Abstract
Background:
Tuberculosis (TB) is a leading cause of illness and death in children globally. Improved bacteriologic and clinical diagnostic approaches in children are urgently needed.
Methods:
In a prospective cohort study, a consecutive series of young (<5 years) children presenting with symptoms suggestive of TB and parenchymal abnormality on chest radiograph to inpatient and outpatient settings in Kisumu County, Kenya during October 2013 – February 2016 were evaluated at baseline and over six months. Up to 14 specimens per child were tested for Mycobacterium tuberculosis complex by fluorescence microscopy, Xpert MTB/RIF, and mycobacterial culture. Using detailed clinical characterization, cases were retrospectively classified according to standardized research case definitions and sensitivity and specificity of microbiological tests on different specimen types were determined.
Results:
Among 300 young children enrolled, 266 had sufficient information to be classified according to the research clinical case definition. Of these, 36% (96/266) had TB disease; 32% (31/96) with bacteriologically confirmed intrathoracic TB. Compared to culture, sensitivity of a single Xpert test ranged from 60-67% and specificity from 97.5-100% for different specimen types.
Conclusions:
Despite extensive specimen collection and laboratory testing, TB could not be bacteriologically confirmed in almost two-thirds of children with intrathoracic TB classified by research clinical case definitions. Improved diagnostic tests are needed to identify children with TB and to exclude other potential causes of illness.
Keywords: tuberculosis, children, diagnosis, Xpert MTB/RIF
Introduction
Globally, tuberculosis (TB) is estimated to cause disease in approximately 1.2 million children and accounts for more than 230,000 deaths in children every year.(1) Most of these deaths are in young children (<5 years) who have not been diagnosed and treated.(2) There are two key limitations to diagnosing TB in children. The first is achieving a bacteriologic confirmation of TB disease using currently available tests. The second is accurately diagnosing TB that is not confirmed bacteriologically but by clinical judgement alone (“clinical TB disease”). Jointly addressing these limitations may hasten time to treatment initiation and decrease mortality among this vulnerable population.
Sputum smear microscopy, a primary diagnostic tool for pulmonary TB in adults, has limited utility in young children, who often have paucibacillary disease and cannot expectorate sputum on demand. Instead, invasive specimens such as gastric aspirate (GA) or induced sputum (IS) are recommended for bacteriologic testing but are rarely collected in resource-limited settings.(3). Even when invasive sampling is performed, the reference standard of mycobacterial culture performed on GA provides bacteriological confirmation in only 30-40% of children clinically diagnosed with TB.(4) The rapid molecular test Xpert MTB/RIF (Xpert; Cepheid, Sunnyvale CA, USA) provides an alternative to mycobacterial culture. Xpert performed on IS or GA from children <5 years has reasonable sensitivity (53-57%) and excellent specificity (98%) compared to culture and is recommended by WHO as a first-line test in children.(5, 6)
In practice diagnosis of TB in young children relies overwhelmingly on clinical diagnosis using clinical and radiologic findings supported by ancillary information such as history of TB contact or immunologic evidence of infection.(7) However, clinical manifestations of TB in children are generally non-specific, making accurate clinical diagnoses challenging.(8) In addition, chest radiograph findings are variable and commonly non-specific, with poor inter-reader concordance.(9) It is widely recognized that a large but uncertain proportion of TB in young children goes undiagnosed and untreated.(10)
In this large cohort of young children with persistent symptoms suggestive of TB disease, we aimed to systematically evaluate specimens and bacteriologic tests for diagnosis of TB disease and clinical characteristics of children classified as having or not having TB disease. We first examined the performance of Xpert and mycobacterial culture across various specimen types for diagnosis of TB disease. Second, we used standardized diagnostic research case definitions to provide detailed clinical characterization of children in this cohort who have bacteriologically-confirmed TB, those who meet a composite case definition of TB disease but in whom bacteriologic confirmation was not achieved despite rigorous testing, or those who are unlikely to have TB.
Methods
Study participants:
In this prospective diagnostic cohort study, we recruited a consecutive series of children <5 years of age from inpatient and outpatient settings in Kisumu County, Kenya, between October 2013 and August 2015 as detailed previously (Supplemental Materials).(11) Briefly, children were eligible for enrolment who had persistent clinical features (fever, cough, malnutrition despite treatment for other potential causes) plus lung parenchymal abnormality on chest radiograph, if they had persistent cervical lymphadenopathy, or if they had been diagnosed with TB elsewhere. Children were excluded from the study if they were on TB treatment or isoniazid preventive therapy (IPT) or had received treatment for TB in the past year or IPT in the last 6 months. This study was approved by the institutional review boards (IRB) of the U.S. Centers for Disease Control and Prevention (CDC), the Kenya Medical Research Institute, and the Jaramogi Oginga Odinga Teaching and Referral Hospital. Children’s Hospital Boston/Harvard Medical School relied on the review and oversight of the CDC IRB. Written informed consent was obtained from parents or legal guardians of participants.
