Abstract
Lenvatinib, a multi-tyrosine kinase inhibitor that inhibits vascular endothelial growth factor and fibroblast growth factor receptors pathway, activated the immune response in tumor microenvironment. However, the combination of lenvatinib and anti-PD-1 has been reported in early phase studies. Hence, this study aims to explore the efficacy and toxicity of lenvatinib combined with nivolumab in the real-world setting. Advanced HCC patients who underwent lenvatinib combined with nivolumab (L + N group) treatment at Taipei Veterans General Hospital (Taipei, Taiwan) were reviewed between January 2016 and December 2020. Treatment response and outcomes were collected and analyzed. A control group with lenvatinib (L group) was also included for comparison. Forty patients were included in L + N group and 47 in L group. The L + N group demonstrated a higher objective response rate than L group (45.0% vs. 23.4%, p = 0.03). The L + N group also achieved longer PFS (7.5 vs. 4.8 months, p = 0.05) and OS (22.9 vs. 10.3 months, p = 0.01) than L group. Patients with HBV infection and REFLECT criteria fit demonstrated a trend of better prognosis. The PFS for those with PR, SD and PD groups were 11.2, 6.4, and 2.2 months and OS were non-reached, 14.6 and 4.7 months, respectively. Portal vein thrombosis (HR 4.3, 95% C.I. 1.5–12.8) and AFP > 400 ng/mL (HR 3.3, 95% C.I. 1.1–9.3) were poor prognostic factors and nivolumab used remained a protective factor (HR 0.2, 95% C.I. 0.1–0.7). Dermatitis (35.0%), pruritis (27.5%), and hypothyroidism (27.5%) were the common toxicities. Few patients developed grade 3/4 toxicities, including dermatitis (15%), gastrointestinal bleeding (7.5%), hypertension (5.0%), pneumonitis (2.5%) and stomatitis (2.5%). This is the first real-world data reporting the promising efficacy and tolerable toxicities of lenvatinib combined with nivolumab in advanced HCC. Further randomized trials are prompted.
Supplementary information
The online version contains supplementary material available at 10.1007/s10637-022-01248-0.
Keywords: Lenvatinib, Nivolumab, Anti-PD-1, Hepatocellular carcinoma (HCC), Vascular endothelial growth factor (VEGF), Fibroblast growth factors receptors (FGFR)
Introduction
Hepatocellular carcinoma (HCC) is the fourth most common cause of cancer-related death worldwide and second most common in Taiwan [1, 2]. For patients with advanced HCC, the prognosis is poor. Sorafenib, a multiple kinase inhibitor, has been the only approved drug for a decade, but the efficacy is limited [3]. Recently, several drugs including lenvatinib, regorafenib, cabozantinib, and ramucirumab have been approved for the treatment of advanced HCC [4–7].Particularly, the combination of atezolizumab (anti-PD-L1) and bevacizumab (anti-VEGF monoclonal antibody)provided longer survival benefit than sorafenib and has become a new standard therapy in first-line treatment for advanced HCC [8]. However, little is known for the combination of multi-kinase inhibitor and anti-PD-1.
Lenvatinib is a multi-tyrosine kinase inhibitor that affects `1–4, platelet-derived growth factor receptor-alpha (PDGFRa), RET, and KIT [9]. By blocking these pathways, it reduces angiogenesis and suppresses tumor growth. Its potent inhibition of FGFR pathway is considered the primary mechanism for controlling liver cancer. In REFLECT study, lenvatinib demonstrated better response and longer PFS, and non-inferiority of survival than that of sorafenib [4].
Immune checkpoint inhibitors have been widely studied in various cancers. Nivolumab and pembrolizumab, two anti-PD-1 agents, become breakthrough therapies in the second-line treatment of advanced HCC [10, 11]. However, in a phase 3 clinical trial (CheckMate-459), nivolumab failed to prove its superiority to sorafenib in the first-line treatment [12]. Therefore, to explore a potential drug for combination becomes the future development for nivolumab.
At present, more and more evidence has shown that VEGF pathway inhibitors have immunomodulatory effects [13]. The combination of VEGF pathway inhibitors and anti-PD-L1 improves treatment efficacy and becomes standard treatment in HCC [8]. Many multi-kinase inhibitors that block VEGF pathway have been proved to have immunomodulatory effects, including lenvatinib [14]. Lenvatinib, a potent FGFR inhibitor, not only suppressed the progression of HCC, but also activated the immune response in tumor microenvironment [15]. Therefore, lenvatinib is potential for the combination of anti-PD-1. However, only a Phase 1 clinical trial showed the efficacy of lenvatinib combined with nivolumab, and no real-world data is reported to respond to the clinical trial [16]. Hence, the purpose of this study is to explore the clinical efficacy and side effects of lenvatinib combined with nivolumab in the real-world settings.
