Abstract
Aims
Radiofrequency catheter ablation (RFCA) is now an established therapeutic option for patients with atrial fibrillation (AF), but the long‐term recurrence rate of AF is still high. Sacubitril/valsartan (Sac/Val) is superior to valsartan in attenuating ventricular remodelling and improving clinical outcomes in heart failure patients, but whether this additional benefit exists in reversing atrial remodelling and reducing AF recurrence of RFCA‐treated AF patients remains uncovered.
Methods and results
Patients that had undergone RFCA were enrolled and randomly assigned 1:1 to valsartan (160 mg/day) or Sac/Val (200 mg/day) treatment group, in addition to other standard treatment of AF. Patients were followed up for 24 weeks. Echocardiography and ambulatory Holter monitoring for 24 h was performed at 24 weeks after RFCA. The primary end point was the change of atrial diameter from baseline to 24 weeks after RFCA. Second end points included the recurrence rate of AF, all‐cause hospitalization and all‐cause death. A total of 64 AF patients were enrolled, 32 of which received Sac/Val and 32 received valsartan treatment. There was no difference in the age (64.8 ± 9.8 vs. 63.7 ± 9.0, P = 0.634), gender (per cent of male: 59.4% vs. 50.0%, P = 0.616), heart rate (84.7 ± 4.1 b.p.m. vs. 80.9 ± 2.6 b.p.m., P = 0.428), systolic (127.5 ± 15.4 mmHg vs. 130.0 ± 17.8 mmHg, P = 0.549) or diastolic (81.7 ± 9.8 mmHg vs. 79.9 ± 12.6, P = 0.537) blood pressure upon admission between valsartan and Sac/Val treatment groups. The percentage of persistent AF was also comparable (43.8% vs. 53.1%, P = 0.617) in both treatment groups. Patients receiving Sac/Val treatment displayed significant decrease in the left atrial diameter (4.3 ± 0.5 cm to 3.8 ± 0.5 cm, P < 0.001), volume index (48.0 ± 6.4 mL/m2 to 41.7 ± 7.0 mL/m2, P < 0.001), and right atrial diameter (4.4 ± 0.8 cm to 3.9 ± 0.7 cm, P = 0.017) from baseline to 24 weeks after RFCA. This effect was not observed in valsartan treatment group. There was a numerical decrease in AF recurrence rate in the Sac/Val group compared with valsartan group (9.4% vs. 15.6%), although this difference did not reach a statistical significance (P = 0.708). No difference in all‐cause hospitalization rate (6.3% in each group) or all‐cause death rate (0% in each group) was observed.
Conclusions
Our data indicate that Sac/Val is superior to valsartan in attenuating atrial structural remodelling in catheter ablation‐treated AF patients.
Keywords: Atrial fibrillation, Sacubitril/valsartan, Atrial remodelling, AF recurrence, Pulmonary vein isolation
Introduction
Atrial fibrillation (AF) is a common type of arrhythmia with a prevalence rate of 1% in general population and 6% in those aged over 60 years. 1 It dramatically increases the risk of ischaemic stroke, heart failure, and death. Radiofrequency catheter ablation (RFCA) to achieve pulmonary vein isolation (PVI) is now an established therapeutic option for patients with symptomatic AF, especially for those who have not responded to, or have contraindications to, anti‐arrhythmic medication. Compared with anti‐arrhythmic therapy, catheter ablation demonstrates superior efficacy in reducing AF recurrence (relative risk reduction of 53%, absolute risk reduction of 29%) and improving symptoms. 2 However, the success rate in maintaining sinus rhythm is around 80% at 3 years and 60% at 10 years, 3 some of which have to receive multiple procedures to achieve this. How to further reduce AF recurrence rate in AF patients after RFCA remains an important clinical issue.
Atrial remodelling, including electrical, structural, and autonomic remodelling, is responsible for the initiation, maintenance, and progression of AF. 4 Notably, left atrial size and volume are independent predictors for AF recurrence after catheter ablation. 5 , 6 A meta‐analysis of 22 studies with 3750 patients shows that increased antero‐posterior diameter of the left atria is associated with more recurrence of AF after RFCA. 7 This implies the reverse of atrial structural remodelling may be a promising therapeutic strategy in reducing the AF recurrence after RFCA.