Specimen collection, bacteriologic testing, and clinical care:
Up to two of each of the following specimens were collected at baseline from each participant and tested by 3 different methods: 1) fluorescence microscopy for acid-fast bacilli, 2) liquid culture by mycobacteria growth indicator tube (MGIT 960, Beckton Dickinson, Franklin Lakes, New Jersey), and 3) Xpert: nasopharyngeal aspirate (NPA), IS, neutralized GA, string test, urine and stool. Lymph node fine needle aspiration biopsy (FNAB) was performed in children with enlarged cervical lymph nodes. In a subset of children, one blood specimen was collected per child and tested by liquid culture (Myco/F Lytic bottles; Becton Dickinson, Franklin Lakes, NJ). Cultures demonstrating growth consistent with Mycobacterium species were identified using the MGIT TBc Identification Test (Beckton Dickinson, Franklin, NJ) and the Genotype Mycobacterium MTBC or CM line probe assay (Hain LifeScience, Nehren, Germany). Xpert and liquid culture were index tests as well as reference tests as part of the case definition for confirmed TB. Laboratory staff had access to index and reference tests but not to clinical data. Detailed specimen collection, processing, and testing techniques are described in Supplemental Materials. Children were treated for TB or provided isoniazid preventive therapy according to the decision of primary clinicians and TB clinic personnel following Kenya national TB diagnosis and treatment guidelines for children.(12) In-person review by study clinicians was done at two weeks, two months, and 6 months after enrolment as recommended for retrospective research clinical case classification.(13)
Chest radiography:
Analog chest radiographs were reviewed by study clinicians and for 143/300 (48%) participants digital chest radiographs (anteroposterior and lateral) were reviewed by a general radiologist to assess them for evidence of lung parenchymal abnormality for enrollment. Retrospective expert reading of digital films was performed by four independent, blinded reviewers. Films were evaluated for quality sufficient for interpretation and final readings were “abnormal–likely TB,” “abnormal–equivocal,” or “normal.” If any abnormalities were present, even if not concerning for TB, radiographs were given a reading of “abnormal-equivocal.” If final readings by two reviewers were discordant, a third reader served as tiebreaker; for three-way ties no final determination was made.
HIV status determination and immunological staging:
History of HIV testing, including results, and antiretroviral treatment (ART) was obtained from caregivers. HIV testing was performed according to Kenya national guidelines.(14, 15) Immunological staging at enrollment by CD4 percentage was performed according to WHO guidelines.(16)
Nutritional assessment:
Nutritional status was assessed using the anthropomorphic indicators weight-for-height (WFH) and weight-for-age (WFA), based on the uniform growth standards for children developed by WHO.(17) Standardized z-scores were calculated using the R-programming package “anthro,” provided by WHO. (18)
TST and IGRA:
TST and QuantiFERON-TB Gold Assay (QFT-GIT, Qiagen, Hilden, Germany) were performed as described in Supplemental Materials. Positive TST was defined as an induration of ≥10mm for HIV-negative and ≥5mm for HIV-positive children and children with moderate or severe malnutrition (WFA or WFH z-score ≤ − 2).(19)
Case classification
We used standard research case definitions that are applied retrospectively and independent of real-time clinical diagnosis and treatment decisions and as detailed in Supplemental Materials (Graham, 2012, 2015). Clinical trajectory was assessed from 2-month follow-up data. Briefly, confirmed TB was defined as having a positive bacteriologic test result on any respiratory specimen; unconfirmed TB as meeting two or more criteria suggestive of TB (specific symptoms, TB exposure, immunologic evidence of TB infection, or response to TB treatment); unlikely TB as not meeting criteria for confirmed TB or unconfirmed TB. Children who met criteria for unconfirmed TB according to baseline data but who were not started on TB treatment and had resolution of symptoms in the absence of treatment had final classification of unlikely TB.
Statistical methods
Frequencies and proportions or medians and interquartile ranges (IQR) were used to describe characteristics of study participants, stratified by clinical case category, for categorical and continuous variables, respectively. Sensitivity of bacteriologic tests performed on each specimen type was determined relative to a composite reference standard classification of having TB disease (confirmed TB + unconfirmed TB).(20, 21) For this analysis, the number of children with a valid test result was considered for each specimen type and test type (smear, Xpert, MGIT). Sensitivity and specificity were determined for Xpert relative to MGIT. Invalid Xpert results (error or indeterminate) and MGIT results showing contamination or non-tuberculous mycobacteria were excluded from measures of performance. We used the binomial Wilson score method to calculate the confidence intervals for sensitivity and specificity.
