Abstract
Background:
Congenital hydrocephalus (CH) comprises a heterogeneous group of birth anomalies with a wide-ranging prevalence across geographic regions and registry type. The aim of the present study was to analyze the early neonatal case fatality rate (CFR) and total birth prevalence of newborns diagnosed with CH.
Methods:
Data were provided by 25 registries from four continents participating in the International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR) on births ascertained between 2000 and 2014. Two CH rates were calculated using a Poisson distribution: early neonatal CFR (death within 7 days) per 100 liveborn CH cases (CFR) and total birth prevalence rate (BPR) per 10,000 births (including live births and stillbirths) (BPR). Heterogeneity between registries was calculated using a meta-analysis approach with random effects. Temporal trends in CFR and BPR within registries were evaluated through Poisson regression modeling.
Results:
A total of 13,112 CH cases among 19,293,280 total births were analyzed. The early neonatal CFR was 5.9 per 100 liveborn cases, 95% confidence interval (CI): 5.4–6.8. The CFR among syndromic cases was 2.7 times (95% CI: 2.2–3.3) higher than among non-syndromic cases (10.4% [95% CI: 9.3–11.7] and 4.4% [95% CI: 3.7–5.2], respectively). The total BPR was 6.8 per 10,000 births (95% CI: 6.7–6.9). Stratified by elective termination of pregnancy for fetal anomalies (ETOPFA), region and system, higher CFR were observed alongside higher BPR rates. The early neonatal CFR and total BPR did not show temporal variation, with the exception of a CFR decrease in one registry.
Conclusions:
Findings of early neonatal CFR and total BPR were highly heterogeneous among registries participating in ICBDSR. Most registries with higher CFR also had higher BPR. Differences were attributable to type of registry (hospital-based vs. population-based), ETOPFA (allowed yes or no) and geographical regions. These findings contribute to the understanding of regional differences of CH occurrence and early neonatal deaths.
Keywords: birth defects, case fatality rate, congenital hydrocephalus, early neonatal deaths, ETOPFA, population surveillance, prevalence, trends
1 |. INTRODUCTION
Congenital hydrocephalus (CH) is defined as an abnormal dilatation of the cerebral ventricles and comprises a heterogeneous group of conditions present at birth (Isaacs et al., 2018). The distension of the brain ventricular system is related to the insufficient cerebrospinal fluid passage from its production point at the ventricular choroid plexuses to its absorption point at the arachnoid villi (Rekate, 2018). Congenital hydrocephalus includes any prenatally and postnatally diagnosed primary hydrocephalus (Morota, 2019). Based on a recent systematic review and a meta-analysis of reported population-based epidemiological studies, CH shows a wide-ranging prevalence according to geographic regions and birth defects registry types (Isaacs et al., 2018). The estimated global prevalence of CH was 8.5 per 10,000 live births. A higher CH prevalence was found in Africa, Asia, and South America when compared to other continents (Dewan et al., 2019; Huang et al., 2018). Likewise, a higher CH prevalence was observed among low- or middle-income countries from Africa or South America compared to high-income countries from Europe or North America (12.3 vs. 7.9 per 10,000 births, respectively) (Dewan et al., 2019; Isaacs et al., 2018). According to a World Health Organization (WHO) report, as overall under-five mortality decreases in almost all countries, the contribution from neonatal death (first 28 completed days of life) emerges as an increasingly prominent component of the overall under-five mortality (Zupan & Åhman, 2006). Furthermore, the vast majority of newborn deaths occur during the neonatal period, especially during the first week (early neonatal death). A 25% case fatality rate (CFR) has been reported for newborns with CH during the early neonatal period (Garne et al., 2010; Rogers & Morris, 1971; Scala et al., 2017). However, adequate data on regional differences and temporal variation in CH occurrence and neonatal deaths are scarce.
2 |. OBJECTIVE
The primary aim of this study was to determine the CH CFR during the early neonatal period (before postnatal day 7) using data from the International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR). A secondary aim was to calculate the CH birth prevalence rate (BPR) per 10,000 births (live births and stillbirths). Rates were calculated by surveillance population coverage type (hospital-based or population-based), geographical regions, policies of elective termination of pregnancy for fetal anomalies (ETOPFA), and temporal variation over the surveillance period.
3 |. METHODS
3.1 |. Study design and settings
This is an observational descriptive study of deaths among newborns with CH based on data from 25 birth defects surveillance registries participating in the ICBDSR. Using previously defined procedures and phenotype definitions for CH (Bakker et al., 2019; ICBDSR, 2021; Nembhard et al., 2020; Politis et al., 2020), the current study focuses on the timeframe between 2000 and 2014 when most of the 25 participating registries shared complete information. Registries’ participation by year is shown in Table 1.
TABLE 1.
Congenital hydrocephalus cases and total births for participating registries by region, time period, and registry type, International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR)
| Registrya | Region | Type of Registry | ETOPFA policy | Period | Total birthsb | Total CH casesc |
|---|---|---|---|---|---|---|
| Argentina RENAC | SA | H | No | 2009–2014 | 1,023,108 | 757 |
| Chile Maule | SA | H | No | 2002–2014 | 172,742 | 35 |
| Colombia Bogotá | SA | H | No | 2000–2014 | 407,394 | 114 |
| Colombia Cali | SA | H | No | 2011–2014 | 27,564 | 3 |
| Czech Republic | EU | P | Yes | 2000–2014 | 1,581,924 | 580 |
| France Paris | EU | P | Yes | 2000–2014 | 397,461 | 495 |
| Germany Saxony Anhalt | EU | P | Yes | 2000–2014 | 260,902 | 159 |
| Iran TROCA | AS | H | Yes | 2000–2012 | 236,882 | 519 |
| Israel SMC | AS | H | Yes | 2000–2014 | 201,660 | 27 |
| Italy Lombardy | EU | P | Yes | 2003–2012 | 133,182 | 92 |
| Italy Tuscany | EU | P | Yes | 2000–2014 | 436,081 | 146 |
| Malta MCAR | EU | P | No | 2000–2013 | 56,623 | 18 |
| Mexico Nuevo Leon | NA | P | No | 2011–2014 | 348,580 | 54 |
| Mexico RYVEMCE | NA | H | No | 2000–2013 | 299,560 | 194 |
| Netherlands Northern | EU | P | Yes | 2000–2014 | 274,223 | 110 |
| Slovak Republic | EU | P | Yes | 2001–2013 | 722,978 | 326 |
| South America ECLAMC | SA | H | No | 2000–2014 | 2,196,092 | 3,124 |
| Spain ECEMC | EU | H | Yes | 2000–2013 | 1,372,874 | 516 |
| Sweden | EU | P | Yes | 2000–2014 | 1,546,347 | 642 |
| UK Wales | EU | P | Yes | 2000–2014 | 503,455 | 431 |
| Ukraine OMNI Net | EU | P | Yes | 2000–2013 | 404,172 | 347 |
| USA Arkansas | NA | P | Yes | 2000–2012 | 508,654 | 303 |
| USA Atlanta | NA | P | Yes | 2000–2008 | 474,708 | 443 |
| USA Texas | NA | P | Yes | 2000–2012 | 5,033,546 | 3,383 |
| USA Utah | NA | P | Yes | 2000–2012 | 672,568 | 294 |
Abbreviations: AS, Asia; CH, congenital hydrocephalus; ETOPFA policy, elective termination of pregnancy for fetal anomalies allowed in the country where the registry is located; EU, Europe; H, Hospital-based system; NA, North America; SA, South America; P, Population-based system.
