Abstract
Aim
The diagnosis of heart failure (HF) has been refined in several steps in recent years, reflecting evolving diagnostic and therapeutic approaches. The European Society of Cardiology (ESC) recently published a modified definition of HF in the 2021 heart failure (HF) guidelines. The impact of this new diagnostic algorithm on the prevalence of HF is not known. The aim of this study was to describe the contemporary prevalence of HF in a representative, completely phenotyped sample from the general population.
Methods and results
This analysis was conducted among 7074 participants (aged 45–78 years, 51.5% women) from the population‐based Hamburg City Health Study. Compared with the 2016 version, HF prevalence increased with the 2021 HF guidelines from 4.31% to 4.83% (12% increase). This increase was driven by a higher number of subjects with HF with reduced/mildly‐reduced ejection fraction (0.47% to 0.52%; 1.37% to 2.12%), while the number of subjects with HF with preserved ejection fraction decreased from 2.46% to 2.19%. Importantly, this did not impact the known risk factor profiles of the phenotypes. Although four drugs are recommended for all subjects with HFrEF in the new guidelines, several adjunctive therapies are recommended for dedicated cases/scenarios (e.g. <1% eligibility for ivabradine/vericiguat/devices).
Conclusion
Heart failure remains common in a contemporary general population sample. The number of patients with HF will increase when the current diagnostic criteria are applied. This offers opportunities to initiate preventive therapies, especially in patients with HFmrEF and HFrEF.
Keywords: 2016 ESC heart failure guidelines, 2021 ESC heart failure guidelines, HFpEF, HFrEF, Diagnosis
Introduction
The prevalence of failure (HF) prevalence is increasing in ageing populations. 1 , 2 At least 26 million people are affected worldwide, making it a disease of pandemic outreach with severe medical and economic consequences. 3 , 4
Nevertheless, most data on HF prevalence originate from older population base studies and electronic health record analyses using simple diagnostic criteria. The availability of new, evidence‐based therapies for heart failure with reduced and mildly reduced ejection fraction, and the diagnostic criteria used in the evidence‐generating studies, led to newer, more precise definitions of heart failure. The most recent diagnostic approach for heart failure was published in the European Society of Cardiology (ESC) 2021 guidelines for the diagnosis and treatment of chronic heart failure. 5 It is unclear whether these revised diagnostic criteria change the prevalence of heart failure in contemporary populations.
Therefore, it was the aim of our study to apply the 2021 ESC HF guidelines in a representative sample from the general population and to compare the prevalence of heart failure and its subtypes to other diagnostic criteria. Thereby, we sought to provide a reliable description of prevalence, characteristics, and eligibility for treatment of the different HF phenotypes in the population.
Methods
This study and the Hamburg City Health Study (HCHS, www.uke.de/hchs) were conducted in agreement with the Declaration of Helsinki. HCHS was approved by the local ethics committee (PV5131, State of Hamburg Chamber of Medical Practitioners), and this study was approved by the review board of the HCHS. All participants provided written informed consent.
Setting
This study was conducted within the framework of the HCHS. HCHS is an ongoing, prospective, population‐based cohort study, aiming to enrol 45 000 randomly selected subjects from the metropolitan area of the city of Hamburg, Germany. 6 Recruitment is ongoing. All subjects are invited for a baseline study visit at the HCHS Epidemiological Study Centre at the University Medical Centre Hamburg‐Eppendorf following the published HCHS protocol. The baseline visit includes a detailed clinical examination, laboratory assessment and imaging [including transthoracic echocardiography (TTE)]. 6
This study is based on the data from the first 10 000 subjects enrolled in HCHS between 2016 and 2019. Among these first 10 000 subjects, those with sufficient image quality for standardized assessment of cardiac function (e.g. left ventricular ejection fraction and diastolic function) and with available clinical and laboratory parameters for HF classification were considered for this study (Figure 1 ).
Figure 1.
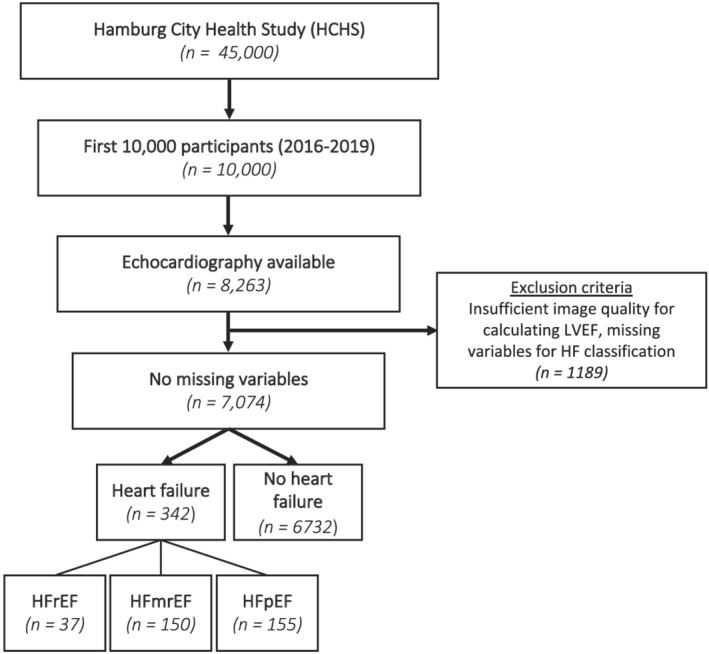
Study PRISMA. From a total of 8263 subjects with available TTE examination, 1189 were excluded due to insufficient echocardiographic image quality or missing variables for HF classification. Following the 2021 ESC HF guidelines 342 participants were classified in the HF group. Of those, 37 were classified as HFrEF, 150 as HFmrEF, and 155 as HFpEF. ESC, European Society of Cardiology; LVEF, left ventricular ejection fraction; HCHS, Hamburg City Health Study; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; TTE, transthoracic echocardiography.
Baseline parameters
Demographic and clinical parameters were either assessed via questionnaires (e.g. self‐reported) or via standardized interviews; and all these variables were obtained based on standard operating procedures and by trained medical personnel. 6 Dyspnoea was evaluated by a standardized self‐reported questionnaire which is provided in the supplements. Oedema were evaluated at the level of the lower leg based on physical examination by medical professionals. By applying pressure on the affected area it was checked whether pitting oedema were present. A discrimination in different grades was not performed.
All TTE examinations were performed and analysed by professional trained cardiologists and sonographers (technicians) at the baseline visit on dedicated ultrasound machines (Siemens Acuson SC2000 Prime, Siemens Healthineers, Erlangen, Germany) following standard operating procedures at the HCHS Epidemiological Study Center Hamburg‐Eppendorf, Hamburg, Germany. 6 This included assessment of left ventricular ejection fraction and diastolic function based on two‐dimensional echocardiography. Based on these exams, image analyses were performed separately on an off‐line workstation. All TTE standard views were assessed in two‐dimensional echocardiography, including a three‐dimensional four‐chamber view for chamber quantification. For continuous quality assessment, every 100th TTE exam was analysed twice by an ESC TTE certified cardiologist. Qualitative and quantitative image analyses were performed using an off‐line workstation with the commercially available and established Siemens syngo SC2000 software (Siemens syngo SC 2000 Version 4.0, Siemens Healthineers, Erlangen, Germany) in agreement with the current recommendations of the American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI). 7 , 8 Left sided volumes and ejection fraction (LVEF) were calculated from two‐dimensional and three‐dimensional loops using the method of disks summation. Left‐sided diameters were measured in parasternal long‐axis view. Mitral inflow pattern was assessed in apical four‐chamber view by placing pulsed‐wave (PW) Doppler sample volume between mitral leaflet tips. PW tissue Doppler imaging (TDI) e′ velocity was measured in apical four‐chamber view by placing the sample volume at the lateral and septal basal regions. Tricuspid annular plane systolic excursion (TAPSE) was obtained by M‐mode echocardiography in the apical four‐chamber view. Right ventricular fractional area change was assessed in a right ventricular focused four‐chamber view.
