Abstract
Aims
We evaluated the clinical outcomes and trajectory of cardiac reverse remodelling according to the timing of sacubitril/valsartan (Sac/Val) use in patients with heart failure (HF) with reduced ejection fraction (HFrEF).
Methods and results
Patients with de novo HFrEF who used Sac/Val between June 2017 and October 2019 were retrospectively enrolled. Patients were grouped into the earlier use group (initiation of Sac/Val < 3 months after the first HFrEF diagnosis) and the later use group (initiation of Sac/Val ≥ 3 months after the first HFrEF diagnosis). Primary outcome was a composite of HF hospitalization and cardiac death. Secondary outcomes were HF hospitalization, cardiac death, all‐cause death, significant ventricular arrhythmia (ventricular tachycardia or ventricular fibrillation), and echocardiographic evidence of cardiac reverse remodelling including left ventricular ejection fraction (LVEF) change during follow‐up. Among 115 enrolled patients, 67 were classified in the earlier use group, and 48 were classified in the later use group. Mean period of HFrEF diagnosis to Sac/Val use was 52.1 ± 14.3 days in the earlier use group, and 201.8 ± 127.3 days in the later use group. During the median follow‐up of 721 days, primary outcome occurred in 21 patients (18.3%). The earlier use group experienced significantly fewer primary outcome than the later use group (10.4% vs. 29.2%, P = 0.010). The Kaplan–Meier survival curve showed better event‐free survival in the earlier use group than in the later use group (log rank = 0.017). There were no significant differences in cardiac death, all‐cause death, and ventricular arrhythmia between two groups (1.5% vs. 2.1%, P = 0.811; 1.5% vs. 4.2%, P = 0.375; 3.0% vs. 0%, P = 0.227, respectively). Despite a significantly lower baseline LVEF in the earlier use group (21.3 ± 6.4% vs. 24.8 ± 7.9%, P = 0.012), an early prominent increase of LVEF was noted before 6 months (35.2 ± 11.9% vs. 27.8 ± 8.8%, P = 0.007). A delayed improvement of LVEF in the later use group resulted in similar LVEF at last follow‐up in both groups (40.7 ± 13.4% vs. 39.4 ± 10.9%, P = 0.686). Although the trajectory of left ventricular remodelling showed similar pattern in two groups, left atrial (LA) reverse remodelling was less prominent in the later use group during the follow‐up period (final LA volume index: 43.6 ± 14.3 mL/m2 vs. 55.2 ± 17.1 mL/m2, P = 0.011).
Conclusions
Earlier use of Sac/Val was related with better clinical outcome and earlier left ventricular reverse remodelling. Remodelling of LA was less prominent in the later use group implying delayed response in diastolic function.
Keywords: Angiotensin receptor‐neprilysin inhibitor, Sacubitril/valsartan, Heart failure with reduced ejection fraction, Left ventricular ejection fraction, Reverse remodelling, Clinical event
Introduction
Heart failure (HF) with reduced ejection fraction (HFrEF) is a complex and progressive clinical syndrome associated with high rates of mortality and re‐hospitalization, decreased functional capacity, reduced quality of life, and substantial socio‐economic burden. 1 , 2 One‐year mortality reaches 20% and 5 year mortality reaches 53–67% after the diagnosis of HF. 3 , 4 The prevalence of HFrEF continues to increase despite advances in therapeutic modalities for cardiovascular diseases and due to an increasingly aging population. 5 , 6 Currently, the incidence of HF worldwide appears to be 1–2% of adults. 7 , 8 However, in recent years, the novel sacubitril/valsartan (Sac/Val), an angiotensin receptor‐neprilysin inhibitor (ARNI), has been proven safe and effective in the management of patients with HFrEF.
The initial results of the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM‐HF) trial showed that Sac/Val is more effective than angiotensin‐converting enzyme inhibitor (ACEi) in reducing the risk of HF hospitalization and cardiac death by 20% in ambulatory patients with HFrEF. 9 In addition, the Comparison of Sacubitril–Valsartan versus Enalapril on Effect on NT‐proBNP in Patients Stabilized from an Acute Heart Failure Episode (PIONEER‐HF) study demonstrated that after stabilization of acute decompensated HF, the in‐hospital use of Sac/Val significantly reduced N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) markers compared with the in‐hospital use of enalapril, ACEi. 10 After switching the enalapril to Sac/Val, the NT‐proBNP levels of the group that was previously administered enalapril improved and final levels were observed to be similar in both groups. 11
Additionally, Sac/Val has been associated with cardiac reverse remodelling with left ventricular (LV) systolic function improvement and a rapid reduction in NT‐proBNP levels. 12 However, the clinical benefits of earlier use of Sac/Val vs. later use of Sac/Val after discharge have not been well established.
This study aimed to evaluate the benefits of earlier use of Sac/Val (<3 months) compared with its later use (≥3 months) in terms of clinical outcomes, including cardiac death or HF hospitalization, and cardiac reverse remodelling.
Methods
Study population
Between June 2017 and October 2019, a total of 343 patients who used Sac/Val after the diagnosis of HFrEF [ejection fraction (EF) < 40%] were consecutively included. Patients age under 19 years old, prior history of HFrEF, no formal echocardiographic information, and ACEi use < 28 days before Sac/Val use were excluded from the analysis (Figure 1 ). During follow‐up, 40 patients were further excluded, of whom 5 underwent heart transplantation surgery or LV assist device insertion, 8 did not perform follow‐up echocardiography, 13 used Sac/Val for <90 days, and 14 were lost to clinical follow‐up. Among the final 115 patients, 67 patients were classified into the earlier use group (initiation of Sac/Val < 3 months after the first HFrEF diagnosis), and 48 were classified into the later use group (initiation of Sac/Val ≥ 3 months after the first HFrEF diagnosis). From June 2017 to August 2020, we followed the cardiovascular centre of the single tertiary university hospital wherein the subjects were diagnosed.
Figure 1.