Each registry has different case ascertainment period, however, we have defined as before prenatal day 7 for all registries for this study. More information could be found at Bakker et al., 2019.
Total births: total number of births (live births + stillbirths).
Total CH cases: total number of CH cases (live births + stillbirths + ETOPFA).
Established in 1974, the ICBDSR is a voluntary nonprofit organization affiliated with WHO (ICBDSR, 2021). Its aim is to prevent birth defects and reduce the burden of their consequences by assembling birth defect surveillance and research programs around the world. Currently, 42 birth defects surveillance registries from 36 countries are members of the ICBDSR, and contribute aggregated data on children and fetuses affected with at least 1 of 39 different birth defects to the ICBDSR for surveillance purposes (a list of all registries, specific birth defects, and their surveillance attributes can be found at www.icbdsr.org).
Using ICBDSR case definition criteria, CH was defined as a congenital malformation characterized by dilatation of the cerebral ventricles not associated with primary brain atrophy, with or without head enlargement, diagnosed at birth. The ICBDSR definition corresponds to the International Classification of Diseases, 10th Revision (ICD-10) Code “Q03” and International Classification of Diseases, ninth Revision/British Pediatric Association (ICD-9/BPA) Code “742.3”. The following cases were excluded: concurrent encephalocele or spina bifida, macrocephaly without dilatation of ventricular system, skull of macerated fetus, hydranencephaly, holoprosencephaly, and postnatally acquired hydrocephalus (ICBDSR, 2021). Congenital hydrocephalus cases were classified as non-syndromic or syndromic according to their clinical presentation. Non-syndromic CH were those with only CH and no other co-occurring major birth defects. Because few registries provided the number of cases with recognized syndromes or multiple congenital anomalies (MCA), we grouped those in a category Syndromic/MCA CH.
3.2 |. Statistical analysis
In the present study, early neonatal CFR per 100 liveborn CH cases (CFR) was defined as the total number of liveborn CH cases who died before postnatal day 7 divided by the total number of live births with CH (Bakker et al., 2019; ICBDSR, 2021). Total CH BPR per 10,000 births was calculated as the total number of CH cases (live births + stillbirths + ETOPFA for congenital hydro-cephaly) divided by the total number of births (live births + stillbirths) within a specified time period. We estimated the BPR and 95% confidence intervals (CI) using a Poisson approximation of binomial distribution. Heterogeneity between registries was calculated with the I2 quantity (a value of 0% indicates no observed heterogeneity, and larger values indicate higher heterogeneity) (Higgins, Thompson, Deeks, & Altman, 2003), using a meta-analysis approach with random effects. Forest plots were used to show the heterogeneity (Bradburn, Deeks, & Altman, 1998).
A random-effects Poisson regression model including ETOPFA, registry type, and geographic region was used to account for BPR and CFR variability between registries:
| (1) |
For the CFR calculation, n was the number of cases of CH death before day 7 and the offset variable (exposure) was the total number of live birth cases. For the BPR calculation, n was the total number of cases of CH (live births + stillbirths + ETOPFA for congenital hydro-cephaly) and the offset variable (exposure) was the total number of births (live births + stillbirths) within a specified time period. The coefficients from each independent dummy variable are bi. ETOPFA is a dummy variable representing ETOPFA allowed (yes or no) in each program. System is a dummy variable indicating the registry type: population-based system versus hospital-based (reference category). Regioni are three dummy variables for each region (Asia, North America, and South America), with Europe as the reference category. Separate Poisson regression models for each registry were used to evaluate the temporal trends in CFR and BPR. Data analysis were performed with software Stata 15 © StataCorp.
Each registry follows local procedures for ethics approval. For this study, no additional ethics committee approval was required since only aggregated data were used.
4 |. RESULTS
A total of 13,112 CH cases (10,472 live births, 796 stillbirths, and 1,844 ETOPFA) among 19,293,280 total births was analyzed. Congenital hydrocephalus cases and total births for participating registries by region, time period, and registry type are shown in Table 1. Stratification of CH cases by pregnancy outcomes, early neonatal deaths, and phenotypic characteristics for participating registries are presented in Table 2.
TABLE 2.
