Abstract
β-catenin signaling, and angiogenesis are associated with colospheroid (CSC), development. CSCs, spheroids derived from colon cancer cells, are responsible for metastasis, drug resistance, and disease recurrence. Whether dysregulating β-catenin and inhibiting angiogenesis reduces CSC growth is unknown. In this study, the molecular mechanism of CSC growth inhibition was evaluated using a novel combination of melatonin (MLT) and andrographolide (AGP). These drugs have anti-carcinogenic, antioxidant, and anti-metastatic properties. CSCs were obtained from two metastatic colon cancer cell lines (HT29 and HCT-15). The viability and stemness were monitored (FDA PI staining and immunoblot for CD44, CD133, Nanog, Sox2 and Oct4). The drug combination synergistically diminished stemness via increased ROS levels, reduced mitochondrial membrane potential and ATP level. MLT+AGP induced cell death by inhibiting β-catenin expression and its downregulatory signals, Cyclin D1, c-Myc. MLT+AGP treated cells exhibited translocation of phospho-β-catenin to the nucleus and de-phosphorylated-β-catenin. Downregulation of β-catenin activation and its transcription factors (TCF4, LEF1) and GTP binding/G-protein related activity were found in the dual therapy. Angiogenic inhibition is consistent with downregulation of VEGF mRNA transcripts (VEGF189), phosphorylated VEGF receptor protein expression, matrigel invasion, and capillary tube inhibition. In vivo, the intravenous injection of MLT+AGP slowed HT29 metastatic colon cancer. Histopathology indicated significant reduction in microvascular density and tumor index. Immunohistochemistry for caspase 7, and β-catenin found increased apoptosis and downregulation of β-catenin signals. The mechanism(s) of decreased colospheroids growth were the inhibition of the Wnt/β-catenin pathway. Our results provide rationale for using MLT in combination with AGP for inhibition of CRCs.
Keywords: Melatonin (MLT), Andrographolide (AGP), Colospheroids (CSCs), Xenograft, Wnt/β-catenin signals, Angiogenesis
1. INTRODUCTION:
Colorectal cancer (CRC) treatment failure is due to metastasis, heterogeneity, drug resistance, and other factors1. These failures could be explained by the characteristics of spheroids (CSCs)2 and their ability to arrest in the G0 phase, thereby giving rise to new tumors3. Moreover, CSCs have a strong self-renewal capacity causing tumorigenesis4,5. Currently, no therapy renders CSCs completely dysfunctional, highlighting the need for improved therapeutic approaches.
The CSC self-renewal regulation involves Notch, phosphoinositide-3-kinase/Ak-mouse thymoma, and Hedgehog including Wnt/β-catenin signaling molecules6,7. Hyperactivation of β-catenin-T cell factor (TCF)/lymphoid enhanced factor (LEF)-regulated gene transcription (the end point of Wnt signaling) is a hallmark of CRC development. Wnt promotes transcription of LGR5, Oct4, Sox2, and Nanog for CSC maintenance, tumor growth, invasion, and metastasis8,9. Therefore, CSC inhibition and Wnt/ β-catenin signaling regulation are promising targets for cancer treatment.
Angiogenesis promoted by factors such as Vascular Endothelial Growth Factor (VEGF) accompanies CSCs self-renewal and tumor initiation9. Increased vascular density and higher recruitment of endothelial progenitor cells is observed in tumors enriched with CSCs10. Therefore, identification of CSC mechanisms involved in angiogenesis could provide a therapeutic approach to CRC.
We reported that bicyclic diterpenoid andrographolide (AGP) has a significant synergistic effect when combined with the neurohormone melatonin (MLT) (Non provisional patent; #PCT/US2021/030084). This combination has a therapeutic impact on metastatic CRC (mCRC), CSCs, and 5-FU resistant cell viability through the unfolded protein response (UPR) mediated endoplasmic reticulum (ER) stress mechanism and angiogenesis inhibition11. Studies document that combined MLT and AGP disrupts patient-derived organoid (PDOD) membrane integrity and decreases Ki-67 expression12. Herein, we examine the impact of this combinational therapy on CSC proliferation derived from two mCRC cell lines. We demonstrate that combined MLT and AGP inhibits (i) spheroid phenotype (ii) ATP-level (iii) angiogenesis(iv) invasion (v) β-catenin and its downregulatory signals (vi) Wnt pathway by transcriptional activity of β-catenin/TCF and (vii) xenograft tumor formation. The combined treatment increased ROS production and apoptosis. These effects were observed without adverse side effects. Thus, these agents inhibited Wnt/β-catenin to reduce spheroid survival derived from metastatic cancer cells.
2. MATERIALS AND METHODS:
2.1. Chemicals
Melatonin was provided by Russel J Reiter, AGP, dimethyl sulfoxide (DMSO), MTT, fluorescein diacetate (FDA), /propidium iodide (PI), were purchased from Sigma-Aldrich.
2.2. Cell cultures, colospheroids formation assay
HT29, HCT-15, HCT-116 cells were generously provided by Lin Jiayuh, Ph. D, endothelial cells from Kim G Hankey, Ph. D., and HEK-293 cells from Rania Younis, Ph. D., at the University of Maryland School of Medicine. Cells were cultured and cell-derived colospheroid formation assay was performed as described11.
2.3. Drug treatment
To monitor the drug impact, spheroids were permitted to grow for 5d (HT29-s derived from HT29) and 3d (HCT-15-s derived from HCT-15). To determine the spheroid morphology, viability, apoptosis, signaling mechanism, and functional study, 80% confluent spheroids were treated with the IC50 value of 0.18 mM of MLT and 9.3 µM of AGP single or in combination for 48 h11. This regimen was used for all experiments. Stock MLT (1M) and AGP (100 mM) were prepared in DMSO, and control wells received DMSO at a final concentration of 0.01%.
2.4. Subcutaneous tumor xenografts
All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Notre Dame (protocol 18-09-4843). This study was conducted using 4- to 6-week-old athymic nude mice (002019-NU/J) housed in sterile filter-capped cages with ad libitum food and water. Male and female mice were subcutaneously (s.c.) injected with the HT29 CRC cell line (2X106). Cultured and resuspended cells at a concentration of 2X106/0.2 mL in RPMI 1640. Cell viability was assessed using trypan blue, and then 0.2 mL of the cell suspension was transplanted s.c. into the right flanks of the mice. The mice were randomly grouped (n=8, 4 males and 4 females per group) and treated intraperitoneally with vehicle (20% polyethylene glycol, 5% DMSO and 75% sterile PBS), melatonin (40 mg/kg, 5 times a week for 2 weeks), and andrographolide (20 mg/kg, 5 times a week for 2 weeks) either alone or in combination. Beginning two weeks after s.c. injection, tumor size was measured twice a week for two weeks with slide calipers and tumor volume (TV) was calculated as (W2XL)/2, where W is width and L is length of the tumor. Relative tumor volume (RTV) was calculated according to the following formula: RTV=TVn/TV0 where TVn is the tumor volume at the day of measurement and TV0 is the tumor volume on the first day of measurement. Weight was measured twice weekly. Mice were euthanized by CO2 exposure followed by cervical dislocation. Tumors were then removed, weighed, dissected, and processed for histological, immunohistochemical, immunoblot, and RNA analyses.
2.5. Spheroid viability and morphological assessment
HT29-s and HCT-15-s viability was determined by FDA/PI staining as described earlier13 . Colospheroids development was monitored, and images were taken using an inverted fluorescence microscope (Olympus IX-71) (Pennsylvania, USA).
2.6. DAPI staining
HT29-s and HCT-15-s were treated with or without MLT and AGP for 48 h. Apoptotic features were evaluated using DAPI staining (Vector Laboratories, Burlingame, CA) as described earlier14.
