Abstract
Background & Aims:
Apelin is the endogenous ligand of its G-protein coupled receptor, APJ, and apelin serum levels are increased in human liver diseases. We evaluated whether apelin-APJ regulates ductular reaction and liver fibrosis during cholestasis.
Approach & Results:
We measured the expression of apelin and APJ, and serum apelin levels in human primary sclerosing cholangitis (PSC) samples. Following bile duct ligation (BDL) or sham surgery, male wild-type mice were treated with ML221 (APJ antagonist) or saline for 1 wk. WT and apelin−/− mice underwent BDL or sham for 1 week. Mdr2−/− mice were treated with ML221 for 1 wk. Apelin levels were measured in serum and cholangiocyte supernatants, and cholangiocyte proliferation/senescence and liver inflammation, fibrosis and angiogenesis were measured in liver tissues. The regulatory mechanisms of apelin-APJ in: (i) biliary damage and liver fibrosis were examined in human biliary cells (HIBEpiCs) treated with apelin; and (ii) HSC activation in apelin-treated human hepatic stellate cell lines (HHSteCs). Apelin serum levels and biliary expression of apelin and APJ increased in PSC samples. Apelin levels were higher in serum and cholangiocyte supernatants from BDL and Mdr2−/− mice. ML221 treatment or apelin−/− reduced BDL- and Mdr2−/−-induced cholangiocyte proliferation/senescence, liver inflammation, fibrosis and angiogenesis. In vitro, apelin induced HIBEpiC proliferation and Nox4 expression, ROS levels and ERK phosphorylation in HIBEpiCs. Pretreatment of HIBEpiCs with ML221, DPI (Nox4 inhibitor), NAC (ROS inhibitor) or PD98059 (ERK inhibitor) reduced apelin-induced cholangiocyte proliferation. Activation of HHSteCs was induced by apelin, but reduced by NAC.
Conclusions:
Apelin-APJ axis induces cholangiocyte proliferation via Nox4/ROS/ERK dependent signaling and induces HSC activation via intracellular ROS. Modulation of the apelin-APJ axis may be important for managing cholangiopathies.
Keywords: Apelin, APJ, Cholangiocyte proliferation, Liver fibrosis, Reactive oxygen species
Apelin is the endogenous ligand of its G-protein coupled receptor, APJ (1). The APLN gene encodes a secreted 77-amino acid precursor called preproapelin. The preproapelin is cleaved into a family of apelin fragments, including apelin-36, apelin-17, apelin-13, apelin-12. Apelin and APJ are widely expressed in the central nervous system as well as peripheral organs, including heart, brain, kidney, liver, and adipose tissue (2).
The apelin-APJ axis plays a key role in organ fibrosis, although the effect remains controversial. The apelin-APJ has been shown to attenuate renal and myocardial fibrosis, while it has been shown to promote liver fibrosis (3, 4). Studies have shown that: (i) serum apelin levels are elevated in patients with chronic liver diseases; (ii) there is a significant correlation between apelin serum levels and Child-Turcotte-Pugh’s (CTP) and MELD score; and (iii) the expression of APJ is increased in the liver of cirrhotic patients (5). Apelin serum levels and apelin/APJ liver expression are overexpressed in the liver of cirrhotic rats (6). APJ blockade prevents the progression of fibrosis in CCl4-treated rats (7).
In cholestatic liver diseases, such as primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC), cholangiocytes (target of these diseases) are the key link between biliary injury and subepithelial fibrosis (8). Also, apelin activation of APJ regulates proliferation via extracellular signal-regulated kinase 1/2 (ERK1/2)-dependent signaling mechanisms (9). Inhibition of apelin signaling with the APJ antagonist, ML221, inhibits cholangiocarcinoma growth (10).
During the progression of fibrosis, quiescent hepatic stellate cells (HSCs) are activated to induce a transformation into a myofibroblast-like phenotype, leading to cell proliferation, expression of pro-fibrogenic mediators and production of extracellular matrix (ECM) constituents (11). Apelin is overexpressed in HSCs of cirrhotic rats and causes increased synthesis of collagen-I and platelet-derived growth factor receptor β (PDGFRβ) in HSCs in vitro (12). Thus, we aimed to evaluate whether apelin-APJ regulates cholangiocyte proliferation and liver fibrosis during the development of cholestasis and the underlying mechanisms involved.
Materials and methods
Materials
Reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise indicated. The antibodies for cytokeratin-19 (CK-19) (ab 52625), desmin (ab185033) and apelin (ab 59469) were purchased from Abcam (Cambridge, MA); F4/80 (70076s), p-ERK (4370) and ERK (4695) were purchased from Cell Signaling Technology (Beverly, MA); α-SMA (α-smooth muscle actin, c6198) were purchased from Sigma-Aldrich; apelin receptor (sc-33823) was purchased from Santa Cruz (Dallas, TX). All selected primers were purchased from Qiagen (Germantown, MD); specific information about the primers was listed in Supplementary Table 1. The iScript cDNA Synthesis Kit (170–8891) and iTaq Universal SYBR Green Supermix (172–5124) were purchased from Bio-Rad (Hercules, CA).
Animal models
All animal experiments were performed in accordance to protocols approved by the Baylor Scott & White Healthcare Institutional Animal Care and Use Committee. C57BL/6 wild-type (WT) mice (control for apelin−/− mice) were purchased from Charles River (Wilmington, MA). Male Mdr2−/− mice were originally purchased from Jackson Laboratories (Bar Harbor, ME); the colonies were then established in our facility. FVB/NJ mice (WT, control for Mdr2−/− mice) were purchased from Jackson Laboratories. These mice were maintained in a temperature-controlled environment (20–22°C) with 12:12-hr light/dark cycles and fed with standard mouse chow along with free access to drinking water ad libitum. Male WT mice underwent bile duct ligation (BDL) or sham and were treated with the apelin inhibitor, ML221 (13) (Tocris Bio-Techne, Minneapolis, MN, 150 µg/kg, 3×weekly via tail vein injections) for 1 wk. The apelin−/− breeding pair was a gift from Dr. Hyung Chun (Yale University, New Haven, CT). Male apelin−/− mice (12 wk age) underwent BDL or sham for 1 wk (14). Male Mdr2−/− mice (12 wk age) were treated with ML221 (150 μg/kg, 3×weekly via tail vein injections) (Mdr2−/− + ML221) for 1 wk (10). Animal groups were summarized in Supplementary Table 2. Following surgery and selected treatments, we collected serum, total liver samples, cholangiocytes, and cholangiocyte supernatant (after 6 hr incubation at 37°C) from the selected groups of animals.
Human samples
The liver tissue samples (OCT-embedded blocks, late stage PSC patients n=7) and serum (healthy human n=8, PSC patients n=26) were obtained under the Institutional Review Board approved protocol at Indiana University Purdue University Indianapolis. Healthy human liver OCT-embedded blocks (n=4) were purchased from Xeno Tech (Kansas, KS). Patient characteristics were listed in Supplementary Table 3.
Isolated mouse cholangiocytes and hepatic stellate cells (HSCs)
Mouse cholangiocytes were obtained by immunoaffinity separation using a mouse IgG2a monoclonal antibody (a gift from Dr. R. Faris, Brown University, Providence, RI), against an unidentified antigen expressed by all rodent cholangiocytes (14). Mouse HSCs were isolated by laser capture microdissection (LCM) (14). Following immunofluorescent staining, desmin-positive cells were dissected from the slides by the LCM system Leica LMD7000 (Buffalo Grove, IL). RNA from HSCs were extracted with the Arcturus PicoPure RNA isolation kit (Thermo Fisher Scientific, Mountain View, CA).
Immunofluorescent staining
Frozen liver sections (6 μm) were placed on glass slides and fixed in 4% paraformaldehyde and permeabilized by 0.1% Triton X-100 at room temperature. Afterwards, sections or cells were blocked with 5% bovine serum albumin and incubated with the selected primary antibodies overnight at 4°C: (i) apelin/APJ co-stained with CK-19 for the expression of apelin and APJ in cholangiocytes; (ii) p-ERK co-stained with CK-19 for the phosphorylation of ERK in proliferating cholangiocytes; (iii) desmin co-stained with α-SMA for the proliferation of HSC, and co-stained with APJ for the expression of APJ in HSCs; (iv) APJ for the immunoreactivity of APJ in human hepatic stellate cell lines (HHSteCs, Sciencell, Carlsbad, CA); and (v) CD31 co-stained with CK-19 for the evaluation of angiogenesis. Next, the samples were incubated with appropriate Cy3/Cy2/Cy5-conjugated secondary antibodies (1:100, Jackson ImmunoResearch Laboratories) for 1 hr at room temperature. Stained sections were observed under an Olympus Fluoview 300 confocal microscope (Olympus, Center Valley, PA) with 4′,6-diamidino-2-phenylindole (DAPI) (Thermo Fisher Scientific) as a nuclear counterstain.
Immunohistochemical staining
We performed immunohistochemical analyses in rodent and human liver sections by standard techniques (14). Briefly, following deparaffinization and antigen retrieval, paraffin-embedded liver sections (5 μm) were incubated with the selected primary and secondary antibodies. Following staining, slides were mounted with a cover slip. Then, liver sections were examined by Image Pro-Analyzer software (Olympus, Tokyo, Japan). Changes in intrahepatic bile duct mass (IBDM, stained with CK-19) (14, 15) and Kupffer cell number (by staining for F4/80) (14) in liver sections were semi-quantitatively measured by Visiopharm software (10 different fields analyzed from three different samples from five different animals).
