Abstract
Background:
Global warming, lifestyle, or working in a high temperature environment leads to have increased health risk factors. This meta-analysis was conducted to determine the impact of high ambient temperature on male reproductive function.
Methods:
Scientific articles were screened in the database including MEDLINE, EMBASE, National center for biotechnology information (NCBI) or Web of Science with relating keywords. Impact data of high ambient temperature on semen parameters were extracted and analyzed by STATA software according to the Random Effects Model. The high ambient temperature exposure group and Non-exposure group were compared using the standard mean difference (SMD). Publications were evaluated for publication bias by Egger test.
Results:
Nine articles were finally selected from databases examining the effect of high ambient temperature on male reproductive health of 356 men from Iran, Italy, Thailand, China, Egypt. High ambient temperature showed a significant decrease in the seminal parameters, semen volume during each ejaculation (SMD = −0.74; 95% CI −1.11, −0.36), sperm concentration (SMD = −1.07; 95% CI −1.42, −0.72), total sperm count (SMD = −1.52; 95% CI −2.96, −0.08), sperm motility (SMD = −1.93; 95% CI −2.83, −1.04), sperm progressive motility (SMD = −1.65; 95% CI −2.39, −0.91) and normal morphology (SMD = −2.41; 95% CI −3.30, −1.52).
Conclusion:
High ambient temperature negatively affects sperm quality, including decreased semen volume, sperm count, sperm concentration, motility and normal morphology. This might lead to protective strategies to avoid the adverse effects of high ambient temperature on male fertility.
Keywords: Meta-analysis, Global warming, Temperature, Heat stress, Semen, Spermatogenesis
Introduction
Global warming will become more extreme in the coming decades (1). Environmental factors impact human reproductive disorders during their development (2). In particular, heat stress is considered to be the most influential cause of reproductive function in mammals (3). There are various causes the elevate scrotal temperature such as occupational exposure, lifestyle, or varicocele (4–10).
Heat stress in animals affects not only one part of the body but also can extend to the entire body, although the heat effect was in contact with a single particular organ or anatomical part (11). During spermatogenesis, the optimal temperature of the testicles is 2 to 4°C lower than body temperature (12). In 2020, Yan-Qing Wu showed that heat stress caused an increase in testicular tissue temperature, decreasing sperm concentration and motility in humans who experienced scrotal warming (13). Heat stress damages human sperm by reducing sperm motility and viability (14). Each 1°C increase in testicular temperature leads to a 14% decrease in spermatogenesis (15). High ambient temperature drastically reduces sperm motility through decreased mitochondrial activity and ATP synthesis (16). Testicular tissue heated stress leads to apoptosis via mitochondrial pathways or DNA damage (17). Besides, damaged sperm in the vas deferens DNA breaks under the influence of high ambient temperature can lead to male infertility (18). Therefore, heat stress is a high-risk factor affecting testicular tissue, reducing sperm quality, and increasing the risk of infertility.
Many other factors are elevating testicular temperature and they can be grouped according to habits, lifestyles (7; 19), occupational factors (i.e. prolonged exposure to high temperatures) (20; 21), and climate change (22). The association between the effect of high ambient temperature on human sperm parameters has also been investigated, but these results are indecisive and controversial. Research conducted in the steel industry environment in Iran has shown that heat stress significantly reduces sperm parameters: quantity, morphology, and motility (5). In a study on 30 healthy male volunteers who had heated stress by belt warming, there were changes in morphology, concentration, motility, DNA integrity, and levels of FSH, LH (23). However, some reports have demonstrated no effect between temperature and male reproductive quality, Saikhun reported that: the semen parameters of two groups (used sauna and control groups) had no statistically significant difference (24). The average sperm velocity had non-significant differences even though the participants had a long exposure to high temperatures (25). Besides, Hjollund NHI concluded scrotal temperature was not significantly associated with sperm motility, morphology, pH of semen, and level of testosterone (26).
This study collected 9 separate publications published over 26 years from 1992 to 2017 on the effects of high ambient temperature on the male reproductive health of a total of 336 men. It was based on the search PRISMA checklist (27) and the PICO model was by the research question and search term. The current study aimed to evaluate the relationship between the temperature and the quality of spermatogenesis based on an accurate review of the best available studies at the research time.
Methods
Search strategy and identification of relevant studies
The search protocol of this study was carried out according to the PRISMA checklist and the PICO model. Population: a male who had been exposed to heat stress. Intervention: Heat stress. Comparator: Non-exposure group and Heat exposure group. Outcomes: High ambient temperature on sperm parameters. Our search strategy is based on relevant keywords related to our topic, scientific articles were manually searched via the PubMed, Embase, and Google scholar websites with the terms: “heat stress”, “high ambient temperature”, “thermal shock”, “testicular heating”, “scrotal heating”, “high temperature”, “work exposure”, “steel industry”, “ceramic industry”, “sauna”, “male infertility”, “semen parameters”, “semen quality”, “sperm abnormal”, “sperm characteristics”, “human sperm”, “impaired spermatogenesis”. As a result, this study has obtained 916 scientific articles and examined the details of each article based on information on its title, abstract, and case report, excluding those that were exhaustive examinations, or duplicated ones. These analyses identified 178 eligible full-text articles for further selection. Finally, 9 articles were selected for the main analysis in this meta-study. The other 169 articles were excluded from the study if they are reviews, in vitro studies, studies with data only in the graphical format, studies not reporting data of interest, or those that have no data available. The reference list of primary research reports was cross-checked in an attempt to identify additional studies.
