Abstract
Cholera toxin (CT) is one of the most well-known immunostimulants. Mammalian studies have shown that CT can generate immune responses against antigen. However, it has not exhibited a definite effect on poultry yet. In this study, focusing on a cost-effective method, the effect of different concentrations of CT obtained from Vibrio cholerae biotype El Tor and serotype Inaba was investigated on the immunogenicity of infectious bronchitis vaccine. After culturing and concentrating CT, different concentrations of CT (0.1, 1, 2, and 5 micrograms) were combined with avian infectious bronchitis vaccine strain H120 produced by Razi Vaccine and Serum Research Institute (RVSRI) and, at 7 days of age, inoculated via the eye drop administration in 42 specific-pathogen-free chickens (seven groups of six chicks that included four experimental groups, two negative control groups (PBS and toxin), and one positive control group). Blood samples were taken weekly from the wing veins of the chickens, and the immunoglobulin G (IgG) titer was checked by enzyme-linked immunosorbent assay. The results showed that 2 µg of CT in comparison with other concentrations caused a significant increase in the antibody titer against avian infectious bronchitis in the blood serums of the chickens. One-way ANOVA test showed that all the results of this study were significant at P<0.05 level. Our data show that CT has the potential to further stimulate the immune system of chickens and may increase the immunogenicity of the infectious bronchitis vaccine. However, more research is needed to examine all aspects of the use of this toxin in animal vaccines.
Keywords: Adjuvant, Infectious bronchitis, Vaccine, Vibrio cholerae toxin
1. Introduction
Infectious bronchitis virus (IBV) belongs to the genus Gammacoronavirus of the subfamily Coronavirinae in the family Coronaviridae. Moreover, it causes infectious bronchitis (IB), a highly contagious viral disease in chickens. IB has spread worldwide and has a significant economic impact on the poultry industry ( 1 ). Morbidity of IBV has been almost always 100%; however, mortality can vary from 0% to 82% depending on the age and the immune status of the birds, as well as the strain of the virus and the involvement of secondary bacterial or viral pathogens.
Live-attenuated vaccines are the most common vaccines used in commercial poultry, especially broilers chickens. The main goal of this strategy is to produce local mucosal and humoral protective immunity ( 2 ). Symptoms, such as mild respiratory symptoms, decreased egg production, low eggshell quality, and numerous viral genetic changes during passage in the herd are among the effects of vaccination with live-attenuated IBV vaccines ( 3 ). Moreover, due to the existence of many known serotypes and genotypes, IBV vaccination is a challenge ( 4 , 5 ). Therefore, the improvement of the efficacy of live-attenuated vaccines by using adjuvants in vaccine formulations to enhance innate and adaptive immune responses is worth studying.
Oil-based emulsions and aluminum derivatives are mainly used in inactivated poultry vaccines to increase immunogenicity; however, adjuvants can significantly increase the cost of these vaccines ( 6 , 7 ). The use of adjuvants in live-attenuated vaccines has been evaluated in several poultry studies. Research shows that chitosan nanoparticles' use as adjuvants increases mucosal responses and protects chickens against the Newcastle disease virus challenge ( 8 ). Kjærup, Dalgaard ( 9 ) successfully tested particles, such as chitosan and fructo-oligosaccharides, as adjuvants in live IBV vaccines. The turning point of these studies was the use of live IBV vaccine with different types of adjuvants, which increased antibody titers and improved protection against viral challenge, compared to non-adjuvant live vaccines in chickens ( 10 ).
Numerous studies have addressed the formulation of cholera toxin B subunit (CTB) -adjuvanted vaccine in humans and animals. Although cholera toxin (CT)-adjuvanted vaccines are not licensed for human use due to the high toxicity of CT, the immune system has been successfully stimulated by CT (especially its B subunit) in many animal models (Stratmann 2015). The mechanism of the CT adjuvanticity effect is unclear; however, it may be related to the following factors: (1) the induction of antigen presentation by different antigen-presenting cells (APCs); (2) the occurrence of complex stimulatory effects on T cell proliferation and cytokine production; (3) greater permeability induction in the intestinal epithelium, which leads to the increased absorption of co-administered antigens; and (4) increased isotype differentiation in B cells, which leads to increased immunoglobulin A (IgA). The mechanisms that lead to the antigens presentation by different APCs are probably more important ( 11 ).
Some studies show that CT increases naive T cells in vitro and leads them to Th2 responses or even Th1/Th2 responses; moreover, it increases the production of interleukins, such as IL-4, IL-5, IL-6, and IL-10 (Brown et al., 1999; Climple et al., 1995; Wilson et al.) In addition, CT significantly increases the antigen presentation by dendritic cells (DCs), macrophages, and B cells ( 12 - 14 ). CT also increases the expression of MHC and CD80 molecules. In particular, CT also induces IL-1 secretion from both DC and macrophages ( 15 ). The detrimental effect of the use of this toxin and its derivatives as adjuvants in animal vaccines has not been reported so far ( 6 , 16 ). There is no documented paper regarding the effectiveness of CT in the avian immune system and its effect on the immunization of the IBV vaccine in poultry.
