Abstract
Aims
Data on B‐type natriuretic peptide (BNP) levels and adverse outcomes in patients with moderate mixed aortic valve disease (MAVD), defined as moderate aortic stenosis (AS) and regurgitation (AR), are scarce. Therefore, this study investigated the impact of BNP on the clinical outcomes in such patients.
Methods and results
Clinical data from 81 patients (mean age, 74.1 ± 6.8 years; 50.6%, men) treated for moderate MAVD and left ventricular ejection fraction (LVEF) ≥ 50% during 2010–2018 were retrospectively analysed. Specific echocardiographic data of the study patients were LVEF of 57.8 ± 5.0%, aortic valve index of 0.64 ± 0.04 cm2/m2, peak aortic valve velocity of 3.38 ± 0.29 m/s, and AR vena contracta width of 4.2 ± 0.7 mm. The median BNP level was 61.4 pg/mL (interquartile range, 29.7–109.9). The primary endpoint was a composite of all‐cause death, heart failure hospitalization, and aortic valve replacement, and its cumulative incidence at 5 years was 57.7%. Multivariable analysis revealed that age (hazard ratio, 1.079; 95% confidence interval, 1.028–1.133; P = 0.002) and BNP levels (hazard ratio, 1.028; 95% confidence interval, 1.003–1.053; P = 0.027) were significantly related to the endpoint; specifically, BNP > 61.4 pg/mL had significantly higher incidence rates of the endpoint than those with a BNP ≤ 61.4 pg/mL (70.3% vs. 45.5% at 5 years; P = 0.018). Compared with patients with BNP ≤ 61.4 pg/mL, those with BNP > 61.4 pg/mL had significantly worse left ventricular global longitudinal strain (−17.1 ± 3.6% vs. −18.7 ± 2.6%; P = 0.029), along with higher left ventricular mass index (116.9 ± 27.8 g/m2 vs. 103.5 ± 19.7 g/m2; P = 0.014), relative wall thickness (0.45 ± 0.07 vs. 0.42 ± 0.05; P = 0.022), left atrial volume index (46.0 ± 28.4 mL/m2 vs. 31.4 ± 10.3 mL/m2; P = 0.003), pulmonary artery systolic pressure (32.6 ± 9.7 mmHg vs. 28.2 ± 4.7 mmHg; P = 0.011), and prevalence of moderate or greater tricuspid regurgitation (15.0% vs. 0.0%; P = 0.012).
Conclusions
Patients with moderate MAVD are at higher risk of unfavourable clinical outcomes, and age and BNP are independently related to the occurrence of adverse events. High BNP levels may reflect extravalvular cardiac damage in patients with moderate MAVD.
Keywords: Mixed aortic valve disease, Aortic stenosis, Aortic regurgitation, B‐type natriuretic peptide, Aortic valve replacement
Introduction
In patients with mixed aortic valve disease (MAVD), defined as a combination of aortic stenosis (AS) and regurgitation (AR), the left ventricle is exposed to greater overall stress than either AS or AR, due to concurrent excessive pressure and volume load. Patients with moderate‐to‐severe MAVD have poorer clinical outcomes than those with only moderate‐to‐severe AS, 1 and peak aortic valve (AV) velocity in these patients is significantly associated with unfavourable outcomes. 2 , 3 According to current treatment guidelines, AV replacement (AVR) is indicated if the peak AV velocity is more than 4.0 m/s in symptomatic patients with preserved left ventricular ejection fraction (LVEF). 4
A recent study has reported that patients with moderate MAVD have unfavourable clinical outcomes, which are significantly worse than those in patients with either moderate AS or AR alone, and approximately equal to those in patients with severe AS alone in terms of adverse events. 5 The previous study has also reported that relative wall thickness is associated with adverse outcomes, suggesting that extravalvular cardiac damage is crucial for risk stratification in moderate MAVD. However, there are inadequate data on clinical outcomes and challenges in risk stratification using clinical findings on extravalvular cardiac damage, including serum B‐type natriuretic peptide (BNP), in such patients. Therefore, this study aimed to investigate clinical outcomes in moderate MAVD and the association between BNP and adverse events in these patients.
Methods
Patient population
This study retrospectively reviewed echocardiography data of patients with moderate MAVD and preserved LVEF (LVEF ≥ 50%) who were treated at our centre between January 2010 and December 2018. Based on published guidelines, 6 , 7 moderate AS was defined as an AV area (AVA) index of >0.6 and ≤0.85 cm2/m2 and a peak AV velocity of ≥3.0 and <4.0 m/s. AR severity was determined using integrative approaches based on semiquantitative parameters, including vena contracta width and AR jet width/left ventricular outflow tract (LVOT) width, and qualitative parameters, including pressure half‐time and presence of descending aortic diastolic flow reversal. Moderate AR was semiquantitatively defined as a vena contracta width of ≥3.0 and <6.0 mm and AR jet width/LVOT width of ≥25% and <65%. Exclusion criteria were age > 85 years, more than moderate mitral regurgitation or stenosis based on current guidelines, 7 , 8 previous AVR, LVEF < 50%, hypertrophic cardiomyopathy, congenital heart disease (except bicuspid AV), and LVOT obstruction. We also excluded patients who underwent AVR within 3 months of index echocardiography to avoid inaccurate evaluation of clinical outcomes. Serum BNP and creatinine levels were measured within 1 week of index echocardiography. Information on patient characteristics, echocardiographic data, and follow‐up were obtained from medical records and echocardiography reports. The study protocol was approved by the Institutional Review Board of New Tokyo Hospital and complied with the Declaration of Helsinki. The requirement for informed consent was waived, given the retrospective nature of the study.
