Abstract
Purpose:
To assess the association of a wrist-worn, device-based metric of 24-hour movement with cognitive function and subjective cognitive complaints among older adults, ages 60 years and older.
Methods:
Cross-sectional analysis of the 2011-2012 and 2013-2014 National Health and Nutrition Examination Survey (NHANES) cycles. A wrist-worn ActiGraph GT3X+ accelerometer captured total 24-hour movement activity; analyzed as Monitor-Independent Movement Summary units (MIMS-units); and quantified into placement based on an age- and sex- standardized percentile. Cognitive tests in the domains of memory, language/verbal fluency, and executive performance were administered. Test-specific cognitive z-scores were generated. Subjective cognitive complaints included perceived difficulty remembering and confusion/memory loss.
Results:
The analytical sample included 2,708 U.S. older adults (69.5±0.2 years, 55% female, 20.9% non-White). Multivariable linear regressions revealed those in quartiles 3 (50th-74th percentile) and 4 (≥75th percentile) for their age and sex had higher cognitive function z-scores across all domains compared to those in quartile 1. Logistic regressions demonstrated those in quartiles 3 and 4 also had lower odds of reporting difficulty remembering (AOR=0.52, 95% CI: 0.31-0.89; AOR=0.57, 95% CI: 0.37-0.88) and confusion/memory loss (AOR=0.49, 95% CI: 0.27-0.91; AOR=0.49, 95% CI: 0.27-0.98), respectively, compared to those in quartile 1.
Conclusions:
In a representative sample of U.S. older adults, higher cognitive functioning occurs among those that perform total 24-hour movement activity at or above the 50th percentile for their age and sex. Future studies should consider movement behaviors across a 24-hour period on cognitive health outcomes in older adults. More research exploring prospective associations of MIMS-units and time-use behaviors across midlife and older adulthood that may affect cognitive functioning across diverse populations is needed.
Keywords: PHYSICAL ACTIVITY, COGNITIVE FUNCTION, NHANES, MONITOR-INDEPENDENT MOVEMENT SUMMARY UNITS, MIMS-UNITS
INTRODUCTION
Time-use behaviors, e.g., sedentary behavior and physical activity (1–3), are modifiable risk factors associated with numerous health outcomes and all-cause mortality (4–9). Inadequate physical activity has been estimated to have a 1.6% worldwide population attributable risk for dementia (10) and harmonized data suggests inactivity may contribute to 21% (over 1.1 million) of Alzheimer’s disease (AD) cases in the U.S. (12.7% worldwide) (11). With nearly 15 million cases of mild cognitive impairment and AD in the U.S. projected by 2060 (12) and limited pharmacological treatments to delay AD progression, there is a need for understanding behaviors that may optimize cognitive resilience to prevent or delay AD onset.
Leveraging accelerometer data that capture movement across the entirety of the day, can increase our understanding of what comprises a healthy 24-hour period for cognitive health. Higher daily step counts have been found to be associated with higher total brain volume (13), higher fractional anisotropy from cerebral white matter (14), and lower rate of subjective cognitive decline (15). Higher device-based daily physical activity was also associated with higher gray matter volume in older adults enrolled in UK Biobank (16). However, there are age and sex differences in activity levels of older adults (17, 18) and potential biases using absolute intensity cut points across demographic subgroups (19–21). As an alternative approach, the use of standardized percentiles of activity can provide relative information about how a person’s movement behaviors across the day are associated with cognitive health compared to others of the same sex and age.
Between 2011-2014, the National Health and Nutrition Examination Survey (NHANES) deployed a 24-hour continuous monitoring protocol using wrist-worn accelerometers to capture activity in U.S. children and adults (22, 23). Data are provided in Monitor-Independent Movement Summary units (MIMS-units) – a quantification of total continuous movement, with larger MIMS-units indicating higher amounts of movement performed throughout a 24-hour period. Given the need to further understand how 24-hour movement relates to brain health, we leveraged previously developed age- and sex- specific population-referenced percentile curves (18) to examine how this novel, wrist-worn movement metric relates to cognitive function and subjective cognitive complaints among a nationally representative sample of U.S. older adults, ages 60 years and older. We hypothesize that higher MIMS-units percentile will be associated with better cognitive function and lower odds of subjective cognitive complaints.
METHODS
Data source
NHANES is a representative sample of the non-institutionalized U.S. population with a complex, multistage probability design (23). Participants completed in-person home interviews and health measurements were performed in a mobile examination center (MEC). In the 2011-2012 and 2013-2014 cycles, participants were invited to wear a physical activity monitor (PAM) for 7 consecutive days following their visit to the MEC (Day 1). Participants were asked to return the device, via mail, on the 9th day. NHANES was approved by the National Center for Health Statistics (NCHS) Institutional Review Board and all participants provided written informed consent. University Institutional Review Board approval for this analysis was exempt as NHANES data are deidentified and publicly available.
Movement Activity
24-hour movement was measured with the ActiGraph GT3X+ (ActiGraph) device. Following the MEC visit, participants were asked to wear the PAM on their non-dominant wrist for 24 hours for 7 consecutive days, including during water-based activities such as bathing and swimming. The publicly available NHANES PAM data are provided in daily MIMS triaxial units. Briefly, MIMS-units are derived from an algorithm based on the raw (80 Hz) acceleration data and are an aggregated triaxial sum of the processed signals from each axis (X, Y, Z) (24). Full details on the derived MIMS-units and the PAM protocol have been previously described (18, 22, 24) with the comparison to other raw accelerometer-derived metrics detailed by John et al. (24).
The PAM header file, which includes device wear information, and the day summary file, which includes one record per day for each participant, were used for these analyses. Using the steps by Belcher et al. (18), we removed the two partial days (1st and 9th day of wear; exam date and requested return date). Among participants ≥60 years, those without device data, dominant wrist or unknown placement were excluded from these analyses. A day was considered usable if it had 1440 minutes of valid wear time, <5% non-wear time, and <17 hours of recorded sleep. Those with 0 useable days were excluded. One day of data collection has been previously found to be sufficient for stable, population-level analyses (25); hence, we included participants with at least one useable day (n=2,897) (see SDC 1, Appendix, Figure S1, Flow diagram for analytical sample of study). However, of note, 98.6% of the sample had ≥3 useable days. Daily MIMS-units were averaged across all usable days. We then matched participants averaged daily MIMS-units to the percentile for their age and sex (18).
