Abstract
Aims
Cognitive dysfunction occurs frequently in patients with heart failure (HF), but early detection remains challenging. Serum glial fibrillary acidic protein (GFAP) is an emerging biomarker of cognitive decline in disorders of primary neurodegeneration such as Alzheimer's disease. We evaluated the utility of serum GFAP as a biomarker for cognitive dysfunction and structural brain damage in patients with stable chronic HF.
Methods and results
Using bead‐based single molecule immunoassays, we quantified serum levels of GFAP in patients with HF participating in the prospective Cognition.Matters‐HF study. Participants were extensively phenotyped, including cognitive testing of five separate domains and magnetic resonance imaging (MRI) of the brain. Univariable and multivariable models, also accounting for multiple testing, were run. One hundred and forty‐six chronic HF patients with a mean age of 63.8 ± 10.8 years were included (15.1% women). Serum GFAP levels (median 246 pg/mL, quartiles 165, 384 pg/mL; range 66 to 1512 pg/mL) did not differ between sexes. In the multivariable adjusted model, independent predictors of GFAP levels were age (T = 5.5; P < 0.001), smoking (T = 3.2; P = 0.002), estimated glomerular filtration rate (T = −4.7; P < 0.001), alanine aminotransferase (T = −2.1; P = 0.036), and the left atrial end‐systolic volume index (T = 3.4; P = 0.004). NT‐proBNP but not serum GFAP explained global cerebral atrophy beyond ageing. However, serum GFAP levels were associated with the cognitive domain visual/verbal memory (T = −3.0; P = 0.003) along with focal hippocampal atrophy (T = 2.3; P = 0.025).
Conclusions
Serum GFAP levels are affected by age, smoking, and surrogates of the severity of HF. The association of GFAP with memory dysfunction suggests that astroglial pathologies, which evade detection by conventional MRI, may contribute to memory loss beyond ageing in patients with chronic HF.
Keywords: Glial fibrillary acidic protein, GFAP, Chronic heart failure, Cognitive decline, Memory dysfunction, Brain atrophy
Introduction
Heart failure (HF) has become one of the most prevalent chronic diseases in industrialized countries. 1 The extent and severity of secondary organ affections significantly contribute to HF‐related health care costs and long‐term outcome. 2 About every second patient with HF exhibits cognitive deficits. 3 , 4 Within the last years, there has been increasing interest in serum markers related to cognitive deficits and predicting cognitive decline. The intermediate filament glial fibrillary acidic protein (GFAP) holds promise in this regard. It is expressed mainly in mature astrocytes and its up‐regulation is a hallmark of disease‐related reactive astrogliosis in the central nervous system. 5 A growing body of evidence suggests that blood GFAP levels can be used to detect even subtle injury to the central nervous system. 6 In a recent population‐based sample (n = 1327), a diagnosis of clinical Alzheimer's disease was 62% or 149% more likely, if GFAP levels were in the third quartile (>232 pg/mL) or the fourth quartile (>337 pg/mL), 7 compared with lower serum levels of GFAP within the first quartile (<160 pg/mL). Accordingly, serum GFAP above median predicted a 130% faster cognitive decline over time compared with those in the lowest quartile. 7 Serum GFAP has been studied in numerous neurological disorders such as Alzheimer's disease, 8 , 9 frontotemporal dementia, 10 and neuromyelitis optica, 11 but its utility to detect and predict secondary cognitive impairment in cardiac diseases remains unknown. While one recent study demonstrated that GFAP predicted neurological outcome after out‐of‐hospital cardiac arrest, 12 the role of GFAP in chronic HF remains unknown.
We hypothesized that serum GFAP levels relate to cognitive function in patients with HF but may depend on comorbidities. To address these issues in a cross‐sectional approach, we made use of the deep clinical phenotyping approach adopted in the context of the Cognition.Matters‐HF study, which investigated patients with chronic stable HF without focal neurological impairment.
Methods
Study design
The design and applied methodology of the Cognition.Matters‐HF study have been reported in detail previously. 13 , 14 In brief, the Cognition.Matters‐HF study was a prospective, monocentric cohort study conducted in compliance with the Declaration of Helsinki and approved by the local ethics committee (#245/10). 14 The study included adult patients with confirmed chronic HF according to the then‐current guidelines of the European Society of Cardiology, 1 whereas patients with newly diagnosed or decompensated HF were not eligible. The selection criteria are summarized in Supporting Information Table S1 . Of note, patients exhibiting apparent neurological or psychiatric disease, history of clinical stroke, or carotid artery stenosis over 50% were not eligible.
Clinical evaluation
Physical examination, electrocardiography, echocardiography, and 6 min walk test were performed according to standard operating procedures (for details, refer to the supporting information, Supplemental Methods). 13 , 14 Neurological evaluation included extensive clinical examination. Psychological testing was performed between 9 AM and 11 AM using a comprehensive test battery based on taxonomy of attention dimensions and is summarized in Table S2 . T‐standardized output values accounting for the modifying effect of age, gender, and educational level were reported with a mean of 50 and standard deviation of 10.
Cerebral magnetic resonance imaging
Brain magnetic resonance imaging (MRI) was performed at a 3‐Tesla scanner (Siemens MAGNETOM Trio, Siemens Healthcare, Erlangen, Germany) as described previously. 14 Briefly, the applied MRI protocol enabled the estimation of global and regional measures of brain structure degeneration by visual rating (Table S3 ). Visual rating of cerebral atrophy ranged on a scale from 1 to 8. For the assessment of medial temporal lobe atrophy, Scheltens score ranging from 0 (normal) to 4 (severe atrophy) was applied, and the mean scores of both sides (left and right) were reported.
