Abstract
A reverse transcriptase PCR (RT-PCR) assay was developed for the detection of acute bee paralysis virus (ABPV) and black queen cell virus (BQCV), two honeybee viruses. Complete genome sequences were used to design unique PCR primers within a 1-kb region from the 3′ end of both genomes to amplify a fragment of 900 bp from ABPV and 700 bp from BQCV. The combined guanidinium thiocyanate and silica membrane method was used to extract total RNA from samples of healthy and laboratory-infected bee pupae. In a blind test, RT-PCR successfully identified the samples containing ABPV and BQCV. Sensitivities were approximately 1,600 genome equivalents of purified ABPV and 130 genome equivalents of BQCV.
The interest in viruses as honeybee pathogens has often been academic, with many viruses persisting as inapparent infections. However, increasing knowledge of the interactions between honeybee viruses and parasites, notably the mite Varroa jacobsoni, has led to suggestions that they may be involved in honeybee mortality (1, 4, 10, 11).
A total of 18 honeybee viruses have been identified and physically characterized (1). Most of these viruses have physical features resembling those of picornaviruses, and they are referred to as picorna-like viruses (6–8). In addition, the complete genome sequences of three picorna-like honeybee viruses, namely acute bee paralysis virus (ABPV), black queen cell virus (BQCV), and sacbrood virus have been determined. Of these three viruses, only sacbrood virus causes symptoms that can confidently be attributed to viral infection (1). ABPV was originally discovered as an inapparent infection in laboratory experiments (5) and is widespread as a latent and inapparent infection (1, 17). ABPV spreads by way of the salivary gland secretions of adult bees and in food stores to which these secretions are added (9). It has a single-stranded RNA genome of 9,470 nucleotides excluding the poly(A) tail (14). ABPV has been identified as a major factor contributing to the mortality of honeybees in colonies infested by V. jacobsoni (10).
BQCV was originally found in dead honeybee queen larvae and pupae (8) and has been shown to be the most common cause of death of queen larvae in Australia (3). The virus has isometric particles that are 30 nm in diameter and a single-stranded RNA genome of 8,550 nucleotides excluding the poly(A) tail (19). BQCV is often present in bees infested with the microsporidian parasite Nosema apis (1, 4) and may be implicated in the mortality of bees infected with this parasite.
Several methods have been used to detect honeybee viruses, including immunodiffusion, enzyme-linked immunosorbent assay, enhanced chemiluminescent Western blotting, and reverse transcriptase PCR (RT-PCR) (1, 2, 25). The most commonly used of these methods is still the immunodiffusion test because it is rapid, inexpensive, and specific (1). However, the serological methods have the drawbacks of limited availability of antisera and questions regarding the specificity of some antisera as a result of antiserum production from preparations containing virus mixtures. Raising antisera is also a time-consuming process with a large amount of a given virus needed to raise the antiserum. Consequently, the use of the serological methods would be limited to laboratories that can produce large amounts of pure virus to raise a library of suitable antisera. By contrast, a diagnostic technique using RT-PCR can be rapidly implemented in independent laboratories after the basic protocol and primer sequences are made available.
RT-PCR has been used to detect a variety of RNA viruses including the picorna-like insect viruses (12, 13, 15, 16, 18, 24–26). The technique is reliable, specific, and sensitive. However, RT-PCR experiments on insects are usually hampered by the problem of inhibitory components, which compromise reverse transcription and PCR reactions (13). To overcome this problem, many RNA extraction methods have been developed or modified in order to remove these inhibitors (23).
In the method described here, total RNA was extracted from infected and healthy bee pupae using the Nucleospin RNAII total RNA isolation kit (Macherey-Nagel). The kit uses the combined guanidinium thiocyanate and silica membrane method. This method has been successfully used in aphids, plants, and mosquitoes (13, 15, 20, 24).
ABPV and BQCV were propagated in apparently healthy white- to purple-eyed drones or worker bee pupae and subsequently purified as described by Leat et al. (19). To determine the concentration of the virus stock, viral RNA was extracted with phenol, precipitated with ethanol, and quantified with a UV spectrophotometer (21). Since the length and molecular weight of each genome are known, the number of genome copies could be calculated. Individual healthy or infected bee pupae were weighed and placed in 1.5-ml Eppendorf tubes; then an appropriate volume of grinding buffer (0.1 M NaCl, 0.1 M glycine, 10 mM EDTA [pH 9.5]) was added and the tube contents were ground with a disposable pestle. The volume of grinding buffer used was about 200 μl per 30 mg of bee material. Total RNA was extracted from approximately 250 μl of the homogenate following the manufacturer's instructions. Pure RNA was finally eluted in 40 μl of RNase-free water.