2.7. SDS-PAGE and immunoblot
SDS-PAGE and immunoblot was performed as published15,16. The details of primary antibodies are in Table-S1. Images were captured using a Syngene G Box digital image (Frederick, MD) and quantified with densitometry as described17.
2.8. Immunofluorescence and Imaging.
HT29-s (~8,000 spheroids) were grown on poly-L-lysine (0.01%) coated coverslips. Immunofluorescence was performed in treated and untreated spheroids as described13. Anti-phospho/total-β-catenin (1:30) antibody were used for overnight incubation at 4°C. Alexa Flour 488 labeled goat anti-mouse IgG (H+L) (1:300) (Invitrogen) and DAPI were used. Keyence Fluorescence microscopy was used to take the images with 200X magnification.
2.9. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Gene expression was evaluated as described18. Primer sequences are listed in Table S2.
2.10. β-catenin overexpression
β-catenin overexpression plasmid was purchased from Addgene (h- β-catenin-pcDNA 3; #16828). HCT-116 cells (8.3×104 cells/well in 24-well plates) were transfected with 1.5 μg plasmid DNA as instructed (#L3000-008; Invitrogen). After transfection (48 h), the media was changed with 1 mg/ml G418 for 48 h, followed by 0.75 mg/ml for a week and maintained at 250 μg/ml. For activation experiments, transfected cells were dosed as indicated in section 2.3. β-catenin overexpression and its signals were validated by immunoblot.
2.11. RhoA activity assay
RhoA activity was assessed by immunoprecipitation following manufacturer instructions (#BK036; Cytoskeleton, Inc. Denver, CO). Briefly, protein extracts from HT29-s were prepared using ice cold cell lysis buffer with protease inhibitor cocktails. Protein concentration was 0.5mg/ml; 300 μg of protein was incubated with 50 µg of Rho binding domain on a rocker for 1hr at 4°C. The immunoprecipitated rhotekin-RBD beads were pelleted down and assayed by immunoblot using anti-RhoA monoclonal antibody as instructed.
2.12. Dual Luciferase assay
Wnt/β-catenin activity was measured using a dual luciferase assay kit (BPS Bioscience; #60500 and #60683–1). Briefly, HEK293T cells were seeded onto 96-well plates at a density of 3X104 cells/well and incubated overnight at 37°C. The cells were then transfected either with 60 ng reporter DNA or negative control reporter DNA. After 42 hrs, transfected cells were treated with or without MLT/AGP as previously described.
2.13. Histological staining and Microvessels counts (MVC)
Treated and untreated xenograft tissue sections were stained with H&E using our UMGCCC core facilities and analyzed microscopically to quantify the mitotic index and MVC as previously described17. Five random fields from each group were evaluated under 200X magnification. Microscopic images from each area were collected using an Olympus BX53 with the use of Bioquant Lifescience 2017 program.
2.14. Immunohistochemistry.
To detect Ki-67, Caspase 7, and β-catenin expression in xenograft treated and untreated tissue, immunohistochemistry was performed as described19. Antibody details are in Table S3.
2.15. Methods for other biological parameters are described in Supplementary methodology
2.16. Statistical Analysis
Statistical analyses were performed with Graph Pad Prism 5.0c (Graph Pad Software Inc., San Diego, CA). Data obtained for all in vitro studies were analyzed using one-way ANOVA. In vivo animal tumor studies were analyzed using 2-way ANOVA. Multiple comparisons were conducted using Tukey’s and Bonferroni post hoc tests. P values were considered significant if they were less than 0.05 and are indicated throughout using asterisks: * = P < 0.05, ** = P < 0.01, ***P < 0.001.
3. RESULTS
3.1. Impact of MLT and AGP on spheroid morphology.
To generate the spheroids, HT29 and HCT-15 cells were cultured11. Fig. 1A shows the development of spheroid formation named as HT29-s and HCT-15-s. We further determined the impact of MLT and AGP on spheroid morphology and viability. Fig. 1B demonstrates that co-treatment disrupted spheroid formation (HT29-s; upper panel) and reduced the overall spheroid size (HCT-15-s; lower panel), consistent with previous studies11. FDA-PI staining revealed increased PI staining in MLT+AGP treated group, indicating increased cell death relative to untreated spheroids or monotherapy (Fig. 1C–D). To determine whether co-treatment induced cell death was due to apoptosis, nuclear morphology was examined using DAPI staining. Co-treatment revealed apoptotic features including apoptotic bodies, cell shrinkage, and chromatin condensation, which were not observed in untreated or monotherapy groups (Fig. 1E). Together, these results indicate that co-treatment suppresses spheroids viability and promotes apoptosis.
Figure 1. Impact of MLT alone or in combination on colospheroids.
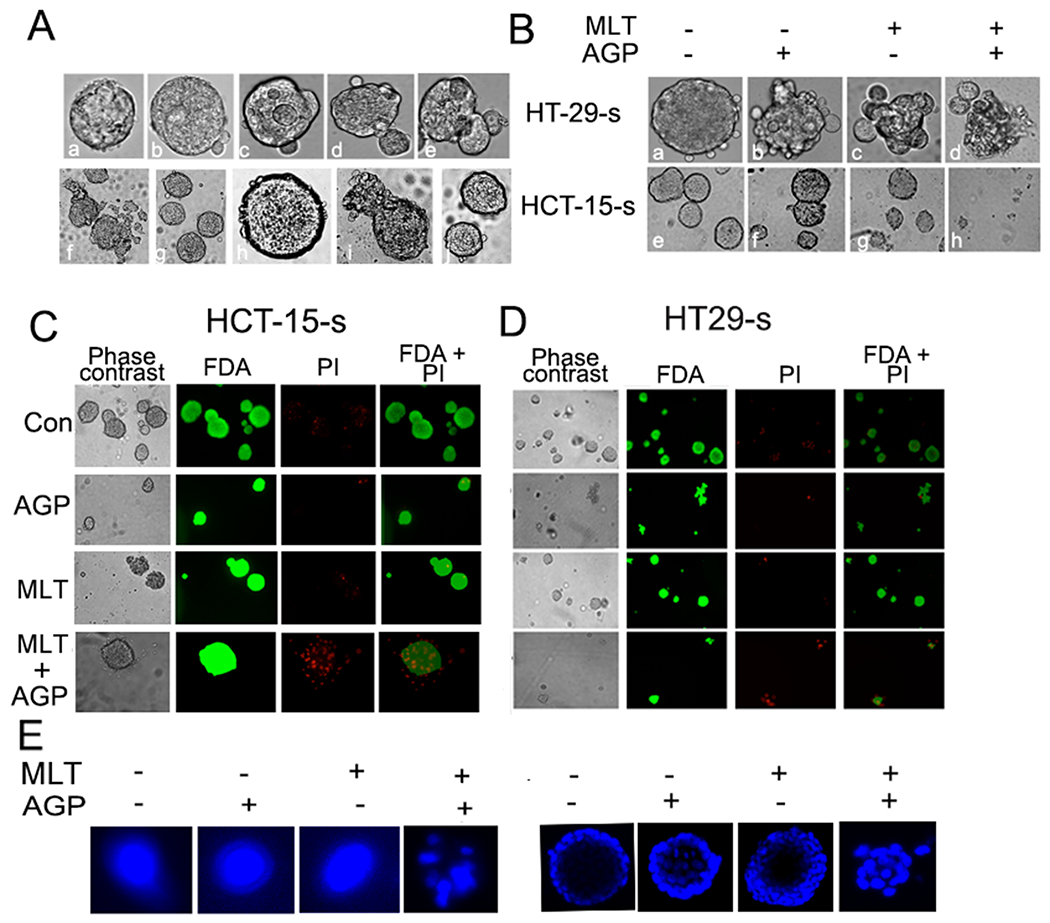
A. HT29 and HCT-15 cells were used for CSCs chronological development from HT29 and HCT-15 mCRC cells, HT29-s (upper lane) and HCT-15-s (lower lane). Mature and dividing CSCs were found at day 5 and day 3, respectively. Mature CSCs were treated with or without the IC50 dose, MLT (0.18 mM) and AGP (9.3 μM) at 48 h. Untreated group maintained their spheroid formation (B-upper HT29-s and lower HCT-15-s). Membrane disintegration and reduced size were observed in the treated group in comparison with the untreated or single treatment groups using phase contrast microscope. Magnification: 10X and scale bar 100 micron. C-D. Fluorescence microscopy images showing the viability of HCT-15-s and HT29-s cultured in vitro as indicated, phase contrast image, FDA stain, PI stain, overlay of FDA and PI stain. E. Apoptotic features were identified by condensation and fragmentation in MLT+AGP treated colospheroids using DAPI staining. Images were captured using inverted fluorescence microscope.