Laboratory analysis
We collected non‐fasting venous blood samples from all patients for routine clinical chemistry investigations at the certified facility of the University Hospital Würzburg. Participants were positioned seated for at least 5 min before puncture. Serum samples were processed immediately, that is, remained at room temperature for 30 min and were centrifuged for 10 min at 2000g. Serum was aliquoted in dedicated fluid tissue tubes (Micronic, Lelystad, Netherlands) and stored in the standardized interdisciplinary biomaterial bank at −80°C until analysis. 15 Serum GFAP was measured using the Simoa GFAP‐kit (102336; Quanterix™, Billerica, MA, USA) on a Simoa HD‐1 analyser instrument (Quanterix™, Billerica, MA, USA) according to the manufacturer's instructions. These measurements were performed blinded to the patients' other results at the Department for Neurology of the University Hospital Ulm. 16
Data analysis
Statistical analysis was performed using the statistical software SPSS (Version 26). To test for normal distribution, we used the Shapiro–Wilk test. Variables were natural log normalized if required. The few missing values (less than 1% missing) were imputed by the mean value of respective variable. For nominal and ordinal data, χ 2 test, or Fisher's exact test were used, according to the nature of the data. For correlation analysis, the Spearman rho coefficient (ρ) was computed. To identify metric correlates of GFAP levels, univariate linear regression analysis was used, and a trend test across quartiles was reported. All tests were performed two‐sided. When identifying determinants of GFAP, to reduce the overoptimism introduced by multiple testing, a Bonferroni correction of P values was used for the 87 clinical parameters that were used for analysis. Thus, identified correlates were then included into a multivariable regression model. First, we analysed correlates using an ‘enter’ approach. We reduced multicollinearity by showing variance inflation factors of <10 in each model including Durbin–Watson statistics. Second, we fed the remaining correlates into another regression model, which then was reduced to the final model through backward elimination, and reproduced by forward entry again. The explained variance of a model was indicated by the coefficients of determination (R 2).
Results
Patient characteristics
Because serum for GFAP determination was not available in 2 out of the 148 patients of the Cognition.Matters‐HF cohort, all analyses refer to 146 patients. Their mean age was 63.8 (standard deviation 10.8) years, and 15.1% were female. The characteristics of the study sample are shown in Tables 1 and S2 and have partially been published before. 14 Mean left ventricular ejection fraction was 43 (8) %, and 72% of patients were in New York Heart Association functional class II or III. Coronary artery disease was the predominant cause for HF in 65%, and 84% of all patients received optimal HF pharmacotherapy according to established guidelines at study inclusion. 1 Serum concentrations of GFAP ranged from 66 to 1512 pg/mL, with mean of 297 (190) pg/mL and median of 246 pg/mL. For histograms, refer to Figure S1 .
Table 1.
Descriptive clinical characteristics of study participants according to serum glial fibrillary acidic protein (GFAP) quartiles
| n | Total | <165 pg/mL (n = 36) | 165 to 244.9 pg/mL (n = 36) | 245 to 383.9 pg/mL (n = 38) | ≥384 pg/mL (n = 36) | T | P | P (adjusted) | |
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 146 | 63.8 (10.8) | 53.6 (8.7) | 63.2 (9.7) | 67.6 (8.1) | 70.4 (8.8) | 8.26 | <0.001 | <0.001 |
| Female sex | 146 | 22 (15.1%) | 3 (8.3%) | 8 (22.2%) | 3 (7.9%) | 8 (22.2%) | 0.128 | ns | |
| Body mass index (kg/m2) | 145 | 29.1 (5.2) | 30.5 (7.5) | 29.8 (4.1) | 28.5 (4.5) | 27.8 (3.6) | −2.50 | 0.014 | ns |
| Parameters of heart failure (HF) | |||||||||
| Duration of HF (years) | 145 | 6.31 (6.03) | 4.6 (4.9) | 6.5 (6.3) | 6.1 (6.3) | 8.1 (6.3) | 2.25 | 0.026 | ns |
| Previous hospitalizations due to HF | 146 | 0.6 (0.9) | 0.7 (1.0) | 0.5 (0.7) | 0.8 (0.9) | 0.5 (0.9) | −0.40 | 0.688 | ns |
| New York Heart Association functional class | 0.422 | ns | |||||||