Results
A total of 300 symptomatic children were enrolled. Most children were enrolled based on meeting symptom (fever, cough, and/or malnutrition) and radiography criteria; 13 children had lymphadenopathy but most (10/13, 77%) of these children had additional symptoms or positive bacteriologic results from a respiratory specimen. Among 266 children with sufficient information for retrospective case classification, 31 (12%) had confirmed TB, 65 (24%) had unconfirmed TB, and 170 (64%) had unlikely TB (Figure S3).
Sensitivity and specificity of different specimen types and tests for bacteriologic diagnosis
Among all children with TB disease (confirmed TB and unconfirmed TB), smear microscopy sensitivity was poor (<10%; Table 1) across all specimen types. Sensitivity of a single Xpert was comparable for GA (14.9%, 95%CI: 9.1-23.5), NPA (14.6%, 95%CI: 8.9-23.0) and stool (14.4%, 95%CI: 8.6-23.2); because confirmed TB was defined by positive Xpert or culture, specificity was by definition 100%. While lymph node FNAB was indicated for few children (n=13), Xpert sensitivity was relatively high (40.0%, 95%CI: 11.8-76.9). Sensitivity was improved by testing up to two samples of each specimen type (Table 1). Relative to culture, sensitivity of a single Xpert ranged from 60-100% and specificity from 97.5%-100% for different specimen types (Table 2). Reasons for exclusion of results from sensitivity and specificity calculations are detailed in Table S4.
Table 1.
Sensitivity of tests of different specimens in children with TB disease (confirmed TB and unconfirmed TB)
| Test and Specimen Type |
One Sample | Up to Two Samples | ||||||
|---|---|---|---|---|---|---|---|---|
| Positive | N* | Sensitivity | Positive | N** | Sensitivity | |||
| Xpert | % | (95% CI) | % | (95% CI) | ||||
| Gastric aspirate | 14 | 94 | 14.9 | (9.1 – 23.5) | 16 | 94 | 17.0 | (10.8 – 25.9) |
| String | 9 | 93 | 9.7 | (5.2 – 17.4) | 11 | 93 | 11.8 | (6.7 – 19.9) |
| Induced sputum | 11 | 91 | 12.1 | (6.9 – 20.4) | 14 | 91 | 15.4 | (9.4 - 24.2) |
| NPA | 14 | 96 | 14.6 | (8.9 – 23.0) | 17 | 96 | 17.7 | (11.4 – 26.5) |
| Stool | 13 | 90 | 14.4 | (8.6 – 23.2) | 13 | 91 | 14.3 | (8.5 - 22.9) |
| Urine | 4 | 89 | 4.5 | (1.8 - 11.0) | 4 | 89 | 4.5 | (1.8 - 11.0) |
| FNAB | 2 | 5 | 40.0 | (11.8 – 76.9) | 2 | 5 | 40.0 | (11.8 – 76.9) |
| Liquid culture (MGIT) | ||||||||
| Gastric aspirate | 21 | 88 | 23.9 | (16.2 – 33.7) | 22 | 93 | 23.7 | (16.2 - 33.2) |
| String | 10 | 87 | 11.5 | (6.4 – 19.9) | 12 | 91 | 13.2 | (7.7 - 21.6) |
| Induced sputum | 12 | 86 | 14.0 | (8.2 – 22.8) | 19 | 91 | 20.9 | (13.8 - 30.3) |
| NPA | 21 | 83 | 25.3 | (17.2 – 35.6) | 22 | 93 | 23.7 | (16.2 - 33.2) |
| Stool | 7 | 77 | 9.1 | (4.5 – 17.6) | 7 | 84 | 8.3 | (4.1 - 16.2) |
| Urine | 2 | 78 | 2.6 | (0.7 – 8.9) | 2 | 83 | 2.4 | (0.7 – 8.4) |
| Blood | 1 | 30 | 3.3 | (0.6 – 16.7) | N/A | N/A | ||
| FNAB | 3 | 5 | 60.0 | (23.1 – 88.2) | N/A | N/A | ||
| Smear microscopy for AFB | ||||||||
| Gastric aspirate | 5 | 94 | 5.3 | (2.3 – 11.9) | 6 | 94 | 6.4 | (3.0 - 13.2) |
| String | 2 | 93 | 2.2 | (0.6 – 7.5) | 3 | 93 | 3.2 | (1.1 - 9.1) |
| Induced sputum | 4 | 91 | 4.4 | (1.7 – 10.8) | 4 | 91 | 4.4 | (1.7 - 10.8) |
| NPA | 6 | 96 | 6.3 | (2.9 - 13.0) | 6 | 96 | 6.3 | (2.9 - 13.0) |
| Stool | 4 | 90 | 4.4 | (1.7 – 10.9) | 4 | 90 | 4.4 | (1.7 - 10.9) |
| Urine | 1 | 90 | 1.1 | (0.2 – 6.0) | 1 | 90 | 1.1 | (0.2 - 6.0) |
Number of children with valid results for test performed on first specimen collected.
