Abstract
Aims
The current study explores whether degree of inflammation, reflected by C‐reactive protein (CRP) level, modifies the effect of intravenous (IV) corticosteroid administered in the emergency department (ED) on clinical outcomes in patients with acute heart failure (AHF).
Methods and results
We selected patients diagnosed with AHF in the ED, with confirmed N‐terminal pro‐B‐type natriuretic peptide > 300 pg/mL and CRP > 5 mg/L in the ED from the Epidemiology of Acute Heart Failure in the Emergency Departments (EAHFE) registry. In these 1109 patients, 121 were treated by corticosteroid. The corticosteroid therapy hazard ratio (HR) for 30 day all‐cause mortality was 1.26 [95% confidence interval (CI) 0.75–2.09, P = 0.38]. Although not statistically significant, HRs tended to decrease with increasing CRP level, with point estimates favouring corticosteroid at CRP levels above 20. In patients with CRP > 40 mg/L, with adjusted HRs of 0.56 (95% CI 0.20–1.55, P = 0.27) for 30 day all‐cause mortality, 0.92 (95% CI 0.52–1.62, P = 0.78) for 30 day post‐discharge ED revisit, hospitalization, or death, and adjusted odds ratio of 0.61 (95% CI 0.17–2.14, P = 0.44) for in‐hospital all‐cause mortality.
Conclusions
The present analysis suggests that corticosteroids might have the potential to improve outcomes in AHF patients with inflammatory activation. Larger, prospective studies of anti‐inflammatory therapy should be considered to assess potential benefit in patients with the highest degree of inflammation.
Keywords: Acute heart failure, Inflammation, Corticosteroids, Emergency department, Mortality
Background
Abnormalities in the inflammatory cascade are well known to be associated with both the onset and progression of heart failure (HF) and related to outcome and cardiac remodelling, even though microbial infection is not involved in most cases. 1 , 2 , 3 , 4 , 5 Indeed, various inflammatory biomarkers have been investigated as prognostic markers in patients with acute HF (AHF). 6 , 7 , 8 , 9 , 10 However, only a few studies have evaluated the effect of direct anti‐inflammatory therapies in AHF patients, 11 , 12 mostly focusing on diuresis. This is likely due to the failure of some anti‐inflammatory therapies to improve clinical outcomes in patients with chronic HF. 13 , 14 Corticosteroids are potent agents used to reduce inflammation and hence could be considered as potential anti‐inflammatory therapy, to be administered intravenously (IV) in the emergency department (ED).
Aims
In this sub‐analysis of the Epidemiology of Acute Heart Failure in the Emergency Departments (EAHFE) registry, we sought to investigate whether for AHF patients in the ED the effects of systemic IV corticosteroid therapy on mortality and adverse events differ according to level of C‐reactive protein (CRP).
Methods
The present study is a sub‐analysis of the EAHFE registry, 15 , 16 which has prospectively enrolled 18 370 patients with exacerbation of chronic HF in 45 Spanish EDs in six 1–2 month recruitment waves between 2007 and 2018. The AHF diagnosis was initially based on the Framingham clinical criteria 17 and confirmed by natriuretic peptide measurement or echocardiography in the majority of patients. Patients with ST‐segment myocardial infarction were excluded. For this sub‐analysis, data from 9 EDs that had enrolled 7041 patients in the EAHFE registry were reviewed, and patients with N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) > 300 pg/mL and CRP > 5 mg/L in the ED with a confirmed diagnosis of HF and suggestive inflammation were included. CRP has been used as the inflammatory marker for the current analysis. CRP is performed routinely in hospital emergency rooms and was available for the majority of patients in the EAHFE registry.
Patients on chronic systemic corticosteroids, with infections triggering episodes of AHF, and whose corticosteroids use in the ED was unknown were excluded. Patients included in the analysis were divided into two groups: patients receiving corticosteroids in the ED (corticosteroid‐treated group) and patients who did not receive corticosteroids (corticosteroid‐untreated group). The primary outcome for this sub‐analysis was 30 day all‐cause mortality. Secondary outcomes were in‐hospital all‐cause mortality and a 30 day post‐discharge composite outcome comprising ED revisit, hospitalization, or death. Subgroup analyses examining increasing CRP thresholds were carried out. The EAHFE registry protocol was approved by a central ethics committee at the Hospital Universitario Central de Asturias (Oviedo, Spain) and conformed with the Declaration of Helsinki principles. All participating patients gave informed consent to be included in the registry and contacted for follow‐up.