RT-PCRs were performed using the Titan RT-PCR system (Roche Diagnostics GmbH, Roche Molecular Biochemicals, Mannheim, Germany). Each 20 μl of reaction mixture contained 9 μl of template, 0.2 mM of each deoxynucleoside triphosphate, 0.5 μM forward primer, 0.5 μM reverse primer, 5 mM dithiothreitol, 0.4 μl of Titan polymerase mix, and 4 μl of 5× RT-PCR buffer (1.5 mM MgCl2). Reverse transcription and amplification were performed in a Hybaid OMN-E thermocycler. The 9-μl template contained either RNA extracted with the Nucleospin RNAII total RNA isolation kit or whole virus particles treated at 90°C for 5 min prior to amplification. The RT-PCR profile used was as follows: a reverse transcription stage at 50°C for 30 min, followed by an initial denaturation stage at 94°C for 2 min. This was then followed by 35 amplification cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s, and a final extension step at 72°C for 7 min. The resulting PCR products were visualized by electrophoresis through 1% agarose gel containing ethidium bromide.
Oligonucleotides for detection of ABPV and BQCV were designed within a 1-kb region measured from the 3′ ends of their respective genomes (ABPV accession number, AF150629 [14]; BQCV accession number, AF183905 [19]). The ABPV primers were ABPV1 (5′-TTA TGT GTC CAG AGA CTG TAT CCA I-3′) and ABPV2 (5′-GCT CCT ATT GCT CGG TTT TTC GGT I-3′), corresponding to nucleotides 8460 to 8484 and 9336 to 9360, respectively. These primers amplify a 900-bp fragment. The BQCV primers were BQCV1 (5′-TGG TCA GCT CCC ACT ACC TTA AAC I-3′) and BQCV2 (5′-GCA ACA AGA AGA AAC GTA AAC CAC I-3′), corresponding to nucleotides 7850 to 7874 and 8526 to 8550, respectively. These primers amplify a 700-bp fragment. Since different strains of ABPV and BQCV may vary slightly in genome sequence, deoxyinosine residues (identified as I in the sequences above) were incorporated into the 3′ end of each primer to ensure that it would anneal to the template in case of a strain-specific mismatch at this position.
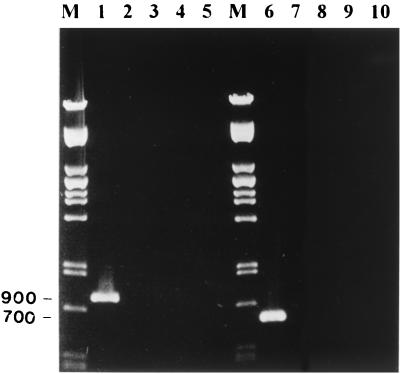
Primers were compared to all of the honeybee virus sequences available in the databases. The maximum degree of sequence identity was 64%, which occurred between ABPV1 primer and BQCV, cloudy wing virus (CWV) and Kashmir bee virus (KBV), and also BQCV2 primer and ABPV. Primers were tested against four honeybee viruses available in our laboratory: ABPV, BQCV, CWV, and KBV. Purified virus stocks were used directly for RT-PCR as described above. Figure 1 (lanes 1 through 5) shows that ABPV primers amplified a fragment of the predicted molecular weight (900 bp) from the ABPV genome. The primers failed to produce any PCR products when tested against BQCV, CWV, and KBV. Similarly, BQCV primers were specific to BQCV and failed to amplify any fragments from ABPV, CWV, or KBV (Fig. 1, lanes 6 through 10). The BQCV PCR amplicon was 700 bp as predicted.
FIG. 1.
Test of the RT-PCR amplification specificity of the ABPV primer set (lanes 1 through 5) and the BQCV primer set (lanes 6 through 10). Each set was tested against four different honeybee viruses. M, Pst lambda DNA marker; lane 1, ABPV; lane 2, BQCV; lane 3, CWV; lane 4, KBV; lane 5, water (negative control); lane 6, BQCV; lane 7, ABPV; lane 8, CWV; lane 9, KBV; lane 10, water (negative control).