Number of children with valid results for first or second specimen collected
Xpert invalid results (error or indeterminate) and MGIT results showing contamination or nontuberculous mycobacteria were excluded.
AFB = acid fast bacteria, MGIT = mycobacterial growth indicator tube
FNAB = fine needle aspirate biopsy; NPA = nasopharyngeal aspirate
Respiratory specimens were collected overnight in the order NPA, IS, GA, string test.
Table 2.
Performance of specimens tested by Xpert relative to culture in children
| Sensitivity | Specificity | |||||||
|---|---|---|---|---|---|---|---|---|
| Specimen type | Positive | MGIT Pos |
% | (95% CI) | Negative | N** | % | (95% CI) |
| One specimena | ||||||||
| Gastric aspirate | 13 | 21 | 61.9 | (40.9 - 79.2) | 251 | 252 | 99.6 | (97.8 - 99.9) |
| String | 6 | 10 | 60 | (31.3 - 83.2) | 258 | 260 | 99.2 | (97.2 - 99.8) |
| Induced sputum | 8 | 12 | 66.7 | (39.1 - 86.2) | 256 | 257 | 99.6 | (97.8 - 99.9) |
| Nasopharyngeal aspirate | 13 | 21 | 61.9 | (40.9 - 79.2) | 245 | 246 | 99.6 | (97.7 - 99.9) |
| Stool | 6 | 7 | 85.7 | (48.7 - 97.4) | 230 | 236 | 97.5 | (94.6 - 98.8) |
| Urine | 2 | 2 | 100 | (34.2 - 100.0) | 231 | 233 | 99.1 | (96.9 - 99.8) |
| FNAB | 2 | 3 | 66.7 | (20.8 - 93.9) | 10 | 10 | 100 | (72.2 - 100.0) |
| Up to two specimens | ||||||||
| Gastric aspirate | 14 | 22 | 63.6 | (43.0 - 80.3) | 267 | 269 | 99.3 | (97.3 - 99.8) |
| String | 8 | 12 | 66.7 | (39.1 - 86.2) | 265 | 267 | 99.3 | (97.3 - 99.8) |
| Induced sputum | 13 | 19 | 68.4 | (46.0 - 84.6) | 264 | 265 | 99.6 | (97.9 - 99.9) |
| Nasopharyngeal aspirate | 15 | 22 | 68.2 | (47.3 - 83.6) | 269 | 270 | 99.6 | (97.9 - 99.9) |
| Stool | 6 | 7 | 85.7 | (48.7 - 97.4) | 257 | 263 | 97.7 | (95.1 - 99.0) |
| Urine | 2 | 2 | 100 | (34.2 - 100.0) | 240 | 242 | 99.2 | (97.0 - 99.8) |
| FNAB | 2 | 5 | 40.0 | (11.8 - 76.9) | 6 | 6 | 100.0 | (61.0 - 100.0) |
Number of children with positive MGIT result on first specimen collected.
Number of children with valid MGIT result on first and/or second specimen collected. MGIT results that were contaminated or showed mycobacteria other than tuberculosis were excluded
FNAB = fine needle aspirate biopsy, MGIT = mycobacterial growth indicator tube
Clinical case definitions
Data informing bacteriologic confirmation, clinical symptoms, and history of close TB contact were available for all children. Digital chest radiograph readings were available for 270 (90%) and results for immunological evidence of TB infection for 283 (94%) children. Sufficient two-month follow-up data to assess the clinical disease trajectory were available for 269 (90%) children.
A total of 31/300 (10%) children met criteria for confirmed TB with a positive bacteriologic test for M. tuberculosis complex (Xpert and/or MGIT) on a respiratory specimen (IS, GA, NPA, string test, stool). Five children had at least one specimen positive for non-tuberculous mycobacteria (NTM) but none met criteria for pulmonary NTM disease (see Supplemental Materials). A total of 43/266 (17%) children had a chest radiograph read as “likely TB,” 59/283 (21%) had immunological evidence of TB infection (positive IGRA and/or positive TST), 292/300 (97%) had at least one case definition symptom, and 89/300 (30%) had history of close TB contact. Among the 85/300 (28%) participants who initiated TB treatment, sufficient follow-up data at 60 days were available for 74 (87%); 43 (58%) had complete and 14 (19%) had partial or no resolution of symptoms; 3 (4%) did not have case definition symptoms at baseline, and 14 (19%) died.