Sacubitril/valsartan (Sac/Val), an angiotensin receptor‐neprilysin inhibitor (ARNI), consists of the molecular components of valsartan (angiotensin II type 1 receptor blocker, ARB) and sacubitril (the neprilysin inhibitor). 8 In addition to the function of valsartan, Sac/Val inhibits the degradation of biologically active natriuretic peptides, which in turn stimulate natriuresis, diuresis and vasodilation. It has been shown that Sac/Val is superior to traditional renin‐angiotensin‐aldosterone system (RAAS) inhibitors in the treatment of cardiovascular diseases, such as reducing the incidences of major cardiovascular events and levels of biomarkers for heart failure in patients with heart failure (HF) and reduced ejection fraction HF (HFrEF). 9 , 10 , 11 Notably, several studies have shown that Sac/Val significantly alleviated cardiac remodelling in myocardial infarction or AF animal models 12 , 13 , 14 and AF patients. 15 However, whether Sac/Val provides more beneficial effects than ARBs in reversing atrial remodelling and reducing AF recurrence after RFCA remains unclear.
Materials and methods
Patients
A cohort of 76 consecutive patients that had undergone their first AF ablation procedure was prospectively recruited. Included individuals were ≥18 years of age, and were diagnosed with paroxysmal or persistent AF. Paroxysmal AF was defined as self‐terminating episodes of AF lasting <7 days. Persistent AF was defined as AF sustained ≥7 days, and/or requiring electrical or pharmacological cardioversion. 16 Patients were excluded if they had any contraindications to Sac/Val or valsartan; were pregnant; had any contraindications to RFCA; systolic blood pressure less than 100 mmHg; had a history of angioedema; had experienced a stroke, myocardial infarction (MI), or peripheral artery disease requiring intervention in the 6 months prior to screening; hyperthyroidism; had decompensated heart failure or gastrointestinal bleeding within 4 weeks prior to the intervention; had life‐expectancy <12 months. This study was reviewed and approved by the ethics committee at Union Hospital, Tongji Medical Collage, Huazhong University of Science and Technology, and was conducted in accordance with the Declaration of Helsinki and its amendments. The patients/participants provided their written informed consent to participate in this study prior to the procedures.
Study design
This was a single‐centre, randomized, open‐labelled study to compare the effects of Sac/Val with valsartan on atrial remodelling and AF recurrence in AF patients that had undergone RFCA. Information for demographic characteristics, medical history, physical examination, routine blood test and transthoracic echocardiography were collected at baseline. Transesophageal echocardiography was performed to rule out intra‐cardiac thrombus. Immediately after AF ablation, patients were randomized 1:1 to Sac/Val or valsartan treatment group using an online random generator (www.random.org), receiving Sac/Val (200 mg/day) or Valsartan (160 mg/day) in addition to other standard treatment of AF, respectively.
Echocardiography
Cardiac structure and function were assessed by 2‐dimensional echocardiography during screening and week 24. Left and right atrial internal diameter, left atrial volume index, left and right ventricular end‐diastolic diameter and ejection fraction were recorded or calculated. Echocardiograms were obtained at the cardiac imaging department according to a standardized protocol 17 and reviewed in a blinded fashion.
Electrophysiological study and atrial fibrillation ablation
All the procedures were performed using radiofrequency energy and a 3D navigation system (CARTO 3; Biosense‐Webster/NAVX; Abbott medical). The detailed procedure of AF ablation was provided in the Supporting Information.
Post‐ablation treatment and follow‐up
Twelve‐lead surface electrocardiogram (ECG) was used to confirm sinus rhythm after RFCA. Oral anticoagulation was started the day after RFCA. Antiarrhythmic drug therapy was not prescribed. After hospital discharge, all patients were scheduled in the outpatient department at 4, 12, and 24 weeks after RFCA. They were asked for any symptoms of AF, documented arrhythmia recurrences and current medication. Echocardiography and ambulatory Holter monitoring for 24 h were performed 24 weeks after RFCA during follow‐up. An AF episode lasting longer than 30 s outside a blanking period of 3 months after RFCA was considered as recurrent AF.