Criteria for inclusion and exclusion
The studies which fulfilled the criteria given below were considered eligible and selected for this meta-analysis: (a) evaluating the association of high ambient temperature on sperm parameters; (b) including cases and control groups; (c) studying the sperm parameters with habits; (d) studying sperm parameters in the context of occupational or environmental exposure to temperature; (e) studies representing original data; (f) studies were written in English. Articles have been removed from our reviewed list: (a) being a review, case report, or conference summary; (b) containing no original data or are not related to the subject of study; (c) having no available data regarding sperm and high ambient temperature.
Collect data for analysis (Data extraction)
Researchers investigated and found differences between independently produced documents - the following information was gathered in a standard format: first author’s last name; year of publication; country of origin; the number of subjects; mean age, data related to the effect of high ambient temperature on male standard semen parameters such as semen volume (mL), concentration (106 sperm/mL), sperm motility (%), normal morphology, abnormal morphology, and defective sperm. Indicator values are described as means and standard or median deviation and interquartile range (IQR). If data is provided as median and IQR, convert it to mean and standard deviation before meta-analysis (28; 29).
Statistical analysis
Random-effects models were used to synthesize the association in the middle between high ambient temperature and sperm parameters. The values of the Heat exposure and Non-exposure groups were compared using the standard mean difference (SMD). The heterogeneity study was described using the Higgins metric I2 (30). An I2 value of 0% was considered to be no observed heterogeneity, while a value greater than 50% was considered substantial heterogeneity. The inverse variance method was applied to pool the mean difference. If data were provided as the median and using interquartile range (IQR) these measures were transformed into mean and standard deviation (SD). Fixed-effects and random-effects models with 95% confidence intervals (CI) were used to estimate the mean effect of high outdoor temperature on men’s semen parameters. Egger test and funnel plots were used to assess the publication bias of selected articles in this study. Three authors independently reviewed and selected final full-text articles from the identifying relevant article list for the meta-analysis step. The meta-analysis was performed using Stata SE version 15 software (Stata Corp, College Station, TX, USA).
Results
Characterization of eligible studies
The results of 916 scientific articles were initially found, of which 738 studies were removed from the list due to the non-responsive title and summary. The remaining 178 full-text articles continued to be reviewed in detail. As a result, the other 169 articles were excluded if they had duplicate content, only graphic data, review studies, animal studies, or in vitro study (Fig. 1). Finally, this study has analyzed 9 separate articles (5; 7; 23–25; 31–34) over 26 years from 1992 to 2017 in terms of the effects of the high ambient temperature on the male reproductive health of a total of 336 men search was completed by June 30th, 2020 (Table 1). In these 9 scientific reports on the impact of high ambient temperature exposure on human semen parameters, there are 6 articles reported on semen volume, 7 articles having information on sperm concentration, 5 articles relating to total sperm count, results in sperm motility found in 6 articles, and details of sperm morphological covered in 6 articles.
