Abstract
Childhood obesity continues to rise in the United States, and with it the off-label use of metformin for weight loss. The influence of age and obesity on the drug’s disposition and exposure has not previously been studied using a mechanistic framework. Here, an adult physiologically based pharmacokinetic (PBPK) model of metformin was scaled to pediatric populations without obesity, with overweight / obesity, and with severe obesity; a published virtual population of children and adolescents with obesity was leveraged during model evaluation. When the pediatric model was simulated in groups 10 – 18 y of age, oral clearance (CL/F) following 1,000 mg of metformin was higher (~1200 mL/min) in those with obesity and severe obesity compared to the groups without and with overweight (~1000 mL/min). In addition, simulated AUC in older children and adolescents with obesity and severe obesity was comparable to that in adults with a similar dose-exposure relationship. Overall, simulations using the pediatric PBPK model support the use of adult doses of metformin in older children and adolescents with obesity. Moreover, the virtual population of children and adolescents with obesity offers a valuable tool to facilitate development of pediatric PBPK models for studying populations with obesity and, in turn, contribute information to inform drug labeling in this special population.
Keywords: pharmacokinetics, obesity, metformin, children, adolescents, PBPK modeling
INTRODUCTION
Today, nearly 20% of children and adolescents in the United States have obesity, with 6% considered to have severe obesity.1 Childhood obesity increases the likelihood of having obesity during adulthood and developing other serious health conditions, including type 2 diabetes and cardiovascular disease.2 Although lifestyle modification is the first-line therapy for the treatment of pediatric obesity, the long-term success of these efforts alone often fails, and the addition of pharmacotherapy becomes the next best option.3 Metformin is a glucose-lowering agent approved by the United States Food and Drug Administration (FDA) to treat type 2 diabetes in persons ≥10 y of age4 and is being increasingly used off label for weight loss in children and adolescents with obesity.5
Metformin is a hydrophilic drug with high solubility and low permeability (BCS Class III), positively charged at physiologic pH, and requires membrane transporters for absorption, distribution, and excretion.6 Absorption of the drug is saturable and occurs primarily in the upper small intestine with an oral bioavailability of ~40 – 60%7; food reduces the rate and extent of absorption.8 Largely unbound in circulation, metformin rapidly distributes to tissues with a large apparent volume of distribution (V/F; ~600 L6). The drug is not metabolized or secreted in bile. Instead, metformin is primarily excreted unchanged in urine via active tubular secretion and, to a lesser extent (~20%), glomerular filtration, with renal clearance ~500 mL/min.6 Using these properties as a foundation, Hanke et al.9 developed a physiologically based pharmacokinetic model (PBPK) of metformin in adults; several active transport processes were implemented, and the model was rigorously evaluated in humans using plasma concentrations, the fraction of drug excreted in urine, and tissue concentrations measured via positron emission tomography.
Physiological changes associated with obesity are likely to alter drug pharmacokinetics (PK) and pharmacodynamics10,11 and, as a result, may require dose adjustments; however, labeling information in children and adolescents with obesity remains limited. To date, metformin PK has been studied in three pediatric populations: a small group of 9 y old girls without overweight (n=6),12 adolescents with overweight / obesity (n=22),13 and children and adolescents with severe obesity and insulin resistance (n=30).14 Based on population PK modeling, the area under the concentration-time curve (AUC) decreased with body weight in adolescents with obesity, corresponding to an increase in CL/F explained by developmental and excess body weight13; similarly, body weight was found to be a covariate of CL/F in children and adolescents with severe obesity.14 In addition, metformin CL/F in pediatric patients with obesity13,14 appears to be ~2 times higher than that in children without obesity, but comparable to values reported in adults without and with obesity and with type 2 diabetes mellitus.6,7
Here, to explore the influence of obesity on metformin PK in children and adolescents using a mechanistic framework, PBPK modeling was employed. Specifically, a published adult PBPK model of metformin9 was scaled to pediatric populations without and with obesity; the pediatric model was evaluated using the virtual population of children and adolescents with obesity developed by Gerhart et al.15 that incorporates age- and obesity-related physiological changes. Overall, the current analysis aims to provide a deeper understanding of the influence of age and obesity on metformin PK and offer guidance on dosing, as well as expand the previous work15 to evaluate the assumptions of the virtual population for the primarily renally eliminated drug metformin, especially in the context of severe obesity.
METHODS
Clinical data
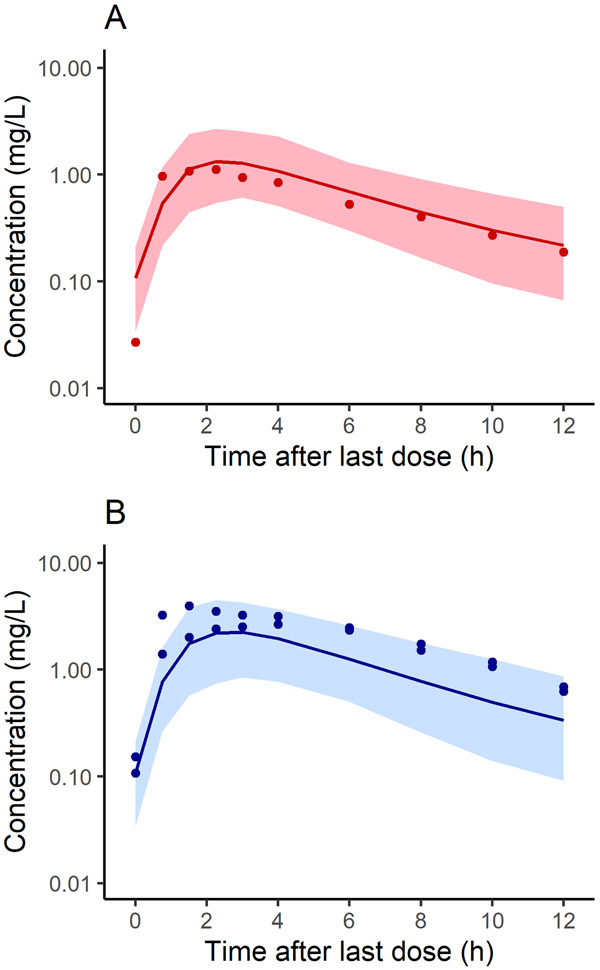
The adult PBPK model of metformin was evaluated using data digitized from studies of girls without overweight,12 adolescents with overweight / obesity,13 and individual-level data obtained from a study of children and adolescents with severe obesity.14 Details on participant characteristics and experimental design are summarized in Table 1. Observed concentrations for the 9 y old girls without overweight12 included individual-level data for two girls who received the highest (850 mg; 37 mg/kg) and lowest (425 mg; 13 mg/kg) doses of metformin when normalized to body weight as well as the mean of four girls who received intermediate weight-normalized doses (850 mg; 21 – 29 mg/kg) (Figure 1). For the group of adolescents with overweight / obesity,13 median concentrations were used for model evaluation. Data from these two studies were digitized using PlotDigitizer 2.6.8 (plotdigitizer.sourceforge.net).