Number of congenital hydrocephalus cases by pregnancy outcomes (live births, stillbirth, ETOPFA), early neonatal deaths, and phenotypic characteristics for participating registries, International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR), 2000–2014
| Registry | Live birth cases | Stillbirth cases | ETOPFA cases | Early neonatala death cases | Non-syndromic cases | Non-syndromic early neonatala death cases | Syndromic/MCA cases | Syndromic/MCA early neonatala death cases |
|---|---|---|---|---|---|---|---|---|
| Argentina RENAC | 719 | 38 | - | 162 | 426 | 49 | 293 | 113 |
| Chile Maule | 30 | 5 | - | 4 | 15 | 1 | 15 | 3 |
| Colombia Bogotá | 108 | 6 | - | 0 | 88 | 0 | 20 | 0 |
| Colombia Cali | 3 | 0 | - | 0 | 3 | 0 | 0 | 0 |
| Czech Republic | 295 | 30 | 255 | 17 | - | - | - | - |
| France Paris | 285 | 8 | 202 | 0 | 225 | 0 | 60 | 0 |
| Germany Saxony Anhalt | 87 | 10 | 62 | 2 | 50 | 0 | 37 | 2 |
| Iran TROCA | 287 | 109 | 123 | 21 | - | - | - | - |
| Israel SMC | 27 | 0 | - | 3 | 25 | 3 | 2 | 0 |
| Italy Lombardy | 47 | 8 | 37 | 0 | 26 | 0 | 21 | 0 |
| Italy Tuscany | 52 | 7 | 87 | 2 | - | - | - | - |
| Malta MCAR | 16 | 2 | - | 0 | 9 | 0 | 7 | 0 |
| Mexico Nuevo Leon | 54 | 0 | - | 6 | - | - | - | - |
| Mexico RYVEMCE | 165 | 29 | - | 6 | 108 | 3 | 57 | 3 |
| Netherlands Northern | 63 | 9 | 38 | 7 | 31 | 2 | 32 | 5 |
| Slovak Republic | 261 | 9 | 56 | 38 | 143 | 7 | 118 | 31 |
| South America ECLAMC | 2,877 | 247 | - | 151 | 1,458 | 57 | 1,419 | 94 |
| Spain ECEMC | 295 | 13 | 208 | 5 | 37 | 0 | 258 | 5 |
| Sweden | 325 | 5 | 312 | 6 | 222 | 0 | 103 | 6 |
| UK Wales | 208 | 14 | 209 | 10 | 103 | 3 | 105 | 7 |
| Ukraine OMNI Net | 196 | 40 | 111 | 12 | 131 | 3 | 65 | 9 |
| USA Arkansas | 271 | 25 | 7 | 14 | - | - | - | - |
| USA Atlanta | 338 | 55 | 50 | 10 | - | - | - | - |
| USA Texas | 3,225 | 99 | 59 | 108 | - | - | - | - |
| USA Utah | 238 | 28 | 28 | 11 | 99 | 2 | 139 | 9 |
Abbreviations: ETOPFA, elective terminations of pregnancy for fetal anomalies; MCA, multiple congenital anomalies.
Early neonatal = before postnatal day 7.
Among 10,472 CH live births, 595 died during the early neonatal period. The overall CH CFR was 5.9% (95% CI 5.4–6.3) according random-effects Poisson regression model (Table 3). The CFR was higher in surveillance registries without ETOPFA permissive policy, South America region, and in hospital-based registries (Table 3). A high CFR heterogeneity (overall I2 = 95.7%, p < .001) among registries was observed (Figure 1).
TABLE 3.
Case fatality rates and birth prevalence rates for congenital hydrocephalus by registry characteristics, International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR), 2000–2014
| Registry characteristic | Case fatality ratea | Total birth prevalence rateb | ||
|---|---|---|---|---|
| Rate per 100 liveborn cases | (95% CI) | Rate/10,000 births | (95% CI) | |
| ETOPFA policyc | ||||
| No | 8.52 | (7.63–9.49) | 9.49 | (9.21–9.78) |
| Yes | 4.22 | (3.73–4.76) | 6.00 | (5.87–6.14) |
| Region | ||||
| South America | 8.74 | (7.81–9.76) | 10.54 | (10.22–10.87) |
| Europe | 5.07 | (4.12–6.17) | 5.04 | (4.89–5.21) |
| Asia | 8.19 | (5.25–12.19) | 12.45 | (11.43–13.54) |
| North America | 3.61 | (3.07–4.23) | 6.39 | (6.21–6.58) |
| System | ||||
| Hospital based | 8.08 | (7.26–8.96) | 8.50 | (8.28–8.73) |
| Population based | 4.14 | (3.63–4.70) | 6.00 | (5.87–6.12) |
| ICBDSR 2000–2014 | 5.86 | (5.39–6.35) | 6.82 | (6.70–6.93) |
Abbreviations: CI, confidence interval using Poisson distribution; ETOPFA, elective termination of pregnancy for fetal anomalies; ICBDSR, International Clearinghouse for Birth Defects Surveillance and Research.
Congenital hydrocephalus case fatality rate per 100 liveborn cases (CFR) was calculated as the total number of liveborn congenital hydrocephalus cases who died before postnatal day 7 (early neonatal death) divided by the total number of live births with congenital hydrocephalus.
Birth prevalence rate per 10,000 births (BPR/10,000) was calculated as the total number of CH cases (live births + stillbirths + ETOPFA for congenital hydrocephalus when allowed) divided by the total number of births (live births + stillbirths) in a specified period.
ETOPFA policy, elective termination of pregnancy for fetal anomalies allowed in the country where the registry is located.
FIGURE 1.
