Abstract
Coronavirus disease 2019 (COVID-19), caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) continues to impact our lives by causing widespread illness and death and poses a threat due to the possibility of emerging strains. SARS-CoV-2 targets angiotensin-converting enzyme 2 (ACE2) before entering vital organs of the body, including the brain. Studies have shown systemic inflammation, cellular senescence, and viral toxicity-mediated multi-organ failure occur during infectious periods. However, prognostic investigations suggest that both acute and long-term neurological complications, including predisposition to irreversible neurodegenerative diseases, can be a serious concern for COVID-19 survivors, especially the elderly population. As emerging studies reveal sites of SARS-CoV-2 infection in different parts of the brain, potential causes of chronic lesions including cerebral and deep-brain microbleeds and the likelihood of developing stroke-like pathologies increases, with critical long-term consequences, particularly for individuals with neuropathological and/or age-associated comorbid conditions. Our recent studies linking the blood degradation products to genome instability, leading to cellular senescence and ferroptosis, raise the possibility of similar neurovascular events as a result of SARS-CoV-2 infection. In this review, we discuss the neuropathological consequences of SARS-CoV-2 infection in COVID survivors, focusing on possible hemorrhagic damage in brain cells, its association to aging, and the future directions in developing mechanism-guided therapeutic strategies.
Keywords: SARS-CoV-2, COVID-19, Coronavirus, Brain fog, Hemorrhage, Senescence, Ferroptosis, Genome instability
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by the zoonotic SARS-CoV-2 infection, has passed a death toll of 5.49 million worldwide, with over 307 million total cases, according to the most recent update on COVID-19 (https://ourworldindata.org/coronavirus-data) COVID-19 has profoundly affected people of all ages, sexes, geographical locations, ethnicities, and religions. Based on the latest statistics of COVID-19 victims, the elderly and individuals with underlying chronic comorbid conditions, such as diabetes, hypertension, respiratory/cardiovascular disorders, and kidney injuries, or following treatment with immunosuppressant medications, are at the highest risk for COVID-19-associated severe complications (Bailly et al., 2021, de Almeida-Pititto et al., 2020, Salazar, 2021, Flythe et al., 2021, Vahidy et al., 2021). As we learn more about continuously evolving SARS-CoV-2 viral variants, and their infection routes and long-term implications on human health, it is becoming obvious that viral infection in different brain regions, including deeper neuroanatomical sites, precedes the clinical manifestations of other non-neurological symptoms, such as respiratory distress, cardiac arrest, gastrointestinal discomforts, and kidney injury (Taquet et al., 2021a, Taquet et al., 2021b, Prescott, 2021, Nalbandian et al., 2021). Loss of smell and taste in COVID-19 has been linked to neuronal damage in the pre-frontal cortex and olfactory bulb areas, recovery from which takes several months after the initial COVID-19 infection (de Melo et al., 2021, Renaud et al., 2021). Emerging studies have highlighted unique patterns of brain injury involving microhemorrhages and multi-infarcts in the brains of both COVID-19 victims and survivors, using neuroimaging techniques (Fitsiori et al., 2020, Dixon et al., 2020). Notably, the pathological hallmarks identified after SARS-CoV-2 infection overlap with those observed after several fatal neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), and stroke (Yu et al., 2021, Sulzer et al., 2020, Modin et al., 2020). Chronic cardiovascular, neuropathological, and neuropsychiatric disturbances have been observed in the majority of COVID-19 survivors, especially those with comorbid diseases (Han et al., 2021, Nakamura et al., 2021). In this review, we critically discuss the implications of SARS-CoV-2-induced neuropathological events with a focus on the potential mechanistic pathways involved. Finally, based on our recent findings in hemorrhagic stroke pathology, we propose a possible therapeutic strategy to ameliorate chronic neurodegenerative and neuropsychiatric symptoms.
1.1. The first known encounter with coronavirus
The first documented appearance of coronavirus occurred in Germany in 1912, when veterinarians were baffled with the symptoms of a heavily swollen belly with febrile temperature in cats. Although medical practitioners were not aware of coronavirus at that time, their observed symptoms were very similar to those of today’s coronavirus infections in humans, bronchitis in chickens, and intestinal disease in pigs that kill all newborn piglets. In the late 1960 s, researchers first isolated the viral strain from adult patients, who presented with common cold symptoms. Tyrrell and Bynoe (1966) then showed that the isolated viral strain, named B814, could be serially passaged and cause infection when inoculated intranasally in human volunteers (Tyrrell et al., 1966). At about the same time, Hamre and Procknow isolated another strain, named 229E, from the respiratory tracts of medical students with cold symptoms (Hamre and Procknow, 1966). Both strains were sensitive to ether, and required a lipid coat for their virulence, furthermore, these viruses were not related to the known strains of myxo- or paramyxoviruses. While the actual identification of these viral strains was not possible then, McIntosh et al., 1967a, McIntosh et al., 1967b reported the discovery of a very similar strain recovered from tracheal organ cultures, which they named “OC”, an acronym of organ culture (McIntosh et al., 1967a, McIntosh et al., 1967b). Almeida and Tyrrell (1967) subsequently showed that electron microscopic structures of B814 and chicken bronchitis virus were very similar (Almeida et al., 1967). Subsequently, several other animal viruses, including infectious bronchitis virus, swine transmissible gastroenteritis virus, swine enteric diarrhea virus, mouse hepatitis virus, and the newly identified swine acute diarrhea syndrome coronavirus, were shown to have similar morphologies (Lin et al., 2016, McIntosh et al., 1967a, McIntosh et al., 1967b, Witte et al., 1968, Zhou et al., 2018), and this new group was named “coronaviruses”, based on the feature that these virus particles were covered with widely spaced, club-shaped surface projections resembling a crown-like structure (Tyrrell et al., 1975), which were further sub-classified. Alpha and beta coronaviruses usually infect mammals, while gamma and delta types infect birds. However, the viruses in some instances can cross-infect mammals (Woo et al., 2012).
1.2. The pathogenicity of positive-sense RNA viruses
The majority of the RNA virus sub-types, which infect humans, are zoonotic in nature, indicating that they can cross-infect between vertebrates and humans. Even many of these RNA viruses, not considered zoonotic earlier, might have undergone evolutionary adaptations to be the zoonotic variant (ME et al., 2013). Being one of the fatal zoonotic viruses, the coronavirus exhibits a relatively larger (80–150 nm in diameter) virion size than most other RNA viruses, having approximately 30 kb in the genome (> three times larger than that of HIV and hepatitis C virus and twice that of influenza virus). Importantly, unlike most other RNA viruses, coronaviruses encode RNA-proofreading enzymes, which may explain the lack of activity of the most common antiviral nucleoside analogs (like ribavirin) after coronavirus infection (Eyer et al., 2018, Tong et al., 2020). Additionally, the proofreading activity enables the virus to survive in extreme environments by preventing self-weakening or inactivating mutations (Robson et al., 2020). The critical factor behind the rapid evolution of this deadly virus is its dynamic recombination and swapping large segments of its RNA genome. This mechanism acts as a “double-edged sword” because it produces more contagious novel variants by swapping genomic segments between two distantly related viral species when they are in the same host cell and enables the new variant to avoid the immune defense generated against the original infection. It is worth mentioning in this context, that positive-sense RNA viruses recombine at a much faster rate than negative-sense RNA viruses (Simon-Loriere and Holmes, 2011). Furthermore, efficient genome recombination enables coronaviruses to expand the range of species for crossover infectivity (Oude Munnink et al., 2021, Calvet et al., 2021).
1.3. The evolution of SARS-CoV-2
Coronaviruses likely appeared in the biosphere some 10,000–300 million years ago, leading to the generation of dozens of strains through evolution. Some of these strains are highly pathogenic (Graham et al., 2013). Among the seven strains that infect humans, four cause mild upper respiratory tract and common cold symptoms, while the other three strains, namely, severe acute respiratory syndrome coronavirus (SARS-CoV), middle east respiratory syndrome coronavirus (MERS-CoV), and the emerging SARS-CoV-2 cause deadly infections and high mortality. Regarding the origins of coronavirus, bats and rodents have played critical roles as natural reservoirs for viral evolution and spillover to humans. Notably, strains 229E and NL63 associated with common colds, as well as MERS, SARS, and SARS-CoV-2, are hosted by bats, while strains OC43 and HKU1 (associated with mild cold symptoms) are of rodent origin (Su et al., 2016, Forni et al., 2017). It appears interspecies transfer of coronavirus from the bats to humans does not occur directly, rather requires an intermediary vector that transfers the virus to humans. The civet cat, sold in the live animal market of Guangdong Province in China could be such an intermediate vector for SARS-CoV infection in humans (Li et al., 2005, Hu et al., 2017). SARS-related coronaviruses (SARSr-CoV), discovered in the remote bat cave in Yunnan Province in China, were found to have 96% genetic similarity with the human SARS-CoV. These strains have high similarity between the receptor-binding domain (RBD) and hypervariable N-terminal domain (NTD) of the S1 gene (which encodes viral spike protein subunit 1), as well as in the ORF3b and ORF8 (a and b) regions (Hu et al., 2017).
Why outbreaks of coronavirus involving bats as the primary host are so lethal to humans, and yet the bat population remains unaffected is an open question. Studies have shown that bats are known to be immunologically resistant to deadly viruses such as CoV, which are highly virulent for non-volant terrestrial mammals (Schountz et al., 2017). Bats have evolutionarily developed a unique mechanism to control their inflammation-induced DNA damage. Transcriptomics analyses in bat cell lines have shown constitutive expressions of interferon (IFN) alpha-inducing IFN-targeted genes involving an antiviral defense mechanism (Zhou et al., 2016). Moreover, other studies have shown that dampened NLRP3 and STING-mediated inflammasome activations are keys to their longevity and natural viral reservoir status (Ahn et al., 2019, Xie et al., 2018, Pavlovich et al., 2018). Brook et al. (2020) reported that when invading viruses face constitutive immune activation in bat cells, they become more virulent, while infecting their secondary hosts who have completely different immune systems than their own system (Brook et al., 2020). It is important to note that the receptor-binding domain (RBD) of the S1 protein of SARS-CoV-2 is significantly different in terms of structural features compared to that of its bat counterpart, RaTG13 (Zhang et al., 2020).
As previously mentioned, identification of the intermediate host is critical for controlling viral spillover from bats to humans. By metagenomic analysis of infected pangolin lung samples, Liu et al. (2019) identified the Malayan pangolin as the intermediate host for CoV and Sendai virus (Liu et al., 2019). Subsequently, Zhang et al. found that pangolin CoV had 91.02% and 90.55% genomic similarities with SARS-CoV-2 (COVID-19) and bat CoV RaTG13, respectively (Zhang et al., 2020). Moreover, pangolin CoV and COVID-19 strains have five identical key amino acids in the S1 protein, which binds to the human angiotensin-converting enzyme 2 (ACE2) receptor for viral entry. The most concerning observation were that, unlike pangolin CoV and bat CoV, SARS-CoV-2 retained the S1/S2 furin cleavage site (Zhang et al., 2020). Because the furin enzyme is abundant in the human respiratory tract and other parts of the body, SARS-CoV-2 uses it to jump across species (Andersen et al., 2020). Furin is also required for cleavage-mediated activation of several important proteins in the brain, e.g., brain-derived neurotrophic factor (BDNF) and neurotrophin 3 (NT3), which are essential for neuronal maturation and synaptic plasticity (Seidah et al., 1996, Koshimizu et al., 2009). These findings may suggest the underlying mechanism of SARS-CoV-2 infection in human brain cells. Recent sequence data indicate that the most recent ancestor of SARS-CoV-2 emerged between late November 2019 and early December 2019 (http://virological.org/t/356). Therefore, there could be a long unrecognized transmission period when the virus might have transmitted from zoonotic animals to humans and acquired the polybasic furin cleavage site at S1-S2 junctions (Andersen et al., 2020). This hypothesis is partially supported by the observation that the lineage of the A SARS-CoV HKU1 strain has furin recognition sites and predicted O-linked glycan sites (Chan et al., 2008).
Furthermore, genomic and phylogenetic analyses indicated that the SARS-CoV-2’s RBD motif for binding the ACE2 receptor did not originate from RNA recombination. Rather, it may be an ancestral trait shared by bat CoV (Boni et al., 2020). They found that the SARS-CoV-2 lineage might have been segregated from SARS-CoV many centuries ago and from its bat counterpart 40–70 years ago. The divergence dates between SARS-CoV-2 and bat sarbecovirus (RaTG13) were calculated as 1948 (95% highest posterior density (HPD): 1879–1999), 1969 (95% HPD: 1930–2000), and 1982 (95% HPD: 1948–2009). This suggests that the lineage responsible for the COVID-19 pandemic has been present in the species for decades (Boni et al., 2020).
2. SARS-CoV-2 infection: ACE2-dependent and -independent pathways
ACE1 and ACE2 are members of the Renin-Angiotensin-Aldosterone System, which regulates the blood pressure and functionality of the body’s vital organs. ACE1 converts angiotensin I to angiotensin II, which regulates aldosterone secretion and vasoconstriction-mediated increases in blood pressure. ACE2 confers its beneficial effects by degrading angiotensin I and II to angiotensin 1–9 and angiotensin 1–7, respectively, that are known to counterbalance the deleterious effects of angiotensin II in inducing inflammation (Versmissen et al., 2020). In addition, lower ACE2 expression levels are associated with worse pulmonary and cardiovascular outcomes (Bellomo et al., 2020, Imai et al., 2005) and myocardial inflammation (Oudit et al., 2009). Furthermore, low ACE2 expression has been correlated with a predisposition toward hypertensive and diabetic disorders, kidney injury, and cardiovascular complications (Li et al., 2020a, Li et al., 2020b, Lu et al., 2012, Patel et al., 2012). The binding of the SARS-CoV-2 spike protein to ACE2 receptors in the respiratory tract leads to ACE1/ACE2 imbalance, acute increases in blood pressure, acute hypoxia, endothelial dysfunction, and lung injury (Bank et al., 2021).
Furthermore, ACE2 expression is ubiquitous in various cell types related to the respiratory, cardiovascular, urogenital systems, and also in the gastrointestinal tract, central nervous system (CNS), and liver (Hamming et al., 2004). ACE2 is also expressed by circulating leukocytes (Trojanowicz et al., 2017). ACE2 appears to play a central role in the cellular response to various endogenous and exogenous stressors. Intestinal cells express the highest level of ACE2 (Hamming et al., 2004). ACE2 depletion has been linked to a disturbed gut microbiome due to impaired amino acid transport, reduced level of tryptophan in the blood, and activation of intestinal inflammation involving mammalian targets of rapamycin signaling (Hashimoto et al., 2011, Hashimoto et al., 2012, Singer and Camargo, 2011). ACE2 was implicated in the regulation of BDNF expression in cognitive processes, and ACE2 overexpression has been shown to protect AD mice from cognitive decline and amyloid pathology (Evans et al., 2020). Furthermore, ACE2 regulates the level of corticotrophin-releasing hormone at the hypothalamus and serotonin levels during neurogenesis (Klempin et al., 2018, Wang et al., 2018). It has been reported in several studies that the majority of COVID-19 infected patients (~40%) develop neurological symptoms, severe hypoxia, and neuro-inflammation (Mao et al., 2020, Muccioli et al., 2020). Following these reports, studies have characterized how SARS-CoV-2 crosses the blood-brain barrier (BBB) to reach different brain regions (Reynolds and Mahajan, 2021). This study also reported that the main components of the BBB, astrocytes, and brain microvascular endothelial cells, expressed ACE2 receptors that allowed SARS-CoV-2 to bind and alter tight junctions, leading to increased permeability of the BBB and neuro-inflammation.