Table 1.
Population demographics for metformin pediatric pharmacokinetic studies.
| Non-overweight a | Overweight / obesity b | Severe obesity, insulin resistant c |
|
|---|---|---|---|
| n | 6 | 22 | 30 |
| Dose (mg) | 850, qd (n=5) | 1,000, bid (n=19) | 1,000, bid (n=28) |
| 425, qd (n=1) | 500, bid (n=3) | 500, bid (n=2) | |
| Formulation | Immediate-release tablet | Immediate-release tablet | Immediate-release tablet d |
| PK study | |||
| Prandial state | Fed | Fasted e | Fasted e |
| Steady state | Yes | Yes | Yes |
| Duration, h | 12 | 8 | 12 |
| Population | Northern Spanish / Catalan (Barcelona, Spain) | White ('s-Hertogenbosch, the Netherlands) | 57% White, 40% African American, 3% Asian (United States); 10% Hispanic / Latino |
| Age, y | 9.5 (9.3 – 9.9) | 14 (11 – 18) | 11 (7.7 – 14) |
| Weight, kg | 33.3 (23.0 – 40.7) | 79.3 (54.7 – 105) | 77.0 (50.5 – 118) |
| Female, % | 100 | 73 | 57 |
| BMI, kg/m2 | 18.5 (15.7 – 21.0) | 29.1 (22.9 – 39.3) | 33.5 (24.2 – 43.6) |
| eGFR, mL/min/1.73 m2 | -- | 112 (94.1 – 136) f | 129 (91.7 – 188) g |
Data are expressed as mean (range) for continuous variables.
Clinical trial (isrctn.com ISRCTN49334271) in non-overweight girls with history of low birth weight and early-normal onset of puberty who were receiving 850 mg/day for 8 months; for the PK study, all girls received 850 mg metformin with dinner, except for 1 girl who accidentally received half that dose (425 mg).12
Clinical study (ClinicalTrials.gov NCT01487993) in 22 adolescents classified as either overweight or obese receiving 1,000 mg (or 500 mg for three subjects) of metformin twice daily for 37 weeks; blood samples for the PK study were collected during an oral glucose tolerance test.13
Six-month clinical trial (ClinicalTrials.gov NCT00005669) in children and adolescents with severe obesity receiving 1,000 mg (or 500 mg for two patients) of metformin; blood samples for the PK study were collected during a hyperglycemic clamp.14
Metformin capsules were manufactured and packaged by the National Institutes of Health Pharmaceutical Development Section.
Pharmacokinetic study was performed during a glucose tolerance test.
Published values were calculated using the Bedside Schwartz Equation
Published values were calculated using the CKiD Schwartz Equation.
bid, twice daily; BMI, body mass index; eGFR, estimated glomerular filtration rate; PK, pharmacokinetic; qd, once daily.
Figure 1.
Population simulations (n=100) of plasma concentration in non-overweight young girls at steady state following a 425 mg (A) or 850 mg (B) dose of metformin. Shown are simulated mean concentrations (solid line) and the 90% prediction interval (shaded region) for each population overlaid with the corresponding observed data. Demographics for the virtual populations were matched to reflect the observed data. Observed data were collected over 12 h from 9 y old non-overweight girls who received once daily doses of metformin for 8 months for early-normal puberty.12
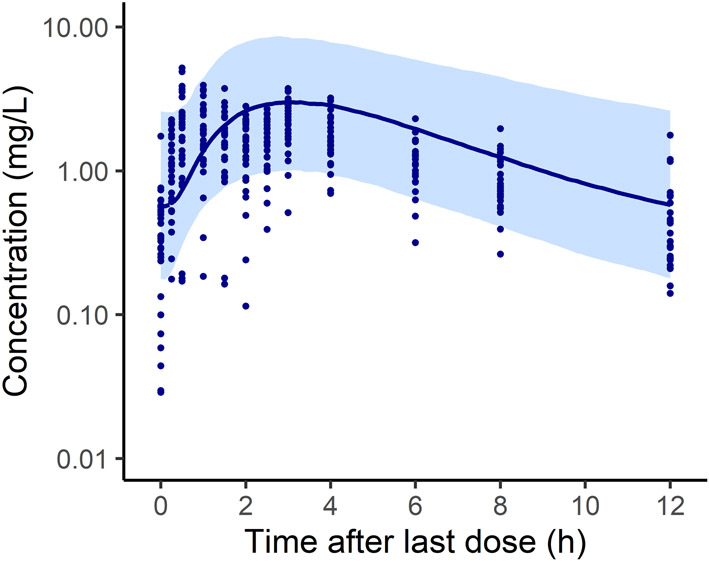
In the study by Sam et al.,14 30 children and adolescents with severe obesity and insulin resistance received 1,000 mg (or 500 mg for two subjects) of metformin twice daily during a 6-month clinical trial; for the PK arm of the study, performed during a hyperglycemic clamp, serial blood samples were collected from each participant at 0, 0.25, 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8 and 12 h after the dose and analyzed for metformin concentration using ultra performance liquid chromatography. Clinical measures and metformin concentration-time profiles for each participant were available, providing a rich dataset for model evaluation; observed concentration data are shown in Figure 2 and Supplementary Figures S1 - S3.