| I | 146 | 39 (26.7%) | 13 (36.1%) | 12 (33.3%) | 7 (18.4%) | 7 (19.4%) | |||
| II | 146 | 88 (60.3%) | 20 (55.6%) | 20 (55.6%) | 26 (68.4%) | 22 (61.1%) | |||
| III | 146 | 19 (13.0%) | 3 (8.3%) | 4 (11.1%) | 5 (13.2%) | 7 (19.4%) | |||
| 6 min walk test distance (m) | 138 | 391.5 (99.3) | 425.1 (94.0) | 376.5 (91.8) | 393.1 (120.4) | 369.7 (79.9) | −2.00 | 0.048 | ns |
| Pre‐existent conditions | |||||||||
| Atrial fibrillation/atrial flutter | 146 | 33 (22.6%) | 5 (13.9%) | 7 (19.4%) | 10 (26.3%) | 11 (30.6%) | 0.338 | ns | |
| Coronary artery disease | 146 | 100 (68.5%) | 22 (61.1%) | 30 (83.3%) | 26 (68.4%) | 22 (61.1%) | 0.139 | ns | |
| History of myocardial infarction | 146 | 80 (54.8%) | 20 (55.6%) | 27 (75.0%) | 18 (47.4%) | 15 (41.7%) | 0.026 | ns | |
| Cardiovascular risk factors | |||||||||
| Diabetes mellitus type II a | 146 | 42 (28.8%) | 9 (25.0%) | 15 (41.7%) | 12 (31.6%) | 6 (16.7%) | 0.117 | ns | |
| Arterial hypertension b | 146 | 116 (79.5%) | 22 (61.1%) | 31 (86.1%) | 30 (78.9%) | 33 (91.7%) | 0.009 | ns | |
| Hyperlipidaemia c | 146 | 105 (71.9%) | 25 (69.4%) | 27 (75.0%) | 28 (73.7%) | 25 (69.4%) | 0.931 | ns | |
| (Former) smoking | 146 | 88 (60.3%) | 32 (88.9%) | 21 (58.3%) | 22 (57.9%) | 13 (36.1%) | <0.001 | 0.008 | |
| Transthoracic echocardiography | |||||||||
| Left atrial end‐systolic volume index (mL/m2) | 143 | 42.0 (17.5) | 35.4 (13.2) | 39.2 (15.5) | 40.2 (14.3) | 52.1 (20.4) | 4.21 | <0.001 | 0.004 |
| Left ventricular ejection fraction (%) | 146 | 42.5 (8.2) | 43.0 (9.1) | 43.2 (6.3) | 42.1 (8.9) | 41.7 (8.4) | −0.82 | 0.412 | ns |
| Laboratory results | |||||||||
| Alanine aminotransferase (U/L) | 145 | 28.1 (14.1) | 34.6 (16.3) | 31.5 (17.7) | 23.4 (8.3) | 23.3 (8.2) | −4.25 | <0.001 | 0.003 |
| Haemoglobin (g/dL) | 145 | 14.3 (1.4) | 15.0 (1.2) | 14.2 (1.3) | 14.3 (1.3) | 13.9 (1.7) | −3.21 | 0.002 | ns |
| Urea (mg/dL) | 146 | 42.6 (16.4) | 34.9 (10.7) | 39.2 (11.7) | 45.0 (17.3) | 51.1 (19.7) | 4.79 | <0.001 | <0.001 |
| N terminal pro brain natriuretic peptide (pg/mL) | 146 | 1330 (2041) | 497 (438) | 1.084 (1.587) | 1.220 (1.744) | 2.492 (3.002) | 4.14 | <0.001 | 0.005 |
| Estimated GFR (mL/min/1.73 m2) | 140 | 66.5 (19.4) | 80.6 (15.8) | 69.1 (14.0) | 62.8 (18.9) | 53.7 (18.5) | −6.91 | <0.001 | <0.001 |
| Brain magnetic resonance imaging | |||||||||
| White matter hyperintensity volume (mm3) | 146 | 4.4 (6.2) | 2.5 (2.5) | 4.1 (3.3) | 3.5 (2.9) | 7.2 (10.8) | 2.96 | 0.004 | ns |
| Cerebral atrophy score (0–8) | 146 | 3.1 (1.2) | 2.5 (1.0) | 3.1 (1.2) | 3.6 (1.4) | 3.7 (1.1) | 4.20 | <0.001 | 0.004 |
| Hippocampal atrophy/Scheltens score (0–4) | 146 | 2.0 (0.9) | 1.9 (0.8) | 1.8 (0.8) | 2.2 (0.9) | 2.2 (1.0) | 1.95 | 0.053 | ns |
| Cognitive domains | |||||||||
| Intensity of attention (T score) | 145 | 41.8 (7.5) | 43.2 (6.3) | 40.8 (7.8) | 39.9 (7.9) | 43.5 (7.6) | 0.02 | 0.983 | ns |
| Selectivity of attention (T score) | 145 | 45.3 (6.2) | 47.7 (4.3) | 46.4 (6.0) | 42.7 (6.8) | 44.4 (6.3) | −3.05 | 0.003 | ns |
| Visual/verbal memory (T score) | 145 | 45.3 (7.9) | 49.3 (7.9) | 45.9 (7.5) | 43.0 (7.0) | 43.4 (8.1) | −3.61 | <0.001 | 0.037 |
| Working memory (T score) | 145 | 46.2 (8.5) | 48.4 (7.7) | 44.4 (9.0) | 45.9 (8.0) | 46.3 (9.2) | −0.74 | 0.462 | ns |
| Visual/verbal fluency (T score) | 145 | 44.8 (7.1) | 45.4 (7.3) | 44.5 (7.2) | 44.6 (7.3) | 44.9 (7.1) | −0.24 | 0.813 | ns |
E, early diastolic mitral valve inflow velocity; e', early diastolic mitral annular relaxation velocity; GFR, glomerular filtration rate (MDRD formula); n, valid values; P, two‐sided P value; P (adjusted), adjusted P value after Bonferroni correction.
Metric data are displayed as mean (standard deviation), for detection of trends, univariable regression with quartiles as independent variable was applied. Chi‐squared test was used to find differences between expected and observed frequencies; if the expected value was below five, we used Fischer's exact test.
History of diabetes mellitus type II or HbA1c > 6.5%.
Sitting blood pressure > 140/90 mmHg or history of hypertension before onset of heart failure.
Hyperlipidaemia or statin treatment.