Methods
Patients and study design
Between January 2016 and December 2020, patients with advanced HCC who underwent lenvatinib combined nivolumab (L + N group) at Taipei Veterans General Hospital (Taipei, Taiwan) were retrospectively reviewed. Patients who have Child–Pugh Score C, aged less than 20-year-old, or those without effective assessment were excluded. A total of 40 patients were enrolled after screening. To compare the efficacy between combination therapy and lenvatinib monotherapy, additional 47 HCC patients underwent lenvatinib (L group) as 1st line therapy with the same inclusion criteria were enrolled. Lenvatinib was given 8 or 12 mg based on body weight and nivolumab was given 1–3 mg/kg every 2 weeks. Patient characteristics, such as age, gender, etiologies, liver function, tumor stage, tumor marker, and previous local therapy and systemic therapy history were collected and analyzed. Child–Pugh score and ALBI grade were used to describe liver function, and Barcelona clinic liver cancer (BCLC) staging system to cancer staging. The diagnosis of HCC was defined as histological confirmation or clinical interpretation based on the American Association for the Study of Liver Diseases (AASLD) criteria [17].
Outcome assessment
Treatment response was assessed by computed tomography scans or magnetic resonance imaging every 2–3 months. The treatment response including complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) were reviewed by two independent specialists according to RECIST and mRECIST criteria (19). Treatment adverse events were graded according to the common terminology criteria for adverse events (CTCAE) version 5.0. Overall survival (OS) was defined as the period from the beginning of treatment to death; progression-free survival (PFS) from the beginning of the treatment to disease progression or death.
Statistical analysis
For comparison of continuous variables, the student's t-test was used. For categorical variables between groups, the Chi-square test or Fisher's exact test was performed. We used logistic regression analysis to find the risk factors for death. In the univariate analysis, variables with p < 0.1 underwent multi-variates analysis using forward stepwise model. A p < 0.05 was considered as an independent prognostic factor. The Kaplan–Meier curves were compared by the Log-rank test. A p value < 0.05 was defined as a statistically significant difference. All the statistical analyses were performed by IBM® SPSS®, version 21.0 (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.).
Results
Patient characteristics
Between January 2016 to December 2020, 40 HCC patients received lenvatinib in combination with nivolumab in our center were included. Among them, twenty-nine patients were male. The mean age was 58.5 ± 13.8; 77.5% (29/40) had HBV, 10.0% (4/40) had HCV, and 37.5% (15/40) had alcoholism history. Child–Pugh score A accounted for 62.5% of patients and Child–Pugh score B for 37.5%. Fifty percent of patients were diagnosed to have portal vein thrombosis and 25% already had distant metastasis. Regard to previous sorafenib treatment, 50% of patients has been experienced and 50% were sorafenib naïve. Additionally, 47 HCC patients with lenvatinib monotherapy were enrolled. Lenvatinib group had similar liver function and tumor stage as combination group, but older age and less HBV infection. Notably, all the patients in lenvatinib group were first-line treatment. The subsequent treatment was given in 84.4% (27/32) and 77.8% (21/27) in L + P and L group, respectively (p = 0.52). The detailed demographic and clinical characteristics were shown in Table 1.
Table 1.