Considering the disadvantages of the live IBV vaccines, such as low immunogenicity and various genetic changes that are one of the causes of failure of IBV vaccination ( 1 , 4 ), numerous reports of CT effectiveness in immunogenicity against the co-administered antigen in animal models, and the positive effect of the use of adjuvants on live vaccines, this study aimed to suggest CT as an adjuvant in IBV vaccine and evaluate its effect on the humoral immunogenicity of the live IBV vaccine in chickens based on cost-effectiveness and ease of production. There is a need for developing and producing new adjuvants to be used together with IBV in order to develop immunity in poultry against IBV. The development and production of such adjuvants will help poultry farmers to benefit more from IBV in poultry and suffer less financial losses.
2. Materials and Methods
2.1. Preparation of the Cholera Toxin
Vibrio cholera biotype El Tor serotype Inaba (obtained from the Iranian Research Organization for Science and Technology PTCC-1611) was inoculated in one liter of Craig's medium containing 30.0 g of casein acid hydrolysate, 4.0 g of yeast extract, 0.5 g of K2HPO4, and 20 Ml of glucose solution 20% ( 17 ) and placed in an incubator at 30ºC for 48 h. The medium was then centrifuged at 17572 G for 20 min. Since CT is an exotoxin, the supernatant was removed and filtered using 0.22 μm syringe filters to remove unwanted contaminants and then saturated by 90% ammonium sulfate (Supelco) and put in a freezer at -20˚C overnight. The saturated supernatant was centrifuged (Sigma) at 10397 G for 15 min, and the precipitate was dissolved in phosphate buffer (PB) at a ratio of 1:1000.
2.2. Evaluation of Toxin Purity by SDS-PAGE Method
Various dilutions (1:5, 1:10, and 1:100) of the concentrated protein were prepared in the previous step. A control protein (bovine serum albumin or [BSA] from Sigma-Aldrich) with a known concentration (0.4 g/ml) and molecular weight (66.2 kDa) was also used for further software analyses using TotalLab TL120 (version 2009). When the electrophoresis was completed, the polyacrylamide gel was stained using silver nitrate (Panreac) as the silvering agent. The protein marker used in this study was purchased from Cytomatingene Co. (Iran). TotalLab TL120 was used for software analysis of the protein bands obtained through SDS-PAGE. This software uses the molecular marker and the control protein BSA to analyze all the bands obtained from the electrophoresis gel. These analyses included the study of the molecular weights of the bands and also the determination of their concentrations with the help of the concentration of the control protein.
2.3. Determination of the CT Protein Concentration by GM1-ELISA Method
The modified method of the US Centers for Disease Control and Prevention ( 18 ) was used in order to evaluate the toxin concentration. Each well was coated with 100 μl of the GM1 (Sigma-Aldrich) solution (1:2000 in PBS). Phosphate-buffered saline (PBS) containing 0.05% Tween was used as the negative control. The microtiter plate was placed in a refrigerator overnight. The wells were washed with the Tween-containing PBS three times. Each time, the wells were filled with 200 μl of PBS solution containing Tween (0.05%) and left at room temperature for 3 min, emptied, and dried. Following that, 150 μl of 1% BSA solution (in PBS) was added to each well, and the plate was put in an incubator at 37˚C for 30 min. The wells were washed again as mentioned above, and the prepared samples (each 100 μl) were added to the wells, and the plate was placed in an incubator at 37˚C for 1 h.
To determine the concentration of the concentrated toxin more precisely, 100 μl of the standard toxin (Sigma-Aldrich) with concentrations of 12.5, 25, 50, and 100 μg/ml were used, respectively. At the end of the incubation period, the plate was washed again three times in the way mentioned above. The wells were filled again with 150 μl of 1% BSA blocking solution and incubated for 30 min at room temperature and then washed again. Following that, 150 μl of the stored rabbit serum containing the CT antibody (diluted to 1:100 in PBS that included 0.1% BSA) was added to each well, and the plate was incubated at 37˚C for 1 h. The plate was then washed in the mentioned way. Afterward, 100 μl of the goat anti-rabbit immunoglobulin G (IgG) antibody conjugated with HRP (Abcam) at the dilution of 1:5000 (diluted in PBS containing 0.1% BSA) was added to each well. The plate was refrigerated overnight. The next day, after the plate reached room temperature, it was incubated at 37˚C for 1 h and then washed. Tetramethyl benzidine (TMB) (Biolegend Co., USA) was used for substrate staining in enzyme-linked immunosorbent assay (ELISA). In the next stage, 100 μl of the TMB solution (prepared according to the manufacturer's guidelines) was added to each well. The stop solution (100 μl of 1 normal sulfuric acid) was added to each well. Finally, plate absorbance was read in an ELISA microplate reader at 450 nm.
