-19 vaccine) among cases and controls. In addition, we estimated VE for partial vaccination defined as one dose recipients, with an interval between vaccination and COVID-19 testing/hospitalization of ≥21 days. We estimated the VE by the interval between two doses (<6, 6-8, 9-11, ≥12 weeks) among fully vaccinated individuals. In addition, we estimated VE for the Delta variant and its sub-lineages as a subgroup analysis. Individuals with past SARS-CoV-2 infection were also included in the VE analysis as vaccination was regardless of prior infection status.
Covariates with a P-value < 0.20 for crude odds ratio (OR) were selected for multiple logistic regression (Afifi et al., 2004). After assessing for multi-collinearity, relevant covariates were identified as confounders by comparing the −2 log-likelihood ratio values of the models with and without the potential confounder(s) as per the conceptual framework based on directed acyclic graphs (Westreich and Greenland, 2013). To account for the heterogeneity of effect by study sites, we used random effect multiple logistic regression model to calculate the adjusted odds ratio (aOR) with 95% CI after adjusting for relevant confounders. We calculated VE as (1-aOR) × 100%. Statistical analyses were done using Stata (version SE 17.0) software (StataCorp, Texas, USA).
Results
Background characteristics of cases and controls
We recruited 1143 cases and 2541 controls from 11 study hospitals. Cases were older (61.1 [SD: 10.5] vs 56.5 [SD: 8.7] years, P < 0.001) and included significantly more females, rural residents, non-earning, with lower formal education and those reporting pre-existing comorbidities than the controls (Table 1 ). Of the 2541 controls, 1652 (65%) were asymptomatic and the remaining were symptomatic (fever [347, 14%], headache [161, 6%], sore throat [138, 5%], weakness [91,4%], cough [78, 3%], bodyache [32, 1.3%], breathlessness [25, 1%], diarrhea [11, 0.4%] and loss of smell/taste/altered taste [6, 0.2%]).
Table 1.
Background characteristics of cases and controls, India, May-July 2021.
| Characteristics | Cases (%) | Controls (%) | P-value |
|---|---|---|---|
| Median age in years (IQR) |
(n = 1143) 60 (52-69) |
(n = 2541) 55 (50-62) |
<0.001 |
| Gender | (n = 1143) | (n = 2541) | |
| Male | 654 (57.2) | 1590 (62.6) | 0.007 |
| Female | 489 (42.8) | 950 (37.4) | |
| Residence | (n = 1138) | (n = 2520) | |
| Rural | 560 (49.2) | 941 (37.3) | <0.001 |
| Urban | 578 (50.8) | 1579 (62.7) | |
| Occupation | (n = 1143) | (n = 2538) | |
| Unemployed/student/homemaker | 574 (50.2) | 901 (35.5) | <0.001 |
| Retired | 139 (12.2) | 333 (13.1) | |
| Agriculture | 121 (10.6) | 262 (10.3) | |
| Professional/technical/administrative/management | 89 (7.8) | 374 (14.7) | |
| Skilled manual | 85 (7.4) | 272 (10.7) | |
| Unskilled manual | 65 (5.7) | 158 (6.2) | |
| Sales and services | 53 (4.6) | 167 (6.6) | |
| Clerical | 17(1.5) | 71 (2.8) | |
| Formal education | (n = 1142) | (n = 2541) | |
| Illiterate | 225 (19.7) | 426 (16.8) | <0.001 |
| Primary school | 195 (17.1) | 248(9.8) | |
| Middle school | 210 (18.4) | 347 (13.7) | |
| Secondary school | 185 (16.2) | 528 (20.8) | |
| Higher secondary school (11th and 12th std) | 122 (10.7) | 335 (13.2) | |
| Graduation | 152 (13.3) | 467 (18.4) | |
| Post-graduation and above | 53 (4.6) | 190 (7.5) | |
| Smoking status | (n = 1114) | (n = 2522) | |
| Never smoked | 915 (82.1) | 2083 (82.6) | 0.43 |
| Former smoker | 118 (10.6) | 237 (9.4) | |
| Current smoker | 81 (7.3) | 202 (8.0) | |
| Any pre-existing comorbidities |
(n = 1143) 781 (68.3) |
(n = 2541) 1336 (52.6) |
<0.001 |
| Type of pre-existing comorbidity | |||
| Hypertension |
(n = 781) 528 (67.6) |
(n = 1335) 678 (50.8) |
<0.001 |
| Diabetes mellitus |
(n = 781) 501 (64.1) |
(n = 1333) 651 (48.8) |
<0.001 |
| Heart disease |
(n = 778) 135 (17.3) |
(n = 1335) 195 (14.6) |
0.09 |
| Asthma |
(n = 780) 50 (6.4) |
(n = 1333) 49 (3.7) |
0.004 |
| Lung disease other than asthmaa |
(n = 767) 49 (6.4) |
(n = 1335) 30 (2.2) |
<0.001 |
| Any malignancy |
(n = 780) 45 (5.8) |
(n = 1336) 260 (19.5) |
<0.001 |
| Liver disease |
(n = 780) 33 (4.2) |
(n = 1336) 35 (2.6) |
0.04 |
| Any immunodeficiency disorder |
(n = 779) 29 (3.7) |
(n = 1332) 45 (3.4) |
0.68 |
| Kidney disease |
(n = 781) 63 (8.1) |
(n = 1334) 69 (5.2) |
0.008 |
| Active tuberculosis |
(n = 780) 10 (1.3) |
(n = 1330) 10 (0.7) |
0.23 |
Includes all conditions affecting the lungs with the exclusion of asthma
IQR = interquartile range.
Vaccination and other COVID-19-related behaviors among cases and controls
Overall, 328 (29%) cases and 1425 (56%) controls reported receiving at least one dose of any vaccine (P < 0.001). The vaccination history of 142 (43%) vaccinated cases and 802 (56%) vaccinated controls was verified using vaccination records. Majority of the vaccinated cases (n = 257, 78%) and controls (n = 1147, 81%) had received AZD1222/Covishield. Compared to controls, a significantly higher proportion of cases reported participation in social/religious events (10% vs. 3%, P < 0.001), did not always use masks (47% vs 27%, P < 0.001), and were exposed to COVID-19 individual within 14 days of testing for SARS-CoV-2 (21% vs 11%, P < 0.001) (Table 2 ).
Table 2.
