Abstract
Background:
Beta Thalassemia Trait (BTT) and Iron Deficiency Anemia (IDA) were two common clinical problems with clinical hypochromic and microcytic manifestations, and their differentiation from each other was very important and needs innovative formulas and laboratory tests. Since the consideration of anemia as a pair with BTT leads to beta-thalassemia major birth in 25% of cases, offering prospective parents detailed information about the likelihood of their offspring developing BTT is essential. The present study aimed to investigate the performance of common equations in differentiation of BTT from IDA.
Methods:
In the present cross-sectional study, twenty common equations were selected in the differentiation of BTT from IDA. To evaluate the equations, the tests of 292 individuals (73 individuals with BTT and 219 individuals with IDA) were compared with the initial diagnosis of hypochromic and microcytic anemia using the formulas. Descriptive and value indices and Roc curve were utilized for all equations to analyze the results.
Results:
Among twenty differential equations, Bordbar, Kerman I, II and Srivastava equations had the highest area under Roc curve (AUC) of 0.841, 0.838, 0.836, and 0.830 respectively, but Kandhro I. equation had the lowest AUC (0.378).
Conclusion:
Given the importance of AUC and value indices of differential equations in clinical decision making, and results of evaluating common equations in differentiation of BTT from IDA. It is essential to improve the values of the equations by re-examining the parameters involved in them.
Keywords: Red blood cell, Thalassemia, Anemia, Mentzer index, Differentiation equation
Introduction
Thalassemia is an inherited blood disorder in which the survival of red blood cells decreases due to the imbalance in the production of beta globulin chain. This disorder has two forms, minor (Beta Thalassemia Trait (BTT) and major, among which the latter needs urgent treatment (1). Its prevalence in Iran is more than 10% for cities around the Persian Gulf and the Caspian Sea, and 4%–8% in other cities of Iran and 2% in West Azerbaijan Province (2,3). Iron deficiency anemia (IDA) is a common clinical problem defined by WHO as hemoglobin of less than 12 and 13 gr per deciliter for adult men and women respectively (4). The prevalence of iron deficiency anemia varies in different cities of Iran and has been reported from 21.5% to 42.7% (5).
Beta thalassemia is the most common type of hereditary and monogenic hemoglobinopathy. Methods of diagnosis of hemoglobinopathies include High-Performance Liquid Chromatography, Hemoglobin Electrophoresis, Screening of PCR Mutations, and DNA tests which are all costly, require sophisticated equipment and skilled workforce. Therefore, it is not possible to access them in any city (6). Since 25% of infants of male and female individuals with BTT are born with beta thalassemia major, the lack of diagnosis and screening increases the number of infants with beta thalassemia major; and these infants will have severe anemia and Hepatosplenomegaly and will need serious medical interventions such as Regular Transfusion Program and Chelation Therapy in at least the first two years of life (7). Therefore, offering prospective parents detailed information about the likelihood of their offspring developing BTT is essential.
A beta thalassemia major prevention plan has been implemented throughout Iran since 1998. According to the plan, the couple specifies their health in terms of having the BTT by testing their blood before marriage. If the test of both individuals is less than 80 fl in terms of the Mean Corpuscular Volume (MCV) and less than 27 Pg in terms of the Corpuscular Mean of Hemoglobin (MCH) (both of them with microcytic hypochromic anemia), they are referred to the Beta Thalassemia counseling center of the health center; and the complementary and differentiating tests indicate that their anemia is due to the BTT or IDA (8,9). BTT and IDA are two most common microcytic hypochromic anemia. These two types of anemia have similar manifestations and can be differentiated from each other by laboratory tests and though blood indices. Diagnosis of the IDA is performed by testing iron metabolism such as serum iron, Total Serum Iron-Binding Capacity, and measuring the amount of serum ferritin. However, the diagnosis of BTT is performed based on the HPLC (High-Performance Liquid Chromatography) and Hemoglobin A2 (HbA2) level (over 3.5%). However, the laboratory diagnosis and differentiation of the BTT from IDA requires advanced equipment and high cost (10).
There are different equations for differentiation of the BTT from IDA, including equations by Mentzer (Men), Ehsani (Eh), England & Fraser (EF), Green & King (GK), RBC counts, RDW, RDWI, Ricerca (Ric), Shine & Lal (Sh -L), Sirdah (Sir), Sirvastava (Sriv), and M/H Ratio (10,11). Each of these differentiators have different sensitivity (SEN), specificity (Spe), and value indices; and different papers have reported different results (12–15).