2.4. Purity from the Endotoxins of Concentrated Toxins, such as Lipopolysaccharides
Limulus amebocyte lysate (LAL) kits (manufactured by Cape Cod Co., USA) were used to detect lipopolysaccharide (LPS) in the concentrated toxin. According to the guidelines of the manufacturers, the test would be positive if the gel at the bottom of the tube remained intact indicating the sample was contaminated with LPS; otherwise, the test would be negative indicating the absence of LPS.
2.5. Assessment of the Activity of the Concentrated Toxin
The modified method of the Centers for Disease Control and Prevention ( 18 ) was used to assess the activity of the concentrated toxin. A series of dilutions (from 100 µg/ml to 0.1 pg/ml) of the concentrated toxin were added to Vero cell cultures and incubated at 37ºC for 24 h. After 24 h, cell destruction was evaluated under an optical microscope. In this study, standard toxin with a concentration of 100 µg/ml was used as a positive control.
2.6. Combination of the Concentrated Toxin with IBV H120 Vaccine Strain for Poultry
After performing tests to confirm the identity of Vibrio cholera toxin, the concentrated toxin was combined with the H120 vaccine strain of the Massachusetts serotype (Razi Vaccine and Serum Research Institute or RVSRI) and prepared for eyedrop vaccination to assess the adjuvant effects of the concentrated toxin. This vaccine is offered as a live highly attenuated vaccine in lyophilized form. For this purpose, the H120 strain IBV vaccine (2500 doses) was dissolved and completely mixed in 2 mL (1 dose/µl) of PBS. The concentrated CT at the four concentrations mentioned below was added to the named vaccine (1 dose for each chicken) and mixed well. The concentrations considered for the CT were 0.1, 1, 2, and 5μg in the vaccine dose injected into the chicks (Table 1).
Table 1.
Experimental and control groups in this study
| Group name | Agent | Concentration |
|---|---|---|
| Negative control (1) | PBS | - |
| Negative control (2) | CT | 20 µg in PBS (1:50) |
| Positive control | Pure IBV vaccine strain H120 | - |
| Toxin group (1) | CT (in IBV vaccine) | 0.1 µg |
| Toxin group (2) | CT (in IBV vaccine) | 1 µg |
| Toxin group (3) | CT (in IBV vaccine) | 2 µg |
| Toxin group (4) | CT (in IBV vaccine) | 5 µg |
2.7. Inoculation of the Chickens with H120 Strain IBV Vaccine Combined with Concentrated CT
To assess the vaccine containing the CT adjuvant in chickens, 42 Leghorn specific-pathogen-free chickens (Venky’s poultry products) were divided into seven groups of six chickens (Table 1). After hatching, the chickens were kept at Mashhad Vaccine and Serum Research Institute for a week without injecting any vaccines or administering medications and were just given feed and water. Supplements containing essential amino acids (KILco) were added to their drinking water 72 h after hatching. A positive control group (IBV H120 vaccine strain) and two negative control groups (only PBS and toxin diluted in PBS) were used to assess antibody levels in the entire studied population.
In the first week after hatching, the chickens were vaccinated at the 50 μl dose via eye drop vaccination to make sure that the chickens were individually inoculated. Each chicken received 50 μl of the vaccine containing the CT adjuvant (25 μl in each eye) via eye drop administration using an accurate micropipette. Two weeks later (when the chickens were 21 days old), they received a booster in the same way. Since the IBV vaccine for poultry is in the form of live viruses, and there is the possibility of contamination of the negative control groups with the virus, these groups were kept in a different place separately from the other chickens.
2.8. Blood Sampling and ELISA Test
Blood samples were taken from the wing veins of eight chickens (randomly selected) to assess antibody levels in the chickens before inoculating them with the vaccine. The chickens were divided into seven groups prior to eyedrop inoculation. Specific tapes with different colors were attached to the legs of the chickens in each group so that the groups could be differentiated from each other. Blood samples were taken weekly from wing veins for six weeks after inoculation (when they were 14, 21, 28, 35, 42, and 49 days old) to assess blood antibody levels. In total, 252 blood samples were collected and centrifuged at 2500 rpm for 15 min. The obtained blood serums were kept at -20˚C for further studies.
The IgG levels in the chicken serums were measured using the kit for diagnosis of IB (manufactured by the BioChek Company, the Netherlands) in all blood serum samples taken from the experimental and control groups following the guidelines of the manufacturers.
2.9. Recording Clinical and Functional Symptoms of the Herd
All chickens in the seven groups were weighed once a week. The amount of feed consumption was also measured once a week for all groups to estimate feed conversion ratio (FCR).