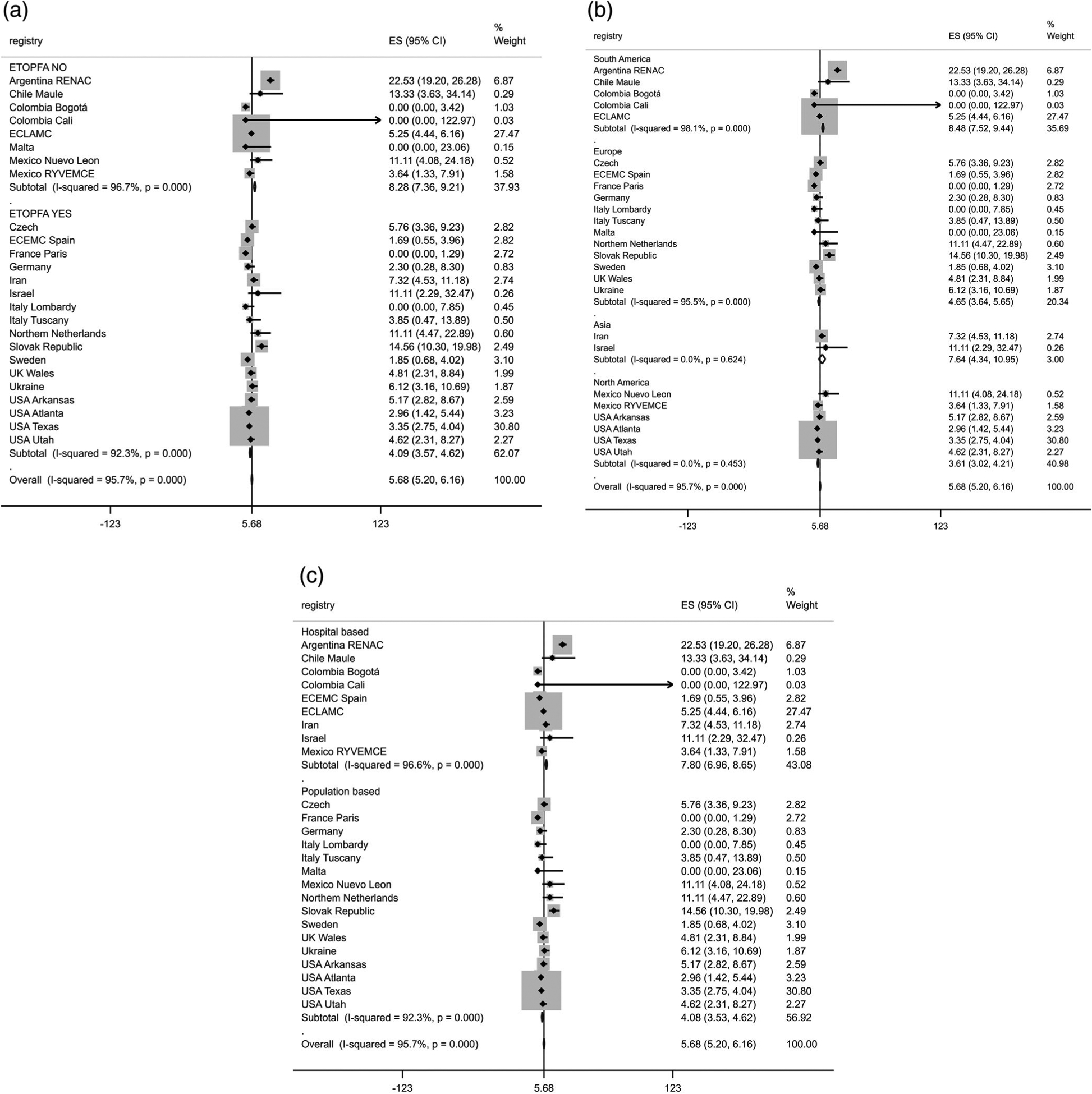
Forest plot of congenital hydrocephalus case fatality rate by (a) elective termination of pregnancy for fetal anomalies (ETOPFA), (b) region and (c) registry type, International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR), 2000–2014. ES (95% CI), case fatality rate per 100 liveborn cases. ETOPFA NO, elective termination of pregnancy for fetal anomaly policy not allowed in the country where the registry is located. ETOPFA YES, elective termination of pregnancy for fetal anomaly policy allowed in the country where the registry is located
The CH BPR per 10,000 births was 6.8 (95% CI 6.7–6.9) with random-effects Poisson regression model. The CH BPR was higher in surveillance registries where ETOPFA is not allowed, South America region, and hospital-based registries (Table 3). A high heterogeneity of the CH BPR per 10,000 births (overall I2 = 99.4%, p < .001) among surveillance registries was observed (Figure 2).
FIGURE 2.
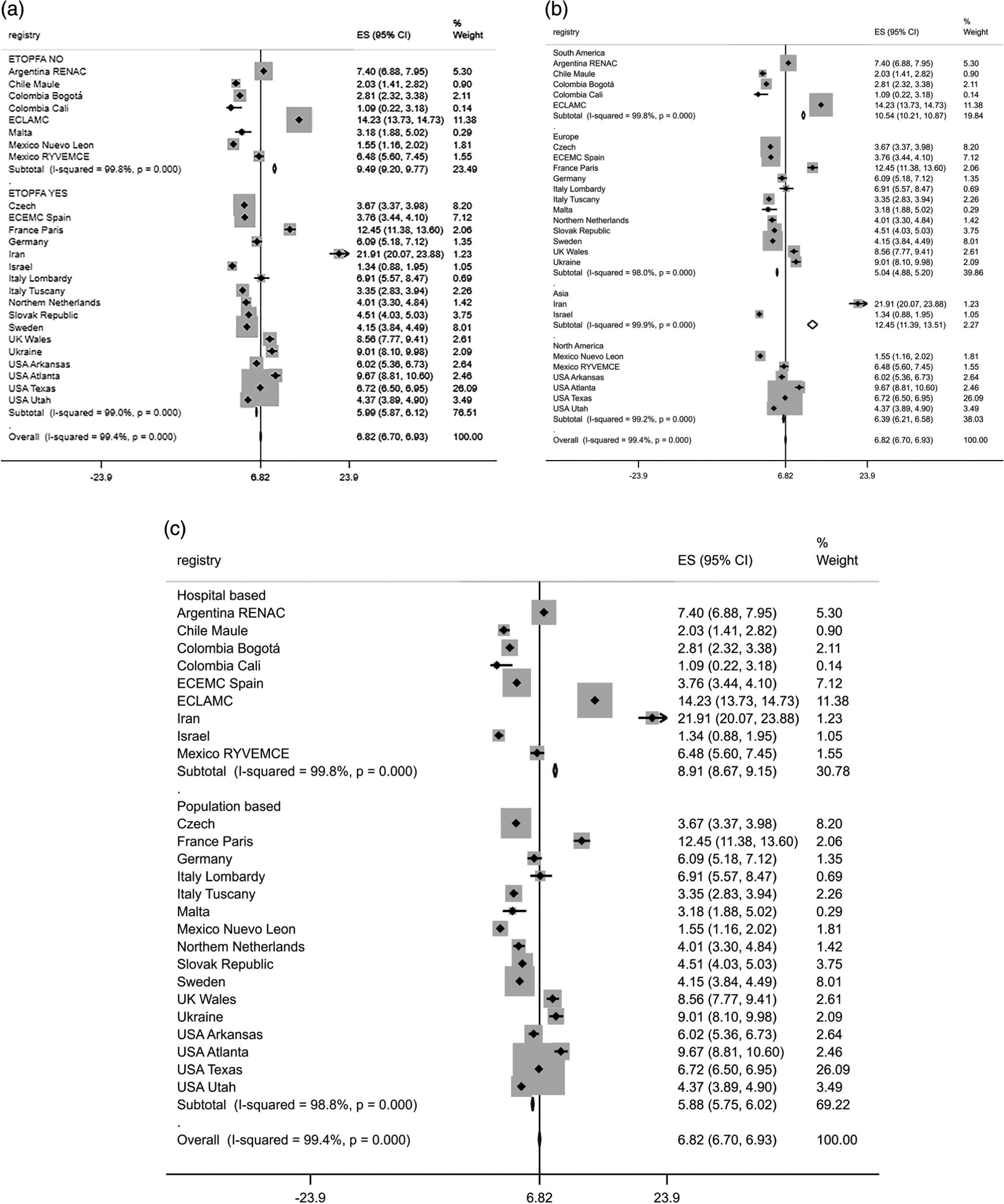
Forest plot of congenital hydrocephalus birth prevalence rate per 10,000 births by (a) elective termination of pregnancy for fetal anomalies (ETOPFA), (b) region and (c) registry type, International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR), 2000–2014. ES (95% CI), case fatality rate per 100 liveborn cases. ETOPFA NO, elective termination of pregnancy for fetal anomaly policy not allowed in the country where the registry is located. ETOPFA YES, elective termination of pregnancy for fetal anomaly policy allowed in the country where the registry is located
Pooled data by registry characteristics (ETOPFA policy, region, and registry type) showed higher CFR alongside BPR rates (Table 3). When using Poisson regression with random effects to account for variation between registries, including the effects of ETOPFA, region and system, registries from Asia and South America had statistically significant higher BPR and borderline statistically significant higher CFR than Europe. Lower CFR (p = .037) and BPR (p = .360) were found in registries from areas where ETOPFA was allowed (Table A1).