Clinical characteristics
| Characteristic | Lenvatinib + Nivolumab (n = 40) | Lenvatinib (n = 47) | p value | ||
|---|---|---|---|---|---|
| Age (year, mean ± SD) | 58.5 ± 13.8 | 70.6 ± 13.3 | < 0.01 | ||
| Male | 29 | 72.5.% | 32 | 68.1% | 0.65 |
| Etiologies | |||||
| HBV | 31 | 77.5% | 26 | 55.3% | 0.03 |
| HCV | 4 | 10.0% | 11 | 23.4% | 0.10 |
| Alcohol | 15 | 37.5% | 12 | 26.7% | 0.28 |
| Child–Pugh score class | 0.23 | ||||
| A | 25 | 62.5% | 35 | 74.5% | |
| B | 15 | 37.5% | 12 | 25.5% | |
| ALBI grade | 0.40 | ||||
| Grade 1 | 12 | 30.0% | 18 | 38.3% | |
| Grade 2 | 21 | 52.5% | 25 | 53.2% | |
| Grade 3 | 7 | 17.5% | 4 | 8.5% | |
| BCLC stage | 0.69 | ||||
| B | 17 | 42.5% | 22 | 46.8% | |
| C | 23 | 57.5% | 25 | 53.2% | |
| PVT | 20 | 50.0% | 14 | 29.8% | 0.05 |
| Metastasis | 10 | 25.0% | 17 | 36.2% | 0.26 |
| AFP > 400 (ng/mL) | 10 | 25.0% | 18 | 38.3% | 0.07 |
| Previous drugs | |||||
| Sorafenib | 20 | 50.0% | 0 | 0% | < 0.01 |
| Regorafenib | 2 | 5.0% | 0 | 0% | 0.21 |
| Cabozatinib | 1 | 5.0% | 0 | 0% | 0.46 |
| Line of treatment | < 0.01 | ||||
| 1st line | 17 | 42.5% | 47 | 100% | |
| 2nd line | 16 | 40.0% | 0 | 0% | |
| ≧3rd line | 7 | 17.5% | 0 | 0% | |
| Previous local therapy | |||||
| Surgery | 10 | 25.0% | 15 | 31.9% | 0.48 |
| RFA | 6 | 15.0% | 17 | 36.2% | 0.03 |
| TACE | 23 | 57.5% | 26 | 55.3% | 0.84 |
ALBI grade albumin-bilirubin grade, PVT portal vein thrombosis, AFP alpha- fetoprotein, RFA radiofrequency ablation, TACE transarterial chemoembolization
Treatment response
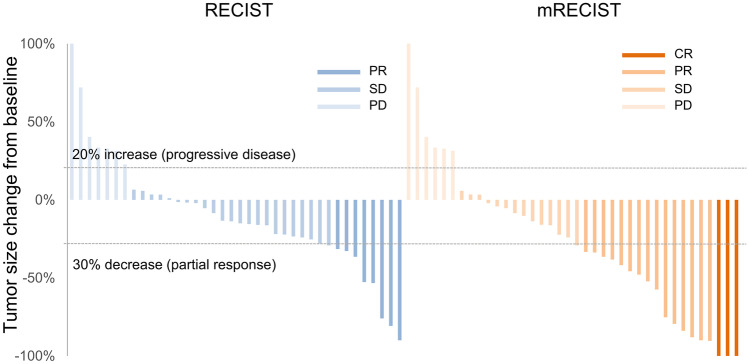
In the L + N group, the ORR was 45.0% and DCR 82.5% by mRECIST, and 20.0%, 82.5% by RECIST 1.1 (Fig. 1). Notably, three patient (7.5%) were defined as CR with mRECIST (one of the cases was shown in supplemental Fig. 2). Compared with the L group, the L + N group had a higher objective response rate (45.0% vs. 23.4%, p = 0.03). In the L + N group, patients with sorafenib naïve, HBV and HCV infection achieved numerically higher response rate without statistically significant (Table 2).
Fig. 1.
Maximum Change from Baseline in the Sum of Longest Diameters Lenvatinib plus nivolumab demonstrated remarkable tumor shrinkage and disease control by RECIST (left) and mRECIST criteria (right). PD was defined as 20% increase in tumor size, while partial response had a 30% decrease. (CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease)
Table 2.