Statistical analysis
Continuous variables are reported as mean ± standard deviation (SD) and categorical data as number and percentage. Associations between corticosteroid therapy in the ED and clinical outcomes were estimated with logistic regression (for in‐hospital mortality) and Cox regression models (for 30 day all‐cause mortality and 30 day post‐discharge combined endpoint consisting of ED revisit, hospitalization, or death). Results are expressed as odds ratios (ORs) and hazard ratios (HRs) with 95% confidence intervals (CIs), respectively. Kaplan–Meier plots of cumulative event‐free survival are presented. Adjustments were made for the EAHFE‐3D scale, which predicts the short‐term prognosis of patients with AHF. 18 The EAHFE‐3D scale is derived from the following variables (maximum 165 points): age ≥ 75 years (30 points), baseline New York Heart Association III–IV (15 points), systolic blood pressure < 110 mmHg (20 points), room air oxygen saturation < 90% (30 points), hyponatraemia (20 points), inotropic or vasopressor treatment (30 points), and need for non‐invasive mechanical ventilation (20 points). To account for missing data, multiple imputation by chained equations was used with 10 imputed datasets. Rubin's algorithm was used for averaging parameter estimates across the imputed datasets. A two‐sided P‐value < 0.05 was considered statistically significant. Statistical analyses were conducted using R Version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria) packages: mice v3.11.0 19 and rms v6.2‐0. 20
Results
Of 7041 patients enrolled in the 9 EDs participating centres, 298 were excluded because of being on chronic corticosteroid therapy, 2374 had infection triggering the AHF episode, 22 had no record of corticosteroid use in the ED, and 3238 had CRP below 5 mg/L or unknown. Thus, 1109 patients were available for the present sub‐analysis. Of them, 121 patients (10.9%) received at least one IV bolus of corticosteroids during the ED stay. Baseline characteristics by corticosteroids usage at baseline are presented in Table 1 . Overall, patients receiving IV corticosteroids were more likely to have higher systolic blood pressure, lower room air oxygen saturation, higher rates of cerebrovascular disease, peripheral artery disease, chronic obstructive pulmonary disease (COPD), and dementia, lower rates of heart valve disease, and lower Barthel's index. The current AHF episode was more commonly triggered by hypertensive crisis. There was also a significant difference in triage level (severity). Regarding laboratory variables, patients receiving IV corticosteroids had lower urea and higher CRP. Baseline characteristics in the subgroup of 281 patients with elevated CRP (>40 mg/L) show similar differences (Supporting Information, Table S1 ). In all patients with CRP > 5 mg/L and NT‐proBNP > 300 pg/mL, the cumulative risk of 30 day all‐cause mortality did not differ statistically significantly between patients treated and not treated with IV corticosteroids, with mortality rates of 14.0% and 11.1%, respectively [HR 1.26 (95% CI 0.75–2.09, P = 0.38)]. Results followed a similar pattern for the subgroup of patients with NT‐proBNP > 1500 pg/mL: HR 1.16 (95% CI 0.67–2.03, P = 0.60) (Figure 1 ). HRs for patients receiving IV corticosteroids relative to patients not treated with IV corticosteroids for 30 day mortality tended to decrease as the level of CRP increased in populations with NT‐proBNP both >300 and >1500 pg/mL (Figure 2 and Supporting Information, Figure S1 ), with point estimates for HRs suggesting a possible protective effect of corticosteroids in subgroups of patients defined by a CRP threshold > 20 mg/L. In patients with very highly elevated inflammatory markers (i.e. CRP > 40 mg/L), the hazards of 30 day mortality were 7.7% and 9.2% lower in corticosteroid‐treated patients, in patients with NT‐proBNP > 300 and >1500 pg/mL, respectively, although these findings were not statistically significant (Figure 1 ). Table 2 shows the unadjusted and adjusted effects of the association between corticosteroid use and the outcomes in patients with elevated CRP (>40 mg/L). Adjusted HRs for patients receiving corticosteroids relative to patients not treated with corticosteroids were all <1 for all 3 outcomes, though not statistically significant [30 day all‐cause mortality: adjusted HR 0.56 (95% CI 0.20–1.55), P = 0.27; 30 day post‐discharge combined endpoint: adjusted HR 0.92 (95% CI 0.52–1.62), P = 0.78; in‐hospital all‐cause mortality: adjusted OR 0.61 (95% CI 0.17–2.14), P = 0.44].
Table 1.