Domain-Specific Cognitive Function
For participants ≥60 years, a series of cognitive assessments were conducted in the domains of memory (Consortium to Establish a Registry for Alzheimer’s disease Word Learning [CERAD W-L] delayed recall test) (26); language/verbal fluency (Animal Fluency test) (27); and executive performance (Digit Symbol Substitution test [DSST]) (28). Of those with useable accelerometer data, participants ineligible for cognitive assessment or those where no tests were done were excluded. Therefore, our analytic sample included 2,708 older adults (see Figure S1, SDC 1, Appendix, Flow diagram for analytical sample of study). Each cognitive test was reported as a raw score, with higher scores indicating higher cognitive functioning (e.g., better cognitive test performance), and was additionally standardized to the analytic sample mean and standard deviation to estimate a domain z-score (mean=0, standard deviation=1).
Subjective Cognitive Complaints
Two items pertaining to subjective cognitive complaints were measured using two self-report questions: 1) “Are you limited in any way because of difficulty remembering or because you experience(s) periods of confusion?” and 2) “During the past 12 months, have you experienced confusion or memory loss that is happening more often or is getting worse?”. Item responses included ‘Yes’, ‘No’, ‘Don’t Know’, ‘Refused’, or ‘Missing’. Refused (n=0) or ‘Don’t know’ (n=3) response choices were set to ‘Missing’.
Covariates
Self-reported covariates included age, sex, race/ethnicity, educational attainment, income (family income to poverty ratio ≤1.50: the ratio at which some federal programs offer subsidies to families), smoking status (current smoker, ever smoker, never smoker), and physical function difficulties (self-reported some difficulty or greater with any of the following tasks: walking from one room to another on the same level, getting in or out of bed, eating, or dressing). Measured covariates included depressive symptom severity (Patient Health Questionnaire [PHQ-9] (29): no symptoms [0 score], minimal symptoms [1-4 score], mild symptoms [5-9 score], moderate to severe symptoms [≥10 score]), body mass index (BMI) (underweight [<18.5 kg/m2], normal weight [18.5-<25 kg/m2], overweight [25-<30 kg/m2], obese [≥30 kg/m2]), hypertension (ever been told by a doctor that you have high blood pressure, measured systolic blood pressure ≥140 mmHg, or measured diastolic blood pressure ≥90 mmHg), and diabetes (ever been told by a doctor that you have diabetes, glucose level ≥126 mg/dL, or HbA1c ≥6.5%).
Statistical Analysis
Using the continuous movement percentile, we categorized participants into quartiles (Q1: <25th percentile, Q2: 25th-49th percentile, Q3: 50th-74th percentile, Q4: ≥75th percentile). We used descriptive statistics, including weighted percentages with standard errors, to describe characteristics of the sample. Using multivariable linear regression models, we investigated the association between movement quartiles (exposure) and domain-specific cognitive function z-scores (outcome) with Q1 (<25th percentile) as referent. For trend analysis, we divided the continuous movement percentile by 10 so that each unit increase was associated with 10 percentile points. We used multivariable logistic regression models to examine the relative odds of subjective cognitive complaints across movement quartiles (referent: Q1). For all regression analyses, we built a series of models adjusting for age, sex, race/ethnicity (Model 1), education, income (Model 2), and depressive symptoms (Model 3), and an additional model controlling for intermediate vascular risk factors: BMI category, hypertension, diabetes, smoking status, and physical function (Model 4). Per the NCHS recommendations, variance estimates were derived using Taylor Series Linearization and missing values were treated as not missing completely at random. All analyses were conducted in SAS version 9.4 (SAS Institute) using the “Survey” procedure to account for the complex survey design and 4-year sampling weights. We also modeled these associations as nonparametric regressions using the package mgcv (30) in RStudio 4.0.4. No adjustments were made for multiple comparisons as this is an observational study with well-defined hypotheses and several of the outcomes are correlated (31).
Sensitivity Analyses
We conducted sensitivity analyses to examine the potential for selection bias. We examined cognitive z-scores derived from the analytical sample compared to the entire NHANES sample ≥60 years, to examine if those without activity data may have been less healthy or less physically active. We also examined potential differences in sample characteristics among those included in the analytical sample and those not included in these analyses.
RESULTS
Participants (n=2,708) wore the PAM on average 5.9±0.1 days and had a median daily MIMS-unit value of 11,274.0±68.9. A total of 2,518 participants (93% of the analytical sample) had all three cognitive tests (see Figure S2, SDC 1, Appendix, Pattern of cognitive test completion of the analytical sample). Characteristics of the sample are presented in Table 1, overall and by movement quartiles. Compared to participants in Quartile 1, participants in Quartile 4 had a lower prevalence of mild and moderate to severe depressive symptom severity, hypertension, diabetes, obesity, and being a current smoker.
Table 1.