2.10. Statistical Analysis
SPSS software (version 26) was used to assess the significant relationship between the experimental and control groups in terms of IgG levels in blood serum samples using ELISA findings. Moreover, one-way ANOVA was utilized to assess the significant relationships among the findings obtained from ELISA. A p-value less than 0.05 was considered statistically significant.
3. Results
3.1. SDS-PAGE Analysis Results
The CTB and CTA subunits have molecular weights of 11.6 and 28 kDa, respectively ( 19 ). These bands were clearly visible in the studied concentrations in the concentrated proteins in the environment when performing the SDS-PAGE test and after polyacrylamide gel staining (Figure 1). The image prepared from the SDS-PAGE gel was entered into TotalLab TL120 for statistical analysis. After identifying the columns and bands in each column, the color in the background of all the columns was set to zero by the software to determine the actual intensity of each band. In the SDS-PAGE test, quantitative attribution was not possible to determine the concentration of the band present in both columns (1:5 and 1:10) because of the very high dilutions. However, the sizes of the other bands could be analyzed by the software to the expected degree of accuracy. Quantitative analysis of the bands (1:100) by the named software showed the concentration of toxin to be 118 μg/ml, which is very close to the results of the GM1-ELISA test (Table 2).
Figure 1.
Craig's medium 15% polyacrylamide gel after silver nitrate staining. In all four columns for the concentrated toxin samples, the 11.6 and 28 kDa bands were observed for the B and A subunits of cholera toxin, respectively. B = BSA, M = Protein marker, D1 = 1:5, D2 = 1:10, D3 and D4 = 1:100 Toxin dilutions.
Table 2.
Exact amount of cholera toxin condensed from the bacterial culture medium of Vibrio cholerae based on ELISA-GM1 test results
| Sample | Dilute | (µg/ml) Toxin concentration |
|---|---|---|
| Concentrated toxin from Craig's medium | 1:1000 | 100.3 |
| 1:2000 | 19.4 | |
| 1:4000 | 1.1 | |
| Supernatant of bacterial culture in Craig's medium | 1:1 | 99.8 |
3.2. Concentration of CT through GM1-ELISA Test
The standard ELISA curve was drawn using Curve Expert (version 1.4) considering the data on optical absorbance of the standard toxin. The amount of concentrated toxin in each sample was calculated by the mentioned software. For the 1:1000 dilution of the concentrated toxin in Craig's medium, the concentration of 100.3 μg/ml was obtained and taking the dilution factor into account, the amount of produced toxin per liter was 100 mg. The concentration of the toxin present in the supernatant of the bacterial culture was 99.8 μg /ml, and considering the dilution factor, the total quantity of the toxin per liter of the culture medium was 99 mg, which was very close to the concentration of the concentrated toxin (Table 2).
3.3. LAL Test Results
The test tubes were held upside-down. The contents of the tubes related to the positive control sample were still intact, whereas those of the tubes containing the test sample were completely out of the gel state and moved downward. According to the guidelines of the manufacturers, the employed kit was sensitive to 0.03 endotoxin units per mL (EU/mL); therefore, the result of the LAL test indicated that the produced toxin in this study was free of endotoxin, such as LPS.
3.4. Activity of the Concentrated CT
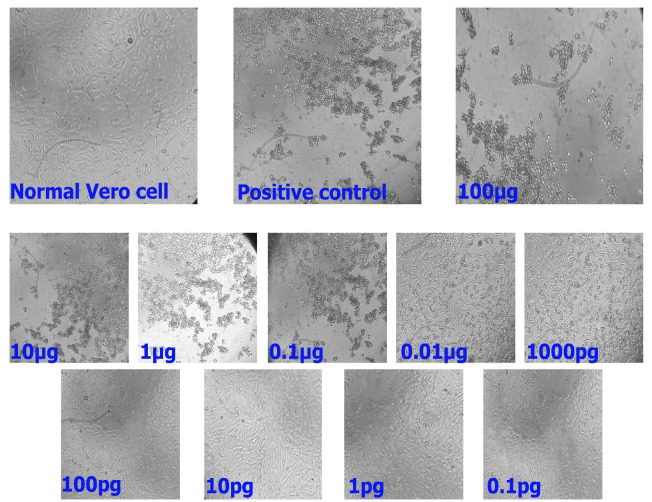
The toxin cultivation plate was removed from the incubator after 24-h incubation, and the cells were examined under a light microscope to assess the activity of the toxin. It was observed visually that the toxin was active and had caused the destruction of the Vero cells. The CT maintained its destructive effect up to a concentration of 1000 pg/ml; however, it lost its effects at higher dilutions, and the cultured cells were not damaged at all by its activity (Figure 2).
Figure 2.
Destructive effects of cholera toxin on Vero cells. The cholera toxin produced by the method described was added to Vero cells from a concentration of 100 µg/ml to 0.1pg/ml. The toxin retains its destructive effect on Vero cells up to a concentration of 1000 pg/ml.