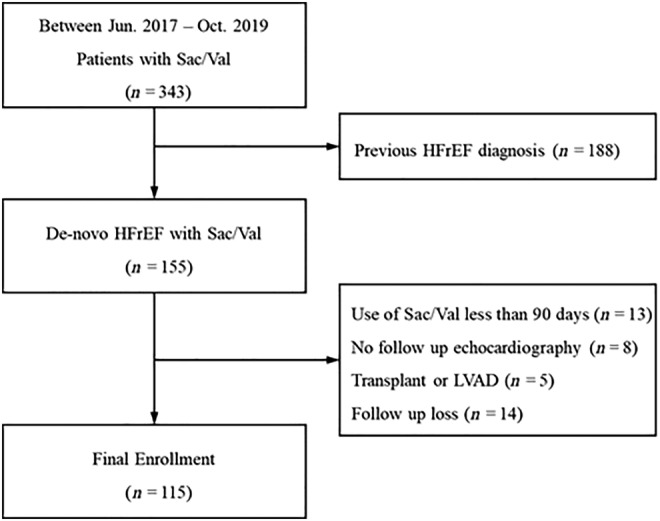
Flowchart for inclusion of study participants. HFrEF, heart failure with reduced ejection fraction; LVAD, left ventricular assist device; Sac/Val, sacubitril/valsartan.
Demographic and clinical data, including medical history of hypertension (HTN), diabetes mellitus (DM), chronic kidney disease (CKD), or atrial fibrillation (AF), and echocardiographic data were obtained from the electronic medical records. All clinical outcomes were adjudicated by two independent cardiologists (HJL and ICK). The Institutional Review Board of Keimyung University approved this study (IRB approval number: 2019‐06‐038) and waived the requirement for informed consent because of the retrospective nature of the research. The investigation conforms with the principles outlined in the Declaration of Helsinki.
The diagnosis of HFrEF was made when a patient showed symptoms (e.g. breathlessness, ankle swelling, and fatigue) and physical signs of congestion (e.g. elevated jugular venous pressure, pulmonary crackles, and peripheral oedema) and had a left ventricular ejection fraction (LVEF) of <40% on transthoracic echocardiography (TTE). 6 Cardiac reverse remodelling was defined as an absolute increase in LVEF ≥ 10% (or absolute LVEF at follow‐up ≥ 50%), associated with a relative reduction in indexed LV end‐diastolic diameter ≥ 10% (or absolute value at follow‐up ≤ 33 mm/m2). 13 , 14 HTN was defined based on diagnosis, medication prescription, systolic blood pressure (SBP) (≥140 mmHg), and diastolic blood pressure (DBP) (≥90 mmHg). DM was defined based on diagnosis, medication prescription, haemoglobin A1C level (≥6.5%), and fasting or random blood glucose level (≥126 and 200 mg/dL, respectively). CKD was defined as an estimated glomerular filtration rate < 60 mL/min/1.73 m2. AF was defined as documented on 12‐lead electrocardiograms.
Transthoracic echocardiography
Complete TTE was performed in all patients using commercially available scanners (GE Vivid E95, GE Healthcare, Waukesha, WI, USA; Philips EPIQ 7, Philips Medical Systems, Andover, MA, USA; Siemens Acuson SC2000 Prime, Siemens Medical Solutions, Mountain View, CA, USA), including 2D, pulsed‐wave, continuous‐wave, and colour Doppler imaging. All studies were performed with patients at rest in the left lateral position. LV dimensions and left atrial (LA) anteroposterior diameters were measured using the M‐mode in the parasternal short‐axis view, although the thickest segment was evaluated throughout the examination. The LA volume index (LAVI) and LVEF were measured using the modified Simpson's method from images with apical two‐chamber and four‐chamber views. Continuous‐wave Doppler was used to assess the aortic outflow peak velocity and the peak acceleration velocity where it is present. The E/e′ ratio was calculated based on the early mitral velocity (E) obtained using pulsed‐wave Doppler and the mitral annular velocity (e′) at the interventricular septal annulus obtained using tissue Doppler imaging. TTE was performed at the physician's discretion.
Study outcome
The primary outcome was a composite of cardiac death and HF hospitalization. The secondary outcomes were HF hospitalization, cardiac death, all‐cause death, significant ventricular arrhythmia (ventricular tachycardia or ventricular fibrillation), echocardiographic evidence of cardiac reverse remodelling [e.g. changes in LV end‐diastolic dimension (LVEDD), LV end‐systolic dimension (LVESD), LV volume index (LVVI), and LAVI], and LVEF during follow‐up. For the fair comparison, we have divided the follow‐up period in 6 month interval. If there were more than two examinations between the interval, the examination that is the closest to the index date was selected.
Statistical analysis
Statistical analysis was performed using SPSS software Version 25 (IBM SPSS Statistics for Windows, IBM Corp., Armonk, NY, USA). Unless otherwise indicated, all continuous variables were presented as mean ± standard deviation and were compared using an independent t‐test. Categorical variables were compared using the χ 2 test. Simple and multivariable Cox regression analyses were used to evaluate the significance of the variables. The covariates that differ significantly between the group with and without the primary outcome were used in the analyses of independent predictors of outcome. Tests on the proportional hazards assumption for each covariate were performed using the Kaplan–Meier estimate of survival distribution. Differences were considered statistically significant at P ≤ 0.05.
Results
Baseline characteristics of the study population
Baseline characteristics according to the earlier or later use of Sac/Val are displayed in Table 1 . Patients in the earlier use group were younger (58.0 ± 14.5 years vs. 65.0 ± 14.1 years, P = 0.010), were heavier (72.1 ± 15.9 kg vs. 66.0 ± 15.5 kg, P = 0.042), had lower occurrences of CKD (14.9% vs. 35.4%, P = 0.011), and had a higher prevalence of HTN (56.7% vs. 33.3%, P = 0.013) than those in the later use group. DBP (73.3 ± 13.8 mmHg vs. 68.2 ± 12.6 mmHg, P = 0.046), mean arterial pressure (89.3 ± 14.1 mmHg vs. 84.8 ± 12.3 mmHg, P = 0.072), haemoglobin levels (14.2 ± 1.8 vs. 13.0 ± 2.4, P = 0.004), and alanine aminotransferase (ALT) levels (32.7 ± 30.3 vs. 20.6 ± 14.9, P = 0.011) were significantly higher in the earlier use group than in the later use group.
Table 1.