Assessment of liver histology, serum chemistry, biliary senescence and liver fibrosis
Liver histology was evaluated by standard H&E staining in paraffin-embedded liver sections (4–6 μm). Following H&E staining, images were captured by Olympus Image Pro-Analyzer software 7.0 (Olympus, Tokyo, Japan) and evaluated by a board-certified pathologist. Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALK PHOS), and total bilirubin were measured by a Dimension RxL Max Integrated Chemistry system (Dade Behring Inc., Deerfield IL) at the Department of Chemistry, Baylor Scott & White Healthcare, Temple TX. Biliary senescence was evaluated in frozen liver sections (10 μm) by staining for senescence-associated-β-galactosidase (SA-β-gal) using the commercially available cellular senescence assay kit (Millipore Sigma, Billerica, MA) (14, 15). Collagen deposition in liver sections (5 μm) was assessed by Sirius Red staining (10 different fields were analyzed from three different samples from three different animals) (14, 15). Liver fibrosis was also measured by hydroxyproline levels in total liver samples using the hydroxyproline Assay Kit (14).
Determination of apelin and reactive oxygen species (ROS) levels
Commercially available Enzyme Linked Immunosorbent Assay (ELISA) kits for measuring apelin levels in human and mouse serum, cell culture medium, cell lysate and cholangiocyte supernatant were purchased from Sigma-Aldrich (16). The commercially available kits for measuring ROS levels in liver samples and cultured cells were purchased from Abcam (Cambridge, MA) and Cell Biolabs, Inc. (San Diego, CA). ELISA and ROS kits were performed according to the manufacturer’s instructions.
Immunoblots
Immunoblots were performed by standard techniques (15). Protein bands were visualized by Fujifilm Image Reader LAS-4000 (Fujifilm,Japan) chemiluminescence densitometric analysis was performed using Image J. β-actin was used as an internal control. For the phosphorylation of ERK, data was presented as p-ERK/ERK.
Total RNA Isolation and Real-time PCR Assays
RNA was isolated from liver tissues and cell lysates using mirVanaTM miRNA Isolation Kit (Ambion, Mountain View, CA) according to the manufacturer’s protocol. Quantitation of RNA was done using Nanodrop 2000 (Thermo Fisher Scientific, Mountain View, CA). cDNA synthesis was carried out using the Affinity Script Multi Temperature cDNA Synthesis Kit (Bio-Rad, Hercules, CA) or TaqMan™ MicroRNA Reverse Transcription Kit (Thermo Fisher Scientific). Real-time PCR was performed using RT2 SYBR Green (Bio-Rad,) or TaqMan™ Universal PCR Master Mix (Thermo Fisher Scientific) for a ViiA 7 Real-Time PCR System according to the manufacturer’s protocol (Applied Biosystems) (14). Glyceraldehyde-3-phosphate dehydrogenase was used as housekeeping; data were analyzed using the PCR array data analysis template (Applied Biosystems). All samples were tested in triplicate, and average values were used for quantification.
In vitro studies
The in vitro studies were performed in human primary cholangiocyte cell line (HIBEpiCs; Sciencell, Carlsbad, CA) and HHSteCs. All cells were grown in 5% CO2 in a humidified incubator maintained at 37°C. When the cells were treated with ML221 (10 μM) (10), DPI (10 μM, Nox4 inhibitor) (17), NAC (5 mM, ROS inhibitor) (18) or PD98059 (10 μM, ERK inhibitor) (19); these compounds were added to the medium 1 hr prior to apelin (10 μM) treatment.
Cell proliferation assay:
Cell proliferation was measured using the MTS CellTiter 96 Aqueous One Solution Reagent assays according to the manufacturer’s instructions (Promega Corporation, Madison, WI). Briefly, cells were seeded in 96-well culture plates and treated accordingly in different groups for 24 hr (apelin, apelin+ML221, apelin+DPI, apelin+NAC, apelin+PD98059). After treatments, MTS was added to each well for 1 hr. The absorbance was measured at 490 nm. Cell proliferation was evaluated directly based on optical density.
Detection of ROS:
Free radicals and other reactive species were detected using the Cellular ROS/Superoxide Detection Assay Kits (Abcam) according to the manufacturer’s instructions. Fluorescence Microplate Assay: Cells were seeded in 96-well plates. After 24 hr, the culture medium was removed. The cells were then incubated with the ROS/Superoxide Detection Mix and treatments (apelin, apelin+ML221, apelin+DPI, apelin+NAC) for 60 min at 37°C. After incubation, intracellular ROS levels were determined using a fluorescence microplate reader. Fluorescence/Confocal Microscopy Assay: Cells were seeded onto 35 mm Thermo Scientific Nunc Glass Bottom Dishes (Thermo Fisher Scientific, Mountain View, CA). After 24 hr, the culture medium was removed. The cells were then incubated with the ROS/Superoxide Detection Mix and treatments (apelin, apelin+ML221, apelin+DPI, apelin+NAC) for 60 min at 37°C. After incubation, Fluorescence images were captured using Leica AF 6000 Modular Systems.
Statistical analysis
All data are expressed as the mean ± SEM. Differences between groups were analyzed by unpaired Student’s t test when two groups were analyzed and one-way ANOVA when more than two groups were analyzed, followed by an appropriate post hoc test. The level of significance was set at P < 0.05.
Results
APJ mediated apelin expression is increased in cholangiocytes during cholestasis
To address the relevance of apelin to human cholestatic liver disease, we measured the immunoreactivity of apelin and APJ in liver sections from healthy controls and patients with late stage PSC. Liver sections of PSC patients showed increased biliary immunoreactivity of apelin and APJ compared with controls (Figure 1A–B). The mRNA expression of APLN (gene for apelin) and APLNR (gene for APJ) was increased in a human cholangiocyte cell line (hPSCL, from an unidentified 46-year old male patient with stage 4 PSC without cholangiocarcinoma; a gift of Dr. Nicholas LaRusso, Mayo Clinic, Rochester, MN) compared with HIBEpiC (control for hPSCL) (20) (Figure 1C). Apelin serum levels were higher in PSC patients compared with healthy controls (Figure 1D). The levels of apelin in serum and cholangiocyte supernatant were increased in BDL mice compared with WT mice (Figure 1E). The expression of Apln and Aplnr was increased in cholangiocytes from BDL and Mdr2−/− mice compared with control mice (Supplementary Figure 1A). By immunofluorescence, there was enhanced immunoreactivity for apelin and APJ (co-stained with CK-19) in cholangiocytes from BDL mice compared with WT mice (Supplementary Figure 1B–C). Apelin serum levels were increased in Mdr2−/− mice compared with WT mice (Figure 1F).
Figure 1. APJ mediated apelin expression is increased in cholangiocytes during cholestasis.
A : Immunofluorescence of apelin (co-stained with CK19) in liver sections from PSC patients (n=7), upper three panels: Orig. magn. ×40, scale bar 50 μm; lower panel: Orig. magn. ×40 zoom5, scale bar 10 μm (red CK19, green apelin, blue DAPI). B: Immunofluorescence of APJ (co-stained) with CK19 in in liver sections from PSC patients (n=7), upper three panels: Orig. magn. ×40, scale bar 50 μm; lower panel: Orig. magn. ×40 zoom5, scale bar 10 μm (red CK19, green APJ, blue DAPI). C: The mRNA expression of APLN and APLNR in cholangiocytes from PSC patients (hPSCL) (mean ± SD, n = 3), *P<0.05 vs. HIBEpiCs. D: Apelin level in PSC patients’ serum (mean ± SD, n = 26), *P<0.05 vs. Control; E: Apelin level in BDL mouse serum and cholangiocyte supernatant (mean ± SD, n = 3), *P<0.05 vs. WT, # P<0.05 vs. BDL. F: Apelin level in Mdr2−/− mouse serum (mean ± SD, n = 3), *P<0.05 vs. WT, # P<0.05 vs. Mdr2−/−.
The mRNA expression of Apln and Aplnr was decreased in BDL and Mdr2−/− mice treated with ML221 compared with that of controls (Supplementary Figure 1A, 1D). The levels of apelin in serum and cholangiocyte supernatant (Figure 1D) and the expression of apelin and APJ (Supplementary Figure 1B–C) were decreased in BDL+ML221 compared with BDL mice. Apelin serum levels were decreased in Mdr2−/− + ML221 compared with Mdr2−/− mice (Figure 1F).
APJ blockade attenuates liver injury
BDL-induced liver injury was demonstrated by increased serum levels of AST, ALT, ALP and total bilirubin (Supplementary Table 4). However, after ML221 treatment in BDL mice, serum AST, ALT, ALP and total bilirubin levels were decreased compared with BDL mice (Supplementary Table 4).