Participant Characteristics of the Analytical Sample (N = 2,708)
| Overall N = 2708 |
MIMS Quartile 1 (<25th percentile) n = 658 |
MIMS Quartile 2 (25th-49th percentile) n = 672 |
MIMS Quartile 3 (50-74th percentile) n = 631 |
MIMS Quartile 4 (≥75th percentile) n = 747 |
|
|---|---|---|---|---|---|
|
|
|||||
| % (SE) | % (SE) | % (SE) | % (SE) | % (SE) | |
| MIMS-units, Median (SE) | 11274.0 (68.9) | 7725.6 (151.7) | 10331.0 (118.5) | 12320.0 (114.1) | 15304.0 (243.5) |
| Sociodemographic Factors | |||||
| Age (years), Mean (SE) | 69.5 (0.2) | 69.7 (0.3) | 69.3 (0.3) | 69.2 (0.3) | 69.6 (0.4) |
| Female | 55.1 (1.0) | 55.5 (2.4) | 55.2 (2.5) | 53.7 (2.3) | 56.0 (2.6) |
| Race/ethnicity | |||||
| Non-Hispanic White | 79.1 (1.9) | 80.6 (2.0) | 81.4 (1.6) | 79.5 (2.7) | 75.1 (2.7) |
| Non-Hispanic Black | 8.8 (1.2) | 10.6 (1.3) | 9.0 (1.3) | 7.7 (1.3) | 8.0 (1.4) |
| Mexican American | 3.4 (0.7) | 2.4 (0.6) | 3.1 (0.6) | 3.6 (1.0) | 4.5 (1.0) |
| Other Hispanic | 3.8 (0.7) | 1.8 (0.4) | 3.1 (0.6) | 4.7 (0.9) | 5.6 (1.3) |
| Non-Hispanic Asian | 3.0 (0.5) | 2.3 (0.5) | 2.1 (0.4) | 3.2 (0.7) | 4.6 (0.8) |
| Other Race/Multi-Racial | 1.8 (0.5) | 2.3 (0.7) | 1.3 (0.8) | 1.3 (0.5) | 2.1 (1.6) |
| Education level | |||||
| < High school | 16.9 (1.4) | 16.7 (2.5) | 16.9 (1.7) | 17.2 (2.0) | 16.6 (1.7) |
| High school/GED | 22.3 (1.3) | 24.0 (2.4) | 21.1 (2.3) | 19.6 (2.2) | 24.4 (2.6) |
| Some college/ Associates | 31.4 (1.4) | 33.8 (3.0) | 33.2 (2.7) | 29.1 (1.9) | 29.3 (1.9) |
| College degree or more | 29.5 (2.1) | 25.5 (3.4) | 28.7 (3.4) | 34.0 (2.9) | 29.7 (3.0) |
| Missing | 0.0 (0.0) | — | 0.0 (0.0) | 0.1 (0.1) | — |
| Income ≤150% of Poverty | 27.1 (1.5) | 29.1 (2.5) | 28.0 (2.2) | 26.1 (2.4) | 25.3 (2.3) |
| Missing | — | — | — | — | — |
| Co-Morbidities | |||||
| Depressive symptoms severitya | |||||
| No symptoms | 34.8 (1.4) | 27.3 (2.2) | 32.6 (3.0) | 38.2 (3.3) | 40.8 (2.6) |
| Minimal symptoms | 42.3 (1.8) | 40.6 (3.1) | 43.9 (3.1) | 41.8 (3.2) | 42.9 (3.2) |
| Mild symptoms | 14.2 (0.9) | 18.8 (2.3) | 15.4 (1.4) | 12.5 (1.8) | 10.2 (1.2) |
| Moderate to severe symptoms | 7.3 (0.9) | 11.6 (2.1) | 6.2 (1.2) | 6.4 (1.2) | 5.2 (.2) |
| Missing | 1.4 (0.2) | 1.7 (0.6) | 1.9 (0.6) | 1.0 (0.4) | 1.0 (0.3) |
| Hypertensionb (yes) | 63.9 (1.4) | 71.5 (2.5) | 66.4 (2.9) | 63.0 (2.7) | 55.4 (2.6) |
| Missing | 0.8 (0.2) | 0.6 (0.3) | 0.7 (0.4) | 0.6 (0.4) | 1.3 (0.7) |
| Diabetesc (yes) | 20.3 (1.0) | 29.6 (2.2) | 24.4 (2.3) | 16.0 (1.5) | 11.7 (1.7) |
| Missing | 0.0 (0.0) | — | — | 0.1 (0.1) | — |
| Body Mass Index | |||||
| Underweight | 1.5 (0.3) | 1.4 (0.6) | 1.4 (0.7) | 1.1 (0.4) | 1.9 (0.8) |
| Normal weight | 24.2 (1.1) | 16.0 (2.2) | 16.0 (1.9) | 28.4 (2.7) | 36.2 (2.7) |
| Overweight | 34.7 (1.1) | 29.1 (1.7) | 33.6 (2.0) | 37.9 (2.1) | 38.0 (2.2) |
| Obese | 38.2 (1.3) | 50.5 (2.6) | 47.0 (3.2) | 31.9 (2.2) | 23.8 (2.0) |
| Missing | 1.4 (0.3) | 2.9 (0.7) | 1.9 (0.8) | 0.7 (0.3) | 0.2 (0.1) |
| Smoking status | |||||
| Never smoker | 50.0 (1.5) | 45.5 (3.3) | 46.9 (2.8) | 52.5 (2.3) | 55.0 (3.0) |
| Ever smoker | 39.1 (1.5) | 37.8 (2.2) | 42.1 (2.5) | 38.9 (2.5) | 37.6 (2.8) |
| Current smoker | 10.8 (0.6) | 16.8 (2.3) | 10.8 (1.5) | 8.5 (1.4) | 7.4 (1.2) |
| Missing | 0.1 (0.0) | — | 0.1 (0.1) | 0.1 (0.1) | 0.1 (0.0) |
| Physical function difficultiesd (yes) | 17.3 (1.0) | 29.4 (2.3) | 16.7 (1.9) | 12.5 (1.4) | 11.3 (1.8) |
| Missing | 0.2 (0.1) | 0.2 (0.1) | 0.4 (0.3) | — | 0.4 (0.3) |
| Domain-Specific Cognitive Function | |||||
| Memorye | |||||
| Z-score, Mean (95% CI) | 0.15 (0.07, 0.22) | −0.01 (−0.12, 0.10) | 0.17 (0.07, 0.28) | 0.20 (0.09, 0.31) | 0.22 (0.09, 0.35) |
| Raw score, Mean (95% CI) | 6.2 (6.0, 6.4) | 5.8 (5.5, 6.1) | 6.2 (6.0, 6.5) | 6.3 (6.0, 6.6) | 6.3 (6.0, 6.6) |
| Missing | 1.1 (0.4) | 0.4 (0.3) | 1.5 (0.8) | 0.5 (0.2) | 2.0 (0.8) |
| Language/Verbal Fluencyf | |||||
| Z-score, Mean (95% CI) | 0.27 (0.21, 0.35) | 0.11 (−0.01, 0.24) | 0.29 (0.14, 0.43) | 0.34 (0.22, 0.46) | 0.36 (0.26, 0.45) |
| Raw score, Mean (95% CI) | 17.9 (17.5, 18.3) | 17.0 (16.4, 17.7) | 18.0 (17.2, 18.8) | 18.3 (17.6, 19.0) | 18.4 (17.9, 18.9) |
| Missing | 1.4 (0.3) | 0.7 (0.3) | 2.0 (0.9) | 0.7 (0.3) | 2.0 (0.5) |
| Executive Performanceg | |||||
| Z-score, Mean (95% CI) | 0.35 (0.28, 0.42) | 0.15 (0.03, 0.26) | 0.35 (0.23, 0.47) | 0.49 (0.38, 0.59) | 0.42 (0.32, 0.53) |
| Raw score, Mean (95% CI) | 51.9 (50.6, 53.1) | 48.3 (46.3, 50.3) | 51.8 (49.7, 53.9) | 54.1 (52.3, 55.9) | 53.0 (51.2, 54.8) |
| Missing | 3.2 (0.3) | 5.8 (1.0) | 3.0 (0.7) | 2.0 (0.6) | 1.9 (0.6) |
| Subjective Cognitive Complaints | |||||
| Difficulties thinking/remembering | 13.4 (0.8) | 20.4 (2.2) | 12.2 (1.4) | 10.5 (1.5) | 10.7 (1.3) |
| Missing | 0.1 (0.1) | — | 0.5 (0.4) | — | — |
| Experience confusion/memory loss | 9.6 (0.7) | 14.7 (1.6) | 9.9 (1.3) | 7.4 (1.1) | 6.6 (1.3) |
| Missing | 0.1 (0.0) | 0.1 (0.1) | 0.1 (0.1) | — | — |
Abbreviations: MIMS, Monitor-Independent Movement Summary units; SE, standard error
Measured by PHQ-9: No symptoms (0 score), Minimal symptoms (1-4 score), Mild symptoms (5-9 score), Moderate to severe symptoms (≥10 score)
Measured as ever been told by a doctor that you have high blood pressure, measured systolic blood pressure ≥140 mmHg, or measured diastolic blood pressure ≥90 mmHg
Measured as ever been told by a doctor that you have diabetes, glucose level score ≥126 mg/dL, or HbA1c ≥6.5%
Measured as self-reported some difficulty or greater with walking from one room to another on the same level, getting in or out of bed, eating, or dressing.