Baseline characteristics of the overall population, earlier use group (<3 months), and later use group (≥3 months)
| Overall population (n = 115) | Earlier use (n = 67) | Later use (n = 48) | P value a | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 60.9 ± 14.7 | 58.0 ± 14.5 | 65.0 ± 14.1 | 0.010 |
| Male gender, n (%) | 77 (67.0) | 47 (70.1) | 30 (62.5) | 0.390 |
| Height, cm | 164.6 ± 10.2 | 165.5 ± 10.7 | 163.4 ± 9.6 | 0.272 |
| Weight, kg | 69.5 ± 15.9 | 72.1 ± 15.9 | 66.0 ± 15.5 | 0.042 |
| Body mass index, kg/m2 | 25.4 ± 4.1 | 26.1 ± 3.9 | 24.5 ± 4.1 | 0.034 |
| Haemodynamic data | ||||
| Systolic blood pressure, mmHg | 120.2 ± 17.4 | 121.7 ± 18.1 | 118.1 ± 16.3 | 0.274 |
| Diastolic blood pressure, mmHg | 71.1 ± 13.5 | 73.3 ± 13.8 | 68.2 ± 12.6 | 0.046 |
| Mean arterial pressure, mmHg | 87.5 ± 13.5 | 89.3 ± 14.1 | 84.8 ± 12.3 | 0.072 |
| Heart rate, b.p.m. | 79.3 ± 15.9 | 79.7 ± 15.2 | 78.6 ± 17.0 | 0.711 |
| Comorbidities | ||||
| Diabetes mellitus, n (%) | 35 (30.4) | 24 (35.8) | 11 (22.9) | 0.138 |
| Hypertension, n (%) | 54 (47.0) | 38 (56.7) | 16 (33.3) | 0.013 |
| Chronic kidney disease, n (%) | 27 (23.5) | 10 (14.9) | 17 (35.4) | 0.011 |
| Dyslipidaemia, n (%) | 26 (22.6) | 16 (23.9) | 10 (20.8) | 0.700 |
| Stroke, n (%) | 10 (8.7) | 6 (9.0) | 4 (8.3) | 0.907 |
| Ischaemic heart disease, n (%) | 22 (19.1) | 10 (14.9) | 12 (25.0) | 0.176 |
| Laboratory data | ||||
| WBC, 103/μL | 7.15 ± 1.80 | 7.07 ± 1.50 | 7.27 ± 2.19 | 0.575 |
| Haemoglobin, g/dL | 13.7 ± 2.1 | 14.2 ± 1.8 | 13.0 ± 2.4 | 0.004 |
| Platelet, 103/μL | 227.3 ± 57.5 | 225.7 ± 55.5 | 229.6 ± 61.0 | 0.734 |
| Total bilirubin, mg/dL | 0.82 ± 0.55 | 0.9 ± 0.62 | 0.72 ± 0.41 | 0.133 |
| BUN, mg/dL | 19.5 ± 8.5 | 18.8 ± 7.0 | 20.4 ± 10.3 | 0.351 |
| Creatinine, mg/dL | 1.04 ± 0.4 | 1.00 ± 0.35 | 1.09 ± 0.47 | 0.247 |
| eGFR, mL/min/1.73 m2 | 81.1 ± 26.2 | 82.8 ± 21.0 | 78.5 ± 32.2 | 0.434 |
| AST, U/L | 25.5 ± 12.5 | 26.8 ± 14.5 | 23.3 ± 8.7 | 0.205 |
| ALT, U/L | 27.7 ± 25.7 | 32.7 ± 30.3 | 20.6 ± 14.9 | 0.011 |
| Total cholesterol, mg/dL | 164.6 ± 46.1 | 164.8 ± 43.4 | 164.1 ± 51.0 | 0.959 |
| Triglyceride, mg/dL | 160.6 ± 72.0 | 167.5 ± 79.3 | 148.9 ± 57.9 | 0.404 |
| HDL, mg/dL | 47.4 ± 14.2 | 48.4 ± 16.1 | 45.5 ± 10.2 | 0.521 |
| LDL, mg/dL | 91.3 ± 38.3 | 89.5 ± 32.5 | 94.4 ± 47.6 | 0.676 |
| NT‐proBNP, pg/mL | 4581.5 ± 5714.9 | 4007.9 ± 4274.7 | 5390.3 ± 7267.4 | 0.292 |
| Treatment at Sac/Val initial use | ||||
| Days to Sac/Val use b , days | 114.6 ± 110.9 | 52.1 ± 14.3 | 201.8 ± 127.3 | 0.000 |
| Beta‐blocker, n (%) | 104 (90.4) | 61 (91.0) | 43 (89.6) | 0.793 |
| Spironolactone, n (%) | 86 (74.8) | 53 (79.1) | 33 (68.8) | 0.207 |
| Other diuretics, n (%) | 84 (73.0) | 54 (80.6) | 30 (62.5) | 0.031 |
| Digoxin, n (%) | 15 (13.0) | 9 (13.4) | 6 (12.5) | 0.884 |
| Amiodarone, n (%) | 6 (5.2) | 3 (4.5) | 3 (6.3) | 0.673 |
| Anti‐coagulant, n (%) | 48 (41.7) | 25 (37.3) | 23 (47.9) | 0.256 |
| Anti‐platelet, n (%) | 30 (26.1) | 14 (20.9) | 16 (33.3) | 0.134 |
| Echocardiography data | ||||
| Ejection fraction, % | 22.8 ± 7.2 | 21.3 ± 6.4 | 24.8 ± 7.9 | 0.012 |
| LVEDD, mm | 6.19 ± 0.72 | 6.27 ± 0.71 | 6.08 ± 0.72 | 0.163 |
| LVESD, mm | 5.42 ± 0.88 | 5.55 ± 0.81 | 5.23 ± 0.93 | 0.055 |
| LAD, mm | 4.71 ± 0.83 | 4.83 ± 0.84 | 4.55 ± 0.79 | 0.071 |
| IVSd, mm | 0.92 ± 0.17 | 0.93 ± 0.15 | 0.90 ± 0.19 | 0.267 |
| PWDd, mm | 0.94 ± 0.18 | 0.96 ± 0.18 | 0.92 ± 0.16 | 0.322 |
| LVVI, mL/m2 | 98.3 ± 31.1 | 102.0 ± 29.6 | 93.2 ± 32.8 | 0.151 |
| LAVI, mL/m2 | 64.8 ± 21.7 | 65.5 ± 19.5 | 63.9 ± 24.6 | 0.708 |
| E, m/s | 0.89 ± 0.27 | 0.91 ± 0.27 | 0.86 ± 0.26 | 0.299 |
| A, m/s | 0.66 ± 0.30 | 0.64 ± 0.30 | 0.71 ± 0.30 | 0.173 |
| E/A | 1.83 ± 1.22 | 2.03 ± 1.36 | 1.49 ± 0.86 | 0.027 |
| Deceleration time, ms | 152.4 ± 51.7 | 144.9 ± 53.6 | 164.0 ± 47.0 | 0.085 |
| E/e′ | 17.3 ± 14.3 | 19.1 ± 17.3 | 14.6 ± 7.8 | 0.073 |
A, peak velocity of late transmitral flow; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; E, peak velocity of early diastolic transmitral flow; e′, peak velocity of early diastolic mitral annular motion as determined by pulse wave Doppler; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; IVSd, interventricular septum thickness at end‐diastole; LAD, left atrial anteroposterior dimension; LAVI, left atrial volume index; LDL, low‐density lipoprotein; LVEDD, left ventricular end‐diastolic dimension; LVESD, left ventricular end‐systolic dimension; LVVI, left ventricular volume index; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; PWDd, posterior wall thickness at end‐diastole; Sac/Val, sacubitril/valsartan; WBC, white blood cell.