B‐type natriuretic peptide analysis
Blood samples were collected into EDTA Vacutainer tubes (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) for the BNP assay. Plasma was immediately separated at −4°C and samples were frozen at −70°C until use. Plasma BNP was quantified onsite using a chemiluminescence immunoassay (Shionogi Co. Ltd, Tokyo, Japan).
Echocardiographic measurements
Echocardiography was performed on the Vivid E9 system (General Electric Healthcare, Little Chalfont, UK), the iE33 system (Philips Healthcare, Andover, MA, USA), or the EPIQ7 system (Philips Healthcare) according to relevant guidelines. 6 , 7 , 8 , 9 Echocardiographic data were stored on a dedicated workstation for offline analysis. Left ventricular end‐diastolic and end‐systolic volumes, LVEF, and left atrial volume were measured using the biplane Simpson disc method. Left ventricular global longitudinal strain was assessed using speckle‐tracking imaging and an external third‐party software program (TomTec Imaging Arena, Munich, Germany). Left ventricular mass index and relative wall thickness were calculated from two‐dimensional echocardiography. Peak AV velocity, and peak and mean AV pressure gradients were measured in the continuous‐wave Doppler mode from apical approaches or right parasternal approaches, if possible, and were calculated using the simplified Bernoulli equation. Velocity ratio was calculated as peak LVOT jet velocity/peak AV velocity. We assessed AV haemodynamics using echocardiographic data at 1 year follow‐up in patients who were alive and did not undergo AVR. 6 Rapid progression of peak AV velocity was defined as Δ peak AV velocity ≥ 0.3 m/s/year. 6
Follow‐up and study endpoints
Follow‐up data were obtained from interviews with patients, their relatives, or their physicians. Particular care was taken to obtain information regarding death, heart failure (HF) hospitalization, and AVR. AVR was recommended by cardiologists or cardiac surgeons in our hospital and was based on current guidelines. 4
The primary endpoint of the study was defined as a composite of all‐cause death, HF hospitalization, and AVR. Secondary endpoints were individual components of the composite primary endpoint. If a patient was hospitalized due to HF at the time of index echocardiography, the first event after discharge from that hospitalization was considered as an event.
Statistical analysis
Categorical variables are presented as frequencies and were analysed using the χ 2 or Fisher's exact test, as appropriate. Continuous variables are presented as mean ± standard deviation or median with interquartile range (IQR) and were compared using the t‐test or Wilcoxon rank‐sum test, as applicable. Cumulative incidence of the predefined composite endpoint was determined using the Kaplan–Meier method and the date of the index echocardiography was defined as the initial time point (t = 0). Univariate and multivariate Cox proportional hazard regression analyses were used to identify factors significantly associated with the primary endpoint. All statistical tests were two‐tailed. A P‐value < 0.10 on univariate analysis was used to select variables for multivariate analysis. To avoid overfitting, the number of variables entered into multivariate models was limited to a maximum of one for every nine or ten events. 10 , 11 Among candidate variables in univariate analyses, those with high multicollinearity, judged based on the variance inflation factor, were excluded from multivariate analysis. A P‐value < 0.05 was considered statistically significant for multivariate analysis. Data analysis was performed using SPSS for Windows Version 25.0 (SPSS Inc, Chicago, IL, USA).
Results
Patient characteristics
We reviewed the records of 81 patients who met the inclusion criteria (Table 1 ). The mean age of patients was 74.1 ± 6.8 years, and 41 (50.6%) were men. New York Heart Association (NYHA) functional classes I and II were seen in 57 (70.4%) and 24 (29.6%) patients, respectively. The median BNP level of the study patients was 61.4 pg/mL (IQR, 29.7–109.9). Echocardiographic data are shown in Table 2 . Specific AV data of the cohort were AVA index of 0.64 ± 0.04 cm2/m2, peak AV velocity of 3.38 ± 0.29 m/s, vena contracta width of 4.2 ± 0.7 mm, and AR jet width/LVOT width of 35.4 ± 5.6%.
Table 1.