Measured via Consortium to Establish a Registry for Alzheimer’s disease (CERAD) Word Learning (CERAD W-L) delayed recall test, max score = 10
Measured via Animal Fluency test
Measured via Digit Symbol Substitution test (DSST)
Domain-Specific Cognitive Function
Associations (β estimates and 95% confidence intervals [CI]) of the movement quartile with domain-specific cognitive assessments are presented in Figure 1 (Model 3 adjusting for age, sex, race/ethnicity, education, income, and depressive symptom severity) and Table S1 (see Appendix, SDC 1, Table 1, linear regression estimates examining MIMS percentile and domain-specific cognitive function z-score). Raw test scores are presented in Table S2 (see Appendix, SDC 1, Table 2, linear regression estimates examining MIMS percentile and domain-specific cognitive function raw test score). Compared to the lowest quartile (Q1: <25th percentile), being in a higher movement quartile was associated with higher memory performance (Figure 1A), language/verbal fluency (Figure 1B), and executive performance (Figure 1C). Being in Q4 compared to Q1 was associated with recalling 0.52 (95 % CI: 0.28, 0.75) more words on the memory test (max score 10), 1.13 (95% CI: 0.47, 1.79) more words on the language/verbal fluency test, and 4.39 (95% CI: 2.38, 6.41) more words on the executive performance test. Similarly, each 10-percentile higher was incrementally associated with higher z-scores (memory: 0.03 [95% CI: 0.02, 0.04], language/verbal fluency: 0.03 [95% CI: 0.01, 0.04], executive performance: 0.04 [0.02, 0.05]). There was a slight attenuation of results when additionally controlling for intermediate vascular risk factors (Model 4), with Q2 memory and executive performance and Q3 language/verbal fluency no longer statistically supported.
Figure 1:
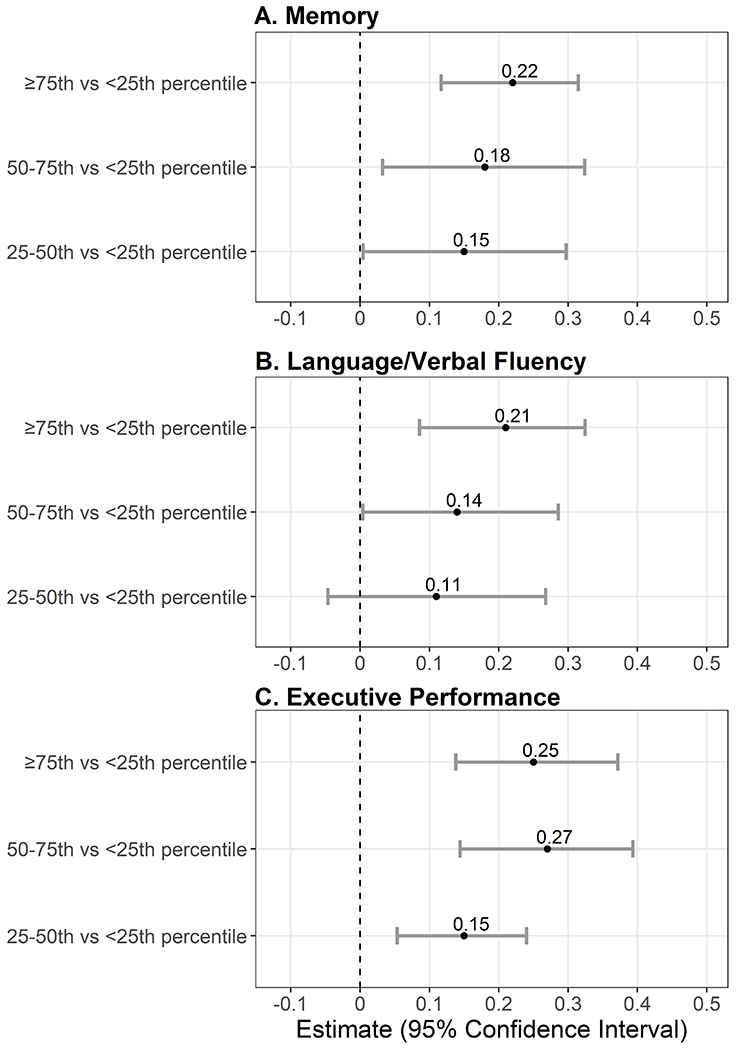
Linear regression models examining MIMS percentile quartile (referent = <25th percentile) and domain-specific cognitive function z-scores (N = 2,708). Panel A, Memory measured via Consortium to Establish a Registry for Alzheimer’s disease (CERAD) Word Learning (CERAD W-L) delayed recall test. Panel B, Language/verbal fluency measured via Animal Fluency test Panel C, Executive performance measured via Digit Symbol Substitution test (DSST). Models are adjusted for age, sex, race/ethnicity, education, income, and depressive symptom severity.