3.2. Characterization of CRC spheroids and reduced expression of surface biomarkers:
It is known that CD44 and CD133 are the robust markers for CRC CSCs. Nanog is a transcription factor required for maintaining the undifferentiated state and self-renewal of spheroids20,21. The results demonstrate CD44, CD 133, and Nanog protein expression of CSCs was higher as compared with parental cells (Fig. 2A, 2C). A significant elevation of biomarkers (Fig. 2B and 2D) indicated spheroid formation. To investigate the impact of monotherapy versus dual therapy on spheroid cell surface markers, CD44, CD133, and Nanog protein expression were examined. A significant downregulation of protein expression was found in the co-treatment group as compared with untreated, MLT, or AGP alone groups (Fig. 2E–I) in at least one set of spheroid lysates.
Figure. 2. Combination of MLT and AGP reduces colospheroids stemness.
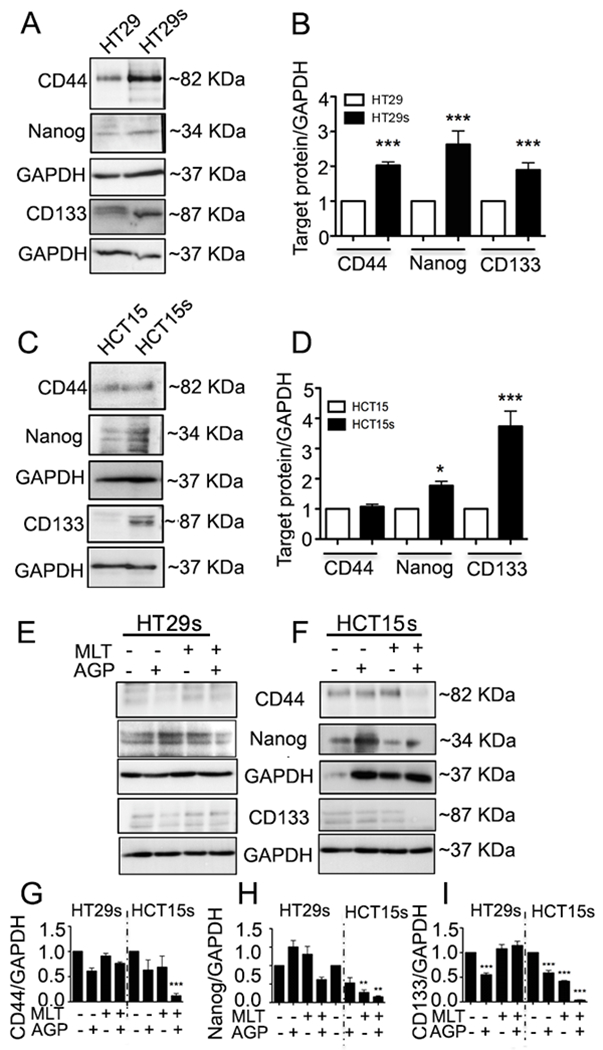
A-D. Characterization of stemness. CSCs lysates were analyzed by immunoblot for CD44, Nanog, CD133, and GAPDH expression. Left panel is a representative photograph from an experiment that was repeated three times. HT29-s (A) and HCT-15-s (C). Right panel (B-D) represents quantitative estimations of protein levels determined by densitometry measurements of immunoblots from three independent experiments after normalization with GAPDH (* P<0.05, ***P<0.001). E-F. Treatment of spheroids with or without AGP (9.3 μM) and MLT (0.18 mM) for 48 h suppresses CSC-s stemness. G-I. Quantitative measurements (G, CD44; H, Nanog; I, CD, 133; **P<0.01, ***P<0.001).
3.3. Impact of MLT AGP combination on Wnt signaling pathway.
The aberrant Wnt/β-catenin signals and crosstalk with EGFR signals promotes and maintains CRC stemness.5,22,23. Studies have demonstrated that AGP kills cancer cells through the downregulation of the cell surface protein, EGFR24. To explore the mechanisms by which MLT and AGP exert their synergistic effects on colospheroid stemness, HT29s and HCT-15-s lysates were monitored for EGFR, β-catenin, and its downregulatory protein expression. Phospho EGFR and phospho-β-catenin levels were significantly downregulated (Fig. 3B and C) in the combinatorial group compared to the untreated or single treatment groups. The significant reduction of phospho-EGFR was also observed in AGP treated HT29-s lysates. Stabilized β-catenin enhances the activity of Oct425. Moreover, Sox2 is a regulator of chemoresistance and interferes with Wnt signaling by binding β-catenin26. Therefore, the expression of additional colospheroid surface markers, Sox2 and Oct4 was monitored. Dual treatment downregulated Sox2 and Oct4 expression in both spheroid lysates compared with the untreated or single drug treatments (Fig. 3A, 6th and 7th lane, 3D and E) This was consistent with other stemness markers (Fig. 2E–I). To determine β-catenin involvement in colospheroids growth and maintenance, immunofluorescence studies were carried out. Confocal microscopy revealed a higher staining of membrane bound phospho-β-catenin in untreated colospheroids whereas decreased expression was found in the MLT+AGP treated condition compared with the single compounds (Fig. 3F–G). Since the nuclear translocation of β-catenin is a key event in activation of the Wnt/ signaling pathway, we checked the two compounds effect together on the β-catenin expression in plasma membrane, nuclear and cytoplasmic spheroid fractions using the Chemicon kit (International, #2900). β-catenin translocated from the cytoplasm to the nucleus with reduced expression (Fig. 3H–I).
Figure. 3. Co-treatment of MLT and AGP suppresses β-catenin signaling.