Statistical analysis
The primary end point of this study was the change of atrial diameter from baseline to 24 weeks after RFCA. Second and exploratory end points included the recurrence rate of AF, all‐cause hospitalization and all‐cause death. Statistical analysis was performed using SPSS (Version 17.0, SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as mean ± standard deviation; while categorical data were presented as absolute values and percentages. Statistically significant differences were determined using a student's t‐test or a Chi‐square test, as appropriate. A P value of 0.05 was considered as statistically significant.
Results
Characteristics of patients
A total of 76 patients were enrolled in the study, 12 of whom was discontinued after randomization due to various reasons (Figure 1 ). Among the rest 64 patients, 32 received Sac/Val and 32 received valsartan treatment, in addition to the standard treatment of AF after ablation. The mean age of the population was 64.3 ± 9.3 years and 54.7% were male, with no significant differences between the two groups. The percentage of persistent AF was comparable between valsartan and Sac/Val treatment groups (43.8% vs. 53.1%, P = 0.617). There is no difference in the mean heart rate, systolic or diastolic blood pressure upon admission between the two groups (Table 1 ).
Figure 1.
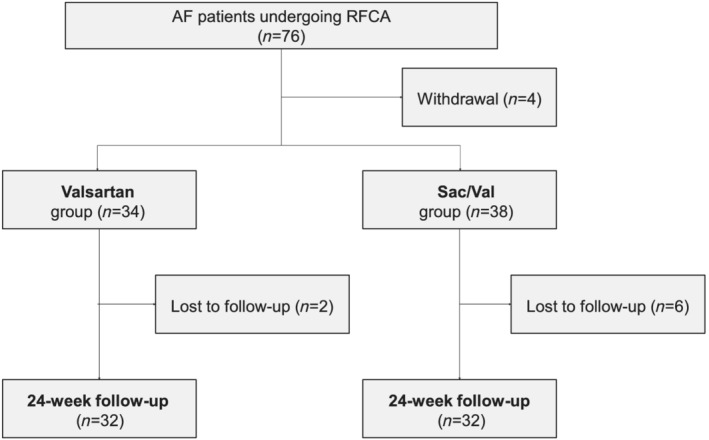
Study flowchart. AF, atrial fibrillation; RFCA, radiofrequency catheter ablation; Sac/Val, Sacubitril/Valsartan.
Table 1.
Demographic and clinical characteristics of the patients at baseline
| Variable | Valsartan (n = 32) | Sac/Val (n = 32) | P value* |
|---|---|---|---|
| Demographics | |||
| Age (years) | 64.8 ± 9.8 | 63.7 ± 9.0 | 0.634 |
| Male gender (%) | 19/32 (59.4) | 16/32 (50.0) | 0.616 |
| BMI (kg/m2) | 23.8 ± 3.8 | 24.3 ± 3.4 | 0.582 |
| Persistent AF (%) | 14/32 (43.8) | 17/32 (53.1) | 0.617 |
| History | |||
| Hypertension (%) | 19/32 (59.4) | 20/32 (62.5) | >0.999 |
| Diabetes mellitus (%) | 6/32 (18.8) | 6/32 (18.8) | >0.999 |
| Hypercholesterolemia (%) | 11/32 (34.4) | 6/32 (18.8) | 0.257 |
| Chronic Heart Failure (%) | 10/32 (31.3) | 11/32 (34.4) | 0.788 |
| History of MI (%) | 2/32 (6.3) | 3/32 (9.4) | >0.999 |
| Prior PCI/CABG (%) | 2/32 (6.3) | 3/32 (9.4) | >0.999 |
| PVD (%) | 17/32 (53.1) | 13/32 (40.6) | 0.616 |
| Stroke (%) | 5/32 (15.6) | 3/32 (9.4) | 0.709 |
| COPD (%) | 5/32 (15.6) | 7/32 (21.9) | >0.999 |
| CKD (%) | 9/32 (28.1) | 8/32 (25.0) | >0.999 |
| Data on hospital admission | |||
| Heart rate (b.p.m.) | 84.7 ± 4.1 | 80.9 ± 2.6 | 0.428 |
| Systolic BP (mmHg) | 127.5 ± 15.4 | 130.0 ± 17.8 | 0.549 |
| Diastolic BP (mmHg) | 81.7 ± 9.8 | 79.9 ± 12.6 | 0.537 |
| Haemoglobin (g/L) | 127.7 ± 15.1 | 127.3 ± 20.2 | 0.939 |
| Creatinine (mg/dL) | 76.5 ± 21.3 | 75.8 ± 15.1 | 0.893 |