Characteristics of patients in the EAHFE registry with NT‐proBNP > 300 pg/mL and CRP > 5 mg/L according to whether they did or did not receive systemic corticosteroids in the emergency department
| Total (n = 1109) | Missing values (%) | Not treated (n = 988) | Treated (n = 121) | P‐value | |
|---|---|---|---|---|---|
| Demographic data | |||||
| Age (years) | 81.2 (10.1) | 0.1 | 81.1 (10.1) | 81.6 (10.1) | 0.63 |
| Male | 500 (45) | 0.3 | 434 (44) | 66 (55) | 0.037 |
| Vitals at ED during acute episode | |||||
| Systolic blood pressure (mmHg) | 137.9 (29.1) | 1.8 | 136.8 (28.3) | 146.6 (33.6) | <0.001 |
| Heart rate (b.p.m.) | 91.3 (26.4) | 2.8 | 91.0 (27.0) | 93.4 (20.8) | 0.36 |
| Room air oxygen saturation (%) | 92.1 (8.1) | 2.8 | 92.6 (7.2) | 87.7 (12.2) | <0.001 |
| Comorbidities | |||||
| Hypertension | 898 (83) | 2.1 | 798 (82) | 100 (86) | 0.35 |
| Diabetes mellitus | 487 (44) | 0.2 | 439 (44) | 48 (40) | 0.40 |
| Dyslipidaemia | 504 (46) | 0.3 | 442 (45) | 62 (52) | 0.19 |
| Ischaemic heart disease | 322 (29) | 0.3 | 286 (29) | 36 (30) | 0.91 |
| Chronic kidney failure (creatinine > 2 mg/dL) | 309 (28) | 0.2 | 278 (28) | 31 (26) | 0.67 |
| Cerebrovascular disease | 142 (13) | 0.2 | 119 (12) | 23 (19) | 0.040 |
| Atrial fibrillation | 593 (54) | 0.2 | 539 (55) | 54 (45) | 0.058 |
| Peripheral artery disease | 84 (8) | 0.2 | 68 (7) | 16 (13) | 0.020 |
| Heart valve disease | 269 (24) | 0.2 | 251 (25) | 18 (15) | 0.016 |
| Chronic obstructive pulmonary disease | 250 (23) | 0.2 | 206 (21) | 44 (37) | <0.001 |
| Dementia | 93 (8) | 0.3 | 70 (7) | 23 (19) | <0.001 |
| Active neoplasia | 166 (15) | 0.3 | 150 (15) | 16 (13) | 0.68 |
| Hepatic cirrhosis | 19 (2) | 0.3 | 17 (2) | 2 (2) | 1.00 |
| Prior episodes of AHF | 690 (64) | 3.1 | 614 (64) | 76 (67) | 0.54 |
| Baseline status | |||||
| Left ventricular ejection fraction (%) | 50.3 (16.7) | 44.5 | 50.3 (16.3) | 50.1 (19.6) | 0.92 |
| Triage level according to severity | 10.6 | <0.001 | |||
| Red | 47 (5) | 34 (4) | 13 (12) | ||
| Orange | 343 (35) | 299 (34) | 44 (39) | ||
| Yellow | 521 (53) | 480 (55) | 41 (37) | ||
| Green | 80 (8) | 66 (8) | 14 (12) | ||
| NYHA class | 5.7 | 0.80 | |||
| I | 244 (23) | 220 (24) | 24 (21) | ||
| II | 557 (53) | 497 (53) | 60 (53) | ||
| III | 226 (22) | 199 (21) | 27 (24) | ||
| IV | 19 (2) | 16 (2) | 3 (3) | ||
| Barthel's index (points) | 77.6 (27.6) | 8.2 | 78.2 (27.2) | 72.4 (30.1) | 0.037 |
| Triggering factor for the current AHF episode | |||||
| Rapid atrial fibrillation | 216 (19) | 0 | 197 (20) | 19 (16) | 0.32 |
| Anaemia | 110 (10) | 0 | 102 (10) | 8 (7) | 0.26 |
| Hypertensive emergency | 63 (6) | 0 | 48 (5) | 15 (12) | 0.002 |
| Non‐compliance | 41 (4) | 0 | 38 (4) | 3 (2) | 0.62 |
