Abstract
Amebiasis and giardiasis are major health problems caused by Entamoeba histolytica and Giardia Lamblia which are the two most common intestinal protozoan parasites with worldwide distribution, especially in developing countries. Both protozoa are spread by the fecal-oral route; that is to say, by eating or drinking contaminated food or water. The present study aimed to assess the prevalence of E. histolytica and G.lamblia in children with clinical signs of diarrhea referring to Ibn Al-Atheer Hospital in Mosul, Iraq, from January 2019 to December 2020. A total of 2,296 samples were examined by the direct swab of stool method. The patients were within the age range of less than 1 year and above 12 years. The results demonstrated that in 2019 and 2020, the prevalence rates of E. histolytica infections were (152, 13.2%) and (181, 15.7%); moreover, the prevalence rates of G.lamblia were obtained at (10, 0.86%) and (12, 1.04%) (P<0.01). The prevalence of intestinal parasite infections was significantly associated with age and gender, and the prevalence of both E. histolytica and G.lamblia infections were higher in males. Furthermore, the highest and lowest prevalence rates of E. histolytica and G.lamblia infections were reported in the age groups of under 1 year and above 12 years (P<0.05). The high prevalence of protozoan infection in the age group of under 1 year can be attributed to their lack of developed immunity system and resistance. Due to disease transmission, the enhancement of health conditions is of utmost importance in controlling the prevalence of intestinal protozoan parasites.
Keywords: Prevalence, Entamoeba histolytica, Giardia Lamblia, Mosul city
1. Introduction
The high prevalence of intestinal protozoan parasites is one of the major health problems, affecting more than three billion people across the globe ( 1 - 4 ). The majority of infected people are children and infants, and based on World Health Organization (WHO), 450 million children and infants are infected by these parasites worldwide ( 5 , 6 ). Giardia Lamblia and Entamoeba histolytica are the most common causes of these intestinal parasites. Like other pathogens, they are widely and commonly distributed in developing countries. They cause dangerous diseases and ultimately lead to public health problems ( 7 ).
Amoebiasis, or amoebic dysentery, which frequently causes diarrhea or dysentery in children of developing countries is an infection caused by Entamoeba histolytica. Every year, 100,000 children die of Amoebiasis ( 8 - 10 ). Entamoeba histolytica is commonly transmitted by fecal-oral route; that is to say, by eating or drinking the food or water contaminated with the protozoan cyst. Moreover, it can be transmitted to the human body by soil, fresh vegetables, direct person-to-person contact, residing in endemic places, and swimming in infected water ( 11 , 12 ). The great prevalence of Entamoeba histolytica infections is highly connected with risk factors, such as poverty, inadequate nutrition, and lack of health services.
The common symptoms of Entamoeba histolytica infections are weight loss, bloody diarrhea, severe dysentery, physical tiresome, fatigue, and abdominal pain. The asymptomatic infection of Entamoeba histolytica seems to be more dangerous than infection with symptoms since negligence will cause severe amoebic dysentery and its side effects. In many cases, trophozoites in the intestinal lumen (non-invasive infection) are restricted to asymptomatic carriers. In some patients, trophozoites invade the intestinal mucosa (intestinal disease) or are transmitted through the bloodstream to extra-intestinal sites, such as the liver, brain, and lungs (extra-intestinal disease), with pathological manifestations.
Aamoeba can invade the liver and lead to a liver abscess which is life-threatening if left untreated. Sometimes, ingested red blood cells are found in the amoeba cell cytoplasm. Entamoeba histolytica can be detected in the large intestine (colon) without causing disease ( 13 ). The infection might be resolved spontaneously without threatening the life of the patient in the presence of hygiene and other necessary health elements.