| eGFR (mL/min/1.73 m2) | 81.9 ± 21.6 | 80.7 ± 22.3 | 0.823 |
| Serum potassium (mmol/L) | 3.8 ± 0.5 | 4.0 ± 0.3 | 0.095 |
b.p.m., beats per minute; BMI, body mass index; BP, blood pressure; CABG, coronary artery bypass grafting; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; MI, myocardial infarction; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease.
P < 0.05, valsartan group versus Sac/Val group.
Atrial fibrillation ablation
Bidirectional block of PVI was achieved in all patients, and posterior box isolation (POBI) was achieved in 83.25% (14/17) persistent AF patients in Sac/Val group and in 78.57% (11/14) persistent AF patients in valsartan group. In addition, there was no significant difference between valsartan group and Sac/Val group in the rate of mitral isthmus (MI) bidirectional block or the cavotricuspid isthmus (CTI) bidirectional block (Supporting Information, Table S1 ).
Atrial structural remodelling
Upon admission, the cardiac structure (including LA diameter, LA volume index, RA diameter, as well as left and right ventricle end‐diastolic diameters) and function (ejection fraction) were comparable between valsartan and Sac/Val group (Table 2 ). Notably, in patients receiving Sac/Val treatment, a significant decrease in the left atrial diameter (4.3 ± 0.5 cm to 3.8 ± 0.5 cm, P < 0.001) and volume index (48.0 ± 6.4 mL/m2 to 41.7 ± 7.0 mL/m2, P < 0.001) from baseline to 24 weeks after RFCA was observed. This effect was not observed in valsartan treatment group. There is also a significant decrease in the RA diameter (from 4.4 ± 0.8 cm to 3.9 ± 0.7 cm, P = 0.017) in the Sac/Val group, but not in the valsartan group. This indicates Sac/Val is superior to valsartan in attenuating the atrial remodelling after AF ablation.
Table 2.
Echocardiographic parameters at baseline and 24 weeks
| Parameter | Baseline | 24 weeks | P value* | P value # | P value $ | ||
|---|---|---|---|---|---|---|---|
| Valsartan (n = 32) | Sac/Val (n = 32) | Valsartan (n = 32) | Sac/Val (n = 32) | ||||
| LA dimeter (cm) | 4.3 ± 0.5 | 4.3 ± 0.5 | 4.1 ± 0.4 | 3.8 ± 0.5 | 0.279 | <0.001 | 0.006 |
| RA dimeter (cm) | 4.2 ± 0.6 | 4.4 ± 0.8 | 4.2 ± 0.6 | 3.9 ± 0.7 | 0.951 | 0.017 | 0.053 |
| LAVi (mL/m2) | 46.8 ± 6.5 | 48.0 ± 6.4 | 45.3 ± 5.8 | 41.7 ± 7.0 | 0.356 | <0.001 | 0.027 |
| LVDd (cm) | 4.7 ± 0.6 | 4.9 ± 0.7 | 4.8 ± 0.5 | 4.9 ± 0.5 | 0.839 | >0.999 | 0.460 |
| RVDd (cm) | 3.6 ± 0.5 | 3.7 ± 0.7 | 3.6 ± 0.5 | 3.5 ± 0.5 | 0.963 | 0.244 | 0.565 |
| LVEF (%) | 60.6 ± 6.8 | 60.9 ± 6.6 | 62.8 ± 3.7 | 63.8 ± 5.0 | 0.115 | 0.054 | 0.332 |
LA, left atrial; RA, right atrial; LAVi, left atrial volume index; LVDd, left ventricle end‐diastolic diameter; LVEF, left ventricular ejection fraction; RVDd, right ventricle end‐diastolic diameter.