3.5. IgG Levels in Blood Serum of the Chickens
IgG logarithmic titer (log10) against IBV in the eight serum samples for the 0-7-day chickens and before vaccine inoculation was 1.54±0.23. According to the manufacturer's instructions, an antibody titer of less than 2.92 is considered negative. During the study, the antibody titers were almost the same in the negative control groups (Table 3). The antibody titers were 2.31±0.28 in the positive control group, 2.38±0.27 in the toxin group (1), 2.36±0.2 in the toxin group (2), 2.49±0.45 in the toxin group (3), and 2.11±0.33 in the toxin group (4) in the 7-14-day chickens (the first week after inoculation). No significant changes in antibody level against IBV were found in any of the groups (P<0.05). The first considerable change (P<0.05) in the IgG level occurred in the 14–21-day chickens (the second week after inoculation) so that the titer of this antibody increased to 3.07±0.27 in the positive control group, 3.05±0.11 in the toxin group (1), 3.11±0.34 in the toxin group (2), 3.35±0.29 in the toxin group (3), and 3.02±0.31 in the toxin group (4). A relatively ascending trend was observed in the antibody titer against IBV in the 21-28-day chickens (the first week after the booster dose) in all the experimental groups (P<0.05). The second dramatic change in antibody titer levels (P<0.05) was recorded in the 28-35-day chickens (the second week after the booster dose).
Table 3.
Mean of changes in antibody titers against IBV in the blood serum of all experimental and control groups during research. In the first week (7 days) the vaccination of chickens began. In the third week (21 days), the booster dose was inoculated. An increase in antibody titers against IBV is evident up to 6 weeks after the initial inoculation of the CT Adjuvanted IB vaccine in the chicken. According to the manufacturer's instructions, an antibody titer of less than 2.92 is considered negative.
| Sampling days (Mean±SD) | |||||||
|---|---|---|---|---|---|---|---|
| Groups | 7 | 14 | 21 | 28 | 35 | 42 | 49 |
| Negative Control (1) | 1.54±0.23 | 1.88±0.08 | 2.41±0.16 | 2.46±0.05 | 2.32±0.09 | 2.49±0.15 | 2.38±0.12 |
| Negative Control (2) | 1.79±0.03 | 2.49±0.21 | 2.51±0.2 | 2.41±0.23 | 2.44±0.14 | 2.43±0.22 | |
| Positive Control | 2.31±0.28 | 3.07±0.27 | 3.12±0.15 | 3.21±0.16 | 3.27±0.16 | 3.26±0.25 | |
| Toxin (1) | 2.38±0.27 | 3.05±0.11 | 3.09±0.2 | 3.34±0.17 | 3.36±0.24 | 3.37±0.25 | |
| Toxin (2) | 2.36±0.2 | 3.11±0.34 | 3.17±0.17 | 3.47±0.07 | 3.48±0.07 | 3.47±0.07 | |
| Toxin (3) | 2.49±0.45 | 3.35±0.29 | 3.36±0.34 | 3.52±0.09 | 3.55±0.22 | 3.53±0.21 | |
| Toxin (4) | 2.11±0.33 | 3.02±0.31 | 2.95±0.24 | 3.30±0.03 | 3.31±0.11 | 3.32±0.25 | |
A relatively ascending trend was noticed in the antibody titer against IBV in the third week after inoculation of the booster dose (when the chickens were 35-42-days old). The change in antibody titer in this period was not as remarkable as that in the 28-35-day chickens. Antibody titer did not increase in the 42-49-day chickens, and antibody level stabilized in the blood serums of the chickens. Logarithmic changes in antibody titer against IBV during the experiment are shown in table 3. Figure 3 illustrates these changes graphically.
Figure 3.
Rate of antibody logarithmic changes against IBV in the chickens' blood serum of experimental and control groups during the study based on cholera toxin dose. The results show that 2 µg of toxin significantly increases the antibody titer against IBV.
3.6. Weight Measurements and FCR
Based on the average recorded weights in each group during the experiment (7 weeks), the maximum and minimum average weights were 603.3g in the toxin group (1) and 501.9 g in the negative control group at the end of the 49th day (Table 4). The lowest recorded FCR during the seven weeks of the experiment was 1.23 in the negative control group (1). A relative decrease was noticed in the FCR at week four, compared to the third week in the negative control group (2) and the positive control group with 1.84 and 1.83, respectively, compared to other groups during the same week (Table 5). In general, the calculated FCR values for all the chickens in the control and experimental groups were consistent with the standard FCR value for Leghorn chickens.
Table 4.