| Acute coronary syndrome | 39 (4) | 0 | 36 (4) | 3 (2) | 0.69 |
| Chronic treatments at home | |||||
| Loop diuretics | 740 (67) | 0.1 | 660 (67) | 80 (66) | 0.95 |
| Thiazide diuretics | 160 (14) | 0.2 | 137 (14) | 23 (19) | 0.16 |
| Mineralocorticoid‐receptor antagonist | 194 (18) | 0.1 | 174 (18) | 20 (17) | 0.86 |
| Renin angiotensin system inhibitors | 596 (54) | 0.2 | 529 (54) | 67 (55) | 0.79 |
| Beta‐blockers | 517 (47) | 1.2 | 469 (48) | 48 (40) | 0.098 |
| Antibiotics in the previous week | 26 (2) | 0 | 21 (2) | 5 (4) | 0.29 |
| Results of blood tests at ED | |||||
| Haemoglobin (g/dL) | 11.8 (2.2) | 0.4 | 11.8 (2.2) | 12.0 (2.1) | 0.50 |
| Haematocrit (%) | 36.7 (6.3) | 0.5 | 36.7 (6.3) | 37.3 (5.6) | 0.29 |
| White blood cell count (/mm3) | 9252.3 (5299.1) | 0.8 | 9270.3 (5484.3) | 9106.1 (3466.0) | 0.75 |
| Platelets (×109/L) | 239.2 (278.9) | 0.9 | 242.7 (294.3) | 211.3 (73.5) | 0.24 |
| Glucose (mg/dL) | 149.7 (76.5) | 1.2 | 149.2 (75.0) | 154.5 (87.9) | 0.47 |
| Urea (mg/dL) | 76.7 (60.5) | 7.6 | 78.2 (62.7) | 63.4 (33.4) | 0.016 |
| Creatinine (mg/dL) | 1.4 (0.9) | 0.2 | 1.4 (0.9) | 1.3 (0.6) | 0.16 |
| Sodium (mmol/L) | 138.6 (5.7) | 2.3 | 138.5 (5.5) | 138.9 (7.2) | 0.57 |
| Potassium (mmol/L) | 4.5 (0.7) | 5.6 | 4.5 (0.7) | 4.4 (0.6) | 0.077 |
| Raised troponin (>99th percentile) | 503 (72) | 37.2 | 442 (71) | 61 (82) | 0.052 |
| NT‐proBNP (pg/mL) | 8584.1 (12 036.5) | 0 | 8713.7 (12 278.9) | 7525.8 (9815.0) | 0.31 |
| CRP (mg/L) | 36.7 (49.1) | 0 | 35.5 (47.7) | 46.3 (58.5) | 0.022 |
| Severity of the AHF episode | |||||
| MEESSI‐AHF risk score | 48.7 | 0.47 | |||
| Low risk | 180 (32) | 164 (33) | 16 (24) | ||
| Intermediate risk | 254 (45) | 219 (44) | 35 (53) | ||
| High risk | 78 (14) | 69 (14) | 9 (14) | ||
| Very high risk | 57 (10) | 51 (10) | 6 (9) | ||
| Prognostic scale | |||||
| EAHFE‐3D scale | 41.6 (23.6) | 11.6 | 40.7 (23.3) | 48.4 (25.0) | 0.001 |
AHF, acute heart failure; CRP, C‐reactive protein; EAHFE, Epidemiology of Acute Heart Failure in the Emergency Departments; ED, emergency department; MEESSI‐AHF, Multiple Estimation of risk based on the Emergency department Spanish Score In patients with AHF; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association.
Values are mean (standard deviation), n (%).
Figure 1.
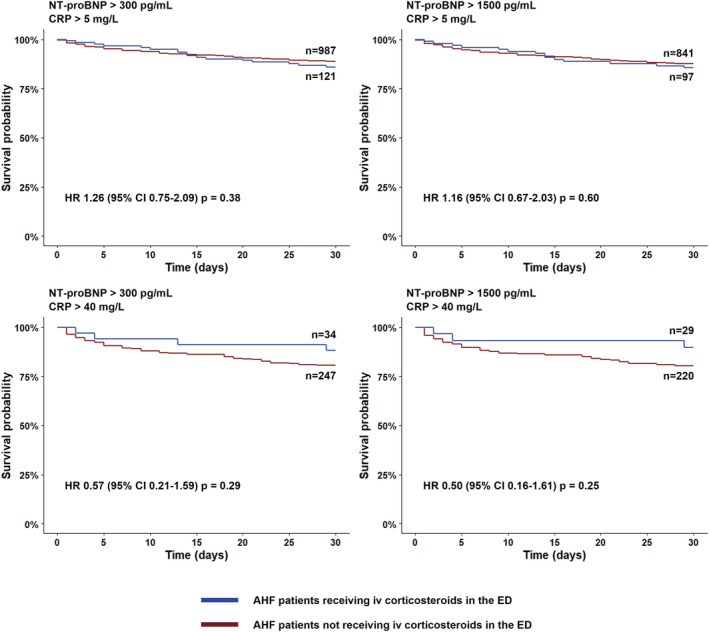
Kaplan–Meier plots showing cumulative risk of 30 day all‐cause mortality in acute heart failure patients treated with corticosteroids in the emergency department vs. without corticosteroids in the emergency department. AHF, acute heart failure; CI, confidence interval; CRP, C‐reactive protein; ED, emergency department; HR, hazard ratio; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Figure 2.