At the baseline visit, blood was obtained from all subjects for measurement of biomarkers. N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) was measured using an immunoassay (Alere NT‐proBNP for ARCHITECT, Abbott Diagnostics, IL) with a measurement range between 8.2–35 000 pg/mL.
Heart failure classification
The HF classification was based on the recent 2021 ESC guideline on HF, and its predecessor from 2016 as a comparator (Supporting Information, Figures S1 and S2 ), using a combination of clinical signs, echocardiographic alterations, and NT‐proBNP concentrations. 25
In general, HF was considered present if subjects showed symptoms/signs (e.g. dyspnoea or oedema) as well as echocardiographic criteria and/or elevated NT‐proBNP.
Heart failure was then further divided into the phenotypes HFrEF, HFmrEF, and HF with preserved ejection fraction (HFpEF). When LVEF was <41% and symptoms or signs of HF were present, subjects were classified in the HFrEF group. The combination of a LVEF of 41–49% and symptoms or signs of HF led to the classification into the HFmrEF group. Subjects were classified as having HFpEF if they presented with preserved LVEF (LVEF ≥50%), symptoms or signs of HF, and either at least two or more echocardiographic signs of cardiac structural of functional abnormalities or the combination of NT‐proBNP levels exceeding 125 pg/mL (sinus rhythm) or 365 pg/mL (atrial fibrillation) and at least one or more echocardiographic signs of cardiac structural or functional abnormalities. Echocardiographic signs of cardiac structural or functional abnormalities were defined as: left ventricular hypertrophy: LV mass indexed to body surface area (BSA) ≥95 g/m2 for women, ≥115 g/m2 for men, left atrial enlargement: defined as left atrial volume index >34 mL/m2 (sinus rhythm) and >40 mL/m2 (atrial fibrillation), E/e′ ratio >9, and tricuspid regurgitation velocity (Vmax) > 2.8 m/s.
Subjects without signs/symptoms were only considered as having HF if they either had a history of HF or were on guideline‐directed medical therapy (GDMT). GDMT included for HFrEF and HFmrEF beta‐blockers, ACE‐inhibitors (ACEi), angiotensin receptor blockers (ARB), angiotensin receptor neprilysin inhibitors (ARNI), mineralocorticoid receptor antagonists (MRA), SGLT2 inhibitors, and loop diuretics, while for HFpEF only SGLT2 inhibitors and loop diuretics were defined as medication.
Subjects with LVEF <50%, but no symptoms or signs of HF were considered to have asymptomatic left ventricular systolic dysfunction (ALVSD).
Statistical analysis
Continuous variables are presented as median and interquartile range, and categorical variables are presented as absolute numbers and percentages. Subjects with missing data were excluded when calculating percentages. The concordance between the five classifications (HFrEF, HFmrEF, HFpEF, ALVSD, and no HF) was determined using Cohen's kappa coefficients and proportion of agreement. The concordance was defined as poor (0–0.20), fair (0.21–0.40), moderate (0.41–0.60), good (0.61–0.80), and optimal (0.81–1). The reclassification percentage was determined as: 100 − proportion of agreement. Data analysis was performed using R version 4.0.3.
Results
Study cohort
Among the first 10 000 subjects enrolled into HCHS, 7074 were included in this analysis (Figure 1 ). The 7074 subjects represented a middle‐aged European population with 3642 (51.5%) women, a median age of 62 (IQR: 55–69) years and a median body mass index (BMI) of 25.9 [IQR: 23.4–28.9] kg/m2 (Figure 1 , Table 1 ). Hypertension was present in 4339 (64.4%) subjects, 530 (8.1%) had diabetes, 431 (6.5%) coronary artery disease (CAD), and 381 (6.0%) showed atrial fibrillation (AF). The median NT‐proBNP was 77.0 [IQR: 43.0–137.0] pg/mL, and the median LVEF was 58.5% [IQR: 55.6–61.9].
Table 1.
Baseline characteristics of the study population
| All | No HF | ALVSD | HFrEF | HFmrEF | HFpEF | |
|---|---|---|---|---|---|---|
| n (%) | 7074 | 6653 | 79 | 37 | 150 | 155 |
| Demographics + biometrics | ||||||
| Age, years | 62 [55, 69] | 62 [55, 69] | 61 [54, 70] | 69 [65, 74] | 69 [63, 73] | 70 [65, 73] |
| Female | 3642 (51.5) | 3489 (52.4) | 26 (32.9) | 5 (13.5) | 40 (26.7) | 82 (52.9) |
| White ethnicity | 6949 (98.9) | 6535 (98.9) | 76 (96.2) | 35 (97.2) | 149 (99.3) | 154 (99.4) |
| Black ethnicity | 27 (0.4) | 25 (0.4) | 1 (1.3) | 0 (0.0) | 0 (0.0) | 1 (0.6) |
| Asian ethnicity | 50 (0.7) | 46 (0.7) | 2 (2.5) | 1 (2.8) | 1 (0.7) | 0 (0) |
| BMI, kg/m2 | 25.9 [23.4, 28.9] | 25.8 [23.4, 28.7] | 25.9 [23.5, 29.4] | 26.8 [24.7, 29.4] | 28.1 [25.6, 31.9] | 28.6 [24.7, 32.7] |
| SBP, mmHg | 136.5 [124.5, 149.8] | 136.5 [124.5, 149.5] | 135.2 [126.5, 152.1] | 128.5 [119.8, 139.2] | 140.5 [132.0, 152.0] | 144.0 [128.2, 158.2] |
| DBP, mmHg | 81.5 [75.5, 88.0] | 81.5 [75.5, 88.0] | 84.5 [78.8, 92.4] | 77.5 [69.2, 84.0] | 83.5 [76.5, 90.0] | 79.8 [71.4, 87.6] |
| Co‐morbidities + risk factors | ||||||
| Hypertension | 4339 (64.4) | 3981 (62.9) | 43 (55.1) | 36 (97.3) | 136 (94.4) | 143 (92.9) |
| Diabetes | 530 (8.1) | 441 (7.1) | 11 (14.1) | 9 (25.7) | 33 (22.6) | 36 (25.5) |
| Current smoking | 1413 (20.1) | 1339 (20.2) | 16 (20.3) | 8 (22.2) | 23 (15.3) | 27 (17.4) |
| Obesity (BMI > 25 kg/m2) | 1243 (18.7) | 1100 (17.6) | 16 (21.1) | 8 (22.9) | 55 (38.2) | 64 (41.6) |
| Coronary artery disease | 431 (6.5) | 315 (5.0) | 3 (3.8) | 19 (67.9) | 47 (34.3) | 47 (32.9) |
| COPD | 444 (6.9) | 395 (6.5) | 0 (0) | 3 (10.3) | 14 (10.9) | 32 (23.7) |
| OSAS | 373 (5.8) | 330 (5.4) | 2 (2.8) | 1 (3.6) | 18 (14.3) | 22 (16.1) |