Subjective Cognitive Complaints
For subjective cognitive complaints, 13.4% (SE 0.8) of participants reported difficulties in thinking or remembering and 9.6% (SE 0.7) reported experiencing confusion/memory loss. Associations (β estimates and 95% CI) of the quartile with subjective cognitive complaints are presented in Figure 2 and Table S3 (see Appendix, SDC 1, Table 3, Logistic regression estimates examining MIMS percentile and subjetive cognitive complaints). Being in a higher movement quartile (compared to Q1) was associated with lower odds of reporting difficulties in thinking or remembering (Figure 2A) and experiencing confusion/memory loss (Figure 2B) across all models. Being in Q4 compared to Q1 was associated with a 0.57 (95% CI: 0.37, 0.88) lower odds of reporting difficulties in thinking or remembering and a 0.49 (95% CI: 0.27, 0.91) lower odds of reporting experiencing confusion/memory loss. Similarly, each 10-percentile higher was associated with lower odds of reporting difficulties in thinking or remembering and experiencing confusion/memory loss. There was a slight attenuation of results when additionally controlling for intermediate vascular risk factors (Model 4) for reporting difficulties in thinking or remembering. Reporting experiencing confusion/memory loss was no longer statistically supported in the model adjusted for intermediate vascular risk factors.
Figure 2:
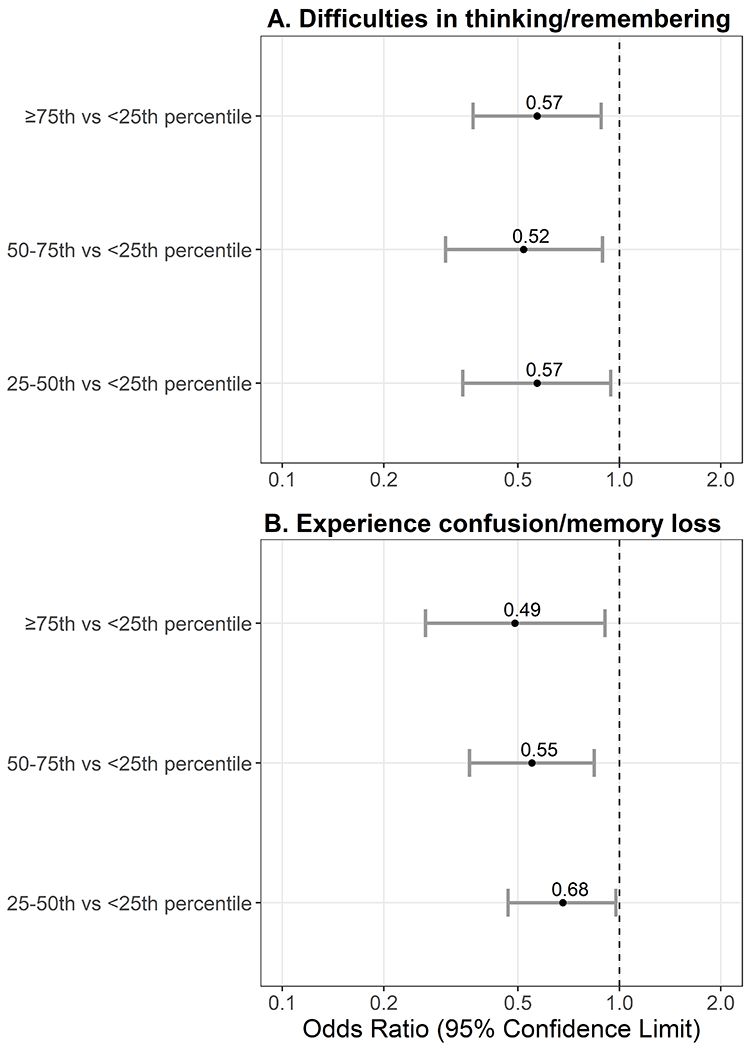
Logistic regression models examining MIMS quartile (referent = <25th percentile) and odds of reporting subjetive cognitive complaints (N = 2,708). Panel A measured by, “Are you limited in any way because of difficulty remembering or because you experience(s) periods of confusion?” Panel B measured by, “During the past 12 months, have you experienced confusion or memory loss that is happening more often or is getting worse?”. Models are adjusted for age, sex, race/ethnicity, education, income, and depressive symptom severity.
Sensitivity Analyses
Inferences were not meaningfully changed when incorporating cognitive scores from the entire NHANES sample ≥60 years, thus we used the analytical sample. However, the analytical sample had higher daily MIMS-units, was more likely to be White, have ≥high school education, experience obesity, and were less likely to have income ≤150% of poverty, self-report physical function difficulty, and have subjective cognitive complaints than those that were not included in these analyses (Table S4, see Appendix, SDC 1, Differences in participant characteristics of the analytical sample and NHANES participants ≥60 years not included in the analyses). The nonparametric regressions models were significant at P < 0.001 and show a similar trend – those in the lowest percentiles for their age and sex performed worse than the mean on all three cognitive measures (Figure 3), thus we present the nonparametric regressions as an additional visual representation of these data but focus our interpretation on the linear estimates.
Figure 3:
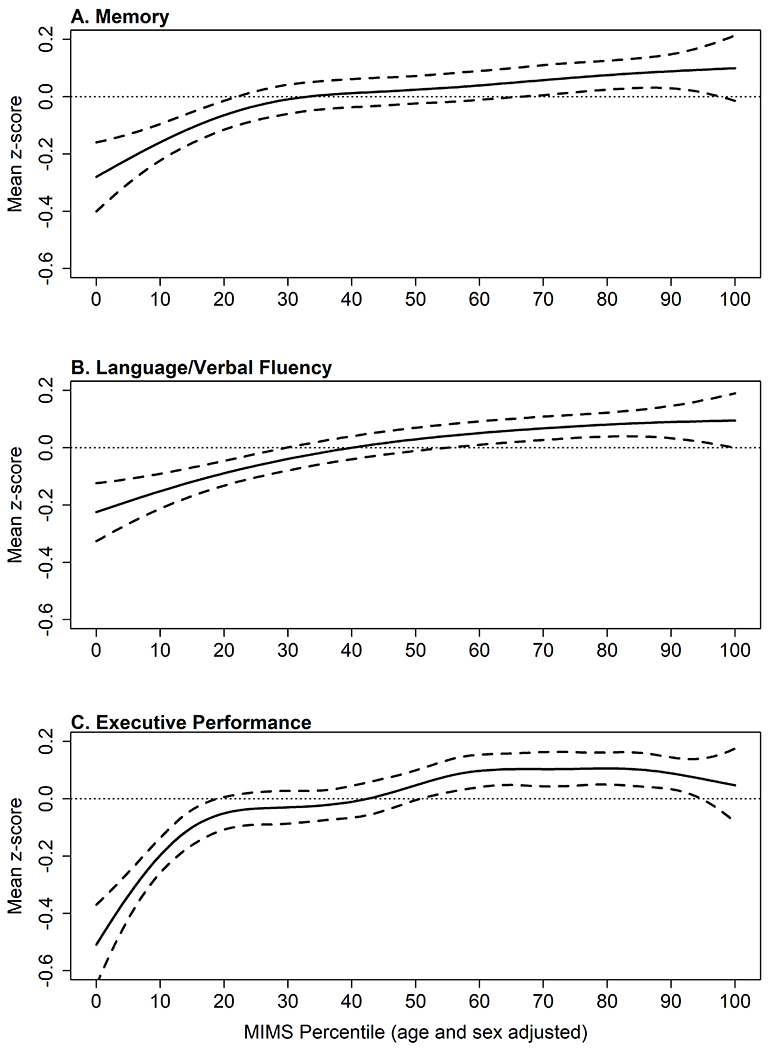
Nonparametic regression models with 95% confidence intervals examining the association of MIMS percentile and change from the domain-specific z-score (N = 2,708). Panel A, Memory measured via Consortium to Establish a Registry for Alzheimer’s disease (CERAD) Word Learning (CERAD W-L) delayed recall test. Panel B, Language/verbal fluency measured via Animal Fluency test Panel C, Executive performance measured via Digit Symbol Substitution test (DSST). Models are adjusted for age, sex, race/ethnicity, education, income, and depressive symptom severity.