Giardia Lamblia protozoa are one of the flagellates that can infect a wide range of hosts. In industrialized countries, the prevalence of Giardia Lamblia parasite ranges from 2%-5%, while in developing countries, it is quite different since it is not less than 30%. Infection with this parasite occurs as a result of taking the mature cysts of the parasite by mouth in eating food via the oral-fecal route ( 14 ). Due to poor sanitary conditions and low water quality control, Giardia Lamblia is widely prevalent in developing countries. Epidemiological studies have pointed out that most children under the age of 12 become infected with this parasite ( 15 ). Different intestinal symptoms of G. lamblia infection include diarrhea, steatorrhea, abdominal cramps, bloating, flatulence, pale greasy and malodorous stools, and weight loss.
Sometimes, the patient may also suffer from nausea or vomiting. Severe and active infection of Giardiasis may lead to lactose intolerance, which lasts for several months after recovering from this parasite ( 4 ). The reproduction of this protozoan in the intestinal of the host, leading to malnutrition and anemia by interfering with the absorption of vitamins, iron, proteins, minerals, lipids, and carbohydrates ( 16 ). Children who suffer from malnutrition are usually the most infected people, with impaired growth and weight loss being the common symptoms of their infection. Overall parasitic infection, in addition to intestinal symptoms, may also cause anemia, as well as physical and mental problems, such as delayed growth and weight loss in children.
In light of the aforementioned issues, the present study aimed to determine the prevalence of Entamoeba histolytica and Giardia Lamblia according to age and gender in children referring to Ibn al-Atheer Hospital in Mosul, Iraq, during 2019-2020.
2. Materials and Methods
2.1. Sampling and Study Design
A total of 2,296 stool samples of children with clinical signs of diarrhea and primary enteritis were collected from Ibn Al-Atheer hospital from January 2019 to December 2020. These samples were collected using clean plastic bottles coded with specific personal information, such as name, age, and collection date.
2.2. Stool Sample Processing
The characteristics of stools were recorded, including textures (mucous, serous, greasy, and bloody), as well as multiple colors (yellow, brown, semi-brown, and greenish). The samples were examined under a light microscope using a direct saline method prepared with 0.9 percent sodium chloride. a small amount of freshly passed stool was taken by a tip of a wood applicator, mixed with a drop of physiological saline or lugol's iodine solution, and put on a glass slide. This slide was carefully examined microscopically by the direct smear process for the presence of cysts or trophozoite ( 17 ).
2.3. Data Analysis
Data were analyzed in SPSS software (version 23) using ratio and rate for qualitative variables, as well as the mean and standard deviation for quantitative variables. To compare the qualitative variables, the chi-square test and t-test were employed to compare quantitative variables and assess the associations between potential risk factors and prevalence of intestinal parasitic infections. A p-value of less than 0.05 was considered to be statistically significant.
3. Results and Discussion
In this study, a total of 2,296 patients who visited Ibn Al-Atheer Hospital in Mosul underwent stool exams (from January 2019 to December 2020). Results in figures 1 and 2 indicate the numbers of infections with parasites of E. histolytica and G. lamblia in this period. A number of 1,150 samples were examined in 2019, and the number of E. histolytica infections was obtained at 152(13.2%) which was higher than infections with G. lamblia (10; 0.86%), and the differences were significant at the probability level (P<0.01). In 2020, 1, 146 samples were examined, among which 181 (15.79%) samples were positive for E. histolytica infection, which was also higher than infection with G. lamblia (n=12; 1.04%), and the differences were significant at the probability level (P<0.01).
Figure 1.
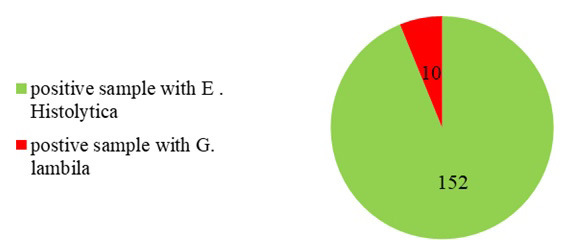
Number of infection with E. histolytica and G. lamblia in children in Mosul in 2019
Figure 2.