The innovation of the present study is the simultaneous comparison of 20 differential equations. Many related research studies have used a few numbers equations than our study to diagnose beta-thalassemia from iron deficiency anemia. Moreover, the present study was the first study in the region.
The present study aimed to evaluate the ability of 20 differential equations consisting of simple blood parameters in the separation of individuals with the BTT from those with the IDA by calculating the sensitivity, specificity, Youden’s index, and other value indices and according to the Roc Curve.
Materials and Methods
Source of Equations
The present study was cross-sectional research that investigated articles published in databases, namely PubMed, Scopus, ProQuest, Web of Science and Google Scholar and selected 20 equations for the differentiation of BTT from IDA by considering the possibility of acquiring blood indexes of differential equation in terms of laboratory and its breadth.
Data Collection
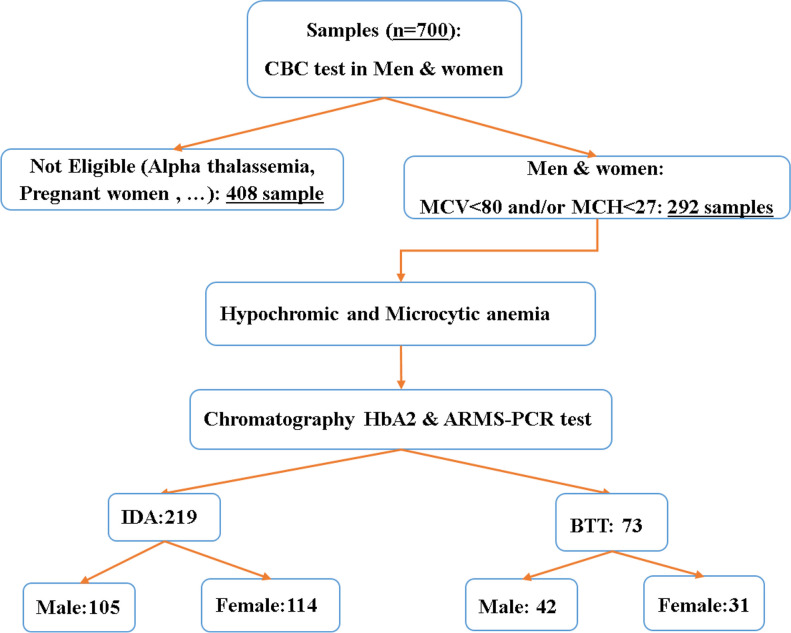
The data of study was obtained from cases of couples who participated in a project for the prevention of beta-thalassemia major and underwent blood sampling for BTT screening before marriage since 2012–2019 (700 couples). This is the process of identifying couples. First, the man’s blood counts are tested. If Mean Corpuscular Volume (MCV) and Corpuscular Mean of Hemoglobin (MCH) of male were more than 80fl and 27pg, the couple was excluded from the beta-thalassemia diagnosis process (408 samples).
Otherwise, the woman’s blood counts will also be tested. If the result of testing both individuals is as the MCV of less than 80fl and MCH of less than 27Pg (both with microcytic hypochromic anemia) (292 samples) (7), the couple is referred to the Beta Thalassemia counseling center of health center in Khoy city(West Azerbaijan, Iran) (Fig. 1). A survey of the archive of the center revealed that 292 couples were both hypochromic and microcytic anemia. Exactly 73 individuals had BTT and 219 had IDA according to complementary and genetic testing (amplification refractory mutation system (ARMS)-PCR). For original data, please contact corresponding author.
Fig. 1:
The study selection flow diagram
Inclusion and Exclusion criteria
The individuals with alpha thalassemia during the genetic testing were excluded from the study since the study was on differential indices of the BTT. There were also no pregnant women among the couples.
Ethical Approval
The protocol of study was also reviewed and approved by the Ethical Committee of the Research Deputy of Khoy University of Medical Sciences, Khoy, Iran. Informed consent had been taken from all patients.
Statistical Analysis
To evaluate and compare each of the equations, the method of calculation and their numerical values were extracted for assigning to the BTT. Every equation is bivariate (BTT or IDA). Table 1 mentions cutoff points for BTT. The values of sensitivity, specificity, and Youden’s index (Y.I) and other value indices were also calculated as Table 2.