Fig. 1:
The selection process of articles for this meta-analysis study
Table 1:
Summary of studies included in the meta-analysis collected until June 30, 2020
| No | Study ID | Year | Country | Participation | n | Heat exposure method | Non-exposure group | Heat exposure group | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||||||||||
| n | Age | Scrotal temperature | n | Age | Scrotal temperature | |||||||||||||||||
| 1 | Hamerezaee 2017 | 2017 | Iran | Workers in the steel industry | 44 | Workers had been exposed to heat stress for approximately 4 h daily | 14 | 32.1±7.2 | 34.79±0.41 | 30 | 33.2±6.8 | 35.85±1.26 | ||||||||||
| 2 | Garolla A2012 | 2012 | Italy | Volunteers have used the sauna | 20 | Volunteers underwent two sauna sessions per week for 3 months, at 80–90°C, each lasting 15 minutes. | 10 | 33.2±4.7 | 34,5±0.68 | 10 | 33.2±4.7 | 37,5±0.48 | ||||||||||
| 3 | Saikhun J 1998a | 1998 | Thailand | Volunteers have used the sauna | 8 | Volunteers used the sauna at a temperature of 80±90°C in the same sauna room for 30 min per day for 1 week. | 8 | 30 (22–53) | 35.2±0.2 | 8 | 30 (22–53) | 37.6±0.2 | ||||||||||
| Saikhun J 1998b | 1998 | Thailand | Volunteers have used the sauna | 8 | Volunteers used a sauna at a temperature of 80±90°C in the same sauna room for 30 min per day for 2 weeks. | 8 | 30 (22–53) | 35.2±0.2 | 8 | 30 (22–53) | 37.6±0.2 | |||||||||||
| 4 | Zhang MH 2017a | 2017 | China | Volunteers were exposed to an electric warming bag attached to the under-pants | 30 | Volunteers were exposed to the condition of 40 to 43°C scrotal heating belt warming 40 min each day for successive 2 days per week, for 1 month. | 30 | 34.3 ± 4.5 | N/A | 29 | 34.3 ± 4.5 | 43.0 ± 0.5 | ||||||||||
| Zhang MH 2017b | 2017 | China | Volunteers were exposed to an electric warming bag attached to the under-pants | 30 | Volunteers were exposed to the condition of 40 to 43°C scrotal heating belt warming 40 min each day for successive 2 days per week, for 2 months. | 30 | 34.3 ± 4.5 | N/A | 30 | 34.3 ± 4.5 | 43.0 ± 0.5 | |||||||||||
| Zhang MH 2017c | 2017 | China | Volunteers were exposed to an electric warming bag attached to the under-pants | 30 | Volunteers were exposed to the condition of 40 to 43°C scrotal heating belt warming 40 min each day for successive 2 days per week, for 3 months. | 30 | 34.3 ± 4.5 | N/A | 30 | 34.3 ± 4.5 | 43.0 ± 0.5 | |||||||||||
| 5 | Momen MN 2010 | 2010 | Egypt | Workers exposed to a high temperature | 130 | Workers from the continuous steel-casting plant, where exposure to heat occurs for about 5 hours. | 90 | 32.6±4.3 | 34.38±0.22 | 40 | 31.9±3.9 | 34.48±0.27 | ||||||||||
| 6 | Rao M 2015 | 2015 | China | Volunteers testicular warming in a water bath | 20 | Volunteers received testicular warming in a 43°C water bath 10 times, for 30 min each time. | 10 | 32.2±7.0 | N/A | 10 | 34.1±6.1 | 43°C | ||||||||||
| 7 | Figa-Talamanca 1992 | 1992 | Italy | Workers in the ceramics industry | 60 | Workers in the ceramics industry had been exposed to high ambient temperature. | 14 | 40.7±10.2 | N/A | 46 | 42.3±7.9 | 42°C | ||||||||||
| 8 | Zhang MH 2015 a | 2015 | China | Volunteers locally at scrotal heat stress belt warming | 25 | Volunteers locally at 40–43°C scrotal heating belt warming 40 min each day for successive 2 days per week, continuously 3 months. | 25 | 34.6 ± 4.5 | N/A | 25 | 34.6 ± 4.5 | 40.0 to 43.0 | ||||||||||
| 9 | Zhang MH 2015 b | 2015 | China | Volunteers locally at scrotal heat stress belt warming | 19 | Volunteers were exposed to the condition of 40–43°C scrotal heating belt warming 40 min each day for successive 2 days per week, continuously for 3 months. | 19 | 34.75±2.6 | N/A | 19 | 34.75±2.6 | 43°C | ||||||||||
Association between high ambient temperature and semen volume
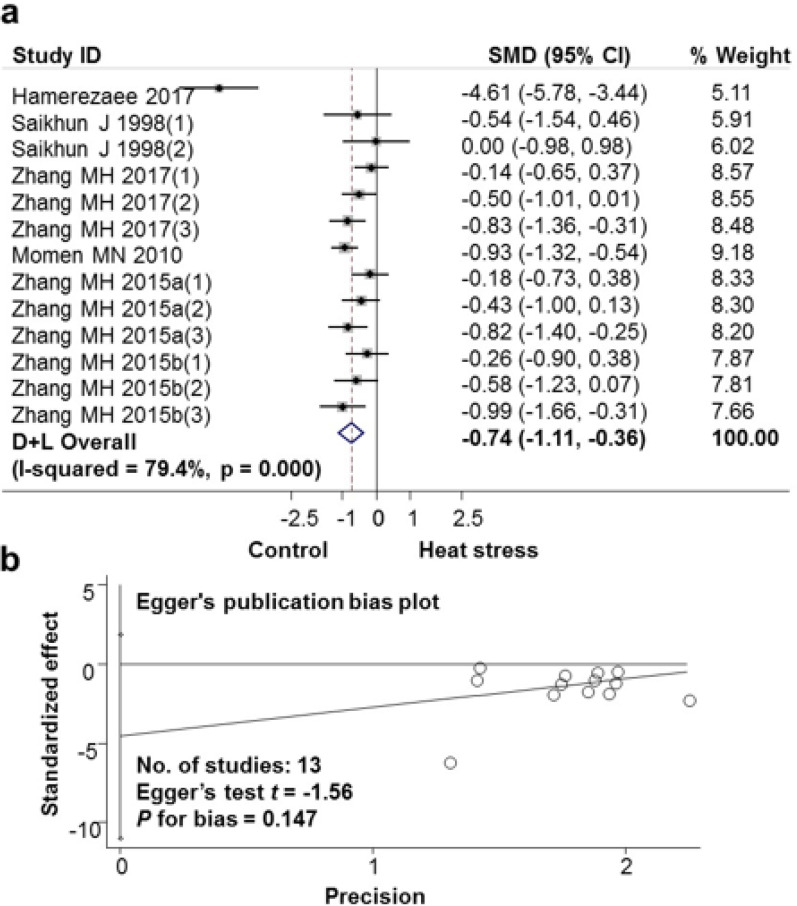
The impact of high ambient temperature exposure on subjects’ semen volume was analyzed, the results are shown in Fig. 2a and Table 2. Data were analyzed according to the Random Effects Model. The difference between studies was large (I-squared heterogeneity index=79.4%, P<0.001). Each study weighed (% weight) from 5.11 to 9.18. The standard mean difference (SMD) difference between the Heat exposure group and the Non-exposure group was −0.74 (95% CI: −1.11, − 0.36). Exposure to heat stress reduced semen volume per ejaculation. In Fig. 2b, the publication bias of these selected articles on the effects of high ambient temperature exposure on semen volume was analyzed by the Egger test. There was no publication bias with P for bias = 0.147.