Vaccination and other COVID-19-related behaviors among cases and controls, India, May-July 2021.
| Vaccination status and COVID-19 related behaviour | Cases (%) | Controls (%) | P-value |
|---|---|---|---|
| Taken any COVID=19 vaccine |
(n = 1143) 328 (28.7) |
(n = 2541) 1425 (56.1) |
<0.001 |
| Vaccine type | (n = 328) | (n = 1425) | |
| BBV152/Covaxin | 71 (21.6) | 266 (18.7) | 0.14 |
| AZD1222/Covishield | 257 (78.4) | 1147 (80.5) | |
| Others | 0 | 12 (0.8) | |
| Vaccine doses - any vaccine | (n = 1143) | (n = 2541) | |
| Unvaccinated | 815 (71.3) | 1116 (43.9) | <0.001 |
| One dose | 226 (19.8) | 813 (32.0) | |
| 2 doses | 102 (8.9) | 612 (24.1) | |
| Vaccine doses - BBV152/Covaxin | (n = 886) | (n = 1382) | |
| Unvaccinated | 815 (92.0) | 1116 (80.6) | <0.001 |
| One dose | 30 (3.4) | 91 (6.6) | |
| 2 doses | 41 (4.6) | 175 (12.7) | |
| Vaccine doses - AZD1222/Covishield | (n = 1072) | (n = 2263) | |
| Unvaccinated | 815 (76.0) | 1116 (49.3) | <0.001 |
| One dose | 196 (18.3) | 720 (31.8) | |
| 2 doses | 61 (5.7) | 427 (18.9) | |
| Source of vaccination details | (n = 328) | (n = 1418) | |
| Recall | 186 (56.7) | 616 (43.4) | <0.001 |
| Text message from vaccination centre | 105 (32.0) | 458 (32.3) | |
| Vaccination certificate | 26 (7.9) | 282 (19.9) | |
| Hospital records | 11 (3.3) | 38 (2.7) | |
| CO-WIN registry | 0 (0.0) | 24 (1.7) | |
| Participated in social/religious event within 14 days of COVID-19 testing |
(n = 1141) 113 (9.9) |
(n = 2539) 80 (3.1) |
<0.001 |
| Frequency of mask use within 14 days of COVID-19 testing | (n = 1142) | (n = 2537) | |
| Never | 26 (2.3) | 22 (0.9) | <0.001 |
| Sometimes | 505 (44.2) | 684 (27.0) | |
| Always | 611 (53.5) | 1831 (72.2) | |
| COVID-19 risk perception | (n = 1142) | (n = 2540) | |
| Low risk | 619 (54.2) | 1440 (56.7) | <0.001 |
| Medium risk | 409 (35.8) | 725 (28.5) | |
| High risk | 114 (10.0) | 375 (14.8) | |
| Exposed to COVID-19 positive individual within 14 days of COVID-19 testing | (n = 1141) | (n = 2537) | |
| No | 652 (57.1) | 2222 (87.6) | <0.001 |
| Yes | 234 (20.5) | 270 (10.6) | |
| Don't know | 255 (22.3) | 45 (1.8) | |
| Prior RT-PCR positive for COVID-19 |
(n = 152) 32 (21.0) |
(n = 1219) 278 (22.8) |
0.63 |
RT- PCR = reverse transcriptase–PCR.
Next generation sequencing among cases
Of the 1143 cases, nasal/throat swabs of 708 (62%) (non-vaccinated =521, vaccinated with single dose =127, vaccinated with two doses = 60), with Cyclic threshold (Ct) values below 30 (Range of Ct value for 1143 cases: 11.32 to 36.1) were selected for NGS. SARS-CoV-2 sequences with a genomic coverage of >95% were obtained for 510 (72%) samples (Supplementary Table S1). Of the 510 samples (367 unvaccinated, 93 single dose, and 50 two doses vaccinated individuals), 508 (99.6%) showed the presence of the Delta variant and its sub-lineages, including B.1.617.2 (70%; 67% unvaccinated; 63% single dose; 88% two doses), AY.26 (21%; 22% unvaccinated; 23% single dose; 10% two doses) and others (Supplementary Table S2).
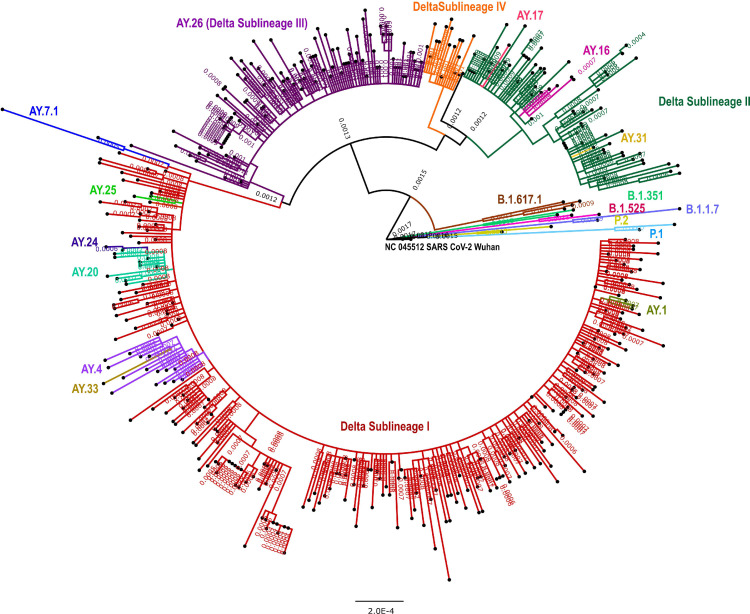
The sequences having >98% SARS-CoV-2 genome coverage (n = 448, including 319 non-vaccinated, 84 one dose, 48 two doses) were used for the generation of a phylogenetic tree along with 12 representative sequences. Four broader sub-lineages of the Delta variant were observed. The majority of the AY pangolin lineages (AY.7.1, AY.25, AY.24, AY.20, AY.4 and AY.33) were grouped in the sub-lineage-I (Figure 1 ). We did not observe any specific differentiation in phylogenetic tree branching between vaccinated and unvaccinated individuals. Amino acid changes by lineages are given in Supplementary Table S3.
Figure 1.