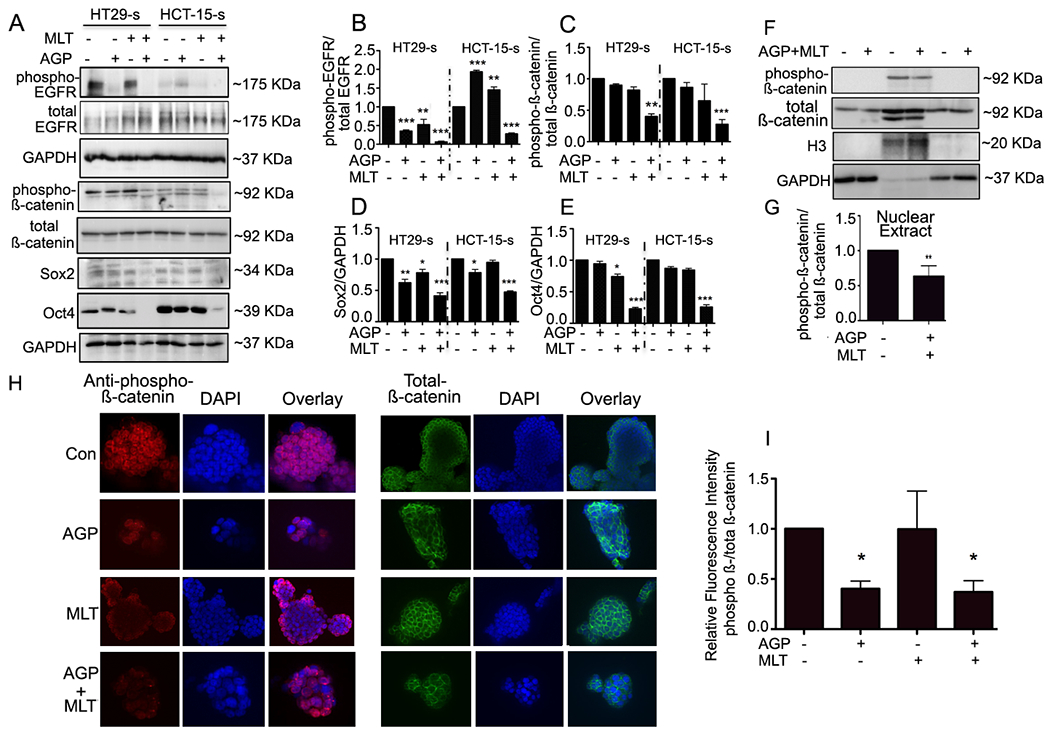
A. Immunoblots from treated or untreated CSCs extracts were used for monitoring β-catenin signaling protein expression. GAPDH was used as a loading control. Quantification of B. Phospho EGFR, C. Phospho β-catenin, D. Sox2, E. Oct4. Statistical significance was determined by one way-ANOVA followed by the Bonderroni test. (*P<0.05, **P<0.01, ***P<0.001). F. HT29-s were grown on poly-L-Lysine coated coverslips. Phospho- (left panel)-and total β-catenin (right panel) protein expression were evaluated by confocal microscopy. Nuclei were stained using DAPI. Fluorescence intensity was determined and compared with untreated CSCs (G-H). I. Quantification. H. Analysis of phospho- and total β-catenin distribution. Equal number spheroids equivalents were electrophoresed and assayed by immunoblot. H3 and GAPDH levels served as the nuclear and cytoplasmic loading control, respectively. I. Representative quantification for phospho/total β-catenin using densitometry (**P<0.01).
3.4. Activation of Wnt/β-catenin and Rho-kinase in colospheroids and dual therapy impact.
To determine if spheroid growth inhibition by dual therapy is mediated by β-catenin activity inhibition, luciferase assay was performed. Fig. 4A shows activity was significantly decreased in the combinatorial group compared to untreated and single treatments. As Rho GTPases are key mediators of Wnt signals27, and RhoA inhibition led to reduced migration in colon cancers28, the impact of dual therapy on the Rho-kinase activity was examined using spheroid lysates. MLT and AGP showed complete loss of kinase activity compared with +/− single compound (Fig. 4B). This evidence suggests that Rho-kinase activity inhibition is essential for colospheroid reduction, which agrees with a previous study29.
Figure. 4. Impact of MLT and AGP on β-catenin and Rho-activity in HT29-s.
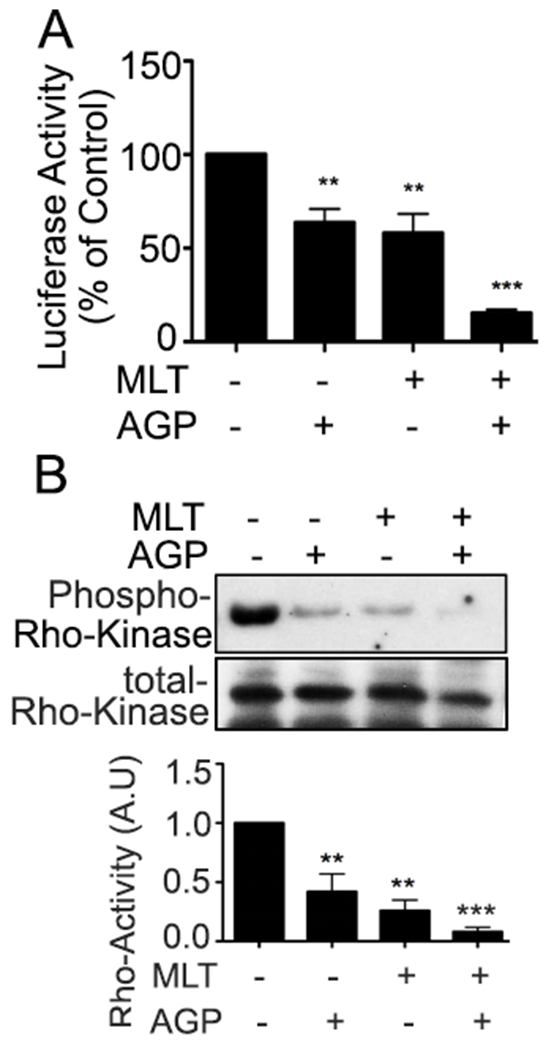
The impact of MLT and AGP on luciferase reporter activity. Spheroids were treated with or without AGP/MLT as previously described. The results are presented as ratio of firefly luminescence to Renilla luminescence. The y-axis represents the luciferase activity of treated colospheroids relative to untreated spheroids (**P< 0.01, ***P<0.001). B. A pull-down assay was used to detect RhoA activity as described. Quantification of relative phospho-Rho kinase level/total Rho kinase by densitometry measurements of immunoblots from three independent experiments. (**P<0.01, ***P<0.001).
3.5. β-catenin/Tcf signaling Inhibition associates with colospheroids death.
We assessed the Wnt target genes lgr5, tcf4, and lef1. Although these molecules are transcription factors in Wnt/β-catenin signaling, they are also Wnt target genes. Consistent with our previous observations (section 3.4), LGR5, TCF4 and LEF1 mRNA and protein expressions were inhibited by MLT+AGP treatment. Moreover, the expression of Axin-1, an essential component of Wnt signal, was inhibited with this dual treatment (Fig. 5A–C). The results suggest dual therapy inhibited spheroid proliferation involving Wnt- β-catenin signals.
Figure 5. MLT AGP inhibits β-catenin/TCF signaling.