Treatment response by mRECIST criteria
| All patients | ||||||||
|---|---|---|---|---|---|---|---|---|
| CR | PR | SD | PD | ORR | pv | DCR | pv | |
| Lenvatinib + nivolumab | 7.5% | 37.5% | 37.5% | 17.5% | 45.0% | 0.03 | 83.5% | 0.50 |
| Lenvatinib | 6.4% | 17.0% | 28.7% | 23.4% | 23.4% | 76.6% | ||
| Lenvatinib + nivolumab group | ||||||||
| CR | PR | SD | PD | ORR | pv | DCR | pv | |
| Sorafenib | ||||||||
| Naïve | 15.0% | 40.0% | 30.0% | 15.0% | 55.0% | 0.20 | 85.0% | 0.68 |
| Experienced | 0% | 35.0% | 45.0% | 20.0% | 35.0% | 80.0% | ||
| HBV | ||||||||
| Positive | 6.5% | 41.9% | 35.5% | 16.1% | 48.4% | 0.42 | 83.9% | 0.65 |
| Negative | 11.1% | 22.2% | 44.4% | 22.2% | 33.3% | 77.8% | ||
| HCV | ||||||||
| Positive | 0.0% | 50.0% | 25.0% | 25.0% | 50.0% | 0.83 | 75.0% | 0.68 |
| Negative | 8.3% | 36.1% | 38.9% | 16.7% | 44.4% | 82.5% | ||
| REFLECT criteria | ||||||||
| Fit | 5.3% | 42.1% | 47.4% | 5.3% | 47.4% | 0.78 | 94.7% | 0.10 |
| Unfit | 9.5% | 33.3% | 28.6% | 28.6% | 42.9% | 71.4% | ||
CR complete response, PR partial response, SD stable disease, PD progressive disease, ORR objective response rate, DCR disease-control rate, pv p-value
Progression-free survival and overall survival
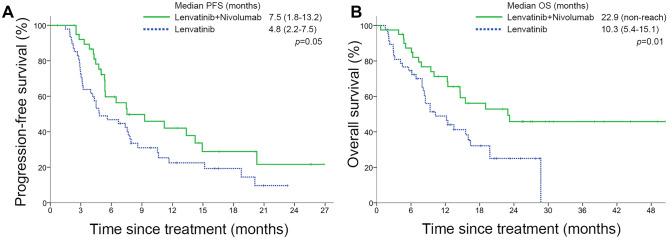
The L + N group achieved longer PFS (7.5 vs. 4.8 months, p = 0.05) and OS (22.9 vs. 10.3 months, p = 0.01) than L group (Fig. 2). Notably, the survival curve of L + N group presented with a long tail. The median follow-up time of all patients was 12.3 months (6.2–21.0).
Fig. 2.
Kaplan–Meier curves for A progression-free survival and B overall survival stratified by treatment
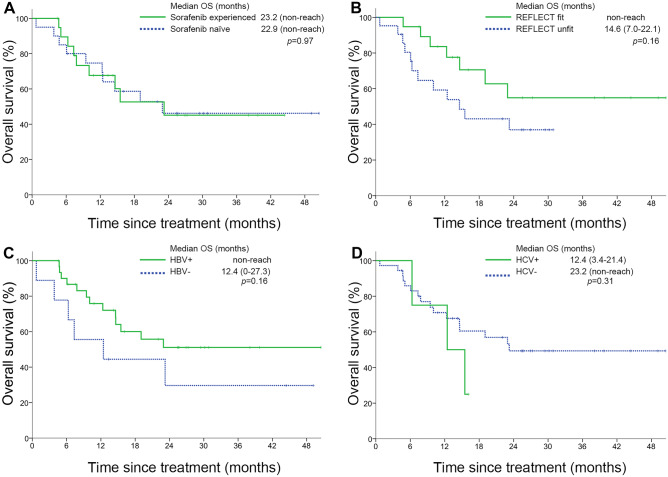
In L + P group, there was no significant difference regardless of sorafenib experienced and HCV infection (Fig. 3A, D). Patients with HBV infection (non-reached vs. 12.4 months, p = 0.16) and REFLECT criteria fit (non-reached vs. 14.6 months, p = 0.16) demonstrated a trend of better prognosis (Fig. 3B, C). For those patients who got response according to RECIST 1.1 criteria achieved longer tumor control and survival. The median OS were significantly different among patients with PR, SD, and PD (non-reached vs. 14.6 vs. 4.7 months, p = 0.03). The median PFS were 11.2 months, 6.4 months, and 2.2 months for patients with PR, SD, and PD respectively(p < 0.0001) (supplemental Fig. 1A, B).
Fig. 3.
Kaplan–Meier curves for overall survival stratified by A sorafenib experienced, B REFLECT criteria, C HBV infection, D HCV infection
Prognostic factors
Among all the 87 HCC patients, HCV, PVT, and AFP > 400 ng/mL were poor prognostic factors and nivolumab used was protective factor in the univariate analysis. In multi-variates analysis, only PVT (HR 4.3, 95% C.I. 1.5–12.8) and AFP > 400 ng/mL (HR 3.3, 95% C.I. 1.1–9.3) were poor prognostic factors and nivolumab used remained a protective factor (HR 0.2, 95% C.I. 0.1–0.7) (Table 3).
Table 3.