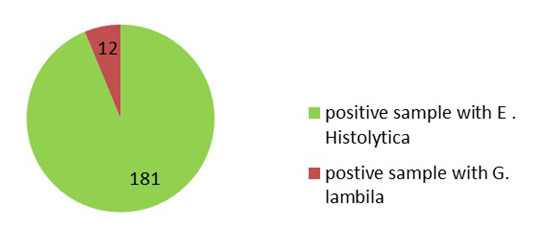
Number of infections with E. histolytica and G. lamblia in children in Mosul in 2020
Figures 3 and 4 display the relationship between infection with E. histolytica and G. lamblia according to gender for the years 2019 and 2020. The results show that the prevalence of the infection in males was more than that in females in 2019 and 2020. In 2019, the prevalence of the infected males with E. histolytica was reported as 89 (7.7%) which was higher than females (63; 5.47%). Moreover, the prevalence of infection with G. lamblia in males (6; 0.52%) was also higher than in females (4; 0.34%) (P<0.05).
Figure 3.
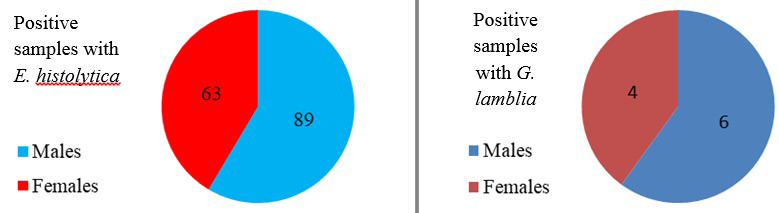
Infection with E. histolytica and G. lamblia according to gender in children in Mosul in 2019
Figure 4.
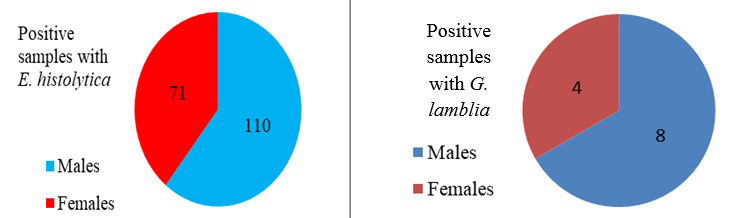
Infection with E. histolytica and G. lamblia according to gender in children in Mosul in 2020
Data analysis in 2020 revealed an increase in the prevalence of E. histolytica in males (110; 9.59%), as compared to females (71; 6.19%) (P<0.01). The prevalence of G. lamblia infection in males was also higher in males (8; 0.69%), in comparison with females (4; 0.34%) (P<0.05).
These results were consistent with those obtained in other studies which pointed out that diarrhea showed a prevalence in males, as compared to that in females ( 18 - 21 ). In addition, the findings of the current study were in line with those obtained by Obadiah ( 22 ) who reported that the prevalence rates of E. histolytica were 48.8% and 34.44% in males and females, respectively. Furthermore, there are other reports pointing to the higher prevalence of this disease in males than in females (27.7% and 24.3% respectively) ( 23 ). The high prevalence of E. histolytica in males can be attributed to the fact that females are less susceptible to parasitic infections. This is due to the low immunity of males, who generally show an increased intensity of infection, as compared to females ( 24 , 25 ).
These differences in responses to infection are due to variations in environmental factors and physiological ones that are originally hormonal ( 26 ). These differences in the infection among the two genders can also be ascribed to variations in endocrine-immune interactions. Furthermore, sex steroids, especially androgens in males and estrogens in females, modify many host immunity aspects, and androgens reduce immune competence. The steroid hormones affect disease resistance genes and behaviors that make males more susceptible to infection ( 25 ). In females, the low prevalence might be also due to more concern for hygiene care ( 13 ).
The results of the assessment of the relationship between age and infection with E. histolytica and G. lamblia (2019, 2020) are depicted in figures 5 and 6. In 2019, the highest and lowest rates of infection were reported in the age ranges of under 1 year and above 12 years (574; 49.9%) (14; 1.2%), respectively. The highest and lowest prevalence rates of E. histolytica infection were reported in the age ranges of und 1 year and above 12 years (65, 5.6%) and (2, 0.17%) (P<0.01). Furthermore, G. lamblia had the highest infection in the age range of under 1 year (3, 0.26%), and there was no infection in the age group of above 12 years, and the differences were not significant.