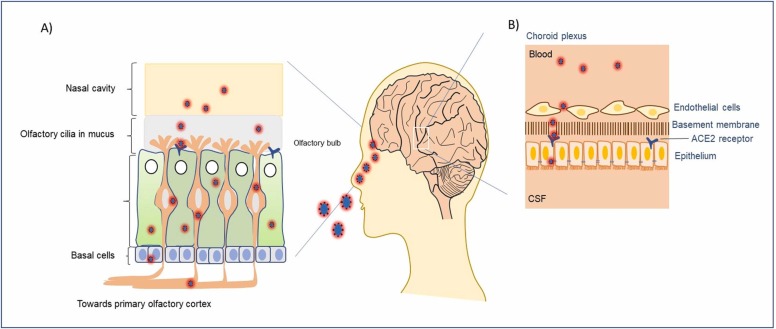
In this context, it is important to note that nasal epithelial cells, the first contact point of SARS-CoV-2 infection ( Fig. 1A), express variable levels of ACE2 receptors depending on the age group. Younger children have consistently lower infection percentages than older children and adults (Castagnoli et al., 2020, Anonymous, 2020). Interestingly, a study of ACE2 levels in children has reported that young children of ages < 10 years had much lower expression of ACE2, when compared to their older counterparts (10–17 years of age), young adults (18–24 years of age), or adults (≥ 25 years of age) (Patel and Verma, 2020). Further case studies in pediatric COVID patients with clear subacute neuropsychiatric symptoms have exhibited the presence of autoantibodies targeting transcription factor 4 (TCF4), antineural as well as anti–SARS-CoV-2 antibodies in their cerebrospinal fluid (CSF) which could be a critical indicator for the immunotherapeutic treatment outcomes (Bartley et al., 2021).
Fig. 1.
The interaction of SARS-CoV-2 with olfactory cilia for entering the central nervous system (CNS). A) Different layers of cells in the nasal cavity that contain receptors for the very first viral entry into the human host. Some of the olfactory cells express ACE2 receptors that endocytose bound viral particles, before their way to the olfactory bulb in the brain. B) Schematic of the cellular barrier between cerebrospinal fluid and circulating blood in the choroid plexus region. Both endothelial and basement epithelial cells express the ACE2 receptors facilitating the internalization of virions in the brain fluid system.
In addition to ACE2, there are several other critical factors, particularly surface serine proteases, that determine viral entry into host cells via membrane fusion between the virion and host cell membranes. This process has been reported for several pandemic viral outbreaks, such as the highly pathogenic avian influenza virus (1918 Spanish flu virus), Sendai virus, and SARS-CoV (Kido et al., 1999, Kido et al., 1992, Kim et al., 2001, Murakami et al., 2001, Okumura et al., 2010). Besides these proteases, transmembrane serine protease 2 (TMPRSS2) and human airway trypsin-like protease (HAT) also serve as critical modulators of viral entry by activating viral surface glycoproteins while binding to ACE2 receptors in human lung airway epithelial cells (Böttcher et al., 2006). Although TMPRSS2 is required following binding of the viral spike protein to the ACE2 receptor, not all airway epithelial cells homogeneously co-express both factors, instead, there is a certain distribution pattern of these two factors in a cell-type dependent manner. Gene expression and immunohistochemical analyses have shown that both nasal and broncho-epithelial type II cells highly co-express ACE2 and TMPRSS2, which is key to SARS-CoV-2 pathogenesis (Bertram et al., 2012, Zou et al., 2020, Qi et al., 2020). Importantly, nasal ciliary cells expressing surface ACE2 receptors serve as the primary entry points for SARS-CoV-2, as well as for the early-stage viral replication (Ahn et al., 2021). A previous report found no detectable expression of ACE2 in upper airway passage cells (Hamming et al., 2004). Furthermore, it was reported that approximately 0.8% of type II pneumocytes co-express ACE2 and TMPRSS2 in fibrotic human lung cells, while 0.3% of ethmoidal sinusitis-associated upper respiratory epithelial cells express both ACE2 and TMPRSS2 (Ziegler et al., 2020). Another study using single-cell RNA sequencing reported that TMPRSS2 had a comparatively broader cell type distribution pattern compared with that of ACE2, suggesting that ACE2 could be the limiting factor in regulating viral entry at the early stages of infection (Sungnak et al., 2020). Notably, SARS-CoV-2 was also found to enter TMPRSS2- cells using alternative proteases, such as cathepsin B/L, which is expressed in 70–90% of ACE2+ cells (Sungnak et al., 2020, Hoffmann et al., 2020).
It is still not determined whether low or high ACE2 expression could be beneficial in avoiding COVID-19. From emerging findings, it was suggested that low ACE2 levels in the upper respiratory tract could be beneficial; however, the same phenomena could be detrimental in the case of lower respiratory tract cells, allowing potential risk for severe acute respiratory syndromes and lung injury following COVID-19. It has been observed in several studies involving humans that some diseases can significantly lower ACE2 expression (e.g., type II diabetes (Mizuiri et al., 2008) and hypertension (Koka et al., 2008)), while smoking (Maggi et al., 2021) and inflammatory bowel disease (Garg et al., 2015) can increase ACE2 levels. This raises the question of how individuals with lower ACE2 expression contract COVID-19. A recent finding might be a possible answer to this question. Calver et al. (2021) have reported that the S1 subunit of the SARS-CoV-2 spike protein-bound αvβ3 and αvβ6 integrins through their interactions with RGDs, and αvβ3 levels were upregulated upon SARS-CoV-2 infection (Calver et al., 2021). It has also been shown that TGFβ increased ACE2 expression following SARS-CoV-2 infection (Nader et al., 2021), suggesting alternative pathways to viral internalization, despite low ACE2 levels in some individuals.
3. SARS-CoV-2 genomic mutations and impact on host immunity
Understanding the genetic constitution of SARS-CoV-2 has important clinical significance in developing a therapeutic regimen. Some emerging studies involving next-generating sequencing have made important discoveries in this field. ORF1a and ORF1b genes together constitute most of the CoV genome. ORF1a undergoes canonical translation to produce polyprotein 1a (pp1a). Additionally, programmed frameshift translation in ORF1a continuing to the end of ORF1b reading frame, yields pp1ab protein (Baranov et al., 2005). Both pp1a and pp1ab then undergo proteolytic cleavage to produce 11 or 15 non-structural proteins (nsps), respectively. ORF1a-linked nsps have been shown to regulate viral gene expression, while ORF1b-related nsps support viral replications in the host cells (Gulyaeva and Gorbalenya, 2021). One-third of SARS-CoV-2 genome from the 3′ end includes important viral structural proteins, such as the S gene encoding spike surface glycoprotein, the E gene for translation of the envelope protein, the M gene for the membrane glycoprotein, and the N gene for the nucleocapsid phosphoprotein, which are essential for viral genome packaging. Spike glycoprotein is composed of two subunits, namely the S1 subunit, which helps anchor the virion to the host-cell surface ACE2 receptor, and the S2 subunit, which mediates viral entry into the host cell by membrane fusion (Miller and Koev, 2000). This 3′ end of the genomic region also includes several unnamed open reading frames (ORFs) that are specifically linked to SARS-CoV or its subgenus-associated pathogenesis (Cui et al., 2019). These types of ORFs are termed “accessory” ORFs (Liu et al., 2014). The study of SARS-CoV-2 genomic organization has revealed that ORFs 3a, 6, 7a, 7b, 8, and 9b have strong protein-coding signatures; ORF3c is an alternate-frame gene encoding a novel functional protein, and ORFs 2b, 3d/3d-2, 3b, 9c, and 10 lack any protein-coding sequences (Jungreis et al., 2021). ORF8 consists of ORF8a and ORF8b sub-genomic regions. Mutational analysis of ORF8 suggests its critical role in within-individual fitness but not in person-to-person transmission (Jungreis et al., 2021). Moreover, in vitro studies using a SARS-CoV-2 strain having a 382 nucleotide deletion in ORF8, isolated from a patient in Singapore in March 2020, has shown that the ∆382 variant induces very similar host transcriptional responses as wild-type SARS-CoV-2 when transfected in human nasal epithelial cells, suggesting that ORF8 may be dispensable for viral entry and replication in host cells (Gamage et al., 2020).
Mutational analysis indicated that evolutionary selection pressure of Sarbecoviruses could be the prime etiological factor behind the rapid evolution of SARS-CoV-2 proteins. Amino acid changing missense mutations preferentially impacted the non-conserved genomic regions than conserved ones (16.4% vs. 9.4%), reflecting inter- vs. within-strain evolutionary agreement. Considerable deviations from such agreements suggest enhanced or suppressed evolutionary selection, for example, both S1 and nsp3 have exhibited fewer than expected amino acid mutations (S1: 13% observed vs. 17% expected; nsp3: 10% observed vs. 15% expected), despite their extremely high inter-strain variations, representing decelerated evolution, while the dramatically high mutational load on the N gene (21% observed vs. 11% expected) indicates accelerated evolutionary selection toward host adaptation (Jungreis et al., 2021). Analyses of mutations in the N protein have shown that all 29 pathogenic mutations were clustered within the R185–G204 region overlapping the predicted B cell epitope (Grifoni et al., 2020). These adaptations may be associated with the immune escape capacity of the SARS-CoV-2. The RBD harbors multiple mutation hot spots that determine the host range of SARS-CoV-like viruses. For SARS-CoV-2, there are six such amino acids, L455, F486, Q493, S494, N501, and Y505 (Wan et al., 2020). Structure-function studies of the RBD motif of SARS-CoV-2 have shown that it has a higher binding affinity for ACE2 receptors from humans, cats, ferrets, and related species with similar ACE2 receptor sequences (Wrapp et al., 2020, Walls et al., 2020, Letko et al., 2020).
Notably, SARS-CoV-2 has been still undergoing mutational adaptions, resulting in the generation of numerous variants, however, with different virulence properties. Based on the capacity of each variant in causing severe disease and/or death, World Health Organization (WHO) has categorized the most detrimental ones as the variants of concern (VOC), e.g. the delta variant (B.1.617.2), first observed in India. The delta variant of clade 21 A exhibits mutation clusters in ORF1a (D691A, G1125C, M2796L, K2894E, V4102I, etc.), ORF1b (S598G, K774N, D884N, P1095S, P1975L, etc.), ORF3 (S26L, V259L), ORF7a (P45L, V82A, T120I), ORF7b (T40I), ORF8 (D119-, F120-), S (T19R, D138-, P139-, G142D, E156-, F157-, R158G, K417N/T, L452R, T478K, E484K, N501Y, T547I, D614G, V615I/F, P681R, A831V, T95I, P1263L, etc.), N (D63G, L161F, R203M, G215C, D377Y), and M (I82T, S94G, D209Y) (https://nextstrain.org/ncov/gisaid/global). Most of the observed mutations in the conserved and highly conserved domains of the spike protein, as well as other viral structural proteins. are likely to provide stronger virulent properties to SARS-CoV-2. More recently, WHO’s Technical Advisory Group on Virus Evolution (TAG-VE) has declared the variant B.1.1.529/BA.1 or Omicron (clade 21 K) as the VOC on November 26th, 2021. Although Omicron was found to share common mutations in its spike protein with Beta (B.1.351), Delta, and Gamma (P.1) variants (Garcia-Beltran et al., 2021a, Garcia-Beltran et al., 2021b), genome surveillance and phylogenetic analysis indicate that Omicron has been derived from the Alpha lineage (Garcia-Beltran et al., 2021a, Garcia-Beltran et al., 2021b). Up to 34 missense mutations were detected in the spike protein of Omicron, out of which 15 mutations were within the RBD motif. The majority of these mutations clustered near the N-terminal domain of the spike protein responsible for antibody binding indicates the possible mechanism of escaping the vaccine-mediated neutralization by Omicron (Garcia-Beltran et al., 2021a, Garcia-Beltran et al., 2021b). Omicron pseudovirus harboring mutations like S371L, S373P, T478K, S375F, Q498R, Q493R, and N501Y could bind the host ACE2 receptor 2-fold more efficient than the Delta variant and 4-fold stronger than wildtype pseudovirus. Computational analysis revealed structural reorganization of the spike protein domain from disordered to ordered structure within the RBD region, suggesting a mechanism for stable interaction with host ACE2 receptors (Kumar et al., 2021). Recent investigations on the vaccine (double-dose)-initiated neutralizing antibody response against SARS-CoV-2 indicated undetectable viral neutralization reflecting potent immune escape capacity of the Omicron, however, individuals having booster dose of mRNA vaccines showed about 4–6 fold less viral load of Omicron than wildtype, suggesting optimal elicitation of polyclonal human neutralizing antibodies can provide substantial resilience to Omicron and future SARS-CoV-2 variants (Garcia-Beltran et al., 2021a, Garcia-Beltran et al., 2021b, Naranbhai et al., 2021, Schmidt et al., 2021).
In addition to gene expression patterns, it is important to understand the architecture of the RNA genome inside the virion to develop antisense RNA-based therapeutic approaches because the RNA structure significantly influences the efficacy of antisense RNA treatment (Patzel et al., 2005, Vickers et al., 2000). SARS-CoV-2 carries the largest RNA genome and the size of the virion is only 80 nm in diameter, it has been challenging to characterize the deeper tertiary genetic architecture (Zhu et al., 2020, Cai et al., 2020). In another study, Cao et al. (2021) were able to reconstruct the genome architecture and identify multiple highly accessible single-stranded regions by exploiting virion RNA in situ conformation sequencing technology, suggesting the possibility for a potential therapeutic platform based on antisense oligos (Cao et al., 2021).
4. SARS-CoV-2 primary entry points in the CNS and diverse neuropathological complications
Emerging studies showed that nasal intramucosal ciliated cells are the primary targets of SARS-CoV-2 entry into the human body. It has been found in nonhuman primates and COVID-19 patients that intramucosal multi-ciliated epithelial cells express SARS-CoV-2 entry-related genes, only in the apical side of this cell type, which may play critical roles in virus internalization, replication, and shedding of active virions in host target organs/tissues (Ahn et al., 2021). Furthermore, Meinhardt et al. (2021) provided substantial evidence that the olfactory transmucosal layer, representing the mucosal-neural interface, serves as the gateway for virions to travel to various neuroanatomical regions of the nasopharynx and CNS (Meinhardt et al., 2021).
As we analyze the pathogenic nature of previous SARS-CoV pandemic strains, such as MERS-CoV and SARS-CoV, there is considerable experimental and clinical evidence of the neuro-invasive nature of CoV, resulting in subsequent neuropathological and neuropsychiatric complications (Glass et al., 2004, Li et al., 2016, Netland et al., 2008). Notably, some of the symptoms observed in non-brain parts of the body in COVID-19 patients might be related to SARS-CoV-2 infection in the associated brain regions of these patients, such as in the medulla oblongata, which controls primary respiratory and cardiovascular functions (Meinhardt et al., 2021). Previous studies have suggested that altered psychological status, disinhibition, perseveration, and paranoia could be manifestations of widespread cytokine storm-mediated neuro-inflammation and encephalitis in post-COVID patients (Yousuf et al., 2021, Moriguchi et al., 2020, Wang et al., 2020, Erickson and Banks, 2018). Another study of post-COVID-19 patients indicated that 33% of the discharged patients presented dysexecutive syndromes, including disorientation, motor function deficits, and poor coordination with verbal commands (Helms et al., 2020). Rhea et al. (2021) have reported that the intravenously injected radiolabeled S1 subunit of the spike protein crossed the BBB by adsorptive transcytosis to reach the brain parenchymal regions, in mice. In addition, intranasally administered S1 protein has exhibited the ability to penetrate different brain regions in mice; however, at a rate 10 times lower than the intravenous route. Furthermore, the ACE2 receptor has been found to play roles in S1 uptake in brain and lung tissues, but not in the kidney, spleen, or liver. Conditional immune activation in these mice has revealed the reduced uptake efficiency of the S1 protein in the hippocampus and olfactory bulb (Rhea et al., 2021).