Table 1:
Equation of differentiation of BTT from IDA along with reference and cutoff point of the BTT
| ID | Discriminan-Formula | Formula(Cut off) | ID | Discriminant Formula | Formula(Cut off) |
|---|---|---|---|---|---|
| 1 | Matos-Carvalho (M&K) (13) | (1.91×RBC)+(0.44×MCHC) (>23.85) | 11 | Green&King (G&K) (24) | (MCV2*RDW)/)Hb *100) (<72) |
| 2 | Srivastava(Sriv) (16) | MCH/RBC (<3.8) | 12 | KandhroI (Kan.I)(25) | (RBC/HCT+0.5×RDW) (cutoff<8.2) (17) |
| 3 | Shine&Lal (SH&L) (17) | MCV2*MCH*0/01 (<1530) | 13 | Kandhro II(Kan.II) (25) | RDW*5/RBC (<16.8) |
| 4 | KermanI (Ker.I) (18) | MCV*MCH/RBC (<300) | 14 | Sirdah (26) | MCV-RBC-(3 × Hb) (<27) |
| 5 | KermanII(Ker.II) (18) | Ker.I *10/MCHC (<85) | 15 | Sirachainan (Sir) (27) | 1.5×Hb-0.05*MCV (cutoff>14) |
| 6 | RDW (19) | MCV* RDW/RBC (<220) | 16 | Bordbar (28) | (|80-MCV|×|27-MCH|) (>44.76) |
| 7 | Mentzer (Men) (20) | MCV/RBC(<13) | 17 | Keikhaei(Kei) (29) | )Hb×RDW×100(/(RBC2×MCHC) (<21) |
| 8 | Ricerca(Ric) (21) | (RDW/RBC) (<3.3 | 18 | Huber-Herklotz (H&H )(30) | (MCH×RDW×0.1/RBC)+ RDW (cutoff<22) (23) |
| 9 | Ehsani (Eh) (22) | MCV-(10×RBC) (<15) | 19 | Zaghloul(Z.I) (31) | Hb+Hct+RBC(Males>55.7, Females> 51.6) |
| 10 | England&Fraser(E&F) (23) | MCV-RBC-(5*Hb)-3.4 (<0) | 20 | Zaghloul(Z.II) (31) | Hb+Hct+RBC–RDW (Males>40.4, Females> 35.6) |
Table 2:
Definition of basic Indices
| ID | Index (abbreviation) | Formula |
|---|---|---|
| 1 | Sensitivity (SEN) | TP/TP+FN× 100 |
| 2 | Specificity (Spe) | TN/TN+FP× 100 |
| 3 | Positive Likelihood Ratio (PLR) | SEN / (1−SPE) |
| 4 | Negative Likelihood Ratio (NLR) | (1−SEN) / SPE |
| 5 | Youden Index (Y.I) | (SEN+SPE) – 1 |
| 6 | Accuracy (Acc) | (TP+TN) / (TP+TN+FP+FN) |
| 7 | Positive Predictive Value (PPV | TP / TP+FP × 100 |
| 8 | Negative Predictive Value (NPV ) | TN / TN+ FN × 100 |
The normality of the variables was evaluated by Kolmogorov-Smirnov test. Accordingly, Mann-Whitney (Non-parametric) test (P-value) for MCV(<0.001), MCH(<0.001), HBA2(<0.001) and RDW (<0.001) and t-test for RBC(0.063), HGB(0.199), HCT(0.287) and MCHC (0.217) was performed (significance level=0.05). SPSS v.20 (IBM Corp., Armonk, NY, USA) was used for statistical analysis of data and drawing the Roc curve and reporting the area under the curve (AUC).
Results
Descriptive analysis
Data of 292 individuals, including 147 men and 145 women (73 individuals with the BTT including 42 men and 31 women) were analyzed; and the individuals’ mean (sd) age was 24.56 (6.02) years. Table 3 reported the demographic characteristics of patients. Table 4 indicated description of blood characteristics for overall, BTT and IDA as well as sex groups along with the significant difference between descriptive indices of two groups using the t-test or Mann-Whitney (Non-parametric) test.