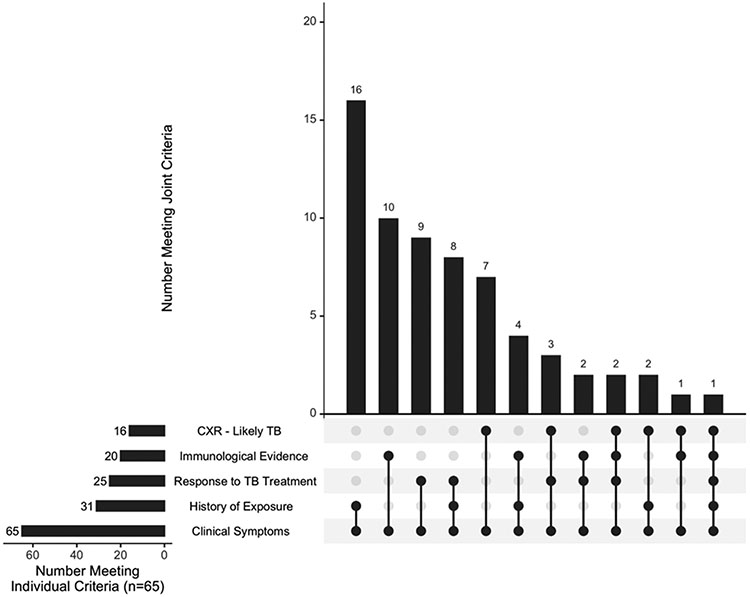
In total, 96/266 (36%) children were classified as having TB disease (confirmed plus unconfirmed TB) (Table 3).(19) One child had bacteriologic confirmation from a non-pulmonary sample (urine) only and was categorized as unconfirmed TB because the case definitions are for intrathoracic TB and require a positive respiratory specimen for confirmed TB. A total of 34/300 (11%) participants were “unclassifiable” due to missing information (Table S3). All 65 children with unconfirmed TB satisfied the symptom criterion; the most common criterion met in addition was history of recent close TB contact (n=16, 25%), immunological evidence of TB infection (n=10, 15%), and response to TB treatment (n=9, 14%) (Figures 1 and S1, Table S1).
Table 3.
Characteristics of study participants by clinical case category
| Characteristic | Total (n=266) |
confirmed TB (n=31) |
unconfirmed TB (n=65) |
unlikely TB (n=170) |
|---|---|---|---|---|
| Age (years), median (IQR) | 2.1 (1.1, 3.7) | 1.9 (1.0, 3.3) | 2.0 (0.9, 3.3) | 2.3 (1.3, 3.9) |
| Female Sex, n (%) | 135 (51) | 17 (55) | 34 (52) | 84 (49) |
| Bacteriological confirmation a | 32 (12) | 31 (100) | 1 (2) | 0 (0) |
| Symptoms/Signs b | 259 (97) | 28 (90) | 65 (100) | 166 (98) |
| Cough only | 115 (44) | 10 (36) | 27 (42) | 78 (47) |
| Fever only | 28 (11) | 1 (4) | 6 (9) | 21 (13) |
| Malnutrition only | 8 (3) | 0 (0) | 2 (3) | 6 (4) |
| Cough and Fever | 62 (24) | 8 (29) | 16 (25) | 38 (23) |
| Cough and Malnutrition | 16 (6) | 2 (7) | 7 (11) | 7 (4) |
| Fever and Lethargy | 5 (2) | 1 (4) | 1 (2) | 3 (2) |
| Cough, Fever, and Lethargy | 14 (5) | 3 (11) | 2 (3) | 9 (5) |
| Fever, Malnutrition, and Lethargy | 2 (1) | 1 (4) | 0 (0) | 1 (1) |
| Cough, Fever and Malnutrition | 2 (1) | 0 (0) | 1 (2) | 1 (1) |
| Cough, Fever, Malnutrition and Lethargy | 7 (3) | 2 (7) | 3 (5) | 2 (1) |
| History of TB exposure c | 85 (32) | 24 (77) | 31 (48) | 30 (18) |
| Chest Radiographs d | 254 (95) | 30 (97) | 59 (91) | 165 (97) |
| Consistent with TB | 43 (17) | 13 (43) | 16 (27) | 14 (8) |
| Abnormal - Equivocal | 145 (57) | 12 (40) | 30 (51) | 103 (62) |
| Normal | 35 (14) | 1 (3) | 9 (15) | 25 (15) |
| Discordant* | 31 (12) | 4 (13) | 4 (7) | 23 (14) |
| Initiated TB Treatment | 66 (25) | 31 (100) | 29 (45) | 6 (4) |
| Responded to TB Treatmente | 43 (75) | 17 (74) | 25 (86) | 1 (20) |
| Immunologic evidence of TB Infection e | 58 (22) | 21 (75) | 20 (33) | 17 (10) |
| Both TST and IGRA positive | 23 (40) | 12 (57) | 3 (15) | 8 (47) |
| Only TST positive | 24 (41) | 3 (14) | 12 (60) | 9 (53) |
| Only IGRA positive | 11 (19) | 6 (29) | 5 (25) | 0 (0) |
| Nutrition Status at Enrollment | 266 (100) | 31 (100) | 65 (100) | 170 (100) |
| WFA z score from −2 to −3 | 40 (15) | 5 (16) | 13 (20) | 22 (13) |
| WFA z score less than −3 | 65 (24) | 8 (26) | 22 (34) | 35 (21) |
| WFH z score from −2 to −3 | 31 (12) | 5 (16) | 9 (14) | 17 (10) |
| WFH z score less than −3 | 54 (20) | 8 (26) | 15 (23) | 31 (18) |
| HIV Status Known | 262 (98) | 29 (94) | 64 (98) | 169 (99) |
| HIV Positive | 64 (24) | 7 (24) | 24 (38) | 33 (20) |
| HIV Exposed Uninfectedg | 96 (37) | 11 (38) | 33 (52) | 52 (31) |
| Immunological Status Known (among HIV+) | 58 (91) | 6 (86) | 20 (83) | 32 (97) |
| Severe Immunosuppression | 28 (48) | 3 (50) | 9 (45) | 16 (50) |