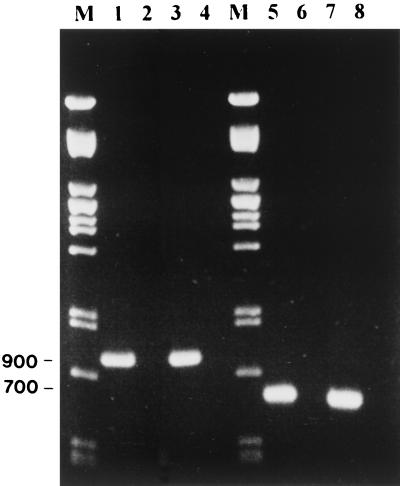
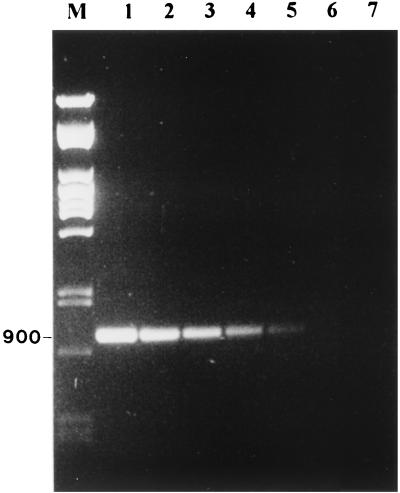
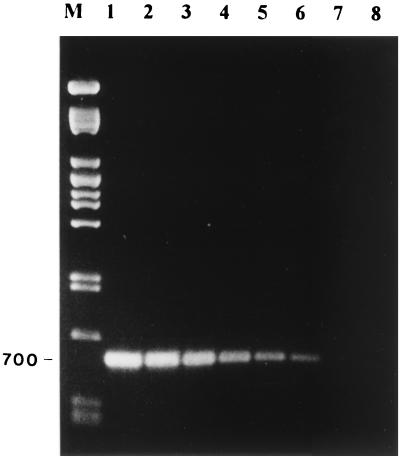
Using these primers, the RT-PCR was able to detect ABPV and BQCV in laboratory-infected bee pupae. Figure 2 (lanes 1 through 4) shows that RT-PCR was able to detect ABPV in laboratory-infected bee pupae. Figure 2 (lanes 5 through 8) shows similar results with BQCV, in which the predicted 700-bp fragment from BQCV was amplified. In both cases, no PCR fragments were amplified from the total RNA of healthy bees. In addition, a blind test was performed on 30 samples of healthy bees and bees infected with ABPV or BQCV. RT-PCR successfully identified the samples containing ABPV and BQCV. To determine the sensitivity of the RT-PCR, stocks of ABPV and BQCV containing 1.6 × 107 and 1.3 × 108 genome equivalents/μl, respectively, were diluted and tested. The total reaction mixture of the RT-PCR (20 μl) was loaded on an agarose gel, and samples were considered positive if a DNA band of the predicted molecular weight was visible. Figures 3 and 4 show the detection sensitivity of RT-PCR on these samples. The detection limit of ABPV and BQCV was estimated to be about 1,600 and 130 genome equivalents, respectively. These results are similar to other reports on RT-PCR detection limits. Canning et al. (12) achieved a sensitivity of 1,000 genome copies of the barley yellow dwarf virus. Van der Wilk et al. (26) reported a sensitivity of 300 to 400 viral particles of purified tobacco rattle virus. Wilde et al. (27) also achieved a sensitivity of this order, reporting positive results from 500 genomic copies of rotavirus RNA. The 10-fold difference in sensitivity between ABPV and BQCV primers could be attributed to the difference in the size of the amplified fragments. Shorter fragments have been shown to be more sensitive than longer ones. In potato Y virus, Singh and Singh (22) achieved a 100-fold difference in sensitivity when amplifying two fragments, which were 1,016 and 704 bp in size, from the 3′ end of the genome.
FIG. 2.