Patient characteristics
| Variables | All patients (n = 81) | BNP ≤ 61.4 pg/mL (n = 41, 50.6%) | BNP > 61.4 pg/mL (n = 40, 49.4%) | P‐value |
|---|---|---|---|---|
| Age, years | 74.1 ± 6.8 | 72.2 ± 8.1 | 76.3 ± 4.4 | 0.005 |
| Men, n | 41 (50.6) | 24 (58.5) | 17 (42.5) | 0.185 |
| Body mass index, kg/m2 | 23.4 ± 2.7 | 23.3 ± 2.2 | 23.5 ± 3.2 | 0.805 |
| Hypertension, n | 55 (67.9) | 25 (61.0) | 30 (75.0) | 0.235 |
| Diabetes mellitus, n | 14 (17.3) | 6 (14.6) | 8 (20.0) | 0.569 |
| Dyslipidaemia, n | 41 (50.6) | 21 (51.2) | 20 (50.0) | 1.000 |
| eGFR a , mL/min/1.73 m2 (n = 74) | 63.7 ± 20.8 | 70.9 ± 20.1 | 55.1 ± 18.3 | 0.001 |
| Chronic kidney disease, n | 41 (50.6) | 14 (34.1) | 27 (67.5) | 0.004 |
| Haemodialysis, n | 7 (8.6) | 1 (2.4) | 6 (15.0) | 0.057 |
| BNP, pg/mL (median) | 61.4 (29.7–109.9) | 29.7 (21.9–45.9) | 115.1 (78.2–237.4) | <0.001 |
| Atrial fibrillation/flutter, n | 17 (21.0) | 8 (19.5) | 9 (22.5) | 0.790 |
| Previous myocardial infarction, n | 10 (12.3) | 2 (4.9) | 8 (20.0) | 0.048 |
| Previous PCI, n | 27 (33.3) | 10 (24.4) | 17 (42.5) | 0.102 |
| Previous CABG, n | 4 (4.9) | 1 (2.4) | 3 (7.5) | 0.359 |
| Peripheral arterial disease, n | 14 (17.3) | 3 (7.3) | 11 (27.5) | 0.020 |
| Chronic lung disease, n | 27 (33.3) | 10 (24.4) | 17 (42.5) | 0.102 |
| Previous stroke, n | 5 (6.2) | 4 (9.8) | 1 (2.5) | 0.359 |
| NYHA functional class | 0.054 | |||
| I, n | 57 (70.4) | 33 (80.5) | 24 (60.0) | |
| II, n | 24 (29.6) | 8 (19.5) | 16 (40.0) | |
| III/IV, n | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Medication | ||||
| Beta‐blocker, n | 12 (14.8) | 4 (9.8) | 8 (20.0) | 0.226 |
| ACEi/ARB, n | 38 (46.9) | 16 (39.0) | 22 (55.0) | 0.184 |
| Mineralocorticoid receptor antagonist, n | 12 (14.8) | 4 (9.8) | 8 (20.0) | 0.226 |
| Loop diuretic, n | 16 (19.8) | 5 (12.2) | 11 (27.5) | 0.100 |
| Aspirin, n | 42 (51.9) | 16 (39.0) | 26 (65.0) | 0.026 |
| Oral anticoagulant, n | 13 (16.0) | 7 (17.1) | 6 (15.0) | 1.000 |
ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BNP, B‐type natriuretic peptide; CABG, coronary artery bypass grafting; eGFR, estimated glomerular filtration rate; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
Continuous data are presented as means ± standard deviations, except BNP (median and interquartile range); categorical data are given as the counts (percentages).
eGFR was indicated in patients without haemodialysis.
Table 2.
Echocardiographic findings
| Variables | All patients (n = 81) | BNP ≤ 61.4 pg/mL (n = 41, 50.6%) | BNP > 61.4 pg/mL (n = 40, 49.4%) | P‐value |
|---|---|---|---|---|
| LVEF, % | 57.8 ± 5.0 | 58.5 ± 4.7 | 57.0 ± 5.3 | 0.161 |
| Left ventricular global longitudinal strain, % | −17.9 ± 3.2 | −18.7 ± 2.6 | −17.1 ± 3.6 | 0.029 |
| LVEDV index, mL/m2 | 73.6 ± 18.1 | 72.3 ± 18.1 | 75.0 ± 18.4 | 0.513 |
| LVESV index, mL/m2 | 31.4 ± 9.8 | 30.3 ± 9.7 | 32.5 ± 10.1 | 0.315 |
| SV index, mL/m2 | 48.4 ± 7.5 | 48.1 ± 7.4 | 48.7 ± 7.8 | 0.729 |
| Interventricular septal thickness, mm | 10.3 ± 1.4 | 10.0 ± 1.2 | 10.7 ± 1.6 | 0.030 |
| Posterior wall thickness, mm | 10.1 ± 1.3 | 9.7 ± 1.2 | 10.5 ± 1.3 | 0.006 |
| Left ventricular mass index, g/m2 | 110.1 ± 24.7 | 103.5 ± 19.7 | 116.9 ± 27.8 | 0.014 |
| Relative wall thickness | 0.44 ± 0.06 | 0.42 ± 0.05 | 0.45 ± 0.07 | 0.022 |
| Left atrial volume index, mL/m2 | 38.6 ± 22.2 | 31.4 ± 10.3 | 46.0 ± 28.4 | 0.003 |
| Ascending aorta dimension, mm | 34.6 ± 4.1 | 35.2 ± 4.3 | 33.9 ± 4.0 | 0.161 |
| PASP, mmHg | 30.4 ± 7.8 | 28.2 ± 4.7 | 32.6 ± 9.7 | 0.011 |
| AVA index, cm2/m2 | 0.64 ± 0.04 | 0.65 ± 0.05 | 0.64 ± 0.03 | 0.432 |
| Peak AV velocity, m/s | 3.38 ± 0.29 | 3.37 ± 0.30 | 3.38 ± 0.27 | 0.861 |
| Peak AVPG, mmHg | 45.9 ± 7.9 | 45.8 ± 8.4 | 46.0 ± 7.5 | 0.903 |
| Mean AVPG, mmHg | 25.1 ± 5.2 | 25.2 ± 5.5 | 25.0 ± 4.9 | 0.857 |
| Velocity ratio | 0.32 ± 0.06 | 0.31 ± 0.06 | 0.33 ± 0.06 | 0.135 |
| Bicuspid aortic valve, n | 11 (13.6) | 7 (17.1) | 4 (10.0) | 0.519 |
| Vena contracta width, mm | 4.2 ± 0.7 | 4.0 ± 0.6 | 4.3 ± 0.8 | 0.028 |
| AR jet width/LVOT width, % | 35.4 ± 5.6 | 35.5 ± 5.8 | 35.3 ± 5.6 | 0.902 |
| Moderate MR, n | 4 (4.9) | 0 (0.0) | 4 (10.0) | 0.055 |
| Moderate MS, n | 0 (0) | 0 (0.0) | 0 (0.0) | Not applicable |
| Moderate/severe TR, n | 6 (7.4) | 0 (0.0) | 6 (15.0) | 0.012 |
AR, aortic regurgitation; AV, aortic valve; AVA, aortic valve area; AVPG, aortic valve pressure gradient; BNP, B‐type natriuretic peptide; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; LVOT, left ventricular outflow tract; MR, mitral regurgitation; MS, mitral stenosis; PASP, pulmonary artery systolic pressure; SV, stroke volume; TR, tricuspid regurgitation.