| Peripheral artery disease | 206 (3.2) | 174 (2.8) | 4 (5.1) | 2 (7.4) | 9 (7.1) | 17 (11.7) |
| History of heart failure | 213 (3.0) | 124 (1.9) | 0 (0) | 17 (50) | 25 (17.4) | 47 (32.0) |
| Dyspnoea at | ||||||
| Severe physical activity | 416 (6.5) | 310 (5.1) | 0 (0) | 8 (28.6) | 31 (23.7) | 67 (48.6) |
| Mild physical activity | 154 (2.4) | 120 (2.0) | 0 (0) | 1 (3.6) | 12 (9.2) | 21 (15.2) |
| At rest | 69 (1.1) | 57 (0.9) | 0 (0) | 1 (3.6) | 2 (1.5) | 9 (6.5) |
| Oedema (lower leg) | 71 (1.7) | 55 (1.4) | 0 (0) | 2 (13.3) | 3 (3.3) | 11 (12.6) |
| Dyspnoea and oedema | 15 (0.2) | 12 (0.2) | 0 (0) | 0 (0) | 1 (0.8) | 2 (2.1) |
| E/e′ > 9 | 1336 (20.5) | 1199 (19.4) | 15 (22.7) | 13 (46.4) | 43 (35.2) | 66 (47.5) |
| Medication + device therapy | ||||||
| Loop diuretics | 139 (2.1) | 76 (1.2) | 0 (0) | 9 (24.3) | 14 (9.5) | 40 (26.7) |
| Thiazide | 165 (2.4) | 140 (2.2) | 2 (2.5) | 2 (5.4) | 9 (6.1) | 12 (8.0) |
| Beta‐blocker | 1121 (16.6) | 927 (14.6) | 0 (0) | 28 (75.7) | 77 (52.4) | 89 (59.3) |
| ACE inhibitors/ARBs | 1391 (20.6) | 1226 (19.3) | 0 (0) | 21 (56.8) | 78 (53.1) | 66 (44.0) |
| ARNI | 3 (0) | 2 (0) | 0 (0) | 1 (2.7) | 0 (0) | 0 (0) |
| Ivabradin | 5 (0.1) | 4 (0.1) | 0 (0) | 1 (2.7) | 0 (0) | 0 (0) |
| MRAs | 48 (0.7) | 26 (0.4) | 0 (0) | 8 (21.6) | 6 (4.1) | 8 (5.3) |
| Vericiguat | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| SGLT2i | 10 (0.1) | 8 (0.1) | 0 (0) | 1 (2.7) | 0 (0) | 1 (0.7) |
| Hydralazine | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Isosorbide dinitrate | 3 (0) | 1 (0) | 0 (0) | 1 (2.7) | 0 (0) | 1 (0.7) |
| Digoxin/Digitoxin | 8 (0.1) | 2 (0) | 0 (0) | 1 (2.7) | 2 (1.4) | 3 (2.0) |
| ICD | 27 (0.5) | 18 (0.4) | 0 (0) | 2 (7.4) | 6 (5.1) | 1 (0.9) |
| Pacemaker | 23 (0.4) | 11 (0.2) | 0 (0) | 3 (9.1) | 4 (2.9) | 5 (3.3) |
| Laboratories | ||||||
| NT‐proBNP, pg/mL | 77.0 [43.0, 137.0] | 74.0 [42.0, 127.0] | 80.0 [40.0, 166.8] | 637.0 [239.5, 1638.5] | 189.0 [80.0, 460.2] | 280.5 [161.5, 616.2] |
| LDL, mg/dL | 121.0 [96.0, 146.0] | 122.0 [97.0, 146.0] | 117.0 [97.0, 148.0] | 98.0 [66.5, 118.8] | 104.5 [71.8, 132.2] | 105.0 [85.0, 133.2] |
| GFR, mL/min/1.73 m2 | 93.2 [83.9, 101.0] | 93.6 [84.6, 101.3] | 92.9 [83.8, 100.2] | 83.6 [66.5, 89.6] | 80.6 [67.7, 92.5] | 86.8 [68.9, 93.5] |
| Haemoglobin, g/dL | 14.3 [13.6, 15.1] | 14.3 [13.6, 15.1] | 14.7 [13.9, 15.6] | 15.0 [14.4, 15.3] | 14.6 [13.6, 15.4] | 13.9 [13.0, 14.9] |
| HbA1c, % | 5.5 [5.3, 5.8] | 5.5 [5.3, 5.8] | 5.6 [5.4, 5.8] | 5.7 [5.5, 6.3] | 5.8 [5.5, 6.2] | 5.7 [5.4, 6.1] |
Continuous variables are presented as median and interquartile range, and categorical variables are presented as absolute numbers and percentages.
ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin‐receptor neprilysin inhibitor; BMI, body mass index; DBP, diastolic blood pressure; DBP, diastolic blood pressure, GFR, glomerular filtration rate; ICD, implantable cardioverter‐defibrillator; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; OSAS, obstructive sleep apnoea; SGLT2i, Sodium‐glucose‐co‐transporter 2 inhibitor; SBP, systolic blood pressure.
Prevalence of heart failure and co‐morbidities
According to the definition provided in the 2021 ESC guidelines on acute and chronic heart failure, 5 342 (4.8%) subjects were diagnosed with HF (Figure 2 ). Among those, 37 (10.8%) had HFrEF, 150 (43.9%) HFmrEF, and 155 (45.3%) HFpEF; 79 (1.1%) of the study population had ALVSD. We observed major sex differences across the HF phenotypes, with only five (13.5%) female subjects were diagnosed with HFrEF, but 40 (26.7%) with HFmrEF and 80 (52.9%) with HFpEF (Figure 3 ). Arterial hypertension was present in most subjects with HF, irrespective of the underlying HF phenotype (92.9% in HFpEF, 94.4% in HFmrEF 97.3% in HFrEF). Obesity was most common among subjects with HFpEF (64 subjects, 41.6%). Seventy‐seven (24.1%) subjects with HF had diabetes, with only little variation between the HF phenotypes (25.5% in HFpEF, 22.6% in HFmrEF 26.5% in HFrEF). Atrial fibrillation was most prevalent among those with HFrEF (11 subjects, 33.3%), and the lowest prevalence was observed among those with HFmrEF (33 subjects, 24.1%). Subjects classified as HFrEF had the highest burden of CAD (19 subjects, 67.9%), moderate to severe mitral regurgitation (six subjects, 16.7%), aortic stenosis (three subjects, 9.7%), and aortic regurgitation (five subjects, 14.3%). Additionally, left bundle branch blocks were most common among HFrEF Subjects (41.9% vs. 10.6% in HFmrEF and 15.1% in HFpEF). HFpEF on the other hand was linked with the highest prevalence of chronic obstructive pulmonary disease (32 subjects, 23.7%), obstructive sleep apnoea syndrome (OSAS) (22 subjects, 16.1%), and peripheral artery disease (17 subjects, 11.7%). The highest NT‐proBNP levels with a median of 637 [IQR: 239.5–1638.5] pg/mL were measured in subjects with HFrEF, whereas those with HFmrEF had the lowest NT‐proBNP levels with 189.0 [IQR: 80.0–460.2] pg/mL. NT‐proBNP levels in subjects with HFpEF were 280.5 [IQR: 161.5–616.2] pg/mL. Accordingly, subjects with HFmrEF demonstrated the lowest levels of left ventricular hypertrophy with a median left ventricular mass index of 100.6 [IQR: 82.6–121.1] g/m2 (125.6 [IQR: 95.8–147.2] g/m2 in HFrEF and 111.4 [IQR: 97.6–128.8] g/m2 in HFpEF) and few echocardiographic signs of diastolic dysfunction, for example, left atrial volume index (28.3 [IQR: 22.7–36.5] mL/m2 vs. 35.0 mL/m2 [IQR: 28.0–45.0] mL/m2 in HFrEF and 38.5 [IQR: 32.3–46.1] mL/m2 in HFpEF) and E/e′ (7.7 [IQR: 6.6–10.0] vs. E/e′ (8.8 [IQR: 7.0–11.0] in HFrEF and E/e′ (8.8 [IQR: 7.3–11.2] in HFpEF).
Figure 2.
