Abstract
Endometrial hyperplasia is defined as a common clinical disorder caused by the increased levels of estrogens with low levels of progesterone; therefore, this hormonal imbalance leads to an increase in the proliferation rate of the endometrial cells. Endometrial carcinoma is one of the most important malignancies affecting women all over the world "accounting for 37.7% of all other disorders affecting the female reproductive system". The expression of the Ki-67 protein is related to the proliferative behavior of malignant tumor cell populations of their own, allowing it to be used as a marker of tumor aggressiveness. The present study was conducted to examine the expression of the proliferation marker, Ki-67 in various endometrial lesions. Ki-67 expression was evaluated in 60 endometrial samples that resulted as either endometrial curetting or hysterectomy specimens, diagnosed with simple hyperplasia (n=10), complex hyperplasia (n=20), atypical hyperplasia (n=6), and endometrial carcinoma (n=24). In patients with endometrial carcinoma, there was an increased expression of Ki-67, compared to proliferative endometrium and simple hyperplasia (P-value=0.0001). There was no such discrepancy between atypical hyperplasia and endometrial carcinoma cases. The expression of Ki-67 showed a positive association with the degree of endometrial cancer (P-value=0.0013), however, not with the age of the patients (P-value>0.05). There is a wide range of variations in the proliferation rate within the development of different endometrial lesions, including benign and malignant lesions. Our findings may be of value in differential diagnosis and prognosis of endometrial hyperplasia and endometrial carcinoma.
Keywords: Endometrial lesions, Ki-67, Manual IHC
1. Introduction
Endometrial hyperplasia is defined as a common clinical disorder caused by increased levels of estrogens with low levels of progesterone; as a result of this hormonal imbalance, an increase occurs in the proliferation rate of the endometrial cells. Endometrial hyperplasia is an important risk factor for endometrial cancer growth and development ( 1 ). Endometrial carcinoma is one of the most important malignancies affecting women all over the world "accounting for 37.7% of all other disorders affecting the female's reproductive system" ( 2 ). Women are more likely to be diagnosed between the ages of 45 and 65 years with endometrial carcinoma ( 3 , 4 ). The main risk factor for endometrial cancer is the incidence of endogenous or exogenous estrogen without satisfactory restriction by a progestin (e.g., postmenopausal estrogen therapy without progestin). Other risk factors include treatment with tamoxifen, obesity, and nulliparity ( 5 ).
Ki-67, also called pKi-67, is a nuclear protein that is encoded by the MKI67 gene. The Ki-67 antigen was initially recognized within the 1980s. It is solely expressed by proliferating cells and is closely related to cellular expansion. Consequently, monoclonal counteracting antibodies against the Ki-67 antigen are widely used in cancer diagnosis ( 6 , 7 ). Ki-67 acts as a nuclear antigen associated with proliferation, located on chromosome 10q25, which is only found in the dividing cells (Gap 1, synthesis, Gap 2, and mitosis phases), not in the G 0 phase ( 8 , 9 ). The gene encoding Ki-67 is a continuous sequence of 29,965-bp length comprised of the gene consisting of 15 exons and 14 introns ( 10 , 11 ). The over-expression of the Ki-67 protein is accompanying the proliferative activity of the malignant cells, allowing it to be used as a marker and as a good indicator for tumor aggressiveness ( 9 ). In a number of previously published studies, it has been documented that the prognostic rate of pKi67 and its latent role have been considered a reliable marker for breast, lung, prostate, cervical, central nervous system, and uterus cancers ( 12 ).
2. Materials and Methods
2.1. Samples
This retrospective study included 60 endometrial samples at the age range of 40-65 years old. The samples arising from either endometrial curettage or specimens of hysterectomy diagnosed with simple hyperplasia (n=10), complex hyperplasia (n=20), atypical hyperplasia (n=6), and endometrial carcinoma (n=24). They were collected from April 2017 to May 2019 in some private laboratories in the city of Hila, Iraq.
2.2. Immunohistochemistry of Ki-67 in Endometrial Tissues
Immunohistochemistry (EnVision system) was applied to determine the expression of Ki-67 in endometrial tissues, using primary antibody Ki67 (Monoclonal Mouse Anti-Human Ki-67 protein, Dako Denmark A/S). Endometrial biopsy which showed proliferative endometrium was considered for the control group. Clinical information was extracted from the archives of hematoxylin and eosin-stained sections and representative blocks, as well as some private pathology laboratories. The score for over-expression of Ki-67 ranged from 0% to 100% and the positive cutoff was ≥ 14%. The Ki-67 proliferation index was divided into three groups, including low (Ki-67≤15%), medium (Ki-67, 16-30%), and high (Ki-67>30%) ( 13 , 14 ).