SARS-CoV-2 ancestors and other viruses have shown that their primary routes involved the disruption of the BBB to gain access to the brain. Emerging findings highlighted additional invasion routes for SARS-CoV-2 in the human brain. Choroid plexus cells are critically important in maintaining CSF balance by producing CSF in ependymal cells lining the ventricles in the brain (Fig. 1B). In addition, this cell type also helps in maintaining the barrier between the blood and CSF (Lun et al., 2015). Notably, neural stem cells at the lateral ventricles are displaced from the ventricular zone to the sub-ventricular zone (SVZ) by ependymal cells (Mirzadeh et al., 2008). Hence, we speculate that SARS-CoV-2 infection in choroid plexus cells may eventually affect the neural stem cell niche in the human brain, leading to long-lasting neuropathological complications. Single-cell transcriptomics analysis revealed that the choroid plexus-CSF barrier was disrupted and choroid plexus cells relayed inflammatory signaling to the parenchymal glia, astrocytes, oligodendrocytes, and microglial cells of the upper cortex in the form of infiltrating T cells and the CCL/CXCL family of chemokines, which can impair cognitive functions (Yang et al., 2021, Baruch et al., 2014). The predominance of SARS-CoV-2 virion-mediated neurotropism in the choroid plexus epithelial cells has also been previously suggested by the results of an in vitro brain organoid study (Jacob et al., 2020). These molecular-level modulations can be correlated with the clinical observations in the post-COVID-19 patient follow-ups because they continue to present symptoms of headache and anosmia even months after their recovery from COVID-19 (Sampaio Rocha-Filho and Voss, 2020, Caronna et al., 2020). Persistent headaches may be attributed to the reduced volumes of CSF due to the damage of choroid plexus cells, while continuing cases of anosmia and hyposmia may be due to loss of the pre-frontal and olfactory bulb neurons. Even brain tissue loss has been suspected in COVID-19 patients (Douaud et al., 2021). Reduction in brain volume could arise from many pathological effects, including stress (Echouffo-Tcheugui et al., 2018); hence, it is possible that patients who contracted COVID-19 experience high degrees of stress at the neuroanatomical level. It has been found that cortical upper layers (layers 2 and 3) are involved in pyramidal excitatory neuronal synaptic signaling to different cognitive regions of the brain, which can be affected by SARS-CoV-2 infection (Yang et al., 2021, Gidon et al., 2020). Moreover, subpopulations of activated microglia and astrocytes in the post-mortem brains of COVID-19 patients resemble the pathological hallmarks of neurodegenerative diseases, such as AD (Sala Frigerio et al., 2019, Keren-Shaul et al., 2017, Mathys et al., 2019). Although there is no conclusive evidence on the interrelations between various neurodegenerative diseases and COVID-19, there are some indications that SARS-CoV-2 infection may increase the risk of most common brain diseases, including accelerated brain aging, PD, AD, amyotrophic lateral sclerosis (ALS), and cerebrovascular disorders. For example, a case report has shown the occurrence of acute hypokinetic-rigid syndrome along with hyposmia in a 58-year-old male patient (Méndez-Guerrero et al., 2020). Apart from observations that advanced stage PD patients have the highest risk for worsening motor dysfunctions, the influence of COVID-19 on the mortality of PD patients remains inconclusive due to significant variations (Fearon and Fasano, 2021). Another case study on two patients with ALS who contracted COVID-19 reported that the patients recovered to their baseline respiratory status, despite having respiratory muscle weakness from ALS (Lee et al., 2021).
Notably, single-cell RNA sequence analysis showed that SARS-CoV-2 infection-induced inflammation could activate several pathways that modulated β-amyloid transportation and aggregation in neuronal cells. In particular, the study has shown upregulation of protein-tyrosine phosphatase 1B (PTP1B) in the COVID-19 patient frontal cortex microglial cluster-associated transcriptome (Yang et al., 2021). PTP1B or PTPN1 activation can indirectly suppress the glycogen synthase kinase 3β signaling pathway, to increase the pathophysiology of amyloidosis and cognitive decline in AD patients (Ricke et al., 2020). Another critical factor related to neuronal processing and trafficking of β-amyloid, low-density lipoprotein receptor-related protein 1B, has been found to increase in the brains of COVID-19 patients (Yang et al., 2021, Cam and Bu, 2006). Receptor interacting serine/threonine kinase 1 (RIPK1) is a critical player in TNFR1 signaling-mediated necroptosis during apoptosis-deficient conditions, which makes RIPK1 a specific therapeutic target for broad-spectrum neurodegenerative, inflammatory and lysosomal storage diseases (Degterev et al., 2019, Wallach et al., 2016). Upregulation of RIPK1 in the brains of COVID-19 patients has suggested possible activation of necrotic pathways (Yang et al., 2021).
Modulation of COVID-19 patient brain gene expression profiles related to synaptic transmission and vesicular trafficking with downregulation of SNAP25, VAMP2, and ATP6VOC has recently been reported (Yang et al., 2021). A genome-wide association study reported slightly overlapping but unique patterns of neuronal perturbations due to SARS-CoV-2 infections, especially showing strong effects in the glial cell population, indicating the possibility of increased vulnerability to various irreversible neurodegenerative and neuropsychiatric pathologies in long-COVID patients (Yang et al., 2021).
Based on these findings, we speculate that SARS-CoV-2 infection can stimulate the activation of many pathogenic mechanisms that may lead to complex forms of neurocognitive as well as neurodegenerative conditions in COVID-19 survivors ( Fig. 2). Further systematic studies in larger cohorts of patients are warranted to delineate the underlying pathomechanisms of possible neurodegenerations during long-lasting COVID.
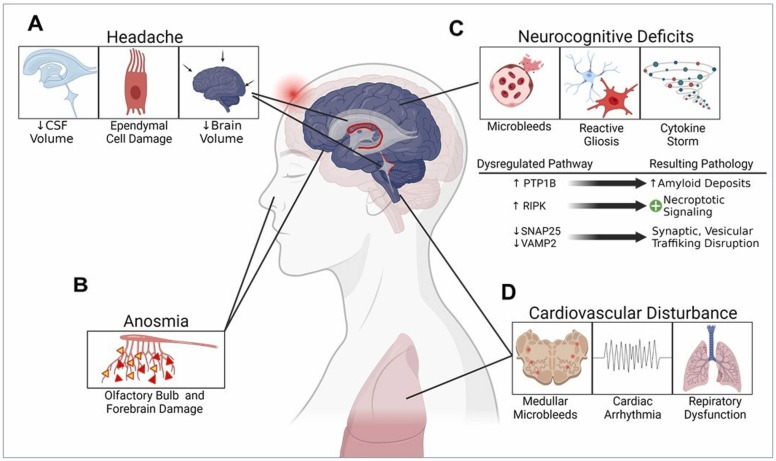
Fig. 2.
SARS-CoV-2-associated long-COVID pathogenic mechanisms in the COVID-19 survivors. A) Chronic headaches can be triggered due to the damage of ependymal cells, reduced cerebrospinal fluid (CSF) content, and the resulting shrunken brain volume. B) Viral infection-induced neuronal damage in the olfactory bulb and forebrain areas leads to acute anosmia. C) Initiation of neurocognitive decline resulting from the hemorrhagic microbleed-induced reactive gliosis, neuroimmune activation, and cytokine storms. Several signaling cascades related to synaptic transmission, vesicular trafficking, amyloidogenesis, and necroptotic pathways are dysregulated in this process. D) Similar mechanisms associated with the microbleeds in the deeper brain regions like medullar oblongata lead to cardiac arrhythmia and abnormal respiratory functions.
4.1. “Brain Fog”: the impact of COVID-19 on long-term neuropsychiatric sequelae and its association with ageing
Long-term and/or an indefinite period of diverse neuropsychiatric disorders have been reported in non-hospitalized COVID-19 survivors. Most importantly, studies have shown that at least 20–30% of these patients experienced a lingering psychological condition, known as “brain fog,” characterized by many cognitive impairments, including memory loss, difficulty in concentrating, forgetting daily activities, difficulty in selecting the right words, taking longer than usual time to complete a regular task, disoriented thought processes, emotional numbness, or poor endurance occasionally accompanied by headaches, insomnia, dysgeusia, and anosmia (Graham et al., 2021, Garrigues et al., 2020). Furthermore, hopelessness, social isolation, insomnia, socio-economic burden, COVID-19 infection, imbalance in daily routines along with pre-existing motor and/or cognitive deficits can significantly affect the emotional and behavioral status, known as “hidden sorrow,” of COVID-19 survivors in long-lasting COVID-19 patients (Pinto et al., 2020, Albuquerque et al., 2021). Notably, researchers have reported that COVID-19 survivors who experience “brain fog” or “dysexecutive syndrome” gradually developed multi-organ abnormalities, such as cardiac and hepatic dysfunctions (Nauen et al., 2021). Likely, patients with pre-existing anxiety syndromes and/or hidden grief or trauma may be the most at-risk population for developing “brain fog”. A psychiatric study involving both children and young adults has identified the developmental pathways of elevated levels of anxiety and dysregulated worry from childhood to adolescence as a consequence of the COVID-19 pandemic, indicating the importance of detecting vulnerable young populations who are at the risk of developing uncontrolled anxiety and psychological stress (Zeytinoglu et al., 2021). Several reports have also indicated the increased incidences of suicidal behavior, which seems a well-established psychosocial consequence of severe pandemic situations (Banerjee et al., 2021). Increased levels of depression, coping motives, and alcohol consumption due to the COVID-19 pandemic-associated psychological distress have also been reported in an online survey of 833 US residents (McPhee et al., 2020). Retrospective cohort studies and case reports have reported that patients with psychiatric disorders, including schizophrenia, had increased mortality from COVID-19, in the US (Wang et al., 2021, Taquet et al., 2021a, Taquet et al., 2021b, Nemani et al., 2021, Palomar-Ciria et al., 2020). Notably, a growing body of evidence has highlighted the critical cross-talk between oxidative stress, neuro-inflammation, and loss of CNS volume in schizophrenia, anxiety, depression, mood disorders, and stress (Müller et al., 2015, Fond et al., 2020, Felger, 2018, Lee and Giuliani, 2019, Ng et al., 2008). These findings reasonably corroborate why patients with pre-existing neuropsychiatric dysfunctions have higher susceptibilities to COVID-19 mortality. However, further in-depth mechanistic studies are needed to understand the causes, to develop more effective therapeutic strategies.
4.2. Chronic neurological dysfunctions after recovery from COVID-19
Several clinical and observational studies have reported several clinical symptoms that persisted in COVID-19 survivors, even after attaining negative PCR results. The Centers for Disease Control and Prevention (CDC) has listed more than 15 such new or ongoing abnormalities that can last for weeks or even months after the first SARS-CoV-2 infection. Respiratory distress, chronic and migraine headaches, broad-spectrum cognitive deficits, abrupt mood swings, loss of smell, altered taste perceptions, insomnia, musculoskeletal pain, and diarrhea are some of the frequently occurring symptoms in post-COVID-19 patients. Long-COVID also pertains to the new onset of symptoms that are either not fully resolved or were never experienced, after a 30-day recovery period. A meta-analysis reported that the most common sustained symptom was tiredness or fatigue (58%), followed by headaches (44%), attention-deficit/disorders (27%), ageusia (23%), anosmia (21%), memory losses (16%), and anxiety and depression (12–13%) (Lopez-Leon et al., 2021). In addition, chronic systemic inflammation in COVID-19 patients may cause peripheral neuropathy (Bureau et al., 2020). A study on a cohort of 714 COVID-19 patients reported that a significant portion of these patients exhibited severe post-traumatic stress disorder (Bo et al., 2021). Although dementia or long-term memory loss has not been reported yet in COVID-19 survivors, multiple pathomechanistic indications regarding the involvement of amyloidosis, AD, and PD-like pathological hallmarks have suggested that individuals predisposed to such neurodegenerative conditions may experience an advanced stage of AD-related dementia at an earlier age than expected. Persistence of extended micro-hemorrhagic lesions may also enhance the cellular stress level, along with chronic oxidative damage, leading to long-term motor and cognitive impairments, especially in the aged population. Chronic cardiovascular abnormalities manifested as chest pains in post-COVID-19 patients can be the predominant illness, possibly due to microbleed-associated neuronal damage in the brain stem and medulla oblongata regions (Fig. 2). In summary, post-COVID-19 brain injuries can happen at multi-dimensional levels, which involve complex pathogenic mechanisms.
5. SARS-CoV-2-induced thrombosis, hemolysis, and microbleeds
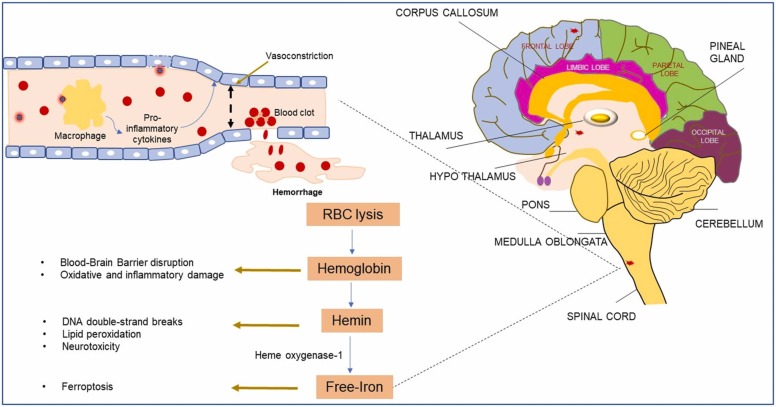
Emerging studies and case reports have highlighted the high incidences of venous thromboembolic events, disseminated intravascular coagulation, and elevated serum D-dimer levels, which are predictors of blood clotting, as critical factors influencing COVID-19-related mortality. In a retrospective cohort study including 400 hospitalized COVID-19 patients (including 144 critically ill patients) 9.5% of the patients had radiographically confirmed thromboembolic complications (Al-Samkari et al., 2020). Other studies have independently confirmed hyperactivation of blood coagulation pathways, leading to venous thromboembolism and thrombosis in 15–30% of patients who did not respond to the anti-coagulation and anti-prophylactic therapies (Nahum et al., 2020, Lodigiani et al., 2020). It has been reported that patients with lupus and antiphospholipid syndrome, who have circulating antiphospholipid autoantibodies, could be predisposed to severe thrombosis when they contract COVID-19 (Zuo et al., 2020). This study reported significantly higher levels of anti-phosphatidylserine/prothrombin antibodies in serum samples of COVID-19 patients. Moreover, purified IgG from these patients induced severe venous thrombosis in two different mice models, suggesting that at least half of the COVID-19 patients became positive for extremely pathogenic auto anti-phospholipid antibodies. Meinhardt et al. reported that SARS-CoV-2 infection could lead to micro-thromboembolic events in the CNS involving the brain endothelial cells and the microvasculature (Meinhardt et al., 2021). Dixon et al. (2020) found that clinical characteristics of cerebral microhemorrhages in COVID-19 patients were correlated with the patterns of microbleeds in non-COVID patients with severe neuropathological conditions (Dixon et al., 2020). They showed that microbleeds had predilections for the brainstem, cortical white matter, and corpus callosum of patients. Another neuroimaging study by Fitsiori et al. (2020) also reported these unusual patterns of microbleeds, especially in the corpus callosum, as well as in the subcortical and para-hippocampal regions, middle cerebellar peduncles, and internal capsule in moderate to critically ill COVID-19 patients ( Fig. 3) (Fitsiori et al., 2020).
Fig. 3.
SARS-CoV-2 infection-induced microbleeds in the brain and its potential consequences. A) Mechanism of virion recognition by endothelial ACE2 receptors, which in turn induce activation of a cytokine storm, leading to vessel rupture and downstream genotoxic and cytotoxic effects. B) Illustration of different brain regions in the sagittal section, indicating the formation of microbleeds as red spots in certain locations.
5.1. Ageing factor in post-COVID-19 recovery: potential involvement of ferroptosis and cellular senescence pathways
Biological aging is a known key contributor to most progressive degenerative diseases, including neurodegeneration. The prevalence of stroke is also more than 70% in individuals aged above 65 years. This is of particular importance because COVID-associated death and microhemorrhagic lesions have been found to comprise three-quarters of the COVID-related death in subjects of more than 65 years of age in the United States as well as globally, according to the CDC’s recent updates, while the death ratio comes to 1 in 1400 people aged less than 65 years, suggesting that chronological aging is also a crucial contributor to patient fatality. Several studies have consistently reported significantly high risks of ischemic/hemorrhagic stroke-like events, mostly cryptogenic in nature, and hypoxia, acute necrotizing hemorrhagic encephalopathy, and acute cerebrovascular adverse events, possibly due to hypercoagulability as the leading causes of high mortality in COVID-19 patients (Yaghi et al., 2020, Kvernland et al., 2021, Dhamoon et al., 2021, Margos et al., 2021, Katz et al., 2020, Bridwell et al., 2020). Studies have also reported cerebral microbleeds as a clinicopathological sign for long-term neurodegenerative conditions, which are relevant in the context of the long-lasting COVID scenario among COVID-19 survivors. Traumatic microbleeds can be detected up to 5 years from their origins, using neuroimaging (Rizk et al., 2020). Microbleeds are also common in the elderly population, indicating that it is a predictor of brain aging (Fisher et al., 2010, Sumbria et al., 2018). Hence, the formation of high numbers of microbleeds in COVID-19 patients may suggest accelerated brain aging in long-COVID patients. Moreover, microbleeds have been pathologically linked to neurodegeneration, cognitive impairment, and dementia (Akoudad et al., 2016, Sepehry et al., 2016).