Prognostic factors for death (n = 87)
| Variables | Univariate HR (95% CI) | p value | Multi-variates HR (95% CI) | p value | ||
|---|---|---|---|---|---|---|
| General | ||||||
| Age ≥ 60 | 1.1 | (0.5–2.8) | 0.79 | |||
| Male | 0.7 | (0.3–1.7) | 0.40 | |||
| HBV | 0.6 | (0.2–1.5) | 0.27 | |||
| HCV | 3.3 | (0.9–13.0) | 0.08 | 2.3 | (0.5–10.0) | 0.26 |
| CPS class B | 2.1 | (0.8–5.5) | 0.13 | |||
| Nivolumab used | 0.4 | (0.2–1.0) | 0.05 | 0.2 | (0.1–0.7) | 0.01 |
| HCC status | ||||||
| PVT | 2.9 | (1.1–7.3) | 0.03 | 4.3 | (1.5–12.8) | 0.01 |
| Metastasis | 1.0 | (0.4–2.6) | 0.94 | |||
| AFP > 400 (ng/mL) | 2.6 | (1.1–6.4) | 0.03 | 3.3 | (1.1–9.3) | 0.03 |
CPS Child–Pugh score, PVT portal vein thrombosis, AFP alpha- fetoprotein
Toxicity
In all grades of toxicities, dermatitis (35.0%), pruritis (27.5%), and hypothyroidism (27.5%) were most common. Few patients developed grade 3/4 toxicities including dermatitis (15.0%), GI bleeding (7.5%), hypertension (5.0%), pneumonitis (2.5%) and stomatitis (2.5%). Notably, only 5.0% of patients developed grade 1/2 hand-foot skin reaction. Severe adverse events were noted in 10% of patients, that included 2 gastric intestinal bleeding, 1 duodenal perforation and 1 pneumonitis (Table 4).
Table 4.
Adverse events of the lenvatinib combined nivolumab group
| Adverse events | Grade 1/2 | Grade 3/4 | All grades |
|---|---|---|---|
| Dermatitis | 20.0% | 15.0% | 35.0% |
| Pruritus | 27.5% | 0.0% | 27.5% |
| Fatigue | 20.0% | 0.0% | 20.0% |
| Hypertension | 15.0% | 5.0% | 20.0% |
| Diarrhea | 17.5% | 0.0% | 17.5% |
| Dysphonia | 12.5% | 0.0% | 12.5% |
| Stomatitis | 10.0% | 2.5% | 12.5% |
| GI bleeding | 0.0% | 7.5% | 7.5% |
| Pneumonitis | 5.0% | 2.5% | 2.5% |
| HRSR | 5.0% | 0.0% | 5.0% |
| Laboratory test | |||
| Hypothyroidism | 27.5% | 0.0% | 27.5% |
| Proteinuria | 20.0% | 0.0% | 20.0% |
| Neutropenia | 17.5% | 0.0% | 17.5% |
| Thrombocytopenia | 15.0% | 0.0% | 15.0% |
| Anemia | 10.0% | 0.0% | 10.0% |
| SAE* | 0.0% | 10.0% | 10.0% |
HRSR hand-foot skin reaction, SAE severe adverse event
*2 gastric intestinal bleeding, 1 duodenal perforation, 1 pneumonitis
Discussion
To our knowledge, this is the first real-world report regarding the combination use of lenvatinib and nivolumab in advanced HCC which showed promising results with an ORR of 45.0% by mRECIST, PFS of 7.5 months, and OS of 14.6 months. These data suggested that lenvatinib plus nivolumab a potential combination in advanced HCC.
Cumulative evidence disclosed that the activation of FGF pathway signaling had an essential role in developing and worsening HCC [18]. Matsuki et al. performed in vitro studies in human HCC cell lines and in vivo studies in mice xenograft models showing that FGF19 –FGFR4 axis enhanced HCC proliferation and growth [19]. These findings may explain the high response rate of lenvatinib, a FGFR 1–4 inhibitor, in advanced HCC [4].