Considering temporal variation within each registry, a decreasing trend in CFR and BPR was observed for two registries (Slovak Republic and Iran at borderline statistical significance). A third registry (USA Texas) showed a decrease in CFR but a slight increase in BPR (Table A2).
Only 10 surveillance registries provided an adequate number of cases to distinguish non-syndromic versus syndromic CH CFR. The CFR for non-syndromic CH was 4.4% (95% CI: 3.7–5.2) and 10.4% (95% CI: 9.3–11.7) for syndromic CH. The ratio of CFRs was 2.6 times higher (95% CI: 1.6–3.7) for syndromic than for non-syndromic CH liveborn cases. The ratio showed a low degree of inconsistency across programs (overall I2 = 23.1%, p = .231).
5 |. DISCUSSION
This study assessed CH early neonatal CFR and total BPR across 25 registries located in 18 countries using a standardized protocol for data collection and case inclusion. Findings from this multi-country, multi-registry study have indicated that CH early neonatal CFR and total BPR are highly heterogenous between registries. Most registries with high early neonatal CFR also showed higher total birth prevalence rate. Registries from Asia and South America regions, hospital-based registries, and registries where ETOPFA is not allowed showed the highest CH early neonatal CFR and total BPR.
In our study, the early neonatal CFR among newborns with CH (5.7%) was lower than (24.4%) reported by EUROCAT in Europe (Garne et al., 2010), although in this study the sample size was small. Registry-based differences in CH early neonatal deaths may indicate differences in regional characteristics. Regional factors which could impact CFR include the following: health system characteristics (e.g., the timing of CH detection [prenatally, at birth, or early neonatal], length of follow up-after birth, or differences in case), different ETOPFA policies, and populational level differences (e.g., genetic, environmental, cultural or socio-economic features) (Dewan et al., 2019). There may also be differences in etiology by region, that is, CH may contain a wide variety of diagnoses such as aqueductal stenosis, intraventricular hemorrhage, and obstructive/communicating hydrocephalus and may include patients with brain tumors (Drake, 2005).
The differences we observed by region and type of registry also were correlated with whether ETOPFA was allowed. ETOFA was legal in the countries encompassing 11 of 12 European, 0 of 5 South American, 4 of 6 North American and the 2 Asian registries. It was also legal in the areas encompassing 14 of 15 population-based and 3 of 10 hospital-based registries. Thus, one of many factors involved in CFR or BPR levels could be that severe cases likely are not terminated during pregnancy in countries where ETOPFA is not allowed, leading to a higher BPR and CFR in live births, compared to countries where ETOPFA is allowed (Best et al., 2020; Liu et al., 2002; Nembhard et al., 2020). However, since ETOPFA was included in the BPR calculation in our study, it cannot explain BPR changes, at least solely. Moreover, a country’s ETOPFA policy is not likely the sole determinant of rate of neonatal death. Using perinatal deaths as a sole health indicator has limited utility since there are other contributing factors, such as access to prenatal screening, the availability of induced abortion, and the intensive care of very ill infants (Garne, 2001).
Certain strategies can be considered as efforts to reduce rates of neonatal death from CH, including prenatal screening and health care access, reinforcement of primary care in health systems and primary prevention health policies. Garne et al. (2010) reported a high (34%) infant mortality rate, mainly during the first postnatal week, of CH cases with associated malformations or chromosome anomalies and emphasized the importance of obtaining detailed clinical description when diagnosing hydrocephalus (Garne et al., 2010). Similar to other major birth defects, a proportion of mortality of infants with CH could be reduced through timely secondary prevention actions and medical care (Bakker et al., 2019). Therefore, it is highly encouraged to provide care and services for persons with birth defects and disabilities through a holistic multidisciplinary and multi-sectorial approach (Zarante et al., 2019), providing universal coverage, and home- and community-based follow-up strategies to maximize health and well-being.
The CH BPR on live births was higher in Asian and South American registries (12/10,000 and 11/10,000, respectively), intermediate in North American registries (6/10,000), and lower in European registries of ICBDSR (5/10,000), in accordance with previously reported data by authors for Europe (5/10,000), North America (8/10,000), and Asia (20/10,000) (Dewan et al., 2019; Garne et al., 2010; Huang et al., 2018; Jeng, Gupta, Wrensch, Zhao, & Wu, 2011; Liu et al., 2018). This geographic heterogeneity could be in part due to differences in demographic characteristics among study populations (Mahmoud, Dinar, Abdulla, Babikir, & Sulieman, 2014).
Risk factors that have been reported to be associated with CH include certain maternal factors, such as maternal age, and maternal chronic diseases (e.g., hypertension, diabetes, obesity), certain environmental factors, (e.g., altitude, paternal occupation, low socioeconomic status), and prenatal medication use (e.g., antidepressants, antibiotics, analgesics) (Kalyvas et al., 2016; Munch, Rasmussen, Wohlfahrt, Juhler, & Melbye, 2014; Walsh et al., 2017). Some of these risk factors combined with lack of prenatal screening and limited ETOPFA could explain the higher CH birth rates observed in regions like Iran and South America (Garne, 2001). In our study, the lowest CH rates were detected in Europe, likely reflecting increased access to prenatal screening and ETOPFA.