Continuous data are presented as means ± standard deviations; categorical data are given as the counts (percentages).
Clinical outcomes
The composite primary endpoint occurred in 62 (76.5%) of the 81 patients during a median follow‐up duration of 4.1 years (IQR, 2.3–5.8). The Kaplan–Meier estimate for the composite primary endpoint was 57.7% at 5 years (Figure 1A ). All‐cause death had occurred in 17 (21.0%) of the 81 patients and was estimated to be 20.5% at 5 years (Figure 1B ); specifically, cardiac death occurred in 7 (8.7%) patients while non‐cardiac death was seen in 10 (12.3%) patients. The probability of HF hospitalization was 10% at 5 years (Figure 1C ) and the cumulative incidence of AVR was 40.9% at 5 years (Figure 1D ).
Figure 1.
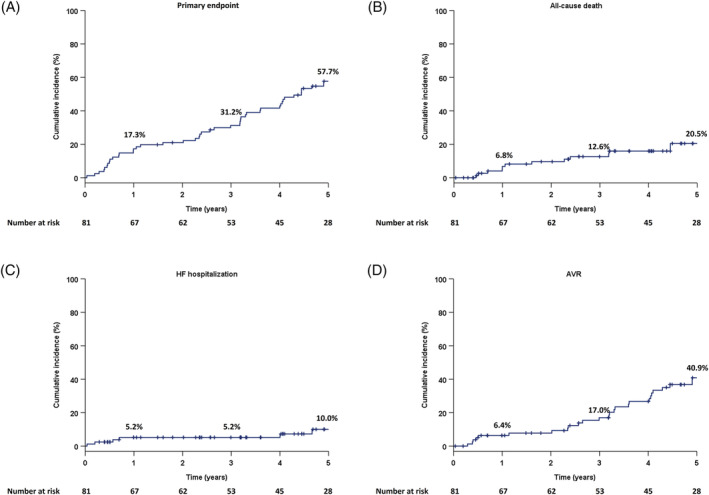
Cumulative incidence of the composite primary endpoint and each secondary endpoint in the study population: (A) the primary endpoint (a composite of all‐cause death, HF hospitalization, and AVR), (B) all‐cause death, (C) HF hospitalization, and (D) AVR. AVR, aortic valve replacement; HF, heart failure.
Aortic valve replacement data
Aortic valve replacement was performed in 37 (45.7%) patients during follow‐up (Table 3 ); of these, 18 (48.6%) patients underwent surgical AVR while transcatheter AVR was performed in 19 (51.4%) patients. Concomitant procedures during surgical AVR included ascending aorta replacement in one (1/18; 5.6%) patient and concomitant CABG in three (3/18; 16.7%) patients. Transfemoral approaches were used in 16 (84.2%) of the 19 patients who underwent transcatheter AVR. The severity of AV disease at AVR was moderate MAVD in 5 (5/37; 13.5%) patients, severe AS in 31 (31/37; 83.8%) patients, and severe AR in 1 (1/37; 2.7%) patient. Among the five patients with moderate MAVD, two underwent concomitant CABG and one patient underwent simultaneous ascending aortic replacement. Isolated AVR was performed in the other two patients—one underwent surgical AVR due to the development of HF symptoms accompanied by Δ peak AV velocity of 0.43 m/s within 6 months before AVR, along with left ventricular dilation, declining LVEF, and increasing BNP levels, while the other patient had a bicuspid AV and underwent transcatheter AVR due to prior HF hospitalization, high AV calcification (2518.0 Agatston unit) based on multidetector computed tomography, and a peak AV velocity close to 4.0 m/s at 6 months after index echocardiography.
Table 3.
Aortic valve replacement data
| Variables | |
|---|---|
| AVR, n | 37 (45.7) |
| SAVR, n | 18/37 (48.6) |
| SAVR and ascending aorta replacement, n | 1/18 (5.6) |
| SAVR and CABG, n | 3/18 (16.7) |
| SAVR, ascending aorta replacement, and CABG, n | 0/18 (0.0) |
| TAVR, n | 19/37 (51.4) |
| Transfemoral approach, n | 16/19 (84.2) |
| Aortic valve disease severity at the time of AVR | |
| AVR for moderate MAVD, n | 5/37 (13.5) |
| AVR for severe AS, n | 31/37 (83.8) |
| AVR for severe AR, n | 1/37 (2.7) |
AR, aortic regurgitation; AS, aortic stenosis; AVR, aortic valve replacement; CABG, coronary artery bypass grafting; MAVD, mixed aortic valve disease; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement.