Mean of weight recorded for all members of the experimental and control groups during the research period
| Sampling days (Mean ± SD) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Groups | 0 | 7 | 14 | 21 | 28 | 35 | 42 | 49 |
| Negative Control (1) | 37.87±2.5 | 67.99±1.1 | 120.47±4.7 | 194.41±14.9 | 282.23±16.9 | 419.02±18 | 512.3±28.6 | 578.6±42.3 |
| Negative Control (2) | 38.84±2.1 | 66.5±2.6 | 116.92±10.3 | 193.39±21.3 | 266.86±33.4 | 379.69±28.3 | 440.4±43.9 | 501.95±26.4 |
| Positive Control | 38.11±1.7 | 71.98±2.7 | 165.43±7.8 | 290.2±18.6 | 365.73±31.3 | 465.48±17.6 | 539.6±12.9 | 597.12±26.9 |
| Toxin (1) | 38.24±1.2 | 61.01±3.6 | 162.98±15.3 | 276.61±19.2 | 350.33±45.6 | 455.5±27.8 | 529.9±37.5 | 603.3±38.7 |
| Toxin (2) | 39.37±2.5 | 56.14±3.7 | 165.41±9.9 | 228.5±22.3 | 344.23±29.2 | 467.5±51.6 | 529.58±22.8 | 599.8±52.9 |
| Toxin (3) | 39.54±2.6 | 66.93±4.0 | 176.6±12.6 | 265±21 | 363.83±31.6 | 454.26±33.9 | 540.35±19.4 | 589.3±34.7 |
| Toxin (4) | 38.54±3.1 | 64.68±5.2 | 174.26±14.3 | 253.23±15.8 | 368.5±33 | 464.7±25.4 | 535.53±38.8 | 577.73±40.2 |
Table 5.
Feed conversion ratio weekly mean in experimental and control groups during the study
| Feed conversion ratio/weeks | |||||||
|---|---|---|---|---|---|---|---|
| Groups | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Negative Control (1) | 1.23 | 1.97 | 2.33 | 2.45 | 2.84 | 2.93 | 2.95 |
| Negative Control (2) | 1.42 | 1.62 | 2.31 | 1.84 | 2.44 | 2.75 | 2.83 |
| Positive Control | 1.44 | 1.86 | 2.71 | 1.83 | 2.26 | 2.33 | 2.1 |
| Toxin (1) | 1.29 | 1.44 | 2.15 | 2.35 | 2.53 | 2.77 | 2.73 |
| Toxin (2) | 1.34 | 1.52 | 2.03 | 2.43 | 2.4 | 2.68 | 2.73 |
| Toxin (3) | 1.66 | 1.63 | 2.26 | 2.39 | 2.63 | 2.75 | 2.81 |
| Toxin (4) | 1.35 | 1.72 | 2.3 | 2.41 | 2.59 | 2.66 | 2.74 |
3.7. Analysis of the Statistical Results
The results of one-way ANOVA showed significant differences in the IgG levels among the studied groups at a significance level of P<0.05. However, no significant (P<0.05) difference was found in the IgG levels between the toxin group (4) (5 µg/dose) and the positive control group.
4. Discussion
The use of inactivated IBV vaccines is not sufficient due to short-term protection and limited immune responses ( 20 ). Studies show that the use of adjuvants increases the specific response to antigen and the protection period ( 21 ). Both live and inactivated vaccines are used to immunize IB. Live vaccines are widely used in veterinary medicine and are commonly used to vaccinate young animals. Under laboratory conditions, 100% efficacy has been proven for live vaccines. However, in the field studies, more than 10% of vaccinated animals are unprotected after receiving the live vaccine ( 4 ). The addition of adjuvants to live-attenuated vaccines has a variety of purposes, including reducing antigen dose, improving immunity, and increasing vaccine efficacy. CT is an adjuvant with a high potential for immune stimulation ( 11 , 22 - 25 ) derived from the bacterium Vibrio cholerae. Various studies have shown that the use of this adjuvant in combination (or as a subunit conjugate) with animal vaccines increases the specific antibody responses against the vaccine antigen. George-Chandy, Eriksson ( 23 ) showed that CTB-antigen conjugate could reduce the dose of antigen required for stimulating the immunity system of rats by up to 10,000 times. However, the CTB-antigen combination had no considerable effect on the stimulation of the immune system in rats.
The use of CT as an oral adjuvant in poultry has been discussed up to the present time, and no definitive results have been achieved ( 26 ). Meinersmann and Porter ( 25 ) aimed to assess the adjuvant effects of CT on its oral administration to chickens. They showed that a combination of tetanus toxoid and CT strongly suppressed the immune responses of chickens to antigens.
CT is a potent mucosal adjuvant in mammals and strongly enhances mucosal IgA and systematic IgG immune response; moreover, it prevents tolerance to oral intake of soluble antigens ( 27 ). Various studies have been performed to evaluate the effect of different adjuvants on the efficacy and immunogenicity of the live IBV vaccine. Tohidi, Ghaniei ( 28 ) showed that the use of bacterial-like particles significantly increased the titer of serum antibodies against IBV in chickens.