Table 3:
Demographic characteristics of study samples
| Variable | Frequency (%) | Total (%) | ||
|---|---|---|---|---|
|
| ||||
| BTT: 73(25) | IDA:219(75) | |||
| Sex | Male | 42(28.58) | 105(71.42) | 147(50.34) |
| Female | 31(21.37) | 114(78.63) | 145(49.66) | |
| Education | Primary | 35(26.92) | 95(73.08) | 130(44.52) |
| Secondary | 25(25.00) | 75(75.00) | 100(34.25) | |
| University | 13(20.97) | 49(79.03) | 62(21.25) | |
| Family marriage | Yes | 13(32.50) | 27(67.50) | 40(13.70) |
| No | 60(23.81) | 192(76.19) | 252(86.30) | |
| Residents | City | 20(13.33) | 130(86.67) | 150(51.37) |
| Village | 53(37.32) | 89(62.68) | 142(48.63) | |
Table 4:
Mean ± standard deviation of blood-related indices of the overall, BTT & IDA as well as sex groups.
| Index | Overall | BTT | IDA | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Males | Female | P | Overall | Males | Females | P | Overall | |||
| RBC | 5.63±0.71 | 6.44±.83 | 5.71±.43 | <0.001 | 6.13±.77 | 5.91±.46 | 5.09±.49 | <0.001 | 5.48±.63 | <0.001 |
| HGB | 13.4±1.91 | 13.25±1.11 | 11.51±1.02 | <0.001 | 12.51±1.37 | 15.07±1.52 | 12.52±1.48 | <0.001 | 13.74±1.97 | <0.001 |
| HCT | 42.23±4.58 | 43.89±3.34 | 38.20±2.81 | <0.001 | 41.46±4.20 | 45.60±3.42 | 39.63±3.75 | <0.001 | 42.48±4.67 | 0.112 |
| MCV* | 75.11±7.10 | 68.28±6.67 | 67.25±4.83 | 0.772 | 67.83±5.93 | 77.45±4.91 | 77.59±6.33 | 0.453 | 77.52±5.69 | <0.001 |
| MCH* | 23.99±3.19 | 20.69±2.54 | 20.41±2.15 | 0.877 | 20.57±2.37 | 25.62±2.62 | 24.68±2.45 | 0.001 | 25.13±2.57 | <0.001 |
| MCHC | 31.76±2.12 | 30.20±1.27 | 30.15±1.67 | 0.864 | 30.18±1.44 | 33.01±2.08 | 31.59±1.79 | <0.001 | 32.26±2.06 | <0.001 |
| RDW* | 14.70±2.46 | 15.65±1.73 | 15.88±1.50 | 0.487 | 15.75±1.63 | 14.15±3.00 | 14.58±2.14 | 0.003 | 14.37±2.59 | <0.001 |
| HbA2* | 3.17±1.39 | 5.16±.90 | 4.93±1.28 | 0.340 | 5.06±1.07 | 2.68±.88 | 2.42±.66 | 0.004 | 2.53±.78 | <0.001 |
P: P-value *: Mann-Whitney test of Means and others t-test
Analytical analysis
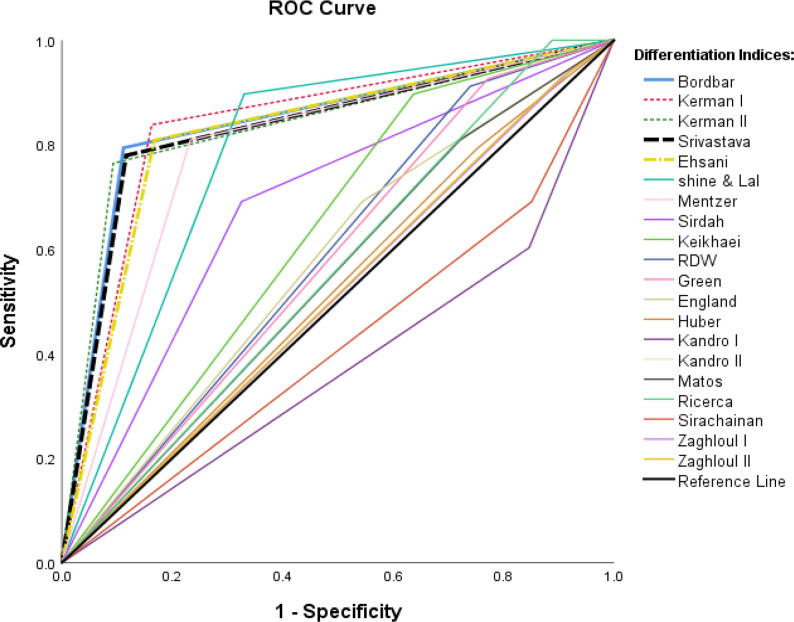
Furthermore, values of SEN, SPE, PPV, NPV, LR+, LR-, AUC and YI were reported for 20 differential equations (Table 5). The final criterion for selecting the best differential index was the size of the area under the ROC Curve (AUC).