P < 0.05, valsartan group baseline versus 24 weeks after AF ablation.
P < 0.05, Sac/Val group baseline versus 24 weeks after AF ablation.
P < 0.05, valsartan group versus Sac/Val group at 24 weeks after AF ablation.
Atrial fibrillation recurrence
We also compared the AF recurrence rate in the Sac/Val and valsartan only groups (Table 3 ). There was a numerical decrease in AF recurrence rate in the Sac/Val group compared with valsartan group (9.4% vs. 15.6%), although this difference did not reach a statistical significance (P = 0.708). The rate for all‐cause hospitalization and all‐cause death were similar in both groups.
Table 3.
Study end points at 24 weeks
| Outcome | Valsartan (n = 32) | Sac/Val (n = 32) | P value* |
|---|---|---|---|
| AF recurrence rate (%) | 5/32 (15.6) | 3/32 (9.4) | 0.708 |
| All‐cause hospitalizations (%) | 2/32 (6.3) | 2/32 (6.3) | >0.999 |
| All‐cause death (%) | 0/32 | 0/32 | N/A |
AF, atrial fibrillation; N/A, not applicable.
P < 0.05, valsartan group versus Sac/Val group.
Blood pressure and side effects
The blood pressure 24 weeks after ablation was similar between Sac/Val and valsartan treatment groups (systolic: 116.9 ± 14.1 mmHg vs. 119.0 ± 13.6 mmHg, P = 0.556; diastolic: 75.7 ± 12.5 mmHg vs. 78.2 ± 8.9 mmHg, P = 0.362). Three participants in each group reported symptomatic hypotension during 1‐month follow‐up and Sac/Val or valsartan treatment was discontinued afterwards. Other side effects, which include vertigo, asthenia, cutaneous allergy and gastrointestinal disorder, were not reported in any of the participants.
Discussion
In this study, we demonstrated that Sac/Val treatment, but not valsartan, reduced left and right atrial diameter and volume index in RFCA‐treated AF patients. To the best of our knowledge, this is the first study that showed Sac/Val was superior to valsartan in reversing atrial structural remodelling in AF patients receiving RFCA. This indicated additional clinical benefits of the dual‐acting ARB and neprilysin inhibitor in the treatment of AF.
The upstream pharmacotherapies for AF aim to reverse or arrest the maladaptive pathophysiological processes that lead to atrial remodelling, 18 , 19 which are believed to be beneficial for reducing the onset or recurrence of AF. The most widely accepted upstream pharmacotherapies so far are RAAS inhibitors, mainly ACEIs and ARBs. While increasing evidence have reported the consistent reduction in new‐onset AF with RAAS inhibitors, 20 , 21 the role in the prevention of AF recurrence turns out to be much less impressive. Although some small prospective studies demonstrate that treatment with ACEIs along 22 or in combination with amiodarone 23 result in a statistically significant reduction in the recurrence of AF, the large prospective GISSI‐AF clinical trial suggests that treatment with valsartan is not associated with a reduction in the incidence of recurrent AF or the reversal of left atrial remodelling. 24 , 25 This is consistent with the findings in our study, suggesting the urgent need of novel agents in the upstream pharmacotherapies for AF.