Maximum likelihood tree of the 450 SARS-CoV-2 genomes in this study: A Maximumlikelihood tree of the 448 SARS-CoV-2 sequences retrieved in this study, along with the representative SARS-Cov-2 sequences (n = 12) from different clades. Tamura-Nei model with a bootstrap replication of 1000 cycles was used to assess statistical robustness. Four major sub-lineages of the Delta variant and other pangolin lineages observed in this study are marked on branches in different colors. Sub-lineage-I-IV is marked in red, dark green, violet and orange colors on the nodes. Kappa sequence is marked in brown color. The representative pangolin lineages are also marked on branches in different colors. FigTree v1.4.4 and Inkscape were used to visualize and edit the generated tree.
Vaccine effectiveness
Overall, 94 (8%) cases and 554 (22%) controls reported complete vaccination, 815 (71.3%) cases and 1116 (43.9%) controls were unvaccinated and the remaining 201 (18%) cases and 714 (22%) controls reported partial vaccination (Table 3 ). After adjusting for age, any pre-existing comorbidities, participation in social/religious events, frequency of mask use, and rural/urban residence, the effectiveness of partial vaccination (65%; 95% CI: 57-71%) was significantly lower than complete vaccination (83%; 95% CI: 78-87%). Vaccine effectiveness was highest (94%; 95% CI: 86-97%) for an interval of 6-8 weeks between two doses of either vaccine and was significantly higher than that for <6 weeks (P = 0.003) and ≥12 weeks (P = 0.034) (Table 3).
Table 3.
Effectiveness of COVID-19 vaccines against severe SARS-CoV-2 infection in individuals aged > 45 years by dose and time since vaccination, and interval between two doses, India, May - July 2021.
| Vaccination status | Casesc (%) | Controlsd(%) | Crude odds ratio (95% CI) | Adjusted odds ratioe(95% CI) | Vaccine effectiveness (%) (95% CI) | Power(%) |
|---|---|---|---|---|---|---|
| AZD1222 (Covishield)/ BBV152 (Covaxin) | (n = 1143) | (n = 2541) | ||||
| Unvaccinated | 815 (71.3) | 1116 (43.9) | 1 | 1 | ||
| Partial vaccinationa | 201 (17.6) | 714 (28.1) | 0.34 (0.29-0.41) | 0.35 (0.29-0.43) | 65 (57-71) | 100 |
| Complete vaccinationb | 94 (8.2) | 554 (21.8) | 0.20 (0.16-0.26) | 0.17 (0.13-0.22) | 83 (78-87) | 100 |
| Interval between 2 doses ( ≥14 days after second dose) - AZD1222(Covishield)/ BBV152 (Covaxin) | (n = 909) | (n = 1670) | ||||
| Unvaccinated | 815 (89.7) | 1116 (66.8) | 1 | 1 | ||
| <6 weeks | 75 (8.2) | 383 (22.9) | 0.24 (0.18 - 0.31) | 0.23 (0.17-0.31) | 77 (69 - 83) | 100 |
| 6-8 weeks | 8 (0.9) | 114 (6.8) | 0.08 (0.04 - 0.18) | 0.06 (0.03 - 0.14) | 94 (86-97) | 100 |
| 9-11 weeks | 7 (0.8) | 42 (2.5) | 0.19 (0.09 - 0.44) | 0.15 (0.06 - 0.34) | 85 (66 - 94) | 98.2 |
| ≥12 weeks | 4 (0.4) | 15 (0.9) | 0.28 (0.09 - 0.86) | 0.28 (0.09 - 0.88) | 72 (12-91) | 32.3 |
| AZD1222/Covishield | (n = 1072) | (n = 2263) | ||||
| Unvaccinated | 815 (76.0) | 1116 (49.3) | 1 | 1 | ||
| Partial vaccinationa | 172 (16.0) | 641 (28.3) | 0.32 (0.27-0.4) | 0.33 (0.27-0.41) | 67 (59-73) | 100 |
| Complete vaccinationb | 58 (5.4) | 388 (17.1) | 0.18 (0.13-0.24) | 0.15 (0.11-0.21) | 85 (79-89) | 100 |
| Interval between 2 doses ( ≥14 days after second dose) - AZD1222/Covishield | (n = 873) | (n = 1504) | ||||
| Unvaccinated | 815 (93.4)) | 1116 (74.2) | 1 | 1 | ||
| <6 weeks | 41 (4.7) | 236 (15.7) | 0.21 (0.15 - 0.30) | 0.19 (0.13 - 0.28) | 81 (72 - 87) | 100 |
| 6-8 weeks | 7 (0.8) | 102 (6.8) | 0.08 (0.04 - 0.18) | 0.06 (0.03 -0.14) | 94 (86 - 97) | 100 |
| 9-11 weeks | 6 (0.7) | 36 (2.4) | 0.19 (0.08 - 0.47) | 0.15 (0.06 - 0.37) | 85 (63 - 94) | 95.7 |
| ≥12 weeks | 4 (0.5) | 14 (0.9) | 0.30 (0.10 - 0.93) | 0.30 (0.10 - 0.95) | 70 (5 - 90) | 25.6 |
| BBV152/Covaxin | (n = 886) | (n = 1382) | ||||
| Unvaccinated | 815 (92.0) | 1116 (80.6) | 1 | 1 | ||
| Partial vaccinationa | 29 (3.3) | 71 (5.1) | 0.57 (0.36-0.90) | 0.61 (0.38-0.98) | 39 (2-62) | 72.9 |
| Complete vaccinationb | 36 (4.1) | 156 (11.3) | 0.30 (0.20-0.44) | 0.29 (0.19-0.43) | 71 (57-81) | 100 |
| Interval between 2 doses ( ≥14 days after second dose) - BBV152/Covaxin | (n = 851) | (n = 1272) | ||||
| Unvaccinated | 815 (95.8) | 1116 (87.7) | 1 | 1 | ||
| <6 weeks | 34 (4.0) | 137 (10.8) | 0.33 (0.22-0.49) | 0.33 (0.21-0.50) | 67 (50-79) | 100 |
| 6-8 weeks | 1 (0.1) | 12 (0.9) | 0.11 (0.01-0.85) | 0.07 (0.01-0.66) | 93 (34-99) | 61.4 |
| 9-11 weeks | 1 (0.1) | 6 (0.5) | 0.20 (0.02-1.70) | 0.14 (0.02-1.16) | 86 (0-98) | 15.4 |
| ≥12 weeks | 0 (0.0) | 1 (0.1) | - | - | - | - |
Partial vaccination: One dose with an interval between second dose and COVID-19 testing/hospitalization ≥21 days.