Earlier use group vs. later use group.
Duration between heart failure with reduced ejection fraction diagnosis and the initiation of Sac/Val.
Mean durations between HFrEF diagnosis and the initiation of Sac/Val use were 52.1 ± 14.3 and 201.8 ± 127.3 days in the earlier and later use groups, respectively. The physician adherence to other guideline‐directed medical therapies, such as beta‐blockers (91.0% vs. 89.6%, P = 0.793) and spironolactone (79.1% vs. 68.8%, P = 0.207), was equally maintained in both groups. Continuous use of diuretic therapy was higher in the earlier use group (80.6% vs. 62.5%, P = 0.031).
The EF in the earlier use group was significantly lower than that in the later use group (21.3 ± 6.4% vs. 24.8 ± 7.9%, P = 0.012). However, there were no differences in LV and LA chamber sizes. The ratio of early to late peak velocity of diastolic transmitral flow (E/A) was higher in the earlier use group (2.03 ± 1.36 vs. 1.49 ± 0.86, P = 0.027).
To investigate the prognostic value of the timing of Sac/Val use in HF aggravation, Cox regression analysis was applied on variables that were significantly different in terms of primary outcome (cardiac death or HF hospitalization) (Table 2 and Supporting Information, Table S1 ). In the univariate analysis, age, ischaemic heart disease, beta‐blocker use, spironolactone use, and days from HF diagnosis to initial Sac/Val use were significantly associated with the primary outcome. In the multivariate analysis, ischaemic heart disease was a definite positive predictor [hazard ratio (HR) 2.743, P = 0.027], while beta‐blocker or spironolactone use was a definite negative predictor (HR 0.170, P = 0.003; HR 0.377, P = 0.042, respectively). In particular, delayed use of Sac/Val was an independent positive predictor of HF aggravation (HR 2.737, P = 0.035) (Table 3 ).
Table 2.
Outcome data of the overall population, earlier use group (<3 months), and later use group (≥3 months)
| Overall population (n = 115) | Earlier use (n = 67) | Later use (n = 48) | P value a | |
|---|---|---|---|---|
| Primary outcome | ||||
| Cardiac death or HF hospitalization b , n (%) | 21 (18.3) | 7 (10.4) | 14 (29.2) | 0.010 |
| Secondary outcome | ||||
| HF hospitalization, n (%) | 21 (18.3) | 7 (10.4) | 14 (29.2) | 0.010 |
| Readmission before Sac/Val c , n (%) | 11 (9.6) | 1 (1.5) | 10 (20.8) | 0.001 |
| Readmission after Sac/Val d , n (%) | 12 (10.4) | 6 (9.0) | 6 (12.5) | 0.540 |
| Cardiac death, n (%) | 2 (1.7) | 1 (1.5) | 1 (2.1) | 0.811 |
| All‐cause death, n (%) | 3 (2.6) | 1 (1.5) | 2 (4.2) | 0.375 |
| VT or VF, n (%) | 2 (1.7) | 2 (3.0) | 0 (0) | 0.227 |
| Cardiac reverse remodelling, n (%) | 24 (20.9) | 16 (23.9) | 8 (16.7) | 0.348 |
| EF change e , % | 16.1 ± 14.4 | 17.9 ± 15.4 | 413.4 ± 12.5 | 0.098 |
EF, ejection fraction; HF, heart failure; VF, ventricular fibrillation; VT, ventricular tachycardia.
Earlier use group vs. later use group.
Two cases of cardiac death have experienced prior HF hospitalization.
Hospitalization for HF within 6 months after diagnosis.
Hospitalization for HF 6 months after diagnosis.
EF change from EF of echocardiography at diagnosis to that of final echocardiography.
Table 3.
Independent predictors for the primary outcome
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age | 1.043 | 1.008–1.079 | 0.016 | |||
| IHD | 4.333 | 1.811–10.366 | 0.001 | 2.743 | 1.119–6.728 | 0.027 |
| Beta‐blocker | 0.164 | 0.063–0.427 | 0.000 | 0.170 | 0.054–0.540 | 0.003 |
| Spironolactone | 0.385 | 0.162–0.914 | 0.031 | 0.377 | 0.147–0.963 | 0.042 |
| Delayed use of Sac/Val a | 2.871 | 1.158–7.113 | 0.023 | 2.737 | 1.076–6.962 | 0.035 |
CI, confidence interval; HR, hazard ratio; IHD, ischaemic heart disease; Sac/Val, sacubitril/valsartan.
Use of Sac/Val 3 months after diagnosis of heart failure with reduced ejection fraction, as in the later use group.