Lenvatinib, like other multiple kinase inhibitors, has been found to have immunomodulatory effects [14]. VEGFA and bFGF significantly upregulated the expression of immune-checkpoint markers and inhibited secretion of IFN-γ and granzyme B, which suppressed T cell cytotoxicity. This immunosuppressive effect was reverted by lenvatinib [20]. Another study showed the activation of FGFR signaling downregulated JAK/STAT pathway leading to the decrease of IFN-γ secretion. With the use of lenvatinib inhibited FGFR signaling restoring the IFN-γ stimulation [21]. In addition, several studies demonstrated that lenvatinib increased the percentage of activated CD8 + T cells that secreting IFN-γ and granzyme B [15, 21, 22]. However, the antitumor activity of lenvatinib was attenuated in immunodeficient mice by CD8 + T cell depletion [15]. On the other hand, lenvatinib decreased the proportion of monocytes and macrophages population, and tumor-associated macrophages (TAMs) [15, 21, 22]. Taking together, lenvatinib promoted anti-tumor immunity by increased IFN-γ–producing CD8 T-cell and decreased TAMs, which makes lenvatinib potential to combine with immunotherapy.
Lenvatinib combined with anti-PD-1 induced greater antitumor activity and had longer survival in animal model of renal cell carcinoma [21]. Two HCC syngeneic mouse model showed that the combination therapy increased more percentage of IFN-γ + and granzyme B + CD8 + T cells and decreased the macrophages population [15, 22]. Combined therapy also reducedPD-1 + T cells and modulated inflammatory factors which had an extensive immunomodulatory effect in the tumor microenvironment in HCC mouse model. Additionally, the immunomodulatory effect was more potent when combined with lenvatinib than sorafenib [20].Therefore, the combination of lenvatinib and anti-PD-1 synergistically modulated the TME and enhanced antitumor immunity.
Lenvatinib combined anti-PD-1 has been approved in endometrial carcinoma and renal cell carcinoma. In KEYNOTE-775/Study 309, lenvatinib and pembrolizumab demonstrated an ORR of 30%, PFS 6.6 months (HR 0.60) and OS 17.4 months (HR 0.68), which was superior to doxorubicin or paclitaxel in platinum-experienced endometrial carcinoma [23]. In renal cell carcinoma, the combination of lenvatinib and pembrolizumab was highly effective with an ORR of 38% in the phase Ib/II trial KEYNOTE-146 [24]. In the phase III CLEAR trial, lenvatinib combined with pembrolizumab showed a longer PFS (23.9 vs. 9.2 months; HR 0.39) and OS (HR 0.66) than sunitinib [25]. In HCC, a phase Ib study of lenvatinib plus nivolumab showed the ORR of 54% and PFS of 7.4 months [16]. Another phase I-II study of lenvatinib plus pembrolizumab had the ORR of 36% and PFS of 8.6 months [26]. These clinical trials revealed promising results that is comparable with the synergistic effect in animal model.
For the treatment outcomes of other combined regimens in advanced HCC, a global open-labeled phase III trial (IMbrave150) revealed atezolizumab plus bevacizumab had a higher ORR (27.3% vs. 11.9%), longer PFS (6.8 vs. 4.3 months) and OS (non-reached vs. 13.2 months) then sorafenib [8]. In the phase II study of CheckMate-040, the combination nivolumab and ipilimumab yielded an ORR of 27–32% andOS (12.5–22.8 months) among different dose of combination in HCC patients who progressed from sorafenib [27]. In our study, lenvatinib combined nivolumab showed an objective response rate of 45% and a median PFS of 7.3 months. Therefore, our study demonstrated comparable efficacy to the treatments mentioned above.
The most adverse effects were graded 1–2 in this study. Skin reactions such as dermatitis and pruritis, and hypothyroidism were most common and could be caused by lenvatinib or nivolumab. Severe AEs including two gastric intestinal bleeding and one duodenal perforation were more likely related to lenvatinib; one patient suffered grade 5 pneumonitis was likely related to nivolumab. Overall, the toxicity profile was comparable to those reported in the phase Ib study of lenvatinib combined nivolumab and phase I-II study of lenvatinib combined pembrolizumab [16, 28].
There were several limitations in our study. First, this is a retrospective study, the information bias and selection bias may exist. However, imbalanced factors of age and HBV infection were not independent risk factors. Therefore, this study still provided a proof of concept. Second, whether the results apply to non-Asian populations is unclear because of the different etiologies between Asian and Western countries. Nevertheless, the response and prognosis were not significantly different regardless of virus infection.