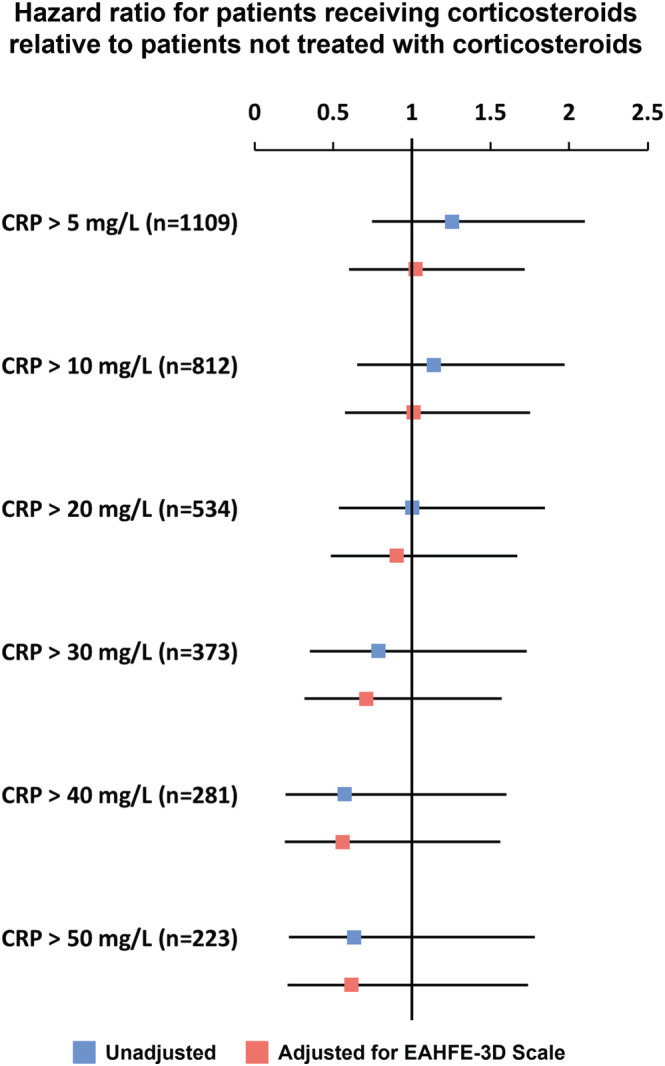
Unadjusted and adjusted 30 day all‐cause mortality according to the CRP levels in patients with N‐terminal pro‐B‐type natriuretic peptide > 300 pg/mL. CRP, C‐reactive protein; EAHFE, Epidemiology of Acute Heart Failure in the Emergency Departments.
Table 2.
Unadjusted and adjusted outcomes in patients receiving corticosteroids (N‐terminal pro‐B‐type natriuretic peptide > 300 pg/mL and C‐reactive protein > 40 mg/L)
| Event number (%) | Unadjusted model | Adjusted model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Not treated | Treated | OR or HR | Lower CI | Upper CI | P‐value | OR or HR | Lower CI | Upper CI | P‐value | |
| 30 day all‐cause mortality (HR) | 48 (19.4%) | 4 (11.8%) | 0.57 | 0.21 | 1.59 | 0.29 | 0.56 | 0.20 | 1.55 | 0.27 |
| 30 day post‐discharge combined endpoint a (HR) | 74 (43.8%) | 11 (42.3%) | 0.92 | 0.49 | 1.73 | 0.79 | 0.92 | 0.52 | 1.62 | 0.78 |
| In‐hospital all‐cause mortality (OR) | 33 (13.4%) | 3 (8.8%) | 0.63 | 0.18 | 2.17 | 0.46 | 0.61 | 0.17 | 2.14 | 0.44 |
Adjustments were performed for Epidemiology of Acute Heart Failure in the Emergency Departments (EAHFE)‐3D scale, which predicts the short‐term prognosis of patients with acute heart failure. EAHFE‐3D scale contains the following variables (maximum 165 points): age ≥ 75 years (30 points), baseline New York Heart Association III–IV (15 points), systolic blood pressure < 110 mmHg (20 points), room air oxygen saturation < 90% (30 points), hyponatraemia (20 points), inotropic or vasopressor treatment (30 points), and need for non‐invasive mechanical ventilation (20 points).