The initial and final doses of Sac/Val in both groups were not different (112.7 ± 53.2 mg/day vs. 108.3 ± 41.7 mg/day, P = 0.638; 197.8 ± 129.5 mg/day vs. 193.8 ± 123.2 mg/day, P = 0.868, respectively) (Supporting Information, Figure S1 ).
Study outcome
During a median follow‐up of 721 days from the diagnosis to clinical event or study endpoint (interquartile range 459–895 days), the primary outcome occurred in 7 patients (10.4%) in the earlier use group and in 14 patients (29.2%) in the later use group (P = 0.010) (Table 2 ). Everyone who died of heart disease has previously been hospitalized again. The Kaplan–Meier survival curve also showed a significant difference in the primary outcome between the two groups (log rank = 0.017) (Figure 2 ). Before the use of Sac/Val, primary outcome was significantly higher in the later use group (1.5% vs. 20.8%, P = 0.001), whereas the difference was not observed after the Sac/Val use (9.0% vs. 12.5%, P = 0.540).
Figure 2.
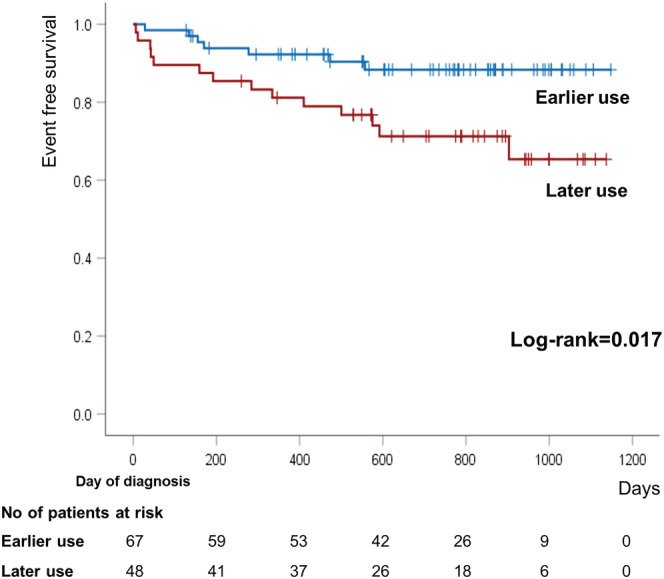
Kaplan–Meier curve for primary outcome, a composite of cardiac death and heart failure hospitalization, according to study groups.
There were no differences in cardiac death, all‐cause death, and ventricular arrhythmia between two groups (1.5% vs. 2.1%, P = 0.811; 1.5% vs. 4.2%, P = 0.375; 3.0% vs. 0%, P = 0.227, respectively). Echocardiographic parameters, including EF, LVEDD, LVESD, LVVI, and LAVI, were compared at baseline and at 6 month intervals during follow‐up (Figure 3 ). The number of echocardiographic examinations in two groups was not different (2.19 ± 1.06 vs. 2.27 ± 0.87, P = 0.681). In the earlier use group, EF remarkably increased during the first 6 months (35.2 ± 11.9% vs. 27.8 ± 8.8%, P = 0.007), but the improvement decreased after 6 months. In the later use group, a prominent improvement in EF was observed after 6 months (37.2 ± 11.2% vs. 34.0 ± 8.8%, P = 0.174). Similar patterns were noted for LV chamber size (i.e. LVEDD, LVESD, and LVVI). Earlier prominent reverse remodelling was noted in the earlier use group, and delayed reverse remodelling was observed in the later use group. In the final echocardiography, two groups showed similar number of patients with cardiac reverse remodelling (23.9% vs. 16.7%, P = 0.348). The pattern of LAVI change was different from that of other parameters in that there was a continuous reduction in LAVI in the earlier use group, whereas maintenance of blunted reduction was observed in the later use group (LAVI after 18 months; 43.6 ± 14.3 mL/m2 vs. 55.2 ± 17.1 mL/m2, P = 0.011) (Figure 3 ).
Figure 3.
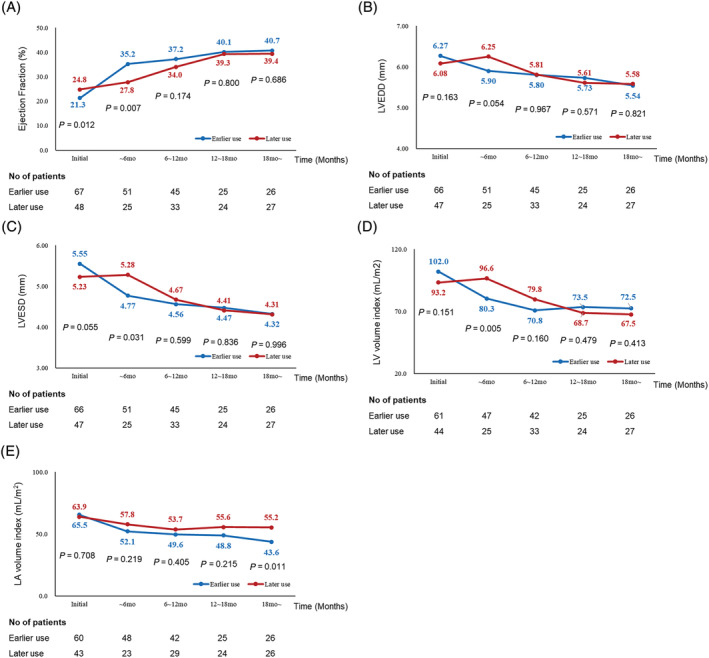
Changes in echocardiographic parameters during follow‐up. (A) Ejection fraction, (B) left ventricular end‐diastolic dimension (LVEDD), (C) left ventricular end‐systolic dimension (LVESD), (D) left ventricular volume index (LVVI), and (E) left atrial volume index (LAVI).