The superior effect of Sac/Val to ACEIs or ARBs on cardiac remodelling has been demonstrated in patients with various cardiovascular diseases. AF patients who had received Sac/Val treatment exhibited better left atrial function than those with valsartan, as evidenced by the increased LA peak systolic strain, left atrial appendage (LAA) emptying flow velocity and LAA ejection fraction. 15 In patients with hypertension, Sac/Val treatment resulted in a greater reduction LV mass index, an indicator for ventricular hypertrophy and remodelling, as compared with olmesartan. 26 This was in part attributable to the greater reduction in blood pressure. In patients with HFrEF, greater reductions were observed with Sac/Val (compared with enalapril) in left atrial volume index, left ventricular end‐systolic and end‐diastolic volume indexes. 27 These findings are in line with our data that Sac/Val is superior to ARB in attenuating cardiac remodelling, although in different cardiovascular settings.
To uncover the underlying molecular mechanisms by which Sac/Val regulates atrial remodelling is not feasible in clinical studies due to technical limit, but several animal studies have provided some hints. In a rabbit rapid atrial pacing model, Li et. al, demonstrated that Sac/Val alleviates atrial electrical remodelling as evidenced by reduced AF inducibility and restoration of atrial effective refractory period (AERP), as compared with vehicle. 12 This is likely due to the attenuated calcium overload and alleviated current density reduction of ICa−L in a calcineurin/NFAT dependent way. More importantly, Sac/Val provides more beneficial effects than valsartan alone in attenuating atrial fibrosis and susceptibility to AF. 15 , 28 In both studies, Sac/Val treatment group exhibited less distorted LA architecture, reduced deposition of collagen, attenuated distribution and expression of fibrotic protein markers, and decreased atrial arrhythmias inducibility, compared with valsartan. This effect is partly attributable to the inhibition of p‐Smad2/3, p‐p38 mitogen activated protein kinase (MAPK), and p‐JNK pathways. Collectively, these data explain our clinical findings that Sac/Val is superior to valsartan in attenuating atrial remodelling.
Study limitation
A conclusion cannot be drawn whether Sac/Val results in a greater reduction in the AF recurrence rate than valsartan. Although there was a numerical decrease in the Sac/Val group, a statistical significance was not reached. This may be due to the small sample size or the relatively short follow‐up period. Further studies are needed to clarify the role of Sac/Val in reducing AF recurrence.
In conclusion, Sac/Val administration significantly reversed atrial remodelling of RFCA‐treated AF patients, which was absent in the valsartan treatment group. This indicates additional clinical benefit of the dual‐acting ARB and neprilysin inhibitor in the treatment of AF after RFCA. Further clinical trials with large sample size are warranted to investigate whether Sac/Val reduces the AF recurrence rate after ablation.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81600187 to M. Z. and 81800891 to N. W.).
Conflict of interest
None declared.
Supporting information
Table S1. Procedural data and electrophysiological findings.
Yang, L. , Zhang, M. , Hao, Z. , Wang, N. , and Zhang, M. (2022) Sacubitril/valsartan attenuates atrial structural remodelling in atrial fibrillation patients. ESC Heart Failure, 9: 2428–2434. 10.1002/ehf2.13937.
Contributor Information
Nan Wang, Email: 117562072@qq.com.
Min Zhang, Email: zm429800@163.com.