CI, confidence interval; HR, hazard ratio; OR, odds ratio.
Thirty‐day post‐discharge combined endpoint indicates emergency department revisit, hospitalization, or death.
Discussion
Acute heart failure patients with elevated inflammatory markers have a poor prognosis. 6 , 7 , 8 , 9 , 10 In the current analysis, we explored the effects of anti‐inflammatory therapy (IV corticosteroids) administered in the ED to AHF patients according to levels of CRP. We have found numerical trends suggesting that some short‐term outcomes in AHF may be affected by corticosteroid therapy in unadjusted and adjusted analyses in patients with highly elevated signs of inflammation such as CRP > 40 mg/L. Although corticosteroids have been classically viewed as anti‐inflammatory agents, corticosteroids can cause sodium and water retention, potentially leading to worsening of HF. However, it has been reported that the administration of corticosteroids to patients with severe AHF produced a potent diuretic effect and improved fluid overload and renal function. 21 , 22 In addition, a recent publication from our group showed that there is no evidence of harm related to the new onset of systemic corticosteroid therapy during an episode of AHF in the ED. 23 Several limitations of this sub‐analysis should be noted. Firstly, this was a retrospective analysis, corticosteroid therapy was not randomized, and the distribution of some variables differed between the corticosteroid‐treated and corticosteroid‐untreated groups. Even the best regression model cannot fully resolve this issue, and some confoundings may still exist. In addition, it is also unclear why the physician used corticosteroids, potentially due to diagnostic uncertainty. Secondly, given the non‐prospective design of the study, details regarding the IV corticosteroid administration including the dose and duration were not available. Thirdly, CRP levels were not followed after baseline. Therefore, it is unclear whether corticosteroids lowered CRP levels in this sub‐analysis and whether prognosis was dependent on changes in CRP. Fourthly, the corticosteroid‐treated group was quite limited in size. In fact, some of the CIs estimated in this study were rather wide, reflecting insufficient statistical power to detect significant trends. Fifthly, in this study, CRP was used as an inflammatory marker in patients with AHF, although not necessarily a well‐established marker in these patients. Future studies using other novel inflammatory markers might be required. Sixthly, patients treated with corticosteroids more often had COPD and had a lower mean oxygen saturation. Despite adjustment for the EAHF‐3D scale, which takes oxygen saturation into account, the potential benefits of corticosteroids at high CRP levels could be due to effects on COPD rather than HF. Finally, this is a post hoc cohort analysis limited to hypothesis generation that requires confirmation in future trials.
Conclusions
Although not statistically significant, the current analysis suggests that corticosteroids might have the potential to improve outcomes in AHF patients with inflammatory activation. Taken together with previous studies of potentially improved diuresis, 21 , 22 the results suggest that future randomized trials on anti‐inflammatory therapy are needed to assess potential benefit in patients with the highest degree of inflammation.
Conflict of interest
O.M. reported receiving grants from ISCIII_Spanish Ministry of Health (FIS18/00393). K.T., B.A.D., C.E., and G.C. are employees of Momentum Research Inc. Momentum Research Inc. has received grants for research from Abbott Laboratories, Cirius Therapeutics Inc., Corteria Pharmaceuticals, Roche Diagnostics Inc., Sanofi, Windtree Therapeutics Inc., and XyloCor Therapeutics. A.M. reported receiving personal fees from Novartis, Orion, Roche, Sanofi, Otsuka, Philips, and Servier; grants and personal fees from Adrenomed and Abbott; and grants from 4TEEN4 outside the submitted work. The other authors declared no potential conflicts of interest with respect to the research authorship and/or publication of this article.
Funding
This study was partially supported by grants from the Instituto de Salud Carlos III supported with funds from the Spanish Ministry of Health and Federación Española de Enfermedades Raras (FEDER) (PI15/01019, PI15/00773, PI18/00393, PI18/00456) and Fundació La Marató de TV3 (2015/2510). The ‘Emergencies: Processes and Pathologies’ research group of the IDIBAPS receives financial support from the Catalonian Government for Consolidated Groups of Investigation (GRC 2009/1385, 2014/0313, and 2017/1424). The ICA‐SEMES Research Group has received unrestricted support from Orion Pharma and Novartis. No funding was provided for the current analysis. The present study was designed, analysed, and written exclusively by the authors independently of these pharmaceutical companies.