Discussion
The main findings of the current study are as follows: (i) earlier use of Sac/Val (<3 months) in patients with de novo HFrEF was associated with reduced clinical events including cardiac death or HF hospitalization; (ii) early prominent systolic function improvement and cardiac reverse remodelling were noted in the earlier use group, whereas delayed catch‐up of systolic function improvement and cardiac reverse remodelling were observed in the later use group. Unlike ventricular remodelling, the decrease in LAVI was prominent in the earlier use group throughout the study period, without a ‘later catch‐up’ phenomenon; (iii) delayed Sac/Val use, ischaemic heart disease, and non‐use of beta‐blockers and spironolactone were independent predictors of HF aggravation (central illustration). 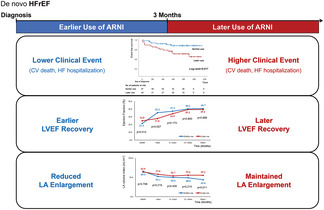
Central illustration. The benefits of the earlier use of sacubitril/valsartan in de novo HFrEF patients. ARNI, angiotensin receptor‐neprilysin inhibitor; CV, cardiovascular; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; LA, left atrial; LVEF, left ventricular ejection fraction.
The effect of the sacubitril/valsartan on clinical outcomes and the benefits of its earlier use
Compared with the ACEi enalapril, Sac/Val has a proven efficacy in reducing cardiac death or HF hospitalization and a good safety profile in the PARADIGM‐HF trial in ambulatory HFrEF patients. 9 In addition, the PIONEER‐HF trial showed that the in‐hospital Sac/Val use after stabilization of acute decompensated HF significantly reduced NT‐proBNP levels rapidly by 29%. After switching the enalapril treatment to the Sac/Val, the group that previously received enalapril showed a late catch‐up of NT‐proBNP levels in the group that previously received Sac/Val, which then showed no difference between the two groups. 11
As a result of these two landmark randomized clinical trials, Sac/Val has become an important HFrEF medication used to improve clinical outcomes. Recent updates of HF guidelines recommend the use of Sac/Val as an initial treatment after stabilization of acute decompensated HF during hospitalization. 15 However, in practice, it is not always possible to start optimal medications for HFrEF immediately after diagnosis, and there are time variations in the prescription period of Sac/Val. We evaluated the benefits of earlier Sac/Val use on clinical outcomes after the initial HFrEF diagnosis. Cardiac death and HF hospitalization were significantly lower in the earlier use group than in the later use group. Moreover, the occurrence of clinical events was noted mainly after the diagnosis of HFrEF in the earlier use group before the initiation of Sac/Val; however, continuous events were noted throughout the follow‐up period in the later use group. Earlier and later use groups showed significant difference in primary outcomes prior to Sac/Val, but not after Sac/Val use (1.5% vs. 20.8%, P = 0.001; 9.0% vs. 12.5%, P = 0.540, respectively).
The effect of sacubitril/valsartan on cardiac remodelling and the benefit of earlier use of sacubitril/valsartan
In HFrEF patients, Sac/Val has shown benefits of cardiac reverse remodelling with a decrease in NT‐proBNP levels. A previous study reported a strong association between NT‐proBNP change and subsequent cardiac reverse remodelling. 12 Meta‐analyses have also shown the benefit of Sac/Val on cardiac reverse remodelling and LV systolic function improvement. 16 We observed early and significant improvement of systolic function in the earlier use group with LV chamber size reduction. The later use group showed delayed improvement; however, the difference in EF between the two groups decreased after 6 months and nearly levelled after 18 months. The LV end‐diastolic and end‐systolic dimensions also showed similar trends.
However, LAVI showed a significant difference between the two groups throughout the follow‐up period, and a ‘later catch‐up’ phenomenon, where differences between groups decrease after long‐term Sac/Val use, was not observed. Unlike ventricular remodelling, the decrease in LAVI was more prominent in the earlier use group, suggesting a long‐term consequence of impaired diastolic function. 17 In patients with de novo HFrEF, the earlier optimization of medications, including Sac/Val, might provide an earlier and prolonged benefit on diastolic function. It should be considered that other complex mechanisms could affect diastolic function and related echocardiographic parameters; thus, a comprehensive approach to management of comorbidities is necessary in HFrEF therapy. 18 , 19 , 20 Further studies are necessary to investigate the effect of earlier Sac/Val use on diastolic dysfunction.
The HF guidelines have Class I recommendations regarding the use of adequate doses of the optimal medication. Nevertheless, it is substantial to be discharged from the hospital without sufficient use of the medication due to various factors (patient factor and doctor factor). 21 , 22 In such cases, routine follow‐ups should be conducted and individualized optimal medical therapy should be continued. Based on study findings, it is important to start Sac/Val as soon as possible, even in HF patients who were discharged from the hospital without its use.
Independent predictors of primary outcome
Among the relevant parameters of the primary outcome, multivariate analysis was performed to evaluate the independent predictors of clinical outcomes. The aetiology of ischaemic heart disease, non‐use of beta‐blockers and spironolactone, and delayed use of Sac/Val were independent predictors of cardiac death and HF hospitalization.
Study limitations
To the best of our knowledge, this is the first study to evaluate the benefit of the earlier use of Sac/Val on clinical outcomes and cardiac reverse remodelling in de novo HFrEF patients. However, this study had certain limitations.
First, this is a retrospective study with small number of inclusions. Due to the retrospective nature of this study, inherent limitations exist. Baseline characteristics between the two groups were different; thus, Cox regression analysis was performed to evaluate the independent predictors of the primary outcome and how much they influence. Delayed use of Sac/Val was independently associated with worse outcomes in patients with ischaemic heart disease and non‐use of beta‐blockers or spironolactone.
Second, unmeasured confounders may exist, which could influence on timing of prescription and outcomes. The timing of Sac/Val use was determined according to the physician's discretion. Frail patients with older age, smaller body mass index, and CKD were more likely to receive Sac/Val later. To overcome this limitation, we have performed Cox regression analysis. Use of Sac/Val was the independent predictor among the variables that showed significance on the univariate analysis.
Third, the timing of follow‐up echocardiography was not controlled and was performed at the physician's discretion. However, there was no significant difference in the number of echocardiography procedures performed in each period between the two groups.
Conclusions
In patients with de novo HFrEF, the earlier use of Sac/Val is associated with reduced clinical events and earlier improvement of LV systolic function. Late improvement of LVEF was noted after Sac/Val initiation in the later use group. Thus, the earlier use of Sac/Val after the stabilization of HF symptoms need to be emphasized to reduce adverse clinical events and to achieve earlier LV reverse remodelling as well as prolonged effect on LA reverse remodelling implying diastolic function preservation.