Increasing evidence of cerebral microbleeds and hemosiderin deposits, in both cardiovascular and neurodegenerative disorders, suggests the utilization of microbleed size and location as biomarkers for the diagnoses and prognoses of these patients, especially in case of stroke (Pétrault et al., 2019). Further evidence exhibits SARS-CoV-2-induced microhemorrhages and intracerebral endotheliitis in the brainstem, deep brain gray and white matter areas, cerebellum, and neocortex (Kirschenbaum et al., 2021). Because the brainstem includes the medulla oblongata, which regulates cardiovascular functions of the body, microhemorrhages in the brainstem can induce long-term cardiovascular dysfunctions, which have been widely observed in COVID-19 survivors as symptoms of long-lasting COVID. Together, these findings may provide the basis for investigating motor and cognitive deficits in COVID-19 patients’ post-mortem brain samples as well as from follow-up studies of COVID survivors.
Previously, we showed the biodegradation of hemoglobin into multiple toxic by-products, including hemin, as the primary contributing factor in eryptosis and ferroptosis (Derry et al., 2020). We further provided evidence that hemin can cause direct DNA double-strand breaks (DSBs) in the genome, the most lethal form of genome damage, in both endothelial and neuronal cells (Dharmalingam et al., 2020). We found that acute exposure to hemin induced cellular senescence or senescence-like states as an early response, possibly due to widespread genome damage together with acute oxidative stress and inflammation. While unresolved and/or persistent senescence is pathological, in this instance, we proposed that hemin-induced early senescence protected the cells from iron toxicity, which acutely increases during further degradation of hemin to bile salts. Deregulation of this tightly regulated hemin detoxification pathway could cause iron-mediated ferroptosis and cell death. Thus, several molecular factors orchestrate the hemin degradation mechanism, leading to the production of free iron, which then can induce both oxidative stress and iron-mediated ferroptosis (Seiwert et al., 2020). In this context, upregulation of heme oxygenase-1 (HO-1) gene expression in response to hemin exposure is protective against several diseases related to hemolysis and oxidative stress (Karatzaferi et al., 2019, Martín et al., 2019). However, hemin-induced HO-1 expression could not prevent SARS-CoV-2 infection, using an in vitro cell model (Maestro et al., 2021). Furthermore, observation of viral infection-induced multi-infarcts and microhemorrhages in distinct brain regions in the form of hemosiderin deposits, which primarily act as an iron storage complex in combination with partially digested lysosomes and ferritins, has been correlated with the upregulation of ferritin light and heavy chain proteins in the CNS of COVID-19 patients (Yang et al., 2021), suggesting iron overloading in these affected brain regions.
Ferroptosis was first discovered in 2012 as a nonapoptotic iron-mediated cell death mechanism (Dixon et al., 2012). In its simplified form as shown in Fig. 4, the chemical basis of ferroptosis is the induction of reactive oxygen species (ROS) through the participation of pro-oxidant iron in a redox mechanism via the Fenton reaction. The pathological hallmark of such oxidative stress is the disruption of lipid membranes, either by direct attack from ROS under the downregulated condition of glutathione peroxidase 4 (GPx4)-associated antioxidant defense (known as canonical ferroptosis) (Hadian and Stockwell, 2020, Li et al., 2020a, Li et al., 2020b) or by overloading of the intracellular iron (II) pool in combination with hyperactivation of HO-1 (called non-canonical ferroptosis) (Hassannia et al., 2018). Furthermore, lipid peroxidation can be induced by enzymes like non-heme iron dioxygenase and lipoxygenase (Kagan et al., 2017, Haeggström and Funk, 2011) or by free radicals (Gaschler and Stockwell, 2017). Elevated levels of malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE) and reduced expression of GPx4 serve as markers for ferroptosis (Ayala et al., 2014).
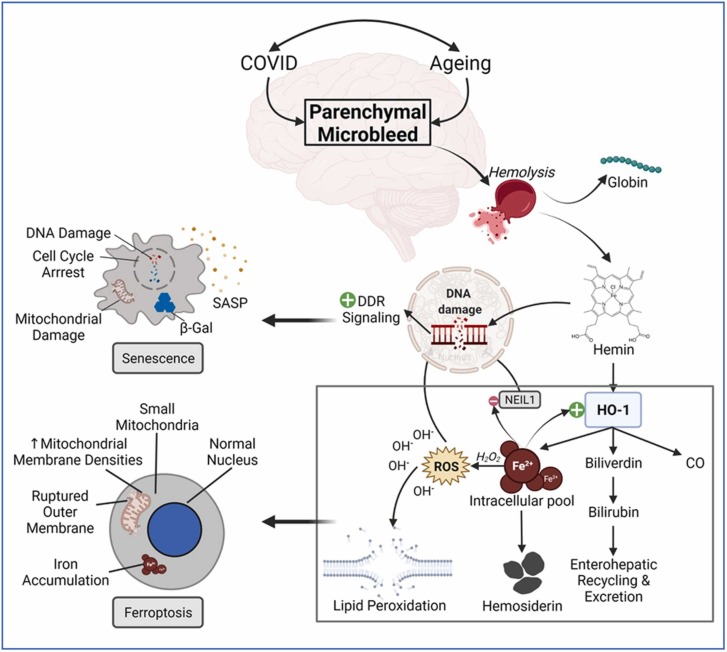
Fig. 4.
Pathomechanistic implications of ferroptosis in age-related neuronal damage in the long COVID-19 survivors. Microbleeds are frequently detected in the aged brain in neuroimaging examinations. However, COVID-19 drastically increases the number of these microhemorrhagic lesions in the brain parenchyma. Following the hemolysis, hemoglobin disintegrates into globin and hemin parts. The schematic of ferroptosis illustrates how hemin sequentially undergoes biodegradation to form the pro-oxidant free iron ions, which in turn get engaged in Fenton’s reaction to produce reactive oxygen species (ROS), resulting in the oxidative stress-induced genome damage as well as membrane disruption via lipid peroxidation mechanism, and ultimately cell death via ferroptosis. Since acutely overloaded intracellular iron (III) ions can inactivate oxidative DNA damage repair proteins like NEIL1, unrepaired DNA damage persistently sends out damage response signals, leading to cellular senescence. The key cellular hallmarks of a senescent and ferroptotic cell are indicated in the respective illustrations.
Cerebral hemorrhage-derived hemin exposure can induce the expression of Toll-like receptor 4 (TLR4) signaling, which in turn activates pro-inflammatory factors, like IL-6 and phosphorylated STAT3. Upregulation of these factors has been correlated with progressive cognitive impairment in hemorrhagic stroke survivors (Derry et al., 2020, Xiong et al., 2016). Moreover, studies have shown the effects of cytokine storms in inducing a senescent stage, along with the accumulation of oxidative DNA damage (Ren et al., 2009). The persistent senescent stage can in turn lead to continuing inflammatory signal activation (Stojanović et al., 2020). Such chronic inflammation has been found to induce telomere dysfunction, leading to irreversible cellular aging (Jurk et al., 2014). We and others have reported that induction of DNA damage response (DDR) signaling induced a senescence-like state (Dharmalingam et al., 2020, Kang et al., 2015), and an increase in the activity of the transcription factor, GATA4, which maintains cross-talk between the DDR and autophagy to cause senescence and inflammation in a p53-independent and NF-κB-dependent manner, and further activating downstream secretory factors (cytokines, chemokines, proteases, nucleases, and growth factors), collectively called the senescence-associated secretory phenotype (SASP), related to cell death (Kang et al., 2015). Elevated GATA4 level is a potential biomarker of cellular senescence in the aging brain. In this context, several studies have suggested that the deleterious effects of pre-existing senescent cells and chronic inflammation in elderly people who contracted COVID-19, could be a potential factor responsible for the intensive responses to pathogen-associated molecular pattern (PAMP) factors and high mortality in patients > 65 years of age (Nehme et al., 2020, Yanez et al., 2020, Perez-Saez et al., 2021, Ho et al., 2020). Consistent with this possibility, a preclinical study has reported that selective elimination of senescent cells by senolytic agents, like fisetin, after exposure to SARS-CoV-2 S1 protein reduced mortality to 50% and overall inflammation in aged mice, suggesting that fundamental aging mechanisms may be the potential therapeutic target to improve the resilience of the elderly population (Camell et al., 2021). However, this study did not consider the effect of viral spike protein on the brain endothelium and resulting microhemorrhages, which have been frequently observed in COVID-19 patients, irrespective of the age group. Excessive free iron can also induce stress granule formation and sequestration of structurally disordered domains containing pathogenic factors related to various neurodegenerative diseases, psychomotor dysfunctions, and increased dopamine levels in the basal ganglia (Chiueh, 2001, Baradaran-Heravi et al., 2020, Zhu et al., 2016, Kim et al., 2018, Bartels et al., 2019, Ma et al., 2021, Becerril-Ortega et al., 2014). It has also been reported that iron overload inhibited the enzymatic capacity of proteins, like NEIL1, that are involved in genome maintenance in response to oxidative stress (Hegde et al., 2010). Taken together, these findings suggest that managing the free iron overload, resulting from hemin breakdown, in and surrounding the SARS-CoV-2-induced microbleeds/multi-infarct areas, could be used to save affected neuronal and non-neuronal cells by carefully balancing the ferroptosis-senescence axis. Our previous findings suggested that hemin exposure induced oxidative stress and genome damage in an organelle-selective manner, e.g., hemin exposure showed immediate oxidative stress in the mitochondria, followed by the cytosol, and finally in the nucleus (Dharmalingam et al., 2020). Because iron is an essential and critical factor for overall human health, nonspecific chelation of iron may give rise to undesirable adverse events, further worsening complications. We have found that treatment with a dual function nanozyme (PEGylated carbon-nanoparticle conjugated with antioxidant scavenger and iron chelator deferoxamine) reverted neuronal senescence and prevented cells from ferroptosis at the same time, if treated within 1 h of hemin exposure, further suggesting that inflammation is the ultimate and universal pathogenic factor in determining the fate of the cells (Dharmalingam et al., 2020). This finding is consistent with a previous report that chronic inflammation may cause irreversible damage to telomeres (Jurk et al., 2014). Another important parameter is the appropriate stoichiometric combination of the antioxidant and iron chelator, such as the combination of PEGylated antioxidant nanoparticles and deferoxamine, to exert synergistic effects on recovery of senescent parameters, reduction of oxidative stress, and increased cell viability in the affected cell populations (Dharmalingam et al., 2020).
6. Summary and future directions
In conclusion, SARS-CoV-2 infection-induced microhemorrhages in crucial brain regions have the potential to accelerate brain aging in COVID-19 survivors and can also lead to complex and irreversible neurodegenerative conditions, a risk factor for both cognitive and motor functions. Loss of neuronal cells in COVID-19 patient-affected brain areas may be attributed to a combination of senescence and ferroptosis along with acute inflammation-induced oxidative stress-mediated cell death. But the crosstalk between these phenomena needs further exploration. Notably, coagulopathy is found to be major risk factor associated with death or long-term physical disabilities in most COVID-19 patients, which requires individualized treatment strategies based on the patient’s comorbid conditions. Combinatorial therapies involving antioxidant and target-specific iron chelators could be a potential strategy for ameliorating acute neuroinflammation and neuronal loss, a topic for future investigation. In this context, our nanozyme formulation has demonstrated promising therapeutic potential in in vitro and animal studies. Currently, there are only a handful of medications and ongoing clinical trials to ameliorate post-stroke long-term neuropathological conditions. Some of the Federal Drug Administration (FDA) approved drugs acting as the inhibitor of the coagulation factor Xa are apixaban (clinical trial: NCT03907046), dabigatran (Pradaxa®), and rivaroxaban (Xarelto®) (Chen et al., 2020). Because persistently high levels of inflammatory biomarkers in the blood have been detected in the recovery stage of COVID patients (Ali et al., 2021), it will be important to determine whether such chronic inflammations can induce stress granule formation in the brain, as it often does under other stress response conditions (Herman et al., 2019). Statins, minocycline, and melatonin are the most commonly administered anti-inflammatory drugs used to treat the neuroinflammation in the CNS (Kaelber et al., 2016). Under the influence of predisposed or comorbid pathological conditions, blood-degraded hemin and its toxic by-products can result in the pathogenic protein-RNA aggregate formation, leading to proteinopathy-associated neurodegenerative diseases. As mentioned earlier, proteinopathy-related neurocognitive decline or impairment can act as the predominant risk exposure to dementia. Clinically, cholinesterase inhibitors are primarily used to treat mild to moderate dementia conditions (Battle et al., 2021), FDA has recently approved a monoclonal antibody therapy (MAB), Aduhelm (BLA: 761178), that can induce targeted clearance of toxic protein aggregates in preventing neuronal death in Alzheimer’s and related dementias. Thus, further mechanistic investigation of the fate and implications of microhemorrhages in the brains of COVID-19 survivors is urgently needed to better strategize the patient-specific therapeutic combination in treating COVID-19-induced long-term neurological and/or neuropsychiatric disorders. Furthermore, careful medical and clinical follow-ups should be performed to diagnose early symptoms of neuropathological, neuropsychiatric, and/or cardiovascular dysfunctions to prevent patients from developing irreversible motor/cognitive impairments and cardiovascular disorders.
Conflict of interest
The authors declare that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Research in the Hegde laboratory is supported by grants from the National Institute of Neurological Disorders and Stroke (NINDS) and National Institute on Aging (NIA) of the National Institutes of Health (NIH, USA) under award numbers R01NS088645 (M.L.H), (M.L.H), RF1NS112719 (M.L.H), R03AG064266 and R01NS094535 (T.A.K and M.L.H), The Welch Foundation (Houston, TX, USA) grant to T.A.K (BE-0048), and the Houston Methodist Research Institute (Houston, TX, USA) funds to M.L.H. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. The authors thank members of the Hegde Laboratory (Haibo Wang, Velmarini Vasquez, Vikas H MalojiRao, and Pavana Hegde) for discussions and critical comments.