Detection of ABPV and BQCV in honeybees by RT-PCR. Extraction of the total RNA from healthy and laboratory-infected bee pupae and RT-PCR amplification were performed as described above. M, Pst lambda DNA marker; lane 1, ABPV-infected bee pupae; lane 2, healthy bee pupae; lane 3, ABPV virus stock (positive control); lane 4, water (negative control); lane 5, BQCV-infected bee pupae; lane 6, healthy bee pupae; lane 7, BQCV virus stock (positive control); lane 8, water (negative control).
FIG. 3.
Determination of the sensitivity of amplification by RT-PCR of the ABPV. ABPV virus stock containing 1.6 × 107 genome copies/μl was serially 10-fold diluted. One microliter of this stock was used in the RT-PCR mixture. M, Pst lambda DNA marker; lane 1, ABPV; lane 2, 10−1 ABPV dilution; lane 3, 10−2 ABPV dilution; lane 4, 10−3 ABPV dilution; lane 5, 10−4 ABPV dilution; lane 6, 10−5 ABPV dilution; lane 7, water (negative control).
We have developed a sensitive RT-PCR assay for the detection of purified ABPV and BQCV or the detection of these viruses in infected pupae. We also aim to develop RT-PCR assays for the identification of other bee viruses as the genome sequences become available.
FIG. 4.
Determination of the sensitivity of amplification by RT-PCR of the BQCV. BQCV virus stock containing 1.3 × 108 genome copies/μl was serially 10-fold diluted. One microliter of this stock was used in the RT-PCR mixture. M, Pst lambda DNA marker; lane 1, BQCV; lane 2, 10−1 BQCV dilution; lane 3, 10−2 BQCV dilution; lane 4, 10−3 BQCV dilution; lane 5, 10−4 BQCV, lane 6, 10−5 BQCV; lane 7, 10−6 BQCV dilution; lane 8, 10−7 BQCV dilution; lane 9, water (negative control).
Acknowledgments
This work was funded by the following South African organizations: Deciduous Fruit Producers Trust, South African Subtropical Fruit Growers Association, Western Cape Beekeepers Association, and the National Research Foundation.
We also thank B. Ball (Rothamsted Experimental Station, Harpenden, United Kingdom) for providing virus stock.
REFERENCES
- 1.Allen M, Ball B. The incidence and world distribution of the honey bee viruses. Bee World. 1996;77:141–162. [Google Scholar]
- 2.Allen M F, Ball B V, White R F, Antoniw J F. The detection of acute paralysis virus in Varroa jacobsoni by the use of a simple indirect ELISA. J Apic Res. 1986;25:100–105. [Google Scholar]
- 3.Anderson D L. Pathogens and queen bees. Australasian Beekeeper. 1993;94:292–296. [Google Scholar]
- 4.Bailey L, Ball B V, Perry J N. Association of viruses with two protozoal pathogens of the honey bee. Ann Appl Biol. 1983;103:13–20. [Google Scholar]
- 5.Bailey L, Gibbs A J, Woods R D. Two viruses from adult honey bees (Apis mellifera Linnaeus) Virology. 1963;21:390–395. doi: 10.1016/0042-6822(63)90200-9. [DOI] [PubMed] [Google Scholar]
- 6.Bailey L, Gibbs A J, Woods R D. The purification and properties of chronic bee-paralysis virus. J Gen Virol. 1968;2:251–260. doi: 10.1099/0022-1317-2-2-251. [DOI] [PubMed] [Google Scholar]
- 7.Bailey L, Woods R D. Three previously undescribed viruses from the honey bee. J Gen Virol. 1974;25:175–186. doi: 10.1099/0022-1317-25-2-175. [DOI] [PubMed] [Google Scholar]
- 8.Bailey L, Woods R D. Two more small RNA viruses from honey bees and further observations on sacbrood and acute bee-paralysis viruses. J Gen Virol. 1977;25:175–186. [Google Scholar]
- 9.Ball B V. Acute paralysis virus isolates from honeybee colonies infested with Varroa jacobsoni. J Apic Res. 1985;24:115–119. [Google Scholar]
- 10.Ball B V, Allen M F. The prevalence of pathogens in honey bee (Apis mellifera) colonies infested with the parasitic mite Varroa jacobsoni. Ann Appl Biol. 1988;113:237–244. [Google Scholar]