Categorical data are given as the counts (percentages).
Clinical impact of B‐type natriuretic peptide on adverse outcomes
Multivariate Cox regression analysis accommodated for overfitting and multicollinearity in several parameters that were associated with adverse outcomes on univariate Cox regression analysis, namely, age, BNP level, NYHA functional class, left atrial volume index, pulmonary artery systolic pressure, AVA index, and moderate‐to‐severe TR. Age (hazard ratio, 1.079; 95% confidence interval, 1.028–1.133; P = 0.002) and BNP level (hazard ratio, 1.028; 95% confidence interval, 1.003–1.053; P = 0.027) were significantly associated with the composite primary endpoint (Table 4 ).
Table 4.
Univariate and multivariate Cox regression analysis to evaluate predictors for the primary composite endpoint
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| Age | 1.075 (1.026–1.128) | 0.003 | 1.079 (1.028–1.133) | 0.002 |
| Men | 0.800 (0.484–1.322) | 0.384 | ||
| Chronic kidney disease | 1.228 (0.744–2.027) | 0.422 | ||
| BNP (per 10 pg/mL increase) | 1.036 (1.020–1.053) | <0.001 | 1.028 (1.003–1.053) | 0.027 |
| Atrial fibrillation/flutter | 0.915 (0.503–1.666) | 0.771 | ||
| Previous myocardial infarction | 0.800 (0.344–1.861) | 0.604 | ||
| Peripheral arterial disease | 1.136 (0.577–2.240) | 0.712 | ||
| Chronic lung disease | 1.641 (0.981–2.746) | 0.059 | ||
| Previous stroke | 1.165 (0.417–3.251) | 0.771 | ||
| Malignant tumour | 1.336 (0.734–2.431) | 0.343 | ||
| NYHA functional class II | 1.752 (1.044–2.941) | 0.034 | 1.380 (0.779–2.444) | 0.270 |
| LVEF | 0.989 (0.935–1.045) | 0.683 | ||
| Left ventricular global longitudinal strain | 0.984 (0.900–1.075) | 0.716 | ||
| LVEDV index | 0.990 (0.977–1.001) | 0.165 | ||
| LVESV index | 0.987 (0.961–1.013) | 0.311 | ||
| SV index | 0.985 (0.951–1.021) | 0.409 | ||
| Left ventricular mass index | 1.005 (0.994–1.015) | 0.375 | ||
| Relative wall thickness (per 0.01 increase) | 1.001 (0.953–1.045) | 0.952 | ||
| Left atrial volume index | 1.016 (1.005–1.026) | 0.036 | 1.007 (0.994–1.020) | 0.317 |
| PASP | 1.026 (0.995–1.058) | 0.098 | 0.990 (0.952–1.030) | 0.628 |
| AVA index (per 0.01 cm2/m2 increase) | 0.945 (0.889–1.006) | 0.077 | 0.949 (0.890–1.013) | 0.117 |
| Peak AV velocity | 1.869 (0.818–4.272) | 0.138 | ||
| Peak AVPG | 1.022 (0.992–1.053) | 0.146 | ||
| Mean AVPG | 1.026 (0.981–1.074) | 0.264 | ||
| Velocity ratio (per 0.01 increase) | 0.962 (0.918–1.008) | 0.100 | ||
| Bicuspid aortic valve | 1.156 (0.545–2.451) | 0.706 | ||
| Vena contracta width | 0.851 (0.590–1.229) | 0.390 | ||
| AR jet width/LVOT width | 0.985 (0.938–1.034) | 0.540 | ||
| Moderate MR | 2.439 (0.871–6.832) | 0.090 | ||
| Moderate/severe TR | 2.844 (1.194–6.773) | 0.018 | 1.042 (0.313–3.465) | 0.947 |
| Beta‐blocker | 0.788 (0.399–1.558) | 0.494 | ||
| ACEi/ARB | 1.220 (0.740–2.012) | 0.435 | ||
| Mineralocorticoid receptor antagonist | 1.511 (0.785–2.906) | 0.217 | ||
ACEi, angiotensin‐converting enzyme inhibitor; AR, aortic regurgitation; ARB, angiotensin receptor blocker; AV, aortic valve; AVA, aortic valve area; AVPG, aortic valve pressure gradient; BNP, B‐type natriuretic peptide; CI, confidence interval; HR, hazard ratio; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; LVOT, left ventricular outflow tract; MR, mitral regurgitation; NYHA, New York Heart Association; PASP, pulmonary artery systolic pressure; SV, stroke volume; TR, tricuspid regurgitation.
Study patients were stratified into two groups based on a median BNP level of 61.4 pg/mL. Kaplan–Meier estimates for the primary endpoint according to the BNP level demonstrated significantly higher rates of the primary endpoint in patients with a BNP > 61.4 pg/mL vs. those with BNP ≤ 61.4 pg/mL (P = 0.018) (Figure 2A ). There were no significant differences in the incidence rates of each component of the primary endpoint, namely, all‐cause death (P = 0.147), HF hospitalization (P = 0.057), and AVR (P = 0.231) (Figure 2B–D ).