Complete vaccination: Two doses with an interval between second dose and COVID-19 testing/hospitalization ≥14 days.
Case: Laboratory confirmed COVID-19 patients hospitalized with severe COVID-19 (One of the following: fever, cough, dyspnoea, fast breathing plus one of the following: respiratory rate > 30 breaths/min; severe respiratory distress; or SpO2 < 90% on room air).
Control: RT-PCR negative individuals who remained negative up to 7 days after initial RT-PCR test.
Adjusted for age, any pre-existing comorbidities, participation in social/religious events, frequency of mask use, and rural/urban residence.
Vaccine effectiveness of AZD1222/Covishield
Of the 1073 cases and 2264 controls, 6% of cases and 17% of controls reported complete vaccination and 16% of cases, and 28% of controls reported partial vaccination with AZD1222/Covishield. The effectiveness of complete vaccination with AZD1222/Covishield was 85% (95% CI: 79-89%), after adjusting for age, any pre-existing comorbidities, participation in social/religious events, frequency of mask use, and rural/urban residence. It was significantly higher than partial vaccination (67%; 95% CI: 59-73%). VE was highest for an interval of 6-8 weeks for AZD1222/Covishield (94%; 95% CI: 86-97%). VE for an interval of ≥12 weeks was under-powered (Table 3).
Vaccine effectiveness of BBV152/Covaxin
Of 887 cases and 1384 controls, 3.4% cases and 5.3% controls reported complete vaccination, and 16% cases and 28.3% controls reported partial vaccination with BBV152/Covaxin. The effectiveness of complete vaccination with BBV152/Covaxin was 71% (95% CI: 57-81%) after adjusting for age, any pre-existing comorbidities, participation in social/religious events, frequency of mask use, and rural/urban residence. It was significantly higher (P = 0.027) than partial vaccination [39% (95% CI: 2-62%)]. The VE for fully vaccinated individuals with an interval of 6-8 weeks for BBV152/Covaxin was 93% (95% CI: 34-99%). VE for any of the intervals ≥6 weeks was under-powered. (Table 3)
Vaccine effectiveness against the Delta variant and sub-lineages
Vaccine effectiveness against the Delta variant and sub-lineages was 85% (95% CI: 77-90%) for complete dose and 74% (95% CI: 64-81%) for a partial dose of AZD1222/Covishield [P = 0.180], after adjusting for age, any pre-existing comorbidities,participation in social/religious event, frequency of mask use, and rural/urban residence. VE was 66% (95% CI: 42-80%) for complete dose and 41% (95% CI: 0-70%) for vaccination with partial dose of BBV152/Covaxin [P = 0.261]. VE of AZD1222/Covishield against the Delta variant was highest for interval of 6-8 weeks between the two doses (94%, 95% CI: 80-98%) (Table 4 ).
Table 4.
Effectiveness of COVID-19 vaccines against severe SARS-CoV-2 infection by Delta variant in individuals aged >45 years by dose and time since vaccination, and interval between two doses, India, May - July 2021.
| Vaccination status | Casesc (%) | Controlsd(%) | Crude odds ratio (95% CI) | Adjuste dodds ratioe(95% CI) | Vaccine effectiveness (%) (95% CI) | Power(%) |
|---|---|---|---|---|---|---|
| AZD1222 (Covishield)/ BBV152 (Covaxin) | (n = 511) | (n = 2541) | ||||
| Unvaccinated | 368 (72.0) | 1116 (43.9) | 1 | 1 | ||
| Partial vaccinationa | 75 (14.7) | 714 (21.8) | 0.29 (0.22-0.38) | 0.29 (0.22-0.39) | 71 (61-78) | 100 |
| Complete vaccinationb | 46 (9.0) | 554 (21.8) | 0.23 (0.17-0.32) | 0.19 (0.14-0.28) | 81 (72-86) | 100 |
| Interval between 2 doses ( ≥14 days after second dose) of AZD1222 (Covishield)/ BBV152 (Covaxin) | (n = 414) | (n = 1670) | ||||
| Unvaccinated | 368 (88.9) | 1116 (66.8) | 1 | 1 | ||
| <6 weeks | 35 (8.4) | 383 (22.9) | 0.26 (0.18-0.39) | 0.24 (0.16-0.35) | 76 (65-84) | 100 |
| 6-8 weeks | 3 (0.7) | 114 (6.8) | 0.07 (0.02-0.23) | 0.06 (0.02-0.18) | 94 (82-98) | 100 |
| 9-11 weeks | 5 (1.2) | 42 (2.5) | 0.30 (0.12-0.77) | 0.20 (0.08-0.54) | 80 (46-92) | 55.6 |
| ≥12 weeks | 3 (0.7) | 15 (0.9) | 0.45 (0.13-1.62) | 0.41 (0.11-1.49) | 59 (0-89) | 3.4 |
| AZD1222/Covishield | (n = 476) | (n = 2263) | ||||
| Unvaccinated | 368(77.3) | 1116 (49.3) | 1 | 1 | ||
| Partial vaccinationa | 62 (13.0) | 641 (28.3) | 0.26 (0.19-0.35) | 0.26 (0.19-0.36) | 74 (64-81) | 100 |
| Complete vaccinationb | 26(5.5) | 388 (17.2) | 0.19 (0.12-0.29) | 0.15 (0.10-0.23) | 85 (77-90) | 100 |
| Interval between 2 doses( ≥14 days after second dose) of AZD1222/Covishield | (n = 394) | (n = 1504) | ||||
| Unvaccinated | 368 (93.4) | 1116 (74.1) | 1 | 1 | ||
| <6 weeks | 16 (4.1) | 236 (15.7) | 0.20 (0.12-0.34) | 0.17 (0.09-0.29) | 83 (71-91) | 100 |
| 6-8 weeks | 3 (0.8) | 102 (6.8) | 0.08 (0.02-0.26) | 0.06 (0.02-0.20) | 94 (80-98) | 100 |
| 9-11 weeks | 4 (1.0) | 36 (2.4) | 0.28 (0.10-0.81) | 0.19 (0.06-0.57) | 81 (43-94) | 47.3 |
| ≥12 weeks | 3 (0.8) | 14 (0.9) | 0.49 (0.14-1.76) | 0.43 (0.12-1.61) | 67 (0-88) | 1.3 |
| BBV152/Covaxin | (n = 403) | (n = 1384) | ||||
| Unvaccinated | 368 (91.3) | 1116 (80.6) | 1 | 1 | ||
| Partial vaccinationa | 13 (3.2) | 73 (5.3) | 0.60 (0.32-1.13) | 0.59 (0.30-1.13) | 41 (0-70) | 46.7 |
| Complete vaccinationb | 20 (5.0) | 156 (11.3) | 0.37 (0.22-0.61) | 0.34 (0.20-0.58) | 66 (42-80) | 99.3 |
| Interval between 2 doses ( ≥14 days after second dose) of BBV152/Covaxin | (n = 388) | (n=1272) | ||||
| Unvaccinated | 368 (94.8) | 1116 (87.7) | 1 | 1 | ||
| <6 weeks | 19 (4.9) | 137 (10.8) | 0.42 (0.25-0.70) | 0.40 (0.23-0.69) | 60 (31-77) | 96.2 |
| 6-8 weeks | 0 (0.0) | 12 (0.9) | - | - | - | - |
| 9-11 weeks | 1 (0.3) | 6 (0.5) | 0.41 (0.05-3.43) | 0.28 (0.03-2.35) | 72 (0-97) | 3.7 |
| ≥12 weeks | 0 (0.0) | 1 (0.1) | - | - | - | - |
Partial vaccination: One dose with an interval between second dose and COVID-19 testing/hospitalization ≥21 days.