Table 5:
Diagnostic values twenty differential indices.
| ID | Index | AUC(95% CI) | Sen (%) | Spe (%) | PPV (%) | NPV (%) | PLR (%) | NLR(%) | Acc | YI |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Bor | 0.841(0.780–0.902) | 0.791 | 0.885 | 0.695 | 0.927 | 6.903 | 0.235 | 0.862 | 0.676 |
| 2 | Ker.I | 0.838(0.780–0.896) | 0.843 | 0.834 | 0.621 | 0.943 | 5.103 | 0.188 | 0.836 | 0.677 |
| 3 | Ker.II | 0.836(0.772–0.899) | 0.765 | 0.907 | 0.722 | 0.924 | 8.258 | 0.259 | 0.873 | 0.672 |
| 4 | Sriv | 0.830(.769–.894) | 0.786 | 0.885 | 0.687 | 0.927 | 6.851 | 0.242 | 0.861 | 0.671 |
| 5 | Eh | 0.821(0.759–0.882) | 0.814 | 0.834 | 0.612 | 0.933 | 4.930 | 0.222 | 0.829 | 0.649 |
| 6 | Men | 0.788(0.725–0.851) | 0.814 | 0.770 | 0.532 | 0.928 | 3.550 | 0.240 | 0.781 | 0.584 |
| 7 | Sh-L | 0.780 (0.725–.842) | 0.888 | 0.669 | 0.470 | 0.948 | 2.691 | 0.165 | 0.724 | 0.558 |
| 8 | Sirdah | 0.683(0.610–0.756) | 0.691 | 0.674 | 0.398 | 0.875 | 2.122 | 0.457 | 0.678 | 0.365 |
| 9 | Kei | 0.630(0.560–0.700) | 0.897 | 0.362 | 0.308 | 0.917 | 1.407 | 0.283 | 0.491 | 0.259 |
| 10 | RDW | 0.586(0.513–0.659) | 0.911 | 0.260 | 0.280 | 0.903 | 1.232 | 0.338 | 0.416 | 0.172 |
| 11 | G-K | 0.577(0.504–0.651) | 0.926 | 0.227 | 0.275 | 0.907 | 1.199 | 0.322 | 0.395 | 0.154 |
| 12 | E-F | 0.570(0.493–0.647) | 0.647 | 0.495 | 0.285 | 0.818 | 1.282 | 0.712 | 0.531 | 0.142 |
| 13 | Kan.II | 0.556(0.482–0.630) | 1 | 0.111 | 0.262 | 1 | 1.125 | 0 | 0.325 | 0.111 |
| 14 | Ric | 0.556(0.482–0.630) | 1 | 0.111 | 0.263 | 1 | 1.125 | 0 | 0.325 | 0.111 |
| 15 | Matos | 0.544(0.467–0.621) | 0.808 | 0.282 | 0.262 | 0.824 | 1.127 | 0.676 | 0.408 | 0.091 |
| 16 | Huber | 0.520(0.442–0.598 | 0.794 | 0.246 | 0.250 | 0.791 | 1.053 | 0.835 | 0.378 | 0.040 |
| 17 | Z.II | 0.512(0.434–0.590 ) | 0.926 | 0.097 | 0.245 | 0.807 | 1.026 | 0.752 | 0.296 | 0.024 |
| 18 | Z.I | 0.510(0.432–0.588) | 0.941 | 0.082 | 0.242 | 0.818 | 1.025 | 0.712 | 0.286 | 0.023 |
| 19 | Sir | 0.420(0.339–0.501) | 0.691 | 0.151 | 0.203 | 0.611 | 0.814 | 2.040 | 0.279 | −0.157 |
| 20 | Kan.I | 0.378(0.297–0.459) | 0.602 | 0.153 | 0.183 | 0.550 | 0.712 | 2.586 | 0.261 | −0.243 |
M:Males; F:Females
Based on findings and the AUC (Fig. 2), Bor, Ker.I, Ker.II, Sriv and Eh differential equations had the highest precision and performance (higher value indices); and the RDW, GK, Kan.II, Ric, Matos, Huber, Z.I, Z.II, and Kei equations had high sensitivity, but low specificity.