Figure 2.
Composite population simulation (n=1,000) of plasma concentration in children and adolescents with severe obesity following a 1,000 mg dose of metformin. Shown are simulated median concentration (solid line) for the population and the 90% prediction interval (shaded region); the corresponding simulation for the 2 patients who received 500 mg doses is shown in Supplementary Figure S3; the composite virtual population was generated using the mean demographics from the published study (Table 1). Observed data presented in this figure were collected over 12 h from 28 children with severe obesity who had been receiving 1,000 mg twice daily metformin therapy for 6 months.14 Individual-level data are also presented in Supplementary Figures S2 and S4. Extended BMI percentile was calculated as BMI divided by the 95th percentile for a subject's age and sex.
PBPK modeling
PBPK modeling was performed using the PK-Sim® software (version 9.1, Open Systems Pharmacology Suite, open-systems-pharmacology.org). The metformin PBPK model developed and verified in adults [available in the Open Systems Pharmacology PBPK model repository with detailed documentation in the publication9] was scaled to children and adolescents without and with obesity. Information on drug properties and transport processes used in the model are summarized in Supplementary Methods and Table S1.
Following previously established guidelines for scaling PBPK models from adults to pediatric populations, it was assumed that clearance processes, model structure, and variability were similar between adults and children.16 In the pediatric model, drug-specific inputs were maintained the same as in the adult model, and system-specific inputs (including anatomical and physiological variables known to change with age) were modified as appropriate. Variability in the reference concentration for each transporter was incorporated into the pediatric simulations assuming log-normal distribution using the mean and geometric standard deviation implemented in the adult model (Supplementary Table S2). Because metformin is not approved for use in persons <10 years of age4 and clinical data were only available in children ≥8 years of age, no transporter ontogeny was incorporated in the model.17 Modifications to the adult model were explored as needed, and changes were considered when sufficient evidence was available for justification.
For model evaluation, virtual subjects and populations were simulated based on observed demographics and dosing protocols for the corresponding study; each population comprised 100 virtual children and adolescents, unless otherwise specified. For those without obesity, simulations were performed using the virtual individuals and populations embedded in the PK-Sim® software.18 For those with obesity, simulations were performed using either the virtual individuals within PK-Sim® and then scaling organ sizes to reflect the redistribution of body composition known to occur during obesity, as described previously,15 or the virtual population of children and adolescents with obesity developed by Gerhart et al.15 Because densely sampled data were available for each subject within the group with severe obesity,14 simulations were performed for each patient using a demographic-matched virtual subject and, to evaluate PK variability, an “individualized population” within ± 1 y of age; a composite population of 1,000 children and adolescents was also simulated based on the mean demographics for the group.
Briefly, the virtual population of children and adolescents with obesity15 was developed by incorporating the following pathophysiological changes. Compared to those without obesity, body weight was increased to reflect a BMI greater than the 95th percentile and redistributed from adipose tissue to lean body mass organs, including ~15% larger kidney and liver volumes. As a result of larger organ sizes, absolute blood flow (and thus cardiac output) was also higher. In addition, the increased kidney size resulted in a greater glomerular filtration rate. For more details on the development of the virtual population of children and adolescents with obesity, see Gerhart et al.15
Model evaluation
Multiple methods were used to evaluate model performance. First, to compare model simulated concentrations to observed data, average fold error (AFE) was calculated as follows:
| (1) |
where n is the number of samples, and pred and obs are the predicted and observed concentrations. For population simulations, the number of observed values that fell within the 90% prediction interval was determined.
Next, predicted and observed values for specific PK parameters, including maximum concentration at steady state (Cmax), AUC calculated over the dosing interval at steady state, CL/F, and V/F, were evaluated. So that parameters were calculated using the same methodology, both predicted and observed plasma concentration data were analyzed using noncompartmental analysis in Phoenix WinNonlin (version 6.4; Certara, Princeton, NJ, USA). Observed PK parameters calculated for the three pediatric populations are summarized in Supplementary Table S3. Results were compared using AFE and the ratio of predicted / observed values. Overall, model evaluation criteria were considered acceptable when simulated concentrations and/or parameter estimates fell within 2-fold of the observed values.
Dosing simulations
Pediatric dosing regimens for metformin were evaluated based on target exposure (i.e., AUC) simulated in adults. Specifically, a virtual population of 1,000 persons aged 45 – 64 y was simulated using the adult PBPK model for metformin and the system-specific parameters generated by PK-Sim® from a normal distribution. Based on dosing recommendations for adults and pediatric patients with type 2 diabetes, which are often used to guide off label dosing in children and adolescents with obesity, simulations were performed with a starting dose of 500 mg twice daily and an upper dosage of 2,000 mg per day, divided into two 1,000 mg doses, given with meals.4,19 Metformin concentrations simulated over 12 h were exported into Phoenix WinNonlin for noncompartmental analysis; predictions were confirmed against values reported in adults. Assuming that the dose-exposure-response relationship in children and adolescents is similar to that in adults,13,20 AUC at these lower and upper dosages referred to target exposure.