Clinical correlates of glial fibrillary acidic protein levels
To identify clinical correlates of GFAP, we defined four equally sized patient groups according to the quartiles 165, 246, and 384 pg/mL (Table 1 ). After correction for multiple testing, we found positive associations of GFAP with age, smoking (ever), NT‐proBNP, urea, and left atrial volume index (LAVI), estimated glomerular filtration rate (eGFR), and alanine aminotransferase (ALAT). In line, thus identified correlates significantly related to ln (GFAP) in Spearman's correlation (Figure 1 ) and univariable regression analysis (Table 2 ). Although there were minor differences in unadjusted GFAP levels depending on the type of HF‐related medication and drugs for important comorbidities (Table S2 ), none of these substance classes emerged as a major predictor in multivariable analysis (Table 2 ).
Figure 1.
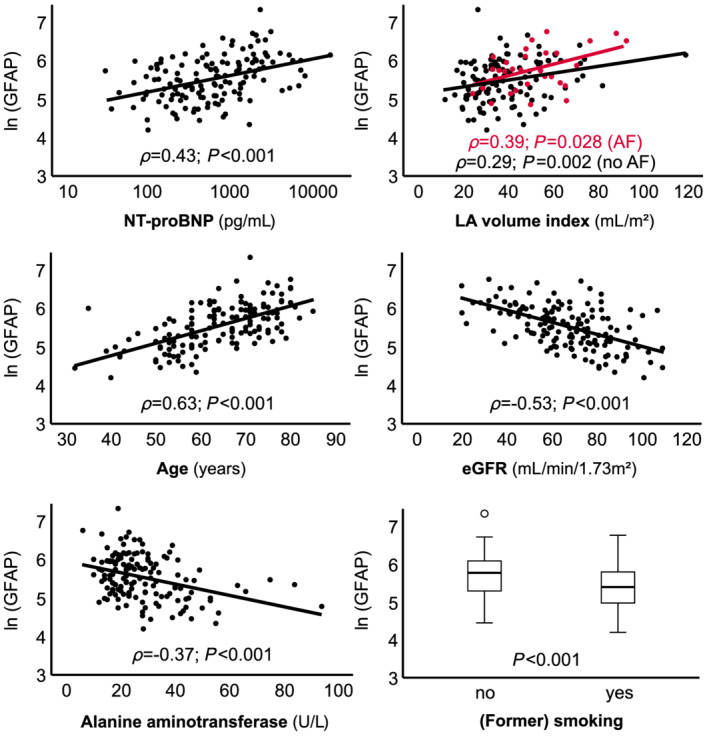
Scatter plot of natural log‐transformed GFAP and clinical parameters. Spearman's regression coefficient (ρ) is shown. AF, atrial fibrillation; eGFR, estimated glomerular filtration rate; GFAP, glial fibrillary acidic protein; LA, left atrium.
Table 2.
Univariable and multivariable correlates of ln (GFAP)
| Univariable analysis | Multivariable analysis—enter approach | Multivariable analysis—backward elimination | |||||||
|---|---|---|---|---|---|---|---|---|---|
| R 2 = 0.55 | R 2 = 0.55 | ||||||||
| n | T | P | VIF | T | P | VIF | T | P | |
| Age (years) | 146 | 9.38 | <0.001 | 1.41 | 5.51 | <0.001 | 1.40 | 5.50 | <0.001 |
| (Former) smoking (y/n) | 146 | 3.96 | <0.001 | 1.06 | 3.10 | 0.002 | 1.05 | 3.17 | 0.002 |
| Left atrial volume index (mL/m2) | 143 | 4.36 | <0.001 | 1.22 | 2.75 | 0.007 | 1.07 | 2.95 | 0.004 |
| Alanine aminotransferase (U/L) | 146 | −4.65 | <0.001 | 1.21 | −2.10 | 0.038 | 1.16 | −2.12 | 0.036 |
| Urea (mg/dL) | 146 | 4.91 | <0.001 | 1.87 | −0.67 | 0.506 | |||
| NT‐proBNP (pg/mL) | 140 | 3.70 | <0.001 | 1.34 | 0.35 | 0.725 | |||
| Estimated GFR (mL/min/1.73 m2) | 146 | −7.47 | <0.001 | 2.01 | −3.94 | <0.001 | 1.23 | −4.69 | <0.001 |
| DWS = 2.040 | DWS = 2.059 | ||||||||
The T value indicates the direction of association and the relative weight of a variable in a model; R 2 indicates the variance explained by the model.
DWS, Durbin–Watson statistics; GFR, glomerular filtration rate (MDRD formula); P = two‐sided P value; R 2, coefficient of determination; VIF, variance inflation factor.
Determinants of glial fibrillary acidic protein in multivariable analyses
To compare the relative effect of the above identified variables, we performed multivariable regression (Table 2 ). Both in the ‘enter’ and the ‘backward elimination’ approach, five independent predictors of ln (GFAP) emerged, hereafter sorted by their relative weight in the model: age, eGFR, smoking status (ever), LAVI, and ALAT. Those models exhibited a R 2 of 0.55 as an indicator for the explained variance. These associations yield the following approximation formula for GFAP levels (ever smoking = 0; never smoking = 1):
Glial fibrillary acidic protein levels and brain morphologic alterations
As displayed in Table 1 and Figure 2 , serum levels of GFAP were associated with cerebral atrophy score. When GFAP was added to age‐adjusted mutivariable models of the above mentioned parameters, the explained variance (R 2) of cerebral atrophy was not increased (Table 3 ). In this model, NT‐proBNP, but not GFAP, significantly predicted global brain atrophy beyond ageing.
Figure 2.