Fig. 2:
Association between high ambient temperatures and semen volume
a. Forest plot for the association between heat stress and semen volume; b. Evaluation publication bias among studies included in the meta-analysis by Egger publication bias plot
Table 2:
Associations between high ambient temperature and sperm parameters
| Sperm parameter | No. of studies | SMD (95% CI) | Egger’s test t | P-value for bias |
|---|---|---|---|---|
| Semen volume | 13 | −0.74 (−1.11, −0.36) | −1.56 | 0.147 |
| Sperm concentration | 13 | −1.07 (−1.42, −0.72) | 0.48 | 0.639 |
| Sperm count | 5 | −1.52 (−2.96, −0.08) | −0.99 | 0.397 |
| Sperm motility | 11 | −1.93 (−2.83, −1.04) | −1.58 | 0.150 |
| Sperm progressive motility | 9 | −1.65 (−2.39, −0.91) | 2.25 | 0.059 |
| Sperm morphology | 13 | −2.41 (−3.30, −1.52) | −2.01 | 0.070 |
Association between high ambient temperature and sperm concentration
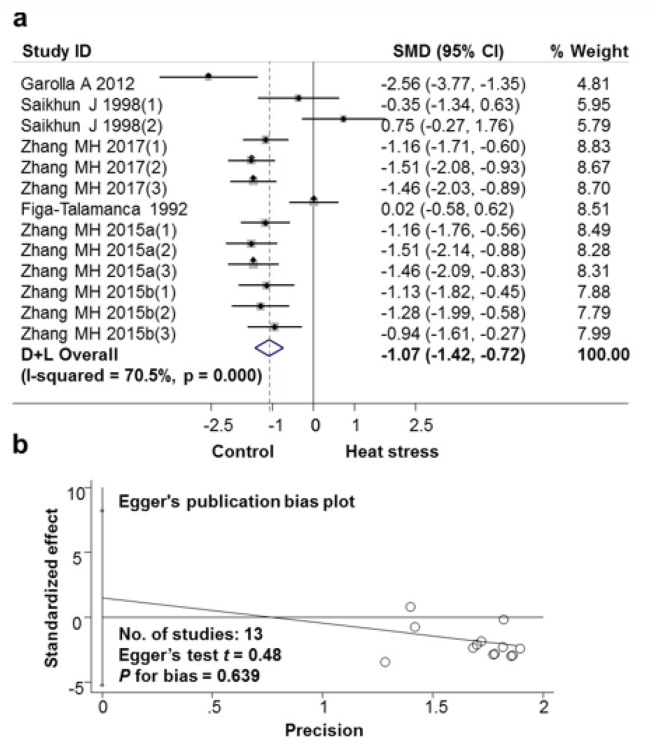
The results of the analysis showed that exposure to high ambient temperatures reduced sperm density in semen volume (Fig. 3a and Table 2). The difference between the studies was large (I-squared heterogeneity=70.5%, P<0.001), weighted from 4.81 to 8.83. The mean SMD calibrated mean dissimilar between the Heat exposure and the Non-exposure group was −1.07 (95% CI: −1.42, −0.72). The analytical results showed no publication bias with P-Value for bias=0.639 (Fig. 3b).
Fig. 3:
Association between high ambient temperatures and sperm concentration
a. Forest plot for the association between heat stress and sperm concentration; b. Evaluation publication bias among studies included in the meta-analysis by Egger publication bias plot
Association between high ambient temperature and sperm count
Figure 4 demonstrates the effect of heat stress exposure on sperm count. Related studies have a large difference among them (I-squared heterogeneity=92.6%, P<0.001). Weight between 18.58 and 21.81. The mean difference in SMD calibration between the Heat exposure group and the Non-exposure group was −1.52 (95% CI: −2.96, −0.08) (Fig. 4a and Table 2). Exposure to heat stress reduces the total number of spermatozoa in semen. The results of the publication bias assessment are presented in Fig. 4b. There was no statistically significant publication bias found among selected articles on the effects of heat stress exposure on sperm count with P for bias=0.397.