2.3. Statistical Analysis
The collected data were analyzed in SPSS software (version 21) using the Chi-square test (P-value at the significance level of <0.05) and the correlation test (R at the significance level of 0.3).
3. Results
3.1. Immunohistochemically Detection of Ki-67
A total of 60 endometrial samples were included in this study from simple hyperplasia (n=10), complex hyperplasia (n=20), atypical hyperplasia (n=6), and endometrial carcinoma (n=24). Expression and localization of Ki-67 in the aforementioned endometrial tissues were examined immunohistochemically. The findings showed differences in the degree of the intensity staining and percentage of positively stained cells among the examined tissues.
The percentage of positive staining results in simple hyperplasia was markedly lower than 40% (4/10 patients) (Figure 1). However, complex hyperplasia (Figures 2 and 3) and typical hyperplasia demonstrated significant over the expression of Ki-67 protein at 80% (16/20 cases) and 100% (6/6 cases), respectively. Similarly, Ki-67 was expressed in 100% (24/24) of cases with endometrial carcinoma (Figure 4). The expression levels of Ki-67 in different endometrial tissues are shown in table 1.
Figure 1.
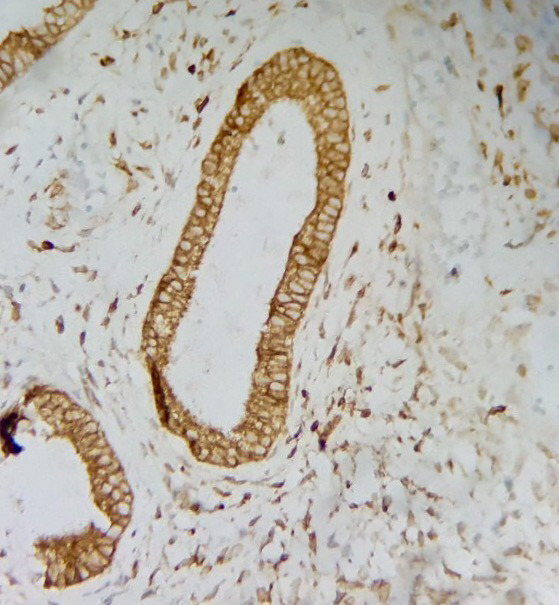
Simple hyperplasia immunohistochemistry of intermediate staining pattern of Ki-67 (40X)
Figure 2.
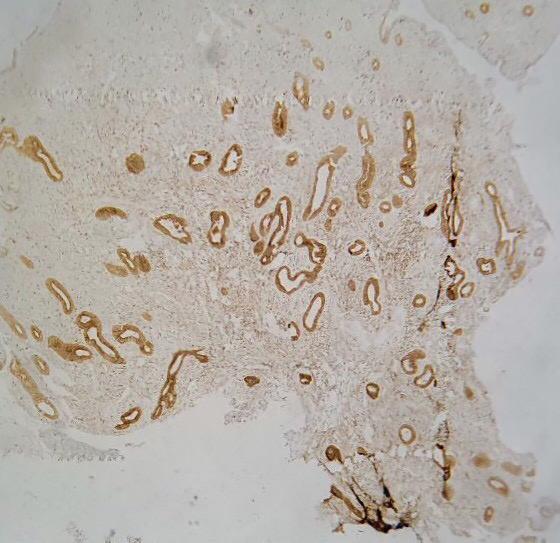
Complex hyperplasia immunohistochemistry of intermediate staining pattern of Ki-67 (40X)
Figure 3.