Using the final pediatric PBPK model, population simulations were performed to evaluate dosing regimens for metformin in older children and adolescents with obesity. To do this, a virtual population of individuals (n=1,000) aged 10 – 18 y were simulated, grouped into the following BMI classifications (n=250/group): non-overweight (5th – 85th BMI percentile), overweight (85th – 95th BMI percentile), obesity (95th – 99th BMI percentile), and severe obesity (≥99th BMI percentile); BMI percentile was calculated based on the Centers for Disease Control and Prevention BMI-for-age growth charts.21 Groups with overweight and without overweight were generated using the virtual populations embedded in PK-Sim; obesity and severe obesity groups were generated using the external virtual population.15 Simulations were performed using the same dosing as the adults (500 and 1,000 mg given twice daily), and exposure was compared to adults. To explore the influence of obesity, PK parameters for metformin were compared across the 4 groups and BMI classifications.
In addition, it has been suggested13,14 that the current maximum daily dose of 2,000 mg/d for pediatric patients with type 2 diabetes be increased to 3,000 mg/d in children and adolescents with obesity. However, because lactic acidosis is a rare but potential serious complication of metformin use, associated with plasma concentrations >5 mg/L,4 it is important to evaluate the safety of this recommendation. Using the groups with obesity and severe obesity (as described above), simulations for doses of 1,000 mg three times per day and 1,500 mg twice daily were compared to those for 1,000 mg twice daily; model predictions were evaluated to confirm that Cmax did not exceed 5 mg/L.
RESULTS
PBPK modeling and model evaluation
When scaling the PBPK model of metformin from adults to pediatric populations without and with obesity, all drug-related parameters from the adult model (Supplementary Table S1) were retained.
First, the model was evaluated in children without obesity based on predictions in demographic-matched virtual subjects (Supplementary Table S4) and population simulations [illustrated in Figure 1 for 425 (A) and 850 mg (B) metformin doses compared with corresponding observed data]. For the girls who received the lowest and intermediate weight-based doses, AFE for predicted plasma concentrations was within 2-fold of the observed values (1.08 and 0.51, respectively), and 90 and 100% of observed concentrations fell within the 90% prediction interval for the virtual population, respectively. For the child who received the highest weight-based dose, while the AFE for plasma concentrations was 0.46, 80% of observed values fell within the 90% prediction interval. Taken together, 90% of all observed data points (27/30) fell within the 90% prediction interval (Figure 1). In addition, in all cases, predicted values for Cmax, AUC, CL/F, and V/F were within 2-fold of the observed values (Supplementary Table S4); briefly, observed and predicted values ranged from 1.1 – 4.0 and 1.2 – 2.1 mg/L for Cmax, 7.0 – 27 and 7.4 – 14 h*mg/L for AUC, and 408 – 785 and 742 – 985 mL/min for CL/F, respectively.
Next, the model was evaluated using data for adolescents with overweight / obesity. Simulated median plasma concentrations for the virtual population followed a similar trend to that for the observed data (data not shown), and the AFE was 1.2. When PK parameters derived using the median simulated plasma concentrations were compared to those calculated using the median observed concentrations, predictions were within 2-fold of the observed values for Cmax, AUC, CL/F, and V/F (Supplementary Table S4).
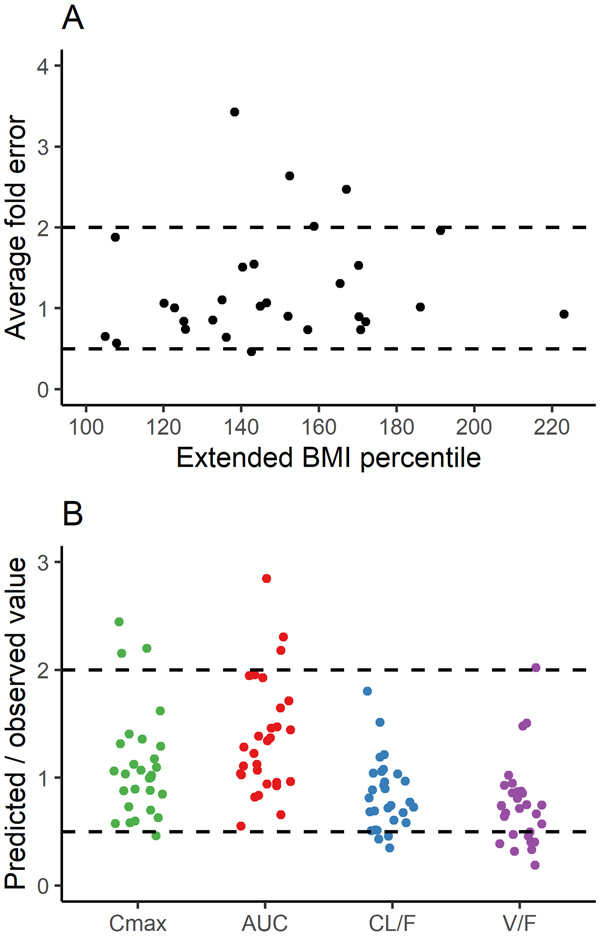
As a final step, the model was evaluated using the rich dataset for children and adolescents with severe obesity and insulin resistance of Sam et al.14 Predicted and observed plasma concentrations for the 30 subjects are illustrated in Supplementary Figure S1 for each patient; the AFE was within 2-fold for 83% (25/30) of subjects (Figure 3A). When predicted and observed values for Cmax, AUC, CL/F, and V/F were compared, the AFE was within 2-fold (Table 2), and the ratios were within 2-fold for 86, 90, 90, and 66% of patients, respectively (Figure 3B). When the model was evaluated for each of these 30 subjects using an “individualized population” (Supplementary Figure S2), plasma concentrations fell within the 90% prediction interval for 82% of samples (288/352). For the composite virtual population simulations, 86% of all samples (304/352) fell within the 90% prediction interval, with 87% (286/329) accounted for by patients who received 1,000 mg (Figure 2 and Supplementary Figure S3B) and 78% (18/23) from those who received 500 mg (Supplementary Figure S3A).
Figure 3.