Supporting information
Table S1. Baseline characteristics of patients in the EAHFE registry with NT‐proBNP > 300 pg/mL and CRP > 40 mg/L according to whether they did or did not receive systemic corticosteroids in the emergency department.
Figure S1. Unadjusted and adjusted 30‐day all‐cause mortality according to the CRP levels in patients with elevated NT‐proBNP (> 1500 pg/mL).
Acknowledgements
We thank Alícia Díaz for her professionalism in data management.
Miró, Ò. , Takagi, K. , Davison, B. A. , Edwards, C. , Freund, Y. , Jacob, J. , Llorens, P. , Mebazaa, A. , and Cotter, G. (2022) Effect of systemic corticosteroid therapy for acute heart failure patients with elevated C‐reactive protein. ESC Heart Failure, 9: 2225–2232. 10.1002/ehf2.13926.
OM and KT have contributed equally to the present paper and should both be considered as first authors.
References
- 1. Goonewardena SN, Stein AB, Tsuchida RE, Rattan R, Shah D, Hummel SL. Monocyte subsets and inflammatory cytokines in acute decompensated heart failure. J Card Fail. 2016; 22: 358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shirazi LF, Bissett J, Romeo F, Mehta JL. Role of inflammation in heart failure. Curr Atheroscler Rep. 2017; 19: 27. [DOI] [PubMed] [Google Scholar]
- 3. Markousis‐Mavrogenis G, Tromp J, Ouwerkerk W, Devalaraja M, Anker SD, Cleland JG, Dickstein K, Filippatos GS, van der Harst P, Lang CC, Metra M, Ng LL, Ponikowski P, Samani NJ, Zannad F, Zwinderman AH, Hillege HL, van Veldhuisen DJ, Kakkar R, Voors AA, van der Meer P. The clinical significance of interleukin‐6 in heart failure: results from the BIOSTAT‐CHF study. Eur J Heart Fail. 2019; 21: 965–973. [DOI] [PubMed] [Google Scholar]
- 4. Omiya S, Omori Y, Taneike M, Murakawa T, Ito J, Tanada Y, Nishida K, Yamaguchi O, Satoh T, Shah AM, Akira S, Otsu K. Cytokine mRNA degradation in cardiomyocytes restrains sterile inflammation in pressure‐overloaded hearts. Circulation. 2020; 141: 667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res. 2015; 116: 1254–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Milo‐Cotter O, Cotter‐Davison B, Lombardi C, Sun H, Bettari L, Bugatti S, Rund M, Metra M, Kaluski E, Kobrin I, Frey A, Rainisio M, McMurray JJV, Teerlink JR, Cotter‐Davison G. Neurohormonal activation in acute heart failure: results from VERITAS. Cardiology. 2011; 119: 96–105. [DOI] [PubMed] [Google Scholar]
- 7. Milo O, Cotter G, Kaluski E, Brill A, Blatt A, Krakover R, Vered Z, Hershkoviz R. Comparison of inflammatory and neurohormonal activation in cardiogenic pulmonary edema secondary to ischemic versus nonischemic causes. Am J Cardiol. 2003; 92: 222–226. [DOI] [PubMed] [Google Scholar]
- 8. Davison BA, Senger S, Sama IE, Koch GG, Mebazaa A, Dickstein K, Samani NJ, Metra M, Anker SD, Cleland JG, Ng LL, Mordi IR, Zannad F, Filippatos GS, Hillege HL, Ponikowski P, van Veldhuisen DJ, Lang CC, van der Meer P, Núñez J, Bayés‐Genís A, Edwards C, Voors AA, Cotter G. Is acute heart failure a distinctive disorder? An analysis from BIOSTAT‐CHF. Eur J Heart Fail. 2021; 23: 43–57. [DOI] [PubMed] [Google Scholar]
- 9. Markousis‐Mavrogenis G, Tromp J, Mentz RJ, O'Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Davison B, Cleland JGF, Givertz MM, van Veldhuisen DJ, Hillege HL, Voors AA, van der Meer P. The additive prognostic value of serial plasma interleukin‐6 levels over changes in brain natriuretic peptide in patients with acute heart failure. J Card Fail. 2021; 27: 808–811. [DOI] [PubMed] [Google Scholar]
- 10. Minami Y, Kajimoto K, Sato N, Hagiwara N. Effect of elevated C‐reactive protein level at discharge on long‐term outcome in patients hospitalized for acute heart failure. Am J Cardiol. 2018; 121: 961–968. [DOI] [PubMed] [Google Scholar]