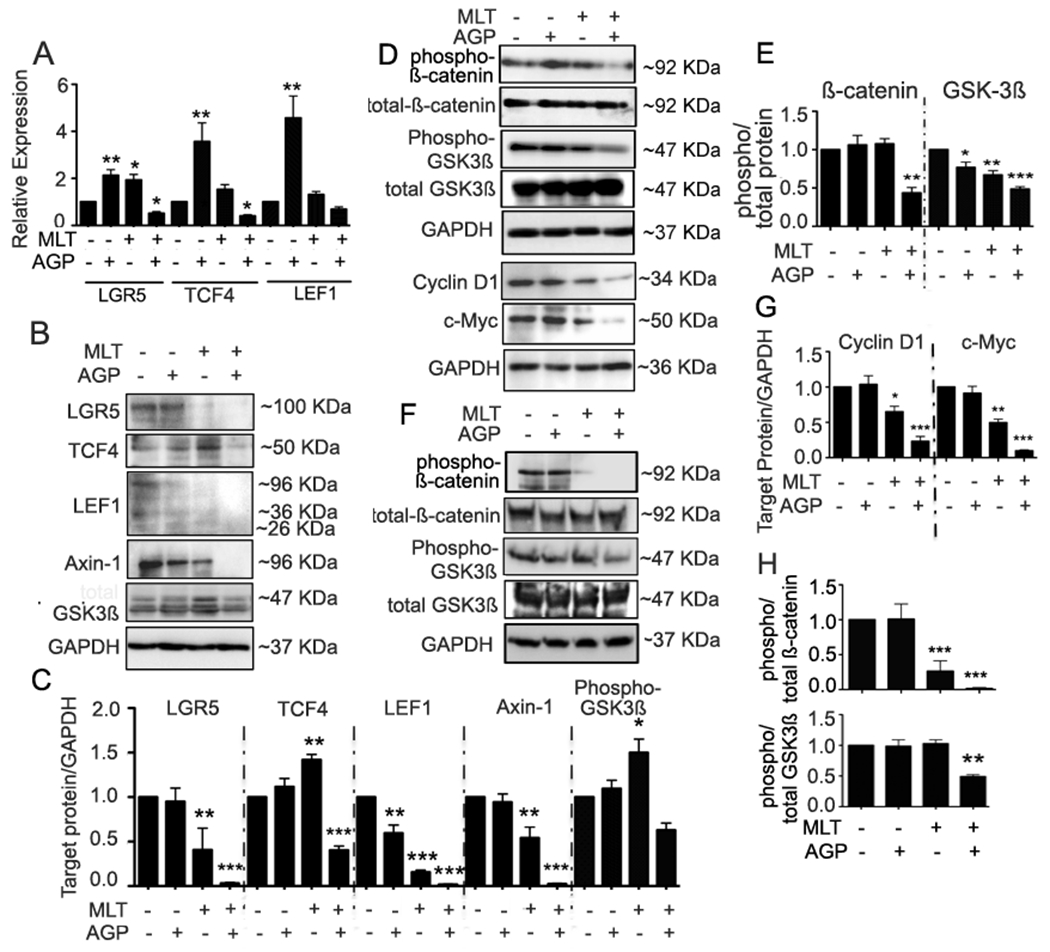
mRNA derived from treated or untreated HT29s were monitored for the β-catenin/Tcf signal associated gene expression by qRT-PCR. A. lgr5, tcf4, and lef1 (Left, middle and right). Bar graphs show quantitative results normalized to GAPDH mRNA levels. Results are from three independent experiments. B. Immunoblot analysis of treated or untreated HT29-s extracts as indicated and C. quantification. LGR5, TCF4, LEF1, Axin-1. D-E. HCT-116 cells were transfected with plasmid for overexpression of β-catenin and then treated with or without AGP/MLT. Cell lysates were analyzed by immunoblot and quantified by densitometry for expression of phospho- β-catenin, GSK3β, and total β-catenin, GSK3β, Cyclin D1, c-Myc. F-G. HCT-116 parental cell lysates were monitored as indicated and quantified. All statistical significance were determined using one-way ANOVA followed by Bonderroni test (*P<0.05, **P< 0.01, ***P<0.001).
To determine the nature of Wnt activity treated cells, β-catenin was overexpressed in HCT116 with maximum expression observed at 48 h. (Suppl Fig. 1). β -catenin is an essential downstream target for GSK3 β action in angiogenesis and impacts the signal transduction pathways involved in the angiogenic phenotype. Therefore, additional experiments were carried out to measure phospho β-catenin, total β-catenin, phospho GSK3β, total GSK3β expression30. Overexpression of β-catenin resulted in significantly reduced levels of Cyclin D1 and cMyc, (Fig. 5D–E). Dual treatment on parental HCT-116 cell extracts showed inhibition of phospho β-catenin and GSK 3β expression compared with the untreated cells (Fig. 5F–G). Taken together, these findings suggest that MLT + AGP act specifically at the level β-catenin axis and its downregulatory signal.
3.6. MLT and AGP synergistically impact on angiogenic inhibition
In earlier studies we found that MLT and AGP synergistically reduced the angiogenic signal on mCRC11. Here, we monitored the angiogenic molecule expression on colospheroids. As VEGF is a transcription factor of β-catenin, first we measured the VEGF 165, VEGF189 transcript level and their receptors (phospho VEGFR1, and VEGFR2) translational level. Results showed transcription and translational level inhibition by the dual treatment (Suppl Fig. 2; Fig. 6A–C). Alternative measures of drug efficacy against angiogenesis are tube formation and sprouting assays of endothelial cells (HUVEC), which entail cell migration and intercellular interactions.31 To monitor the impact of MLT and AGP synergism at the level of tube formation, HUVEC were seeded and examined. After 18 h of treatment, parameters of the tube network, including number of branches at branching point and total capillary tube length, were quantified32. The combination led to a greater decrease in tube network length, size, and junctions than that from monotherapy (Fig. 6D, F, G, Suppl Fig. 5). Capillary proliferation and invasion contribute to colon cancer progression. Matrigel invasion of spheroids in the presence or absence of MLT and AGP was examined. Invasion through the transwell membrane in untreated wells (Fig. 6E) remained the same, whereas those invaded through Matrigel reduced significantly in the presence of dual compound (Fig. 6E, H).
Figure 6. MLT and AGP synergistically downregulates angiogenic signaling in colospheroids.
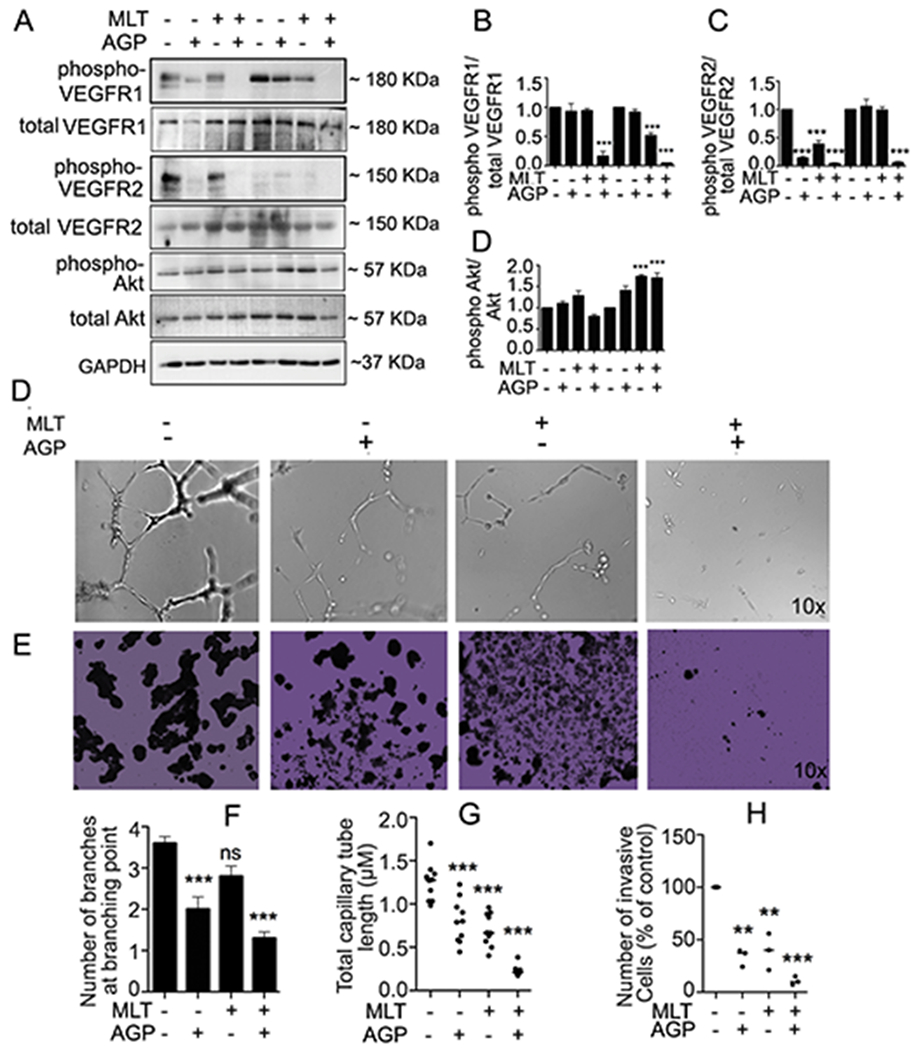
Treated/untreated HT29-s lysates were subjected for protein expression as indicated. Phospho protein was normalized with total protein B-D. All statistically significant differences were determined using one-way ANOVA followed Bonferroni test (*P<0.05, **P< 0.01, ***P<0.001). D, F-G. MLT+AGP suppressed angiogenic branching and total capillary tube length. E. Matrigel invasion inhibition of HT29-s after treatment as indicated. H. The invaded cells were quantified by counting using an optical microscope at 100X magnification and averaged after counting five fields per membrane (**P< 0.01, ***P<0.001).