Apelin induces cholangiocyte proliferation during cholestasis via APJ
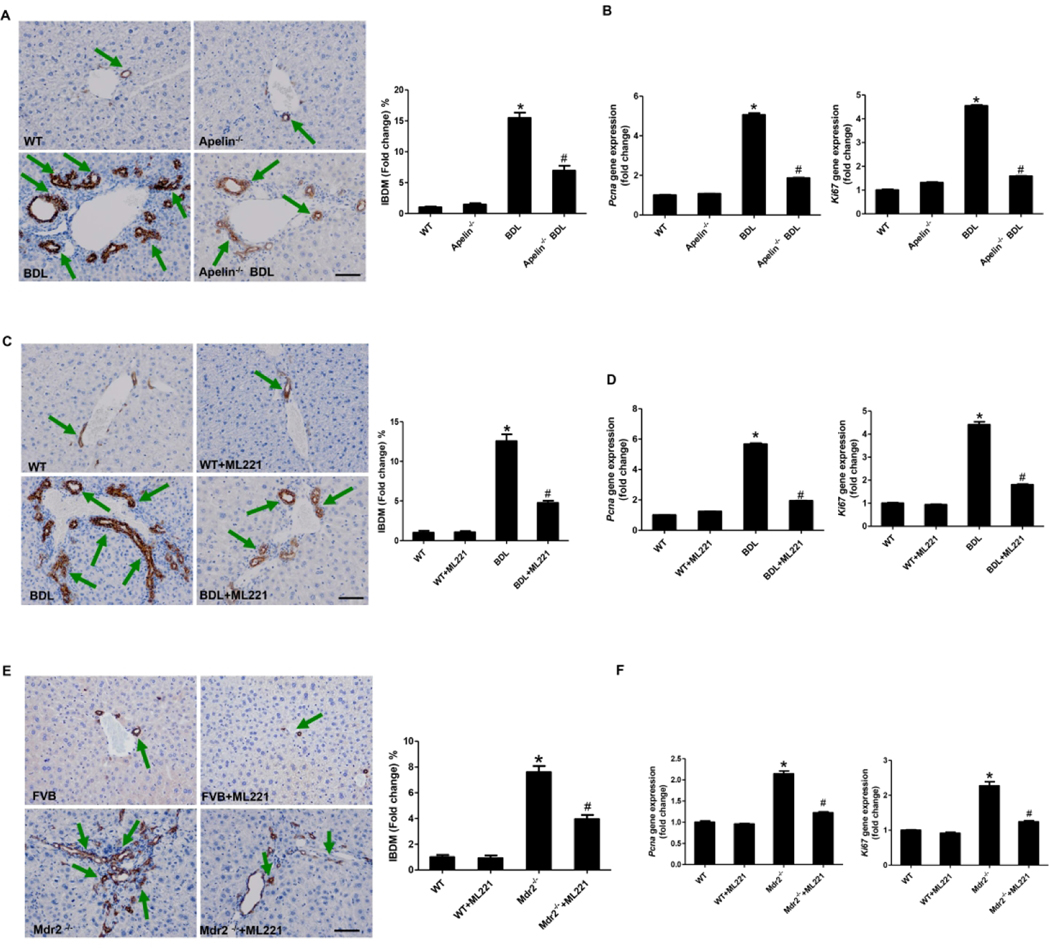
To determine if apelin regulates cholangiocyte proliferation, we performed BDL in apelin knockout (apelin−/−) mice. The levels of apelin in serum and cholangiocyte supernatant were lower in apelin−/− BDL compared with BDL mice (Supplementary Figure 1E). There was increased IBDM in BDL mice compared with WT mice, values that were reduced in apelin−/− BDL mice (Figure 2A). Further, the mRNA expression of Pcna and Ki67 was reduced in cholangiocytes from apelin−/− BDL mice, as well as in BDL mice treated with ML221 compared with BDL mice (Figure 2B, D). In addition, a significant increase of IBDM from Mdr2−/− mice was observed compared with WT mice, which was reduced in Mdr2−/− + ML221 mice (Figure 2E). Additionally, compared with Mdr2−/− mice, the mRNA expression of Pcna and Ki67 in Mdr2−/− + ML221 mouse cholangiocytes was significantly decreased (Figure 2F).
Figure 2. Apelin induces cholangiocyte proliferation during cholestasis via APJ.
A The immunohistochemical of CK19 in apelin−/− BDL mouse liver sections (mean ± SD, n = 3), Orig., magn., 20×; scale bars represent 50 μm, *P<0.05 vs. WT, #P<0.05 vs. BDL. B: The mRNA expression of PCNA and KI67 in cholangiocytes from apelin−/− BDL mice (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. BDL. C Immunohistochemistry for CK19 in BDL+ML221 mouse liver sections (mean ± SD, n = 3), Orig., magn., 20×; scale bars represent 50 μm, *P<0.05 vs. WT, #P<0.05 vs. BDL. D: The mRNA expression of PCNA and KI67 in cholangiocytes from BDL+ML221 mice (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. BDL. E Immunohistochemistry for CK19 in Mdr2−/− mouse liver sections (mean ± SD, n = 3), Orig., magn., 20×; scale bars represent 50 μm, *P<0.05 vs. WT, #P<0.05 vs. Mdr2−/−. F: The mRNA expression of PCNA and KI67 in cholangiocytes from Mdr2−/− mice (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. Mdr2−/−.
Apelin induces cholangiocyte profibrogenesis via APJ during cholestasis
The expression of fibrosis markers Col1a1, Fn1 and Tgfb1 was increased in cholangiocytes from BDL mice compared with WT mice but was decreased in BDL+ML221 mice and apelin−/− BDL mice compared with BDL mice (Supplementary Figure 2A–B). To determine the direct effects of apelin on cholangiocyte profibrogenesis, we treated HIBEpiCs with apelin and measured the mRNA expression of fibrosis markers. Compared with control, the expression of fibrosis markers was increased in apelin treated HIBEpiCs, which was reversed by pretreatment with ML221 (Supplementary Figure 2C).
Apelin knockout or APJ blockade attenuates biliary senescence in cholestatic liver
Biliary senescence is a key hallmark of cholangiopathies including PSC, which contributes to enhanced liver fibrosis (21). By SA-β-gal staining in liver sections, there was enhanced biliary senescence in liver samples from PSC patients compared with controls (Figure 3A). Biliary senescence was increased in BDL mouse liver sections compared with those from WT mice, which was significantly decreased in BDL mice treated with ML221 and apelin−/− BDL mice (Figure 3B). There was enhanced mRNA expression of the senescence markers Cdkn2a, Cdkn1a and Ccl2 in cholangiocytes from BDL mice compared with WT mice, which was decreased in BDL+ML221 mice and apelin−/− BDL mice (Supplementary Figure 3A). The expression of Cdkn2a, Cdkn1a and Ccl2 was increased in cholangiocytes from Mdr2−/− compared with WT mice, which was decreased in Mdr2−/−+ML221 compared with Mdr2−/− mice (Supplementary Figure 3B).
Figure 3. Apelin knockout or APJ blockade attenuates biliary senescence, inflammation and angiogenesis in cholestatic liver.
A: SA-β-gal staining in liver sections from PSC patients (n = 7), Orig., magn., 20×; scale bars represent 50 μm. B: SA-β-gal staining in in mouse liver sections (mean ± SD, n = 3), Orig., magn., 20×; scale bars represent 50 μm, *P<0.05 vs. WT, #P<0.05 vs. BDL. C: The immunohistochemical of F4/80 in mouse liver sections (mean ± SD, n = 3), Orig., magn., 20×; scale bars represent 50 μm, *P<0.05 vs. WT, #P<0.05 vs. BDL. D: Immunofluorescence for CD31 (co-stained with CK19) in mouse frozen liver sections (n=3), Orig., magn., 40×; scale bars represent 50 μm (purple CK19, green CD31, blue DAPI).
Apelin knockout or APJ blockade attenuates inflammation and angiogenesis in cholestatic liver
There was increased F4/80-positive areas in liver section from BDL mice compared with WT mice (Figure 3C); F4/80-positive areas were reduced in liver sections from BDL+ML221 mice and apelin−/− BDL mice compared with BDL mice (Figure 3C). Consistent with the histological findings, the upregulation of Il1b, Il6, Il33 and Tnfa in BDL liver was reduced by apelin knockout or ML221 treatment (Supplementary Figure 3C). The expression of CD31 (a marker of endothelial cells) (14), was increased in liver samples form BDL compared with WT mice, but was decreased in BDL+ML221 mice and apelin−/− BDL mouse liver compared with BDL mice (Figure 3D). Similarly, the mRNA expression of angiogenesis markers (Pecam1, Vegfa and Vwf) was increased in BDL mice compared with WT mice, but decreased in BDL+ML221 mice and apelin−/− BDL mouse liver compared with BDL mice (Supplementary Figure 3D).