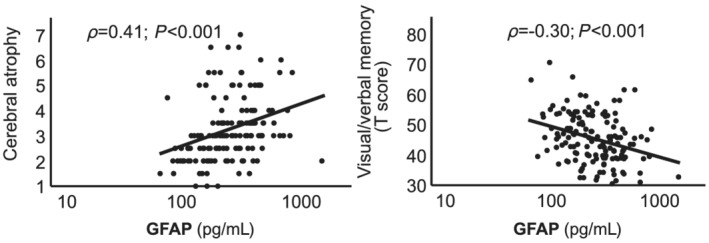
Scatter plot of natural log‐transformed GFAP and parameters of brain atrophy/memory. Spearman's regression coefficient (ρ), cerebral atrophy score (on a scale from 1 to 8) and age‐adjusted, gender‐adjusted, and education‐adjusted T scores of visual/verbal memory are shown. GFAP, glial fibrillary acidic protein.
Table 3.
Univariable and multivariable correlates of cerebral atrophy
| Univariable analysis | Multivariable analysis—enter approach | Multivariable analysis—backward elimination | |||||||
|---|---|---|---|---|---|---|---|---|---|
| R 2 = 0.36 (+0.00) | R 2 = 0.36 (+0.00) | ||||||||
| n | T | P | VIF | T | P | VIF | T | P | |
| Age (years) | 146 | 7.99 | <0.001 | 1.34 | 7.27 | <0.001 | 1.04 | 7.49 | <0.001 |
| NT‐proBNP (pg/mL) | 146 | 1.08 | 3.48 | 0.001 | 1.04 | 3.26 | 0.001 | ||
| + GFAP (pg/mL) | 146 | 2.87 | 0.005 | 1.39 | −1.40 | 0.165 | |||
| DWS = 1.576 | DWS = 1.552 | ||||||||
The T value indicates the direction of association and the relative weight of a variable in a model; R 2 indicates the variance explained by the model.
DWS, Durbin–Watson statistics; P, two‐sided P value; R 2, coefficient of determination; VIF, variance inflation factor.
Glial fibrillary acidic protein levels and cognitive functioning
Aiming to evaluate the value of GFAP as a biomarker of cognitive decline, we found that out of the five tested cognitive domains, only visual/verbal memory was significantly associated with GFAP after correction for multiple testing (Table 1 ). In line, serum GFAP showed high (negative) correlations coefficients (ρ) regarding memory T values. Of note, the T values of cognitive domains are already adjusted for age, and partially for gender and educational level (Figure 2 ).
Using multivariable models, we addressed, whether GFAP could improve models of visual/verbal memory (Table 4 ). We found that brain MRI parameters were related to memory impairment, for example, the hippocampal atrophy score. When GFAP was added to these models, the explained variance was increased. There, GFAP emerged as an independent predictor of memory dysfunction along with hippocampal atrophy.
Table 4.
Univariable and multivariable correlates of age‐adjusted visual/verbal memory T score
| Univariable analysis | Multivariable analysis—enter approach | Multivariable analysis—backward elimination | |||||||
|---|---|---|---|---|---|---|---|---|---|
| R 2 = 0.06 (+0.05) | R 2 = 0.05 (+0.06) | ||||||||
| n | T | P | VIF | T | P | VIF | T | P | |
| White matter hyperintensity volume (mm3) | 146 | −1.04 | 0.302 | 1.09 | −0.12 | 0.908 | |||
| Cerebral atrophy score (0–8) | 146 | −1.59 | 0.115 | 1.20 | −0.18 | 0.860 | |||
| Hippocampal atrophy/Scheltens score (0–4) | 146 | −2.73 | 0.007 | 1.14 | −2.07 | 0.041 | 1.03 | −2.26 | 0.025 |
| +GFAP (pg/mL) | 146 | −3.40 | 0.001 | 1.09 | −2.86 | 0.005 | 1.03 | −3.02 | 0.003 |
| DWS = 2.249 | DWS = 2.254 | ||||||||
The T value indicates the direction of association and the relative weight of a variable in a model; R 2 indicates the variance explained by the model.
DWS, Durbin–Watson statistics; P, two‐sided P value; R 2, coefficient of determination; VIF, variance inflation factor.
Discussion
The current study provides for the first time a detailed characterization of serum GFAP levels in extensively phenotyped patients free from stroke or other focal neurological deficits yet suffering from chronic HF. 14 In this cohort, 68% of participants exhibited cognitive impairment. We quantified serum levels of GFAP to explore, whether it has a mediating role between HF, cognitive impairment, and associated changes in brain morphology. Three key findings emerged: first, we revealed that serum GFAP levels were independently affected by age, smoking, and surrogates of the severity of chronic HF. Second, GFAP correlated to global brain atrophy, but not beyond ageing. Third, higher serum GFAP levels independently predicted worse performance in the cognitive domain of visual/verbal memory.
Serum levels of glial fibrillary acidic protein in heart failure patients compared with neurological diseases
Median GFAP was 246 pg/mL in our sample, which is higher than in healthy adults 6 and within the pathological range of neurological disorders such as neuromyelitis optica and Alzheimer's disease. Neuromyelitis optica is caused by autoantibodies against aquaporin‐4, which destroy astrocytes. In this condition, median GFAP levels in three previous reports were 208, 109, and 274 pg/mL compared with 97, 68, and 61 pg/mL in healthy controls. 11 , 17 , 18 Patients with Alzheimer's disease, exhibiting a more complex brain pathology, displayed median serum GFAP levels of 376 pg/mL, compared with 157 pg/mL in controls. 8 The relatively high GFAP concentration in chronic HF suggests ongoing glial damage, which might be triggered by interleukin 6‐induced neuroinflammation 19 and/or neuronal and glial cell destruction upon hypoperfusion and hypoxia. 20 Future studies are needed to advance these hypotheses and to disclose how GFAP gets access to the systemic circulation in the absence of an overt blood–brain barrier disruption as it is found in neuromyelitis optica. 21 Because astrocyte feet are an integral component of the blood brain barrier, it is conceivable that astrocytic damage in HF patients concomitantly induces subtle leakage of the blood–brain barrier not strong enough to cause extravasation of the MRI contrast agent gadolinium‐diethylenetriaminepentacetate for detection but sufficient for release of GFAP.