| Moderate Immunosuppression | 8 (14) | 1 (17) | 4 (20) | 3 (9) |
| Mild Immunosuppression | 11 (19) | 2 (33) | 2 (10) | 7 (22) |
| No Immunosuppression | 11 (19) | 0 (0) | 5 (25) | 6 (19) |
One participant had a positive Xpert result from a non-respiratory specimen (urine) only. The 2012 and 2015 clinical case definitions are for classification of intrathoracic TB and specify that a confirmed case have at least one positive bacteriologic test result from a respiratory specimen.
No children had only lethargy or only lethargy and malnutrition as symptoms
Defined according to 2012 standardized guidelines as close contact in the past 2 years
Includes chest radiograph readings unresolved due to quality issues and/or three-way ties among readers
Not all patients initiating treatment had follow up data, n=57 (86%); confirmed TB: n=23/31 (74%); unconfirmed TB: n=29/29 (100%); unlikely TB: n=5/6 (83%)
Not all patients had immunological data, n=258 (96%); confirmed TB: n=28; unconfirmed TB: n=61; unlikely TB: n=169. In instances where only TST or IGRA was positive the other test may be negative or missing.
Exposure to HIV in utero. Not all patients had HIV exposure data; n=246 (92%); confirmed TB: n=27; unconfirmed TB: n=61; unlikely TB n=158
Discordant permutations detailed in Supplementary Table S5
Figure 1:
Criteria for final classification as unconfirmed TB, by individual and joint criteria
A total of 232/266 (87%) children with assigned case definition had sufficient follow-up data to assess their clinical trajectory. Among these, 66/232 (28%) initiated treatment of whom 57 had sufficient follow-up to assess response; 43 (75%) had full, 10 (18%) partial, and 4 (7%) no symptom resolution after 60 days of TB treatment. Of the remaining 175 participants who did not initiate TB treatment, 103 (59%) had full, 51 (29%) partial, and 21 (12%) no symptom resolution after 60 days of follow-up. Initially 114/266 (43%) children met criteria for unconfirmed TB at baseline, but 49/114 (43%) had resolution of symptoms without TB treatment and were therefore reclassified as unlikely TB. Symptoms and TB exposure history were the most common criteria met by children reclassified from unconfirmed TB to unlikely TB (Figure S2). A total of 29/114 classified as unconfirmed TB at baseline-initiated treatment and were not eligible for reclassification.
Clinical characteristics and outcomes
Among children for whom there was enough information for classification, 259/266 (97%) met case definition symptom criteria: 216/266 (83%) for persistent cough (“cough”), 120/266 (46%) for persistent unexplained fever (“fever”), 35/266 (14%) for failure to thrive (“malnutrition”) and 28/266 (11%) for persistent unexplained lethargy (“lethargy”) (Table S1).
Among children with results available, close TB contact was reported for 24/31 (77%) children with confirmed TB, 31/65 (48%) with unconfirmed TB and 30/170 (18%) with unlikely TB. Immunological evidence of TB infection was found in 21/28 (75%) of children with confirmed TB, 20/61 (33%) with unconfirmed TB and 17/169 (12%) with unlikely TB. Chest radiograph findings were read as “likely TB” for 13/31 (43%) with confirmed TB, 16/65 (27%) with unconfirmed TB and 14/170 (8%) with unlikely TB. Characteristics of children in each case definition category were compared for those with and without immunological evidence of infection (Table S2).