Another potential explanation for higher BPRs is due to higher consanguinity in certain regions, as we observed for the registry from the north region of Iran where high levels of consanguinity were reported in the literature (Alijahan, Mirzarahimi, Ahmadi Hadi, & Hazrati, 2013; Daliri et al., 2019; Saadat, Ansari-Lari, & Farhud, 2004). Congenital hydrocephalus has been described in almost 100 recognized syndromes, in whose etiology consanguinity plays a role (Alijahan et al., 2013; Rittler, Liascovich, López-Camelo, & Castilla, 2001; Shaheen et al., 2017). However, the Iranian TRoCA registry, located in the Northwest in Tabriz, is the only registry where a meaningful declining temporal trend for the early neonatal CFR and total BPR was observed, which coincided with ETOPFA legalization in 2005 (Hedayat, Shooshtarizadeh, & Raza, 2006).
5.1 |. Limitations
We were not able to assess the degree to which differences in case ascertainment may have impacted the observed differences in early neonatal deaths. In addition, we were not able to evaluate certain individual-level characteristics, such as sociodemographic data, pregnancy exposures, and maternal age. We could not evaluate categories of birth defects related to each CH case in order to evaluate association of co-occurring anomalies. ETOPFA policy differences between regions could introduce some artifact on the rate estimates. A possible underestimation of rates may exist due to registry system characteristics, for example, some population registries rely on the successful linkage of cases between birth defect registries and vital statistics that could result in some missed deaths.
5.2 |. Strengths
This study included a large sample size, allowing for an assessment of CH prevalence and early neonatal deaths within a multi-country context. We were able to examine all birth outcomes that included live births, stillbirths, and ETOPFA (when allowed). ICBDSR registries use well-defined case definition given standardized protocols to determine case status by trained registry personnel. These standard quality control protocols enhanced data quality, allowed for pooling findings, and improved comparability between registries. Finally, most ICBDSR registries have been in operation for many years, allowing us to study trends over a 15-year period.
6 |. CONCLUSIONS
We report a combined CH early neonatal CFR of 5.9 per 100 liveborn cases and a total BPR of 6.8 per 10,000 births, during 2000–2014 for registries participating in ICBDSR; however, rates were highly heterogeneous among registries. No rates showed meaningful temporal variation, with the exception of a CFR decrease in one registry. Most registries with a higher early neonatal CFR also had higher total BPR. Differences between registries are attributable in part to geographic region, type of registry (hospital-based vs. population-based systems), and ETOPFA policy (allowed or not). Our findings contribute to understanding of regional differences of CH occurrence and neonatal deaths.
ACKNOWLEDGMENTS
We thank each ICBDSR member program’s staff for providing information on the characteristics of their program and data on CH case status, and for conducting linkages between birth defects registries and administrative databases to assess neonatal outcomes. We thank Silvina Heisecke from CEMIC-CONICET who reviewed the language and grammar of the final manuscript. Grants: The preparation of the data set from the Czech Republic was supported by the Czech Ministry of Health grant nr. AZV 17-29622A.
Funding information
Ministerstvo Zdravotnictví Ceské Republiky, Grant/Award Number: AZV 17-29622A
APPENDIX A
TABLE A1.
Random effects Poisson regression models to estimate the effects of ETOPFA policy, registry type and region on birth prevalence rate and case fatality rate of congenital hydrocephalus newborn, International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR), 2000–2014
| Total birth prevalence ratea | Case fatality rateb | |||||
|---|---|---|---|---|---|---|
| PRRc | (95% CI) | p-value | PRRc | (95% CI) | p-value | |
| ETOPFA policyd | 0.66 | (0.27–1.6) | .360 | 0.22 | (0.05–0.92) | .037 |
| Registry typee | 2.04 | (0.84–4.99) | .117 | 2.69 | (0.70–10.38) | .151 |
| Asiaf | 3.93 | (1.16–13.28) | .028 | 4.94 | (0.85–28.85) | .076 |
| North Americaf | 1.04 | (0.55–1.94) | .911 | 0.85 | (0.38–1.91) | .690 |
| South Americaf | 1.36 | (0.51–3.63) | .535 | 1.40 | (0.35–5.59) | .630 |
Abbreviation: CI, confidence interval.
Birth prevalence rate per 10,000 births (BPR/10,000) was calculated as the total number of CH cases (live births + stillbirths + ETOPFA for congenital hydrocephalus when allowed) divided by the total number of births (live births + stillbirths) in a specified period.
Congenital hydrocephalus case fatality rate (lethality) per 100 liveborn cases (CFR) was calculated as the total number of liveborn congenital hydrocephalus cases who died before postnatal day 7 (early neonatal death) divided by the total number of live births with congenital hydrocephalus.
PRR: prevalence rate ratio was used as indicator of the effect of ETOPFA, registry type, and region over the rates of BPR and CFR.
ETOPFA policy: registry where elective termination of pregnancy for fetal anomalies (ETOPFA) is allowed (not allowed is the reference category).
Registry type: population-based registry or hospital-based registry (reference category).
Location of the registry, Europe is the reference category.
TABLE A2.