Conflict of interest
Ji‐Hye Oh, Jae‐Man Lee, Hee‐Jung Lee, Jongmin Hwang, Cheol Hyun Lee, Yun‐Kyeong Cho, Hyoung‐Seob Park, Hyuck‐Jun Yoon, Jin‐Wook Chung, Hyungseop Kim, Chang‐Wook Nam, Seongwook Han, Seung‐Ho Hur, Jong‐Chan Youn, and In‐Cheol Kim declare no conflict of interest.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1C1C1014161).
Supporting information
Figure S1. Initial and final doses of Sac/Val in overall population, earlier use group and later use group.
Table S1. Baseline characteristics of HFrEF patients and patients with positive events vs. negative events.
Oh, J.‐H. , Lee, J.‐M. , Lee, H.‐J. , Hwang, J. , Lee, C. H. , Cho, Y.‐K. , Park, H.‐S. , Yoon, H.‐J. , Chung, J.‐W. , Kim, H. , Nam, C.‐W. , Han, S. , Hur, S.‐H. , Youn, J.‐C. , and Kim, I.‐C. (2022) The benefits of the earlier use of sacubitril/valsartan in de novo heart failure with reduced ejection fraction patients. ESC Heart Failure, 9: 2435–2444. 10.1002/ehf2.13940.
References
- 1. Steinberg BA, Fang JC. Long‐term outcomes of acute heart failure: where are we now? J Am Coll Cardiol. 2017; 70: 2487–2489. [DOI] [PubMed] [Google Scholar]
- 2. Murphy SP, Ibrahim NE, Januzzi JL Jr. Heart failure with reduced ejection fraction: a review. JAMA. 2020; 324: 488–504. [DOI] [PubMed] [Google Scholar]
- 3. Tsao CW, Lyass A, Enserro D, Larson MG, Ho JE, Kizer JR, Gottdiener JS, Psaty BM, Vasan RS. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Fail. 2018; 6: 678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, Jiang R, Killian JM, Roger VL. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 2015; 175: 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail. 2020; 22: 1342–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, ESC Scientific Document Group , de Boer RA, Christian Schulze P, Abdelhamid M, Aboyans V, Adamopoulos S, Anker SD, Arbelo E, Asteggiano R, Bauersachs J, Bayes‐Genis A, Borger MA, Budts W, Cikes M, Damman K, Delgado V, Dendale P, Dilaveris P, Drexel H, Ezekowitz J, Falk V, Fauchier L, Filippatos G, Fraser A, Frey N, Gale CP, Gustafsson F, Harris J, Iung B, Janssens S, Jessup M, Konradi A, Kotecha D, Lambrinou E, Lancellotti P, Landmesser U, Leclercq C, Lewis BS, Leyva F, Linhart A, Løchen ML, Lund LH, Mancini D, Masip J, Milicic D, Mueller C, Nef H, Nielsen JC, Neubeck L, Noutsias M, Petersen SE, Sonia Petronio A, Ponikowski P, Prescott E, Rakisheva A, Richter DJ, Schlyakhto E, Seferovic P, Senni M, Sitges M, Sousa‐Uva M, Tocchetti CG, Touyz RM, Tschoepe C, Waltenberger J, Adamo M, Baumbach A, Böhm M, Burri H, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gardner RS, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Piepoli MF, Price S, Rosano GMC, Ruschitzka F, Skibelund AK. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021. 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 7. Brouwers FP, de Boer RA, van der Harst P, Voors AA, Gansevoort RT, Bakker SJ, van der Harst P, Hillege HL, van Veldhuisen DJ, van Gilst WH. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community‐based cohort: 11‐year follow‐up of PREVEND. Eur Heart J. 2013; 34: 1424–1431. [DOI] [PubMed] [Google Scholar]
- 8. Meyer S, Brouwers FP, Voors AA, Hillege HL, de Boer RA, Gansevoort RT, van der Harst P, Rienstra M, van Gelder IC, van Veldhuisen DJ, van Gilst WH, van der Meer P Sex differences in new‐onset heart failure. Clin Res Cardiol 2015;104: 342–350. [DOI] [PubMed] [Google Scholar]
- 9. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 10. Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E. Angiotensin‐neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019; 380: 539–548. [DOI] [PubMed] [Google Scholar]
- 11. DeVore AD, Braunwald E, Morrow DA, Duffy CI, Ambrosy AP, Chakraborty H, McCague K, Rocha R, Velazquez EJ, PIONEER‐HF Investigators . Initiation of angiotensin‐neprilysin inhibition after acute decompensated heart failure: secondary analysis of the open‐label extension of the PIONEER‐HF trial. JAMA Cardiol. 2020; 5: 202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Januzzi JL Jr, Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, Camacho A, Piña IL, Rocha RA, Shah AM, Williamson KM, Solomon SD, PROVE‐HF Investigators . Association of change in N‐terminal pro‐B‐type natriuretic peptide following initiation of sacubitril‐valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA. 2019; 322: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Merlo M, Pyxaras SA, Pinamonti B, Barbati G, Di Lenarda A, Sinagra G. Prevalence and prognostic significance of left ventricular reverse remodeling in dilated cardiomyopathy receiving tailored medical treatment. J Am Coll Cardiol. 2011; 57: 1468–1476. [DOI] [PubMed] [Google Scholar]
- 14. Dal Ferro M, Stolfo D, Altinier A, Gigli M, Perrieri M, Ramani F, Barbati G, Pivetta A, Brun F, Monserrat L, Giacca M, Mestroni L, Merlo M, Sinagra G. Association between mutation status and left ventricular reverse remodelling in dilated cardiomyopathy. Heart. 2017; 103: 1704–1710. [DOI] [PubMed] [Google Scholar]