Complete vaccination: Two doses with an interval between second dose and COVID-19 testing/hospitalization ≥14 days.
Case: Laboratory confirmed COVID-19 patients hospitalized with severe COVID-19 (One of the following: fever, cough, dyspnoea, fast breathing plus one of the following: respiratory rate > 30 breaths/min; severe respiratory distress; or SpO2 < 90% on room air) infected with Delta variant.
Control: RT-PCR negative individuals who remained negative up to 7 days after initial RT-PCR test.
Adjusted for age, any pre-existing comorbidities, participation in social/religious events, frequency of mask use, and rural/urban residence.
Discussion
The results from this large multi-centric study indicate that both AZD1222/Covishield and BBV152/Covaxin significantly reduced the risk of severe COVID-19 and against the Delta variant among the Indian population aged 45 years and above, more so with two doses, albeit higher for AZD1222/Covishield than BBV152/Covaxin. The highest reduction in risk of severe COVID-19 was documented for 6-8 week intervals between the two doses.
Our vaccine effectiveness estimates were expected to be lower than the efficacy estimates from vaccine trials. BBV152/Covaxin was introduced based on evidence of high safety, tolerability, and immune responses among the Indian participants ( Ella et al., 2021a, 2021b). Subsequently, the efficacy in the phase III trial was estimated to be 93% against severe COVID-19 (Ella et al., 2021c). Real-world estimates of VE should be considered in the context of programmatic issues such as storage and cold chain maintenance and off-schedule and incomplete delivery of doses (World Health Organization, 2021). Besides, the field performance of vaccines was influenced by newly emerging SARS-CoV-2 variants. The VE of two doses of Oxford-ChAdOx1-S against severe infection with the Delta variant in the United Kingdom was 67% (Lopez Bernal et al., 2021). Indian studies indicated reduced neutralization capability of BBV152/Covaxin and AZD1222/Covishield vaccine against the Delta variant (Sapkal et al., 2021; Yadav et al., 2021). Our study was conducted when B.1.617 lineages dominated India (INSACOG, 2021).
Our results are consistent with the VE reports of either AZD1222/Covishield or BBV152/Covaxin against hospitalization to be 77% for complete and 70% for partial vaccination among healthcare workers in South India (Victor et al., 2021). Our VE estimates for AZD1222/Covishield against severe disease are higher than 67% (95% CI: 44-81%) for moderate to severe disease and 76% (95% CI: 37-89%) for supplemental oxygen therapy among a cohort study of hospital employees in New Delhi (Satwik et al., 2021). The disparity could be on account of study design, different outcome measures between the two studies, and the fact that hospital employees, although at higher risk for SARS-CoV-2 infection, are also more likely to be vaccinated.
The interval between the two doses can also influence the VE in programmatic conditions. We estimated maximum effectiveness for a 6-8 week interval for AZD1222/Covishield, with a gradual decline beyond 12 weeks. Unlike our observation, other published studies of Oxford-ChAdOx1-S indicated higher efficacy or immunogenicity for >12 weeks intervals between two vaccine doses (Flaxman et al., 2021; Voysey et al., 2021). During the high transmission period when we conducted this study, asymptomatic or known SARS-CoV-2 infection a few weeks before the first or second dose could have boosted the vaccine-induced immunogenicity manifesting as improved vaccine performance at a shorter interval. Our finding has programmatic implications for rolling out the vaccination to reach the maximum eligible Indian population at the earliest, where after an initial interval of 4 weeks for both BBV152/Covaxin and AZD1222/Covishield, the interval was increased to 12-16 weeks for AZD1222/Covishield (Perappadan, 2021). The policy on the interval between the two doses varies across countries and needs further evidence for its standardization (Bobdey et al., 2021).
We highlight the strengths of our study. The study sites included 11 hospitals spread across all parts of the country, thereby strengthening the generalizability of VE estimates. We used a robust case-control study design and an approach to multiple logistic regression guided by the application of a causal framework using directed acyclic graphs to identify and adjust for appropriate known and measured confounders. Further, we used a random effects model for multiple logistic regression to account for the variation in VE by hospital sites. Cases and controls in each study site were recruited from the same hospital. Hence, they are likely to belong to the same catchment area of the particular hospital and come from the same source population. This minimizes the chance of selection bias influencing VE estimates. Although we could not achieve the desired sample size, our study had adequate power to estimate VE for both vaccines separately. Such power could be attributed to substantially higher vaccine coverage than the assumed coverage for calculating the sample size. Molecular characterization of SARS-CoV-2 enabled us to estimate the VE specifically for the Delta variant and its sub-lineages.