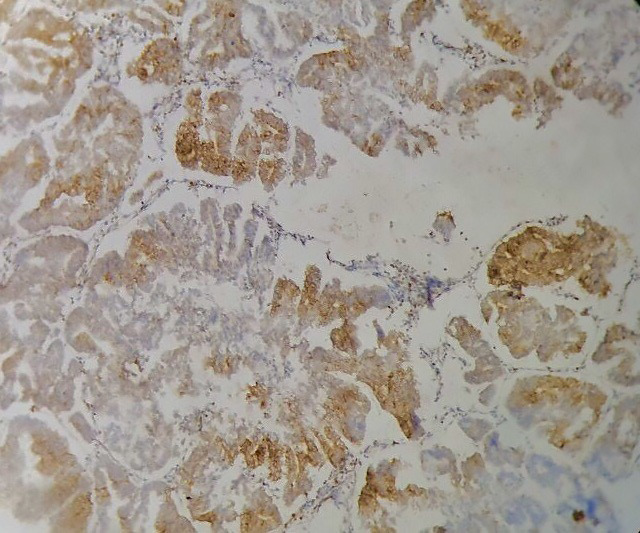
Complex hyperplasia immunohistochemistry of intermediate staining pattern of Ki-67 (40X)
Figure 4.
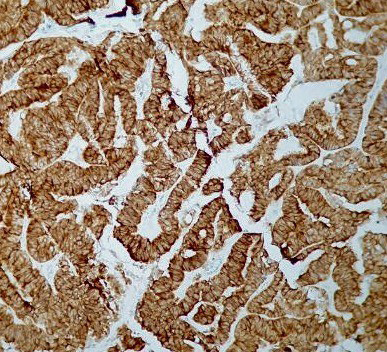
Endometrium carcinoma immunohistochemistry of high staining pattern of Ki-67 (40X)
Table 1.
Expression level of Ki-67 in different endometrial tissues
| Type of tissue | Immunostaining of Ki-67 | Total | ||
|---|---|---|---|---|
| Positive | Negative | |||
| Simple hyperplasia | 4 (40%) | 6 (60%) | 10 (16.7%) | P-value=0.0001 |
| Complex hyperplasia | 16 (80%) | 4 (20%) | 20 (33.3%) | |
| Atypical hyperplasia | 6 (100%) | 0 | 6 (10%) | |
| Endometrial carcinoma | 24 (100%) | 0 | 24 (40%) | |
| Total | 50 (83.3%) | 10 (16.7%) | 60 (100%) | |
3.2. Association between Ki-67 Expression and Clinical Grading of Endometrial Carcinoma
There was an association between the expression of Ki-67, and the clinical grading of endometrial carcinoma is presented in table 2. The incidence of positively-stained tumor cells with Ki-67 tended to be more frequent as the cancer grade increased (P-value=0.0013). No significant correlation was obtained between Ki-67 expression level and other variables, age, and histological type of endometrial cancer (P-value>0.05) (Figure 5).
Table 2.
Immunostaining of Ki-67 in relation to the grade of endometrial carcinoma
| Grade | Immunostaining of Ki-67 | Total | ||
|---|---|---|---|---|
| Positive | Negative | |||
| Grade I | 2 (50%) | 2 (50%) | 4 (16.7%) | P-value=0.0013 |
| Grade II | 6 (75%) | 2 (25%) | 8 (33.3%) | |
| Grade III | 12 (100%) | 0 | 12 (50%) | |
| Total | 20 (83.3%) | 4 (16.7%) | 24 (100%) | |
Figure 5.
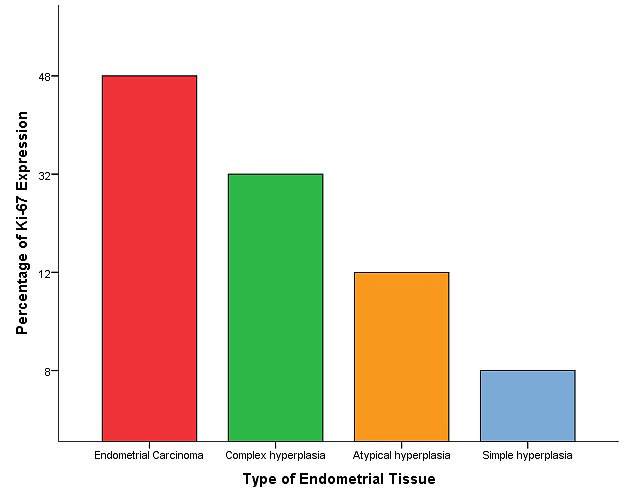
Distribution of positive Ki-67 cases in relation to the type of endometrial tissue
4. Discussion
Ki-67 protein expression is believed to be a valuable marker for cell growth and proliferation ( 15 ). Since the growth of tumor tissue depends on its proliferative activity ( 16 ), the expression of the Ki-67 proliferative marker was assessed in our study to investigate the potential diagnostic and prognostic roles of this marker in endometrial carcinoma. In a panel of histologically distinct endometrial tissues, the examination of Ki-67 expression showed a rise in Ki-67 expression from 40% to 80% in simple to complex hyperplasia and 100% in both atypical and endometrial carcinoma (P-value<0.0001). The results of the current study regarding the Ki-67 over-expression in normal endometrial proliferative glands and stroma to crowding glands showed that the Ki-67 immunostaining is a reliable technique to separate proliferative endometrial lesions with secretory modifications from precursor and neoplastic lesions in atypical hyperplasia and endometrial carcinoma. Furthermore, our findings indicated that the Ki-67 expression could be a marker for endometrial carcinogenesis and tumor cell proliferation. The gradual and progressive increase in the expression of Ki-67 levels among the examined endometrial tissues may be due to the physiological effect of estrogenic stimuli. Therefore, when these two cellular proteins are deregulated, they possibly have a role in endometrial carcinogenesis ( 15 , 17 ). Although the high mean score of Ki-67 in young women is associated with the increased rate of cellular proliferation and explains a highly aggressive type of carcinoma, there has been a lack of consensus regarding the predictive and prognostic influence of Ki-67 in cancer ( 18 - 20 ). This should be noted that the expression levels of Ki-67 were positively associated