- 15. Writing C, Maddox TM, Januzzi JL Jr, Allen LA, Breathett K, Butler J, Davis LL, Fonarow GC, Ibrahim NE, Lindenfeld JA, Masoudi FA, Motiwala SR, Oliveros E, Patterson JH, Walsh MN, Wasserman A, Yancy CW, Youmans QR. Update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021; 77: 772–810. [DOI] [PubMed] [Google Scholar]
- 16. Wang Y, Zhou R, Lu C, Chen Q, Xu T, Li D. Effects of the angiotensin‐receptor neprilysin inhibitor on cardiac reverse remodeling: meta‐analysis. J Am Heart Assoc. 2019; 8: e012272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. El Aouar LM, Meyerfreud D, Magalhaes P, Rodrigues SL, Baldo MP, Brasil Y, Magalhães P, el Aouar SM, el Aouar NA, Mill JG, Campos Filho O. Relationship between left atrial volume and diastolic dysfunction in 500 Brazilian patients. Arq Bras Cardiol. 2013; 101: 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morris DA, Belyavskiy E, Aravind‐Kumar R, Kropf M, Frydas A, Braunauer K, Marquez E, Krisper M, Lindhorst R, Osmanoglou E, Boldt LH, Blaschke F, Haverkamp W, Tschöpe C, Edelmann F, Pieske B, Pieske‐Kraigher E. Potential usefulness and clinical relevance of adding left atrial strain to left atrial volume index in the detection of left ventricular diastolic dysfunction. JACC Cardiovasc Imaging. 2018; 11: 1405–1415. [DOI] [PubMed] [Google Scholar]
- 19. Miyoshi H, Oishi Y, Mizuguchi Y, Iuchi A, Nagase N, Ara N, Oki T. Association of left atrial reservoir function with left atrial structural remodeling related to left ventricular dysfunction in asymptomatic patients with hypertension: evaluation by two‐dimensional speckle‐tracking echocardiography. Clin Exp Hypertens. 2015; 37: 155–165. [DOI] [PubMed] [Google Scholar]
- 20. Teniente‐Valente R, Solorio S, Vargas‐Salado E, Aguirre‐Vazquez C, Hernandez‐Gonzalez MA, Olvera‐Lopez JA, Rodríguez‐Mariscal L, Luna‐Ruiz MA, Contreras JM, Ortiz BO. Improvement of diastolic function after regression of left ventricular hypertrophy. Arch Cardiol Mex. 2008; 78: 392–399. [PubMed] [Google Scholar]
- 21. Wachter R, Senni M, Belohlavek J, Straburzynska‐Migaj E, Witte KK, Kobalava Z, Fonseca C, Goncalvesova E, Cavusoglu Y, Fernandez A, Chaaban S, Bøhmer E, Pouleur AC, Mueller C, Tribouilloy C, Lonn E, A.L. Buraiki J, Gniot J, Mozheiko M, Lelonek M, Noè A, Schwende H, Bao W, Butylin D, Pascual‐Figal D, on behalf of the TRANSITION Investigators , Gniot J, Mozheiko M, Lelonek M, Dominguez AR, Horacek T, del Rio EG, Kobalava Z, Mueller CE, Cavusoglu Y, Straburzynska‐Migaj E, Slanina M, vom Dahl J, Senni M, Ryding A, Moriarty A, Robles MB, Villota JN, Quintana AG, Nitschke T, Manuel Garcia Pinilla J, Bonet LA, Chaaban S, Filali zaatari, MD S, Spinar J, Musial W, Abdelbaki K, Belohlavek J, Fehske W, Bott MC, Hoegalmen G, Leiro MC, Ozcan IT, Mullens W, Kryza R, al‐Ani R, Loboz‐Grudzien K, Ermoshkina L, Hojerova S, Fernandez AA, Spinarova L, Lapp H, Bulut E, Almeida F, Vishnevsky A, Belicova M, Pascual D, Witte K, Wong K, Droogne W, Delforge M, Peterka M, Olbrich HG, Carugo S, Nessler J, McGill TH, Huegl B, Akin I, Moreira I, Baglikov A, Thambyrajah J, Hayes C, Barrionuevo MR, Yigit Z, Kaya H, Klimsa Z, Radvan M, Kadel C, Landmesser U, di Tano G, Lisik MB, Fonseca C, Oliveira L, Marques I, Santos LM, Lenner E, Letavay P, Bueno MG, Mota P, Wong A, Bailey K, Foley P, Hasbani E, Virani S, Massih TA, al‐Saif S, Taborsky M, Kaislerova M, Motovska Z, Praha, Cohen AA, Logeart D, Endemann D, Ferreira D, Brito D, Kycina P, Bollano E, Basilio EG, Rubio LF, Aguado MG, Schiavi LB, Zivano DF, Lonn E, Sayed AE, Pouleur AC, Heyse A, Schee A, Polasek R, Houra M, Tribouilloy C, Seronde MF, Galinier M, Noutsias M, Schwimmbeck P, Voigt I, Westermann D, Pulignano G, Vegsundvaag J, Alexandre da Silva Antunes J, Monteiro P, Stevlik J, Goncalvesova E, Hulkoova B, Juan Castro Fernandez A, Davies C, Squire I, Meyer P, Sheppard R, Sahin T, Sochor K, de Geeter G, Wachter R, Schmeisser A, Weil J, Soares AO, Vasilevna OB, Oshurkov A, Sunderland SJ, Glover J, Exequiel T, Decoulx E, Meyer S, Muenzel T, Frioes F, Arbolishvili G, Tokarcikova A, Karlstrom P, Carles Trullas Vila J, Perez GP, Sankaranarayanan R, Nageh T, Alasia DC, Refaat M, Demirkan B, al‐Buraiki J, Karabsheh S. Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: primary results of the randomised TRANSITION study. Eur J Heart Fail. 2019; 21: 998–1007. [DOI] [PubMed] [Google Scholar]
- 22. Senni M, Wachter R, Witte KK, Straburzynska‐Migaj E, Belohlavek J, Fonseca C, Mueller C, Lonn E, Chakrabarti A, Bao W, Noe A, Schwende H, Butylin D, Pascual‐Figal D, on behalf of the TRANSITION Investigators . Initiation of sacubitril/valsartan shortly after hospitalisation for acutely decompensated heart failure in patients with newly diagnosed (de novo) heart failure: a subgroup analysis of the TRANSITION study. Eur J Heart Fail. 2020; 22: 303–312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Initial and final doses of Sac/Val in overall population, earlier use group and later use group.
Table S1. Baseline characteristics of HFrEF patients and patients with positive events vs. negative events.