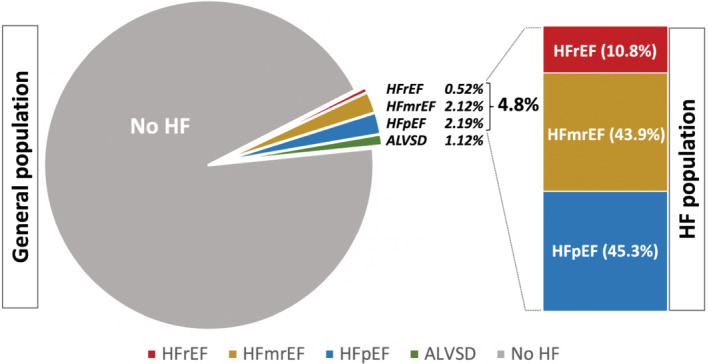
Prevalence of HF in the general population as well as the distribution of the different HF phenotypes. ALVSD, asymptomatic left ventricular systolic dysfunction; HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
Figure 3.
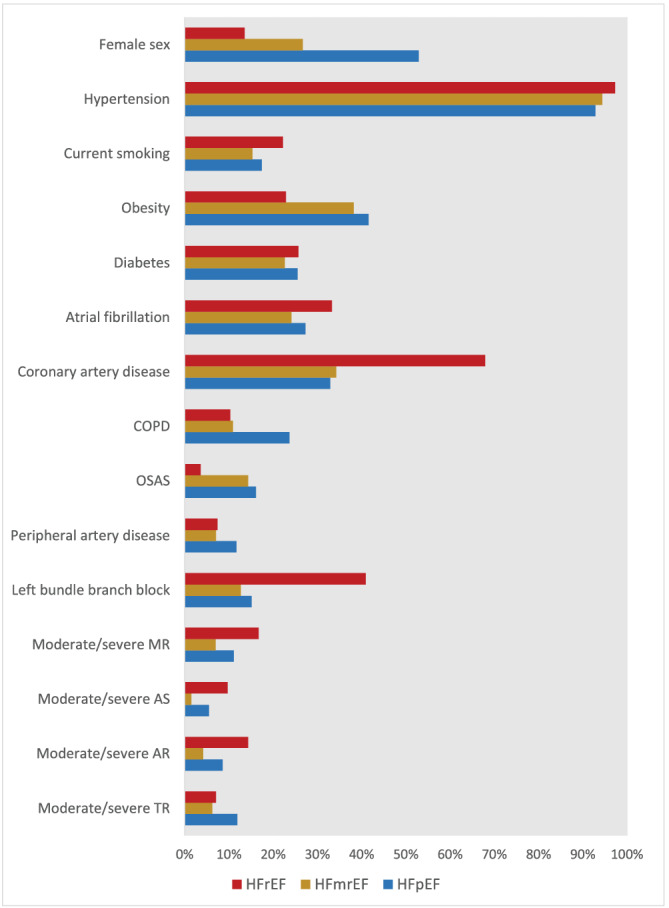
HF phenotypes and their different characteristics. ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin‐receptor neprilysin inhibitor; BMI, body mass index; DBP, diastolic blood pressure; DBP, diastolic blood pressure, GFR, glomerular filtration rate; ICD, implantable cardioverter‐defibrillator; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; OSAS, obstructive sleep apnoea; SGLT2i, Sodium‐glucose‐co‐transporter 2 inhibitor; SBP, systolic blood pressure.
Comparison of the 2016 and 2021 European Society of Cardiology Heart Failure Guidelines on acute and chronic heart failure
The application of the 2021 ESC HF guidelines led to a 12% net increase in HF prevalence from 4.31% according to the 2016 guidelines to 4.83% (Figure 4 ). Nevertheless, the proportion of the different HF phenotypes relevantly changed. There was a sharp increase of HFmrEF prevalence from 1.38% to 2.12% (54% increase) and a minor increase of HFrEF prevalence from 0.47% to 0.52%, while the amount of HFpEF subjects declined from 2.46% to 2.19% (Table 2 ).
Figure 4.
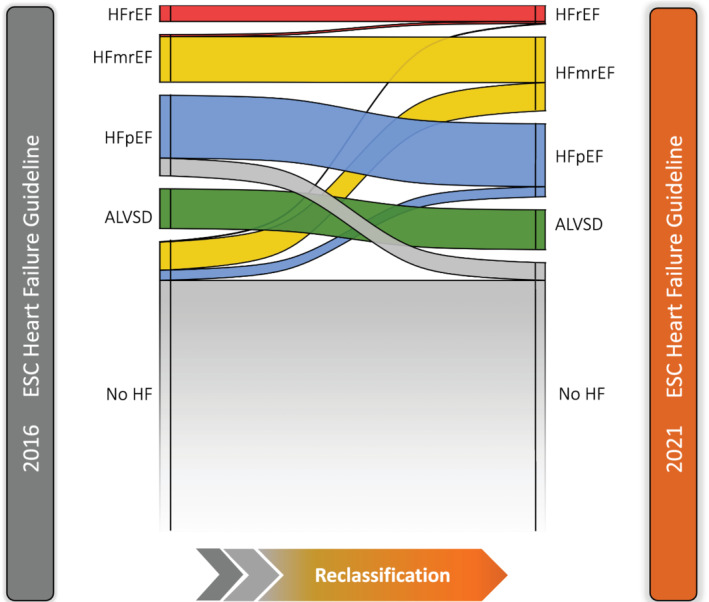
Reclassification of subjects into the different HF phenotypes based on the 2016 ESC vs the 2021 ESC HF guidelines. ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin‐receptor neprilysin inhibitor; BMI, body mass index; DBP, diastolic blood pressure; DBP, diastolic blood pressure, GFR, glomerular filtration rate; ICD, implantable cardioverter‐defibrillator; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; OSAS, obstructive sleep apnoea; SGLT2i, Sodium‐glucose‐co‐transporter 2 inhibitor; SBP, systolic blood pressure.
Table 2.