Fig. 4:
Association between high ambient temperatures and sperm count
a. Forest plot for the association between heat stress and sperm count; b. Evaluation publication bias among studies included in the meta-analysis by Egger publication bias plot
Association between high ambient temperature and sperm motility
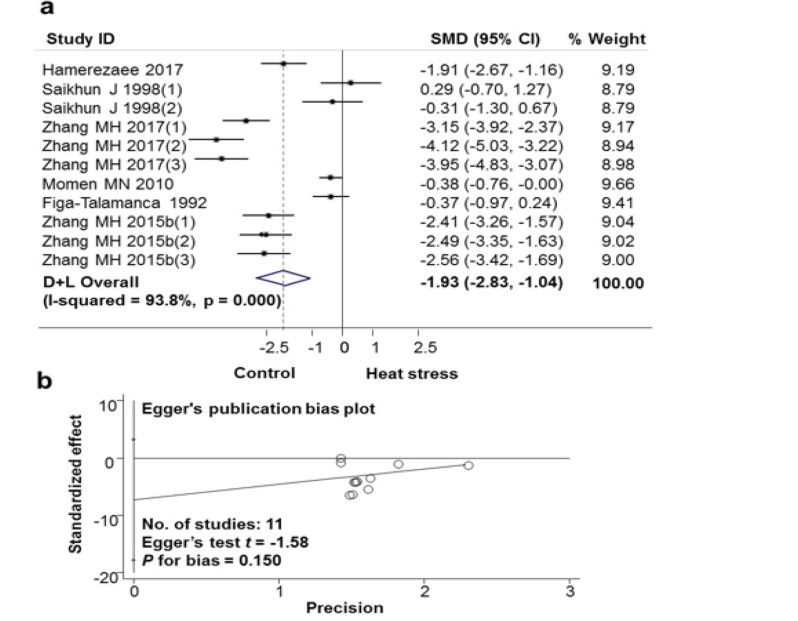
The impact of high ambient temperature exposure on sperm motility was shown in Fig. 5 and Table 2. The meta-analysis showed exposure to heat stress reduced sperm motility in semen (11 studies from 6 scientific articles). The difference among the studies was large (I-squared heterogeneity=93.8%, P>0.001). The mean SMD calibration difference between the Heat exposure group and the Non-exposure group was −1.93 (95% CI: −2.83, −1.04) (Fig. 5a). There was no publication bias with P for bias=0.150 (Fig. 5b). Similarly, the results of analysis of the effects of exposure to heat stress on sperm progressive motility (9 studies from 4 scientific articles) showed that exposure to heat stress decreased the progressive mobility of sperm in the semen accumulation. The mean difference in SMD calibration between the Heat exposure group and the Non-exposure group was −1.65 (95% CI: −2.39, −0.91) (Fig. 6a). Results of the publication bias assessment in these articles of the impact of high ambient temperature exposure on sperm progressive motility, by the Egger test, showed no publication bias with P=0.059 (Fig. 6b).
Fig. 5:
Association between high ambient temperatures and sperm motility
a. Forest plot for the association between heat stress and sperm motility; b. Evaluation publication bias among studies included in the meta-analysis by Egger publication bias plot
Fig. 6:
Association between high ambient temperatures and sperm progressive motility
a. Forest plot for the association between heat stress and sperm motility; b. Evaluation publication bias among studies included in the meta-analysis by Egger publication bias plot
Association between high ambient temperature and sperm morphology
The meta-analysis was also utilized to investigate the association between high ambient temperature and sperm morphology, the results are presented in Fig. 7a and Table 2. The studies have a large difference (I-squared heterogeneity=93.2%, P>0.001), weight from 7.44 to 8.00. The mean SMD calibration difference between the Heat exposure group and the Non-exposure group was −2.41 (95% CI: −3.30, −1.52). Exposure to heat stress reduces the number of normally morphologically shaped sperm in semen accumulation. There was no publication bias among the studies related to sperm morphology status after males had been exposed to elevated ambient temperatures, demonstrated by the Egger test with P for bias =0.070 (Fig. 7b).