Apelin induces liver fibrosis via APJ during cholestatic injury
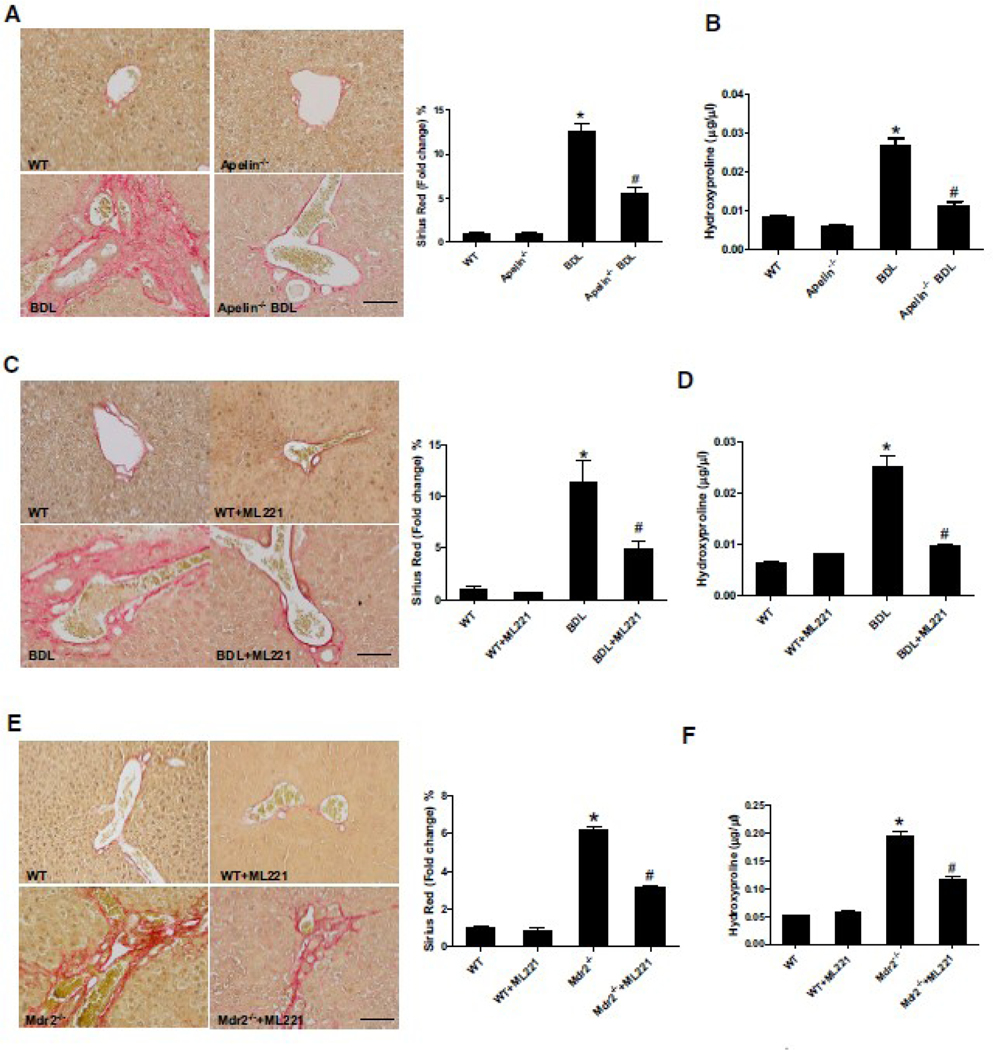
Collagen deposition was increased in BDL mice compared with WT mice, but was significantly decreased in apelin−/− BDL mice (Figure 4A–B). The mRNA expression of the fibrosis markers, Acta2, Fn1, Tgfb1 and Col1a1, was increased in total liver samples from BDL mice compared with WT mice, which was significantly reduced in apelin−/− BDL mice compared with BDL WT mice (Supplementary Figure 4A). BDL-induced liver fibrosis was decreased in BDL mice treated with ML221 (Figure 4C–D). The mRNA expression of fibrosis markers was decreased in BDL+ML221 mouse liver compared with BDL mice (Supplementary Figure 4B). In addition, collagen deposition was increased in Mdr2−/− compared with WT mice, but significantly decreased in Mdr2−/− +ML221 compared with Mdr2−/− mice (Figure 4E–F) Similarly, the mRNA expression of fibrosis markers was increased in Mdr2−/− mouse liver compared with WT mice, which was significantly decreased in Mdr2−/− +ML221 mice compared withMdr2−/− mice (Supplementary Figure 4C).
Figure 4. Apelin induces liver fibrosis via APJ during cholestatic.
A: Sirius red staining in apelin−/− BDL mouse liver sections (mean ± SD, n = 3), Orig., magn., 20×; scale bars represent 50 μm, *P<0.05 vs. WT, #P<0.05 vs. BDL. B: Hydroxyproline levels in apelin−/− BDL mouse liver (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. BDL. C: Sirius red staining in BDL +ML221 mouse liver sections (mean ± SD, n = 3), Orig., magn., 20×; scale bars represent 50 μm, *P<0.05 vs. WT, #P<0.05 vs. BDL. D: Hydroxyproline levels in BDL +ML221 mouse liver (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. BDL. E: Sirius red staining in Mdr2−/− mouse liver sections (mean ± SD, n = 3), Orig., magn., 20×; scale bars represent 50 μm, *P<0.05 vs. WT, #P<0.05 vs. Mdr2−/−. F: Hydroxyproline levels in Mdr2−/− mouse liver (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. Mdr2−/−.
Apelin-APJ induces cholangiocyte proliferation via Nox4/ROS/ERK signaling pathway during cholestasis
Apelin has been shown to regulate Nox4 expression levels and stimulate the formation of reactive oxygen species (ROS) that triggers vascular smooth muscle cell proliferation via ERK (22). We hypothesized that apelin-APJ induces cholangiocyte proliferation via Nox4/ROS/ERK signaling pathway in cholestasis. We demonstrated that the mRNA expression of NOX4 was increased in hPSCL compared with HIBEpiCs (Figure 5A). Similarly, the mRNA expression of Nox4 increased in cholangiocytes from BDL mice compared with WT mice, expression that was reduced in cholangiocytes from BDL+ML221 mice compared with BDL mice (Figure 5B). In vitro, apelin stimulation increased the mRNA expression of NOX4 in HIBEpiCs, whereas ML221 pretreatment reversed apelin-induced NOX4 expression (Figure 5C).
Figure 5. Apelin-APJ induces cholangiocyte proliferation via Nox4/ROS/ERK signaling pathway during cholestasis.
A: The mRNA expression of NOX4 in cholangiocytes from PSC patients (hPSCL) (mean ± SD, n = 3), *P<0.05 vs. HIBEpiCs. B: The mRNA expression of Nox4 in mouse cholangiocytes (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. BDL. CThe mRNA expression of NOX4 in apelin treated HIBEpiCs (mean ± SD, n = 3), *P<0.05 vs. Control, #P<0.05 vs. apelin-13. D: ROS level in mouse cholangiocyte supernatant and cholangiocyte cell lysate (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. BDL. E: Fluorescence Microplate Assay for intracellular ROS regeneration in apelin treated HIBEpiCs (mean ± SD, n = 3), *P<0.05 vs. Control, #P<0.05 vs. apelin-13. F: Confocal Microscopy Assay for intracellular ROS regeneration in HIBEpiCs (green ROS), Orig., magn., 20×; scale bars represent 100 μm.
The levels of ROS in cholangiocyte cell lysate and cholangiocyte supernatant from BDL mice were increased compared with WT mice, but decreased in BDL+ML221 mice and apelin−/− BDL mice compared with BDL mice (Figure 5D). ROS levels were higher in apelin-treated HIBEpiCs (compared with control HIBEpiCs), but decreased in HIBEpiCs pretreated with ML221, DPI, or NAC (Figure 5E–F).
There was increased ERK phosphorylation in cholangiocytes from BDL mice compared with WT mice, which was decreased in cholangiocytes from BDL+ML221 compared with BDL mice (Figure 6A). By immunofluorescence, we confirmed the increased activation of ERK with p-ERK nuclear localization in BDL mouse cholangiocytes, that decreased in BDL+ML221 mouse cholangiocytes compared with BDL mouse cholangiocytes (Figure 6B). Also, the phosphorylation of ERK was deceased in cholangiocytes from apelin−/− BDL compared with BDL mice (Figure 6C). In vitro, the phosphorylation of ERK was increased in HIBEpiCs after apelin treatment, whereas pretreatment with ML221, DPI, NAC, or PD98059 decreased apelin-induced ERK phosphorylation (Figure 6D). Apelin treatment promoted HIBEpiC proliferation, but pretreatment of HIBEpiCs with ML221, DPI, NAC, or PD98059 attenuated apelin-induced biliary proliferation (Figure 6E). Similarly, compared with control, the expression of PCNA and KI67 was increased in apelin treated HIBEpiCs, which was reversed by pretreatment of ML221, DPI, NAC or PD98059 (Figure 6F).
Figure 6. Apelin-APJ induces cholangiocyte proliferation via Nox4/ROS/ERK signaling pathway during cholestasis.
A: The expression of p-ERK in mouse cholangiocytes (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. BDL. B: Immunofluorescence for p-ERK (co-stained with CK19) in frozen liver sections (n=3), upper three panels: Orig. magn. ×40, scale bar 50 μm; lower panel: Orig. magn. ×40 zoom5, scale bar 10 μm (red CK19, green p-ERK, blue DAPI). C: The expression of p-ERK in cholangiocytes from apelin−/− BDL mice (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. BDL. D: Expression of p-ERK in apelin treated HIBEpiCs (mean ± SD, n = 3), *P<0.05 vs. Control, #P<0.05 vs. apelin-13. E: The measurement of proliferation in apelin treated HIBEpiCs by MTS (mean ± SD, n = 3), *P<0.05 vs. Control, #P<0.05 vs. apelin-13. F: The mRNA expression of PCNA and KI67 in apelin treated HIBEpiCs (mean ± SD, n = 3), *P<0.05 vs. Control, #P<0.05 vs. apelin-13.