Electrocardiographic and echocardiographic characteristic of the different HF phenotypes
| All | No HF | ALVSD | HFrEF | HFmrEF | HFpEF | |
|---|---|---|---|---|---|---|
| n (%) | 7074 | 6653 | 79 | 37 | 150 | 155 |
| ECG | ||||||
| Heart rate, b.p.m. | 68.5 [62.0, 76.0] | 68.5 [62.0, 76.0] | 73.2 [65.0, 78.9] | 72.0 [65.0, 83.2] | 72.2 [62.5, 83.6] | 69.0 [59.5, 76.0] |
| PR interval, ms | 162.0 [148.0, 178.0] | 162.0 [148.0, 178.0] | 165.0 [154.0, 188.0] | 168.0 [151.0, 194.5] | 166.0 [148.0, 186.0] | 168.0 [150.0, 184.0] |
| QRS interval, ms | 92.0 [86.0, 100.0] | 92.0 [86.0, 98.0] | 94.0 [88.0, 104.0] | 112.0 [100.0, 142.0] | 96.0 [88.0, 108.0] | 95.0 [90.0, 108.0] |
| QTc interval, ms | 420.0 [406.0, 435.0] | 419.0 [405.0, 434.0] | 430.0 [416.0, 444.2] | 455.5 [436.0, 485.0] | 429.0 [412.0, 450.0] | 430.0 [413.0, 448.0] |
| LBBB | 290 (4.6) | 235 (3.9) | 5 (6.7) | 13 (41.9) | 14 (10.6) | 23 (15.1) |
| RBBB | 365 (5.7) | 336 (5.6) | 5 (6.7) | 3 (9.7) | 10 (7.6) | 11 (7.2) |
| LAH | 255 (4.0) | 220 (3.7) | 6 (8.0) | 4 (12.5) | 12 (9.0) | 13 (8.5) |
| AV blocks | 359 (5.1) | 317 (4.8) | 6 (7.5) | 4 (10.8) | 14 (9.3) | 18 (11.8) |
| Atrial fibrillation | 381 (6.0) | 290 (4.9) | 5 (6.7) | 11 (33.3) | 33 (24.1) | 42 (27.3) |
| Echocardiography | ||||||
| LVEF, % | 58.5 [55.6, 61.9] | 58.8 [56.0, 62.0] | 47.1 [44.3, 48.8] | 37.3 [29.2, 38.7] | 47.7 [45.0, 49.0] | 57.6 [55.2, 59.9] |
| LV mass index, g/m2 | 81.4 [71.3, 94.4] | 80.7 [70.8, 92.8] | 87.5 [75.6, 107.9] | 125.6 [95.8, 147.2] | 100.6 [82.6, 121.1] | 111.4 [97.6, 128.8] |
| LAVI | 24.9 [20.2, 30.1] | 24.7 [20.0, 29.6] | 21.6 [17.8, 30.1] | 35.0 [28.0, 45.0] | 28.3 [22.7, 36.5] | 38.5 [32.3, 46.1] |
| LVEDV, mL | 109.1 [91.2, 131.2] | 108.7 [91.1, 130.5] | 114.5 [90.7, 138.6] | 150.2 [115.2, 247.0] | 113.0 [94.7, 150.7] | 116.7 [95.4, 151.8] |
| LV lateral e′, mm/s | 10.3 [8.4, 12.3] | 10.3 [8.5, 12.4] | 9.2 [7.3, 11.3] | 8.0 [6.1, 10.7] | 8.6 [6.8, 11.2] | 9.7 [7.8, 11.4] |
| LV septal e′, mm/s | 8.5 [7.0, 10.2] | 8.5 [7.1, 10.2] | 7.6 [6.4, 9.7] | 7.0 [5.5, 8.1] | 7.1 [5.9, 9.1] | 7.5 [6.0, 9.1] |
| E/e′ mean ratio | 7.3 [6.2, 8.6] | 7.2 [6.2, 8.6] | 7.8 [6.4, 9.0] | 8.8 [7.0, 11.0] | 7.7 [6.6, 10.0] | 8.8 [7.3, 11.2] |
| E/A ratio | 0.9 [0.8, 1.2] | 0.9 [0.8, 1.2] | 0.8 [0.7, 1.0] | 0.7 [0.6, 0.9] | 0.8 [0.6, 1.1] | 0.9 [0.7, 1.2] |
| TR Vmax, m/s | 2.3 [2.2, 2.5] | 2.3 [2.2, 2.5] | 2.3 [2.2, 2.6] | 2.6 [2.3, 2.9] | 2.4 [2.2, 2.6] | 2.6 [2.3, 2.8] |
| TAPSE, mm | 24.0 [21.3, 27.0] | 24.2 [21.5, 27.0] | 22.7 [19.9, 25.1] | 20.8 [16.2, 23.8] | 22.6 [19.5, 25.9] | 22.8 [19.9, 26.0] |
| Diastolic dysfunction | 623 (9.8) | 323 (5.4) | 79 (100.0) | 37 (100.0) | 150 (100.0) | 34 (25.2) |
| AS, moderate/severe | 50 (0.8) | 36 (0.6) | 1 (1.6) | 3 (9.7) | 2 (1.5) | 8 (5.5) |
| AR, moderate/severe | 197 (2.9) | 173 (2.7) | 0 (0) | 5 (14.3) | 6 (4.2) | 13 (8.6) |
| MR, moderate/severe | 134 (2.0) | 97 (1.5) | 4 (5.6) | 6 (16.7) | 10 (7.0) | 17 (11.1) |
| TR, moderate/severe | 165 (2.6) | 136 (2.3) | 1 (1.6) | 2 (7.1) | 8 (6.2) | 18 (11.9) |
AR, aortic regurgitation; AS, aortic stenosis; AV, atrioventricular; LAH, left anterior haemiblock; LAVI, left atrial volume index; LBB, left bundle branch block; LDL, low‐density lipoprotein; LVEDD, left ventricular end‐diastolic diameter; LV, left ventricle; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; MR, mitral regurgitation; MS, mitral stenosis; RBBB, right bundle branch block; RV, right ventricle; TAPSE, tricuspid annular peak systolic excursion; TR, tricuspid regurgitation.
The extension of LVEF range from below 40% to below 41% for the HFrEF group resulted in a slight increase in HFrEF prevalence (five subjects were reclassified in the HFrEF group due LVEF between 40% and 41%). HFmrEF prevalence increased exclusively due to the inclusion of subjects without elevated NT‐proBNP (56 subjects showed an LVEF of 41–50% without NT‐proBNP levels >125 pg/mL). The decline of HFpEF prevalence was driven by several aspects that were changed in the 2021 ESC HF guidelines: (i) the redefinition of echocardiographic criteria for diastolic dysfunction (26 subjects met the 2016 but not the 2021 criteria for diastolic dysfunction); (ii) the new NT‐proBNP limit for subjects with AF (11 subjects with AF had NT‐proBNP levels <365 pg/mL); (iii) the raised LAVI threshold for subjects with AF (eight subjects suffered from AF but showed LAVI <40 mL/m2). Nevertheless, known risk factor profiles of the different phenotypes remained unchanged.
Eligibility for guideline‐recommended heart failure therapies
The new 2021 ESC HF guidelines recommend four key therapeutics drugs for all subjects with HFrEF: beta‐blockers, renin‐angiotensin system inhibitors (angiotensin‐converting enzyme inhibitors (ACEi); angiotensin receptor blockers (ARBs), and angiotensin‐receptor neprilysin inhibitors (ARNIs), mineralocorticoid receptor antagonists (MRAs), and sodium‐glucose‐co‐transporter 2 inhibitors (SGLT inhibitors). Furthermore, there are multiple additional treatment possibilities, reflecting a tailored therapeutic approach. In the consideration of recent data, we assumed an indication for SGLT2 inhibitors for HFmrEF. 9 , 10 Figure 5 highlights the dominant role of quadruple therapy in all subjects with HFrEF and HFmrEF (up to 54.7% eligibility of subjects within the HF population, 276 out of 10 000 subjects from the general population). Other treatment options such as digoxin, vericiguat or devices apply to selected cases demonstrated by an eligibility below 1%.
Figure 5.
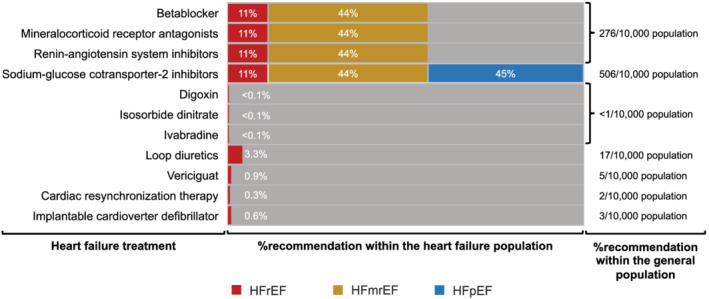
Percentage of recommendations for guideline directed therapy within the HF population and the general population. ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin‐receptor neprilysin inhibitor; BMI, body mass index; DBP, diastolic blood pressure; DBP, diastolic blood pressure, GFR, glomerular filtration rate; ICD, implantable cardioverter‐defibrillator; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; OSAS, obstructive sleep apnoea; SGLT2i, Sodium‐glucose‐co‐transporter 2 inhibitor; SBP, systolic blood pressure.
The application of guideline‐directed medical therapy (GDMT) within our study population reflects the guideline recommendations at the time of enrolment. Beta‐blocker therapy showed the highest prevalence in subjects with HFrEF (75.7%), followed by ACE inhibitors/ARBs (56.8%), and MRAs (21.6%). Only 2.7% of subjects with HFrEF were under therapy with ARNIs and SGLT2 inhibitors. Approximately half of all subjects with HFmrEF took beta‐blockers (53.1%) and/or ACE inhibitors/ARBs (53.8%). While 4.1% of the HFmrEF group took MRAs, none were under therapy with ARNIs or SGLT2 inhibitors.