Poisson regression models to estimate the temporal variations in the birth prevalence rates and case fatality rates for congenital hydrocephalus by participating registries, International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR), 2000–2014
| Registry | Birth prevalence rate trend | 95% confidence interval | p-value | Case fatality rate trend | 95% confidence interval | p-value |
|---|---|---|---|---|---|---|
| Argentina RENAC | 0.52 | 0.33–0.83 | .006 | 0.96 | 0.34–2.73 | .936 |
| Chile Maule | 0.93 | 0.85–1.03 | .186 | 0.69 | 0.34–1.40 | .304 |
| Colombia Bogotáa | 0.96 | 0.88–1.05 | .347 | |||
| Colombia Calia | ||||||
| Czech Republic | 0.97 | 0.95–0.99 | .005 | 0.97 | 0.85–1.09 | .573 |
| France Parisa | 0.99 | 0.97–1.01 | .434 | |||
| Germany Saxony Anhalt | 0.97 | 0.94–1.00 | .093 | 0.77 | 0.39–1.54 | .466 |
| Iran TROCA | 0.89 | 0.85–0.93 | <.001 | 0.58 | 0.33–1.01 | .055 |
| Israel SMC | 0.95 | 0.85–1.05 | .278 | 1.10 | 0.44–2.71 | .842 |
| Italy Lombardya | 1.05 | 0.96–1.14 | .261 | |||
| Italy Tuscany | 0.98 | 0.95–1.02 | .452 | 1.00 | 0.63–1.59 | 1.000 |
| Malta MCARa | 1.03 | 0.92–1.14 | .626 | |||
| Mexico Nuevo Leona | ||||||
| Mexico RYVEMCE | 0.97 | 0.93–1.01 | .166 | 1.09 | 0.92–1.30 | .328 |
| Netherlands Northern | 1.01 | 0.97–1.06 | .540 | 0.91 | 0.75–1.11 | .338 |
| Slovak Republic | 0.96 | 0.94–0.99 | .008 | 0.88 | 0.79–0.97 | .008 |
| South America ECLAMC | 1.05 | 1.04–1.06 | <.001 | 0.99 | 0.95–1.03 | .571 |
| Spain ECEMC | 1.03 | 1.01–1.06 | .005 | 1.08 | 0.84–1.39 | .552 |
| Sweden | 0.95 | 0.93–0.96 | <.001 | 1.06 | 0.91–1.23 | .487 |
| UK Wales | 0.98 | 0.96–1.00 | .065 | 0.88 | 0.73–1.07 | .197 |
| Ukraine OMNI Net | 0.97 | 0.95–1.00 | .059 | 0.94 | 0.79–1.11 | .457 |
| USA Arkansas | 1.00 | 0.96–1.03 | .922 | 0.84 | 0.66–1.07 | .163 |
| USA Atlanta | 1.00 | 0.91–1.11 | .949 | 0.97 | 0.50–1.88 | .939 |
| USA Texas | 1.03 | 1.02–1.04 | <.001 | 0.92 | 0.86–0.98 | .014 |
| USA Utah | 0.94 | 0.90–0.98 | .002 | 1.00 | 0.81–1.23 | .998 |
Iterative process of regression models did not converge. Regression model: ln(n) = α + b1 year + b2 year2 + ln(offset). For birth prevalence rate (BPR), n is the total number of cases with congenital hydrocephalus and the offset variable is the total number of births; for case fatality rate (CFR), n is the number of cases of congenital hydrocephalus death before day 7 and the offset variable is the number of congenital hydrocephalus live births. Year and year2 were dummy variables representing each year during which each registry provided data to ICBDSR.
Footnotes
Publisher's Disclaimer: DISCLAIMER
Publisher's Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. This analysis has been replicated by Jorge Lopez-Camelo.
CONFLICT OF INTERESTS
The authors declare that they have no competing interests in publishing this manuscript. The authors received no funding for this analysis.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Alijahan R, Mirzarahimi M, Ahmadi Hadi P, & Hazrati S (2013). Prevalence of congenital abnormalities and its related risk factors in Ardabil, Iran, 2011. The Iranian Journal of Obstetrics, Gynecology and Infertility, 16(54), 16–25. 10.22038/ijogi.2013.1086 [DOI] [Google Scholar]
- Bakker MK, Kancherla V, Canfield MA, Bermejo-Sanchez E, Cragan JD, Dastgiri S, … Mastroiacovo P (2019). Analysis of mortality among neonates and children with spina bifida: An international registry-based study, 2001–2012. Paediatric and Perinatal Epidemiology, 33(6), 436–448. 10.1111/ppe.12589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best KE, Rankin J, Dolk H, Loane M, Haeusler M, Nelen V, … Khoshnood B (2020). Multilevel analyses of related public health indicators: The European Surveillance of Congenital Anomalies (EUROCAT) Public Health Indicators. Paediatric and Perinatal Epidemiology, 34(2), 122–129. 10.1111/ppe.12655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradburn M, Deeks J, & Altman DG (1998). metan—An alternative meta-analysis command. Stata Techicall Bulletin, 44, 4–15. [Google Scholar]
- Daliri S, Safarpour H, Bazyar J, Sayehmiri K, Karimi A, & Anvary R (2019). The relationship between some neonatal and maternal factors during pregnancy with the prevalence of congenital malformations in Iran: A systematic review and meta-analysis. The Journal of Maternal-Fetal & Neonatal Medicine: The Official Journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians, 32(21), 3666–3674. 10.1080/14767058.2018.1465917 [DOI] [PubMed] [Google Scholar]
- Dewan MC, Rattani A, Mekary R, Glancz LJ, Yunusa I, Baticulon RE, … Warf BC (2019). Global hydrocephalus epidemiology and incidence: Systematic review and meta-analysis. Journal of Neurosurgery, 130(4), 1065–1079. 10.3171/2017.10.JNS17439 [DOI] [PubMed] [Google Scholar]
- Drake JM (2005). Congenital hydrocephalus. Journal of Neurosurgery, 103(2 Suppl), 111, discussion 111–112. 10.3171/ped.2005.103.2.0111 [DOI] [PubMed] [Google Scholar]
- Garne E (2001). Perinatal mortality rates can no longer be used for comparing quality of perinatal health services between countries. Paediatric and Perinatal Epidemiology, 15(3), 315–316. 10.1046/j.1365-3016.2001.00356.x [DOI] [PubMed] [Google Scholar]
- Garne E, Loane M, Addor M-C, Boyd PA, Barisic I, & Dolk H (2010). Congenital hydrocephalus—Prevalence, prenatal diagnosis and outcome of pregnancy in four European regions. European Journal of Paediatric Neurology, 14(2), 150–155. 10.1016/j.ejpn.2009.03.005 [DOI] [PubMed] [Google Scholar]