Thirty of 300 (10%) children died; by case definition the proportion who died were 4/31 (13%) children with confirmed TB, 7/65 (11%) with unconfirmed TB, 10/170 (6%) with unlikely TB, and 9/34 (27%) children who were unclassifiable. Of the 30 children who died, 16 started TB treatment (4/4 (100%) with confirmed TB, 2/7 (29%) with unconfirmed TB, 1/10 (10%) with unlikely TB, and 9/9 (100%) unclassifiable); 14 died before the two-month follow-up visit and two had complete or partial resolution of symptoms at the two-month visit but subsequently died. Twenty-two of 30 (73%) children who died were HIV-positive and of these 13 (59%) had evidence of severe immunosuppression.
Discussion
In this prospective study using rigorous bacteriologic testing in children enrolled based on symptomatology consistent with active TB disease, approximately one third were classified as having intrathoracic TB using standardized research case definitions. However, among these children classified as having TB disease, only approximately one third had bacteriologic confirmation despite testing of up to 14 samples per child. These findings may reflect both limitations in bacteriologic diagnosis of children as well as the accuracy of a composite reference standard for TB disease, and underscore the importance of research efforts aimed at improving TB diagnostics in children.(21)
As expected, culture had the highest sensitivity for most specimen types. However, sensitivity of Xpert relative to culture was greater than 60% for most specimen types. The reason that the yield of NPA relative to IS was higher in this study than in other studies is not clear but does not appear to be due to order of collection; NPA was obtained before IS as described by Zar et al. and other respiratory specimens (GA and string test) followed.(22)
A constellation of clinical features and ancillary test results may point toward TB disease but are not diagnostic and may be affected by conditions such as malnutrition or HIV.(23) Our findings highlight that individual clinical features do not confirm nor refute a diagnosis of TB disease.(7) For example, only 75% of children with bacteriologically confirmed TB had positive TST and/or IGRA, reinforcing that tests of TB infection cannot be used to rule-out TB disease. Further, among children with confirmed TB only 43% of children had chest radiographs read as “likely TB,” highlighting the limitations of chest radiography in this age group; 77% had a reported history of recent close contact with someone with sputum smear positive TB. Cough, fever, and malnutrition were common in children both with and without TB disease, highlighting the recognized challenge of discerning TB symptoms from those of common childhood illnesses.(24)
A limitation of this study is that participants were enrolled based on prolonged symptoms and therefore may not be representative of the full spectrum of TB disease in children. However, standardized case definitions and detailed clinical descriptions allow comparison across pediatric diagnostics studies. In addition, we used case definitions for intrathoracic TB; a small number of children were enrolled based on lymphadenopathy but almost all of these children had additional symptoms suggestive of intrathoracic TB or positive bacteriologic test results from respiratory specimens.
Standardized research clinical case definitions were applied to study participants retrospectively and independent of decisions of the primary medical providers to treat for TB, as specified in guidance for these definitions.(19) Several considerations regarding these definitions are worth highlighting. Almost half (43%) of children who met criteria for unconfirmed TB according to baseline characteristics had full resolution of symptoms in the absence of TB treatment and were re-classified as unlikely TB according to definition guidance. Although some children reclassified from having unconfirmed TB to unlikely TB may indeed have had TB disease that resolved without treatment as described in the pre-treatment era, many may not have had TB disease at all.(25) Further, resolution of symptoms was common among children with unconfirmed TB who received TB treatment but also among those who did not; sensitivity and specificity of the response to treatment criterion for unconfirmed TB disease is therefore likely limited. These findings have important implications for TB diagnostic research. Although the methodology for retrospectively assigning clinical case definitions is standardized, the decision to treat is not. Variability in decisions to treat may affect final classification of children (in particular, unconfirmed TB versus unlikely TB) and impact the derived performance characteristics for diagnostic tests. A detailed description of children in each category as done here may aid in interpretation of findings between studies.
Our findings strongly support the need for improved TB diagnostics in young children, particularly diagnostics that do not rely on isolation of mycobacteria (i.e., “biomarker” based tests).(26) Specifically, improved diagnostics are needed to identify those children who truly have TB disease and those who do not. Our finding that most TB disease in this extensively bacteriologically evaluated cohort is “clinical TB disease” (unconfirmed TB) also reinforces the need to strengthen systems for recognizing and treating this form of disease, even as the TB research community works to identify better diagnostic tests.
Supplementary Material
Acknowledgments
We acknowledge the study participants and their families and the clinical and laboratory and data management staff at participating sites. We acknowledge Lindsay Hatzenbuehler for development of the chest radiography interpretation form, John Wen for data management assistance, and Brian Baker for study management.
Financial Support
This work was supported by the United States Agency for International Development and the Centers for Disease Control and Prevention. A portion of this work was funded by the President's Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention and by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health, Bethesda, MD, USA [grant number K23HD072802 to RS].