Apelin-APJ induces HSC proliferation and activation via ROS during cholestasis
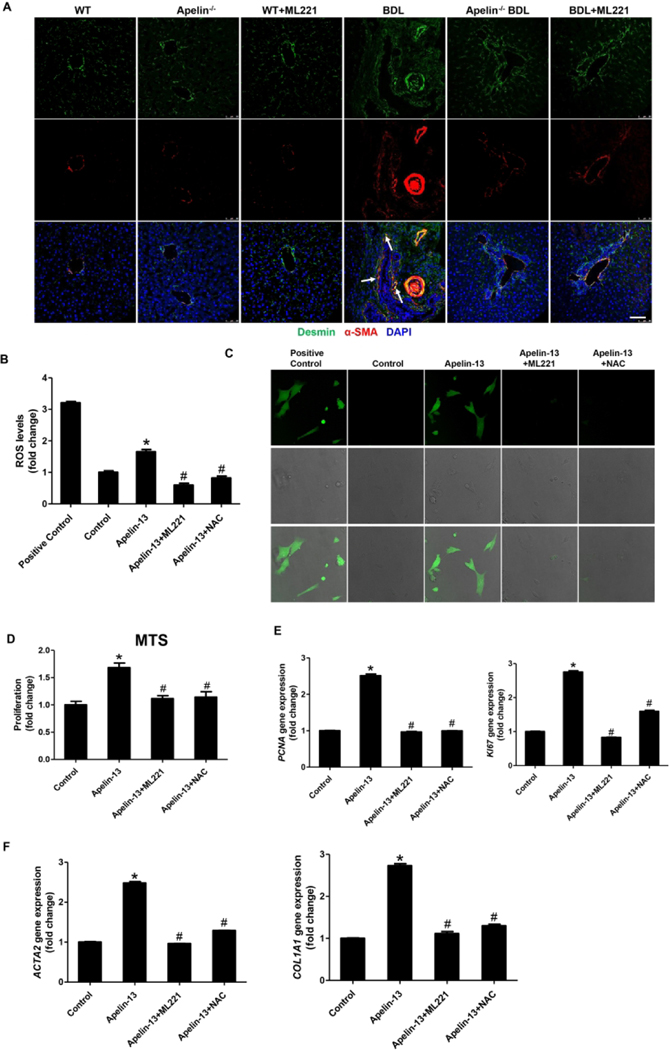
To unravel the mechanism by which the apelin/APJ axis regulates liver fibrosis, we evaluated the role of apelin-APJ in HSC activation. By immunofluorescence, there was enhanced immunoreactivity for desmin and α-SMA in liver sections from BDL mice compared with WT mice (Figure 7A). However, the activation of HSC was decreased in BDL+ML221 mice and apelin−/− BDL mice compared with BDL mice (Figure 7A). Consistently, the mRNA expression of HSC proliferation (PCNA and KI67) and activation (ACTA2 and COL1A1) markers was decreased in LCM-isolated HSCs from BDL+ML221 mice and apelin−/− BDL mice compared with BDL mice (Supplementary Figure 4 D–E). When HHSteCs were incubated with cholangiocyte supernatant, the mRNA expression of PCNA, KI67, ACTA2, and COL1A1 was decreased in HHSteCs treated with supernatant from BDL+ML221 mice compared with HHSteCs treated with supernatant from BDL mice (Supplementary Figure 4 F–G).
Figure 7. Apelin induces hepatic stellate cell proliferation and activation via APJ during cholestasis.
A: Immunofluorescence for α-SMA (co-stained with desmin) in mouse frozen liver sections (n=3), Orig., magn., 40×; scale bars represent 50 μm (red α-SMA, green desmin, blue DAPI). B: Fluorescence Microplate Assay for intracellular ROS regeneration in apelin treated HHSteCs (mean ± SD, n = 3), *P<0.05 vs. Control, #P<0.05 vs. apelin-13. C: Confocal Microscopy Assay for intracellular ROS regeneration in apelin treated HHSteCs (green ROS), Orig., magn., 20×; scale bars represent 100 μm. D: The measurement of proliferation in apelin treated HHSteCs by MTS (mean ± SD, n = 3), *P<0.05 vs. Control, #P<0.05 vs. apelin-13. G: The mRNA expression of PCNA and KI67 in apelin treated HHSteCs (mean ± SD, n = 3) *P<0.05 vs. Control, #P<0.05 vs. apelin-13. H: The mRNA expression of ACTA2 and COL1A1 in apelin treated HHSteCs (mean ± SD, n = 3) *P<0.05 vs. Control, #P<0.05 vs. apelin-13.
By immunofluorescence, we demonstrated that APJ is expressed in mouse HSCs and HHSteCs (Supplementary Figure 5A–B). To validate the paracrine effect of cholangiocyte apelin on HSC proliferation and activation, we treated HHSteCs with apelin in vitro. Studies have shown intracellular ROS generation can induce HSC proliferation and activation (23). In apelin treated HHSteCs, the intracellular ROS generation was higher than controls, whereas HHSteCs pretreated with ML221 or NAC reversed the generation of ROS (Figure 7B–C). Apelin treatment promoted HHSteC proliferation, but pretreatment of HHSteCs with ML221 or NAC attenuated apelin-induced proliferation (Figure 7D). Consistently, the expression of PCNA and KI67 was increased in apelin treated HHSteCs compared with control, which was reversed by pretreatment of ML221 or NAC (Figure 7E). The expression of activation markers of HSC was increased in apelin treated HHSteCs compared with control, which was decreased by ML221 or NAC pretreatment compared with apelin treatment (Figure 7F).
Discussion
Activation of the apelin-APJ axis induces a diverse array of biological effects, including angiogenesis (24) and fluid homeostasis (25). The current study evaluated whether the apelin-APJ axis plays a role in ductular reaction and liver fibrosis during cholestasis. We observed that the expression of the apelin/APJ axis increased in cholangiocytes of patients with PSC. This represents the first evidence linking apelin activation to cholestasis. Apelin levels in serum and in cholangiocyte supernatant and the biliary expression of apelin and APJ were increased in both BDL and Mdr2−/− mice. Treatment of BDL and Mdr2−/− mice with APJ antagonist ML221 or apelin knockout ameliorated biliary hyperplasia and senescence, and liver fibrosis.
Cholangiocyte proliferation is a major hallmark of ductular reaction, which is associated with a paracrine activation of HSCs and increased phenotypes in cholangiopathies such as PSC and PBC (26). Proliferating cholangiocytes interact with other cells in the biliary microenvironment such as vascular endothelial cells, hepatocytes and hepatic cells, promoting liver inflammation and fibrosis (27). In our study, administration of ML221 reduced cholangiocyte proliferation in BDL and Mdr2−/− mice and apelin knockout also decreased cholangiocyte proliferation in BDL mice, which provide the evidence that apelin-APJ is key in modulating biliary homeostasis in cholestasis.
ERK1/2 is a key regulatory kinase regulating cell proliferation in different pathophysiological states including liver proliferation and fibrosis (18, 28). Consistent with our findings, apelin-APJ promotes the proliferation of several cell types including vascular smooth muscle and human breast cancer cells through ERK activation (9, 29). Our previous study demonstrated that ML221 treatment decreased cholangiocarcinoma growth both in vitro and in vivo by changes in ERK phosphorylation (10). In the current study, we observed that blockage of APJ or knockout apelin in BDL mice reduced the activation of ERK in cholangiocytes, which supports the concept of apelin-APJ regulation of ERK activation during cholestasis. In vitro, ML221 and PD98055 reduction of apelin-induced ERK phosphorylation and cholangiocyte proliferation further support the concept that the apelin-APJ axis promotes biliary hyperplasia by activation of ERK signaling pathway.
Many different stimuli including growth factors, cytokines, ligands for heterotrimeric G protein-coupled receptors or transforming agents activate the ERK signaling pathway (30). There is evidence that ROS induce cell proliferation through ERK activation (31). Also, bile acids increase intracellular ROS levels, which trigger ERK phosphorylation and cholangiocytes proliferation (18). In support of these findings, our study has demonstrated increased ROS levels in cholestatic cholangiocytes, which was reduced by ML221 treatment and apelin knockout. Indeed, apelin increases ROS generation in endothelia (32) and induces ROS synthesis during vascular smooth muscle cell (VSMC) proliferation (33) through a ROS-ERK-dependent pathway (22). Our in vitro results showed that NAC and ML221 treatment reduced apelin induced cholangiocytes ROS generation and ERK activation. We propose that apelin-APJ promotes the proliferation of cholangiocytes by inducing intracellular ROS generation and activating the ERK signaling pathway.
When stimulated, many enzymes (e.g., cytochrome P450 mono-oxidase, and NADPH oxidase, Nox) involved in normal cell metabolism, trigger ROS accumulation in the body, leading to oxidative stress (34). In the current study, Nox4 gene expression was significantly increased in cholestatic cholangiocytes from BDL mice and PSC patients. Nox4, one of the major Nox isoforms expressed in the liver, regulates the progression of cholestatic liver injury and liver fibrosis (35). Also, Nox4 plays a crucial role in BDL-induced liver fibrosis (36) and PDGF-induced proliferation of hepatic stellate cells (37). Supporting these studies, APJ blockage or apelin knockout reduced the expression of Nox4 in cholangiocytes from BDL mice as well as in biliary cell lines. A study has shown that in the endoplasmic reticulum stress autophagy, apelin can promote the expression of Nox4 in cardiomyocytes, and promote the production of ROS, leading to myocardial hypertrophy (38).
HSC activation is crucial for the deposition of the ECM during liver fibrosis. There is evidence that HSCs can be activated by cholangiocytes secreting inflammatory factors, chemokines, and neuropeptides, such as TGF-β, incretin, and calcitonin gene-related peptides through paracrine pathways (14, 39). The activation of APJ during hypoxia and inflammation induces a powerful proliferative effect in the human hepatic cell line, LX-2 (40). Furthermore, consistent with our data APJ reduces apelin-induced collagen-I and PDGFRβ expression in HSC lines (12). However, the mechanism of apelin induced HSCs activation is unclear. Thus, we aimed to determine the mechanisms by which biliary-secreted apelin promotes liver fibrosis by a paracrine mechanism. A previous study has shown that ethanol-increase ROS levels induces HSCs activation (41). Apelin has been shown to induce intracellular ROS synthesis in endothelial cells (32). Supporting these studies, we observed apelin-induced ROS production in cholangiocytes and HSCs that was inhibited by the ROS inhibitor, NAC.