References
- Ahn J.H., Kim J., Hong S.P., Choi S.Y., Yang M.J., Ju Y.S., Kim Y.T., Kim H.M., Rahman M.D.T., Chung M.K., Hong S.D., Bae H., Lee C.S., Koh G.Y. Nasal ciliated cells are primary targets for SARS-CoV-2 replication in the early stage of COVID-19. J. Clin. Investig. 2021;131(13) doi: 10.1172/jci148517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn M., Anderson D.E., Zhang Q., Tan C.W., Lim B.L., Luko K., Wen M., Chia W.N., Mani S., Wang L.C., Ng J.H.J., Sobota R.M., Dutertre C.A., Ginhoux F., Shi Z.L., Irving A.T., Wang L.F. Dampened NLRP3-mediated inflammation in bats and implications for a special viral reservoir host. Nat. Microbiol. 2019;4(5):789–799. doi: 10.1038/s41564-019-0371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akoudad S., Wolters F.J., Viswanathan A., de Bruijn R.F., van der Lugt A., Hofman A., Koudstaal P.J., Ikram M.A., Vernooij M.W. Association of cerebral microbleeds with cognitive decline and dementia. JAMA Neurol. 2016;73(8):934–943. doi: 10.1001/jamaneurol.2016.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque S., Teixeira A.M., Rocha J.C. COVID-19 and disenfranchised grief. Front. Psychiatry. 2021;12(114) doi: 10.3389/fpsyt.2021.638874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali K.M., Ali A.M., Tawfeeq H.M., Figueredo G.P., Rostam H.M. Hypoalbuminemia in patients following their recovery from severe coronavirus disease 2019. J. Med. Virol. 2021;93(7):4532–4536. doi: 10.1002/jmv.27002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida J.D., Tyrrell D.A. The morphology of three previously uncharacterized human respiratory viruses that grow in organ culture. J. Gen. Virol. 1967;1(2):175–178. doi: 10.1099/0022-1317-1-2-175. [DOI] [PubMed] [Google Scholar]
- de Almeida-Pititto B., Dualib P.M., Zajdenverg L., Dantas J.R., de Souza F.D., Rodacki M., Bertoluci M.C., Brazilian Diabetes Society Study G. Severity and mortality of COVID 19 in patients with diabetes, hypertension and cardiovascular disease: a meta-analysis. Diabetol. Metab. Syndr. 2020;12(1):75. doi: 10.1186/s13098-020-00586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Samkari H., Karp Leaf R.S., Dzik W.H., Carlson J.C.T., Fogerty A.E., Waheed A., Goodarzi K., Bendapudi P.K., Bornikova L., Gupta S., Leaf D.E., Kuter D.J., Rosovsky R.P. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26(4):450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous Coronavirus disease 2019 in children - United States, February 12-April 2, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69(14):422–426. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala A., Muñoz M.F., Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014;2014 doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly L., Fabre R., Courjon J., Carles M., Dellamonica J., Pradier C. Obesity, diabetes, hypertension and severe outcomes among inpatients with coronavirus disease 2019: a nationwide study. Clin. Microbiol. Infect. 2021 doi: 10.1016/j.cmi.2021.09.010. S1198-1743X(1121)00503-00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee D., Kosagisharaf J.R., Sathyanarayana Rao T.S. ‘The dual pandemic’ of suicide and COVID-19: A biopsychosocial narrative of risks and prevention. Psychiatry Res. 2021;295 doi: 10.1016/j.psychres.2020.113577. 113577-113577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank S., De S.K., Bankura B., Maiti S., Das M., A Khan G. ACE/ACE2 balance might be instrumental to explain the certain comorbidities leading to severe COVID-19 cases. Biosci. Rep. 2021;41(2) doi: 10.1042/BSR20202014. BSR20202014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baradaran-Heravi Y., Van Broeckhoven C., van der Zee J. Stress granule mediated protein aggregation and underlying gene defects in the FTD-ALS spectrum. Neurobiol. Dis. 2020;134 doi: 10.1016/j.nbd.2019.104639. [DOI] [PubMed] [Google Scholar]
- Baranov P.V., Henderson C.M., Anderson C.B., Gesteland R.F., Atkins J.F., Howard M.T. Programmed ribosomal frameshifting in decoding the SARS-CoV genome. Virology. 2005;332(2):498–510. doi: 10.1016/j.virol.2004.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels M., Weckbecker D., Kuhn P.-H., Ryazanov S., Leonov A., Griesinger C., Lichtenthaler S.F., Bötzel K., Giese A. Iron-mediated aggregation and toxicity in a novel neuronal cell culture model with inducible alpha-synuclein expression. Sci. Rep. 2019;9(1):9100. doi: 10.1038/s41598-019-45298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley C.M., Johns C., Ngo T.T., Dandekar R., Loudermilk R.L., Alvarenga B.D., Hawes I.A., Zamecnik C.R., Zorn K.C., Alexander J.R., Wapniarski A.E., DeRisi J.L., Francisco C., Nash K.B., Wietstock S.O., Pleasure S.J., Wilson M.R. Anti-SARS-CoV-2 and autoantibody profiles in the cerebrospinal fluid of 3 teenaged patients with COVID-19 and subacute neuropsychiatric symptoms. JAMA Neurol. 2021 doi: 10.1001/jamaneurol.2021.3821. 10.1001/jamaneurol.2021.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruch K., Deczkowska A., David E., Castellano J.M., Miller O., Kertser A., Berkutzki T., Barnett-Itzhaki Z., Bezalel D., Wyss-Coray T., Amit I., Schwartz M. Aging. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science. 2014;346(6205):89–93. doi: 10.1126/science.1252945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battle C.E., Abdul-Rahim A.H., Shenkin S.D., Hewitt J., Quinn T.J. Cholinesterase inhibitors for vascular dementia and other vascular cognitive impairments: a network meta-analysis. Cochrane Database Syst. Rev. 2021;2(2) doi: 10.1002/14651858.CD013306.pub2. Cd013306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerril-Ortega J., Bordji K., Fréret T., Rush T., Buisson A. Iron overload accelerates neuronal amyloid-β production and cognitive impairment in transgenic mice model of Alzheimer’s disease. Neurobiol. Aging. 2014;35(10):2288–2301. doi: 10.1016/j.neurobiolaging.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Bellomo R., Wunderink R.G., Szerlip H., English S.W., Busse L.W., Deane A.M., Khanna A.K., McCurdy M.T., Ostermann M., Young P.J., Handisides D.R., Chawla L.S., Tidmarsh G.F., Albertson T.E. Angiotensin I and angiotensin II concentrations and their ratio in catecholamine-resistant vasodilatory shock. Crit. Care. 2020;24(1):43. doi: 10.1186/s13054-020-2733-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram S., Heurich A., Lavender H., Gierer S., Danisch S., Perin P., Lucas J.M., Nelson P.S., Pöhlmann S., Soilleux E.J. Influenza and SARS-coronavirus activating proteases TMPRSS2 and HAT are expressed at multiple sites in human respiratory and gastrointestinal tracts. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0035876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bo H.X., Li W., Yang Y., Wang Y., Zhang Q., Cheung T., Wu X., Xiang Y.T. Posttraumatic stress symptoms and attitude toward crisis mental health services among clinically stable patients with COVID-19 in China. Psychol. Med. 2021;51(6):1052–1053. doi: 10.1017/s0033291720000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boni M.F., Lemey P., Jiang X., Lam T.T.-Y., Perry B.W., Castoe T.A., Rambaut A., Robertson D.L. Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic. Nat. Microbiol. 2020;5(11):1408–1417. doi: 10.1038/s41564-020-0771-4. [DOI] [PubMed] [Google Scholar]
- Böttcher E., Matrosovich T., Beyerle M., Klenk H.D., Garten W., Matrosovich M. Proteolytic activation of influenza viruses by serine proteases TMPRSS2 and HAT from human airway epithelium. J. Virol. 2006;80(19):9896–9898. doi: 10.1128/jvi.01118-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridwell R., Long B., Gottlieb M. Neurologic complications of COVID-19. Am. J. Emerg. Med. 2020;38(7):1549. doi: 10.1016/j.ajem.2020.05.024. e1543-1549.e1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook C.E., Boots M., Chandran K., Dobson A.P., Drosten C., Graham A.L., Grenfell B.T., Müller M.A., Ng M., Wang L.-F., van Leeuwen A. Accelerated viral dynamics in bat cell lines, with implications for zoonotic emergence. eLife. 2020;9 doi: 10.7554/eLife.48401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau B.L., Obeidat A., Dhariwal M.S., Jha P. Peripheral neuropathy as a complication of SARS-Cov-2. Cureus. 2020;12(11) doi: 10.7759/cureus.11452. e11452-e11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z., Cao C., Ji L., Ye R., Wang D., Xia C., Wang S., Du Z., Hu N., Yu X., Chen J., Wang L., Yang X., He S., Xue Y. RIC-seq for global in situ profiling of RNA-RNA spatial interactions. Nature. 2020;582(7812):432–437. doi: 10.1038/s41586-020-2249-1. [DOI] [PubMed] [Google Scholar]
- Calver J., Joseph C., John A., Organ L., Fainberg H., Porte J., Mukhopadhyay S., Barton L., Stroberg E., Duval E., Copin M., Poissy J., Steinestrel K., Tatler A., Jenkins G. S31 the novel coronavirus SARS-CoV-2 binds RGD integrins and upregulates avb3 integrins in Covid-19 infected lungs. Thorax. 2021;76(Suppl. 1):A22–A23. doi: 10.1136/thorax-2020-BTSabstracts.37. [DOI] [Google Scholar]
- Calvet G.A., Pereira S.A., Ogrzewalska M., Pauvolid-Corrêa A., Resende P.C., Tassinari W.S., Costa A.P., Keidel L.O., da Rocha A.S.B., da Silva M.F.B., Dos Santos S.A., Lima A.B.M., de Moraes I.C.V., Mendes Junior A.A.V., Souza T.D.C., Martins E.B., Ornellas R.O., Corrêa M.L., Antonio I., Guaraldo L., Motta F.D.C., Brasil P., Siqueira M.M., Gremião I.D.F., Menezes R.C. Investigation of SARS-CoV-2 infection in dogs and cats of humans diagnosed with COVID-19 in Rio de Janeiro, Brazil. PLoS One. 2021;16(4) doi: 10.1371/journal.pone.0250853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cam J.A., Bu G. Modulation of β-amyloid precursor protein trafficking and processing by the low density lipoprotein receptor family. Mol. Neurodegener. 2006;1(1):8. doi: 10.1186/1750-1326-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camell C.D., Yousefzadeh M.J., Zhu Y., Langhi Prata L.G.P., Huggins M.A., Pierson M., Zhang L., O'Kelly R.D., Pirtskhalava T., Xun P., Ejima K., Xue A., Tripathi U., Machado Espindola-Netto J., Giorgadze N., Atkinson E.J., Inman C.L., Johnson K.O., Cholensky S.H., Carlson T.W., LeBrasseur N.K., Khosla S., O'Sullivan M.G., Allison D.B., Jameson S.C., Meves A., Li M., Prakash Y.S., Chiarella S.E., Hamilton S.E., Tchkonia T., Niedernhofer L.J., Kirkland J.L., Robbins P.D. Senolytics reduce coronavirus-related mortality in old mice. Science. 2021 doi: 10.1126/science.abe4832. 10.1126/science.abe4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C., Cai Z., Xiao X., Rao J., Chen J., Hu N., Yang M., Xing X., Wang Y., Li M., Zhou B., Wang X., Wang J., Xue Y. The architecture of the SARS-CoV-2 RNA genome inside virion. Nat. Commun. 2021;12(1):3917. doi: 10.1038/s41467-021-22785-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caronna E., Ballvé A., Llauradó A., Gallardo V.J., Ariton D.M., Lallana S., López Maza S., Olivé Gadea M., Quibus L., Restrepo J.L., Rodrigo-Gisbert M., Vilaseca A., Hernandez Gonzalez M., Martinez Gallo M., Alpuente A., Torres-Ferrus M., Pujol Borrell R., Alvarez-Sabin J., Pozo-Rosich P. Headache: a striking prodromal and persistent symptom, predictive of COVID-19 clinical evolution. Cephalalgia. 2020;40(13):1410–1421. doi: 10.1177/0333102420965157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagnoli R., Votto M., Licari A., Brambilla I., Bruno R., Perlini S., Rovida F., Baldanti F., Marseglia G.L. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020;174(9):882–889. doi: 10.1001/jamapediatrics.2020.1467. [DOI] [PubMed] [Google Scholar]
- Chan C.-M., Woo P.C.Y., Lau S.K.P., Tse H., Chen H.-L., Li F., Zheng B.-J., Chen L., Huang J.-D., Yuen K.-Y. Spike protein, S, of human coronavirus HKU1: role in viral life cycle and application in antibody detection. Exp. Biol. Med. 2008;233(12):1527–1536. doi: 10.3181/0806-RM-197. [DOI] [PubMed] [Google Scholar]
- Chen A., Stecker E., Warden B.A. Direct oral anticoagulant use: a practical guide to common clinical challenges. J. Am. Heart Assoc. 2020;9(13) doi: 10.1161/jaha.120.017559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiueh C.C. Iron overload, oxidative stress, and axonal dystrophy in brain disorders. Pediatr. Neurol. 2001;25(2):138–147. doi: 10.1016/s0887-8994(01)00266-1. [DOI] [PubMed] [Google Scholar]
- Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degterev A., Ofengeim D., Yuan J. Targeting RIPK1 for the treatment of human diseases. Proc. Natl. Acad. Sci. USA. 2019;116(20):9714–9722. doi: 10.1073/pnas.1901179116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry P.J., Vo A.T.T., Gnanansekaran A., Mitra J., Liopo A.V., Hegde M.L., Tsai A.L., Tour J.M., Kent T.A. The chemical basis of intracerebral hemorrhage and cell toxicity with contributions from eryptosis and ferroptosis. Front. Cell. Neurosci. 2020;14 doi: 10.3389/fncel.2020.603043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamoon M.S., Thaler A., Gururangan K., Kohli A., Sisniega D., Wheelwright D., Mensching C., Fifi J.T., Fara M.G., Jette N., Cohen E., Dave P., DiRisio A.C., Goldstein J., Loebel E.M., Mayman N.A., Sharma A., Thomas D.S., Vega Perez R.D., Weingarten M.R., Wen H.H., Tuhrim S., Stein L.K. Acute cerebrovascular events with COVID-19 infection. Stroke. 2021;52(1):48–56. doi: 10.1161/strokeaha.120.031668. [DOI] [PubMed] [Google Scholar]
- Dharmalingam P., Talakatta G., Mitra J., Wang H., Derry P.J., Nilewski L.G., McHugh E.A., Fabian R.H., Mendoza K., Vasquez V., Hegde P.M., Kakadiaris E., Roy T., Boldogh I., Hegde V.L., Mitra S., Tour J.M., Kent T.A., Hegde M.L. Pervasive genomic damage in experimental intracerebral hemorrhage: therapeutic potential of a mechanistic-based carbon nanoparticle. ACS Nano. 2020;14(3):2827–2846. doi: 10.1021/acsnano.9b05821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon L., McNamara C., Gaur P., Mallon D., Coughlan C., Tona F., Jan W., Wilson M., Jones B. Cerebral microhaemorrhage in COVID-19: a critical illness related phenomenon. Stroke Vasc. Neurol. 2020;5(4):315–322. doi: 10.1136/svn-2020-000652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., Patel D.N., Bauer A.J., Cantley A.M., Yang W.S., Morrison B., 3rd, Stockwell B.R. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G., Lee S., Alfaro-Almagro F., Arthofer C., Wang C., Lange F., Andersson J.L.R., Griffanti L., Duff E., Jbabdi S., Taschler B., Winkler A., Nichols T.E., Collins R., Matthews P.M., Allen N., Miller K.L., Smith S.M. Brain imaging before and after COVID-19 in UK Biobank. medRxiv. 2021 doi: 10.1101/2021.06.11.21258690. 10.1101/2021.06.11.21258690. [DOI] [Google Scholar]