Effect of severity of heart failure
To explain high concentrations of GFAP in HF comparable with those in severe neurologic disorders, we analysed cardiological correlates of GFAP. A higher symptom burden of HF (New York Heart Association functional class) and lesser physical performance (i.e. 6 min walk test distance) were not associated to GFAP. However, LAVI, which is an established echocardiographic parameter of diastolic dysfunction, 22 was positively related to GFAP levels in our cohort, irrespective of the presence of atrial fibrillation. Increased LAVI has been shown to be independently associated with cognitive impairment 23 and elevated brain amyloid 24 in patients with sinus rhythm, which was even further aggravated by age. 25 Other studies consistently reported an association between parameters of diastolic function and cognition. 26 , 27 , 28 While causal factors like congestion, hypoperfusion, and HF‐related chronic inflammation are being discussed, 29 the cause‐and‐effect association of this potentially bilateral interaction as well as its prognostic utility must be detailed in future studies. Consistently, in univariable regression, serum levels of NT‐proBNP, an indicator for left ventricular and left atrial stress, 30 were positively correlated to GFAP. Higher NT‐proBNP concentrations were also associated with incident cognitive impairment in a previous study independent of atherogenic and Alzheimer's disease risk factors 31 and in a study of the general population with mild cognitive impairment. 32 Of note, we found no associations to GFAP for parameters of systolic dysfunction like the left ventricular ejection fraction.
Effect of age and comorbidities
In this investigation, patient age positively related to serum GFAP levels, which is in accordance with recent findings in depressive disorders, 33 possible reflecting ‘normal’ neurodegenerative glial damage upon ageing. A recent study investigating human brains found age‐related decline of synaptic transmission and increased expression of GFAP in both sexes. 34 In mice, an age‐related increase in GFAP RNA has been reported, potentially reflecting an increase in the size, number, and/or fibrous character of astrocytes. 35 We additionally found higher GFAP levels in patients with impaired kidney and liver function, which in our cohort may be explained by a HF‐induced typical cardio‐hepato‐renal compromise. These findings have important implications for the interpretation of serum GFAP in neurological disorders by pointing to age and renal and hepatic function as major factors confounding its measurement results.
Relation of glial fibrillary acidic protein to brain morphology and cognitive impairment in heart failure
In this cohort, single GFAP measurements did not add explained variance to models of brain atrophy. On the one hand, this might be due to the long‐term emergence of these brain alterations. On the other hand, while GFAP reflects glial damage, neuronal biomarkers like neurofilament light chain are indeed related to brain degeneration in elderly. 36 We were surprised that GFAP did not relate to white matter hyperintensity volume, as these lesions display areas of reduced glial density. 37 As GFAP is a marker of glial damage with a short half‐life, it might not be suitable to mark slowly progressive cerebral small vessel disease underlying white matter hyperintensities. 38 Thus, future longitudinal assessment of GFAP and brain morphologic alterations might have the potential to increase the relevance of serum GFAP in this regard.
In this sample of HF patients, the glial biomarker GFAP independently predicted age‐adjusted performance in the cognitive domain visual/verbal memory along with hippocampal atrophy. As previously published, cognitive deficits in the Cognition.Matters‐HF cohort were associated with regional brain atrophy of the medial temporal lobe. 14 While hippocampal atrophy, measured by Scheltens score, is known to foster memory deficits, 39 our findings suggest that in chronic HF, memory impairment might partially also be due to ongoing glial activation and damage within the hippocampus, which evades conventional MRI. Accordingly, histological analysis revealed a decreased neurogenesis together with an increased number of reactive astrocytes in the ventral hippocampus in HF rats compared with sham rats. 40 While similar correlations of GFAP to cognitive decline were found in Alzheimer's disease, 8 we here expand these finding to a cardiologically diseased cohort free of focal neurological deficits. This has potential implications for early identification and stratification of HF patients at risk for cognitive decline.
Limitations and conclusion
Limitations of the current study include its cross‐sectional approach. Future studies have to confirm the utility of monitoring GFAP in order to predict changes in cognitive function in HF over time. Strengths of this investigation are the extensive neurological and cardiological work‐up applied, the comprehensive cognitive test battery including five different domains instead of global tests, and the detailed clinical and laboratory phenotyping.
In summary, this detailed evaluation of GFAP from sera of patients with chronic HF support its potential as biomarker for the detection of cognitive deficits in patients with HF, which are related to cardiac dysfunction. Furthermore, the associations of serum GFAP levels in our cohort indicate the important role of glial damage in cardiological disorders. When GFAP is determined in the context of neurological disorders, future analyses have to put more weight on the confounding effect of age and renal and cardiac function to avoid false conclusions, especially in elderly populations at risk for Alzheimer's disease and other dementias.
Conflict of interest
None to declare.