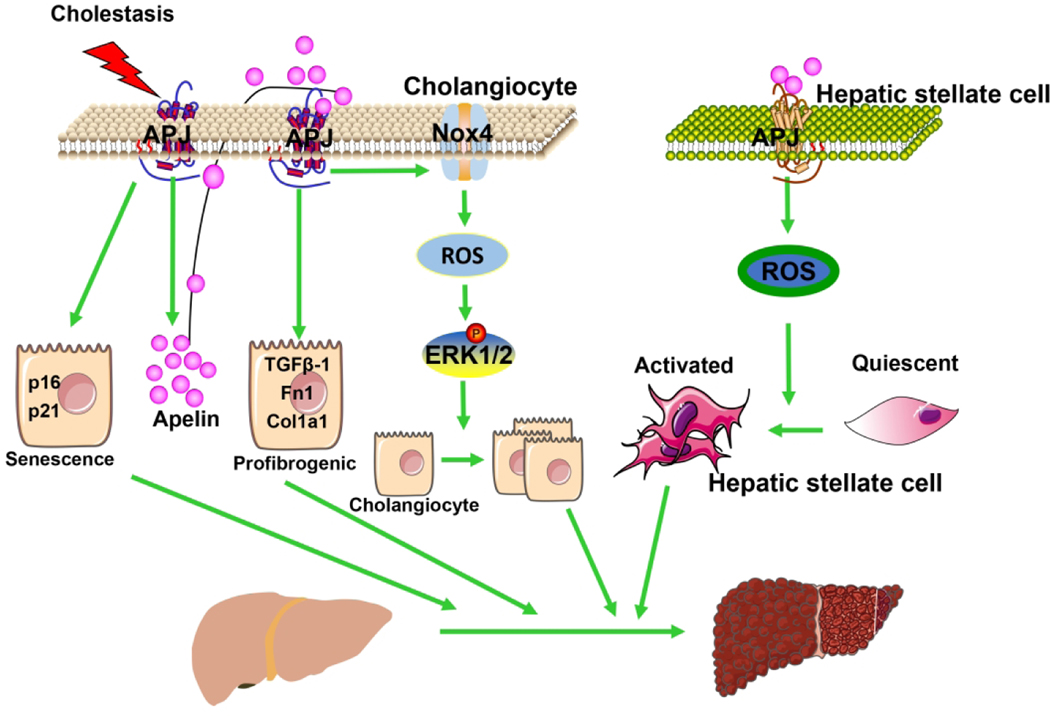
Cholangiocyte senescence is a hallmark of liver fibrosis and has been found in the livers of patients with PSC and PBC as well as mouse models of PSC (15, 42). Study suggest apelin-APJ might become a novel promising therapeutic target for anti-aging (43). Supporting these findings, we demonstrated that ML221 treatment or apelin knockout reduce BDL and Mdr2−/− induced biliary senescence and subsequently liver fibrosis. Inflammation is part of the liver wound healing response that, in chronic conditions, leads to the development of fibrosis and cirrhosis in cholangiopathies such as PBC and PSC (15, 44). There is evidence that pro-inflammatory factors can influence apelin expression and, likewise, apelin can influence pro-inflammatory factor expression (45). Blocking apelin-APJ can reduce expression of proinflammatory factors in the mesentery in a rat model of portal vein ligation (46). Apelin-APJ may be directly involved in the inflammatory response in cholestasis, and it may also reduce liver inflammation by reducing DR. The mechanism of apelin-APJ in liver inflammation needs to be further studied. Published literature indicates that angiogenesis may contribute to the progression of fibrosis during the wound healing process in chronic liver disease (47, 48). Consistent with the finding that apelin-APJ regulates angiogenesis, we observed that ML221 treatment or apelin knockout reduce BDL-induce liver angiogenesis (49). For example, in CCl4-induced cirrhosis, blocking APJ reduces liver angiogenesis and decreases the progression of cirrhosis (7). In conclusion, as shown in our working model, we propose that modulation of the apelin-APJ axis plays an important role regulating in cholangiocyte proliferation and liver fibrosis during cholestasis and may represent a novel therapeutic target for cholestatic liver diseases (Figure 8).
Figure 8. Working model of apelin-APJ regulation of cholangiocyte proliferation and liver fibrosis in cholestasis.
APJ mediates apelin expression increased in cholangiocytes during cholestasis, which promoted cholangiocytes proliferations via APJ/Nox4/ROS/ERK pathway by autocrine and induced HSC activation via ROS generation by paracrine. In addition, apelin-APJ is involved in biliary senescence and cholangiocytes profibrogenic.
Supplementary Material
Supplementary Figure 1. APJ mediated apelin expression is increased in cholangiocytes during cholestasis. A: The mRNA expression of Apln and Aplnr in BDL mouse isolated cholangiocytes (mean ± SD, n = 3), *P<0.05 vs. WT #P<0.05 vs. BDL; B: Immunofluorescence of apelin (co-stained with CK19) in mouse frozen liver sections (n=3), upper three panels: Orig., magn., 20×; scale bars represent 100 μm; lower panel: Orig. magn. ×20 zoom5, scale bar 20 μm (red CK19, green apelin, blue DAPI); C: Immunofluorescence of APJ co-staining with CK19 in mouse frozen liver sections (n=3), upper three panels: Orig., magn., 20×; scale bars represent 100 μm; lower panel: Orig. magn. ×20 zoom5, scale bar 20 μm (red CK19, green APJ, blue DAPI); D: The mRNA expression of Apln and Aplnr in Mdr2−/− mouse isolated cholangiocytes (mean ± SD, n = 3), *P<0.05 vs. WT #P<0.05 vs. Mdr2−/−; E: Apelin level in apelin−/− mouse serum and cholangiocyte supernatant (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. BDL.
Supplementary Figure 2. Apelin induces cholangiocyte profibrogenesis via APJ during cholestasis A: The mRNA expression of fibrosis markers Col1a1, Fn1 and Tgfb1 in cholangiocytes from BDL and apelin−/− BDL mouse (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. BDL. B: The mRNA expression of fibrosis markers Col1a1, Fn1 and Tgfb1 in cholangiocytes from BDL+ML221 mouse (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. BDL. C: The mRNA expression of fibrosis markers COL1A1, FN1 and TGFB1 in apelin-13 treated HIBEpiCs (mean ± SD, n = 3), *P<0.05 vs. Control, #P<0.05 vs. apelin-13.
Supplementary Figure 3. Evaluation of senescent, inflammatory and angiogenesis markers in different in vivo models. A: The mRNA expression of Cdkn2a, Cdkn1a and Ccl2 in BDL mouse liver (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. BDL. B: The mRNA expression of Cdkn2a, Cdkn1a and Ccl2 in Mdr2−/− mouse liver (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. Mdr2−/−. C: The mRNA expression of Il1b, Il6, Il33 and Tnfa in mouse liver (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. BDL. D: The mRNA expression of Pecam1, Vegfa and Vwf in mouse liver (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. BDL.
Supplementary Figure 4. Apelin induces liver fibrosis via APJ during cholestatic injury. A: The mRNA expression of fibrosis markers Acta2, Col1a1, Fn1 and Tgfb1 in apelin−/− BDL mouse liver (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. BDL; B: The mRNA expression of fibrosis markers Acta2, Col1a1, Fn1 and Tgfb1 in BDL +ML221 mouse liver (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. BDL; C: The mRNA expression of fibrosis markers Acta2, Col1a1, Fn1 and Tgfb1 in Mdr2−/− mouse liver (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. Mdr2−/−; D: The mRNA expression of Pcna and Ki67 in LCM-isolated mouse HSCs (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. BDL; E: The mRNA expression of Acta2 and Col1a1 in LCM-isolated mouse HSCs (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. BDL; F: The mRNA expression of PCNA and KI67 in HHSteCs treated with mouse cholangiocyte supernatant (mean ± SD, n = 3), *P<0.05 vs. Control #P<0.05 vs. BDL cho-sup; G: The mRNA expression of ACTA2 and COL1A1 in HHSteCs treated with mouse cholangiocyte supernatant (mean ± SD, n = 3), *P<0.05 vs. Control #P<0.05 vs. BDL cho-sup.
Supplementary Figure 5. APJ is expressed in mouse HSCs and HHSteCs. A: Immunofluorescence of APJ co-staining with desmin in mouse frozen liver sections (n=3), Orig., magn., 40×; scale bars represent 50 μm (green desmin; red APJ, blue DAPI). B: Immunofluorescence of APJ in HHSteCs (n=3), Orig., magn., 40×; scale bars represent 50 μm (green APJ, blue DAPI).
Acknowledgments
This work was supported by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Baylor Scott & White, a VA Research Senior Career Scientist Award, and the NIH grants DK058411, DK07698, DK107310, DK110035, DK062975, DK115184, AA025997 and AA025157 to Drs. Alpini, Meng and Glaser and the NIH grants DK108959 and DK119421 to Dr. Francis and NIH grants DK107682 and AA025208, UH2AA026903, U01AA026917 to Dr. Liangpunsakul and 17SDG33670306 AHA Scientist Development Grant to Dr. Chakraborty. This material is the result of work supported by resources at both Central Texas Veterans Health Care System, Temple, TX and Richard L. Roudebush VA Medical Center, Indianapolis, IN. The content is the responsibility of the author(s) alone and does not necessarily reflect the views or policies of the Department of Veterans Affairs or the United States Government.