Based on the recent publication of the positive EMPEROR‐PRESERVED trial, we also assumed an indication for SGLT2 inhibitors in subjects with HFpEF. 10 This would result in SGLT2 inhibitors being the first and only treatment pillar for HFpEF.
Discussion
The prevalence of heart failure remains high in a contemporary, middle‐aged population. The number of patients diagnosed with heart failure increases when contemporary diagnostic criteria are applied, reflecting a growing eligibility for evidence‐based therapies in patients with HFrEF and HFmrEF. 11
Heart failure is very common in the general population
The prevalence of HF according to the new ESC 2021 HF guidelines was 4.8%, highlighting the relevant disease burden in the middle‐aged general population. Estimates of HF prevalence derived from the ESC HF association (HFA) ATLAS study ranged from 1% to 4% with great heterogeneity across the participating countries as a result of not only diverging populations but also methodological differences. 12 However, HF prevalence is highly dependent on the age structure of the investigated population as HF prevalence increases with age. 1 , 2 , 13 Therefore, the higher HF prevalence in our cohort is likely due to an exclusion of those under the age of 45 years which is in line with other studies like the Rotterdam Heart Study, which showed a median HF prevalence of 6.4% in subjects aged above 55 years (mean age 74 years). 13 According to most studies, HFpEF and HFmrEF versus HFrEF seem to be equally distributed among all HF patients, although these data are mainly derived from hospitalized patients. 14 In our study however, the prevalence of both HFmrEF and HFpEF was almost four‐fold higher compared with HFrEF. These results, which are in line with recent findings in a primary care setting, might indicate a shift in prevalence from HFrEF to HFmrEF and HFpEF. 15 , 16 , 17 Importantly, the use of a standardized protocol including meticulous quality control for all echocardiography measurements in our study strongly supports this observation. Although there are strict guidelines on the measurement of LVEF, this parameter still has a high inter‐observer and even intra‐observer variability. 7 , 18 , 19 Therefore, estimates on the prevalence of HF phenotypes from studies without such a standardized protocol might be misleading, and especially “eye‐balling” of LVEF might skew the estimates. 20
Increase in heart failure prevalence compared with the 2016 European Society of Cardiology Heart Failure Guidelines
The application of the ESC 2021 HF guidelines on a population level resulted in a 12% net increase in HF prevalence compared with the 2016 ESC HF guidelines. This was mainly driven by a drastic increase of 54% of HFmrEF according to the new HF guidelines. HFmrEF is redefined as a phenotype closer to HFrEF than to HFpEF, which is underpinned by renaming HFmrEF to HF with mildly reduced EF instead of mid‐range EF. Redefining HFmrEF resulted in NT‐proBNP and echocardiographic abnormalities beyond a mildly reduced ejection no longer being considered as diagnostic markers for HFmrEF. The removal of elevated NT‐proBNP as a requirement for diagnosing HFmrEF drastically expands the HFmrEF group, offering new treatment options for this group of patients.
For diagnosing HFpEF, especially the role of NT‐proBNP was partly redefined in the new HF guidelines, acknowledging the existence of a HF subgroup without elevated natriuretic peptides on the one hand and demanding higher NT‐proBNP thresholds for patients with AF. 21 , 22 However, several echocardiographic criteria both in subjects with and without elevated natriuretic peptides need to be fulfilled for diagnosing HFpEF, representing a simplified approach based on the recently published HFA‐PEFF diagnostic algorithm. 23 Consequently, HFpEF was more strictly diagnosed according to the new HF guidelines compared with the 2016 HF guidelines, resulting in less cases and suggesting a more valid definition. For diagnosing HFpEF, especially the role of NT‐proBNP was partly redefined in the new HF guidelines, acknowledging the existence of a HF subgroup without elevated natriuretic peptides on the one hand and demanding higher NT‐proBNP thresholds for patients with AF. 21 , 22
Quadruple medication as a new cornerstone for heart failure therapy
The 2021 HF guidelines advocate the use of beta‐blockers, MRAs, renin‐angiotensin system inhibitors, and SGLT2 inhibitors in all subjects with HFrEF. Assuming an indication for HFmrEF, 9 , 10 this would translate into 276 out of 10 000 people in the middle‐aged general population being eligible for this quadruple therapy compared with 47 out of 10 000 people according to the 2016 guidelines. This massive rise in eligibility is owed to the fact that beta‐blockers, MRAs, and renin‐angiotensin system inhibitors are no longer recommended for HFrEF subjects alone but may also be considered for those with HFmrEF. 5 However, these guideline recommendations were not derived from focused randomized controlled trials in HFmrEF patients but were partly based on subgroup analyses of trials including patients with LVEF >40%.
SGLT2 inhibitors are not yet medically approved nor recommended for HFmrEF and HFpEF, but it can be expected that they will be recommended for both entities based on recent data: The recently published Empagliflozin in Heart Failure with a Preserved Ejection Fraction (EMPEROR Preserved) trial demonstrated a reduction of the primary endpoint cardiovascular death or hospitalization for heart failure in HFmrEF and HFpEF subjects treated with Empagliflozin. 10 Especially if the Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure (DELIVER) trial produces similar results for Dapagliflozin, the role for SGLT2 inhibitors in HFmrEF and HFpEF will be strenghtened. 9 , 24 This might almost double the number of patients eligible for SGLT2 inhibitors in the middle‐aged general population.
Aside from the aforementioned medical therapy, the current guidelines suggest several adjunctive therapies such as cardiac implantable devices or drugs such as ivabradine and vericiguat for selected scenarios. This further highlights the tailored treatment approach of the 2021 ESC HF guidelines, which allows for optimization based on the patient's clinical profile, providing multiple options to improve outcomes.
Limitations
Because most of the subjects in this study are of Caucasian ascend and as all live in the city of Hamburg, the applicability of our results to other populations might be limited. Nevertheless, it is still highly likely that out study cohort is well representative of the European/North European population. In this study we generated a diagnostic algorithm that reflects the 2021 ESC HF Guidelines as close as possible. Nevertheless, due to necessary standardizations and without individual assessment of heart failure suspicion in each subject by a medical professional, this algorithm might lead to over‐ or underdiagnosing heart failure. On top of structural changes and elevated NTproBNP, this study used prescription of guideline‐directed heart failure therapy to diagnose heart failure in asymptomatic patients. This allows for the inclusion of heart failure patients who are asymptomatic because of the prescribed guideline‐directed heart failure therapy. However, it might also lead to an overestimation of the heart failure prevalence, as these medications might have been prescribed for other diseases than heart failure. Additionally, some measures (e.g. dyspnoea) were not assessed by clinical tests but by self‐reported questionnaires, which might lead to inaccuracy in reporting. Also, there was no gold‐standard for diagnosing the different HF entities. Especially for HFpEF invasive measurements are recommended, which were not applied in our study; and adding those invasive measures would further refine the classification of the study subjects.
Conclusions
In this study, using the definition provided by the recently published 2021 ESC HF guidelines, the HF prevalence in the middle‐aged general population was high (4.8%). Compared with the 2016 ESC HF guidelines, HF prevalence increased by 12%. HFmrEF was redefined by the 2021 HF guidelines with a 54% increase in prevalence, whereas the proportion of subjects with HFpEF decreased suggesting a more valid definition. The rise in HF prevalence, especially for HFmrEF, offers new possibilities to initiate preventive treatments as the new guidelines propose quadruple therapy as well as several options for adjunctive therapies personalized to the patient, highlighting a tailored treatment approach.