DISCUSSION
This study explores the association of age- and sex- related percentile of a wrist-worn 24-hour total movement activity with cognitive function in a nationally representative sample of U.S. older adults. Alike to BMI percentile curves for children, the standardized wrist worn MIMS-percentiles (18) were used in this study to provide unit of comparison relative to others of the same age and sex. We found a direct association of higher cognitive functioning in all domains (memory, language/verbal fluency, executive function/processing speed) in participants classified in movement quartiles three and four (50-74th and ≥75th) compared to participants in the lowest 25th percentile of movement activity after adjustment for age, sex, race/ethnicity, education, income, and depressive symptom severity. Results for Q3 language/verbal fluency were attenuated after controlling for vascular risk factors (Model 4). While there was not a dose-response association, as participants in the third and fourth quartiles had similar estimates, these results do indicate that the associations for higher cognitive functioning appear to be among participants achieving at or above the 50th movement percentile for their age and sex. Further, participants in the 50th percentile or higher were about half as likely to self-report difficulties in thinking/remembering or experiencing confusion/memory loss compared to participants in the 25th percentile or lower.
Although this study used a novel wrist-worn 24-hour movement metric, the results and inferences align with published research on the cognitive benefits of physical activity measured via self-report and through accelerometry. Using the NHANES self-reported physical activity data, researchers found higher executive performance (DSST) scores in more active older adults compared to the least active adults (32). In another NHANES study, every 30 minutes a day spent in MVPA was associated with higher executive performance, language fluency, and global cognition z-scores for older adults sleeping less than 7 hours per night (33). While self-reported activity and MIMS-units are not directly comparable, these findings suggest the higher cognitive functioning can occur in achievable movement doses and do not occur only amongst the most ‘highly active’ groups. This implies that there may be an optimal threshold for daily movement activity for cognitive functioning for older adults. By using a wrist-worn device, this study has several advantages over the previous use of self-reported activity, such as lower potential for recall bias, lower participant burden, and the ability to more accurately quantify 24-hour movement.
Subjective cognitive complaints are associated with AD abnormalities even without changes to cognitive test performance (34) and have been widely used in surveillance studies for cognitive impairment (35). We found that compared to being in the lowest movement quartile, higher quartiles were associated with lower odds of reporting difficulties in thinking or remembering and experiencing confusion/memory loss. In a longitudinal study conducted in China, researchers found higher daily steps were associated with reduced subjective decline (15). Although not directly comparable, these similar findings suggest more daily movement is correlated with lower subjective cognitive decline.
There was a slight attenuation of the measures of association after adjusting for intermediate vascular risk factors with associations examining language/verbal fluency becoming statistically null. This is similar to findings conducted in a cohort of older adults with self-reported leisure time physical activity and 14-year follow-up (36). Vascular risk factors, including hypertension and diabetes, are both potentially a consequence of low 24-hour movement, as well as risk factors for poor cognitive performance. Therefore, it is hypothesized that these vascular risk factors may lie on the causal pathway between 24-hour movement and cognition. Although a formal mediation analysis was beyond the scope of this study, the attenuation in our measures of association after adjustment for vascular risk factors suggest that the association between 24-hour movement and cognition may be partially mediated by vascular risk factors. Future analyses within a causal mediation framework should be conducted to quantify the indirect, direct, and total effects of 24-hour movement and vascular risk factors on cognition.
Supporting total daily movement across the 24-hour paradigm is imperative, particularly with the emerging evidence of the protective health benefits of light intensity physical activity (37, 38). Using cognitive tests, studies have found higher volume of total activity is associated with better executive functioning and verbal memory (39) with benefits for cognitive functioning found at light intensity physical activity (38). This is important given that older adults’ activity is often irregular and performed at lower intensity levels than younger adults (40, 41) and the estimated prevention of over 230 million AD cases in the U.S. with a 25% reduction in the prevalence of physical inactivity (i.e., not meeting physical activity guidelines) (11).
It is hypothesized that higher levels of physical activity may be associated with structural brain measures, including brain volumes, which are associated with cognitive performance. Higher total accelerometry physical activity has been associated with higher fractional anisotropy from cerebral white matter (14) and higher gray matter volume (16) using brain imaging. Physical activity may increase brain volumes, particularly in the hippocampal region, by inducing central and peripheral growth factors and growth factor cascades in the brain (42), such as brain-derived neurotrophic factor (BDNF) and insulin-like growth factor-1 (IGF-1), which can enhance plasticity and neurogenesis. In addition, physical activity may increase blood flow and blood vessel growth in the brain (42) which can improve cognition (43). Other potential mechanisms of this phenomenon include physical activity related reduction of inflammation, which impairs growth factor signaling both systemically and in the brain (42).
Strengths and Limitations
There are several strengths of our study, primarily the use of device-based measures for movement activity and validated assessments of cognitive function across three domains (26–28). Additionally, the use of a large, nationally representative sample of U.S. older adults and inclusion of sample weights and complex sampling procedures allows for nationally representative estimates. Further, the associations remained consistent across subgroups of BMI, hypertension, and diabetes indicating generalizability of these results across chronic disease conditions. This is one of the first studies to include subjective cognitive complaints with 24-hour total movement and standardized percentiles, which is a strength as it may be an earlier marker for prodromal AD and reflect early changes in cognitive function that may not be captured with objective cognitive assessments.