Fig. 2:
Roc Curves of blood indices
Discussion
The present study aimed to evaluate the ability of 20 equations of differentiating BTT from IDA including simple blood indices. Sensitivity, specificity, Youden’s index, and other value indices, and Roc curve drawing were used for evaluation. Findings of the present study indicated that blood indices, namely RBC, RDW and HbA2 in individuals with BTT, and indices, namely HGB, HCT, MCV, MCH and MCHC in individuals with IDA were higher than the comparison group, but the differences were statistically significant only for HbA2, MCHC, HGB and RBC. Examination of the research findings based on gender also indicated that blood indices of men and women with IDA were similar, but among individuals with the BTT, men had higher RBC and HbA2 than women and the difference was significant (Table 4).
In a similar study (12), only the MCHC index was higher in individuals with BTT; and the difference of the HGB index was not significant between two groups. In another study (15), RBC, HGB, HbA2 and MCV indices were greater in individuals with Beta Thalassemia Trait; and the MCH and RDW were greater in those with IDA, but the study did not report any significant difference. Matos et al. (13) also reported that RBC, HGB, HT and MCHC indices were greater in individuals with BTT than those with IDA. The mean RBC, HGB and HCT were greater in men with IDA than in women, but all seven indices were higher in men than women in BTT group (18).
The present study indicated the best performance based on the AUC belonged to Bor, Ker.I, Ker.II, Sriv and Eh differentiators, and the lowest performance was obtained for Kan.I and Sir Equations. Examination of the value indices of the top 5 differential equations indicated that the highest SEN, NPV and Y.I were seen in Ker.I equation, and the highest SPE, PPV, PLR, NLR and Acc in Ker.II equations. Therefore, the importance of these two indices seems not to be less than Bor (Table 5).
Each of the published articles reported different results depending on the analytical method. Arora et al.(10) selected Men, Matos & Carvalho and Red Cell Distribution Index (RCDI) equations for investigation. Men’s equation with a sensitivity of 97.62%, specificity of 66.67%, and Youden’s index of 64% was better than the other two indices. However, values of the RCDI differentiator (92.86%, 66.67% and 59%) were a little different from Men. Jahangiri et al. (11) conducted a systematic review of 12 famous differential equations and reported M/H index (ratio of microcytic to hypochromic cells) with sensitivity and specificity of 0.92 and 0.86 respectively. In the discussion of the study, the formula alone could not differentiate BTT from IDA. Kabootarizadeh et al. (6) used a neural network model on blood indices, namely RBC, HGB, MCV and MCH, in Ahvazi couples and reported the sensitivity, specificity and precision of the resulting model equal to 92.33%, 93.13% and 92.5% respectively. The study found the model to be suitable, but it was necessary to test it in more complex conditions. Jameel et al. (12) studied 135 individuals with microcytic-hypochromic anemia and concluded that the RDWI differential equation (88% sensitivity and 86% specificity) differentiated BTT from IDA better than RDW (80% sensitivity and 86% specificity). The present study also reported the sensitivity and specificity of RDW equal to 91% and 26% respectively. Ullah et al. (15) conducted a cross-sectional study in Pakistan and investigated five differential equations, Men, RDWI, Sh-L, Srivastava and GK and found a better performance of RDWI with 100% sensitivity and 93% specificity than other equations. In their paper, 570 out of 800 blood samples had IDA and 230 ones had BTT. However, they also discussed the investigation of the issue in larger sample sizes. In a study, among nine famous indices and the innovative differential equation (Sir), the Sir equation and GK and RDWI indices were selected with AUC of 0.914, 0.909, and 0.907 respectively, but there was no significant difference between AUC values of three indices(26). The RDW index had the minimum AUC. Finally, Hashemieh et al. (8) examined the performance of a beta-thalassemia major prevention plan.
Study Limitations
One of the limitations of the study was the small sample size, the other is that despite the advantages and simplicity of the implementation of blood indices, there is a limitation of discriminating formulas since they are not able to differentiate all cases of IDA from BTT.