Abbreviations:
- α-SMA
α-smooth muscle actin
- ACTA2
actin alpha 2
- BDL
bile duct ligation
- CK-19
cytokeratin-19
- CCl4
carbon tetrachloride
- CDKN1A
cyclin dependent kinase inhibitor 1a
- CDKN2A
cyclin dependent kinase inhibitor 2a
- CCL2
C-C motif chemokine ligand 2
- COL1A1
collagen type I alpha 1
- DAPI
4’,6-diamidino-2-phenylindole
- DPI
diphenyleneiodonium chloride
- ELISA
enzyme linked immunosorbent assay
- ERK
extracellular signal-regulated kinase
- Fn1
fibronectin1
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HHSteCs
human hepatic stellate cells
- HIBEpiCs
human intrahepatic biliary epithelial cells
- HSCs
hepatic stellate cells
- hPSCL
human PSC cell lines
- Mdr2−/−
multidrug resistance gene 2 knockout
- NAC
N-Acetyl-L-cysteine
- NADPH
nicotinamide adenine dinucleotide phosphate
- LCM
laser capture microdissection
- IBDM
intrahepatic bile duct mass
- IL
interleukin
- PCNA
proliferating cell nuclear antigen
- PBC
primary biliary cirrhosis
- PDGFRB
platelet-derived growth factor receptor beta
- PECAM-1
platelet endothelial cell adhesion molecule 1
- PSC
primary sclerosing cholangitis
- ROS
reactive oxygen species
- TGFB1
transforming growth factor-β1
- TNFA
tumor necrosis factor alpha
- VEGFA
vascular endothelial growth factor A
- VWF
von Willebrand factor
- WT
wild-type
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Kawamata Y, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun 1998;251:471–476. [DOI] [PubMed] [Google Scholar]
- 2.Hosoya M, Kawamata Y, Fukusumi S, Fujii R, Habata Y, Hinuma S, Kitada C, et al. Molecular and functional characteristics of APJ. Tissue distribution of mRNA and interaction with the endogenous ligand apelin. J Biol Chem 2000;275:21061–21067. [DOI] [PubMed] [Google Scholar]
- 3.Lv X, Kong J, Chen WD, Wang YD. The Role of the Apelin/APJ System in the Regulation of Liver Disease. Front Pharmacol 2017;8:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang S, Chen L, Lu L, Li L. The apelin-APJ axis: A novel potential therapeutic target for organ fibrosis. Clin Chim Acta 2016;456:81–88. [DOI] [PubMed] [Google Scholar]
- 5.Yokomori H, Oda M, Yoshimura K, Machida S, Kaneko F, Hibi T. Overexpression of apelin receptor (APJ/AGTRL1) on hepatic stellate cells and sinusoidal angiogenesis in human cirrhotic liver. J Gastroenterol 2011;46:222–231. [DOI] [PubMed] [Google Scholar]
- 6.Principe A, Melgar-Lesmes P, Fernandez-Varo G, del Arbol LR, Ros J, Morales-Ruiz M, Bernardi M, et al. The hepatic apelin system: a new therapeutic target for liver disease. Hepatology 2008;48:1193–1201. [DOI] [PubMed] [Google Scholar]
- 7.Reichenbach V, Ros J, Fernandez-Varo G, Casals G, Melgar-Lesmes P, Campos T, Makriyannis A, et al. Prevention of fibrosis progression in CCl4-treated rats: role of the hepatic endocannabinoid and apelin systems. J Pharmacol Exp Ther 2012;340:629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alvaro D, Mancino MG, Glaser S, Gaudio E, Marzioni M, Francis H, Alpini G.Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology 2007;132:415–431. [DOI] [PubMed] [Google Scholar]
- 9.Peng X, Li F, Wang P, Jia S, Sun L, Huo H. Apelin-13 induces MCF-7 cell proliferation and invasion via phosphorylation of ERK1/2. Int J Mol Med 2015;36:733–738. [DOI] [PubMed] [Google Scholar]
- 10.Hall C, Ehrlich L, Venter J, O’Brien A, White T, Zhou T, Dang T, et al. Inhibition of the apelin/apelin receptor axis decreases cholangiocarcinoma growth. Cancer Lett 2017;386:179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu J, Liu X, Koyama Y, Wang P, Lan T, Kim IG, Kim IH, et al. The types of hepatic myofibroblasts contributing to liver fibrosis of different etiologies. Front Pharmacol 2014;5:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melgar-Lesmes P, Casals G, Pauta M, Ros J, Reichenbach V, Bataller R,Morales-Ruiz M, et al. Apelin mediates the induction of profibrogenic genes in human hepatic stellate cells. Endocrinology 2010;151:5306–5314. [DOI] [PubMed] [Google Scholar]
- 13.Maloney PR, Khan P, Hedrick M, Gosalia P, Milewski M, Li L, Roth GP, et al. Discovery of 4-oxo-6-((pyrimidin-2-ylthio)methyl)-4H-pyran-3-yl 4-nitrobenzoate (ML221) as a functional antagonist of the apelin (APJ) receptor. Bioorg Med Chem Lett 2012;22:6656–6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu N, Meng F, Invernizzi P, Bernuzzi F, Venter J, Standeford H, Onori P, et al. The secretin/secretin receptor axis modulates liver fibrosis through changes in transforming growth factor-beta1 biliary secretion in mice. Hepatology 2016;64:865–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wan Y, Meng F, Wu N, Zhou T, Venter J, Francis H, Kennedy L, et al. Substance P increases liver fibrosis by differential changes in senescence of cholangiocytes and hepatic stellate cells. Hepatology 2017;66:528–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Than A, Zhang X, Leow MK, Poh CL, Chong SK, Chen P. Apelin attenuates oxidative stress in human adipocytes. J Biol Chem 2014;289:3763–3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Augsburger F, Filippova A, Rasti D, Seredenina T, Lam M, Maghzal G, Mahiout Z, et al. Pharmacological characterization of the seven human NOX isoforms and their inhibitors. Redox Biol 2019;26:101272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reich M, Deutschmann K, Sommerfeld A, Klindt C, Kluge S, Kubitz R, UllmerC, et al. TGR5 is essential for bile acid-dependent cholangiocyte proliferation in vivo and in vitro. Gut 2016;65:487–501. [DOI] [PubMed] [Google Scholar]
- 19.Widjaja AA, Singh BK, Adami E, Viswanathan S, Dong J, D’Agostino GA, Ng B, et al. Inhibiting Interleukin 11 Signaling Reduces Hepatocyte Death and Liver Fibrosis, Inflammation, and Steatosis in Mouse Models of Nonalcoholic Steatohepatitis. Gastroenterology 2019;157:777–792 e714. [DOI] [PubMed] [Google Scholar]
- 20.Tabibian JH, Trussoni CE, O’Hara SP, Splinter PL, Heimbach JK, LaRusso NF. Characterization of cultured cholangiocytes isolated from livers of patients with primary sclerosing cholangitis. Lab Invest 2014;94:1126–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan Y, McDaniel K, Wu N, Ramos-Lorenzo S, Glaser T, Venter J, Francis H, et al. Regulation of Cellular Senescence by miR-34a in Alcoholic Liver Injury. Am J Pathol 2017;187:2788–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Q, Cao J, Chen L. Apelin/APJ system: A novel therapeutic target for oxidative stress-related inflammatory diseases (Review). Int J Mol Med 2016;37:1159–1169. [DOI] [PubMed] [Google Scholar]
- 23.Iwamoto K, Kanno K, Hyogo H, Yamagishi S, Takeuchi M, Tazuma S, ChayamaK. Advanced glycation end products enhance the proliferation and activation of hepatic stellate cells. J Gastroenterol 2008;43:298–304. [DOI] [PubMed] [Google Scholar]
- 24.Cox CM, D’Agostino SL, Miller MK, Heimark RL, Krieg PA. Apelin, the ligandfor the endothelial G-protein-coupled receptor, APJ, is a potent angiogenic factor required for normal vascular development of the frog embryo. Dev Biol 2006;296:177–189. [DOI] [PubMed] [Google Scholar]
- 25.De Mota N, Reaux-Le Goazigo A, El Messari S, Chartrel N, Roesch D, Dujardin C, Kordon C, et al. Apelin, a potent diuretic neuropeptide counteracting vasopressin actions through inhibition of vasopressin neuron activity and vasopressin release. Proc Natl Acad Sci U S A 2004;101:10464–10469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato K, Marzioni M, Meng F, Francis H, Glaser S, Alpini G. Ductular Reactionin Liver Diseases: Pathological Mechanisms and Translational Significances. Hepatology 2019;69:420–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glaser SS, Gaudio E, Miller T, Alvaro D, Alpini G. Cholangiocyte proliferationand liver fibrosis. Expert Rev Mol Med 2009;11:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Q, Chen L, Kong D, Shao J, Wu L, Zheng S. Dihydroartemisinin alleviates bile duct ligation-induced liver fibrosis and hepatic stellate cell activation by interfering with the PDGF-betaR/ERK signaling pathway. Int Immunopharmacol 2016;34:250–258. [DOI] [PubMed] [Google Scholar]
- 29.Liu QF, Yu HW, You L, Liu MX, Li KY, Tao GZ. Apelin-13-induced proliferation and migration induced of rat vascular smooth muscle cells is mediated by the upregulation of Egr-1. Biochem Biophys Res Commun 2013;439:235–240. [DOI] [PubMed] [Google Scholar]