with the grade of endometrial cancer. Grade III tumors were stated to have higher scores than grade II and I tumors (P-value=0.0013). Given the importance of Ki-67 in maintaining cellular growth and proliferation, this correlation between Ki-67 expression patterns across the examined endometrial cancerous tissues is expected. However, no significant correlation was found with the patient's age or histological type of endometrial cancer (P-value>0.05). These findings are broadly consistent with previously reported results documenting a clear correlation between the Ki-67 marker and certain cancer-grade pathological prognostic parameters ( 21 , 22 ). The recorded data in the current study designated the importance of Ki-67 as a proliferation-associated protein that was differentially expressed in the four investigated histologically different endometrial tissues and demonstrated the highest expression level in endometrial carcinoma samples, compared to other samples. Our analysis revealed that both Ki-67 expression and endometrial cancer grade were positively correlated. Nevertheless, no such results were obtained when the patient's age or cancer histology was considered.
5. Conclusion
Our study was successful in adding details to the existing knowledge and shed the light on Ki-67 value as a potential mitotic indicator and promising prognostic biomarker, along with the cancer grade. In this regard, it is necessary to further analyze Ki-67 to attain additional diagnostic and prognostic information.
Authors' Contribution
Study concept and design: R. G. F. and I. A. A. A.
Acquisition of data: R. G. F. and I. A. A. A.
Analysis and interpretation of data: R. G. F.
Drafting of the manuscript: I. A. A. A.
Critical revision of the manuscript for important intellectual content: R. G. F. and I. A. A. A.
Statistical analysis: R. G. F.
Administrative, technical, and material support: R. G. F. and I. A. A. A.
Ethics
Ethical clearance was obtained from the Institutes Ethical Clearance Committee.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long‐term study of “untreated” hyperplasia in 170 patients. Cancer 1985;56(2):403–12. doi: 10.1002/1097-0142(19850715)56:2<403::aid-cncr2820560233>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Shin H, Bray F, Forman D, Mathers C, Parkin D. GLOBOCAN 2008, Cancer incidence and mortality worldwide: IARC CancerBase No. 10. Lyon, France: International Agency for Research on Cancer. 2008 [Google Scholar]
- 4.Gabryś M. Epidemiologia i etiopatogeneza raka błony śluzowej trzonu macicy. Ginekologia onkologiczna Urban & Partner, Wrocław. 2006:683–94. [Google Scholar]
- 5.Ogane N, Yasuda M, Kato H, Kato T, Yano M, Kameda Y, et al. Cleaved caspase‐3 expression is a potential prognostic factor for endometrial cancer with positive peritoneal cytology. Cytopathology. 2018;29(3):254–61. doi: 10.1111/cyt.12550. [DOI] [PubMed] [Google Scholar]
- 6.Bullwinkel J, Baron‐Lühr B, Lüdemann A, Wohlenberg C, Gerdes J, Scholzen T. Ki‐67 protein is associated with ribosomal RNA transcription in quiescent and proliferating cells. J Cell Physiol. 2006;206(3):624–35. doi: 10.1002/jcp.20494. [DOI] [PubMed] [Google Scholar]