Conclusion
This study is by far the first real-world data regarding the combination of lenvatinib and nivolumab in advanced HCC. We reported the promising efficacy and tolerable toxicities that is comparable with the clinical trial. Whether this regimen can become standard of care remains to be confirmed in a large prospective clinical trial.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Fig. 1. Kaplan–Meier curves for (A) progression-free survival and (B) overall survival stratified by treatment response (RECIST criteria) (TIF 4687 KB)
Supplementary file2 Fig. 2. Case with complete response by mRECSIT criteria Ill-defined mass lesion at posterior segment of liver (arrow) and portal vein thrombus (dotted arrow).Two months later, the tumor was regressed. Six months later, both tumor and portal vein thrombus achieved complete response. In the meanwhile, his tumor marker declined to normal range (TIF 3001 KB)
Acknowledgements
The author wishes to acknowledge the support by Ministry of Health and Welfare and the Center of Excellence for Cancer Research (MOHW110‑TDU‑B‑211‑144019), Taipei Veterans General Hospital (V111C-131 to SC Chen).
Authors’ contributions
Conceived and designed the experiments: S‑CC, YC. Performed the experiments: W-CW, T-YL. Analyzed the data: Y-PH, M‑HC. Contributed reagents/materials/analysis tools: C-AL, R‑CL, Y-HH. Contributed to the writing of the manuscript: W-CW, S‑CC. The author(s) read and approved the final manuscript.
Funding
Ministry of Health and Welfare and the Center of Excellence for Cancer Research (MOHW110‑TDU‑B‑211‑144019), Taipei Veterans General Hospital (V111C-131 to SC Chen).
Availability of data and materials
Data available on request from the authors.
Declarations
Ethics approval and consent to participate
This study has been approved by the institutional review board of Taipei Veterans General Hospital (TPEVGH IRB No.: 2021–08-005AC), which is the appropriate regulatory agency to review research on both adults and children. All methods were carried out in accordance with relevant guidelines and regulations. The subject informed consent form is waived by the institutional review board of Taipei Veterans General Hospital, and study‑related information could be not informed the subjects.
Consent for publication
NA.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.International Agency for Research on Cancer. World Health Organization. Cancer today. Retrieved May 30, 2021, from https://gco.iarc.fr/today/home
- 2.The leading causes of death in Taiwan (2018). Retrieved May 30, 2021, from https://www.mohw.gov.tw/cp-16-48057-1.html
- 3.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 4.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet (London, England) 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 5.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med. 2018;379(1):54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (London, England) 2017;389(10064):56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 7.Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 8.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda M, Okusaka T, Mitsunaga S, Ueno H, Tamai T, Suzuki T, Hayato S, Kadowaki T, Okita K, Kumada H. Safety and Pharmacokinetics of Lenvatinib in Patients with Advanced Hepatocellular Carcinoma. Clin Cancer Res. 2016;22(6):1385–1394. doi: 10.1158/1078-0432.CCR-15-1354. [DOI] [PubMed] [Google Scholar]
- 10.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling THR, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet (London, England) 2017;389(10088):2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu AX, Finn RS, Cattan S, Edeline J, Ogasawara S, Palmer DH, Verslype C, Zagonel V, Rosmorduc O, Vogel A et al (2018) KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol 36(suppl 4S):abstr 209
- 12.Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, Kudo M, Han KH, Harding JJ, Merle P, et al. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC) Ann Oncol. 2019;30:v874–v875. doi: 10.1093/annonc/mdz394.029. [DOI] [Google Scholar]
- 13.Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15(5):325–340. doi: 10.1038/nrclinonc.2018.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin YY, Tan CT, Chen CW, Ou DL, Cheng AL, Hsu C. Immunomodulatory Effects of Current Targeted Therapies on Hepatocellular Carcinoma: Implication for the Future of Immunotherapy. Semin Liver Dis. 2018;38(4):379–388. doi: 10.1055/s-0038-1673621. [DOI] [PubMed] [Google Scholar]