Fig. 7:

Association between high ambient temperatures and sperm morphology
a. Forest plot for the association between heat stress and sperm motility; b. Evaluation publication bias among studies included in the meta-analysis by Egger publication bias plot
Discussion
Global warming is dangerous to public health, according to the statistic estimation of Europe, heat-waves in 2003 caused 70,000 deaths (35), and in Russia in 2010 also led to 11,000 deaths (36). There are many causes of global warming such as climate change, wildfires, and smog, along with the lack of crops, which contribute to further mortality (37). Surface temperature will continue to rise in many parts of the world. By the year 2100, about the worst-case climate change scenario, Earth’s surface temperature will continue to increase by 3–4°C unless the treaties on reducing industrial gas emissions around the world are implemented (38). The relationship between the ambient temperature and mortality needs special attention (39). However, when the outside temperature exceeded the permissible limits, whether they were prolonged heatwaves in summer or cold spells in winter, it likely led to an increase in the death rate (40). For low- and middle-income countries where the working condition has many disadvantages, occupational factors affecting reproductive health need to be more attentive because it affects not only the economy but also the development of society (41). In South America and Asia, climate change influences both mental and physical health, and the effects on populations are increasingly negative (42). Many occupational factors have been identified as possible causes of the decline in male fertility. They include materials commonly used in industry and agriculture, for example, heavy metals and various chemical agents. In addition, other physical factors such as elevated Earth’s surface temperature, radiation exposure, and biological factors (i.e. phyto- and xeno-estrogen pollution in the environment) were detrimental to male physiological function. These effects not only reduce sperm concentration but also lead to changes in sexual behavior, mood disturbances, and cause genital cancer (22). Moreover, the difference in sperm quality was significant when combined with other occupational risks such as the forced working posture of workers (43).
There was many strong scientific evidence on the quality of men’s sperm is influenced by the high temperature. The rate of male infertility tended to increase because citizens worked in high-temperature environments for many hours (20). Moreover, heat stress has a direct impact on work performance by increasing the risk of illness and work-related injuries. Boni et al. demonstrated high ambient temperature as a serious threat to reproductive function in humans (44). Similarly, Hamerezaee et al. have drawn similar results about the effects of temperature stress on semen quality when studying steels industry workers in Iran (5). There was another large-scale epidemiological study at a Danish infertility clinic in which subjects underwent sperm examinations or infertility treatments and provided information on occupational exposure. The groups of workers exposed to textile dyes or lead, noise, cadmium, or mercury were all potentially infertile (45). In another case, a study conducted in a fertility clinic in New Orleans found that men working in buildings without air conditioning during summertime would reduce their sperm quality. In contrast, a study in the ceramic industry reported that workers exposed to high temperatures had no significant difference in semen analysis except for the sperm velocity (25). Furthermore, the mean physiological parameters did not differ significantly between subjects adapted and non-adapted at the office and work-place (46).
In addition to reports showing the link between sperm parameters and exposure to the workplace with high ambient temperature, there were also reports of the importance of diet, physical activity, and habit management on the quality of sperms (7). The percentage of sperm motility was significantly reduced if men worked or slept in a warm environment, sat for an average workday of 6 hours, wore tight-fitting underwear when sleeping, or use electrical blankets-cotton (10). Testicular hyperthermia caused by elevating the testicles into the groin during the day would alter the sperm characteristics (47), including the decrease in sperm motility parameters (10). In Finland and the Nordic countries, there was a high demand for studies on the effect of saunas on male fertility. Exposure to high temperatures in a sauna causes significant sperm impairments, including fluctuations in sperm indexes, mitochondrial function, and sperm DNA closure (7). Moreover, in a study of regular sauna bathers (n=10), the male reproductive function was disrupted and could be altered (48). Regarding the biological mechanisms of the effect of heat on male fertility, it was suggested several mechanisms. High ambient temperature occurs, leading to high levels of crystalline nitric oxide (NO), nitric oxide synthase (NOS), macrophage movement inhibitors (MIF), sperm DNA integrity; the condensation of chromosomes, and Caspase-3 increases. While the concentration of Caspase-3 (Cysteine-requiring Aspartate Protease) is an important sieving enzyme for apoptosis. When Caspase-3 is activated and loses the integrity of DNA fragmentation, other markers of end-stage apoptosis are expressed by a different proportion of ejaculated sperm (49).
Conclusion
Various factors such as high ambient temperature in living or occupational environments, and unhealthy living habits, would increase the temperature of the scrotum, therefore, inducing negative effects on sperm quality. These factors may adversely affect male fertility. There is an urgent need to make recommendations and strategies to protect the health of people living or working in high-temperature environments, especially the reproductive health of males.
Journalism ethics considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
This study was supported by the Hue university-level research projects in science and technology, Grant No. DHH 2019-04-88. The authors also acknowledge the partial support by Hue University under the Core Research Program, Grant No. NCM.DHH.2022.02, Regenerative Medicine.
Footnotes
Conflict of interest
There is no conflict of interest.