References
- 1. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: A global burden of disease 2010 study. Circulation. 2014; 129: 837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shi LZ, Heng R, Liu SM, Leng FY. Effect of catheter ablation versus antiarrhythmic drugs on atrial fibrillation: A meta‐analysis of randomized controlled trials. Exp Ther Med. 2015; 10: 816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tilz RR, Heeger CH, Wick A, Saguner AM, Metzner A, Rillig A, Wohlmuth P, Reissmann B, Lemeš C, Maurer T, Santoro F, Riedl J, Sohns C, Mathew S, Kuck KH, Ouyang F. Ten‐year clinical outcome after circumferential pulmonary vein isolation utilizing the Hamburg approach in patients with symptomatic drug‐refractory paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2018; 11: e005250. [DOI] [PubMed] [Google Scholar]
- 4. Casaclang‐Verzosa G, Gersh BJ, Tsang TS. Structural and functional remodeling of the left atrium: Clinical and therapeutic implications for atrial fibrillation. J Am Coll Cardiol. 2008; 51: 1–11. [DOI] [PubMed] [Google Scholar]
- 5. Sohns C, Sohns JM, Vollmann D, Lüthje L, Bergau L, Dorenkamp M, Zwaka PA, Hasenfuß G, Lotz J, Zabel M. Left atrial volumetry from routine diagnostic work up prior to pulmonary vein ablation is a good predictor of freedom from atrial fibrillation. Eur Heart J Cardiovasc Imaging. 2013; 14: 684–691. [DOI] [PubMed] [Google Scholar]
- 6. Berruezo A, Tamborero D, Mont L, Benito B, Tolosana JM, Sitges M, Vidal B, Arriagada G, Méndez F, Matiello M, Molina I, Brugada J. Pre‐procedural predictors of atrial fibrillation recurrence after circumferential pulmonary vein ablation. Eur Heart J. 2007; 28: 836–841. [DOI] [PubMed] [Google Scholar]
- 7. Zhuang J, Wang Y, Tang K, Li X, Peng W, Liang C, Xu Y. Association between left atrial size and atrial fibrillation recurrence after single circumferential pulmonary vein isolation: A systematic review and meta‐analysis of observational studies. Europace. 2012; 14: 638–645. [DOI] [PubMed] [Google Scholar]
- 8. Voors AA, Dorhout B, van der Meer P. The potential role of valsartan + AHU377 (LCZ696) in the treatment of heart failure. Expert Opin Investig Drugs. 2013; 22: 1041–1047. [DOI] [PubMed] [Google Scholar]
- 9. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau J, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM‐HF Committees and Investigators . Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin‐converting enzyme inhibition in patients with chronic systolic heart failure: Rationale for and design of the prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM‐HF). Eur J Heart Fail. 2013; 15: 1062–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 11. Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E, PIONEER‐HF Investigators . Angiotensin‐neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019; 380: 539–548. [DOI] [PubMed] [Google Scholar]
- 12. Li LY, Lou Q, Liu GZ, Lv JC, Yun FX, Li TK, Yang W, Zhao HY, Zhang L, Bai N, Zhan CC, Yu J, Zang YX, Li WM. Sacubitril/valsartan attenuates atrial electrical and structural remodelling in a rabbit model of atrial fibrillation. Eur J Pharmacol. 2020; 881: 173120. [DOI] [PubMed] [Google Scholar]
- 13. von Lueder TG, Wang BH, Kompa AR, Huang L, Webb R, Jordaan P, Atar D, Krum H. Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circ Heart Fail. 2015; 8: 71–78. [DOI] [PubMed] [Google Scholar]
- 14. Torrado J, Cain C, Mauro AG, Romeo F, Ockaili R, Chau VQ, Nestler JA, Devarakonda T, Ghosh S, Das A, Salloum FN. Sacubitril/valsartan averts adverse post‐infarction ventricular remodeling and preserves systolic function in rabbits. J Am Coll Cardiol. 2018; 72: 2342–2356. [DOI] [PubMed] [Google Scholar]
- 15. Suo Y, Yuan M, Li H, Zhang Y, Li Y, Fu H, Han F, Ma C, Wang Y, Bao Q, Li G. Sacubitril/valsartan improves left atrial and left atrial appendage function in patients with atrial fibrillation and in pressure overload‐induced mice. Front Pharmacol. 2019; 10: 1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom‐Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL, ESC Scientific Document Group . 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS): The task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2020; 42: 373–498. [DOI] [PubMed] [Google Scholar]