- Echouffo-Tcheugui J.B., Conner S.C., Himali J.J., Maillard P., DeCarli C.S., Beiser A.S., Vasan R.S., Seshadri S. Circulating cortisol and cognitive and structural brain measures. Fram. Heart Study. 2018;91(21):e1961–e1970. doi: 10.1212/wnl.0000000000006549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson M.A., Banks W.A. Neuroimmune axes of the blood-brain barriers and blood-brain interfaces: bases for physiological regulation, disease states, and pharmacological interventions. Pharmacol. Rev. 2018;70(2):278–314. doi: 10.1124/pr.117.014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C.E., Miners J.S., Piva G., Willis C.L., Heard D.M., Kidd E.J., Good M.A., Kehoe P.G. ACE2 activation protects against cognitive decline and reduces amyloid pathology in the Tg2576 mouse model of Alzheimer’s disease. Acta Neuropathol. 2020;139(3):485–502. doi: 10.1007/s00401-019-02098-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyer L., Nencka R., de Clercq E., Seley-Radtke K., Růžek D. Nucleoside analogs as a rich source of antiviral agents active against arthropod-borne flaviviruses. Antivir. Chem. Chemother. 2018 doi: 10.1177/2040206618761299. 26 2040206618761299-2040206618761299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon C., Fasano A. Parkinson’s disease and the COVID-19 pandemic. J. Park. Dis. 2021;11(2):431–444. doi: 10.3233/jpd-202320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger J.C. Imaging the role of inflammation in mood and anxiety-related disorders. Curr. Neuropharmacol. 2018;16(5):533–558. doi: 10.2174/1570159X15666171123201142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M., French S., Ji P., Kim R.C. Cerebral microbleeds in the elderly: a pathological analysis. Stroke. 2010;41(12):2782–2785. doi: 10.1161/STROKEAHA.110.593657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitsiori A., Pugin D., Thieffry C., Lalive P., Vargas M.I. COVID-19 is associated with an unusual pattern of brain microbleeds in critically Ill patients. J. Neuroimaging. 2020;30(5):593–597. doi: 10.1111/jon.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flythe J.E., Assimon M.M., Tugman M.J., Chang E.H., Gupta S., Shah J., Sosa M.A., Renaghan A.D., Melamed M.L., Wilson F.P., Neyra J.A., Rashidi A., Boyle S.M., Anand S., Christov M., Thomas L.F., Edmonston D., Leaf D.E. Characteristics and outcomes of individuals with pre-existing kidney disease and COVID-19 admitted to intensive care units in the United States. Am. J. Kidney Dis. 2021;77(2):190–203. doi: 10.1053/j.ajkd.2020.09.003. e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fond G., Lançon C., Korchia T., Auquier P., Boyer L. The role of inflammation in the treatment of schizophrenia. Front. Psychiatry. 2020;11:160. doi: 10.3389/fpsyt.2020.00160. 〈https://www.frontiersin.org/article/10.3389/fpsyt.2020.00160〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forni D., Cagliani R., Clerici M., Sironi M. Molecular evolution of human coronavirus genomes. Trends Microbiol. 2017;25(1):35–48. doi: 10.1016/j.tim.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamage A.M., Tan K.S., Chan W.O.Y., Liu J., Tan C.W., Ong Y.K., Thong M., Andiappan A.K., Anderson D.E., Wang Y., Wang L.F. Infection of human nasal epithelial cells with SARS-CoV-2 and a 382-nt deletion isolate lacking ORF8 reveals similar viral kinetics and host transcriptional profiles. PLoS Pathog. 2020;16(12) doi: 10.1371/journal.ppat.1009130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Beltran W.F., Lam E.C., St Denis K., Nitido A.D., Garcia Z.H., Hauser B.M., Feldman J., Pavlovic M.N., Gregory D.J., Poznansky M.C., Sigal A., Schmidt A.G., Iafrate A.J., Naranbhai V., Balazs A.B. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021;184(9):2372–2383. doi: 10.1016/j.cell.2021.03.013. e2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Beltran W.F., St Denis K.J., Hoelzemer A., Lam E.C., Nitido A.D., Sheehan M.L., Berrios C., Ofoman O., Chang C.C., Hauser B.M., Feldman J., Gregory D.J., Poznansky M.C., Schmidt A.G., Iafrate A.J., Naranbhai V., Balazs A.B. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. medRxiv. 2021 doi: 10.1101/2021.12.14.21267755. 10.1101/2021.12.14.21267755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg M., Burrell L.M., Velkoska E., Griggs K., Angus P.W., Gibson P.R., Lubel J.S. Upregulation of circulating components of the alternative renin-angiotensin system in inflammatory bowel disease: a pilot study. J. Renin Angiotensin Aldosterone Syst. 2015;16(3):559–569. doi: 10.1177/1470320314521086. [DOI] [PubMed] [Google Scholar]
- Garrigues E., Janvier P., Kherabi Y., Le Bot A., Hamon A., Gouze H., Doucet L., Berkani S., Oliosi E., Mallart E., Corre F., Zarrouk V., Moyer J.-D., Galy A., Honsel V., Fantin B., Nguyen Y. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J. Infect. 2020;81(6):e4–e6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaschler M.M., Stockwell B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017;482(3):419–425. doi: 10.1016/j.bbrc.2016.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidon A., Zolnik T.A., Fidzinski P., Bolduan F., Papoutsi A., Poirazi P., Holtkamp M., Vida I., Larkum M.E. Dendritic action potentials and computation in human layer 2/3 cortical neurons. Science. 2020;367(6473):83–87. doi: 10.1126/science.aax6239. [DOI] [PubMed] [Google Scholar]
- Glass W.G., Subbarao K., Murphy B., Murphy P.M. Mechanisms of host defense following severe acute respiratory syndrome-coronavirus (SARS-CoV) pulmonary infection of mice. J. Immunol. 2004;173(6):4030–4039. doi: 10.4049/jimmunol.173.6.4030. [DOI] [PubMed] [Google Scholar]
- Graham E.L., Clark J.R., Orban Z.S., Lim P.H., Szymanski A.L., Taylor C., DiBiase R.M., Jia D.T., Balabanov R., Ho S.U., Batra A., Liotta E.M., Koralnik I.J. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”. Ann. Clin. Transl. Neurol. 2021;8(5):1073–1085. doi: 10.1002/acn3.51350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R.L., Donaldson E.F., Baric R.S. A decade after SARS: strategies for controlling emerging coronaviruses. Nat. Rev. Microbiol. 2013;11(12):836–848. doi: 10.1038/nrmicro3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni A., Sidney J., Zhang Y., Scheuermann R.H., Peters B., Sette A. A sequence homology and bioinformatic approach can predict candidate targets for immune responses to SARS-CoV-2. Cell Host Microbe. 2020;27(4):671–680. doi: 10.1016/j.chom.2020.03.002. e672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyaeva A.A., Gorbalenya A.E. A nidovirus perspective on SARS-CoV-2. Biochem. Biophys. Res. Commun. 2021;538:24–34. doi: 10.1016/j.bbrc.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadian K., Stockwell B.R. SnapShot: ferroptosis. Cell. 2020;181(5) doi: 10.1016/j.cell.2020.04.039. 1188-1188.e1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeggström J.Z., Funk C.D. Lipoxygenase and leukotriene pathways: biochemistry, biology, and roles in disease. Chem. Rev. 2011;111(10):5866–5898. doi: 10.1021/cr200246d. [DOI] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamre D., Procknow J.J. A new virus isolated from the human respiratory tract. Proc. Soc. Exp. Biol. Med. 1966;121(1):190–193. doi: 10.3181/00379727-121-30734. [DOI] [PubMed] [Google Scholar]
- Han Y., Yuan K., Wang Z., Liu W.-J., Lu Z.-A., Liu L., Shi L., Yan W., Yuan J.-L., Li J.-L., Shi J., Liu Z.-C., Wang G.-H., Kosten T., Bao Y.-P., Lu L. Neuropsychiatric manifestations of COVID-19, potential neurotropic mechanisms, and therapeutic interventions. Transl. Psychiatry. 2021;11(1):499. doi: 10.1038/s41398-021-01629-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Sakata Y., Fukushima K., Maeda T., Arita Y., Shioyama W., Nakaoka Y., Hori Y., Morii E., Aozasa K., Kanakura Y., Yamauchi-Takihara K., Komuro I. Pulmonary arterial hypertension associated with chronic active Epstein-Barr virus infection. Intern. Med. 2011;50(2):119–124. doi: 10.2169/internalmedicine.50.4143. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Perlot T., Rehman A., Trichereau J., Ishiguro H., Paolino M., Sigl V., Hanada T., Hanada R., Lipinski S., Wild B., Camargo S.M., Singer D., Richter A., Kuba K., Fukamizu A., Schreiber S., Clevers H., Verrey F., Rosenstiel P., Penninger J.M. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487(7408):477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassannia B., Wiernicki B., Ingold I., Qu F., Van Herck S., Tyurina Y.Y., Bayır H., Abhari B.A., Angeli J.P.F., Choi S.M., Meul E., Heyninck K., Declerck K., Chirumamilla C.S., Lahtela-Kakkonen M., Van Camp G., Krysko D.V., Ekert P.G., Fulda S., De Geest B.G., Conrad M., Kagan V.E., Vanden Berghe W., Vandenabeele P., Vanden Berghe T. Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. J. Clin. Investig. 2018;128(8):3341–3355. doi: 10.1172/jci99032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde M.L., Hegde P.M., Holthauzen L.M.F., Hazra T.K., Rao K.S.J., Mitra S. Specific inhibition of NEIL-initiated repair of oxidized base damage in human genome by copper and iron: potential etiological linkage to neurodegenerative diseases. J. Biol. Chem. 2010;285(37):28812–28825. doi: 10.1074/jbc.M110.126664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., Collange O., Boulay C., Fafi-Kremer S., Ohana M., Anheim M., Meziani F. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman A.B., Silva Afonso M., Kelemen S.E., Ray M., Vrakas C.N., Burke A.C., Scalia R.G., Moore K., Autieri M.V. Regulation of stress granule formation by inflammation, vascular injury, and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2019;39(10):2014–2027. doi: 10.1161/atvbaha.119.313034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho F.K., Petermann-Rocha F., Gray S.R., Jani B.D., Katikireddi S.V., Niedzwiedz C.L., Foster H., Hastie C.E., Mackay D.F., Gill J.M.R., O'Donnell C., Welsh P., Mair F., Sattar N., Celis-Morales C.A., Pell J.P. Is older age associated with COVID-19 mortality in the absence of other risk factors? General population cohort study of 470,034 participants. PLoS One. 2020;15(11) doi: 10.1371/journal.pone.0241824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Zeng L.P., Yang X.L., Ge X.Y., Zhang W., Li B., Xie J.Z., Shen X.R., Zhang Y.Z., Wang N., Luo D.S., Zheng X.S., Wang M.N., Daszak P., Wang L.F., Cui J., Shi Z.L. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog. 2017;13(11) doi: 10.1371/journal.ppat.1006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., Crackower M.A., Fukamizu A., Hui C.C., Hein L., Uhlig S., Slutsky A.S., Jiang C., Penninger J.M. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob F., Pather S.R., Huang W.K., Zhang F., Wong S.Z.H., Zhou H., Cubitt B., Fan W., Chen C.Z., Xu M., Pradhan M., Zhang D.Y., Zheng W., Bang A.G., Song H., Carlos de la Torre J., Ming G.L. Human pluripotent stem cell-derived neural cells and brain organoids reveal SARS-CoV-2 neurotropism predominates in choroid plexus epithelium. Cell Stem Cell. 2020;27(6):937–950. doi: 10.1016/j.stem.2020.09.016. e939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungreis I., Sealfon R., Kellis M. SARS-CoV-2 gene content and COVID-19 mutation impact by comparing 44 Sarbecovirus genomes. Nat. Commun. 2021;12(1):2642. doi: 10.1038/s41467-021-22905-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurk D., Wilson C., Passos J.F., Oakley F., Correia-Melo C., Greaves L., Saretzki G., Fox C., Lawless C., Anderson R., Hewitt G., Pender S.L.F., Fullard N., Nelson G., Mann J., van de Sluis B., Mann D.A., von Zglinicki T. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat. Commun. 2014;5(1):4172. doi: 10.1038/ncomms5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelber S., Pantcheva P., Borlongan C.V. Drug- and cell-based therapies for targeting neuroinflammation in traumatic brain injury. Neural Regen. Res. 2016;11(10):1575–1576. doi: 10.4103/1673-5374.193231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan V.E., Mao G., Qu F., Angeli J.P., Doll S., Croix C.S., Dar H.H., Liu B., Tyurin V.A., Ritov V.B., Kapralov A.A., Amoscato A.A., Jiang J., Anthonymuthu T., Mohammadyani D., Yang Q., Proneth B., Klein-Seetharaman J., Watkins S., Bahar I., Greenberger J., Mallampalli R.K., Stockwell B.R., Tyurina Y.Y., Conrad M., Bayır H. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 2017;13(1):81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C., Xu Q., Martin T.D., Li M.Z., Demaria M., Aron L., Lu T., Yankner B.A., Campisi J., Elledge S.J. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science. 2015;349(6255):aaa5612. doi: 10.1126/science.aaa5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatzaferi C., Sandri M., Sakkas G.K., Smith C. Effects of redox disturbances on motility, contractility and muscle tissue pathogenesis. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/3272035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J.M., Libman R.B., Wang J.J., Sanelli P., Filippi C.G., Gribko M., Pacia S.V., Kuzniecky R.I., Najjar S., Azhar S. Cerebrovascular complications of COVID-19. Stroke. 2020;51(9):e227–e231. doi: 10.1161/strokeaha.120.031265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren-Shaul H., Spinrad A., Weiner A., Matcovitch-Natan O., Dvir-Szternfeld R., Ulland T.K., David E., Baruch K., Lara-Astaiso D., Toth B., Itzkovitz S., Colonna M., Schwartz M., Amit I. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell. 2017;169(7):1276–1290. doi: 10.1016/j.cell.2017.05.018. e1217. [DOI] [PubMed] [Google Scholar]
- Kido H., Yokogoshi Y., Sakai K., Tashiro M., Kishino Y., Fukutomi A., Katunuma N. Isolation and characterization of a novel trypsin-like protease found in rat bronchiolar epithelial Clara cells. A possible activator of the viral fusion glycoprotein. J. Biol. Chem. 1992;267(19):13573–13579. [PubMed] [Google Scholar]
- Kido H., Murakami M., Oba K., Chen Y., Towatari T. Cellular proteinases trigger the infectivity of the influenza A and Sendai viruses. Mol. Cells. 1999;9(3):235–244. [PubMed] [Google Scholar]
- Kim D.R., Sharmin S., Inoue M., Kido H. Cloning and expression of novel mosaic serine proteases with and without a transmembrane domain from human lung. Biochim. Biophys. Acta. 2001;1518(1–2):204–209. doi: 10.1016/s0167-4781(01)00184-1. [DOI] [PubMed] [Google Scholar]
- Kim Y., Connor J., Stahl M. Alpha-synuclein protein homeostasis and oligomerization in iron-overloaded cells expressing mutant HFE (P3.059) Neurology. 2018;90(Suppl. 15) P3.059. [Google Scholar]
- Kirschenbaum D., Imbach L.L., Rushing E.J., Frauenknecht K.B.M., Gascho D., Ineichen B.V., Keller E., Kohler S., Lichtblau M., Reimann R.R., Schreib K., Ulrich S., Steiger P., Aguzzi A., Frontzek K. Intracerebral endotheliitis and microbleeds are neuropathological features of COVID-19. Neuropathol. Appl. Neurobiol. 2021;47(3):454–459. doi: 10.1111/nan.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempin F., Mosienko V., Matthes S., Villela D.C., Todiras M., Penninger J.M., Bader M., Santos R.A.S., Alenina N. Depletion of angiotensin-converting enzyme 2 reduces brain serotonin and impairs the running-induced neurogenic response. Cell. Mol. Life Sci. 2018;75(19):3625–3634. doi: 10.1007/s00018-018-2815-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koka V., Huang X.R., Chung A.C., Wang W., Truong L.D., Lan H.Y. Angiotensin II up-regulates angiotensin I-converting enzyme (ACE), but down-regulates ACE2 via the AT1-ERK/p38 MAP kinase pathway. Am. J. Pathol. 2008;172(5):1174–1183. doi: 10.2353/ajpath.2008.070762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshimizu H., Kiyosue K., Hara T., Hazama S., Suzuki S., Uegaki K., Nagappan G., Zaitsev E., Hirokawa T., Tatsu Y., Ogura A., Lu B., Kojima M. Multiple functions of precursor BDNF to CNS neurons: negative regulation of neurite growth, spine formation and cell survival. Mol. Brain. 2009;2(1):27. doi: 10.1186/1756-6606-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Thambiraja T.S., Karuppanan K., Subramaniam G. Omicron and delta variant of SARS-CoV-2: a comparative computational study of spike protein. J. Med. Virol. 2021 doi: 10.1002/jmv.27526. 10.1002/jmv.27526. [DOI] [PubMed] [Google Scholar]
- Kvernland A., Kumar A., Yaghi S., Raz E., Frontera J., Lewis A., Czeisler B., Kahn D.E., Zhou T., Ishida K., Torres J., Riina H.A., Shapiro M., Nossek E., Nelson P.K., Tanweer O., Gordon D., Jain R., Dehkharghani S., Henninger N., de Havenon A., Grory B.M., Lord A., Melmed K. Anticoagulation use and hemorrhagic stroke in SARS-CoV-2 patients treated at a New York healthcare system. Neurocrit. Care. 2021;34(3):748–759. doi: 10.1007/s12028-020-01077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.-H., Giuliani F. The role of inflammation in depression and fatigue. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.01696. 1696-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Arcila-Londono X., Steijlen K., Newman D., Grover K. Case report of ALS patient with COVID-19 infection (5032) Neurology. 2021;96(Suppl. 15):S5032. [Google Scholar]
- Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5(4):562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Cao F., Yin H.L., Huang Z.J., Lin Z.T., Mao N., Sun B., Wang G. Ferroptosis: past, present and future. Cell Death Dis. 2020;11(2):88. doi: 10.1038/s41419-020-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Wang X., Chen J., Zhang H., Deng A. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol. 2020;5(7):825–830. doi: 10.1001/jamacardio.2020.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Wohlford-Lenane C., Perlman S., Zhao J., Jewell A.K., Reznikov L.R., Gibson-Corley K.N., Meyerholz D.K., McCray P.B., Jr. Middle East respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J. Infect. Dis. 2016;213(5):712–722. doi: 10.1093/infdis/jiv499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Shi Z., Yu M., Ren W., Smith C., Epstein J.H., Wang H., Crameri G., Hu Z., Zhang H., Zhang J., McEachern J., Field H., Daszak P., Eaton B.T., Zhang S., Wang L.F. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310(5748):676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- Lin C.M., Saif L.J., Marthaler D., Wang Q. Evolution, antigenicity and pathogenicity of global porcine epidemic diarrhea virus strains. Virus Res. 2016;226:20–39. doi: 10.1016/j.virusres.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D.X., Fung T.S., Chong K.K., Shukla A., Hilgenfeld R. Accessory proteins of SARS-CoV and other coronaviruses. Antivir. Res. 2014;109:97–109. doi: 10.1016/j.antiviral.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Chen W., Chen J.P. Viral metagenomics revealed Sendai virus and coronavirus infection of Malayan Pangolins (Manis javanica) Viruses. 2019;11(11) doi: 10.3390/v11110979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., Kucher N., Studt J.D., Sacco C., Bertuzzi A., Sandri M.T., Barco S. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Leon S., Wegman-Ostrosky T., Perelman C., Sepulveda R., Rebolledo P.A., Cuapio A., Villapol S. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci. Rep. 2021;11(1):16144. doi: 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu N., Yang Y., Wang Y., Liu Y., Fu G., Chen D., Dai H., Fan X., Hui R., Zheng Y. ACE2 gene polymorphism and essential hypertension: an updated meta-analysis involving 11,051 subjects. Mol. Biol. Rep. 2012;39(6):6581–6589. doi: 10.1007/s11033-012-1487-1. [DOI] [PubMed] [Google Scholar]
- Lun M.P., Monuki E.S., Lehtinen M.K. Development and functions of the choroid plexus-cerebrospinal fluid system. Nat. Rev. Neurosci. 2015;16(8):445–457. doi: 10.1038/nrn3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Gholam Azad M., Dharmasivam M., Richardson V., Quinn R.J., Feng Y., Pountney D.L., Tonissen K.F., Mellick G.D., Yanatori I., Richardson D.R. Parkinson’s disease: alterations in iron and redox biology as a key to unlock therapeutic strategies. Redox Biol. 2021;41 doi: 10.1016/j.redox.2021.101896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestro S., Córdoba K.M., Olague C., Argemi J., Ávila M.A., González-Aseguinolaza G., Smerdou C., Fontanellas A. Heme oxygenase-1 inducer hemin does not inhibit SARS-CoV-2 virus infection. Biomed. Pharmacother. 2021;137 doi: 10.1016/j.biopha.2021.111384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi F., Rosellini A., Spezia P.G., Focosi D., Macera L., Lai M., Pistello M., de Iure A., Tomino C., Bonassi S., Russo P. Nicotine upregulates ACE2 expression and increases competence for SARS-CoV-2 in human pneumocytes. ERJ Open Res. 2021;7(2) doi: 10.1183/23120541.00713-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margos N.P., Meintanopoulos A.S., Filioglou D., Ellul J. Intracerebral hemorrhage in COVID-19: a narrative review. J. Clin. Neurosci. 2021;89:271–278. doi: 10.1016/j.jocn.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín P.L., Ceccatto P., Razori M.V., Francés D.E.A., Arriaga S.M.M., Pisani G.B., Martínez A.I., Sánchez Pozzi E.J., Roma M.G., Basiglio C.L. Heme oxygenase-1 induction by hemin prevents oxidative stress-induced acute cholestasis in the rat. Clin. Sci. 2019;133(1):117–134. doi: 10.1042/cs20180675. [DOI] [PubMed] [Google Scholar]
- Mathys H., Davila-Velderrain J., Peng Z., Gao F., Mohammadi S., Young J.Z., Menon M., He L., Abdurrob F., Jiang X., Martorell A.J., Ransohoff R.M., Hafler B.P., Bennett D.A., Kellis M., Tsai L.H. Single-cell transcriptomic analysis of Alzheimer's disease. Nature. 2019;570(7761):332–337. doi: 10.1038/s41586-019-1195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh K., Dees J.H., Becker W.B., Kapikian A.Z., Chanock R.M. Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc. Natl. Acad. Sci. USA. 1967;57(4):933–940. doi: 10.1073/pnas.57.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh K., Becker W.B., Chanock R.M. Growth in suckling-mouse brain of “IBV-like” viruses from patients with upper respiratory tract disease. Proc. Natl. Acad. Sci. USA. 1967;58(6):2268–2273. doi: 10.1073/pnas.58.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhee M.D., Keough M.T., Rundle S., Heath L.M., Wardell J.D., Hendershot C.S. Depression, environmental reward, coping motives and alcohol consumption during the COVID-19 pandemic. Front. Psychiatry. 2020;11(1128) doi: 10.3389/fpsyt.2020.574676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ME J.W., Adair K., Brierley L. RNA viruses: a case study of the biology of emerging infectious diseases. Microbiol. Spectr. 2013;1(1) doi: 10.1128/microbiolspec.OH-0001-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt J., Radke J., Dittmayer C., Franz J., Thomas C., Mothes R., Laue M., Schneider J., Brünink S., Greuel S., Lehmann M., Hassan O., Aschman T., Schumann E., Chua R.L., Conrad C., Eils R., Stenzel W., Windgassen M., Rößler L., Goebel H.H., Gelderblom H.R., Martin H., Nitsche A., Schulz-Schaeffer W.J., Hakroush S., Winkler M.S., Tampe B., Scheibe F., Körtvélyessy P., Reinhold D., Siegmund B., Kühl A.A., Elezkurtaj S., Horst D., Oesterhelweg L., Tsokos M., Ingold-Heppner B., Stadelmann C., Drosten C., Corman V.M., Radbruch H., Heppner F.L. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021;24(2):168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- de Melo G.D., Lazarini F., Levallois S., Hautefort C., Michel V., Larrous F., Verillaud B., Aparicio C., Wagner S., Gheusi G., Kergoat L., Kornobis E., Donati F., Cokelaer T., Hervochon R., Madec Y., Roze E., Salmon D., Bourhy H., Lecuit M., Lledo P.M. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci. Transl. Med. 2021;13(596) doi: 10.1126/scitranslmed.abf8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez-Guerrero A., Laespada-García M.I., Gómez-Grande A., Ruiz-Ortiz M., Blanco-Palmero V.A., Azcarate-Diaz F.J., Rábano-Suárez P., Álvarez-Torres E., de Fuenmayor-Fernández de la Hoz C.P., Vega Pérez D., Rodríguez-Montalbán R., Pérez-Rivilla A., Sayas Catalán J., Ramos-González A., González de la Aleja J. Acute hypokinetic-rigid syndrome following SARS-CoV-2 infection. Neurology. 2020;95(15):e2109–e2118. doi: 10.1212/wnl.0000000000010282. [DOI] [PubMed] [Google Scholar]
- Miller W.A., Koev G. Synthesis of subgenomic RNAs by positive-strand RNA viruses. Virology. 2000;273(1):1–8. doi: 10.1006/viro.2000.0421. [DOI] [PubMed] [Google Scholar]
- Mirzadeh Z., Merkle F.T., Soriano-Navarro M., Garcia-Verdugo J.M., Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3(3):265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuiri S., Hemmi H., Arita M., Ohashi Y., Tanaka Y., Miyagi M., Sakai K., Ishikawa Y., Shibuya K., Hase H., Aikawa A. Expression of ACE and ACE2 in individuals with diabetic kidney disease and healthy controls. Am. J. Kidney Dis. 2008;51(4):613–623. doi: 10.1053/j.ajkd.2007.11.022. [DOI] [PubMed] [Google Scholar]
- Modin D., Claggett B., Sindet-Pedersen C., Lassen M.C.H., Skaarup K.G., Jensen J.U.S., Fralick M., Schou M., Lamberts M., Gerds T., Fosbøl E.L., Phelps M., Kragholm K.H., Andersen M.P., Køber L., Torp-Pedersen C., Solomon S.D., Gislason G., Biering-Sørensen T. Acute COVID-19 and the incidence of ischemic stroke and acute myocardial infarction. Circulation. 2020;142(21):2080–2082. doi: 10.1161/circulationaha.120.050809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., Ueno M., Sakata H., Kondo K., Myose N., Nakao A., Takeda M., Haro H., Inoue O., Suzuki-Inoue K., Kubokawa K., Ogihara S., Sasaki T., Kinouchi H., Kojin H., Ito M., Onishi H., Shimizu T., Sasaki Y., Enomoto N., Ishihara H., Furuya S., Yamamoto T., Shimada S. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muccioli L., Pensato U., Cani I., Guarino M., Cortelli P., Bisulli F. COVID-19-associated encephalopathy and cytokine-mediated neuroinflammation. Ann. Neurol. 2020;88(4):860–861. doi: 10.1002/ana.25855. [DOI] [PubMed] [Google Scholar]
- Müller N., Weidinger E., Leitner B., Schwarz M.J. The role of inflammation in schizophrenia. Front. Neurosci. 2015;9 doi: 10.3389/fnins.2015.00372. 372-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M., Towatari T., Ohuchi M., Shiota M., Akao M., Okumura Y., Parry M.A., Kido H. Mini-plasmin found in the epithelial cells of bronchioles triggers infection by broad-spectrum influenza A viruses and Sendai virus. Eur. J. Biochem. 2001;268(10):2847–2855. doi: 10.1046/j.1432-1327.2001.02166.x. [DOI] [PubMed] [Google Scholar]
- Nader D., Fletcher N., Curley G.F., Kerrigan S.W. SARS-CoV-2 uses major endothelial integrin αvβ3 to cause vascular dysregulation in-vitro during COVID-19. PLoS One. 2021;16(6) doi: 10.1371/journal.pone.0253347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahum J., Morichau-Beauchant T., Daviaud F., Echegut P., Fichet J., Maillet J.M., Thierry S. Venous thrombosis among critically Ill patients with coronavirus disease 2019 (COVID-19. JAMA Netw. Open. 2020;3(5) doi: 10.1001/jamanetworkopen.2020.10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Z.M., Nash R.P., Laughon S.L., Rosenstein D.L. Neuropsychiatric complications of COVID-19. Curr. Psychiatry Rep. 2021;23(5) doi: 10.1007/s11920-021-01237-9. 25-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S., Ahluwalia N., Bikdeli B., Dietz D., Der-Nigoghossian C., Liyanage-Don N., Rosner G.F., Bernstein E.J., Mohan S., Beckley A.A., Seres D.S., Choueiri T.K., Uriel N., Ausiello J.C., Accili D., Freedberg D.E., Baldwin M., Schwartz A., Brodie D., Garcia C.K., Elkind M.S.V., Connors J.M., Bilezikian J.P., Landry D.W., Wan E.Y. Post-acute COVID-19 syndrome. Nat. Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranbhai V., Garcia-Beltran W.F., Chang C.C., Mairena C.B., Thierauf J.C., Kirkpatrick G., Onozato M.L., Cheng J., St, Denis K.J., Lam E.C., Kaseke C., Tano-Menka R., Yang D., Pavlovic M., Yang W., Kui A., Miller T.E., Astudillo M.G., Cahill J.E., Dighe A.S., Gregory D.J., Poznansky M.C., Gaiha G.D., Balazs A.B., Iafrate A.J. Comparative immunogenicity and effectiveness of mRNA-1273, BNT162b2 and Ad26.COV2.S COVID-19 vaccines. J. Infect. Dis. 2021 doi: 10.1093/infdis/jiab593. 10.1093/infdis/jiab593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauen D.W., Hooper J.E., Stewart C.M., Solomon I.H. Assessing brain capillaries in coronavirus disease 2019. JAMA Neurol. 2021;78(6):760–762. doi: 10.1001/jamaneurol.2021.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehme J., Borghesan M., Mackedenski S., Bird T.G., Demaria M. Cellular senescence as a potential mediator of COVID-19 severity in the elderly. Aging Cell. 2020;19(10) doi: 10.1111/acel.13237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemani K., Li C., Olfson M., Blessing E.M., Razavian N., Chen J., Petkova E., Goff D.C. Association of psychiatric disorders with mortality among patients with COVID-19. JAMA Psychiatry. 2021;78(4):380–386. doi: 10.1001/jamapsychiatry.2020.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008;82(15):7264–7275. doi: 10.1128/jvi.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng F., Berk M., Dean O., Bush A.I. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int. J. Neuropsychopharmacol. 2008;11(6):851–876. doi: 10.1017/s1461145707008401. [DOI] [PubMed] [Google Scholar]
- Okumura Y., Takahashi E., Yano M., Ohuchi M., Daidoji T., Nakaya T., Böttcher E., Garten W., Klenk H.D., Kido H. Novel type II transmembrane serine proteases, MSPL and TMPRSS13, proteolytically activate membrane fusion activity of the hemagglutinin of highly pathogenic avian influenza viruses and induce their multicycle replication. J. Virol. 2010;84(10):5089–5096. doi: 10.1128/jvi.02605-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oude Munnink B.B., Sikkema R.S., Nieuwenhuijse D.F., Molenaar R.J., Munger E., Molenkamp R., van der Spek A., Tolsma P., Rietveld A., Brouwer M., Bouwmeester-Vincken N., Harders F., Hakze-van der Honing R., Wegdam-Blans M.C.A., Bouwstra R.J., GeurtsvanKessel C., van der Eijk A.A., Velkers F.C., Smit L.A.M., Stegeman A., van der Poel W.H.M., Koopmans M.P.G. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science. 2021;371(6525):172–177. doi: 10.1126/science.abe5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudit G.Y., Kassiri Z., Jiang C., Liu P.P., Poutanen S.M., Penninger J.M., Butany J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur. J. Clin. Investig. 2009;39(7):618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomar-Ciria N., Blanco Del Valle P., Hernández-Las Heras M.Á., Martínez-Gallardo R. Schizophrenia and COVID-19 delirium. Psychiatry Res. 2020;290 doi: 10.1016/j.psychres.2020.113137. 113137-113137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A.B., Verma A. Nasal ACE2 levels and COVID-19 in children. JAMA. 2020;323(23):2386–2387. doi: 10.1001/jama.2020.8946. [DOI] [PubMed] [Google Scholar]
- Patel V.B., Bodiga S., Basu R., Das S.K., Wang W., Wang Z., Lo J., Grant M.B., Zhong J., Kassiri Z., Oudit G.Y. Loss of angiotensin-converting enzyme-2 exacerbates diabetic cardiovascular complications and leads to systolic and vascular dysfunction: a critical role of the angiotensin II/AT1 receptor axis. Circ. Res. 2012;110(10):1322–1335. doi: 10.1161/circresaha.112.268029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzel V., Rutz S., Dietrich I., Köberle C., Scheffold A., Kaufmann S.H. Design of siRNAs producing unstructured guide-RNAs results in improved RNA interference efficiency. Nat. Biotechnol. 2005;23(11):1440–1444. doi: 10.1038/nbt1151. [DOI] [PubMed] [Google Scholar]
- Pavlovich S.S., Lovett S.P., Koroleva G., Guito J.C., Arnold C.E., Nagle E.R., Kulcsar K., Lee A., Thibaud-Nissen F., Hume A.J., Mühlberger E., Uebelhoer L.S., Towner J.S., Rabadan R., Sanchez-Lockhart M., Kepler T.B., Palacios G. The Egyptian rousette genome reveals unexpected features of bat antiviral immunity. Cell. 2018;173(5):1098–1110. doi: 10.1016/j.cell.2018.03.070. e1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Saez J., Lauer S.A., Kaiser L., Regard S., Delaporte E., Guessous I., Stringhini S., Azman A.S. Serology-informed estimates of SARS-CoV-2 infection fatality risk in Geneva, Switzerland. Lancet Infect. Dis. 2021;21(4):e69–e70. doi: 10.1016/s1473-3099(20)30584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pétrault M., Casolla B., Ouk T., Cordonnier C., Bérézowski V. Cerebral microbleeds: beyond the macroscope. Int. J. Stroke. 2019;14(5):468–475. doi: 10.1177/1747493019830594. [DOI] [PubMed] [Google Scholar]
- Pinto S., Soares J., Silva A., Curral R., Coelho R. COVID-19 suicide survivors-a hidden grieving population. Front. Psychiatry. 2020;11 doi: 10.3389/fpsyt.2020.626807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott H.C. Outcomes for patients following hospitalization for COVID-19. JAMA. 2021;325(15):1511–1512. doi: 10.1001/jama.2021.3430. [DOI] [PubMed] [Google Scholar]