Funding
This work was supported by grants from the Bundesministerium für Bildung und Forschung (01EO1004 and 01EO1504) through the Comprehensive Heart Failure Center, habilitation grant (A.F.) and UNION‐CVD Clinician Scientist Program grant (J.T.) by Interdisciplinary Center of Clinical Research Würzburg.
Supporting information
Table S1. Imaging protocol, sequence parameters and specifications.
Table S2. Extended descriptive clinical characteristics of study participants according to serum glial fibrillary acidic protein (GFAP) quartiles.
Table S3. Spearman's correlation (ρ) to cognitive function and brain morphology.
Figure S1. Serum GFAP concentrations. Histograms and quantil‐quantil (Q‐Q) plots of absolute and ln‐transformed GFAP measurements in the serum of chronic heart failure patients via ultrasensitive, bead‐based single molecular immunoassay. Dots represent single patients (n=146).
Acknowledgement
We thank the multidisciplinary Cognition. Matters‐HF team for excellent support in the conduct of this comprehensive study. Open Access funding enabled and organized by Projekt DEAL. This publication was supported by the Open Access Publication Fund of the University of Wuerzburg.
Traub, J. , Otto, M. , Sell, R. , Homola, G. A. , Steinacker, P. , Oeckl, P. , Morbach, C. , Frantz, S. , Pham, M. , Störk, S. , Stoll, G. , and Frey, A. (2022) Serum glial fibrillary acidic protein indicates memory impairment in patients with chronic heart failure. ESC Heart Failure, 9: 2626–2634. 10.1002/ehf2.13986.
Contributor Information
Guido Stoll, Email: stoll_g@ukw.de.
Anna Frey, Email: frey_a@ukw.de.
References
- 1. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 2. Tanai E, Frantz S. Pathophysiology of heart failure. Compr Physiol. 2015; 6: 187–214. [DOI] [PubMed] [Google Scholar]
- 3. Festa JR, Jia X, Cheung K, et al. Association of low ejection fraction with impaired verbal memory in older patients with heart failure. Arch Neurol. 2011; 68: 1021–1026. [DOI] [PubMed] [Google Scholar]
- 4. Hajduk AM, Kiefe CI, Person SD, Gore JG, Saczynski JS. Cognitive change in heart failure: a systematic review. Circ Cardiovasc Qual Outcomes. 2013; 6: 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Middeldorp J, Hol EM. GFAP in health and disease. Prog Neurobiol. 2011; 93: 421–443. [DOI] [PubMed] [Google Scholar]
- 6. Abdelhak A, Foschi M, Abu‐Rumeileh S, et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat Rev Neurol. 2022; 18: 158–172. [DOI] [PubMed] [Google Scholar]
- 7. Rajan KB, Aggarwal NT, McAninch EA, et al. Remote blood biomarkers of longitudinal cognitive outcomes in a population study. Ann Neurol. 2020; 88: 1065–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oeckl P, Halbgebauer S, Anderl‐Straub S, et al. Glial fibrillary acidic protein in serum is increased in Alzheimer's disease and correlates with cognitive impairment. J Alzheimers Dis. 2019; 67: 481–488. [DOI] [PubMed] [Google Scholar]
- 9. Elahi FM, Casaletto KB, La Joie R, et al. Plasma biomarkers of astrocytic and neuronal dysfunction in early‐ and late‐onset Alzheimer's disease. Alzheimers Dement. 2020; 16: 681–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benussi A, Ashton NJ, Karikari TK, et al. Serum glial fibrillary acidic protein (GFAP) is a marker of disease severity in frontotemporal lobar degeneration. J Alzheimers Dis. 2020; 77: 1129–1141. [DOI] [PubMed] [Google Scholar]
- 11. Watanabe M, Nakamura Y, Michalak Z, et al. Serum GFAP and neurofilament light as biomarkers of disease activity and disability in NMOSD. Neurology. 2019; 93: e1299–e1311. [DOI] [PubMed] [Google Scholar]
- 12. Humaloja J, Lahde M, Ashton NJ, et al. GFAp and tau protein as predictors of neurological outcome after out‐of‐hospital cardiac arrest: a post hoc analysis of the COMACARE trial. Resuscitation. 2022; 170: 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frey A, Homola GA, Henneges C, et al. Temporal changes in total and hippocampal brain volume and cognitive function in patients with chronic heart failure—the COGNITION.MATTERS‐HF cohort study. Eur Heart J. 2021; 42: 1569–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frey A, Sell R, Homola GA, et al. Cognitive deficits and related brain lesions in patients with chronic heart failure. JACC Heart Fail. 2018; 6: 583–592. [DOI] [PubMed] [Google Scholar]
- 15. Geiger J, Both S, Kircher S, Neumann M, Rosenwald A, Jahns R. Hospital‐integrated biobanking as a service—the interdisciplinary Bank of Biomaterials and Data Wuerzburg (ibdw). Open J Bioresourc. 2018; 5: 6. [Google Scholar]
- 16. Steinacker P, Anderl‐Straub S, Diehl‐Schmid J, et al. Serum neurofilament light chain in behavioral variant frontotemporal dementia. Neurology. 2018; 91: e1390–e1401. [DOI] [PubMed] [Google Scholar]
- 17. Schindler P, Grittner U, Oechtering J, et al. Serum GFAP and NfL as disease severity and prognostic biomarkers in patients with aquaporin‐4 antibody‐positive neuromyelitis optica spectrum disorder. J Neuroinflammation. 2021; 18: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chang X, Huang W, Wang L, et al. Serum neurofilament light and GFAP are associated with disease severity in inflammatory disorders with aquaporin‐4 or myelin oligodendrocyte glycoprotein antibodies. Front Immunol. 2021; 12: 647618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Athilingam P, Moynihan J, Chen L, D'Aoust R, Groer M, Kip K. Elevated levels of interleukin 6 and C‐reactive protein associated with cognitive impairment in heart failure. Congest Heart Fail. 2013; 19: 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ciacciarelli A, Sette G, Giubilei F, Orzi F. Chronic cerebral hypoperfusion: an undefined, relevant entity. J Clin Neurosci. 2020; 73: 8–12. [DOI] [PubMed] [Google Scholar]