- 30.Li Q, Chen M, Liu H, Yang L, Yang T, He G. The dual role of ERK signaling inthe apoptosis of neurons. Front Biosci (Landmark Ed) 2014;19:1411–1417. [DOI] [PubMed] [Google Scholar]
- 31.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am JPhysiol Lung Cell Mol Physiol 2000;279:L1005–1028. [DOI] [PubMed] [Google Scholar]
- 32.Liu M, Li H, Zhou Q, Zhao H, Lv D, Cao J, Jiang J, et al. ROS-Autophagy pathway mediates monocytes-human umbilical vein endothelial cells adhesion induced by apelin-13. J Cell Physiol 2018;233:6839–6850. [DOI] [PubMed] [Google Scholar]
- 33.Hashimoto T, Kihara M, Imai N, Yoshida S, Shimoyamada H, Yasuzaki H, Ishida J, et al. Requirement of apelin-apelin receptor system for oxidative stress-linked atherosclerosis. Am J Pathol 2007;171:1705–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ Res 2018;122:877–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paik YH, Kim J, Aoyama T, De Minicis S, Bataller R, Brenner DA. Role of NADPH oxidases in liver fibrosis. Antioxid Redox Signal 2014;20:2854–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang JX, Chen X, Serizawa N, Szyndralewiez C, Page P, Schroder K, Brandes RP, et al. Liver fibrosis and hepatocyte apoptosis are attenuated by GKT137831, a novel NOX4/NOX1 inhibitor in vivo. Free Radic Biol Med 2012;53:289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adachi T, Togashi H, Suzuki A, Kasai S, Ito J, Sugahara K, Kawata S. NAD(P)H oxidase plays a crucial role in PDGF-induced proliferation of hepatic stellate cells. Hepatology 2005;41:1272–1281. [DOI] [PubMed] [Google Scholar]
- 38.Xie F, Wu D, Huang SF, Cao JG, Li HN, He L, Liu MQ, et al. The endoplasmic reticulum stress-autophagy pathway is involved in apelin-13-induced cardiomyocyte hypertrophy in vitro. Acta Pharmacol Sin 2017;38:1589–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ehrlich L, Scrushy M, Meng F, Lairmore TC, Alpini G, Glaser S. Biliaryepithelium: A neuroendocrine compartment in cholestatic liver disease. Clin Res Hepatol Gastroenterol 2018;42:296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melgar-Lesmes P, Pauta M, Reichenbach V, Casals G, Ros J, Bataller R, Morales-Ruiz M, et al. Hypoxia and proinflammatory factors upregulate apelin receptor expression in human stellate cells and hepatocytes. Gut 2011;60:1404–1411. [DOI] [PubMed] [Google Scholar]
- 41.Szuster-Ciesielska A, Plewka K, Daniluk J, Kandefer-Szerszen M. Betulin and betulinic acid attenuate ethanol-induced liver stellate cell activation by inhibiting reactive oxygen species (ROS), cytokine (TNF-alpha, TGF-beta) production and by influencing intracellular signaling. Toxicology 2011;280:152–163. [DOI] [PubMed] [Google Scholar]
- 42.Meng L, Quezada M, Levine P, Han Y, McDaniel K, Zhou T, Lin E, et al. Functional role of cellular senescence in biliary injury. Am J Pathol 2015;185:602–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Q, Chen L, Tang M, Guo Y, Li L. Apelin/APJ system: A novel promising target for anti-aging intervention. Clin Chim Acta 2018;487:233–240. [DOI] [PubMed] [Google Scholar]
- 44.Kennedy L, Francis H, Invernizzi P, Venter J, Wu N, Carbone M, Gershwin ME, et al. Secretin/secretin receptor signaling mediates biliary damage and liver fibrosis in early-stage primary biliary cholangitis. FASEB J 2019;33:10269–10279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Antushevich H, Wojcik M. Review: Apelin in disease. Clin Chim Acta2018;483:241–248. [DOI] [PubMed] [Google Scholar]
- 46.Tiani C, Garcia-Pras E, Mejias M, de Gottardi A, Berzigotti A, Bosch J, Fernandez M. Apelin signaling modulates splanchnic angiogenesis and portosystemic collateral vessel formation in rats with portal hypertension. J Hepatol 2009;50:296–305. [DOI] [PubMed] [Google Scholar]
- 47.Poisson J, Lemoinne S, Boulanger C, Durand F, Moreau R, Valla D, Rautou PE. Liver sinusoidal endothelial cells: Physiology and role in liver diseases. J Hepatol 2017;66:212–227. [DOI] [PubMed] [Google Scholar]
- 48.Xu M, Xu HH, Lin Y, Sun X, Wang LJ, Fang ZP, Su XH, et al. LECT2, a Ligand for Tie1, Plays a Crucial Role in Liver Fibrogenesis. Cell 2019;178:1478–1492 e1420. [DOI] [PubMed] [Google Scholar]
- 49.Chen L, Zhou T, Wu N, O’Brien A, Venter J, Ceci L, Kyritsi K, et al. Pinealectomy or light exposure exacerbates biliary damage and liver fibrosis in cholestatic rats through decreased melatonin synthesis. Biochim Biophys Acta Mol Basis Dis 2019;1865:1525–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. APJ mediated apelin expression is increased in cholangiocytes during cholestasis. A: The mRNA expression of Apln and Aplnr in BDL mouse isolated cholangiocytes (mean ± SD, n = 3), *P<0.05 vs. WT #P<0.05 vs. BDL; B: Immunofluorescence of apelin (co-stained with CK19) in mouse frozen liver sections (n=3), upper three panels: Orig., magn., 20×; scale bars represent 100 μm; lower panel: Orig. magn. ×20 zoom5, scale bar 20 μm (red CK19, green apelin, blue DAPI); C: Immunofluorescence of APJ co-staining with CK19 in mouse frozen liver sections (n=3), upper three panels: Orig., magn., 20×; scale bars represent 100 μm; lower panel: Orig. magn. ×20 zoom5, scale bar 20 μm (red CK19, green APJ, blue DAPI); D: The mRNA expression of Apln and Aplnr in Mdr2−/− mouse isolated cholangiocytes (mean ± SD, n = 3), *P<0.05 vs. WT #P<0.05 vs. Mdr2−/−; E: Apelin level in apelin−/− mouse serum and cholangiocyte supernatant (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. BDL.
Supplementary Figure 2. Apelin induces cholangiocyte profibrogenesis via APJ during cholestasis A: The mRNA expression of fibrosis markers Col1a1, Fn1 and Tgfb1 in cholangiocytes from BDL and apelin−/− BDL mouse (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. BDL. B: The mRNA expression of fibrosis markers Col1a1, Fn1 and Tgfb1 in cholangiocytes from BDL+ML221 mouse (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. BDL. C: The mRNA expression of fibrosis markers COL1A1, FN1 and TGFB1 in apelin-13 treated HIBEpiCs (mean ± SD, n = 3), *P<0.05 vs. Control, #P<0.05 vs. apelin-13.
Supplementary Figure 3. Evaluation of senescent, inflammatory and angiogenesis markers in different in vivo models. A: The mRNA expression of Cdkn2a, Cdkn1a and Ccl2 in BDL mouse liver (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. BDL. B: The mRNA expression of Cdkn2a, Cdkn1a and Ccl2 in Mdr2−/− mouse liver (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. Mdr2−/−. C: The mRNA expression of Il1b, Il6, Il33 and Tnfa in mouse liver (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. BDL. D: The mRNA expression of Pecam1, Vegfa and Vwf in mouse liver (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. BDL.
Supplementary Figure 4. Apelin induces liver fibrosis via APJ during cholestatic injury. A: The mRNA expression of fibrosis markers Acta2, Col1a1, Fn1 and Tgfb1 in apelin−/− BDL mouse liver (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. BDL; B: The mRNA expression of fibrosis markers Acta2, Col1a1, Fn1 and Tgfb1 in BDL +ML221 mouse liver (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. BDL; C: The mRNA expression of fibrosis markers Acta2, Col1a1, Fn1 and Tgfb1 in Mdr2−/− mouse liver (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. Mdr2−/−; D: The mRNA expression of Pcna and Ki67 in LCM-isolated mouse HSCs (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. BDL; E: The mRNA expression of Acta2 and Col1a1 in LCM-isolated mouse HSCs (mean ± SD, n = 3), *P<0.05 vs. WT, #P<0.05 vs. BDL; F: The mRNA expression of PCNA and KI67 in HHSteCs treated with mouse cholangiocyte supernatant (mean ± SD, n = 3), *P<0.05 vs. Control #P<0.05 vs. BDL cho-sup; G: The mRNA expression of ACTA2 and COL1A1 in HHSteCs treated with mouse cholangiocyte supernatant (mean ± SD, n = 3), *P<0.05 vs. Control #P<0.05 vs. BDL cho-sup.
Supplementary Figure 5. APJ is expressed in mouse HSCs and HHSteCs. A: Immunofluorescence of APJ co-staining with desmin in mouse frozen liver sections (n=3), Orig., magn., 40×; scale bars represent 50 μm (green desmin; red APJ, blue DAPI). B: Immunofluorescence of APJ in HHSteCs (n=3), Orig., magn., 40×; scale bars represent 50 μm (green APJ, blue DAPI).