- 11. Van Tassell BW, Canada J, Carbone S, Trankle C, Buckley L, Erdle CO, Abouzaki NA, Dixon D, Kadariya D, Christopher S, Schatz A, Regan J, Viscusi M, Del Buono M, Melchior R, Mankad P, Lu J, Sculthorpe R, Biondi‐Zoccai G, Lesnefsky E, Arena R, Abbate A. Interleukin‐1 blockade in recently decompensated systolic heart failure: results from REDHART (Recently Decompensated Heart Failure Anakinra Response Trial). Circ Heart Fail. 2017; 10: e004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. VanTassell BW, Abouzaki NA, Erdle CO, Carbone S, Trankle CR, Melchior RD, Turlington JS, Thurber CJ, Christopher S, Dixon DL, Fronk DT, Thomas CS, Rose SW, Buckley LF, Dinarello CA, Biondi‐Zoccai G, Abbate A. Interleukin‐1 blockade in acute decompensated heart failure: a randomized, double‐blinded, placebo‐controlled pilot study. J Cardiovasc Pharmacol. 2016; 67: 544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT. Randomized, double‐blind, placebo‐controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor‐α, in patients with moderate‐to‐severe heart failure: results of the anti‐TNF Therapy Against Congestive Heart Failure (ATTACH). Circulation. 2003; 107: 3133–3140. [DOI] [PubMed] [Google Scholar]
- 14. Mann DL, McMurray JJV, Packer M, Swedberg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L, Krum H, Liu P, Nieminen M, Tavazzi L, Van Veldhuisen DJ, Waldenstrom A, Warren M, Westheim A, Zannad F, Fleming T. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004; 109: 1594–1602. [DOI] [PubMed] [Google Scholar]
- 15. Miro O, Rossello X, Gil V, Martin‐Sanchez FJ, Llorens P, Herrero‐Puente P, Jacob J, Bueno H, Pocock SJ. Predicting 30‐day mortality for patients with acute heart failure in the emergency department. Ann Intern Med. 2017; 167: 698–705. [DOI] [PubMed] [Google Scholar]
- 16. Jacob J, Tost J, Miró Ò, Herrero P, Martín‐Sánchez FJ, Llorens P. Impact of chronic obstructive pulmonary disease on clinical course after an episode of acute heart failure. EAHFE–COPD study. Int J Cardiol. 2017; 227: 450–456. [DOI] [PubMed] [Google Scholar]
- 17. Ho KKL, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993; 88: 107–115. [DOI] [PubMed] [Google Scholar]
- 18. Jacob J, Miró Ò, Herrero P, Martín‐Sánchez FJ, Gil V, Tost J, Aguirre A, Escoda R, Alquézar A, Andueza JA, Llorens P. Predicting short‐term mortality in patients with acute exacerbation of chronic heart failure: the EAHFE‐3D scale. Med Intensiva. 2016; 40: 348–355. [DOI] [PubMed] [Google Scholar]
- 19. van Buuren S, Groothuis‐Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw. 2011; 45: 1–67. [Google Scholar]
- 20. Harrell E, Frank M. Package ‘rms’ Title Regression Modeling Strategies. CRAN R‐project 2018.
- 21. Zhang H, Liu C, Ji Z, Liu G, Zhao Q, Gao Y, Wang L, Deng B, Zhen Y, Tian L, Ji L, Liu K. Prednisone adding to usual care treatment for refractory decompensated congestive heart failure. Int Heart J. 2008; 49: 587–595. [DOI] [PubMed] [Google Scholar]
- 22. Liu C, Liu K. Cardiac outcome prevention effectiveness of glucocorticoids in acute decompensated heart failure: COPE‐ADHF study. J Cardiovasc Pharmacol. 2014; 63: 333–338. [DOI] [PubMed] [Google Scholar]
- 23. Miró Ò, Takagi K, Gayat E, Llorens P, Martín‐Sánchez FJ, Jacob J, Herrero‐Puente P, Gil V, Wussler DN, Richard F, López‐Grima ML, Gil C, Garrido JM, Pérez‐Durá MJ, Alquézar A, Alonso H, Tost J, Lucas Invernón FJ, Mueller C, Mebazaa A, CORT‐AHF Study . Effect on outcomes of systemic corticosteroid therapy during early management acute heart failure. JACC Heart Fail. 2019; 7: 834–845. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics of patients in the EAHFE registry with NT‐proBNP > 300 pg/mL and CRP > 40 mg/L according to whether they did or did not receive systemic corticosteroids in the emergency department.
Figure S1. Unadjusted and adjusted 30‐day all‐cause mortality according to the CRP levels in patients with elevated NT‐proBNP (> 1500 pg/mL).