Our study had several limitations. Firstly, misclassification bias could have affected the VE estimates in several ways. (1) It is possible that vaccinated individuals could have had a higher risk of COVID-19 due to (a) potential transmission during travel for vaccination, (b) crowding at the vaccination centers, and (c) risky behaviors post-vaccination due to the self-perceived vaccine protection. This could have led to an underestimation of the VE. (2) Low sensitivity or specificity of RT-PCR testing and asymptomatic status of COVID-19 could have led to differential misclassification of the control status (Tahamtan and Ardebili, 2020). To reduce such bias, we confirmed the RT-PCR status among negative controls after a week. However, we could not confirm all the negative controls. Hence, we could have underestimated the VE. (3) The status of IgG used as a surrogate marker for recent infection, though done, could not differentiate the antibodies generated by the vaccine or the infection. As a result, we could have misclassified the infection status of those infected before joining the study but did not test positive at the time of enrolment, thus leading to the underestimation of VE. (4) We anticipated differential misclassification about the interval between two doses of vaccine, as cases might have recalled vaccination dates better than controls- thereby causing a biased estimate of VE by dosing interval in either direction. (5) The vaccination status, including dates thereof, was based on recall by nearly half of the study participants, and more so among the cases than the controls. This could have led to differential misclassification in vaccination status resulting in VE estimate biased in either direction. The sensitivity analysis of VE estimate by mode of ascertainment of vaccination status, record vs recall, showed a difference in VE for partial vaccination with AZD1222/Covishield and complete vaccination with BBV152/Covaxin (Supplementary Table S4). Secondly, a prior SARS-CoV-2 infection and conferred immunity thereof, could have influenced the VE estimates (Lipsitch et al., 2020). Such prior infections could reduce the chance of re-infection. While being aware of a prior infection can reduce the likelihood of vaccination, unknown prior infections (e.g., asymptomatic) are unlikely to have influenced the decision to vaccinate. Although the bias in estimating VE due to unknown prior infections is reported to be minimal in case-control studies, it is unknown whether this could be true during high transmission periods (World Health Organization, 2021). Thirdly, the sample size could have influenced the VE estimates. Four-fifth of the study participants had received AZD1222/Covishield hence rendering the recruited study size for BBV152/Covaxin inadequate for VE estimates. Estimation of VE for an interval of more than 9 weeks was underpowered and hence needs to be interpreted cautiously. Further, it did not allow us to statistically compare VE across intervals. WHO suggests that any useful COVID-19 vaccine should have VE estimates with a lower bound of the 95% CI above 50% (World Health Organization, 2021). Fourthly, although we adjusted the odds ratio for known and relevant confounders identified based on causal framework analysis, the bias-indicator measuring VE among recently vaccinated individuals (within 6 and 14 days after one dose; results not shown) indicates that our adjusted odds ratio and the resulting VE estimates may remain biased due to unknown, unmeasured and/or residual confounding (Hitchings et al., 2022). However, with moderate to high background seroprevalence, hitherto unknown prior infections may result in protection from a single dose of vaccine during a shorter period post-vaccination when compared to the efficacy trial results (Saadat et al., 2021). Finally, the external validity of the VE findings beyond 45 years needs to be considered cautiously. The younger adults (18-44 years) have recently become eligible for vaccination. However, we expect similar VE results in the younger age group as well.
Our findings highlight significant real-world protection with two vaccine doses against severe COVID-19 and specifically against the currently dominant Delta variant in India. The substantial effectiveness of only one dose, more so for AZD1222/Covishield, supports the policy decision from a public health perspective to initially maximize coverage with a single -dose in the country. Our study's finding for the effectiveness of a single dose of AZD1222/Covishield is in contrast with other studies that report either a lack of effectiveness (Satwik et al., 2021) or significant but lower effectiveness (Victor et al., 2021). This could be due to differences in study design, including a difference in the primary outcome measure of disease severity and potential difference in background seroprevalence among the study population of the studies referenced above. Vaccine effectiveness below 100% suggests the possibility of severe disease among vaccinated and thus further likely transmission in the absence of adequate control measures (Lee et al., 2022; Pritchard et al., 2021).
Our finding of high variation in the Delta lineage across the country suggests fast mutations in Delta due to its immune escape ability in the host genome. Further, it has been suggested that the dominance of the Delta variant and its sub-lineages with higher transmissibility and potential for immune escape can make achieving robust protection against infection even with near universal vaccination coverage much more difficult. (Gupta et al., 2021; Lazarevic et al., 2021) Nevertheless, acceleration of the two-dose vaccination coverage can be critical for an effective and timely reduction in the burden of severe COVID-19 in India.
Conflict of interest
The authors have no competing interests to declare.
Funding statement
This study was funded by the Indian Council of Medical Research, New Delhi, India.
Ethics statement
We obtained written informed consent from all the participants or their legally authorized representatives. Study procedures were approved by the Institutional Human Ethics Committees of all participating institutions.
Acknowledgments
The authors acknowledge the contributions of all the study participants, and the clinical and laboratory staff from all the collaborating institutions. We acknowledge Michaelraj E, Amanda Rozario GA, Gayathri K, Fathima Shireen, and Mohana Balan Parivallal for their support in data management. We appreciate the technical inputs towards the design and implementation of the study provided by the members of the Epidemiology and Surveillance Working Group of the ICMR, constituted by the COVID-19 National Task Force of the Government of India and the WHO India team.