Study limitations include the cross-sectional study design, which does not allow for causal inferences and is potentially subject to reverse causality (44). Changes in brain volume have been found to be associated with total physical activity (45), thus those with higher cognition may be able to perform more movement-based behaviors. Thus, while we chose to use activity as a predictor, cognition could also be used (44). Future research exploring prospective associations across midlife and older adulthood in diverse samples is still needed. Additionally, we are not able to discern the domain or type of activity (i.e., leisure time vs occupational activity), which has been shown to be important for AD (46). However, this is the first study to examine the relation of this novel wrist-worn metric for total movement activity and cognitive function. Although MIMS-units were developed with a non-proprietary, open-source algorithm, to date, there are no validated cut points for these units to be equated to activity intensity thresholds. Thus, we are unable to examine how these movement profiles compare to time spent in specific intensities (i.e., light intensity and MVPA) and, consequently, meeting physical activity recommendations. Despite this, results support the Physical Activity Guidelines for Americans message that some activity is better than none (4). In addition, although participants were instructed to wear the device on their non-dominant wrist, wrist-worn accelerometers capture all wrist accelerations and thus can capture non-functional movement, such as eating and writing, which may not be related to brain health. Due to lack of cut points and age and sex-related MIMS-units difference, we use the derived 24-hour MIMS-percentiles based on these data as our predictor to quantity this movement metric. However, by doing so, we are unable to remove the potential confounding effects of poor sleep (47–49) (e.g., greater nighttime movement) and potentially higher daily MIMS-unit that may result. However, the standardize percentile can provide a reference for clinicians and researchers to use for brain and body health. Finally, although we adjust for several factors that may influence cognitive functioning, the potential for residual confounding, such as genetic risk for AD (e.g., APOE ε4 allele or familial history) or performance-based measures of physical functioning, cannot be eliminated.
CONCLUSIONS
In summary, we found higher percentiles of movement is important for cognitive health in older adults. The greatest associations for higher cognitive functioning and less subjective cognitive complaints occur among those with movement activity ≥50th percentile. These findings further support the importance of movement behaviors to support brain health.
Supplementary Material
SDC 1: Supplemental Digital Content.docx – Appendix
Table S1: Linear regression estimates examining MIMS percentile and domain-specific cognitive function z-score (N = 2,708).
Table S2: Linear regression estimates examining MIMS percentile and domain-specific cognitive function raw test score (N = 2,708).
Table S3: Logistic regression estimates examining MIMS percentile and subjetive cognitive complaints (N = 2,708).
Table S4: Differences in participant characteristics of the analytical sample (N = 2,708) and NHANES participants ≥60 years not included in the analyses.
Figure S1: Flow diagram for analytical sample of study.
Figure S2: Pattern of cognitive test completion of the analytical sample (N = 2,708).
Acknowledgements
We are grateful for the participants of NHANES.
Conflict of Interest and Funding Statement
Some support for this work was provided to EED, PP, and KPG by the Jackson 24H-ACT Study (R01 AG067513 to KPG & PP). The authors declare no conflict of interests. The results of the present study do not constitute endorsement by the American College of Sports Medicine. The findings of this study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Footnotes
This manuscript will undergo copyediting, page composition, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered that could affect the content.
REFERENCES
- 1.Rosenberger ME, Fulton JE, Buman MP, et al. The 24-Hour Activity Cycle: A New Paradigm for Physical Activity. Med Sci Sports Exerc. 2019;51(3):454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pedišić Ž, Dumuid D, S Olds T. Integrating sleep, sedentary behaviour, and physical activity research in the emerging field of time-use epidemiology: definitions, concepts, statistical methods, theoretical framework, and future directions. Kinesiology. 2017;49(2.):252–69. [Google Scholar]
- 3.Falck RS, Davis JC, Khan KM, Handy TC, Liu-Ambrose T. A Wrinkle in Measuring Time Use for Cognitive Health: How should We Measure Physical Activity, Sedentary Behaviour and Sleep? Am J Lifestyle Med. 2021(0):15598276211031495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd ed. Washington, DC: U.S. Department of Health and Human Services; 2018. [Google Scholar]
- 5.Bull FC, Al-Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. WHO guidelines on physical activity and sedentary behaviour. Geneva: World Health Organization, 2020. Licence: CC BY-NC-SA 3.0 IGO. [PubMed] [Google Scholar]
- 7.Saunders TJ, McIsaac T, Douillette K, et al. Sedentary behaviour and health in adults: an overview of systematic reviews. Appl Physiol Nutr Metab. 2020;45(10 (Suppl. 2)):S197–S217. [DOI] [PubMed] [Google Scholar]
- 8.Tremblay MS, Aubert S, Barnes JD, et al. Sedentary Behavior Research Network (SBRN) - Terminology Consensus Project process and outcome. Int J Behav Nutr Phys Act. 2017;14(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. World Report on Ageing and Health: World Health Organization; 2015. [Google Scholar]
- 10.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brookmeyer R, Abdalla N, Kawas CH, Corrada MM. Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States. Alzheimers Dement. 2018;14(2):121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spartano NL, Davis-Plourde KL, Himali JJ, et al. Association of Accelerometer-Measured Light-Intensity Physical Activity With Brain Volume: The Framingham Heart Study. JAMA Netw Open. 2019;2(4):e192745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian Q, Glynn NW, Erickson KI, et al. Objective measures of physical activity, white matter integrity and cognitive status in adults over age 80. Behav Brain Res. 2015;284:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen ST, Stevinson C, Tian T, Chen LJ, Ku PW. Accelerometer-measured daily steps and subjective cognitive ability in older adults: A two-year follow-up study. Exp Gerontol. 2020;133:110874. [DOI] [PubMed] [Google Scholar]
- 16.Hamer M, Sharma N, Batty GD. Association of objectively measured physical activity with brain structure: UK Biobank study. J Intern Med. 2018;284(4):439–43. [DOI] [PubMed] [Google Scholar]
- 17.Jefferis BJ, Sartini C, Lee IM, et al. Adherence to physical activity guidelines in older adults, using objectively measured physical activity in a population-based study. BMC Public Health. 2014;14:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belcher BR, Wolff-Hughes DL, Dooley EE, et al. U.S. Population-referenced Percentiles for Wrist-Worn Accelerometer-derived Activity. Med Sci Sports Exerc. 2021;53(11):2455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watson KB, Carlson SA, Carroll DD, Fulton JE. Comparison of accelerometer cut points to estimate physical activity in US adults. J Sports Sci. 2014;32(7):660–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raiber L, Christensen RAG, Randhawa AK, Jamnik VK, Kuk JL. Do moderate- to vigorous-intensity accelerometer count thresholds correspond to relative moderate- to vigorous-intensity physical activity? Appl Physiol Nutr Metab. 2019;44(4):407–13. [DOI] [PubMed] [Google Scholar]
- 21.Schrack JA, Leroux A, Fleg JL, et al. Using Heart Rate and Accelerometry to Define Quantity and Intensity of Physical Activity in Older Adults. J Gerontol A Biol Sci Med Sci. 2018;73(5):668–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS). National Health and Nutrition Examination Physical Activity Monitor (PAM) Procedures Manual 2011-2012 [cited 2021 6]. Available from: https://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/Physical_Activity_Monitor_Manual.pdf.