Figure 2.
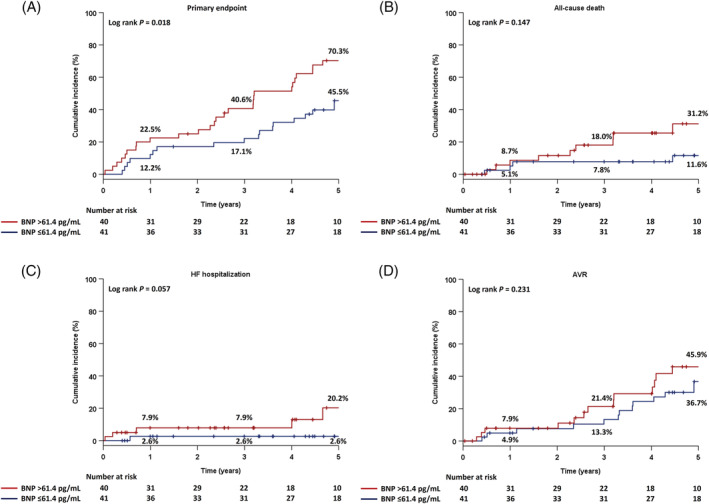
Cumulative incidence of the composite primary endpoint and each secondary endpoint in two groups, classified based on BNP levels as BNP > 61.4 pg/mL and BNP ≤ 61.4 pg/mL: (A) the primary endpoint (a composite of all‐cause death, HF hospitalization, and AVR), (B) all‐cause death, (C) HF hospitalization, and (D) AVR. AVR, aortic valve replacement; BNP, B‐type natriuretic peptide; HF, heart failure.
Clinical and echocardiographic characteristics of patients with a high B‐type natriuretic peptide level
Comparisons between patients with BNP ≤ 61.4 pg/mL and BNP > 61.4 pg/mL are listed in Tables 1 and 2 . Patients with BNP > 61.4 pg/mL were older and had higher rates of chronic kidney disease, previous myocardial infarction, and peripheral arterial disease. With respect to echocardiographic parameters, compared with those with BNP ≤ 61.4 pg/mL, patients with BNP > 61.4 pg/mL had worse left ventricular global longitudinal strain, along with higher left ventricular mass index, relative wall thickness, left atrial volume index, pulmonary artery systolic pressure, and TR grade. However, there were no significant differences between the two groups in several AV haemodynamic parameters, except vena contracta width.
Changes in echocardiographic data at 1 year follow‐up
Changes in clinical and echocardiographic data at 1 year follow‐up were evaluated in 51 patients after excluding patients who had died or had undergone AVR (Table 5 ). The median follow‐up duration was 1.0 years (IQR, 0.9–1.1). There were significantly worsening findings, including LVEF, LV end‐diastolic and end‐systolic volume indices, AVA index, peak AV velocity, and peak and mean AV pressure gradient. Further, rapid progression of peak AV velocity was seen in 19 (37.3%) patients.
Table 5.
Changes of clinical data in 51 patients at 1 year follow‐up (median follow‐up duration, 1.0 years; interquartile range, 0.9–1.1 years)
| Variables | Baseline | One‐year follow‐up | P‐value |
|---|---|---|---|
| LVEF, % | 58.0 ± 5.1 | 56.2 ± 6.4 | 0.025 |
| LVEDV index, mL/m2 | 74.1 ± 17.7 | 78.0 ± 18.2 | 0.042 |
| LVESV index, mL/m2 | 31.5 ± 9.9 | 34.8 ± 12.1 | 0.008 |
| Left ventricular mass index, g/m2 | 110.1 ± 24.2 | 110.4 ± 23.2 | 0.473 |
| Relative wall thickness | 0.43 ± 0.05 | 0.44 ± 0.08 | 0.616 |
| Left atrial volume index, mL/m2 | 35.8 ± 19.9 | 36.4 ± 14.0 | 0.437 |
| PASP, mmHg | 28.5 ± 6.0 | 30.2 ± 7.8 | 0.340 |
| AVA index, cm2/m2 | 0.64 ± 0.05 | 0.62 ± 0.10 | 0.022 |
| Peak AV velocity, m/s | 3.36 ± 0.29 | 3.52 ± 0.46 | 0.002 |
| Peak AVPG, mmHg | 45.4 ± 8.0 | 50.5 ± 12.8 | 0.001 |
| Mean AVPG, mmHg | 25.0 ± 5.4 | 27.4 ± 7.3 | 0.002 |
| BNP, pg/mL (median) | 54.1 (25.7–79.4) | 49.5 (32.0–91.9) | 0.054 |
AV, aortic valve; AVA, aortic valve area; AVPG, aortic valve pressure gradient; BNP, B‐type natriuretic peptide; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; PASP, pulmonary artery systolic pressure.
Continuous data are presented as means ± standard deviations, except BNP (median and interquartile range).
Discussion
Our analyses of moderate MAVD cases revealed that (i) 57.7% of the patients experienced the primary composite endpoint at 5 years; (ii) estimated AVR requirement was 40.9% at 5 years; and (iii) BNP levels were independently associated with the primary endpoint and patients with high BNP levels had extravalvular cardiac damage.