CRediT authorship contribution statement
Tarun Bhatnagar: Conceptualization, Methodology, Formal analysis, Writing – original draft, Writing – review & editing, Project administration, Funding acquisition. Sirshendu Chaudhuri: Conceptualization, Methodology, Validation, Formal analysis, Data curation, Writing – original draft. Manickam Ponnaiah: Conceptualization, Methodology, Writing – review & editing, Project administration. Pragya D Yadav: Conceptualization, Methodology, Writing – original draft, Writing – review & editing, Project administration, Funding acquisition. R Sabarinathan: Software, Formal analysis, Data curation, Writing – review & editing. Rima R Sahay: Validation, Investigation, Resources, Supervision, Project administration, Writing – review & editing. Faheem Ahmed: Validation, Supervision, Project administration, Writing – review & editing. S Aswathy: Validation, Supervision, Project administration, Writing – review & editing. Pankaj Bhardwaj: Validation, Supervision, Project administration, Writing – review & editing. Anil Bilimale: Validation, Supervision, Project administration, Writing – review & editing. M Santhosh Kumar: Software, Validation, Resources, Writing – review & editing, Supervision. M. Logaraj: Validation, Supervision, Project administration, Writing – review & editing. Uday Narlawar: Validation, Supervision, Project administration, Writing – review & editing. C Palanivel: Validation, Supervision, Project administration, Writing – review & editing. Prakash Patel: Validation, Supervision, Project administration, Writing – review & editing. Sanjay K Rai: Validation, Supervision, Project administration, Writing – review & editing. Vartika Saxena: Validation, Supervision, Project administration, Writing – review & editing. Arvind Singh: Validation, Supervision, Project administration, Writing – review & editing. Jeromie WV Thangaraj: Software, Validation, Resources, Writing – review & editing, Supervision. Ashwini Agarwal: Investigation, Resources, Writing – review & editing. Yasir Alvi: Investigation, Resources, Writing – review & editing. : Investigation, Resources, Writing – review & editing. P Ashok: Investigation, Resources, Writing – review & editing. Dinesh Babu: Investigation, Resources, Writing – review & editing. Yogesh Bahurupi: Investigation, Resources, Writing – review & editing. Sangita Bhalavi: Investigation, Resources, Writing – review & editing. Priyamadhaba Behera: Investigation, Resources, Writing – review & editing. Priyanka Pandit Biswas: Investigation, Resources, Writing – review & editing. Jaykaran Charan: Investigation, Resources, Writing – review & editing. Nishant Kumar Chauhan: Investigation, Resources, Writing – review & editing. KB Chetak: Investigation, Resources, Writing – review & editing. Lalit Dar: Investigation, Resources, Writing – review & editing. Ayan Das: Investigation, Resources, Writing – review & editing. R Deepashree: Investigation, Resources, Writing – review & editing. Minakshi Dhar: Investigation, Resources, Writing – review & editing. Rahul Dhodapkar: Investigation, Resources, Writing – review & editing. TS Dipu: Investigation, Resources, Writing – review & editing. Mridu Dudeja: Investigation, Resources, Writing – review & editing. Manisha Dudhmal: Investigation, Resources, Writing – review & editing. Ravisekhar Gadepalli: Investigation, Resources, Writing – review & editing. Mahendra Kumar Garg: Investigation, Resources, Writing – review & editing. AV Gayathri: Investigation, Resources, Writing – review & editing. Akhil Dhanesh Goel: Investigation, Resources, Writing – review & editing. H Basavana Gowdappa: Investigation, Resources, Writing – review & editing. Randeep Guleria: Validation, Writing – review & editing, Supervision. Manoj Kumar Gupta: Investigation, Resources, Writing – review & editing. Farzana Islam: Investigation, Resources, Writing – review & editing. Mannu Jain: Investigation, Resources, Writing – review & editing. Vineet Jain: Investigation, Resources, Writing – review & editing. M Lanord Stanley Jawahar: Investigation, Resources, Writing – review & editing. Rajendra Joshi: Investigation, Resources, Writing – review & editing. Shashi Kant: Validation, Writing – review & editing, Supervision. Sitanshu Sekhar Kar: Validation, Writing – review & editing, Supervision. Deepjyoti Kalita: Investigation, Resources, Writing – review & editing. Meenakshi Khapre: Investigation, Resources, Writing – review & editing. Satyendra Khichar: Investigation, Resources, Writing – review & editing. Sarika Prabhakar Kombade: Investigation, Resources, Writing – review & editing. Sunil Kohli: Investigation, Resources, Writing – review & editing. Abhinendra Kumar: Investigation, Resources, Writing – review & editing. Anil Kumar: Investigation, Resources, Writing – review & editing. Deepak Kumar: Investigation, Resources, Writing – review & editing. Kiran G Kulirankal: Investigation, Resources, Writing – review & editing. KV Leela: Investigation, Resources, Writing – review & editing. Triparna Majumdar: Investigation, Resources, Writing – review & editing. Baijayantimala Mishra: Investigation, Resources, Writing – review & editing. Puneet Misra: Validation, Writing – review & editing, Supervision. Sanjeev Misra: Investigation, Resources, Writing – review & editing. Prasanta Raghab Mohapatra: Investigation, Resources, Writing – review & editing. M Narayana Murthy: Investigation, Resources, Writing – review & editing. Dimpal A Nyayanit: Investigation, Resources, Writing – review & editing. Manish Patel: Investigation, Resources, Writing – review & editing. Monika Pathania: Investigation, Resources, Writing – review & editing. Savita Patil: Investigation, Resources, Writing – review & editing. Binod Kumar Patro: Investigation, Resources, Writing – review & editing. Ramniwas Jalandra: Investigation, Resources, Writing – review & editing. Pragati Rathod: Investigation, Resources, Writing – review & editing. Naimesh Shah: Investigation, Resources, Writing – review & editing. Anita Shete: Investigation, Resources, Writing – review & editing. Deepak Shukla: Investigation, Resources, Writing – review & editing. M Shwethashree: Investigation, Resources, Writing – review & editing. Smita Sinha: Investigation, Resources, Writing – review & editing. MN Sumana: . Ashish Surana: Investigation, Resources, Writing – review & editing. Anjan Trikha: Investigation, Resources, Writing – review & editing. A Tejashree: Investigation, Resources, Writing – review & editing. Mahalingam Venkateshan: Investigation, Resources, Writing – review & editing. G Vijaykrishnan: Investigation, Resources, Writing – review & editing. Sarita Wadhava: Investigation, Resources, Writing – review & editing. Naveet Wig: Validation, Writing – review & editing, Supervision. Nivedita Gupta: Methodology, Resources, Project administration, Writing – review & editing. Priya Abraham: Validation, Writing – review & editing, Supervision. Manoj V Murhekar: Conceptualization, Methodology, Formal analysis, Writing – review & editing, Project administration, Funding acquisition.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.07.033.