Average fold error of predicted to observed metformin plasma concentrations compared across extended BMI percentile (A) and the ratios of predicted to observed values for pharmacokinetic parameters (B) in children with severe obesity. Dashed lines represent the 2-fold error range. Panel A includes comparisons for 30 children and, due to missing data, panel B includes comparisons for 29 children. Extended BMI percentile was calculated as BMI divided by the 95th percentile for a subject's age and sex. Parameters include AUC, or area under the concentration-time curve (calculated from 0 to 12 h at steady state); CL/F, or oral clearance (calculated as dose / AUC); Cmax, or maximum plasma concentration of metformin at steady state; and V/F, or apparent volume of distribution (calculated as CL/F divided by the elimination rate constant).
Table 2.
Observed and predicted pharmacokinetic parameters for children and adolescents with severe obesity.
| Parameter | Observed a | Predicted b | AFE |
|---|---|---|---|
| Cmax (mg/L) | 2.80 ± 0.978 (1.17 – 5.18); 1.56, 4.05 | 2.82 ± 0.577 (1.96 – 4.98); 1.56, 1.87 | 1.02 |
| AUC (h*mg/L) | 14.3 ± 5.00 (7.15 – 28.4); 10.4, 20.4 | 18.6 ± 6.10 (13.4 – 46.7); 10.0, 11.3 | 1.27 |
| CL/F (mL/min) | 1007 ± 326 (457 – 1817); 627, 318 | 737 ± 136 (278 – 973); 649, 574 | 0.786 |
| V/F (L) | 345 ± 150 (180 – 766); 125, 71.7 | 202 ± 30.3 (140 – 274); 185, 145 | 0.672 |
| eGFR (mL/min) | 131 ± 24.1 (91.7 – 188) c; 134, 102 | 84.5 ± 6.79 (70.4 – 94.8); 82.6, 70.4 | 0.654 |
Results for observed and predicted values for parameters are expressed as mean ± SD (range) for 27 children who received 1,000 mg doses of metformin twice daily and single values (in italics) for two subjects who received 500 mg doses twice daily. One patient who received 1,000 mg of metformin twice daily was excluded from the table due to missing data. AFE was calculated for the 29 paired values for PK parameters and 28 paired values for eGFR due to a missing observed estimate.
Observed values were calculated by noncompartmental analysis of observed plasma concentration-time data collected over 12 h following 6 months of metformin therapy.
Predicted values were calculated by noncompartmental analysis of simulated plasma concentration data at times used in the published sampling protocol.
Published values for eGFR (mL/min/1.73 m2) were converted to absolute eGFR (mL/min) using an estimate for body surface area (m2) calculated by the Haycock Equation.22
AFE, average fold error; AUC, area under the concentration-time curve calculated from 0 to 12 h at steady state; CL/F, oral clearance calculated as dose (mg) divided by AUC; Cmax, maximum plasma concentration of metformin at steady state; eGFR, estimated glomerular filtration rate; SD, standard deviation; V/F, apparent volume of distribution calculated as CL/F divided by the elimination rate constant.
Dosing simulations
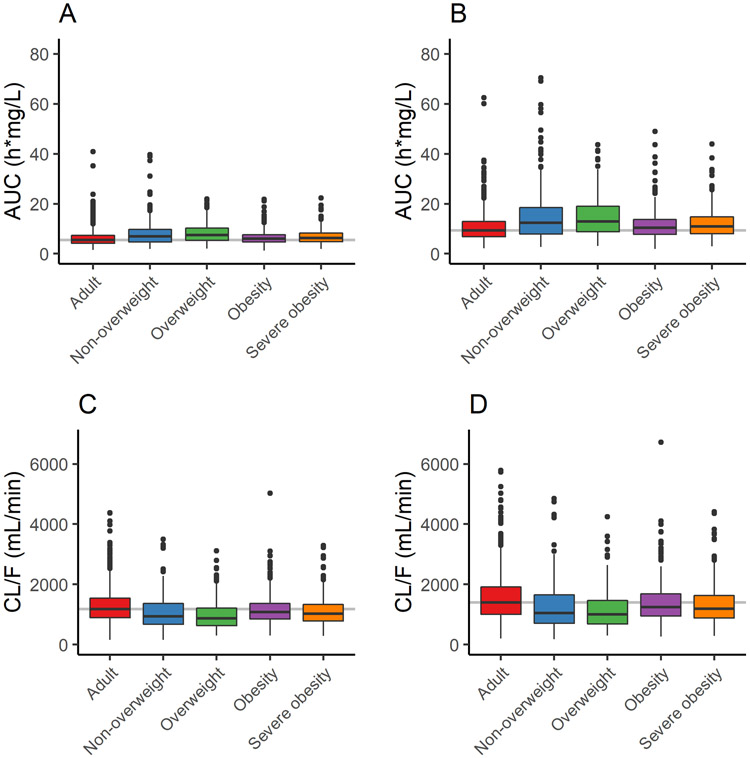
To obtain target exposure for metformin, simulations were performed in 45 – 64 y old adults. Overall, simulated plasma concentration profiles and PK parameters were comparable to values reported in the literature.6,7 Using the usual starting dose of 500 mg twice daily, median AUC was 5.5 h*mg/L, and for the upper dose of 1,000 mg twice daily, median AUC was 9.3 h*mg/L (Figure 4).
Figure 4.
Simulated metformin plasma AUC (A, B) and CL/F (C, D) for adults (n=1,000) and groups of children with non-overweight, overweight, obesity, and severe obesity (n=250/group) at steady state following a 500 mg (A, C) or 1,000 mg (B, D) dose. AUC was calculated from 0 to 12 h at steady state and CL/F was calculated as dose/AUC. Adult values were used as a reference for comparison; median simulated CL/F agrees well with values reported in the literature for healthy adults following oral 1,000 mg twice daily doses (1,265 ± 274 mL/min).6 For pediatric populations, non-overweight was classified as BMI between 5th and 85th percentile, overweight as BMI between 85th and 95th percentile, obesity as BMI between 95th and 99th percentile, and severe obesity as BMI ≥99th percentile. Boxplots show median or 50th percentile (middle band), 25th and 75th percentiles (lower and upper quartiles), range (vertical line), and outliers (symbols). AUC, area under the concentration-time curve; CL/F, oral clearance.