Conclusion
Although none of the equations could have 100% sensitivity and specificity, five differential equations showed better performance; the Bor equation was in the first place. The equations Ker.I and Ker.II indicates that the Bor index could achieve a greater AUC, but other value indices had maximum amounts in Ker differential equations. Therefore, the importance of two indices seems not to be less than Bor. Previous studies and their comparison to achieve better differentiation performance and detect their weaknesses indicated that it was necessary to consider the ethnic and racial composition in the population as well as other factors involved in the field as well as test a variety of differentiators and introduce new methods to provide blood and differential indices.
Journalism Ethics considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
We are deeply grateful for the cooperation of the Ethics Committee of Khoy University of Medical Sciences and also Beta Thalassemia Counseling Center.
Footnotes
Funding
The present article was conducted and approved as a research project by the Ethical Committee of the Deputy Research with a code of ethics (IR.KHOY.REC.1398.004) and was funded by Khoy University of Medical Sciences, Khoy, Iran.
Conflict of interest
The authors declare that there is no conflict of interests.
References
- 1.Choudhry VP. (2017). Thalassemia minor and major: Current management. Indian J Pediatr, 84(8):607–611. [DOI] [PubMed] [Google Scholar]
- 2.Jaripour ME, Hayatigolkhatmi K, Iranmanesh V, et al. (2018). Prevalence of B-Thalassemia Mutations among Northeastern Iranian Population and their Impacts on Hematological Indices and Application of Prenatal Diagnosis, a Seven-Years Study. Mediterr J Hematol Infect Dis, 10(1):e2018042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmadnezhad E, Sepehrvand N, fayyaz Jahani F, et al. (2012). Evaluation and Cost Analysis of National Health Policy of Thalassaemia Screening in West-Azerbaijan Province of Iran. Int J Prev Med, 3(10):687–92. [PMC free article] [PubMed] [Google Scholar]
- 4.Joosten E. (2017). Iron deficiency anemia in older adults: A review. Geriatr Gerontol Int, 18(3):373–379. [DOI] [PubMed] [Google Scholar]
- 5.Esmat B, Mohammad R, Behnam S, et al. (2010). Prevalence of Iron Deficiency Anemia among Iranian Pregnant Women; a Systematic Review and Meta-analysis. J Reprod Infertil, 11(1):17–24. [PMC free article] [PubMed] [Google Scholar]
- 6.Kabootarizadeh L, Jamshidnezhad A, Koohmareh Z. (2019). Differential Diagnosis of Iron-Deficiency Anemia from Î2-Thalassemia Trait Using an Intelligent Model in Comparison with Discriminant Indexes. Acta Informatica Medica, 27(2):78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Origa R. (2000). Beta-Thalassemia. 2000 [updated 2000; cited 2000 Sep 28 [Updated 2018 Jan 25]]; Available from: https://www.ncbi.nlm.nih.gov/sites/books/NBK1426/
- 8.Hashemieh M, Timori Naghadeh H, Tabrizi Namini M, et al. (2015). The Iran Thalassemia Prevention Program: Success or Failure? Iran J Ped Hematol Oncol, 5(3):161–6. [PMC free article] [PubMed] [Google Scholar]
- 9.Samavat A, Modell B. (2004). Iranian national thalassaemia screening programme. BMJ, 329(7475):1134–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arora S, Rana D, Kolte S, Dawson L, et al. (2018). Validation of new indices for differentiation between iron deficiency anemia and beta thalessemia trait, a study in pregnant females. International Journal of Scientific Reports, 4(2):26. [Google Scholar]
- 11.Jahangiri M, Rahim F, Saki Malehi A, et al. (2019). Differential Diagnosis of Microcytic Anemia, Thalassemia or Iron Deficiency Anemia: A Diagnostic Test Accuracy Meta-Analysis. Mod Med Lab J, 3(1): 16–29. [Google Scholar]