Disclaimer
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.WHO. Global Tuberculosis Report. Geneva. 2020. [Google Scholar]
- 2.Dodd PJ, Yuen CM, Sismanidis C, Seddon JA, Jenkins HE. The global burden of tuberculosis mortality in children: a mathematical modelling study. The Lancet Global health. 2017;5:e898–e906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kunkel A, Abel Zur Wiesch P, Nathavitharana RR, Marx FM, Jenkins HE, Cohen T. Smear positivity in paediatric and adult tuberculosis: systematic review and meta-analysis. BMC infectious diseases. 2016;16:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tebruegge M, Ritz N, Curtis N, Shingadia D. Diagnostic Tests for Childhood Tuberculosis: Past Imperfect, Present Tense and Future Perfect? The Pediatric infectious disease journal. 2015;34:1014–1019. [DOI] [PubMed] [Google Scholar]
- 5.Detjen AK, DiNardo AR, Leyden J, et al. Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in children: a systematic review and meta-analysis. The Lancet Respiratory medicine. 2015;3:451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Organization WH. Rapid Communication: Molecular assays as initial tests for the diagnosis of tuberculosis and rifampicin resistance. Geneva 2020 [Google Scholar]
- 7.Perez-Velez CM, Roya-Pabon CL, Marais BJ. A systematic approach to diagnosing intrathoracic tuberculosis in children. The Journal of infection. 2017;74 Suppl 1:S74–S83. [DOI] [PubMed] [Google Scholar]
- 8.Perez-Velez CM, Marais BJ. Tuberculosis in children. The New England journal of medicine. 2012;367:348–361. [DOI] [PubMed] [Google Scholar]
- 9.Hesseling AC, Schaaf HS, Gie RP, Starke JR, Beyers N. A critical review of diagnostic approaches used in the diagnosis of childhood tuberculosis. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2002;6:1038–1045. [PubMed] [Google Scholar]
- 10.Seddon JA, Shingadia D. Epidemiology and disease burden of tuberculosis in children: a global perspective. Infect Drug Resist. 2014;7:153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song R, Click ES, McCarthy KD, et al. Sensitive and Feasible Specimen Collection and Testing Strategies for Diagnosing Tuberculosis in Young Children. JAMA Pediatr. 2021:e206069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Health KMo. Guidelines for Management of Tuberculosis and Leprosy in Kenya. July ed2013. [Google Scholar]
- 13.Graham SM, Ahmed T, Amanullah F, et al. Evaluation of tuberculosis diagnostics in children: 1. Proposed clinical case definitions for classification of intrathoracic tuberculosis disease. Consensus from an expert panel. The Journal of infectious diseases. 2012;205 Suppl 2:S199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanitation MoPHa. National Guidelines for HIV Testing and Counseling in Kenya. In: (NASCOP) NAaSCP, ed. 2nd ed. Nairobi, Kenya: 2010. [Google Scholar]
- 15.health Mo. Guidelines for Prevention of Mother to Child Transmission (PMTCT) of HIV/AIDS in Kenya. In: Programme NASC, ed. 4th ed. Nairobi, Kenya: 2014. [Google Scholar]
- 16.WHO. WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children 2007. [Google Scholar]
- 17.(UNICEF) WHOatUNCsF. Recommendations for data collection, analysis and reporting on anthropometric indicators in children under 5 years old. Geneva, Switzerland: 2019. [Google Scholar]
- 18.Schumacher D anthro: Computation of the WHO Child Growth Standards. R package version 0.9.1 Available at: https://CRAN.R-project.org/package=anthro. [Google Scholar]
- 19.Graham SM, Cuevas LE, Jean-Philippe P, et al. Clinical Case Definitions for Classification of Intrathoracic Tuberculosis in Children: An Update. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;61Suppl 3:S179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kay AW, Gonzalez Fernandez L, Takwoingi Y, et al. Xpert MTB/RIF and Xpert MTB/RIF Ultra assays for active tuberculosis and rifampicin resistance in children. Cochrane Database Syst Rev. 2020;8:CD013359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicol MP, Zar HJ. Advances in the diagnosis of pulmonary tuberculosis in children. Paediatr Respir Rev. 2020;36:52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zar HJ, Workman L, Isaacs W, et al. Rapid molecular diagnosis of pulmonary tuberculosis in children using nasopharyngeal specimens. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;55:1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas TA. Tuberculosis in Children. Pediatr Clin North Am. 2017;64:893–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuevas LE, Petrucci R, Swaminathan S. Tuberculosis diagnostics for children in high-burden countries: what is available and what is needed. Paediatrics and international child health. 2012;32 Suppl 2:S30–37. [DOI] [PubMed] [Google Scholar]
- 25.Marais BJ, Gie RP, Schaaf HS, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2004;8:392–402. [PubMed] [Google Scholar]
- 26.Nicol MP, Gnanashanmugam D, Browning R, et al. A Blueprint to Address Research Gaps in the Development of Biomarkers for Pediatric Tuberculosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;61Suppl 3:S164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.