- 11.Brødsgaard C J, Ritter W, Hansen H, Brødsgaard H F. Interactions among Varroa jacobsoni mites, acute paralysis virus, and Paenibacillus larvae larvae and their influence on mortality of larval honeybees in vitro. Apidologie. 2000;31:543–554. [Google Scholar]
- 12.Canning E S G, Penrose M J, Barker I, Coates D. Improved detection of barley yellow dwarf virus in single aphids using RT-PCR. J Virol Methods. 1996;56:191–197. doi: 10.1016/0166-0934(95)01959-6. [DOI] [PubMed] [Google Scholar]
- 13.Chungue E, Roche C, Lefevre M-F, Barbazan P, Chanteau S. Ultra-rapid, simple, sensitive, and economical silica method for extraction of dengue viral RNA from clinical specimens and mosquitoes by reverse transcriptase-polymerase chain reaction. J Med Virol. 1993;40:142–145. doi: 10.1002/jmv.1890400211. [DOI] [PubMed] [Google Scholar]
- 14.Govan V A, Leat N, Allsop M, Davison S. Analysis of the complete genome sequence of acute bee paralysis virus shows that it belongs to the novel group of insect-infecting RNA viruses. Virology. 2000;277:457–463. doi: 10.1006/viro.2000.0616. [DOI] [PubMed] [Google Scholar]
- 15.Harris E, Roberts T G, Smith L, Selle J, Kramer L D, Valle S, Sandoval E, Balmaseda A. Typing of dengue viruses in clinical specimens and mosquitoes by single-tube multiplex reverse transcriptase PCR. J Clin Microbiol. 1998;36:2634–2639. doi: 10.1128/jcm.36.9.2634-2639.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hung A C F, Shimanuki H. A scientific note on the detection of Kashmir bee virus in individual honey bees and Varroa jacobsoni mites. Apidologie. 1999;30:353–354. [Google Scholar]
- 17.Hung A C F, Shimanuki H, Knox D A. Inapparent infection of acute paralysis virus and Kashmir bee virus in the U.S. honey bees. Am Bee J. 1996;136:874–876. [Google Scholar]
- 18.Johnson K N, Christian P D. Molecular characterization of Drosophila C virus isolates. J Invertebr Pathol. 1999;73:248–254. doi: 10.1006/jipa.1998.4830. [DOI] [PubMed] [Google Scholar]
- 19.Leat N, Ball B, Govan V, Davison S. Analysis of the complete genome sequence of black queen cell virus, a picorna-like virus of honey bees. J Gen Virol. 2000;81:2111–2119. doi: 10.1099/0022-1317-81-8-2111. [DOI] [PubMed] [Google Scholar]
- 20.Naidu R A, Robinson D J, Kimmins F M. Detection of each of the causal agents of groundnut rosette disease in plants and vector aphids by RT-PCR. J Virol Methods. 1998;76:9–18. doi: 10.1016/s0166-0934(98)00118-9. [DOI] [PubMed] [Google Scholar]
- 21.Sambrook J, Fritsh E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 22.Singh M, Singh R P. Potato virus Y detection: sensitivity of RT-PCR depends on the size of the fragment amplified. Can J Plant Pathol. 1997;19:149–155. [Google Scholar]
- 23.Singh R P. Reverse-transcription polymerase chain reaction for the detection of viruses from plants and aphids. J Virol Methods. 1998;74:125–138. doi: 10.1016/s0166-0934(98)00074-3. [DOI] [PubMed] [Google Scholar]
- 24.Stevens M, Hull R, Smith H G. Comparison of ELISA and RT-PCR for the detection of beet yellows closterovirus in plants and aphids. J Virol Methods. 1997;68:9–16. doi: 10.1016/s0166-0934(97)00103-1. [DOI] [PubMed] [Google Scholar]
- 25.Stoltz D, Shen X R, Boggis C, Sisson G. Molecular diagnosis of Kashmir bee virus infection. J Apic Res. 1995;34:153–160. [Google Scholar]
- 26.Van der Wilk F, Korsman M, Zoon F. Detection of tobacco rattle virus in nematodes by reverse transcription and polymerase chain reaction. Eur J Plant Pathol. 1994;100:109–122. [Google Scholar]
- 27.Wilde J, Eiden J, Yolken R. Removal of inhibitory substances from human fecal specimens for detection of group A rotavirus by reverse transcriptase and polymerase chain reactions. J Clin Microbiol. 1990;28:1300–1307. doi: 10.1128/jcm.28.6.1300-1307.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]