3.7. Synergistic effect is associated with ROS generation and loss of ATP generation, Mitochondrial membrane potential.
Low levels of ROS in niches are important for stemness maintenance33. Therefore, the intracellular ROS level was measured in treated/untreated spheroids. Dual treatment increased ROS generation compared with the untreated/single treatment. Pretreatment of CSCs with the ROS inhibitor N-acetyl-L-cysteine 1 h prior resulted in a significant decrease in ROS in the treated group compared with the untreated group (Suppl. Fig. 3A–B). Generation of ROS is associated with changes in mitochondrial membrane potential (MMP) 13. Suppl. Fig. 3C shows dual therapy in HT29-s significantly decreased MMP and reduced ATP level (Suppl. Fig. 3D).
3.8. Impact of MLT and AGP co-treatment on tumor progression.
We next determined whether the in vitro results translate into the in vivo model. We selected HT29 cells, as they display enhanced malignant potential34. Two weeks after tumor cell injection, control mice displayed rapid tumor growth for the next 14 days. The combination therapy significantly inhibited tumor growth to 23.89% compared to control (Fig. 7A, 7B). MLT or AGP alone reduced tumor growth to 63.93% and 65.1%, respectively compared to control (Fig. 7B). At the completion of therapy, tumor weight was maximum in controls (1.23 ± 0.39 g) and decreased by MLT (0.94 ± 0.1 g) or AGP (0.80 ± 0.21 g) therapy. The combination of MLT and AGP further reduced the tumor weight (0.38 ± 0.1 g) (Fig. 7C). There was no therapy-related change in the body weight of mice (Fig. 7D) indicating that therapies appeared to be well tolerated.
Figure 7. Tumor growth inhibition by MLT and AGP in HT29 cell-derived subcutaneous xenografts.
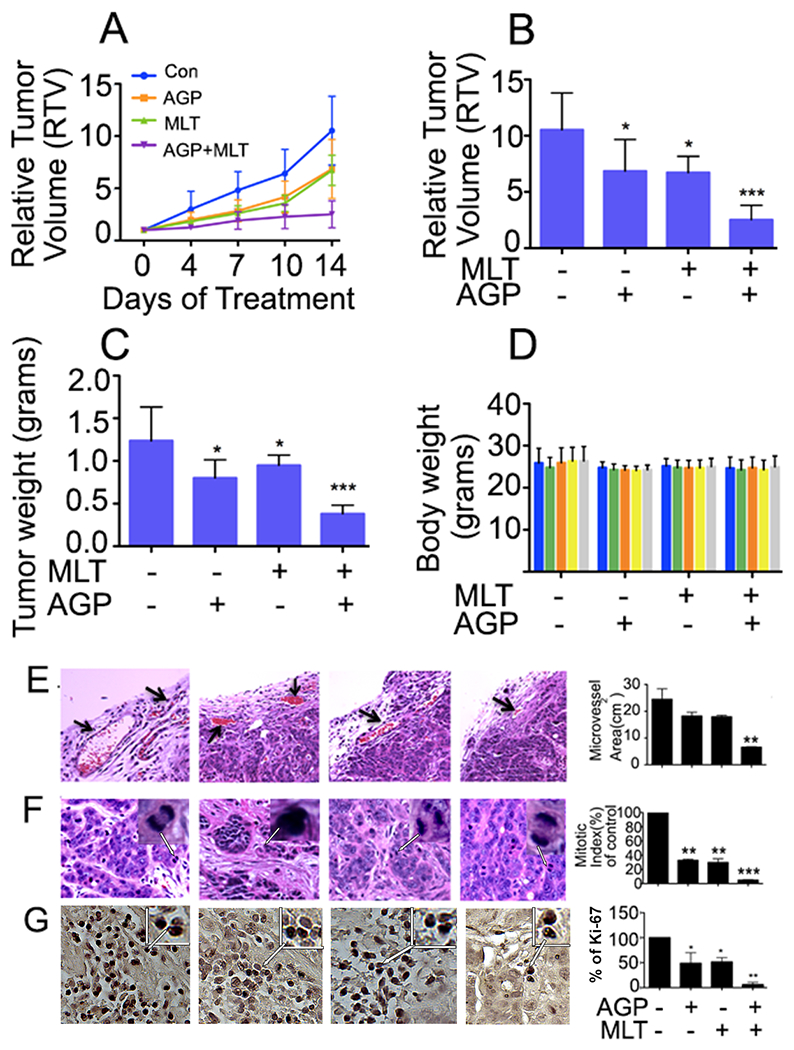
HT29 tumor-bearing nude mice were treated with MLT (40 mg/kg, 5 times a week) and AGP (20 mg/kg, 5 times a week) alone and in combination for 2 weeks. A. Tumor size was measured twice a week using calipers and the data were plotted. RTV changes over a period of 2 weeks after injection of drugs were shown. B. RTV changes after 2 weeks of drug treatments were plotted and compared. C. Mean tumor weight was calculated from final day tumor weights and were plotted. D. Mouse body weight was measured twice a week during the 2-week therapy period and were plotted. Data are representative of mean values ± standard deviations from 8 mice per group. E. H&E-stained tumor sections identifying the microvascular density in treated and untreated tumor tissue. Arrows indicate microvessels. F. H&E staining identifying the mitotic index (arrowheads). The right-side representative histograms are for the microvessels area and percentage of mitotic index from ten representative areas of each tumor. Statistical significance was determined using one way-ANOVA followed Bonferroni test (**P<0.01, ***P<0.001). G. Immunohistochemistry for Ki67 expression in HT29 CRC tumor sections with or without MLT, and AGP (200X). The histogram on the right panel represents the average percentage of Ki67 expression.
3.9. MLT and AGP decreases microvasculature and mitotic index.
Tumor tissues were evaluated to monitor the impact of single/dual therapy on tumor microenvironment and angiogenesis. H&E-stained sections were evaluated and large microvessels were quantified in five vessel-rich microscopic fields per tumor. Dual treatment reduced the microvessel size and number (Fig. 7E). Reduced mitotic index was found in all MLT + AGP treated tissues (Fig. 7F). To assess the synergistic effect of MLT and AGP on the inhibition of vessel formation, the microvessel density in the tumor tissue was examined immunohistochemically after staining for CD31. Microscopic examination revealed significant inhibition of positive CD31 cells in the MLT+AGP treated group (suppl Fig. 4C and F). Cellular proliferation and apoptosis were evaluated by immunohistochemistry for Ki67 and caspase-7 in the presence or absence of drug treatment. Decreased Ki67 positive cells were found in AGP and MLT+AGP treatment (Fig. 7G) whereas caspase 7 was elevated in the dual treatment (Suppl. Fig. 4A). To determine if the β-catenin in vitro results corroborated the in vivo results, we monitored this protein level in tumor tissue. A decreased level was found in MLT and in the MLT+AGP treated tissues (Suppl. Fig. 4B).