Conflict of interest
JPW receives research funding by the German Heart Foundation under the grant agreement No F/29/19 unrelated to the submitted work. CM receives speaker fees from Astra Zeneca, Novartis, and Loewenstein medical unrelated to the submitted work. RBS has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program under the grant agreement No 648131, from the European Union's Horizon 2020 research and innovation program under the grant agreement No 847770 (AFFECT‐EU) and German Center for Cardiovascular Research (DZHK e.V.) (81Z1710103); German Ministry of Research and Education (BMBF 01ZX1408A) and ERACoSysMed3 (031L0239). RBS has received lecture fees and advisory board fees from BMS/Pfizer outside this work. DW received honorary from Abiomed, AstraZeneca, Bayer, Berlin‐Chemie and Novartis, outside of the submitted work. PK receives additional research support for basic, translational, and clinical research projects from European Union, British Heart Foundation, Leducq Foundation, Medical Research Council (UK), and German Centre for Cardiovascular Research, from several drug and device companies active in atrial fibrillation, and has received honoraria from several such companies in the past, but not in the last 3 years. PK is listed as inventor on two patents held by University of Birmingham (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783). SB reports honoraria from Abbott, Siemens, Thermo Fisher, and Roche, outside of the submitted work. BS received honoraria by AstraZeneca and Abiomed; and research funding by the German Research Foundation and the Else Kröner‐Fresenius Stiftung. The other authors report no conflict of interest.
Funding
The HCHS is supported by the Innovative Medicine Initiative (grant number 116074), by the Foundation Leducq (grant number 16 CVD 03), by the euCanSHare Grant Agreement (grant number 825903‐euCanSHare H2020), and by the Deutsche Forschungsgemeinschaft (grant number TH1106/5‐1; AA93/2‐1). Furthermore, it is supported by the participating institutes and departments from the University Medical Centre Hamburg‐Eppendorf, which contribute with individual and scaled budgets to the overall funding. Technical equipment is provided by SIEMENS according to a contract for 12 years, the Schiller AG on a loan basis for 6 years, and Topcon on a loan basis from 2017 until 2022. The Hamburg City Health Study is additionally supported by an unrestricted grant (2017 to 2022) by Bayer. Project‐related analyses are supported by Amgen, Astra Zeneca, BASF, Deutsche Gesetzliche Unfallversicherung (DGUV), Deutsches Krebsforschungszentrum (DKFZ), Deutsches Zentrum für Herz‐Kreislauf‐Forschung (DZHK), Deutsche Stiftung für Herzforschung, Novartis, Seefried Stiftung, and Unilever. The study is further supported by donations from the ‘Förderverein zur Förderung der HCHS e.V.’, TePe® (2014) and Boston Scientific (2016). A current list of the supporters is online available on www.uke.de/hchs. Sponsor funding has in no way influenced the content or management of this study.
Supporting information
Figure S1. Flow chart of the 2021 ESC HF classification. HxHF = history of heart failure; All other abbreviations as in Table 1.
Figure S2. Flow chart of the 2016 ESC HF classification. HxHF = history of heart failure; All other abbreviations as in Table 1.
Table S1. Reclassification table of HF prevalence using the 2021 ESC HF algorithm and the 2016 ESC HF algorithm. Subjects for whom at least one classification could not be determined (1220) were excluded. Abbreviations as in Table 1.
Acknowledgements
The authors acknowledge the participants of the Hamburg City Health Study, the staff at the Epidemiological Study Centre, cooperation partners, patrons and the Deanery from the University Medical Centre Hamburg. Open Access funding enabled and organized by Projekt DEAL.
Wenzel, J.‐P. , Nikorowitsch, J. , bei der Kellen, R. , Magnussen, C. , Bonin‐Schnabel, R. , Westermann, D. , Twerenbold, R. , Kirchhof, P. , Blankenberg, S. , and Schrage, B. (2022) Heart failure in the general population and impact of the 2021 European Society of Cardiology Heart Failure Guidelines. ESC Heart Failure, 9: 2157–2169. 10.1002/ehf2.13948.
References
- 1. Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, Allison M, Hemingway H, Cleland JG, McMurray JJV, Rahimi K. Temporal trends and patterns in heart failure incidence: A population‐based study of 4 million individuals. Lancet. 2018; 391: 572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Riet EES, Hoes AW, Wagenaar KP, Limburg A, Landman MAJ, Rutten FH. Epidemiology of heart failure: The prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail. 2016; 18: 242–252. [DOI] [PubMed] [Google Scholar]
- 3. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017; 3: 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després J‐P, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Das SR, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Heart disease and stroke Statistics‐2016 update: A report from the American Heart Association. Circulation. 2016; 133: 38–360. [DOI] [PubMed] [Google Scholar]
- 5. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, ESC Scientific Document Group , de Boer RA, Christian Schulze P, Abdelhamid M, Aboyans V, Adamopoulos S, Anker SD, Arbelo E, Asteggiano R, Bauersachs J, Bayes‐Genis A, Borger MA, Budts W, Cikes M, Damman K, Delgado V, Dendale P, Dilaveris P, Drexel H, Ezekowitz J, Falk V, Fauchier L, Filippatos G, Fraser A, Frey N, Gale CP, Gustafsson F, Harris J, Iung B, Janssens S, Jessup M, Konradi A, Kotecha D, Lambrinou E, Lancellotti P, Landmesser U, Leclercq C, Lewis BS, Leyva F, Linhart A, Løchen ML, Lund LH, Mancini D, Masip J, Milicic D, Mueller C, Nef H, Nielsen JC, Neubeck L, Noutsias M, Petersen SE, Sonia Petronio A, Ponikowski P, Prescott E, Rakisheva A, Richter DJ, Schlyakhto E, Seferovic P, Senni M, Sitges M, Sousa‐Uva M, Tocchetti CG, Touyz RM, Tschoepe C, Waltenberger J, Adamo M, Baumbach A, Böhm M, Burri H, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gardner RS, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Piepoli MF, Price S, Rosano GMC, Ruschitzka F, Skibelund AK. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 00: 1–128. [Google Scholar]
- 6. Jagodzinski A, Johansen C, Koch‐Gromus U, Aarabi G, Adam G, Anders S, Augustin M, der Kellen RB, Beikler T, Behrendt C‐A, Betz CS, Bokemeyer C, Borof K, Briken P, Busch C‐J, Büchel C, Brassen S, Debus ES, Eggers L, Fiehler J, Gallinat J, Gellißen S, Gerloff C, Girdauskas E, Gosau M, Graefen M, Härter M, Harth V, Heidemann C, Heydecke G, der Kellen RB, Huber TB, Hussein Y, Kampf MO, von dem Knesebeck O, Konnopka A, König HH, Kromer R, Kubisch C, Kühn S, Loges S, Löwe B, Lund G, Meyer C, Nagel L, Nienhaus A, Pantel K, Petersen E, Püschel K, Reichenspurner H, Sauter G, Scherer M, Scherschel K, Schiffner U, Schnabel RB, Schulz H, Smeets R, Sokalskis V, Spitzer MS, Terschüren C, Thederan I, Thoma T, Thomalla G, Waschki B, Wegscheider K, Wenzel JP, Wiese S, Zyriax BC, Zeller T, Blankenberg S. Rationale and Design of the Hamburg City Health Study. Eur J Epidemiol. 2020; 35: 169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015; 16: 233–271. [DOI] [PubMed] [Google Scholar]