- 21. You X, Yan L, Li X, et al. Disruption of blood‐brain barrier integrity associated with brain lesions in Chinese neuromyelitis optica spectrum disorder patients. Mult Scler Relat Disord. 2019; 27: 254–259. [DOI] [PubMed] [Google Scholar]
- 22. Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016; 29: 277–314. [DOI] [PubMed] [Google Scholar]
- 23. Karadag B, Ozyigit T, Ozben B, Kayaoglu S, Altuntas Y. Relationship between left atrial volume index and cognitive decline in elderly patients with sinus rhythm. J Clin Neurosci. 2013; 20: 1074–1078. [DOI] [PubMed] [Google Scholar]
- 24. Johansen MC, Mosley TH, Knopman DS, et al. Associations between atrial cardiopathy and cerebral amyloid: the ARIC‐PET study. J Am Heart Assoc. 2020; 9: e018399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alosco ML, Gunstad J, Jerskey BA, et al. Left atrial size is independently associated with cognitive function. Int J Neurosci. 2013; 123: 544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park CM, Williams ED, Chaturvedi N, et al. Associations between left ventricular dysfunction and brain structure and function: findings from the SABRE (Southall and Brent Revisited) Study. J Am Heart Assoc. 2017; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Futami S, Ishikawa J, Jubishi C, et al. Prevalence and determinants of cognitive impairment in elderly patients with heart failure—pilot study in a geriatric hospital. Circ Rep. 2020; 2: 265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shimizu A, Sakurai T, Mitsui T, et al. Left ventricular diastolic dysfunction is associated with cerebral white matter lesions (leukoaraiosis) in elderly patients without ischemic heart disease and stroke. Geriatr Gerontol Int. 2014; 14: 71–76. [DOI] [PubMed] [Google Scholar]
- 29. Cannon JA, Moffitt P, Perez‐Moreno AC, et al. Cognitive impairment and heart failure: systematic review and meta‐analysis. J Card Fail. 2017; 23: 464–475. [DOI] [PubMed] [Google Scholar]
- 30. Zuber M, Cuculi F, Attenhofer Jost CH, et al. Value of brain natriuretic peptides in primary care patients with the clinical diagnosis of chronic heart failure. Scand Cardiovasc J. 2009; 43: 324–329. [DOI] [PubMed] [Google Scholar]
- 31. Cushman M, Callas PW, McClure LA, et al. N‐Terminal pro‐B‐type natriuretic peptide and risk of future cognitive impairment in the REGARDS cohort. J Alzheimers Dis. 2016; 54: 497–503. [DOI] [PubMed] [Google Scholar]
- 32. Kara K, Mahabadi AA, Weimar C, et al. N‐Terminal pro‐B type natriuretic peptide is associated with mild cognitive impairment in the general population. J Alzheimers Dis. 2017; 55: 359–369. [DOI] [PubMed] [Google Scholar]
- 33. Steinacker P, Al Shweiki MR, Oeckl P, et al. Glial fibrillary acidic protein as blood biomarker for differential diagnosis and severity of major depressive disorder. J Psychiatr Res. 2021; 144: 54–58. [DOI] [PubMed] [Google Scholar]
- 34. Wruck W, Adjaye J. Meta‐analysis of human prefrontal cortex reveals activation of GFAP and decline of synaptic transmission in the aging brain. Acta Neuropathol Commun. 2020; 8: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goss JR, Finch CE, Morgan DG. Age‐related changes in glial fibrillary acidic protein mRNA in the mouse brain. Neurobiol Aging. 1991; 12: 165–170. [DOI] [PubMed] [Google Scholar]
- 36. Khalil M, Pirpamer L, Hofer E, et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat Commun. 2020; 11: 812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhu W, Huang H, Yang S, et al. Cortical and subcortical grey matter abnormalities in white matter hyperintensities and subsequent cognitive impairment. Neurosci Bull. 2021; 37: 789–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol. 2015; 11: 157–165. [DOI] [PubMed] [Google Scholar]
- 39. Zimmerman ME, Pan JW, Hetherington HP, et al. Hippocampal neurochemistry, neuromorphometry, and verbal memory in nondemented older adults. Neurology. 2008; 70: 1594–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Suzuki H, Sumiyoshi A, Matsumoto Y, et al. Structural abnormality of the hippocampus associated with depressive symptoms in heart failure rats. Neuroimage. 2015; 105: 84–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Imaging protocol, sequence parameters and specifications.
Table S2. Extended descriptive clinical characteristics of study participants according to serum glial fibrillary acidic protein (GFAP) quartiles.
Table S3. Spearman's correlation (ρ) to cognitive function and brain morphology.
Figure S1. Serum GFAP concentrations. Histograms and quantil‐quantil (Q‐Q) plots of absolute and ln‐transformed GFAP measurements in the serum of chronic heart failure patients via ultrasensitive, bead‐based single molecular immunoassay. Dots represent single patients (n=146).