The use of montanide gel as an adjuvant has also been shown to significantly increase antibody titers against IBV in chickens ( 10 , 29 , 30 ).
According to the results obtained from the effect of CT on the immunogenicity of vaccines in animal models, this study investigated the effect of different concentrations of CT on the immunogenicity of the IBV strain H120 vaccine. To ensure individual vaccination of chickens, the adjuvanted vaccine was administered as eye drops. The results show that the use of CT has the potential to stimulate the immune system of chickens against IBV and can be used as an effective adjuvant in this vaccine (Table 3). However, its use requires more extensive research to examine all aspects of safety.
Authors' Contribution
Study concept and design: A. K. and M. J. M.
Acquisition of data: A. K.
Analysis and interpretation of data: H. F.
Drafting of the manuscript: M. J. M.
Critical revision of the manuscript for important intellectual content: M. J. M.
Statistical analysis: A. K.
Administrative, technical, and material support: A. K. and M. J. M.
Ethics
All instruments applied in this study were calibrated and maintained in accordance with routine quality control procedures overseen by the ethics committee of the Islamic Azad University, Tehran, Iran.
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgment
The authors appreciate the efforts of all the personnel of the Veterinary and Biotechnology Research Department of the Razi Vaccine and Serum Research Institute (RVSRI). They also thank Dr. E. Tabatabaei Zadeh, an RVSRI faculty member, for his outstanding contributions. Observance of ethical principles of working with laboratory animals has been approved by the National Ethics Committee in Iranian Biomedical Research under license No. RVSRI.REC 98008.
References
- 1.Barberis A, Alloui N, Bennoun O, Agabou A. Infectious Bronchitis in Poultry: Constraints and Biotechnological Developments in Vaccines. Asian J Poult Sci. 2015;9(2):57–69. [Google Scholar]
- 2.Nazmi A, Hauck R, Corbeil LB, Gallardo RA. The effect of diatomaceous earth in live, attenuated infectious bronchitis vaccine, immune responses, and protection against challenge. Poult. Sci 2017;96(8):2623–9. doi: 10.3382/ps/pex093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bwala DG, Clift S, Duncan NM, Bisschop SP, Oludayo FF. Determination of the distribution of lentogenic vaccine and virulent Newcastle disease virus antigen in the oviduct of SPF and commercial hen using immunohistochemistry. Res Vet Sci. 2012;93(1):520–8. doi: 10.1016/j.rvsc.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 4.Cavanagh D. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 2003;32(6):567–82. doi: 10.1080/03079450310001621198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook JK, Jackwood M, Jones R. The long view: 40 years of infectious bronchitis research. Avian Pathol. 2012;41(3):239–50. doi: 10.1080/03079457.2012.680432. [DOI] [PubMed] [Google Scholar]
- 6.Spickler AR, Roth JA. Adjuvants in veterinary vaccines: modes of action and adverse effects. Journal of Veterinary Internal Medicine. 2003;17(3):273–81. doi: 10.1111/j.1939-1676.2003.tb02448.x. [DOI] [PubMed] [Google Scholar]
- 7.Meeusen EN, Walker J, Peters A, Pastoret P-P, Jungersen G. Current status of veterinary vaccines. Clin Microbiol Rev. 2007;20(3):489–510. doi: 10.1128/CMR.00005-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao K, Chen G, Shi X-m, Gao T-t, Li W, Zhao Y, et al. Preparation and efficacy of a live newcastle disease virus vaccine encapsulated in chitosan nanoparticles. PloS One. 2012;7(12):53314. doi: 10.1371/journal.pone.0053314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kjærup RM, Dalgaard TS, Norup LR, Bergman I-M, Sørensen P, Juul-Madsen HR. Adjuvant effects of mannose-binding lectin ligands on the immune response to infectious bronchitis vaccine in chickens with high or low serum mannose-binding lectin concentrations. Immunobiology. 2014;219(4):263–74. doi: 10.1016/j.imbio.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deville S, Arous JB, Bertrand F, Borisov V, Dupuis L. Efficacy of intranasal and spray delivery of adjuvanted live vaccine against infectious bronchitis virus in experimentally infected poultry. Procedia Vaccinol. 2012;6:85–92. doi: 10.1016/j.provac.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sánchez J, Holmgren J. Cholera toxin—a foe & a friend. Indian J Med Res. 2011;133(2):153. [PMC free article] [PubMed] [Google Scholar]