- 15.Kimura T, Kato Y, Ozawa Y, Kodama K, Ito J, Ichikawa K, Yamada K, Hori Y, Tabata K, Takase K, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018;109(12):3993–4002. doi: 10.1111/cas.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kudo M, Ikeda M, Motomura K, Okusaka T, Kato N, Dutcus CE, Hisai T, Suzuki M, Ikezawa H, Iwata T, Kumada H, Kobayashi M (2020) A phase Ib study of lenvatinib (LEN) plus nivolumab (NIV) in patients (pts) with unresectable hepatocellular carcinoma (uHCC): Study 117. J Clin Oncol 38(suppl 4;abstr 513)
- 17.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology (Baltimore, MD) 2018;68(2):723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 18.Miura S, Mitsuhashi N, Shimizu H, Kimura F, Yoshidome H, Otsuka M, Kato A, Shida T, Okamura D, Miyazaki M. Fibroblast growth factor 19 expression correlates with tumor progression and poorer prognosis of hepatocellular carcinoma. BMC Cancer. 2012;12:56. doi: 10.1186/1471-2407-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuki M, Hoshi T, Yamamoto Y, Ikemori-Kawada M, Minoshima Y, Funahashi Y, Matsui J. Lenvatinib inhibits angiogenesis and tumor fibroblast growth factor signaling pathways in human hepatocellular carcinoma models. Cancer Med. 2018;7(6):2641–2653. doi: 10.1002/cam4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng H, Kan A, Lyu N, Mu L, Han Y, Liu L, Zhang Y, Duan Y, Liao S, Li S, et al. Dual Vascular Endothelial Growth Factor Receptor and Fibroblast Growth Factor Receptor Inhibition Elicits Antitumor Immunity and Enhances Programmed Cell Death-1 Checkpoint Blockade in Hepatocellular Carcinoma. Liver cancer. 2020;9(3):338–357. doi: 10.1159/000505695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adachi Y, Kamiyama H, Ichikawa K, Fukushima S, Ozawa Y, Yamaguchi S, Goda S, Kimura T, Kodama K, Matsuki M et al (2021) Inhibition of FGFR reactivates IFNγ signaling in tumor cells to enhance the combined antitumor activity of lenvatinib with anti-PD-1 antibodies. Cancer Res [DOI] [PMC free article] [PubMed]
- 22.Kato Y, Tabata K, Kimura T, Yachie-Kinoshita A, Ozawa Y, Yamada K, Ito J, Tachino S, Hori Y, Matsuki M, et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS ONE. 2019;14(2):e0212513. doi: 10.1371/journal.pone.0212513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marth C, Tarnawski R, Tyulyandina A, Pignata S, Gilbert L, Kaen D, Rubio MJ, Frentzas S, Beiner M, Magallanes-Maciel M, et al. Phase 3, randomized, open-label study of pembrolizumab plus lenvatinib versus chemotherapy for first-line treatment of advanced or recurrent endometrial cancer: ENGOT-en9/LEAP-001. Int J Gynecol Cancer. 2022;32(1):93–100. doi: 10.1136/ijgc-2021-003017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee CH, Shah AY, Rasco D, Rao A, Taylor MH, Di Simone C, Hsieh JJ, Pinto A, Shaffer DR, Girones Sarrio R, et al. Lenvatinib plus pembrolizumab in patients with either treatment-naive or previously treated metastatic renal cell carcinoma (Study 111/KEYNOTE-146): a phase 1b/2 study. Lancet Oncol. 2021;22(7):946–958. doi: 10.1016/S1470-2045(21)00241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, Grünwald V, Hutson TE, Kopyltsov E, Méndez-Vidal MJ, et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N Engl J Med. 2021;384(14):1289–1300. doi: 10.1056/NEJMoa2035716. [DOI] [PubMed] [Google Scholar]
- 26.Llovet J, Shepard KV, Finn RS, Ikeda M, Sung M, Baron AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, et al. A phase Ib trial of lenvatinib (LEN) plus pembrolizumab (PEMBRO) in unresectable hepatocellular carcinoma (uHCC): Updated results. Ann Oncol. 2019;30:v286–v287. doi: 10.1093/annonc/mdz247.073. [DOI] [Google Scholar]
- 27.Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020;6(11):e204564. doi: 10.1001/jamaoncol.2020.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masafumi Ikeda MWS, Kudo M, Kobayashi M, Baron AR, Finn RS, Kaneko S, Zhu AX, Kubota T, Kraljevic S, Ishikawa K, Siegel AB, Kumada H, Okusaka T (2018) A phase 1b trial of lenvatinib (LEN) plus pembrolizumab (PEM) in patients (pts) with unresectable hepatocellular carcinoma (uHCC). J Clin Oncol 36(15_supp):4076
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 Fig. 1. Kaplan–Meier curves for (A) progression-free survival and (B) overall survival stratified by treatment response (RECIST criteria) (TIF 4687 KB)
Supplementary file2 Fig. 2. Case with complete response by mRECSIT criteria Ill-defined mass lesion at posterior segment of liver (arrow) and portal vein thrombus (dotted arrow).Two months later, the tumor was regressed. Six months later, both tumor and portal vein thrombus achieved complete response. In the meanwhile, his tumor marker declined to normal range (TIF 3001 KB)
Data Availability Statement
Data available on request from the authors.