- 17. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ, Chamber Quantification Writing Group, American Society of Echocardiography's Guidelines and Standards Committee, European Association of Echocardiography . Recommendations for chamber quantification: A report from the American Society of Echocardiography's guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005; 18: 1440–1463. [DOI] [PubMed] [Google Scholar]
- 18. Savelieva I, Kakouros N, Kourliouros A, Camm AJ. Upstream therapies for management of atrial fibrillation: Review of clinical evidence and implications for European Society of Cardiology guidelines. Part II: Secondary prevention. Europace. 2011; 13: 610–625. [DOI] [PubMed] [Google Scholar]
- 19. Savelieva I, Kakouros N, Kourliouros A, Camm AJ. Upstream therapies for management of atrial fibrillation: Review of clinical evidence and implications for European Society of Cardiology guidelines. Part I: Primary prevention. Europace. 2011; 13: 308–328. [DOI] [PubMed] [Google Scholar]
- 20. Healey JS, Baranchuk A, Crystal E, Morillo CA, Garfinkle M, Yusuf S, Connolly SJ. Prevention of atrial fibrillation with angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers: A meta‐analysis. J Am Coll Cardiol. 2005; 45: 1832–1839. [DOI] [PubMed] [Google Scholar]
- 21. Kumagai K, Nakashima H, Urata H, Gondo N, Arakawa K, Saku K. Effects of angiotensin II type 1 receptor antagonist on electrical and structural remodeling in atrial fibrillation. J Am Coll Cardiol. 2003; 41: 2197–2204. [DOI] [PubMed] [Google Scholar]
- 22. Belluzzi F, Sernesi L, Preti P, Salinaro F, Fonte ML, Perlini S. Prevention of recurrent lone atrial fibrillation by the angiotensin‐II converting enzyme inhibitor ramipril in normotensive patients. J Am Coll Cardiol. 2009; 53: 24–29. [DOI] [PubMed] [Google Scholar]
- 23. Madrid AH, Bueno MG, Rebollo JM, Marín I, Peña G, Bernal E, Rodriguez A, Cano L, Cano JM, Cabeza P, Moro C. Use of irbesartan to maintain sinus rhythm in patients with long‐lasting persistent atrial fibrillation: A prospective and randomized study. Circulation. 2002; 106: 331–336. [DOI] [PubMed] [Google Scholar]
- 24. GISSI‐AF Investigators , Disertori M, Latini R, Barlera S, Franzosi MG, Staszewsky L, Maggioni AP, Lucci D, Di Pasquale G, Tognoni G. Valsartan for prevention of recurrent atrial fibrillation. N Engl J Med. 2009; 360: 1606–1617. [DOI] [PubMed] [Google Scholar]
- 25. Staszewsky L, Wong M, Masson S, Raimondi E, Gramenzi S, Proietti G, Bicego D, Emanuelli C, Pulitano G, Taddei F, Nicolis EB, Correale E, Fabbri G, Bertocchi F, Franzosi MG, Maggioni AP, Tognoni G, Disertori M, Latini R, GISSI‐AF Investigators . Left atrial remodeling and response to valsartan in the prevention of recurrent atrial fibrillation: The GISSI‐AF echocardiographic substudy. Circ Cardiovasc Imaging. 2011; 4: 721–728. [DOI] [PubMed] [Google Scholar]
- 26. Schmieder RE, Wagner F, Mayr M, Delles C, Ott C, Keicher C, Hrabak‐Paar M, Heye T, Aichner S, Khder Y, Yates D, Albrecht D, Langenickel T, Freyhardt P, Janka R, Bremerich J. The effect of sacubitril/valsartan compared to olmesartan on cardiovascular remodelling in subjects with essential hypertension: The results of a randomized, double‐blind, active‐controlled study. Eur Heart J. 2017; 38: 3308–3317. [DOI] [PubMed] [Google Scholar]
- 27. Desai AS, Solomon SD, Shah AM, Claggett BL, Fang JC, Izzo J, McCague K, Abbas CA, Rocha R, Mitchell GF, EVALUATE‐HF Investigators . Effect of Sacubitril‐valsartan vs Enalapril on aortic stiffness in patients with heart failure and reduced ejection fraction: A randomized clinical trial. JAMA. 2019; 322: 1077–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li SN, Zhang JR, Zhou L, Xi H, Li CY, Zhao L. Sacubitril/valsartan decreases atrial fibrillation susceptibility by inhibiting angiotensin II‐induced atrial fibrosis through p‐Smad2/3, p‐JNK, and p‐p38 signaling pathways. J Cardiovasc Transl Res. 2022; 15: 131–142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Procedural data and electrophysiological findings.