- 12.Braun MC, He J, Wu C-Y, Kelsall BL. Cholera toxin suppresses interleukin (IL)-12 production and IL-12 receptor β1 and β2 chain expression. J Exp Med. 1999;189(3):541–52. doi: 10.1084/jem.189.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klimpel GR, Asuncion M, Haithcoat J, Niesel DW. Cholera toxin and Salmonella typhimurium induce different cytokine profiles in the gastrointestinal tract. Infect Immun. 1995;63(3):1134–7. doi: 10.1128/iai.63.3.1134-1137.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson AD, Bailey M, Williams NA, Stokes CR. The in vitro production of cytokines by mucosal lymphocytes immunized by oral administration of keyhole limpet hemocyanin using cholera toxin as an adjuvant. Eur J Immunol. 1991;21(10):2333–9. doi: 10.1002/eji.1830211007. [DOI] [PubMed] [Google Scholar]
- 15.Akhiani A, NILSSON LÅ, OUCHTERLONY Ö. Intranasal administration of Schistosoma mansoni adult worm antigen in combination with cholera toxin induces a Th2 cell response. Parasite Immunol. 1997;19(4):183–90. doi: 10.1046/j.1365-3024.1997.d01-196.x. [DOI] [PubMed] [Google Scholar]
- 16.Stratmann T. Cholera toxin subunit B as adjuvant––an accelerator in protective immunity and a break in autoimmunity. Vaccines. 2015;3(3):579–96. doi: 10.3390/vaccines3030579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atlas RM. Handbook of Microbiological Media. 4th Edition ed. Washington D C : CRC Press; 2010. pp. 288–482. [Google Scholar]
- 18.CDC. Laboratory methods for the diagnosis of Vibrio cholerae. Atlanta, GA. 1994;500:38–67. [Google Scholar]
- 19.Ohtomo N, Muraoka T, Tashiro A, Zinnaka Y, Amako K. Size and Structure of the Cholera Toxin Molecule and Its Subunits. J Infect Dis. 1976;133(Supplement 1):S31–S40. doi: 10.1093/infdis/133.supplement_1.s31. [DOI] [PubMed] [Google Scholar]
- 20.Smith J, Sadeyen J-R, Cavanagh D, Kaiser P, Burt DW. The early immune response to infection of chickens with Infectious Bronchitis Virus (IBV) in susceptible and resistant birds. BMC Vet Res. 2015;11(1):256. doi: 10.1186/s12917-015-0575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toro H, Pennington D, Gallardo RA, Van Santen VL, Van Ginkel FW, Zhang J, et al. Infectious bronchitis virus subpopulations in vaccinated chickens after challenge. Avian Dis. 2012;56(3):501–8. doi: 10.1637/9982-110811-Reg.1. [DOI] [PubMed] [Google Scholar]
- 22.Eriksson K, Fredriksson M, Nordström I, Holmgren J. Cholera toxin and its B subunit promote dendritic cell vaccination with different influences on Th1 and Th2 development. Infect Immun. 2003;71(4):1740–7. doi: 10.1128/IAI.71.4.1740-1747.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.George-Chandy A, Eriksson K, Lebens M, Nordström I, Schön E, Holmgren J. Cholera toxin B subunit as a carrier molecule promotes antigen presentation and increases CD40 and CD86 expression on antigen-presenting cells. Infect Immun. 2001;69(9):5716–25. doi: 10.1128/IAI.69.9.5716-5725.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lebens M, Terrinoni M, Karlsson SL, Larena M, Gustafsson-Hedberg T, Källgård S, et al. Construction and preclinical evaluation of mmCT, a novel mutant cholera toxin adjuvant that can be efficiently produced in genetically manipulated Vibrio cholerae. Vaccine. 2016;34(18):2121–8. doi: 10.1016/j.vaccine.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Meinersmann RJ, Porter RE. Effect of Vibrio cholerae Toxin on Oral Immunization of Chickens. Avian Dis. 1993;37(2):427–32. [PubMed] [Google Scholar]
- 26.Quéré P, Girard F. Systemic adjuvant effect of cholera toxin in the chicken. Vet Immunol Immunopathol. 1999;70(1):135–41. doi: 10.1016/s0165-2427(99)00060-4. [DOI] [PubMed] [Google Scholar]
- 27.Snider DP. The mucosal adjuvant activities of ADP-ribosylating bacterial enterotoxins. Critical Reviews™ in Immunology. 2017;15(3-4):499–530. doi: 10.1615/CritRevImmunol.v37.i2-6.150. [DOI] [PubMed] [Google Scholar]
- 28.Tohidi E, Ghaniei A, Farzin H, Haghparast A. Humoral immunity provided by a novel infectious bronchitis vaccine supplemented by bacterium-like particles (BLPs) Turk J Vet Anim Sci. 2020;44(3):534–41. [Google Scholar]
- 29.Abodalal SA. Preparation And Evaluation Of Adjuvanted Live Vaccine Against Infectious Bronchitis. Egypt J Agric Res. 2017;95:1327–37. [Google Scholar]
- 30.Di Pasquale A, Preiss S, Tavares Da Silva F, Garçon N. Vaccine Adjuvants: from 1920 to 2015 and Beyond. Vaccines. 2015;3(2):320–43. doi: 10.3390/vaccines3020320. [DOI] [PMC free article] [PubMed] [Google Scholar]