Figure 5.
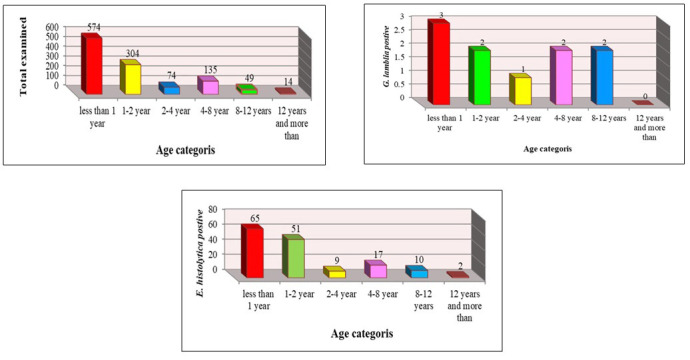
Infection with E. histolytica and G. lamblia in children according to age in Mosul in 2019
Figure 6.
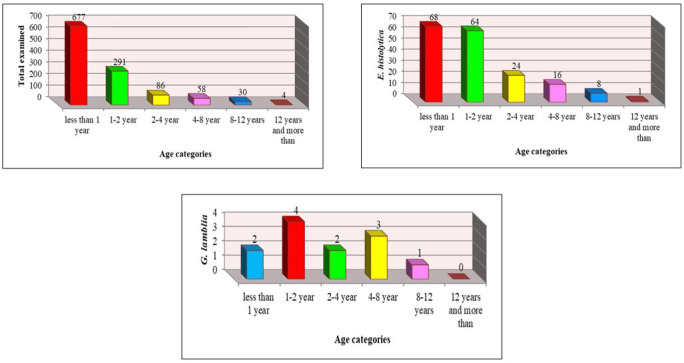
Infection with E. histolytica and G. lamblia in children according to age in Mosul in 2020
Furthermore, the prevalence rates of infection in 2020 were the same as in 2019 with some variations. The highest and lowest prevalence rates of infection were in the age groups of under 1 year (677; 59.07%) and above 12 years (4, 0.34%). The highest and lowest prevalence rates of infection with E. histolytica were in the age groups of under 1 year (68, 5.93%) and above 12 years (1, 0.08%) (P<0.01). In addition, the prevalence of G. lamblia in the age group of 1-2 years was (4, 0.34%) and there was no infection in the age group of above 12 years, and the differences were not significant.
These results were in accordance with those obtained in some studies which reported the prevalence rates of E. histolytica as 15.6% in preschool children in Bangladesh ( 27 ), 21.0% in seven villages of Malaysia ( 28 ), and 11% in children of Delhi, India, and Pakistan, ( 29 , 30 ). These results are also in agreement with those indicated in Al-Karamah Teaching Hospital in Iraq ( 31 ).
This high infection in children in this age group can be attributed to their poor health and resistance, compared to those who are older. Moreover, not fully developed crucial defense systems of these children make it difficult for them to resist disease; accordingly, they become more sensitive to parasites, as compared to older individuals. Furthermore, lack of adequate water, toilet training, poor hygiene, lack of social support, climatic conditions, and large populations all play important roles in the prevalence of E. histolytic and G infection. ( 11 , 13 , 31 ). To prevent infection with E. histolytica and G. lamblia, community health education needs to be improved, with hygiene, cleanliness, and water purification being the important aspects of community health ( 31 , 32 ).
Authors' Contribution
Study concept and design: Z. D. M. Z.
Acquisition of data: Z. D. M. Z.
Analysis and interpretation of data: Z. D. M. Z.
Drafting of the manuscript: Z. D. M. Z.
Critical revision of the manuscript for important intellectual content: Z. D. M. Z.
Statistical analysis: Z. D. M. Z.
Administrative, technical, and material support: Z. D. M. Z.
Ethics
All procedures performed in the study involving human participants were in accordance with the ethical standards of the Al-Noor University College, Bartella, Iraq.