References
- 1.Bongaarts J, Sitruk-Ware R. (2019). Climate change and contraception. BMJ Sexual & Reproductive Health, 45:233–5. [DOI] [PubMed] [Google Scholar]
- 2.Giudice LC. (2021). Environmental impact on reproductive health and risk mitigating strategies. Curr Opin Obstet Gynecol, 33(4):343–349. [DOI] [PubMed] [Google Scholar]
- 3.Hou Y, Wang X, Lei Z, et al. (2015). Heat-stress-induced metabolic changes and altered male reproductive function. J Proteome Res, 14(3):1495–503. [DOI] [PubMed] [Google Scholar]
- 4.Mínguez-Alarcón L, Gaskins AJ, Chiu Y-H, et al. (2018). Type of underwear worn and markers of testicular function among men attending a fertility center. Hum Reprod, 33(9):1749–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamerezaee M, Dehghan SF, Golbabaei F, et al. (2018). Assessment of Semen Quality among Workers Exposed to Heat Stress: A Cross-Sectional Study in a Steel Industry. Saf Health Work, 9(2):232–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Yu C, Bao J, et al. (2017). Impact of temperature on mortality in Hubei, China: a multi-county time series analysis. Scientific Reports, 7:45093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garolla A, Torino M, Sartini B, et al. (2013). Seminal and molecular evidence that sauna exposure affects human spermatogenesis. Hum Reprod, 28(4):877–85. [DOI] [PubMed] [Google Scholar]
- 8.Sharlip ID, Jarow JP, Belker AM, et al. (2002). Best practice policies for male infertility. Fertil Steril, 77(5):873–82. [DOI] [PubMed] [Google Scholar]
- 9.Jung A, Schill WB, Schuppe HC. (2005). Improvement of semen quality by nocturnal scrotal cooling in oligozoospermic men with a history of testicular maldescent. Int J Androl, 28(2):93–8. [DOI] [PubMed] [Google Scholar]
- 10.Jung A, Schuppe HC. (2007). Influence of genital heat stress on semen quality in humans. Andrologia, 39(6):203–15. [DOI] [PubMed] [Google Scholar]
- 11.Setchell BP, Waites GM, Thorburn GD. (1966). Blood flow in the testis of the conscious ram measured with Krypton85: effects of heat, catecholamines and acetylcholine. Circulation research, 18:755–765. [DOI] [PubMed] [Google Scholar]
- 12.Wang C, McDonald V, Leung A, et al. (1997). Effect of Increased Scrotal Temperature on Sperm Production in Normal Men. Fertil Steril, 68:334–9. [DOI] [PubMed] [Google Scholar]
- 13.Wu Y-Q, Rao M, Hu S-F, et al. (2020). Effect of transient scrotal hyperthermia on human sperm: an iTRAQ-based proteomic analysis. Reprod Biol Endocrinol, 18(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao F, Whiting S, Lambourne S, et al. (2021). Melatonin alleviates heat stress-induced oxidative stress and apoptosis in human spermatozoa. Free Radic Biol Med, 164:410–416. [DOI] [PubMed] [Google Scholar]
- 15.Durairajanayagam D, Agarwal A, Ong C. (2015). Causes, effects and molecular mechanisms of testicular heat stress. Reprod Biomed Online, 30(1):14–27. [DOI] [PubMed] [Google Scholar]
- 16.Gong Y, Guo H, Zhang Z, et al. (2017). Heat Stress Reduces Sperm Motility via Activation of Glycogen Synthase Kinase-3α and Inhibition of Mitochondrial Protein Import. Front Physiol, 8:718- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shahat A, Rizzoto G, Kastelic J. (2020). Amelioration of heat stress-induced damage to testes and sperm quality. Theriogenology,158: 84–96. [DOI] [PubMed] [Google Scholar]
- 18.Kim B, Park K, Rhee K. (2013). Heat stress response of male germ cells. Cell Mol Life Sci, 70(15):2623–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durairajanayagam D. (2018). Lifestyle causes of male infertility. Arab J Urol, 16(1):10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Otaibi ST. (2018). Male infertility among bakers associated with exposure to high environmental temperature at the workplace. J Taibah Univ Med Sci, 13(2):103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohammadia M, Heidari H, Charkhloo E, et al. (2020). Heat stress and physiological and perceptual strains of date harvesting workers in palm groves in Jiroft. Work, 66(3):625–636. [DOI] [PubMed] [Google Scholar]
- 22.Sinawat S. (2000). The environmental impact on male fertility. J Med Assoc Thai, 83(8):880–5. [PubMed] [Google Scholar]
- 23.Zhang MH, Zhai LP, Fang ZY, et al. (2018). Effect of scrotal heating on sperm quality, seminal biochemical substances, and reproductive hormones in human fertile men. J Cell Biochem, 119(12):10228–10238. [DOI] [PubMed] [Google Scholar]
- 24.Saikhun J, Kitiyanant Y, Vanadurongwan V, et al. (1998). Effects of sauna on sperm movement characteristics of normal men measured by computer-assisted sperm analysis. Int J Androl, 21(6):358–63. [DOI] [PubMed] [Google Scholar]
- 25.Figa-Talamanca I, Dell’Orco V, Pupi A, et al. (1992). Fertility and semen quality of workers exposed to high temperatures in the ceramics industry. Reprod Toxicol, 6(6):517–23. [DOI] [PubMed] [Google Scholar]