5. DISCUSSION
CRC is the third most common cancer and a major cause of morbidity and mortality worldwide35. In the United States, CRC is the second leading cause of cancer death. Current treatment consists in surgical resection and/or chemotherapy including 5-FU depending on stage. This does not usually remove tumor cells completely resulting in possible recurrence36, due to the presence of colospheroids11 (20–30% of total CRC cell population). Targeting this specific cell subpopulation may be an effective strategy to eradicate CRC and increase the survival of metastatic patients. Our prior studies demonstrated that MLT and AGP synergistically deplete colospheroids phenotypes and decrease Ki-67 expression in PDOD. This combination had little effect on cell viability of normal colon epithelial cells (FHC). The synergistic mechanism promotes mCRC cell death due to the involvement of UPR mediated ER stress transducers and angiogenic inhibition11,12.
Here, we demonstrated that this treatment combination decreased stemness properties through CD44, CD133, Nanog, Sox2, and Oct4 inhibition. The overexpression of these cell surface markers is associated with high Wnt activity37 and its pathway induces the proliferation and differentiation of tumors. Dephosphorylated β-catenin accumulates in the cytoplasm, enters the nucleus, and interacts with T-cell factor/lymphoid enhancer factor (TCF/LEF)38,39. Indeed, our data suggests that this drug combination serves as a regulator of gene expression by interacting with β-catenin-TCF/LEF transcriptional complexes and may present new therapeutic targets in colon cancer cell signaling. Our results demonstrated that MLT and AGP inhibit several β-catenin target proteins in a manner comparable to the Wnt/β-catenin inhibitor PRI-724, which is currently in clinical trials. Many Wnt/β-catenin signaling inhibitors, such as PKF115–584 , are in preclinical and clinical trials and inhibit the growth of HCC cells in xenografts39. To our knowledge, this is the first study that demonstrates the impact of MLT and AGP on HT29 and HCT-15 human CRC stemness by suppressing Wnt signaling. However, other signaling pathways, such as Notch and Hedgehog signaling, are likely to be involved in MLT-AGP-induced effects on CSCs; these warrant further investigation.
Emerging evidence indicates that Rho GTPases play an important role in non-canonical Wnt pathway and its inhibition dysregulates Wnt pathway27. Accumulating evidence supports the role of ROCK in tumor development and progression through regulating key cellular functions associated with malignancy, including tumorigenicity, tumor growth, metastasis, angiogenesis, tumor cell apoptosis/survival and chemoresistance40. Our study demonstrated that inhibition of Rho kinase activity may lead to colospheroids growth inhibition. However, further studies are needed.
Among the most interesting aspects of this report are the tumor findings. The results obtained from the metastatic HT29 xenograft model demonstrate the dual drug efficacy in vivo. MLT and AGP combination treatment of mice bearing HT29 subcutaneous tumors showed a significant reduction in tumor growth rate and tumor weight while body and organ weights were unaffected. The decreased tumor growth is associated with reduced Ki-67 and β-catenin levels, and increased caspase-7 levels. Preclinical studies suggest that the anti-angiogenic strategy is a promising approach for cancer control because drug resistance is less likely41. Moreover, microvascular count (MVC) for the quantification of angiogenesis is a tumor staging measurement, and prognostic for invasiveness and metastasis. In this study, we found a significant reduction in mitotic index and the number of microvessels as a result of the dual therapy as further evidence for the anti-angiogenesis activity of this combination therapies. Future experiments will monitor the drug combination effect on a spheroid cell-derived tumor model.
The effective concentration of MLT is greatly reduced when combined with AGP. In this study, we used 0.18 mM MLT when combined with AGP, which is the IC50 value for MLT when AGP and MLT are combined. This concentration is about 500x less than the concentration used for in vitro studies when used alone. How this concentration relates to effective doses when combined with AGP in humans is not known. The MLT level in human plasma varies significantly with a 24 h cycle, from ~7 pg/ml to ~70 pg/ml or 30.1pM - 301 pM42. Daily MLT (40 mg/kg) combined with a low-dose of interleukin-2 plus significantly increased 1 year survival rate of patients with mCRC, compared with supportive care alone43. Similarly, melatonin with irinotecan achieved a higher percent of disease-control in mCRC patients than irinotecan alone, because a partial response and stable disease were obtained by more patients. In a phase-II study including 14 patients with metastatic breast cancer, an oral 20 mg/day of melatonin starting 7 days before tamoxifen therapy achieved a partial response in 4/14 (28.5%) patients, caused a relief of anxiety in most patients, and did not enhance the toxicity of tamoxifen44. Melatonin as an adjuvant to other therapies appears safe and may be effective in clinical trials in human patients.
The present article documented that the combination of MLT and AGP inhibits CRC stem cell proliferation. The mechanism involves the inhibition of angiogenic and β-catenin signals and is supported by in vitro and in vivo experiments. We believe that the cell inhibition is due to the binding of MLT with the MLT receptor; however, this requires confirmation.
Supplementary Material
ACKNOWLEDGEMENTS
We wish to thank the Department of Pediatrics at the University of Maryland School of Medicine for financial support. This work was supported by the Program in Oncology, University of Maryland Marlene and Stewart Greenebaum Comprehensive Cancer Center core facilities for processing xenograft tissue sections. This research was also funded by Veteran’s Affairs Merit Review Award BX004895 and NIH NINDS R01NS119275 to T.K
Footnotes
AUTHOR AGREEMENT STATEMENT
We declare that this manuscript is original, has not been published before and is not currently being considered for publication elsewhere.
PATENTS
This study has been the subject of non-provisional patent (PCT/US2021/030084) by AB of the University of Maryland School of Medicine.