- 7.Cuylen S, Blaukopf C, Politi AZ, Müller-Reichert T, Neumann B, Poser I, et al. Ki-67 acts as a biological surfactant to disperse mitotic chromosomes. Nature. 2016;535(7611):308–12. doi: 10.1038/nature18610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darzynkiewicz Z, Zhao H, Zhang S, Marietta YL, Ernest YL, Zhang Z. Initiation and termination of DNA replication during S phase in relation to cyclins D1, E and A, p21WAF1, Cdt1 and the p12 subunit of DNA polymerase δ revealed in individual cells by cytometry. Oncotarget. 2015;6(14):11735. doi: 10.18632/oncotarget.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klöppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann N Y Acad Sci. 2004;1014(1):13–27. doi: 10.1196/annals.1294.002. [DOI] [PubMed] [Google Scholar]
- 10.Le Guellec S, Perallon R, Alunni J-P, Charitansky H, Leaha C, Gonzalez AM, et al , editors. Neoadjuvant treatment of breast cancer: implications for the pathologist. Ann Pathol doi: 10.1016/j.annpat.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Li LT, Jiang G, Chen Q, Zheng JN. Ki67 is a promising molecular target in the diagnosis of cancer. Mol Med Rep. 2015;11(3):1566–72. doi: 10.3892/mmr.2014.2914. [DOI] [PubMed] [Google Scholar]
- 12.Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11(2):174–83. doi: 10.1016/S1470-2045(09)70262-1. [DOI] [PubMed] [Google Scholar]
- 13.Dowsett M, Nielsen TO, A’Hern R, Bartlett J, Coombes RC, Cuzick J, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103(22):1656–64. doi: 10.1093/jnci/djr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldhirsch A, Ingle JN, Gelber R, Coates A, Thürlimann B, Senn H-J. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009;20(8):1319–29. doi: 10.1093/annonc/mdp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gurda GT, Baras AS, Kurman RJ. Ki-67 index as an ancillary tool in the differential diagnosis of proliferative endometrial lesions with secretory change. Int J Gynecol Pathol. 2014;33(2):114–9. doi: 10.1097/PGP.0000000000000092. [DOI] [PubMed] [Google Scholar]
- 16.Zhong W, Peng J, Wu D, Han Z, Bi X, Dai Q. Ki-67 and PCNA expression in prostate cancer and benign prostatic hyperplasia. Clin Invest Med. 2008:8–15. doi: 10.25011/cim.v31i1.3136. [DOI] [PubMed] [Google Scholar]
- 17.Shevra C, Ghosh A, Kumar M. Cyclin D1 and Ki-67 expression in normal, hyperplastic and neoplastic endometrium. J Postgrad Med. 2015;61(1):15. doi: 10.4103/0022-3859.147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huvila J, Talve L, Carpén O, Edqvist P-H, Pontén F, Grénman S, et al. Progesterone receptor negativity is an independent risk factor for relapse in patients with early stage endometrioid endometrial adenocarcinoma. Gynecol Oncol. 2013;130(3):463–9. doi: 10.1016/j.ygyno.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 19.Liu T, Gao H, Yang M, Zhao T, Liu Y, Lou G. Correlation of TNFAIP8 overexpression with the proliferation, metastasis, and disease-free survival in endometrial cancer. Tumor Biol. 2014;35(6):5805–14. doi: 10.1007/s13277-014-1770-y. [DOI] [PubMed] [Google Scholar]
- 20.Stefansson I, Salvesen H, Immervoll H, Akslen L. Prognostic impact of histological grade and vascular invasion compared with tumour cell proliferation in endometrial carcinoma of endometrioid type. Histopathology. 2004;44(5):472–9. doi: 10.1111/j.1365-2559.2004.01882.x. [DOI] [PubMed] [Google Scholar]
- 21.Kitson S, Sivalingam VN, Bolton J, McVey R, Nickkho-Amiry M, Powell ME, et al. Ki-67 in endometrial cancer: scoring optimization and prognostic relevance for window studies. Mod Pathol. 2017;30(3):459–68. doi: 10.1038/modpathol.2016.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Truskinovsky AM, Lifschitz-Mercer B, Czernobilsky B. Hyperplasia and carcinoma in secretory endometrium: a diagnostic challenge. Int J Gynecol Pathol. 2014;33(2):107–13. doi: 10.1097/PGP.0b013e3182a2945d. [DOI] [PubMed] [Google Scholar]