- 26.Hjollund NHI, Storgaard L, Ernst E, et al. (2002). Impact of diurnal scrotal temperature on semen quality. Reprod Toxicol, 16(3):215–21. [DOI] [PubMed] [Google Scholar]
- 27.Liberati A, Altman DG, Tetzlaff J, et al. (2009). The PRISMA statement for reporting systematic and meta-analyses of studies that evaluate interventions: explanation and elaboration. J Clin Epidemiol, 62(10):e1–34. [DOI] [PubMed] [Google Scholar]
- 28.Luo D, Wan X, Liu J, et al. (2018). Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res, 27(6):1785–1805. [DOI] [PubMed] [Google Scholar]
- 29.Hozo SP, Djulbegovic B, Hozo I. (2005). Estimating the mean and variance from the median, range, and the size of a sample. BMC medical research methodology, 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins JP, Thompson SG. (2002). Quantifying heterogeneity in a meta-analysis. Stat Med, 21(11):1539–58. [DOI] [PubMed] [Google Scholar]
- 31.Momen MN, Ananian FB, Fahmy IM, et al. (2010). Effect of high environmental temperature on semen parameters among fertile men. Fertil Steril, 93(6):1884–6. [DOI] [PubMed] [Google Scholar]
- 32.Rao M, Zhao X-L, Yang J, et al. (2015). Effect of transient scrotal hyperthermia on sperm parameters, seminal plasma biochemical markers, and oxidative stress in men. Asian J Androl, 17(4):668–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang M-H, Zhang A-D, Shi Z-D, et al. (2015). Changes in levels of seminal nitric oxide synthase, macrophage migration inhibitory factor, sperm DNA integrity and caspase-3 in fertile men after scrotal heat stress. PLoS One, 10(10):e0141320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang M-H, Shi Z-D, Yu J-C, et al. (2015). Scrotal heat stress causes sperm chromatin damage and cysteinyl aspartate-spicific proteinases 3 changes in fertile men. J Assist Reprod Genet, 32(5):747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robine J-M, Cheung SLK, Le Roy S, et al. (2008). Death toll exceeded 70,000 in Europe during the summer of 2003. C R Biol, 331(2):171–8. [DOI] [PubMed] [Google Scholar]
- 36.Shaposhnikov D, Revich B, Bellander T, et al. (2014). Mortality related to air pollution with the Moscow heat wave and wildfire of 2010. Epidemiology, 25(3):359–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun Q, Miao C, Hanel M, et al. (2019). Global heat stress on health, wildfires, and agricultural crops under different levels of climate warming. Environ Int, 128:125–136. [DOI] [PubMed] [Google Scholar]
- 38.Kjellstrom T, McMichael AJ. (2013). Climate change threats to population health and well-being: the imperative of protective solutions that will last. Glob Health Action, 6:20816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rossati A. (2017). Global Warming and Its Health Impact. Int J Occup Environ Med, 8(1):7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calleja-Agius J, England K, Calleja N. (2021). The effect of global warming on mortality. Early Hum Dev, 155 : 105222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Costello A, Abbas M, Allen A, et al. (2009). Managing the health effects of climate change: lancet and University College London Institute for Global Health Commission. Lancet, 373(9676):1693–733. [DOI] [PubMed] [Google Scholar]
- 42.Rataj E, Kunzweiler K, Garthus-Niegel S. (2016). Extreme weather events in developing countries and related injuries and mental health disorders-a systematic review. BMC Public Health, 16:1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boggia B, Carbone U, Farinaro E, et al. (2009). Effects of working posture and exposure to traffic pollutants on sperm quality. J Endocrinol Invest, 32(5):430–4. [DOI] [PubMed] [Google Scholar]
- 44.Boni R. (2019). Heat stress, a serious threat to reproductive function in animals and humans. Mol Reprod Dev, 86(10):1307–1323. [DOI] [PubMed] [Google Scholar]
- 45.Rachootin P, Olsen J. (1983). The risk of infertility and delayed conception associated with exposures in the Danish workplace. J Occup Med, 25(5):394–402. [PubMed] [Google Scholar]
- 46.Golbabaei F, Monazzam M, Hematjo R, et al. (2013). The assessment of heat stress and heat strain in pardis petrochemical complex, Tehran, Iran. Int J Occup Hyg, 5:6–11. [Google Scholar]
- 47.Mieusset R, Bujan L, Mondinat C, et al. (1987). Association of scrotal hyperthermia with impaired spermatogenesis in infertile men. Fertil Steril, 48(6):1006–11. [DOI] [PubMed] [Google Scholar]
- 48.Hussain J, Cohen M. (2018). Clinical Effects of Regular Dry Sauna Bathing: A Systematic Review. Evid Based Complement Alternat Med, 2018:1857413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paasch U, Grunewald S, Fitzl G, et al. (2003). Deterioration of plasma membrane is associated with activated caspases in human spermatozoa. J Androl, 24(2):246–52. [DOI] [PubMed] [Google Scholar]