- 12.Jameel T, Baig M, Ahmed I, et al. (2017). Differentiation of beta thalassemia trait from iron deficiency anemia by hematological indices. Pak J Med Sci, 33(3):665–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matos F, Jr, Dusse L, Borges KBG, et al. (2016). A new index to discriminate between iron deficiency anemia and thalassemia trait. Rev Bras Hematol Hemoter, 38(3):214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sehgal K, Mansukhani P, Dadu T, et al. (2015). Sehgal index: A new index and its comparison with other complete blood count-based indices for screening of beta thalassemia trait in a tertiary care hospital. Indian J Pathol Microbiol, 58(3):310–5. [DOI] [PubMed] [Google Scholar]
- 15.Ullah Z, Khattak AA, Ali SA, et al. (2016). Evaluation of five discriminating indexes to distinguish Beta-Thalassemia Trait from Iron Deficiency Anaemia. J Pak Med Assoc, 66(12):1627–1631. [PubMed] [Google Scholar]
- 16.Srivastava PC, Bevington JM. (1973). Iron deficiency and-or thalassaemia trait. Lancet, 1(7807):832. [DOI] [PubMed] [Google Scholar]
- 17.Shine I, Lal S. (1977). A strategy to detect beta-thalassaemia minor. Lancet, 1(8013):692–4. [DOI] [PubMed] [Google Scholar]
- 18.Sirdah M, Al Mghari K, Abuzaid AH, et al. (2018). Should sex differences be considered when applying mathematical indices and formulas for discriminating Î2- thalassemia minor from iron deficiency? Pract Lab Med, 11:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jayabose S, Giavanelli J, Levendoglu-Tugal O, et al. (1992). Differentiating Iron Deficiency Anemia from Thalassemia Minor by Using an RDW-Based Index. J Pediatr Hematol Oncol, 21:314 [Google Scholar]
- 20.Mentzer WC., Jr (1973). Differentiation of iron deficiency from thalassaemia trait. Lancet, 1(7808):882. [DOI] [PubMed] [Google Scholar]
- 21.Ricerca BM, Storti S, d’Onofrio G, et al. (1987). Differentiation of iron deficiency from thalassaemia trait: a new approach. Haematologica, 72(5):409–13. [PubMed] [Google Scholar]
- 22.Ehsani M, Darvish A, Eslani A, et al. (2005). A new formula for differentiation of iron deficiency anemia (IDA) and thalassemiatrait (TT). Turk J Hematol, 22(Suppl):268. [Google Scholar]
- 23.England JM, Bain BJ, Fraser PM. (1973). Differentiation of iron deficiency from thalassaemia trait. Lancet, 1(7818):1514. [DOI] [PubMed] [Google Scholar]
- 24.Green R, King R. (1989). A new red cell discriminant incorporating volume dispersion for differentiating iron deficiency anemia from thalassemia minor. Blood Cells, 15(3):481–91; discussion 492–5. [PubMed] [Google Scholar]
- 25.Hafeez Kandhro A, Shoombuatong W, Prachayasittikul V, et al. (2017). New bioinformatics-based discrimination formulas for differentiation of thalassemia traits from iron deficiency anemia. Lab Med, 48(3):230–237. [DOI] [PubMed] [Google Scholar]
- 26.Sirdah M, Tarazi I, Al EN, et al. (2008). Evaluation of the diagnostic reliability of different RBC indices and formulas in the differentiation of the beta-thalassaemia minor from iron deficiency in Palestinian population. Int J Lab Hematol, 30(4):324–330. [DOI] [PubMed] [Google Scholar]
- 27.Sirachainan N, Iamsirirak P, Charoenkwan P, et al. (2014). New mathematical formula for differentiating thalassemia trait and iron deficiency anemia in thalassemia prevalent area: a study in healthy school-age children. Southeast Asian J Trop Med Public Health, 45(1):174–82. [PubMed] [Google Scholar]
- 28.Bordbar E, Taghipour M, Zucconi BE. (2015). Reliability of different RBC indices and formulas in discriminating between Î2-thalassemia minor and other microcytic hypochromic cases. Mediterr J Hematol Infect Dis, 7(1): e2015022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keikhaei B. (2010). A new valid formula in differentiating iron deficiency anemia from ß-thalassemia trait. Pakistan Journal of Medical Sciences Online, 26(2):368–373. [Google Scholar]
- 30.Huber A, Ottiger C, Risch L, et al. (2004). Thalassämie-syndrome: Klinik und diagnose syndromes thalassémiques: clinique et diagnostic. Forum Médical Suisse, 4:947–952. [Google Scholar]
- 31.Zaghloul A, Al-bukhari T, Bajuaifer N, et al. (2016). Introduction of new formulas and evaluation of the previous red blood cell indices and formulas in the differentiation between beta thalassemia trait and iron deficiency anemia in the Makkah region. Hematology, 21(6):351–8. [DOI] [PubMed] [Google Scholar]