- Qi F., Qian S., Zhang S., Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem. Biophys. Res. Commun. 2020;526(1):135–140. doi: 10.1016/j.bbrc.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J.-L., Pan J.-S., Lu Y.-P., Sun P., Han J. Inflammatory signaling and cellular senescence. Cell Signal. 2009;21(3):378–383. doi: 10.1016/j.cellsig.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud M., Thibault C., Le Normand F., McDonald E.G., Gallix B., Debry C., Venkatasamy A. Clinical outcomes for patients with anosmia 1 year after COVID-19 diagnosis. JAMA Netw. Open. 2021;4(6) doi: 10.1001/jamanetworkopen.2021.15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J.L., Mahajan S.D. SARS-COV2 alters blood brain barrier integrity contributing to neuro-inflammation. J. Neuroimmune Pharmacol. 2021;16(1):4–6. doi: 10.1007/s11481-020-09975-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhea E.M., Logsdon A.F., Hansen K.M., Williams L.M., Reed M.J., Baumann K.K., Holden S.J., Raber J., Banks W.A., Erickson M.A. The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice. Nat. Neurosci. 2021;24(3):368–378. doi: 10.1038/s41593-020-00771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricke K.M., Cruz S.A., Qin Z., Farrokhi K., Sharmin F., Zhang L., Zasloff M.A., Stewart A.F.R., Chen H.H. Neuronal protein tyrosine phosphatase 1B Hastens amyloid β-associated Alzheimer’s disease in mice. J. Neurosci. 2020;40(7):1581–1593. doi: 10.1523/jneurosci.2120-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizk T., Turtzo L.C., Cota M., Van Der Merwe A.J., Latour L., Whiting M.D., Chan L. Traumatic microbleeds persist for up to five years following traumatic brain injury despite resolution of other acute findings on MRI. Brain Inj. 2020;34(6):773–781. doi: 10.1080/02699052.2020.1725835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson F., Khan K.S., Le T.K., Paris C., Demirbag S., Barfuss P., Rocchi P., Ng W.-L. Coronavirus RNA proofreading: molecular basis and therapeutic targeting. Mol. Cell. 2020;79(5):710–727. doi: 10.1016/j.molcel.2020.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala Frigerio C., Wolfs L., Fattorelli N., Thrupp N., Voytyuk I., Schmidt I., Mancuso R., Chen W.T., Woodbury M.E., Srivastava G., Möller T., Hudry E., Das S., Saido T., Karran E., Hyman B., Perry V.H., Fiers M., De Strooper B. The major risk factors for Alzheimer’s disease: age, sex, and genes modulate the microglia response to Aβ plaques. Cell Rep. 2019;27(4):1293–1306. doi: 10.1016/j.celrep.2019.03.099. e1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar M.R. Is hypertension without any other comorbidities an independent predictor for COVID-19 severity and mortality? J. Clin. Hypertens. 2021;23(2):232–234. doi: 10.1111/jch.14144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio Rocha-Filho P.A., Voss L. Persistent headache and persistent anosmia associated With COVID-19. Headache. 2020;60(8):1797–1799. doi: 10.1111/head.13941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt F., Weisblum Y., Rutkowska M., Poston D., DaSilva J., Zhang F., Bednarski E., Cho A., Schaefer-Babajew D.J., Gaebler C., Caskey M., Nussenzweig M.C., Hatziioannou T., Bieniasz P.D. High genetic barrier to SARS-CoV-2 polyclonal neutralizing antibody escape. Nature. 2021;600(7889):512–516. doi: 10.1038/s41586-021-04005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schountz T., Baker M.L., Butler J., Munster V. Immunological control of viral infections in bats and the emergence of viruses highly pathogenic to humans. Front. Immunol. 2017;8:1098. doi: 10.3389/fimmu.2017.01098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah N.G., Benjannet S., Pareek S., Chrétien M., Murphy R.A. Cellular processing of the neurotrophin precursors of NT3 and BDNF by the mammalian proprotein convertases. FEBS Lett. 1996;379(3):247–250. doi: 10.1016/0014-5793(95)01520-5. [DOI] [PubMed] [Google Scholar]
- Seiwert N., Wecklein S., Demuth P., Hasselwander S., Kemper T.A., Schwerdtle T., Brunner T., Fahrer J. Heme oxygenase 1 protects human colonocytes against ROS formation, oxidative DNA damage and cytotoxicity induced by heme iron, but not inorganic iron. Cell Death Dis. 2020;11(9):787. doi: 10.1038/s41419-020-02950-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepehry A.A., Lang D., Hsiung G.Y., Rauscher A. Prevalence of brain microbleeds in Alzheimer disease: a systematic review and meta-analysis on the influence of neuroimaging techniques. AJNR Am. J. Neuroradiol. 2016;37(2):215–222. doi: 10.3174/ajnr.A4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon-Loriere E., Holmes E.C. Why do RNA viruses recombine? Nat. Rev. Microbiol. 2011;9(8):617–626. doi: 10.1038/nrmicro2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer D., Camargo S.M. Collectrin and ACE2 in renal and intestinal amino acid transport. Channels. 2011;5(5):410–423. doi: 10.4161/chan.5.5.16470. [DOI] [PubMed] [Google Scholar]
- Stojanović S.D., Fiedler J., Bauersachs J., Thum T., Sedding D.G. Senescence-induced inflammation: an important player and key therapeutic target in atherosclerosis. Eur. Heart J. 2020;41(31):2983–2996. doi: 10.1093/eurheartj/ehz919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S., Wong G., Shi W., Liu J., Lai A.C.K., Zhou J., Liu W., Bi Y., Gao G.F. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D., Antonini A., Leta V., Nordvig A., Smeyne R.J., Goldman J.E., Al-Dalahmah O., Zecca L., Sette A., Bubacco L., Meucci O., Moro E., Harms A.S., Xu Y., Fahn S., Ray Chaudhuri K. COVID-19 and possible links with Parkinson’s disease and parkinsonism: from bench to bedside. NPJ Park. Dis. 2020;6(1):18. doi: 10.1038/s41531-020-00123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumbria R.K., Grigoryan M.M., Vasilevko V., Paganini-Hill A., Kilday K., Kim R., Cribbs D.H., Fisher M.J. Aging exacerbates development of cerebral microbleeds in a mouse model. J. Neuroinflamm. 2018;15(1):69. doi: 10.1186/s12974-018-1092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungnak W., Huang N., Bécavin C., Berg M., Queen R., Litvinukova M., Talavera-López C., Maatz H., Reichart D., Sampaziotis F., Worlock K.B., Yoshida M., Barnes J.L. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020;26(5):681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M., Geddes J.R., Husain M., Luciano S., Harrison P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416–427. doi: 10.1016/s2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M., Luciano S., Geddes J.R., Harrison P.J. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2021;8(2):130–140. doi: 10.1016/s2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S., Su Y., Yu Y., Wu C., Chen J., Wang S., Jiang J. Ribavirin therapy for severe COVID-19: a retrospective cohort study. Int. J. Antimicrob. Agents. 2020;56(3) doi: 10.1016/j.ijantimicag.2020.106114. 106114-106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanowicz B., Ulrich C., Kohler F., Bode V., Seibert E., Fiedler R., Girndt M. Monocytic angiotensin-converting enzyme 2 relates to atherosclerosis in patients with chronic kidney disease. Nephrol. Dial. Transpl. 2017;32(2):287–298. doi: 10.1093/ndt/gfw206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell D.A., Bynoe M.L. Cultivation of viruses from a high proportion of patients with colds. Lancet. 1966;1(7428):76–77. doi: 10.1016/s0140-6736(66)92364-6. [DOI] [PubMed] [Google Scholar]
- Tyrrell D.A., Almeida J.D., Cunningham C.H., Dowdle W.R., Hofstad M.S., McIntosh K., Tajima M., Zakstelskaya L.Y., Easterday B.C., Kapikian A., Bingham R.W. Coronaviridae. Intervirology. 1975;5(1–2):76–82. doi: 10.1159/000149883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahidy F.S., Pan A.P., Ahnstedt H., Munshi Y., Choi H.A., Tiruneh Y., Nasir K., Kash B.A., Andrieni J.D., McCullough L.D. Sex differences in susceptibility, severity, and outcomes of coronavirus disease 2019: Cross-sectional analysis from a diverse US metropolitan area. PLoS One. 2021;16(1) doi: 10.1371/journal.pone.0245556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versmissen J., Verdonk K., Lafeber M., van den Akker J.P.C., Hunfeld N.G.M., Hoorn E.J., Danser A.H.J. Angiotensin-converting enzyme-2 in SARS-CoV-2 infection: good or bad? J. Hypertens. 2020;38(6):1196–1197. doi: 10.1097/HJH.0000000000002472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers T.A., Wyatt J.R., Freier S.M. Effects of RNA secondary structure on cellular antisense activity. Nucleic Acids Res. 2000;28(6):1340–1347. doi: 10.1093/nar/28.6.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D., Kang T.B., Dillon C.P., Green D.R. Programmed necrosis in inflammation: toward identification of the effector molecules. Science. 2016;352(6281):aaf2154. doi: 10.1126/science.aaf2154. [DOI] [PubMed] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292. doi: 10.1016/j.cell.2020.02.058. e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94(7) doi: 10.1128/jvi.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Jiang M., Chen X., Montaner L.J. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J. Leukoc. Biol. 2020;108(1):17–41. doi: 10.1002/jlb.3covr0520-272r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.A., de Kloet A.D., Smeltzer M.D., Cahill K.M., Hiller H., Bruce E.B., Pioquinto D.J., Ludin J.A., Katovich M.J., Raizada M.K., Krause E.G. Coupling corticotropin-releasing-hormone and angiotensin converting enzyme 2 dampens stress responsiveness in male mice. Neuropharmacology. 2018;133:85–93. doi: 10.1016/j.neuropharm.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Xu R., Volkow N.D. Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry. 2021;20(1):124–130. doi: 10.1002/wps.20806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte K.H., Tajima M., Easterday B.C. Morphologic characteristics and nucleic acid type of transmissible gastroenteritis virus of pigs. Arch. Gesamte Virus. 1968;23(1):53–70. doi: 10.1007/bf01242114. [DOI] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Lam C.S., Lau C.C., Tsang A.K., Lau J.H., Bai R., Teng J.L., Tsang C.C., Wang M., Zheng B.J., Chan K.H., Yuen K.Y. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 2012;86(7):3995–4008. doi: 10.1128/jvi.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Li Y., Shen X., Goh G., Zhu Y., Cui J., Wang L.F., Shi Z.L., Zhou P. Dampened STING-dependent interferon activation in bats. Cell Host Microbe. 2018;23(3):297–301. doi: 10.1016/j.chom.2018.01.006. e294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X.Y., Liu L., Wang F.X., Yang Y.R., Hao J.W., Wang P.F., Zhong Q., Zhou K., Xiong A., Zhu W.Y., Zhao T., Meng Z.Y., Wang Y.C., Gong Q.W., Liao M.F., Wang J., Yang Q.W. Toll-like receptor 4/MyD88-mediated signaling of hepcidin expression causing brain iron accumulation, oxidative injury, and cognitive impairment after intracerebral hemorrhage. Circulation. 2016;134(14):1025–1038. doi: 10.1161/circulationaha.116.021881. [DOI] [PubMed] [Google Scholar]
- Yaghi S., Ishida K., Torres J., Mac Grory B., Raz E., Humbert K., Henninger N., Trivedi T., Lillemoe K., Alam S., Sanger M., Kim S., Scher E., Dehkharghani S., Wachs M., Tanweer O., Volpicelli F., Bosworth B., Lord A., Frontera J. SARS-CoV-2 and stroke in a New York healthcare system. Stroke. 2020;51(7):2002–2011. doi: 10.1161/strokeaha.120.030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanez N.D., Weiss N.S., Romand J.-A., Treggiari M.M. COVID-19 mortality risk for older men and women. BMC Public Health. 2020;20(1):1742. doi: 10.1186/s12889-020-09826-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A.C., Kern F., Losada P.M., Agam M.R., Maat C.A., Schmartz G.P., Fehlmann T., Stein J.A., Schaum N., Lee D.P., Calcuttawala K., Vest R.T., Berdnik D., Lu N., Hahn O., Gate D., McNerney M.W., Channappa D., Cobos I., Ludwig N., Schulz-Schaeffer W.J., Keller A., Wyss-Coray T. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature. 2021;595(7868):565–571. doi: 10.1038/s41586-021-03710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousuf F., King R., Ainger T., Hessler A. Autoimmune encephalitis following recovery of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (2756) Neurology. 2021;96(Suppl. 15):S2756. [Google Scholar]
- Yu Y., Travaglio M., Popovic R., Leal N.S., Martins L.M. Alzheimer’s and Parkinson’s diseases predict different COVID-19 outcomes: a UK biobank study. Geriatrics. 2021;6(1) doi: 10.3390/geriatrics6010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeytinoglu S., Morales S., Lorenzo N.E., Chronis-Tuscano A., Degnan K.A., Almas A.N., Henderson H., Pine D.S., Fox N.A. A developmental pathway from early behavioral inhibition to young adults’ anxiety during the COVID-19 pandemic. J. Am. Acad. Child Adolesc. Psychiatry. 2021 doi: 10.1016/j.jaac.2021.01.021. 10.1016/j.jaac.2021.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Wu Q., Zhang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr. Biol. 2020;30(7):1346–1351. doi: 10.1016/j.cub.2020.03.022. e1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Tachedjian M., Wynne J.W., Boyd V., Cui J., Smith I., Cowled C., Ng J.H., Mok L., Michalski W.P., Mendenhall I.H., Tachedjian G., Wang L.F., Baker M.L. Contraction of the type I IFN locus and unusual constitutive expression of IFN-α in bats. Proc. Natl. Acad. Sci. USA. 2016;113(10):2696–2701. doi: 10.1073/pnas.1518240113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Fan H., Lan T., Yang X.L., Shi W.F., Zhang W., Zhu Y., Zhang Y.W., Xie Q.M., Mani S., Zheng X.S., Li B., Li J.M., Guo H., Pei G.Q., An X.P., Chen J.W., Zhou L., Mai K.J., Wu Z.X., Li D., Anderson D.E., Zhang L.B., Li S.Y., Mi Z.Q., He T.T., Cong F., Guo P.J., Huang R., Luo Y., Liu X.L., Chen J., Huang Y., Sun Q., Zhang X.L., Wang Y.Y., Xing S.Z., Chen Y.S., Sun Y., Li J., Daszak P., Wang L.F., Shi Z.L., Tong Y.G., Ma J.Y. Fatal swine acute diarrhoea syndrome caused by an HKU2-related coronavirus of bat origin. Nature. 2018;556(7700):255–258. doi: 10.1038/s41586-018-0010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z.J., Wu K.C., Yung W.H., Qian Z.M., Ke Y. Differential interaction between iron and mutant alpha-synuclein causes distinctive Parkinsonian phenotypes in Drosophila. Biochim. Biophys. Acta. 2016;1862(4):518–525. doi: 10.1016/j.bbadis.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., Cao Y., Yousif A.S., Bals J., Hauser B.M., Feldman J., Muus C., Wadsworth M.H., 2nd, Kazer S.W., Hughes T.K., Doran B., Gatter G.J., Vukovic M., Taliaferro F., Mead B.E., Guo Z., Wang J.P., Gras D., Plaisant M., Ansari M., Angelidis I., Adler H., Sucre J.M.S., Taylor C.J., Lin B., Waghray A., Mitsialis V., Dwyer D.F., Buchheit K.M., Boyce J.A., Barrett N.A., Laidlaw T.M., Carroll S.L., Colonna L., Tkachev V., Peterson C.W., Yu A., Zheng H.B., Gideon H.P., Winchell C.G., Lin P.L., Bingle C.D., Snapper S.B., Kropski J.A., Theis F.J., Schiller H.B., Zaragosi L.E., Barbry P., Leslie A., Kiem H.P., Flynn J.L., Fortune S.M., Berger B., Finberg R.W., Kean L.S., Garber M., Schmidt A.G., Lingwood D., Shalek A.K., Ordovas-Montanes J. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181(5):1016–1035. doi: 10.1016/j.cell.2020.04.035. e1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y., Estes S.K., Ali R.A., Gandhi A.A., Yalavarthi S., Shi H., Sule G., Gockman K., Madison J.A., Zuo M., Yadav V., Wang J., Woodard W., Lezak S.P., Lugogo N.L., Smith S.A., Morrissey J.H., Kanthi Y., Knight J.S. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci. Transl. Med. 2020;12(570) doi: 10.1126/scitranslmed.abd3876. [DOI] [PMC free article] [PubMed] [Google Scholar]