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgment
I would like to thank prof. Dr. Ibraheem Ahmed Abdullah for his constant support, recommendations, and guidance that helped to conduct this research project.
References
- 1.Abdullah A. Prevalence of Intestinal Parasites (Entamoeba species and Giardia lamblia) in Duhok and Erbil cities, Northern Iraq. Journal of Microbiology & Experimentation. 2017;4 [Google Scholar]
- 2.Cordova Paz Soldan O, Vargas Vasquez F, Gonzalez Varas A, Perez Cordon G, Velasco Soto JR, Sanchez-Moreno M, et al. Intestinal parasitism in Peruvian children and molecular characterization of Cryptosporidium species. Parasitol Res. 2006;98(6):576–81. doi: 10.1007/s00436-005-0114-7. [DOI] [PubMed] [Google Scholar]
- 3.Costa JO, Resende JA, Gil FF, Santos JFG, Gomes MA. Prevalence of Entamoeba histolytica and other enteral parasitic diseases in the metropolitan region of Belo Horizonte, Brazil. A cross-sectional study. Sao Paulo Med J 2018;136(4):319–23. doi: 10.1590/1516-3180.2018.0036170418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar S, Singh VA. Prevalence of Entamoeba histolytica and Giardia lamblia infection in a Rural Area of Haryana, India. Int J Curr Microbiol Appl Sci. 2016;5(6):204–9. [Google Scholar]
- 5.Abdulhaleem N, Mahmuda A, Al-Zihiry K, Abd Majid R, Lung L, Abdullah W, et al. An overview of the prevalence and distribution of gastrointestinal parasitic infections in post-war Iraq. Trop J Pharm Res. 2017;16:1443. [Google Scholar]
- 6.WHO. World Health Report 2000-Conquering Suffering Enriching Humanity. Geneva: WHO, 2000. World Health Organization, 2004. Guidelines for Drinking-water quality Geneva 2000 [3r] [Google Scholar]
- 7.Al Saqur IM, Al-Warid HS, Albahadely HS. The prevalence of Giardia lamblia and Entamoeba histolytica/dispar among Iraqi provinces. Karbala Int J Mod Sci. 2017;3(2):93–6. [Google Scholar]
- 8.Bayoumi M, Nykwac O, Kardaman M, Ullberg M, Alshammari E, Sandström G, et al. Intestinal Parasitic Infections in School Students in Malakal City, Upper Nile State, South Sudan. Microbiol Infect Dis. 2016;4:1–5. [Google Scholar]
- 9.Ibrahim MA, Hussein RA, Al-Mayah QS. Molecular identification and genetic diversity of Entamoeba species from diarrheic patients in Baqubah / Iraq. Dusunen Adam. 2019;10:951–60. [Google Scholar]
- 10.Saleh FE, A Gad M, A Ashour A, I Soliman M, M El-Senousy W, Z Al-Herrawy A. A Molecular detection of Entamoeba histolytica in fresh vegetables and irrigation. Egypt J Aquat Biol Fish. 2019;22(5 (Special Issue)):551–61. [Google Scholar]
- 11.Callixte C, Ayubu A, Lestari P, Daniel N, Budhy TI. Epidemiological Prevalence of Entamoeba histolytica Infections Among the Patients Attending Nyanza District Hospital, Rwanda in 2018. Int J Epidemiol Res. 2019;6(4):149–53. [Google Scholar]
- 12.Khalaf MS, Rashid SA. Molecular Study of Enamoeba dispar and Entamoeba moshkovskii isolated from amoeboid dysentery in comparison with Entamoeba histolytica infections. J Pharm Sci Res. 2018;10(9):2129–33. [Google Scholar]
- 13.Abioye JOK, Mbagwu TT, Babatunde S. Prevalence of Entamoeba histolytica in Bingham University and Environs. EC Microbiol. 2019;15(4):242–50. [Google Scholar]
- 14.Tektook NK, Al-abodi HR, Pirko EYJBR-t. Prevalence, risk factors and molecular investigation of Giardiasis amonginfants in Al-Shamia City, Al-Qadisiya Governorate, Iraq. Biomed Res. 2019;30:1–4. [Google Scholar]