Appendix. Supplementary materials
References
- Afifi A, Clark V, May S. 4th ed. Chapman & Hall/CRC; Boca Raton, FL: 2004. Regression analysis with multicollinearity. [Google Scholar]
- Bobdey S, Kaushik SK, Menon AS. The conundrum of two-dose interval of ChAdOx1 nCOV-19 corona virus vaccine: way ahead. Med J Armed Forces India. 2021;77:S250–S253. doi: 10.1016/j.mjafi.2021.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ella R, Reddy S, Blackwelder W, Potdar V, Yadav P, Sarangi V, et al. Efficacy, safety, and lot to lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): a, double-blind, randomised, controlled phase 3 trial. Lancet. 2021;398:2173–2184. doi: 10.1016/S0140-6736(21)02000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ella R, Reddy S, Jogdand H, Sarangi V, Ganneru B, Prasad S, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect Dis. 2021;21:950–961. doi: 10.1016/S1473-3099(21)00070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ella R, Vadrevu KM, Jogdand H, Prasad S, Reddy S, Sarangi V, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect Dis. 2021;21:637–646. doi: 10.1016/S1473-3099(20)30942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaxman A, Marchevsky NG, Jenkin D, Aboagye J, Aley PK, Angus B, et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002) Lancet. 2021;398:981–990. doi: 10.1016/S0140-6736(21)01699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Shankar S, Chatterjee K, Chatterjee K, Yadav AK, Pandya K, Suryam V, Agrawal S, Ray S, Phutane V, Datta R. COVISHIELD (AZD1222) VaccINe effectiveness among healthcare and frontline Workers of Indian Armed Forces: interim results of VIN-WIN cohort study. Med J Armed Forces India. 2021;77:S264–S270. doi: 10.1016/j.mjafi.2021.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Kaur H, Yadav PD, Mukhopadhyay L, Sahay RR, Kumar A, et al. Clinical characterization and genomic analysis of samples from COVID-19 breakthrough infections during the second wave among the various states of India. Viruses. 2021;13:1782. doi: 10.3390/v13091782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchings MDT, Lewnard JA, Dean NE, Ko AI, Ranzani OT, Andrews JR, et al. Use of recently vaccinated individuals to detect bias in test-negative case-control studies of COVID-19 vaccine effectiveness. Epidemiology. 2022;33:450–456. doi: 10.1097/EDE.0000000000001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INSACOG [bulletin], New Delhi: Department of Biotechnology, Ministry of Science and Technology, Government of India, 2021 https://dbtindia.gov.in/sites/default/files/INSACOG%20WEEKLY%20BULLETIN%20June%2030.pdf. (accesed on 1 November 2021).
- Jaiswal A, Subbaraj V, Vivian Thangaraj JW, et al. COVID-19 vaccine effectiveness in preventing deaths among high-risk sgroups in Tamil Nadu. India. Indian J Med Res. 2021;153:689–691. doi: 10.4103/ijmr.ijmr_1671_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarevic I, Pravica V, Miljanovic D, Cupic M. Immune evasion of SARS-CoV-2 emerging variants: what have we learnt so far? Viruses. 2021;13:1192. doi: 10.3390/v13071192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LYW, Rozmanowski S, Pang M, Charlett A, Anderson C, Hughes GJ, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infectivity by viral load, S Gene variants and demographic factors, and the utility of lateral flow devices to prevent transmission. Clin Infect Dis. 2022;74:407–415. doi: 10.1093/cid/ciab421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsitch M, Kahn R, Mina MJ. Antibody testing will enhance the power and accuracy of COVID-19-prevention trials. Nat Med. 2020;26:818–819. doi: 10.1038/s41591-020-0887-3. [DOI] [PubMed] [Google Scholar]
- Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health and Family Welfare. Co-WIN Dashboard, https://dashboard.cowin.gov.in/, 2021b (accessed 20 October 2021).
- Ministry of Health and Family Welfare . Government of India; New Delhi: 2021. Frequently Asked Questions – COVID vaccination.https://www.mohfw.gov.in/covid_vaccination/vaccination/faqs.html accessed 17 September 2021. [Google Scholar]
- O'Neill RT. On sample sizes to estimate the protective efficacy of a vaccine. Stat Med. 1988;7:1279–1288. doi: 10.1002/sim.4780071208. [DOI] [PubMed] [Google Scholar]
- Perappadan BS. The Hindu; 2021. Gap between two doses of Covishield extended to 12–16 weeks, says government. [Google Scholar]
- Pritchard E, Matthews PC, Stoesser N, Eyre DW, Gethings O, Vihta KD, et al. Impact of vaccination on new SARS-CoV-2 infections in the United Kingdom. Nat Med. 2021;27:1370–1378. doi: 10.1038/s41591-021-01410-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadat S, Rikhtegaran Tehrani Z, Logue J, Newman M, Frieman MB, Harris AD, et al. Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS-CoV-2. JAMA. 2021;325:1467–1469. doi: 10.1001/jama.2021.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkal GN, Yadav PD, Sahay RR, Deshpande G, Gupta N, Nyayanit DA, et al. Neutralization of Delta variant with sera of CovishieldTM vaccinees and COVID-19-recovered vaccinated individuals. J Travel Med. 2021;28:taab119. doi: 10.1093/jtm/taab119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satwik R, Satwik A, Katoch S, Saluja S. ChAdOx1 nCoV-19 effectiveness during an unprecedented surge in SARS COV-2 infections. Eur J Intern Med. 2021;93:112–113. doi: 10.1016/j.ejim.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serum Institute of India . Serum Institute of India; 2021. Product insert: ChAdOx1 nCoV- 19 corona virus vaccine (recombinant) – COVISHIELD.https://www.seruminstitute.com/pdf/covishield_ChAdOx1_nCoV19_corona_virus_vaccine_insert.pdf [Accessed 2 June 2022] [Google Scholar]
- Tahamtan A, Ardebili A. Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert Rev Mol Diagn. 2020;20:453–454. doi: 10.1080/14737159.2020.1757437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Hindu. India approves COVID-19 vaccines Covishield and Covaxin for emergency use. 2021 Jan 3. https://www.thehindu.com/news/national/drug-controller-general-approves-covishield-and-covaxin-in-india-for-emergency-use/article33485539.ece, 2021 (accessed 17 September 2021).
- Victor PJ, Mathews KP, Paul H, Mammen JJ, Murugesan M. Protective effect of COVID-19 vaccine among health care workers during the second wave of the pandemic in Inda. Mayo Clin Proc. 2021;96:2493–2494. doi: 10.1016/j.mayocp.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397:881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westreich D, Greenland S. The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol. 2013;177:292–298. doi: 10.1093/aje/kws412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Clinical management of COVID-19. Interm Guid. 2020. https://www.who.int/teams/health-care-readiness/covid-19 (accessed 17 September 2021).
- World Health Organization. Evaluation of COVID-19 vaccine effectiveness. Interim Guidance 2021. https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-vaccine_effectiveness-measurement-2021.1, 2021 (accessed 17 September 2021).
- Yadav PD, Sapkal GN, Ella R, et al. Neutralization of Beta and Delta variant with sera of COVID-19 recovered cases and vaccinees of inactivated COVID-19 vaccine BBV152/Covaxin. J Travel Med. 2021;28:taab104. doi: 10.1093/jtm/taab104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.