- Hedayat KM, Shooshtarizadeh P, & Raza M (2006). Therapeutic abortion in Islam: Contemporary views of Muslim Shiite scholars and effect of recent Iranian legislation. Journal of Medical Ethics, 32(11), 652–657. 10.1136/jme.2005.015289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, & Altman DG (2003). Measuring inconsistency in meta-analyses. BMJ, 327(7414), 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y-H, Wu Q-J, Chen Y-L, Jiang C-Z, Gong T-T, Li J, … Zhou C (2018). Trends in the prevalence of congenital hydrocephalus in 14 cities in Liaoning province, China from 2006 to 2015 in a population-based birth defect registry from the Liaoning Women and Children’s Health Hospital. Oncotarget, 9(18), 14472–14480. 10.18632/oncotarget.24239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICBDSR. (2021). International Clearinghouse for Birth Defects Surveillance and Research. ICBDSR. http://www.icbdsr.org/programme-description/
- Isaacs AM, Riva-Cambrin J, Yavin D, Hockley A, Pringsheim TM, Jette N, … Hamilton MG (2018). Age-specific global epidemiology of hydrocephalus: Systematic review, metanalysis and global birth surveillance. PLoS One, 13(10), e0204926. 10.1371/journal.pone.0204926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng S, Gupta N, Wrensch M, Zhao S, & Wu YW (2011). Prevalence of congenital hydrocephalus in California, 1991–2000. Pediatric Neurology, 45(2), 67–71. 10.1016/j.pediatrneurol.2011.03.009 [DOI] [PubMed] [Google Scholar]
- Kalyvas AV, Kalamatianos T, Pantazi M, Lianos GD, Stranjalis G, & Alexiou GA (2016). Maternal environmental risk factors for congenital hydrocephalus: A systematic review. Neurosurgical Focus, 41(5), E3. 10.3171/2016.8.FOCUS16280 [DOI] [PubMed] [Google Scholar]
- Liu J, Jin L, Li Z, Zhang Y, Zhang L, Wang L, & Ren A (2018). Prevalence and trend of isolated and complicated congenital hydrocephalus and preventive effect of folic acid in northern China, 2005–2015. Metabolic Brain Disease, 33(3), 837–842. 10.1007/s11011-017-0172-4 [DOI] [PubMed] [Google Scholar]
- Liu S, Joseph KS, Kramer MS, Allen AC, Sauve R, Rusen ID, … Fetal and Infant Health Study Group of the Canadian Perinatal Surveillance System. (2002). Relationship of prenatal diagnosis and pregnancy termination to overall infant mortality in Canada. JAMA, 287(12), 1561–1567. 10.1001/jama.287.12.1561 [DOI] [PubMed] [Google Scholar]
- Mahmoud MZ, Dinar HA, Abdulla AA, Babikir E, & Sulieman A (2014). Study of the association between the incidences of congenital anomalies and hydrocephalus in Sudanese fetuses. Global Journal of Health Science, 6(5), 1–8. 10.5539/gjhs.v6n5p1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morota N (2019). Prenatal hydrocephalus: Prenatal counseling, post-natal treatment, outcome. In Cinalli G, Ozek MM, & Sainte-Rose C (Eds.), Pediatric hydrocephalus (pp. 1–19). Cham: Springer International Publishing. 10.1007/978-3-319-31889-9_48-1 [DOI] [Google Scholar]
- Munch TN, Rasmussen M-LH, Wohlfahrt J, Juhler M, & Melbye M (2014). Risk factors for congenital hydrocephalus: A nationwide, register-based, cohort study. Journal of Neurology, Neurosurgery, and Psychiatry, 85(11), 1253–1259. 10.1136/jnnp-2013-306941 [DOI] [PubMed] [Google Scholar]
- Nembhard WN, Bergman JEH, Politis MD, Arteaga-Vázquez J, Bermejo-Sánchez E, Canfield MA, … Mastroiacovo P (2020). A multi-country study of prevalence and early childhood mortality among children with omphalocele. Birth Defects Research, 112, 1787–1801. 10.1002/bdr2.1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politis MD, Bermejo-Sánchez E, Canfield MA, Contiero P, Cragan JD, Dastgiri S, … International Clearinghouse for Birth Defects Surveillance and Research. (2020). Prevalence and mortality in children with congenital diaphragmatic hernia: A multi-country study. Annals of Epidemiology, 56, 61–69.e3. 10.1016/j.annepidem.2020.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekate HL (2018). Classification of hydrocephalus. In Cinalli G, Ozek MM, & Sainte-Rose C (Eds.), Pediatric hydrocephalus (pp. 1–17). Cham: Springer International Publishing. 10.1007/978-3-319-31889-9_45-1 [DOI] [Google Scholar]
- Rittler M, Liascovich R, López-Camelo J, & Castilla EE (2001). Parental consanguinity in specific types of congenital anomalies. American Journal of Medical Genetics, 102(1), 36–43. [DOI] [PubMed] [Google Scholar]
- Rogers SC, & Morris M (1971). Infant mortality from spina bifida, congenital hydrocephalus, monstrosity, and congenital diseases of the cardiovascular system in England and Wales. Annals of Human Genetics, 34(3), 295–305. [DOI] [PubMed] [Google Scholar]
- Saadat M, Ansari-Lari M, & Farhud DD (2004). Consanguineous marriage in Iran. Annals of Human Biology, 31(2), 263–269. 10.1080/03014460310001652211 [DOI] [PubMed] [Google Scholar]
- Scala C, Familiari A, Pinas A, Papageorghiou AT, Bhide A, Thilaganathan B, & Khalil A (2017). Perinatal and long-term outcomes in fetuses diagnosed with isolated unilateral ventriculomegaly: Systematic review and meta-analysis. Ultrasound in Obstetrics & Gynecology: The Official Journal of the International Society of Ultrasound in Obstetrics and Gynecology, 49(4), 450–459. 10.1002/uog.15943 [DOI] [PubMed] [Google Scholar]
- Shaheen R, Sebai MA, Patel N, Ewida N, Kurdi W, Altweijri I, … Alkuraya FS (2017). The genetic landscape of familial congenital hydrocephalus. Annals of Neurology, 81(6), 890–897. 10.1002/ana.24964 [DOI] [PubMed] [Google Scholar]
- Walsh S, Donnan J, Morrissey A, Sikora L, Bowen S, Collins K, & MacDonald D (2017). A systematic review of the risks factors associated with the onset and natural progression of hydrocephalus. Neurotoxicology, 61, 33–45. 10.1016/j.neuro.2016.03.012 [DOI] [PubMed] [Google Scholar]
- Zarante I, Hurtado-Villa P, Walani SR, Kancherla V, López Camelo J, Giugliani R, … Durán P (2019). A consensus statement on birth defects surveillance, prevention, and care in Latin America and the Caribbean. Revista Panamericana de Salud Pública, 43, e2. 10.26633/RPSP.2019.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupan J, & Åhman E (2006). Neonatal and perinatal mortality: Country, regional and global estimates. Geneva: World Health Organization. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