Pediatric simulations were performed in groups 10 – 18 y of age classified as non-overweight, overweight, obesity, or severe obesity based on BMI percentile; population demographics are compared in Supplementary Figure S4. As expected, body weight, kidney size, and GFR were higher in the virtual groups with obesity compared to non-obesity (Supplementary Figure S5). Using the same dosing schedules as in the adult simulations, median AUC following the 500 mg dose was 7.0, 7.4, 6.0, and 6.3 h*mg/L for the non-overweight, overweight, obesity, and severe obesity groups, respectively, and 12.4, 13.0, 10.5, 11.0 h*mg/L following the 1,000 mg dose (Figure 4); compared to the corresponding median AUC in adults, values were within 109 – 118% for the obesity and severe obesity groups and within 127 – 140% for the non-overweight and overweight groups. As expected, simulated CL/F across groups was inversely related to AUC and comparable for the two doses (median values were within 11 – 16%), and the increased CL/F with obesity was a function of increased kidney volume. Simulations also showed that metformin exposure was similar across the age range studied.
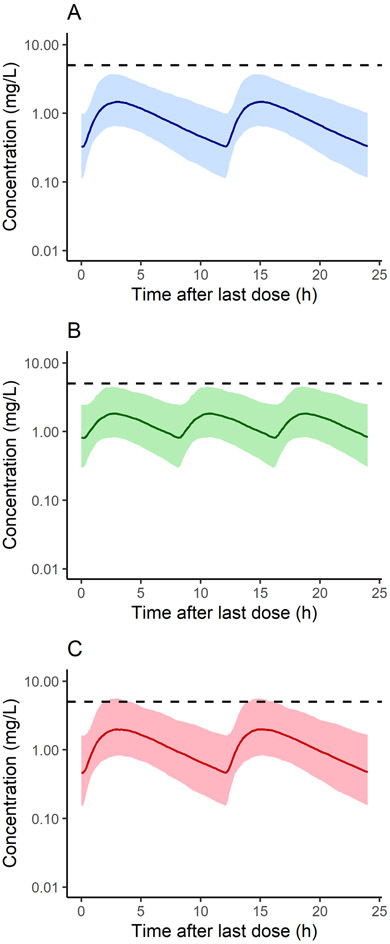
To evaluate whether increasing the maximum daily dose in children and adolescents with obesity to 3,000 mg would result in Cmax > 5 mg/L, simulations were performed using the 500 virtual subjects with BMI >95th percentile (i.e., the obesity and severe obesity groups). Shown in Figure 5 are population simulations of plasma concentration-time profiles for the current maximum daily dosage given as 1,000 mg twice daily (A) and the newly proposed maximum given either as 1,000 mg three times per day (B) or 1,500 mg twice daily (C); neither the median nor 90% prediction interval increased above 5 mg/L for the first 2 dosing schemes, but the upper prediction interval slightly exceeded this cutoff for the third protocol. At steady state, Cmax was >5 mg/L for 1.4% of individuals receiving 1,000 twice daily, 3.6% of those receiving 1,000 mg three times per day, and 8.2% of subjects receiving 1,500 mg twice daily.
Figure 5.
Populations simulations (n=500) of metformin plasma concentration in children with obesity based on the current recommended maximum dose of 2,000 mg/d (A) and the newly proposed maximum of 3,000 mg/d (B-C). Simulations reflect steady state concentrations over the last 24 h following 1,000 mg twice daily (A), 1,000 mg three times per day (B), and 1,500 mg twice daily (C). The virtual population includes children with BMI >95th percentile. The shaded regions represent the 90% model prediction interval, and the dashed line represents the median simulated concentration. The dashed horizontal line represents 5 mg/L, the plasma concentration threshold associated with lactic acidosis.4
DISCUSSION
As childhood obesity continues to rise,1 understanding its effects on drug PK becomes even more important for determining safe and effective dosing in this population. Although the investigation of metformin for weight loss in children began more than 20 years ago,23 dosing recommendations for this indication in pediatrics have not yet been established. Here, to address these gaps in knowledge, an adult PBPK model of metformin9 was scaled to pediatric populations with varying severity of obesity; overall, with all drug-related parameters fixed at the adult values, the pediatric PBPK model adequately described the available clinical data.12-14 The simulated dose-exposure relationship in pediatric patients aged 10 – 18 y with obesity was similar to that in adults following fixed dosing, corresponding to similar CL/F in these populations. In addition, this work provided another opportunity to verify the virtual population of children and adolescents with obesity15 as a tool to facilitate development of PBPK models in pediatric populations with obesity.
Model predictions were comparable to the population mean values for CL/F derived using nonlinear mixed effects (NLME) modeling in the original publications.13,14 Specifically, using a previously published covariate relationship13 and the mean body weight for the virtual population of children and adolescents with obesity (79.8 kg), predicted CL/F was 1,235 mL/min, which agrees well with the mean of 1,229 mL/min estimated by the PBPK model; predictions of V/F were also similar (485 and 468 L, respectively). Moreover, when simulated values for CL/F were plotted as a function of body weight for virtual individuals (Supplementary Figure S6), a positive correlation was observed that agreed well with the same comparison presented as Figure 1 in the original publication.13 For children and adolescents with severe obesity, CL/F predicted by NLME modeling (1,135 mL/min)14 normalized to the mean body weight of the virtual population (71.4 kg) was 1,037 mL/min, which is in-line with the mean CL/F predicted by the PBPK model of 764 mL/min. Moreover, when dosing simulations from Figure 4 in the original publication14 were replicated using the PBPK model (Supplementary Figure S7), plasma concentration-time profiles were comparable. Overall, predictions by the PBPK model compared well with results derived using NLME modeling, suggesting that, by leveraging available system- and drug-related information, PBPK modeling can predict drug responses that can be verified with potentially less data, addressing some of the challenges associated with data collection in children with obesity.