- 23.Johnson CL, Dohrmann SM, Burt VL, Mohadjer LK. National Health and Nutrition Examination Survey: Sample Design, 2011-2014: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2014. [Google Scholar]
- 24.John D, Tang Q, Albinali F, Intille S. An Open-Source Monitor-Independent Movement Summary for Accelerometer Data Processing. J Meas Phys Behav. 2019;2(4):268–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolff-Hughes DL, McClain JJ, Dodd KW, Berrigan D, Troiano RP. Number of accelerometer monitoring days needed for stable group-level estimates of activity. Physiol Meas. 2016;37(9):1447. [DOI] [PubMed] [Google Scholar]
- 26.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159–65. [DOI] [PubMed] [Google Scholar]
- 27.Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms and Commentary. 3rd ed. New York: Oxford University Press; 2006. [Google Scholar]
- 28.Wechsler D WAIS Manual – Third Edition. New York: Psychological Corporation; 1997. [Google Scholar]
- 29.Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psych Annals. 2002(32):509–21. [Google Scholar]
- 30.Wood SN. Generalized additive models: an introduction with R: CRC press; 2017. [Google Scholar]
- 31.Rothman KJ. No Adjustments Are Needed for Multiple Comparisons. Epidemiology. 1990;1(1):43–6. [PubMed] [Google Scholar]
- 32.Loprinzi PD, Edwards MK, Crush E, Ikuta T, Del Arco A. Dose-Response Association Between Physical Activity and Cognitive Function in a National Sample of Older Adults. Am J Health Promot. 2018;32(3):554–60. [DOI] [PubMed] [Google Scholar]
- 33.Wei J, Hou R, Xie L, et al. Sleep, sedentary activity, physical activity, and cognitive function among older adults: The National Health and Nutrition Examination Survey, 2011-2014. J Sci Med Sport. 2021;24(2):189–94. [DOI] [PubMed] [Google Scholar]
- 34.Jessen F, Amariglio RE, van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 2014;10(6):844–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernstein AB, Remsburg RE. Estimated Prevalence of People With Cognitive Impairment: Results From Nationally Representative Community and Institutional Surveys. Gerontologist. 2007;47(3):350–4. [DOI] [PubMed] [Google Scholar]
- 36.Palta P, Sharrett AR, Deal JA, et al. Leisure-time physical activity sustained since midlife and preservation of cognitive function: The Atherosclerosis Risk in Communities Study. Alzheimers Dement. 2019;15(2):273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LaMonte MJ, Lewis CE, Buchner DM, et al. Both light intensity and moderate‐to‐vigorous physical activity measured by accelerometry are favorably associated with cardiometabolic risk factors in older women: the Objective Physical Activity and Cardiovascular Health (OPACH) study. J Am Heart Assoc. 2017;6(10):e007064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stubbs B, Chen L-J, Chang C-Y, Sun W-J, Ku P-W. Accelerometer-assessed light physical activity is protective of future cognitive ability: A longitudinal study among community dwelling older adults. Exp Gerontol. 2017;91:104–9. [DOI] [PubMed] [Google Scholar]
- 39.Spartano NL, Demissie S, Himali JJ, et al. Accelerometer-determined physical activity and cognitive function in middle-aged and older adults from two generations of the Framingham Heart Study. Alzheimers Dement 2019;5:618–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harada ND, Chiu V, King AC, Stewart AL. An evaluation of three self-report physical activity instruments for older adults. Med Sci Sports Exerc. 2001;33(6):962–70. [DOI] [PubMed] [Google Scholar]
- 41.Westerterp KR. Physical activity as determinant of daily energy expenditure. Physiol Behav. 2008;93(4-5):1039–43. [DOI] [PubMed] [Google Scholar]
- 42.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–72. [DOI] [PubMed] [Google Scholar]
- 43.Pereira AC, Huddleston DE, Brickman AM, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104(13):5638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stillman CM, Erickson KI. Physical activity as a model for health neuroscience. Ann N Y Acad Sci. 2018;1428(1):103–11. [DOI] [PubMed] [Google Scholar]
- 45.Arnardottir NY, Koster A, Domelen DRV, et al. Association of change in brain structure to objectively measured physical activity and sedentary behavior in older adults: Age, Gene/Environment Susceptibility-Reykjavik Study. Behav Brain Res. 2016;296:118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stephen R, Hongisto K, Solomon A, Lönnroos E. Physical Activity and Alzheimer’s Disease: A Systematic Review. J Gerontol A Biol Sci Med Sci. 2017;72(6):733–9. [DOI] [PubMed] [Google Scholar]
- 47.Alfini A, Albert M, Faria AV, et al. Associations of actigraphic sleep and circadian rest/activity rhythms with cognition in the early phase of Alzheimer’s disease. Sleep Adv. 2021;2(1):zpab007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bubu OM, Brannick M, Mortimer J, et al. Sleep, Cognitive impairment, and Alzheimer’s disease: A Systematic Review and Meta-Analysis. Sleep. 2016;40(1). [DOI] [PubMed] [Google Scholar]
- 49.Itani O, Jike M, Watanabe N, Kaneita Y. Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med. 2017;32:246–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SDC 1: Supplemental Digital Content.docx – Appendix
Table S1: Linear regression estimates examining MIMS percentile and domain-specific cognitive function z-score (N = 2,708).
Table S2: Linear regression estimates examining MIMS percentile and domain-specific cognitive function raw test score (N = 2,708).
Table S3: Logistic regression estimates examining MIMS percentile and subjetive cognitive complaints (N = 2,708).
Table S4: Differences in participant characteristics of the analytical sample (N = 2,708) and NHANES participants ≥60 years not included in the analyses.
Figure S1: Flow diagram for analytical sample of study.
Figure S2: Pattern of cognitive test completion of the analytical sample (N = 2,708).