Unfavourable clinical outcomes
Egbe et al. have reported the cumulative incidence of adverse events to be 71% at 5 years in patients with moderate MAVD, wherein adverse events were defined as a composite of HF symptom development of NYHA functional class III or IV, AVR, or cardiac death; interestingly, this was not significantly different from adverse event incidence in patients with severe AS (P = 0.49). 5 Although the cumulative incidence of the adverse events in our study was lower than previously reported, patients with moderate MAVD are expected to experience unfavourable outcomes during follow‐up when medically treated.
Aortic valve replacement in patients with moderate mixed aortic valve disease
Aortic valve replacement was the most frequent adverse event among the components of the primary endpoint, and a similar high cumulative incidence of AVR has been reported previously (65% at 5 years). 5 Moreover, at the 1 year follow‐up, patients with moderate MAVD exhibited worsening AV haemodynamics and more than one‐third of the cohort had a rapid progression of peak AV velocity. Current guidelines suggest that AVR should be considered in symptomatic patients with moderate MAVD and preserved LVEF if peak AV velocity is more than 4.0 m/s 4 ; however, AVR is not indicated in patients with peak AV velocity of <4.0 m/s even though moderate MAVD is a progressive disease and AVR might be unavoidable in the near future in such patients. This represents a clinical challenge in the optimal management of such patients and additional studies are needed to evaluate the safety and efficacy of AVR in moderate MAVD.
The association of B‐type natriuretic peptide with extravalvular cardiac damage and clinical outcomes
B‐type natriuretic peptide is helpful in the diagnosis, management, and risk stratification of HF. 12 , 13 Several previous studies have suggested BNP to be a promising blood biomarker that can enhance risk stratification in patients with either AS or AR alone, 14 , 15 , 16 , 17 , 18 , 19 , 20 and recent guidelines refer to the usefulness of BNP in risk stratification and monitoring during follow‐up. 4 , 21 BNP is released from the myocardium when the atria and ventricles are exposed to intracardiac stress. Thus, BNP levels can be expected to increase in patients with MAVD because of pressure overload and wall stress due to AS and diastolic stretch and volume overload due to AR. 22 , 23 However, there are inadequate data on BNP levels in patients with MAVD. 1 , 2 , 3 , 5 , 24 In the present study, patients with higher BNP levels had several worse echocardiographic parameters than those with lower BNP levels, suggesting that high BNP levels reflect comprehensive extravalvular cardiac damage in patients with moderate MAVD. Further, given that BNP is an easily accessible biomarker, it may play an important role in risk stratification if used along with other clinical and echocardiographic findings in patients with moderate MAVD.
Study limitations
The above notwithstanding, a few limitations to our study include its small‐scale and retrospective nature. Further, as there was considerable bias in data accumulation, these results should be validated in multicentre prospective studies with a larger population. Unlike previous studies, we categorized AR severity using semiquantitative and qualitative approaches, rather than using quantitative or integrated approaches 5 , 24 ; however, a previous report has demonstrated that vena contracta width is significantly correlated to such quantitative indices, regardless of the central or eccentric AR jets. 25 Therefore, assessment of AR grade using vena contracta width was deemed suitable. Finally, mitral inflow pattern and left ventricular diastolic dysfunction could not be analysed because more than 20% of the study patients had atrial fibrillation, paced rhythm, or previous mitral valve surgery.
Conclusions
Patients with moderate MAVD are at risk for rapid progression of deleterious AV haemodynamics and unfavourable clinical outcomes. High BNP level was independently related to adverse events and might reflect extravalvular cardiac damage; hence, patients with moderate MAVD require careful follow‐up, especially those with high BNP levels.
Conflict of interest
The authors have no conflicts of interest to declare.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Onishi, H. , Naganuma, T. , Izumo, M. , Ouchi, T. , Yuki, H. , Mitomo, S. , and Nakamura, S. (2022) Prognostic relevance of B‐type natriuretic peptide in patients with moderate mixed aortic valve disease. ESC Heart Failure, 9: 2474–2483. 10.1002/ehf2.13946.