The virtual population of children and adolescents with obesity15 used during model evaluation was developed to reflect a greater total body weight and lean body mass (organ volumes) with obesity compared to without, and thus higher absolute blood flow (and cardiac output) and GFR. As illustrated in Supplementary Figure S5 for the simulated groups 10 – 18 y of age, body weight increased with obesity status; kidney volume was similar between non-overweight and overweight as well as between obesity and severe obesity groups (medians were within 3% for both comparisons), but higher in the groups with obesity and severe obesity compared to the non-overweight group (by ~14 and 10%, respectively); similar trends were observed for GFR. It is likely that kidney volume and GFR are even further increased in the case of severe obesity,24 but more research is needed to fully understand this relationship. Importantly, for the purpose of the current work, the assumptions of the virtual population were adequate to predict metformin PK in children and adolescents with severe obesity.
To explore the effect of age and obesity on metformin exposure and CL/F, model simulations were performed in pediatric groups ranging from non-overweight to severe obesity and compared to those in adults. In agreement with van Rongen et al.,13 predicted AUC in older children and adolescents with obesity was lower (and thus CL/F higher) than in children without obesity but similar to adults (Figure 4). In addition, simulated CL/F was similar between the obesity and severe obesity groups, which is supported by values for CL/F reported in the literature for pediatric populations with overweight / obesity and severe obesity (1,170 and 1,135 mL/min, respectively).13,14 Overall, the observed trends in exposure and CL/F correspond to obesity-related physiologic changes including greater body size / body weight in the obesity and severe obesity groups (within ~14% of that in adults; Supplementary Figure S5) and greater kidney volume and GFR compared to the non-overweight and overweight groups.
Model simulations were performed to evaluate metformin dosing in older children and adolescents with obesity. Since information on exposure-response in this population is limited, the relationship was assumed to be similar to that in adults. Specifically, since the dose-exposure-response to metformin therapy is comparable in adults and pediatric patients with type 2 diabetes20,25 and the dose-exposure relationship in adults is similar to that in children and adolescents with obesity,13 it seems reasonable to assume that the pharmacodynamic response to metformin for treatment of obesity would also be comparable between these age groups. Overall, adult doses of metformin resulted in simulated exposure in older children and adolescents with obesity comparable to that in adults, which is in-line with the knowledge that approved dosing in adolescents and adults are often the same.26
While the adult PBPK model of metformin was successfully scaled to pediatric populations without and with obesity, some limitations are worth mentioning. Although regulatory agencies continue to increase focus on studying drug PK in pediatrics,27 this population is still largely underrepresented in the literature which limits the available data for model evaluation; three pediatric studies provided metformin PK data and the non-overweight population was represented by only six girls. Moreover, some comparisons were complicated by the large interindividual variability characteristic of metformin PK. Fortunately, for the population of most interest for this analysis, children and adolescents with severe obesity, densely sampled PK data were available along with detailed information on subject characteristics and trial design. In addition, as noted in the original publication,14 double peaks in the concentration-time profiles were observed for the majority of patients with severe obesity; since the possible explanations suggested by the authors relate to study design or physiochemical properties of the drug (see Supplementary Methods), and not obesity status, changes to capture these doubles peaks were not incorporated into the PBPK model. Although exploring potential areas of model misspecification was also challenged by sparse data in pediatrics, this investigation led to results that support the hypothesis that metformin undergoes saturable paracellular absorption28 (Supplementary Methods). In addition, the virtual population of children and adolescents with obesity15 included a greater proportion of individuals with BMI between the 95 – 99th percentile compared to those with BMI >99th percentile (severe obesity). To evaluate the model in pediatric populations with severe obesity, virtual children and adolescents were filtered by BMI and the heaviest were selected for the corresponding population simulation; using this approach, the PBPK model adequately captured the clinical data.
In conclusion, simulations using the pediatric PBPK model of metformin support the use of adult dosing regimens in older children and adolescents with obesity. The model offers a framework for future research studying metformin in pediatric populations with obesity that can be expanded, for example, to younger age groups; to predict relevant drug-drug interactions; to account for other disease states; to incorporate a pharmacodynamic component; or to simulate metformin concentrations at the site of action. In addition, results presented herein support the future use of the virtual population of children and adolescents with obesity to facilitate development of pediatric PBPK models for studying populations with obesity and, as a result, contribute information to inform drug labeling in this special population.
Supplementary Material
ACKNOWLEDGEMENTS
This research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) under award 5R01HD096435 (to D.G.) and ZIAHD00641 (to J.A.Y.). J.G.G. received research support from a National Institute of General Medical Sciences (NIGMS) funded T32 program (T32GM122741) and through a Fred Eshelman Pre-Doctoral Fellowship in Pharmaceutical Sciences from the American Foundation for Pharmaceutical Education (AFPE). D.G. received additional research support from the NICHD (1R01HD102949-01A1). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health and US Food and Drug Administration. Phoenix® WinNonlin® was generously provided to the authors by Certara through the Academic Center of Excellence Program.
Footnotes
CONFLICT OF INTEREST
J.A.Y. reports grants for studies of obesity treatment funded by pharmaceutical companies involving other drugs: setmelanotide (Rhythm Pharmaceuticals), diazoxide choline controlled-release (Soleno Therapeutics), and colchicine (Hikma Pharmaceuticals). D.G. receives research support from Nabriva Therapeutics through a contract with the University of North Carolina at Chapel Hill. In addition, D.G. serves as a consultant for Tellus Therapeutics, focusing on neonatal drug development. The remaining authors have no relevant conflicts of interest to disclose.
DATA ACCESIBILITY
The metformin adult PBPK model used in this publication has been published in the Open Systems Pharmacology model repository (https://github.com/Open-Systems-Pharmacology/Metformin-Model).