- 15.Bahrami F, Haghighi A, Zamini G, Khadem-Erfan M, Azargashb E. Prevalence and associated risk factors of intestinal parasitic infections in Kurdistan province, northwest Iran. Cogent Med. 2018;5 [Google Scholar]
- 16.Bazzaz A, Shakir O, Hamad R. Prevalence of Two Gastrointestinal Parasites Entamoeba histolytica and Giardia lamblia within Samarra City, Iraq. Adv Biosci Biotechnol. 2017;08:399–410. [Google Scholar]
- 17.Singh A, Houpt E, Petri WA. Rapid Diagnosis of Intestinal Parasitic Protozoa, with a Focus on Entamoeba histolytica. Interdiscip Perspect Infect Dis. 2009;2009:547090. doi: 10.1155/2009/547090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Ammash MSJ. Study on prevalences of Entameoba histolytica & Giardia lamblia in Samarra city. Kufa J Vet Med Sci. 2016;6 [Google Scholar]
- 19.AL-Kllaby KKA. Epidemiological study for common intestinal pathogens & related with acute diarrhea in children in Najaf governorate: Univ Kufa; 1999 [Google Scholar]
- 20.AL-Mashhadani WSH. Isolation & diagnosis for some Microbial causes to diarrhea & resistant bacterial isolations to antibiotic & product it of betalactamase enzyme. Al-mustansiriya: Coll. Sci., Univ; 2000 [Google Scholar]
- 21.Salman AO. Epidemiology study to intestinal parasites in infective children with diarrhea & intended two children hospital in Baghdad city. Univ Baghdad. 2002 [Google Scholar]
- 22.Obadiah H. Review of the survey of Entamoeba histolytica in Children a Brief Focus on Nigeria Situation. J Physiol Pharmacol Adv. 2012;2(3):150–7. [Google Scholar]
- 23.Reuben R, Katsa M, Suleiman H. Prevalence of Intestinal Amoebiasis in School Age Children in Lafia, Nasarawa State, Nigeria. 2013;2:1–6. [Google Scholar]
- 24.Klein SL. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci Biobehav Rev. 2000;24(6):627–38. doi: 10.1016/s0149-7634(00)00027-0. [DOI] [PubMed] [Google Scholar]
- 25.Klein SL. Hormones and mating system affect sex and species differences in immune function among vertebrates. Behav Process. 2000;51(1):146–66. doi: 10.1016/s0376-6357(00)00125-x. [DOI] [PubMed] [Google Scholar]
- 26.Zuk M, McKean KA. Sex differences in parasite infections: Patterns and processes. Int J Parasitol. 1996;26(10):1009–24. [PubMed] [Google Scholar]
- 27.Ali IK, Hossain MB, Roy S, Ayeh-Kumi PF, Petri WA, Jr. , Haque R, et al. Entamoeba moshkovskii infections in children, Bangladesh. Emerg Infect Dis. 2003;9(5):580–4. doi: 10.3201/eid0905.020548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aza A, Ashley S, Albert J. Parasitic infection in human communities living on the fringes of the Crocker Range Park Sabah, Malaysia. ARBEC. 2003 [Google Scholar]
- 29.Lee KJ, Bae YT, Kim DH, Deung YK, Ryang YS, Kim HJ, et al. Status of intestinal parasites infection among primary school children in Kampongcham, Cambodia. Korean J Parasitol. 2002;40(3):153–5. doi: 10.3347/kjp.2002.40.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tasawar Z, Kausar S, Lashari MH. Prevalence of Entamoeba histolytica in humans. Pak J Pharm Sci. 2010;23(3):344–8. [PubMed] [Google Scholar]
- 31.Rahi AA, Majeed L. Epidemiological study of intestinal protozoa at Wasit province. EAS J Orthop Physiother. 2019;1(3):26–8. [Google Scholar]
- 32.Dhawan VK. Current diagnosis and treatment of amoebiasis. US Infect Dis. 2008;4(1):59–61. [Google Scholar]