- 8. Goldstein SA, Evangelista A, Abbara S, Arai A, Asch FM, Badano LP, Bolen MA, Connolly HM, Cuéllar‐Calàbria H, Czerny M, Devereux RB, Erbel RA, Fattori R, Isselbacher EM, Lindsay JM, McCulloch M, Michelena HI, Nienaber CA, Oh JK, Pepi M, Taylor AJ, Weinsaft JW, Zamorano JL, Dietz H, Eagle K, Elefteriades J, Jondeau G, Rousseau H, Schepens M. Multimodality Imaging of Diseases of the Thoracic Aorta in Adults: From the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr , Vol. 28; 2015. p 119–182. [DOI] [PubMed] [Google Scholar]
- 9. Ahmad Y, Madhavan MV, Stone GW, Francis D, Makkar R, Bhatt DL, Howard JP. Sodium‐glucose cotransporter 2 inhibitors in patients with heart failure: A systematic review and meta‐analysis of randomized trials. Eur Hear Journal Qual Care Clin Outcomes. 2021; 12: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner–La Rocca HP, Choi D‐J, Chopra V, Chuquiure‐Valenzuela E, Giannetti N, Gomez‐Mesa JE, Janssens S, Januzzi JL, Gonzalez‐Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Brunner–la Rocca HP, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021; 385: 1451–1461. [DOI] [PubMed] [Google Scholar]
- 11. Seferović PM, Vardas P, Jankowska EA, Maggioni AP, Timmis A, Milinković I, Polovina M, Gale CP, Lund LH, Lopatin Y, Lainscak M, Savarese G, Huculeci R, Kazakiewicz D, Coats AJS. The heart failure association Atlas: Heart failure epidemiology and management statistics 2019. Eur J Heart Fail. 2021; 23: 906–914. [DOI] [PubMed] [Google Scholar]
- 12. Seferović PM, Vardas P, Jankowska EA, Maggioni AP, Timmis A, Milinković I, Polovina M, Gale CP, Lund LH, Lopatin Y, Lainscak M, Savarese G, Huculeci R, Kazakiewicz D, Coats AJS, Berger R, Jahangirov T, Kurlianskaya A, Troisfontaines P, Droogne W, Hudic LD, Tokmakova M, Glavaš D, Barberis V, Spinar J, Wolsk E, Uuetoa T, Tolppanen H, Kipiani Z, Störk S, in collaboration with the National Heart Failure Societies of the ESC member countries (see Appendix) , Hudić LD, Bauersachs J, Keramida K, Parissis J, Habon T, Gotsman I, Weinstein JM, Ingimarsdottir IJ, Crowley J, Dalton B, Aspromonte N, Nodari S, Volterrani M, Rakisheva A, Mirrakhimov E, Kamzola G, Skouri H, Čelutkiene J, Jovanova S, Vataman E, Cobac IP, van Pol P, de Boer R, Lueder T, Straburzynska‐Migaj E, Moura B, Chioncel O, Fomin I, Begrambekova J, Lopatin Y, Mareev Y, Goncalvesova E, Seferović P, Milinković I, Lainščak M, Pinilla JMG, Lindmark K, Mueller C, Cavusoglu Y, Gardner R, Voronkov L. The heart failure association Atlas: Heart failure epidemiology and management statistics 2019. Eur J Heart Fail. 2021; 23: 906–914. [DOI] [PubMed] [Google Scholar]
- 13. Bleumink GS, Knetsch AM, Sturkenboom MCJM, Straus SMJM, Hofman A, Deckers JW, Witteman JCM, Stricker BHC. Quantifying the heart failure epidemic: Prevalence, incidence rate, lifetime risk and prognosis of heart failure the Rotterdam study. Eur Heart J. 2004; 25: 1614–1619. [DOI] [PubMed] [Google Scholar]
- 14. Koh AS, Tay WT, Teng THK, Vedin O, Benson L, Dahlstrom U, Savarese G, Lam CSP, Lund LH. A comprehensive population‐based characterization of heart failure with mid‐range ejection fraction. Eur J Heart Fail. 2017; 19: 1624–1634. [DOI] [PubMed] [Google Scholar]
- 15. van Riet EES, Hoes AW, Limburg A, Landman MAJ, van der Hoeven H, Rutten FH. Prevalence of unrecognized heart failure in older persons with shortness of breath on exertion. Eur J Heart Fail. 2014; 16: 772–777. [DOI] [PubMed] [Google Scholar]
- 16. Tsao CW, Lyass A, Enserro D, Larson MG, Ho JE, Kizer JR, Gottdiener JS, Psaty BM, Vasan RS. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Hear Fail. 2018; 6: 678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006; 355: 251–259. [DOI] [PubMed] [Google Scholar]
- 18. Hoffmann R, von Bardeleben S, Kasprzak JD, Borges AC, ten Cate F, Firschke C, Lafitte S, Al‐Saadi N, Kuntz‐Hehner S, Horstick G, Greis C, Engelhardt M, Vanoverschelde JL, Becher H. Analysis of regional left ventricular function by cineventriculography, cardiac magnetic resonance imaging, and unenhanced and contrast‐enhanced echocardiography: A multicenter comparison of methods. J Am Coll Cardiol. 2006; 47: 121–128. [DOI] [PubMed] [Google Scholar]
- 19. Hoffmann R, von Bardeleben S, Barletta G, Pasques A, Kasprzak J, Greis C, Becher H. Comparison of two‐ and three‐dimensional unenhanced and contrast‐enhanced echocardiographies versus cineventriculography versus cardiac magnetic resonance for determination of left ventricular function. Am J Cardiol. 2014; 113: 395–401. [DOI] [PubMed] [Google Scholar]
- 20. Cole GD, Dhutia NM, Shun‐Shin MJ, Willson K, Harrison J, Raphael CE, Zolgharni M, Mayet J, Francis DP. Defining the real‐world reproducibility of visual grading of left ventricular function and visual estimation of left ventricular ejection fraction: Impact of image quality, experience and accreditation. Int J Cardiovasc Imaging. 2015; 31: 1303–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Clerico A, Zaninotto M, Passino C, Plebani M. Obese phenotype and natriuretic peptides in patients with heart failure with preserved ejection fraction. Clin Chem Lab Med. 2018; 56: 1015–1025. [DOI] [PubMed] [Google Scholar]
- 22. Kristensen SL, Mogensen UM, Jhund PS, Rørth R, Anand IS, Carson PE, Desai AS, Pitt B, Pfeffer MA, Solomon SD, Zile MR, Køber L, Mcmurray JJV. N‐terminal pro‐B‐type natriuretic peptide levels for risk prediction in patients with heart failure and preserved ejection fraction according to atrial fibrillation status. Circ Heart Fail. 2019; 12: 1–10. [DOI] [PubMed] [Google Scholar]
- 23. Pieske B, Tschope C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, Lancellotti P, Melenovsky V, Morris DA, Nagel E, Pieske‐Kraigher E, Ponikowski P, Solomon SD, Vasan RS, Rutten FH, Voors AA, Ruschitzka F, Paulus WJ, Seferovic P, Filippatos G. How to diagnose heart failure with preserved ejection fraction: The HFA‐PEFF diagnostic algorithm: A consensus recommendation from the heart failure association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019; 40: 3297–3317. [DOI] [PubMed] [Google Scholar]
- 24. Solomon SD, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Lindholm D, Wilderäng U, Öhrn F, Claggett B, Langkilde AM, Petersson M, McMurray JJV. Dapagliflozin in heart failure with preserved and mildly reduced ejection fraction: Rationale and design of the DELIVER trial. Eur J Heart Fail. 2021; 23: 1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez‐Juanatey JR, Harjola V‐P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution. Eur J Heart Fail. 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 26. Nagueh SFF, Smiseth OAA, Appleton CPP, Byrd BFF, Dokainish H, Edvardsen T, Flachskampf FAA, Gillebert TCC, Klein ALL, Lancellotti P, Marino P, Oh JKK, Popescu BAA, Waggoner ADD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016; 17: 1321–1360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow chart of the 2021 ESC HF classification. HxHF = history of heart failure; All other abbreviations as in Table 1.
Figure S2. Flow chart of the 2016 ESC HF classification. HxHF = history of heart failure; All other abbreviations as in Table 1.
Table S1. Reclassification table of HF prevalence using the 2021 ESC HF algorithm and the 2016 ESC HF algorithm. Subjects for whom at least one classification could not be determined (1220) were excluded. Abbreviations as in Table 1.