REFERENCES
- 1.Ogden CL, Fryar CD, Martin CB, et al. Trends in obesity prevalence by race and Hispanic origin, 1999-2000 to 2017-2018. JAMA. 2000;324(12):1208–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinberger J, Daniels SR. Obesity, insulin resistance, diabetes, and cardiovascular risk in children: an American Heart Association scientific statement from the Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young) and the Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism). Circulation. 2003;107(10):1448–53. [DOI] [PubMed] [Google Scholar]
- 3.Boland CL, Harris JB, Harris KB. Pharmacological management of obesity in pediatric patients. Ann Pharmacother. 2015;49(2):220–32. [DOI] [PubMed] [Google Scholar]
- 4.Bristol-Myers Squibb Company. Glucophage® (metformin hydrochloride) tablets [package insert]. (Bristol-Myers Squibb Company, Princeton, NJ, 2017). [Google Scholar]
- 5.Hendricks EJ. Off-label drugs for weight management. Diabetes Metab Syndr Obes. 2017;10:223–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham GG, Punt J, Arora M, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50(2):81–98. [DOI] [PubMed] [Google Scholar]
- 7.Scheen AJ. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 1996;30(5):359–71. [DOI] [PubMed] [Google Scholar]
- 8.Sambol NC, Brookes LG, Chiang J, et al. Food intake and dosage level, but not tablet vs solution dosage form, affect the absorption of metformin HCl in man. Br J Clin Pharmacol. 1996;42(4):510–2. [DOI] [PubMed] [Google Scholar]
- 9.Hanke N, Türk D, Selzer D, et al. A comprehensive whole-body physiologically based pharmacokinetic drug–drug–gene interaction model of metformin and cimetidine in healthy adults and renally impaired individuals. Clin Pharmacokinet. 2020;59(11):1419–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology — drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–67. [DOI] [PubMed] [Google Scholar]
- 11.Smit C, De Hoogd S, Brüggemann RJM, Knibbe CAJ. Obesity and drug pharmacology: a review of the influence of obesity on pharmacokinetic and pharmacodynamic parameters. Expert Opin Drug Metab Toxicol. 2018;14(3):275–85. [DOI] [PubMed] [Google Scholar]
- 12.Sánchez-Infantes D, Díaz M, López-Bermejo A, Marcos MV, de Zegher F, Ibáñez L. Pharmacokinetics of metformin in girls aged 9 years. Clin Pharmacokinet. 2011;50(11):735–8. [DOI] [PubMed] [Google Scholar]
- 13.van Rongen A, van der Aa MP, Matic M, et al. Increased metformin clearance in overweight and obese adolescents: a pharmacokinetic substudy of a randomized controlled trial. Pediatr Drugs. 2018;20(4):365–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sam WJ, Roza O, Hon YY, et al. Effects of SLC22A1 polymorphisms on metformin-induced reductions in adiposity and metformin pharmacokinetics in obese children with insulin resistance. J Clin Pharmacol. 2017;57(2):219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerhart JG, Carreno FO, Edginton AN, et al. Development and evaluation of a virtual population of children with obesity for physiologically-based pharmacokinetic modeling. Clin Pharmacokinet. 2021; doi: 10.1007/s40262-021-01072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maharaj AR, Edginton AN. Physiologically based pharmacokinetic modeling and simulation in pediatric drug development. CPT Pharmacometrics Syst Pharmacol. 2014;3:e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brouwer KLR, Aleksunes LM, Brandys B, et al. Human ontogeny of drug transporters: review and recommendations of the pediatric transporter working group. Clin Pharmacol Ther. 2015;98(3):266–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bayer Technology Services. Computational systems biology software suite. PK-Sim® and MOBI® manual. Version 7.0.0, SB Suite http://www.systems-biology.com/products/pk-sim.html. Accessed April 30, 2021.
- 19.Masarwa R, Brunetti VC, Aloe S, Henderson M, Platt RW, Filion KB. Efficacy and safety of metformin for obesity: a systematic review. Pediatrics. 2021;147(3):e20201610. [DOI] [PubMed] [Google Scholar]
- 20.Jones KL, Arslanian S, Peterokova VA, Park JS, Tomlinson MJ. Effect of metformin in pediatric patients with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2002;25(1):89–94. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Percentile data files with LMS values [Internet]. National Center for Health Statistics. 2009. Available from: https://www.cdc.gov/growthcharts/percentile_data_files.htm July 7, 2021 [Google Scholar]
- 22.Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978;93(1):62–6. [DOI] [PubMed] [Google Scholar]
- 23.Kaplowitz P Is there a role for metformin in the treatment of childhood obesity? Pediatrics. 2017;140(1):e20171205. [DOI] [PubMed] [Google Scholar]
- 24.Ghobadi C, Johnson TN, Aarabi M, et al. Application of a systems approach to the bottom-up assessment of pharmacokinetics in obese patients: Expected variations in clearance. Clin Pharmacokinet. 2011;50(12):809–22. [DOI] [PubMed] [Google Scholar]
- 25.Gao X, Christensen M, Burghen GA, et al. Pharmacokinetics of metformin in pediatric type 2 diabetic and healthy adult subjects. Clin Pharmacol Ther. 2003;(Abstract PII-65). [Google Scholar]
- 26.Momper JD, Mulugeta Y, Green DJ, et al. Adolescent dosing and labeling since the Food and Drug Administration Amendments Act of 2007. JAMA Pediatr. 2013;167(10):926–32. [DOI] [PubMed] [Google Scholar]
- 27.Zisowsky J, Krause A, Dingemanse J. Drug development for pediatric populations: regulatory aspects. Pharmaceutics. 2010;2(4):364–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Proctor WR, Bourdet DL, Thakker DR. Mechanisms underlying saturable intestinal absorption of metformin. Drug Metab Dispos. 2008;36(8):1650–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.