DECLARATION
All authors read and approved the final manuscript.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23(10):1124–1134. [DOI] [PubMed] [Google Scholar]
- 2.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. [DOI] [PubMed] [Google Scholar]
- 3.Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–648. [DOI] [PubMed] [Google Scholar]
- 4.Bjerkvig R, Tysnes BB, Aboody KS, Najbauer J, Terzis AJ. Opinion: the origin of the cancer stem cell: current controversies and new insights. Nat Rev Cancer. 2005;5(11):899–904. [DOI] [PubMed] [Google Scholar]
- 5.Gupta R, Bhatt LK, Johnston TP, Prabhavalkar KS. Colon cancer stem cells: Potential target for the treatment of colorectal cancer. Cancer Biol Ther. 2019;20(8):1068–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanwar SS, Yu Y, Nautiyal J, Patel BB, Majumdar AP. The Wnt/beta-catenin pathway regulates growth and maintenance of colonospheres. Mol Cancer. 2010;9:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song L, Li Y, He B, Gong Y. Development of Small Molecules Targeting the Wnt Signaling Pathway in Cancer Stem Cells for the Treatment of Colorectal Cancer. Clin Colorectal Cancer. 2015;14(3):133–145. [DOI] [PubMed] [Google Scholar]
- 8.Basu S, Haase G, Ben-Ze’ev A. Wnt signaling in cancer stem cells and colon cancer metastasis. F1000Res. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garza Trevino EN, Gonzalez PD, Valencia Salgado CI, Martinez Garza A. Effects of pericytes and colon cancer stem cells in the tumor microenvironment. Cancer Cell Int. 2019;19:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lizarraga-Verdugo E, Avendano-Felix M, Bermudez M, Ramos-Payan R, Perez-Plasencia C, Aguilar-Medina M. Cancer Stem Cells and Its Role in Angiogenesis and Vasculogenic Mimicry in Gastrointestinal Cancers. Front Oncol. 2020;10:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banerjee V, Sharda N, Huse J, et al. Synergistic potential of dual andrographolide and melatonin targeting of metastatic colon cancer cells: Using the Chou-Talalay combination index method. Eur J Pharmacol. 2021;897:173919. [DOI] [PubMed] [Google Scholar]
- 12.Sharda N, Ikuse T, Hill E, et al. Impact of Andrographolide and Melatonin Combinatorial Drug Therapy on Metastatic Colon Cancer Cells and Organoids. Clin Med Insights Oncol. 2021;15:11795549211012672.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banerjee A, Banerjee V, Czinn S, Blanchard T. Increased reactive oxygen species levels cause ER stress and cytotoxicity in andrographolide treated colon cancer cells. Oncotarget. 2017;8(16):26142–26153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Majumdar KN, Banerjee A, Ratha J, Mandal M, Sarkar RN, Saha KD. Leishmanial lipid suppresses tumor necrosis factor alpha, interleukin-1beta, and nitric oxide production by adherent synovial fluid mononuclear cells in rheumatoid arthritis patients and induces apoptosis through the mitochondrial-mediated pathway. Arthritis Rheum. 2008;58(3):696–706. [DOI] [PubMed] [Google Scholar]
- 15.Banerjee A, Basu M, Blanchard TG, et al. Early Molecular Events in Murine Gastric Epithelial Cells Mediated by Helicobacter pylori CagA. Helicobacter. 2016;21(5):395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nita-Lazar M, Banerjee A, Feng C, Vasta GR. Galectins regulate the inflammatory response in airway epithelial cells exposed to microbial neuraminidase by modulating the expression of SOCS1 and RIG1. Mol Immunol. 2015;68(2 Pt A):194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blanchard TG, Lapidus R, Banerjee V, et al. Upregulation of RASSF1A in Colon Cancer by Suppression of Angiogenesis Signaling and Akt Activation. Cell Physiol Biochem. 2018;48(3):1259–1273. [DOI] [PubMed] [Google Scholar]
- 18.Banerjee A, Ahmed H, Yang P, Czinn SJ, Blanchard TG. Endoplasmic reticulum stress and IRE-1 signaling cause apoptosis in colon cancer cells in response to andrographolide treatment. Oncotarget. 2016;7(27):41432–41444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiu J, Piazuelo MB, Ding H, et al. Gastric LTi cells promote lymphoid follicle formation but are limited by IRAK-M and do not alter microbial growth. Mucosal Immunol. 2015;8(5):1047–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang L, Shi P, Zhao G, et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 2020;5(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozawa M, Ichikawa Y, Zheng YW, et al. Prognostic significance of CD44 variant 2 upregulation in colorectal cancer. Br J Cancer. 2014;111(2):365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Neerven SM, Vermeulen L. The interplay between intrinsic and extrinsic Wnt signaling in controlling intestinal transformation. Differentiation. 2019;108:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng CC, Liao PN, Ho AS, et al. STAT3 exacerbates survival of cancer stem-like tumorspheres in EGFR-positive colorectal cancers: RNAseq analysis and therapeutic screening. J Biomed Sci. 2018;25(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan Y, Chiow KH, Huang D, Wong SH. Andrographolide regulates epidermal growth factor receptor and transferrin receptor trafficking in epidermoid carcinoma (A-431) cells. Br J Pharmacol. 2010;159(7):1497–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly KF, Ng DY, Jayakumaran G, Wood GA, Koide H, Doble BW. beta-catenin enhances Oct-4 activity and reinforces pluripotency through a TCF-independent mechanism. Cell Stem Cell. 2011;8(2):214–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seo E, Basu-Roy U, Zavadil J, Basilico C, Mansukhani A. Distinct functions of Sox2 control self-renewal and differentiation in the osteoblast lineage. Mol Cell Biol. 2011;31(22):4593–4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlessinger K, Hall A, Tolwinski N. Wnt signaling pathways meet Rho GTPases. Genes Dev. 2009;23(3):265–277. [DOI] [PubMed] [Google Scholar]
- 28.Jeong D, Park S, Kim H, et al. RhoA is associated with invasion and poor prognosis in colorectal cancer. Int J Oncol. 2016;48(2):714–722. [DOI] [PubMed] [Google Scholar]
- 29.Ohata H, Ishiguro T, Aihara Y, et al. Induction of the stem-like cell regulator CD44 by Rho kinase inhibition contributes to the maintenance of colon cancer-initiating cells. Cancer Res . 2012;72(19):5101–5110. [DOI] [PubMed] [Google Scholar]
- 30.Skurk C, Maatz H, Rocnik E, Bialik A, Force T, Walsh K. Glycogen-Synthase Kinase3beta/beta-catenin axis promotes angiogenesis through activation of vascular endothelial growth factor signaling in endothelial cells. Circ Res. 2005;96(3):308–318. [DOI] [PubMed] [Google Scholar]
- 31.Nacev BA, Liu JO. Synergistic inhibition of endothelial cell proliferation, tube formation, and sprouting by cyclosporin A and itraconazole. PLoS One. 2011;6(9):e24793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guha P, Kaptan E, Bandyopadhyaya G, et al. Cod glycopeptide with picomolar affinity to galectin-3 suppresses T-cell apoptosis and prostate cancer metastasis. Proc Natl Acad Sci U S A. 2013;110(13):5052–5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi X, Zhang Y, Zheng J, Pan J. Reactive oxygen species in cancer stem cells. Antioxid Redox Signal. 2012;16(11):1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen WS, Liu JH, Wei SJ, Liu JM, Hong CY, Yang WK. Colon cancer cells with high invasive potential are susceptible to induction of apoptosis by a selective COX-2 inhibitor. Cancer Sci. 2003;94(3):253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 36.Del Rio M, Molina F, Bascoul-Mollevi C, et al. Gene expression signature in advanced colorectal cancer patients select drugs and response for the use of leucovorin, fluorouracil, and irinotecan. J Clin Oncol. 2007;25(7):773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vermeulen L, De Sousa EMF, van der Heijden M, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12(5):468–476. [DOI] [PubMed] [Google Scholar]
- 38.Lepore Signorile M, Grossi V, Di Franco S, et al. Pharmacological targeting of the novel beta-catenin chromatin-associated kinase p38alpha in colorectal cancer stem cell tumorspheres and organoids. Cell Death Dis. 2021;12(4):316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Wang Z, Ajani JA, Song S. Drug resistance and Cancer stem cells. Cell Commun Signal. 2021;19(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei L, Surma M, Shi S, Lambert-Cheatham N, Shi J. Novel Insights into the Roles of Rho Kinase in Cancer. Arch Immunol Ther Exp (Warsz). 2016;64(4):259–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362(6423):841–844. [DOI] [PubMed] [Google Scholar]
- 42.Kennaway DJ. Measuring melatonin by immunoassay. J Pineal Res. 2020;69(1):e12657. [DOI] [PubMed] [Google Scholar]
- 43.Barni S, Lissoni P, Cazzaniga M, et al. A randomized study of low-dose subcutaneous interleukin-2 plus melatonin versus supportive care alone in metastatic colorectal cancer patients progressing under 5-fluorouracil and folates. Oncology. 1995;52(3):243–245. [DOI] [PubMed] [Google Scholar]
- 44.Lissoni P, Barni S, Meregalli S, et al. Modulation of cancer endocrine therapy by melatonin: a phase II study of tamoxifen plus melatonin in metastatic breast cancer patients progressing under tamoxifen alone. Br J Cancer. 1995;71(4):854–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