References
- 1. Ngiam JN, Chew NWS, Pramotedham T, Tan BY‐Q, Sia C‐H, Loh PH, Ruan W, Tay E, Kong WKF, Yeo T‐C, Poh K‐K. Implications of coexisting aortic regurgitation in patients with aortic stenosis. JACC: Asia. 2021; 1: 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zilberszac R, Gabriel H, Schemper M, Zahler D, Czerny M, Maurer G, Rosenhek R. Outcome of combined stenotic and regurgitant aortic valve disease. J Am Coll Cardiol. 2013; 61: 1489–1495. [DOI] [PubMed] [Google Scholar]
- 3. Egbe AC, Poterucha JT, Warnes CA. Mixed aortic valve disease: midterm outcome and predictors of adverse events. Eur Heart J. 2016; 37: 2671–2678. [DOI] [PubMed] [Google Scholar]
- 4. Writing Committee M, Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, Jneid H, Krieger EV, Mack M, Mcleod C, O'Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A, Toly C. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021; 77: e25‐e197. [DOI] [PubMed] [Google Scholar]
- 5. Egbe AC, Luis SA, Padang R, Warnes CA. Outcomes in moderate mixed aortic valve disease: is it time for a paradigm shift? J Am Coll Cardiol. 2016; 67: 2321–2329. [DOI] [PubMed] [Google Scholar]
- 6. Baumgartner H, Hung J, Bermejo J, Chambers JB, Edvardsen T, Goldstein S, Lancellotti P, LeFevre M, Miller F Jr, Otto CM. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2017; 30: 372–392. [DOI] [PubMed] [Google Scholar]
- 7. Zoghbi WA, Adams D, Bonow RO, Enriquez‐Sarano M, Foster E, Grayburn PA, Hahn RT, Han Y, Hung J, Lang RM, Little SH, Shah DJ, Shernan S, Thavendiranathan P, Thomas JD, Weissman NJ. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017; 30: 303–371. [DOI] [PubMed] [Google Scholar]
- 8. Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quinones M, American Society of E, European Association of E . Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009; 22: 1–23 quiz 101‐2. [DOI] [PubMed] [Google Scholar]
- 9. Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC, Horton K, Ogunyankin KO, Palma RA, Velazquez EJ. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2019; 32: 1–64. [DOI] [PubMed] [Google Scholar]
- 10. Cepeda MS, Boston R, Farrar JT, Strom BL. Comparison of logistic regression versus propensity score when the number of events is low and there are multiple confounders. Am J Epidemiol. 2003; 158: 280–287. [DOI] [PubMed] [Google Scholar]
- 11. Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. (0895‐4356 (Print)). [DOI] [PubMed]
- 12. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Group ESCSD . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 13. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017; 70: 776–803. [DOI] [PubMed] [Google Scholar]
- 14. Weber M, Arnold R, Rau M, Elsaesser A, Brandt R, Mitrovic V, Hamm C. Relation of N‐terminal pro B‐type natriuretic peptide to progression of aortic valve disease. Eur Heart J. 2005; 26: 1023–1030. [DOI] [PubMed] [Google Scholar]
- 15. Parikh V, Kim C, Siegel RJ, Arsanjani R, Rader F. Natriuretic peptides for risk stratification of patients with valvular aortic stenosis. Circ Heart Fail. 2015; 8: 373–380. [DOI] [PubMed] [Google Scholar]
- 16. Clavel MA, Malouf J, Michelena HI, Suri RM, Jaffe AS, Mahoney DW, Enriquez‐Sarano M. B‐type natriuretic peptide clinical activation in aortic stenosis: impact on long‐term survival. J Am Coll Cardiol. 2014; 63: 2016–2025. [DOI] [PubMed] [Google Scholar]
- 17. Chin CW, Messika‐Zeitoun D, Shah AS, Lefevre G, Bailleul S, Yeung EN, Koo M, Mirsadraee S, Mathieu T, Semple SI, Mills NL, Vahanian A, Newby DE, Dweck MR. A clinical risk score of myocardial fibrosis predicts adverse outcomes in aortic stenosis. Eur Heart J. 2016; 37: 713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chin CW, Shah AS, McAllister DA, Joanna Cowell S, Alam S, Langrish JP, Strachan FE, Hunter AL, Maria Choy A, Lang CC, Walker S, Boon NA, Newby DE, Mills NL, Dweck MR. High‐sensitivity troponin I concentrations are a marker of an advanced hypertrophic response and adverse outcomes in patients with aortic stenosis. Eur Heart J. 2014; 35: 2312–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chin CWL, Everett RJ, Kwiecinski J, Vesey AT, Yeung E, Esson G, Jenkins W, Koo M, Mirsadraee S, White AC, Japp AG, Prasad SK, Semple S, Newby DE, Dweck MR. Myocardial fibrosis and cardiac decompensation in aortic stenosis. JACC Cardiovasc Imaging. 2017; 10: 1320–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dahou A, Clavel MA, Capoulade R, O'Connor K, Ribeiro HB, Cote N, Le Ven F, Rodes‐Cabau J, Dumesnil JG, Mathieu P, Pibarot P. B‐type natriuretic peptide and high‐sensitivity cardiac troponin for risk stratification in low‐flow, low‐gradient aortic stenosis: a substudy of the TOPAS study. JACC Cardiovasc Imaging. 2018; 11: 939–947. [DOI] [PubMed] [Google Scholar]
- 21. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Juni P, Pierard L, Prendergast BD, Sadaba JR, Tribouilloy C, Wojakowski W, Group EESD . ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022; 12: 561–632. [DOI] [PubMed] [Google Scholar]
- 22. Vanderheyden M, Goethals M, Verstreken S, De Bruyne B, Muller K, Van Schuerbeeck E, Bartunek J. Wall stress modulates brain natriuretic peptide production in pressure overload cardiomyopathy. J Am Coll Cardiol. 2004; 44: 2349–2354. [DOI] [PubMed] [Google Scholar]
- 23. Iwanaga Y, Nishi I, Furuichi S, Noguchi T, Sase K, Kihara Y, Goto Y, Nonogi H. B‐type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: comparison between systolic and diastolic heart failure. J Am Coll Cardiol. 2006; 47: 742–748. [DOI] [PubMed] [Google Scholar]
- 24. Rashedi N, Popovic ZB, Stewart WJ, Marwick T. Outcomes of asymptomatic adults with combined aortic stenosis and regurgitation. J Am Soc Echocardiogr. 2014; 27: 829–837. [DOI] [PubMed] [Google Scholar]
- 25. Tribouilloy CM, Enriquez‐Sarano M, Bailey KR, Seward JB, Tajik AJ. Assessment of severity of aortic regurgitation using the width of the vena contracta: a clinical color Doppler imaging study. (1524‐4539 (Electronic)). [DOI] [PubMed]


